Abstract
We have previously reported that a burst of vascular endothelial growth factor (VEGF) signaling to tumor-associated endothelium induces a proviral state, during which systemically delivered oncolytic reovirus can replicate in endothelium, thereby inducing immune-mediated vascular collapse and significant antitumor therapy. Using chimeric receptors, we show here that induction of the proviral state proceeds through VEGFR2, but not VEGFR1, signaling in endothelial cells. In contrast, innate immune activation by reovirus-exposed endothelial cells was predominantly through VEGFR1. By screening conventional chemotherapies for their ability to induce similar effects in combination with reovirus both in vitro and in vivo, we observed that the proviral state could also be induced in endothelial cells exposed to VEGF during rebound from paclitaxel-mediated inhibition of VEGF signaling. We translated these in vitro findings in vivo by careful scheduling of paclitaxel chemotherapy with systemic virotherapy, neither of which alone had therapeutic effects against B16 tumors. Systemic availability of reovirus during endothelial cell recovery from paclitaxel treatment allowed for endothelial replication of the virus, immune-mediated therapy, and tumor cures. Therefore, careful scheduling of combination viro- and chemotherapies, which preclinical testing suggests are individually ineffective against tumor cells, can lead to rational new clinical protocols for systemic treatments with oncolytic viruses.
Introduction
The development of oncolytic viruses for cancer therapy was based upon the assumption that they will initiate spreading intratumoral infections following in vivo delivery, even if the efficiency of delivery is initially low.1,2,3 The resultant viral replication and oncolysis would then lead to tumor destruction. In addition to these direct oncolytic effects, it is now clear that both innate, and adaptive, host immune responses against a potently immunogenic virus, as well as against tumor antigens released as a result of viral killing of tumor cells, can contribute significantly to antitumor effects in vivo.4,5,6,7
Reovirus (respiratory enteric orphan virus) displays innate oncolytic activity against a wide range of human and murine tumor cells.8,9 This is, in part at least, because oncogene-mediated perturbation of the double-stranded RNA-activated protein kinase-mediated antiviral response allows for progressive viral replication and cytolysis of the host cell in malignant, as opposed to normal, cells.10,11,12 In addition, we have shown that antitumor therapy in preclinical models is also directly associated with immune activation by virus replication in tumors.13,14,15 As a result of these encouraging preclinical studies, we, and others, have already completed early phase clinical studies of reovirus, both as a single agent, and in combination with other treatment modalities, in patients with advanced cancer, with evidence of significant antitumor activity.8,16,17,18,19
A major challenge for clinical development of oncolytic viruses remains the ability to deliver them systemically, in the presence of an intact immune system, to metastatic tumors.1,20,21,22,23,24,25 In this respect, the tumor vasculature represents both a potential target for antitumor therapies, as well as a major barrier to systemic virotherapy.26,27 Many human tumors overexpress vascular endothelial growth factor (VEGF), particularly VEGF165, to support their own growth.28,29 VEGF has multiple effects on tumor vasculature, through binding to a series of receptors, including two major tyrosine kinase receptors, VEGF receptor-1 (VEGFR1; Flt-1) and VEGFR2 (Flk-1, KDR) expressed by endothelial cells.29,30 These effects include increasing vasodilation, permeabilization, and vascular disorganization and, of particular relevance to tumors, angiogenesis, through binding to vasculature-associated VEGFR2.26,29
We have previously shown that a pulse of VEGF signaling conditions tumor-associated endothelium for productive replication of reovirus.31 This proviral state, characterized by a transient, quasi-transformed phenotype induced by VEGF signaling following a period of VEGF deprivation,31 allowed reovirus to replicate in VEGF stimulated endothelial cells, and rendered the infected endothelial cells susceptible to attack in vivo by innate immune effectors, particularly natural killer (NK) cells.31 These factors combined to generate tumor regressions, mediated through vascular collapse induced by both virus replication and innate immune attack on virus-infected vasculature.31 We also showed that transient inhibition of continuous VEGF signaling to tumor-associated endothelium, followed by a recovery period in which VEGF from the tumor accumulated and signaled to the endothelial cells, could mimic the effects of a VEGF burst on inducing the proviral state.31 Thus, B16-VEGF tumors treated in vivo with either Sunitinib or Avastin became highly susceptible to systemic treatment with reovirus, but only if the VEGF inhibitors were withdrawn 24–48 hours before virus delivery. We showed that removal of the VEGF inhibitor initiated a rebound phase in endothelial cells, during which time tumor-derived VEGF induced the proviral state, which could subsequently be exploited by treatment with systemically delivered reovirus.31
Here, we show that the VEGFR1- and VEGFR2-dependent endothelial conditioning effects of a VEGF burst could be mimicked by transient pretreatment with paclitaxel,32 followed by exposure to reovirus during the rebound period in which paclitaxel was absent but VEGF was supplied by the tumor. These in vitro findings were used to develop a combination treatment in vivo, in which a proportion of VEGF-producing tumors were cured by carefully timed treatment with both paclitaxel and intravenous reovirus. These data will allow us to refine the design of our early phase trials using combinations of systemic reovirus with clinically approved chemotherapy19,33 to generate rational, mechanism-driven clinical protocols.
Results
Reovirus replication in endothelial cells is mediated through VEGFR2
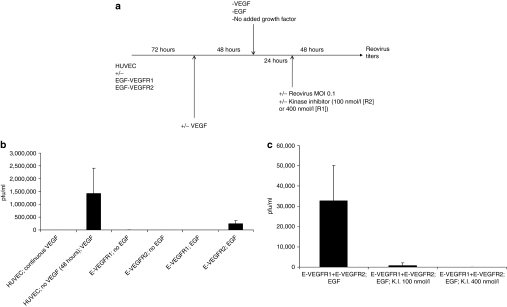
Human umbilical vein endothelial cells (HUVEC) were transduced with chimeric receptors in which the extracellular endothelial growth factor (EGF)-binding domain of the EGF receptor was fused to the transmembrane and intracellular signaling domain of either VEGFR1 or VEGFR2.34,35 Consistent with our previous findings, HUVEC growing in normal growth medium (containing VEGF) did not support reovirus replication (Figure 1a,b, column 1).31 However, removal of VEGF for 48 hours, followed by its reinstatement and viral infection, induced a proviral state resulting in reovirus replication (Figure 1a,b, column 2).31 When signaling was provided through either VEGFR1 or VEGFR2 by EGF, reovirus replication was only supported by treatment of HUVEC/EGF-VEGFR2 cells with both EGF and virus (Figure 1b, columns 5 and 6) (P < 0.001). Differences in the levels of reovirus replication between HUVEC treated with VEGF (Figure 1b, column 2), and HUVEC/EGF-VEGFR2 (Figure 1b, column 6), are most likely due to the different levels of surface expression of the endogenous VEGFR2, and transduced EGF-VEGFR2 chimeric receptor, respectively.
Figure 1.
Signaling through VEGFR2 induces a proviral state for reovirus replication. (a) Human umbilical vein endothelial cell (HUVEC) cells, transduced with either the EGF-VEGFR1 or EGF-VEGFR2 chimeric receptors, as described in Materials and Methods section, were treated as shown. Briefly, 72 hours following transduction, cells were washed and replated in medium containing, or lacking (“VEGF deprivation”), VEGF. Forty-eight hours later, the media were changed again and cells were either kept in the absence of VEGF, or were exposed either to VEGF165 (6 ng/ml) or to EGF (10 ng/ml) (“VEGF/EGF burst”). Twenty-four hours later, cells were exposed to reovirus either in the continued presence, or absence, of VEGF or endothelial growth factor (EGF). Forty-eight hours later, viral titers were determined by plaque assay. (b) The protocol of a above was carried out using (i) HUVEC cells grown continually in VEGF; (ii), HUVEC deprived of VEGF followed by a VEGF burst; (iii), HUVEC/EGF-VEGFR1 grown continually in the absence of VEGF but with no EGF burst,50 HUVEC/EGF-VEGFR2 grown continually in the absence of VEGF but with no EGF burst; (iv), HUVEC/EGF-VEGFR1 grown continually in the absence of VEGF and treated with an EGF burst; (v), HUVEC/EGF-VEGFR2 grown continually in the absence of VEGF and treated with an EGF burst. Titers of reovirus released following each experimental set of conditions, in triplicate wells, are shown. (c) The protocol of a above was repeated using HUVEC cells transduced with both the EGF-VEGFR1 and EGF-VEGFR2 chimeric receptors (at the same levels as used individually in b) and treated with VEGF deprivation followed by EGF burst in the absence of any kinase inhibitor (i); in the presence of kinase inhibitor at 100 nmol/l which selectively inhibits VEGFR2 (ii); or in the presence of kinase inhibitor at 400 nmol/l which inhibits both VEGFR1 and VEGFR2 signaling (iii). Titers of reovirus released following each experimental set of conditions, in triplicate wells, are shown.
In the experiment of Figure 1b,c, HUVEC expressing both EGF-VEGFR1 and EGF-VEGFR2 supported replication of reovirus upon exposure to EGF (Figure 1c, column 1, 3.27 × 104 plaque-forming unit/ml ± 1.2 × 104), but at significantly lower levels than from HUVEC transduced with similar levels of EGF-VEGFR2 alone (Figure 1b, column 6, 2.47 x 105 plaque-forming unit/ml ± 1.8 × 104). In addition, productive infection was significantly reduced by a kinase inhibitor at concentrations (100 nmol/l) which preferentially inhibit VEGFR2 signaling (Figure 1c, column 2).36
NK cell activation by reovirus-exposed endothelial cells is mediated through VEGFR1
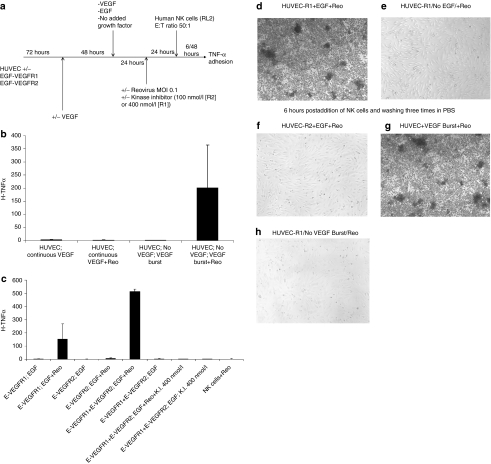
We reported that destruction of reovirus-infected endothelial cells contributes to vascular collapse and tumor regressions in vivo.31 Consistent with this, HUVEC did not activate NK cells (Figure 2a) either in the absence of reovirus (Figure 2b, column 1), following exposure to reovirus (Figure 2b, column 2), or following a VEGF burst (Figure 2b, column 3). However, HUVEC infected with reovirus following a VEGF burst stimulated secretion of tumor necrosis factor-α (TNF-α) from cocultured NK cells (Figure 2b, column 4) (P = 0.02 compared to all three other treatments).
Figure 2.
Signaling through VEGFR1 combines with reovirus infection to activate natural killer (NK) cells. (a) The protocol of Figure 1a was repeated with the addition of RL2 human NK cells 24 hours following infection of cultures with reovirus. (b) Forty-eight hours following the addition of NK cells, supernatants were assayed for tumor necrosis factor-α (TNF-α) by enzyme-linked immunosorbent assay (ELISA) from: (i) human umbilical vein endothelial cell (HUVEC) grown continually in vascular endothelial growth factor (VEGF)-containing medium with no reovirus infection; (ii) HUVEC grown continually in VEGF-containing medium with reovirus infection; (iii) HUVEC deprived of VEGF (48 hours) followed by VEGF burst but no reovirus; (iv) HUVEC deprived of VEGF (48 hours) followed by VEGF burst and reovirus infection. Levels of TNF-α released into the culture supernatants are shown from triplicate wells per treatment. (c) Forty-eight hours following the addition of NK cells, supernatants were assayed for TNF-α by ELISA from: (i) HUVEC/EGF-VEGFR1 cells grown in the absence of VEGF, given an EGF burst but no reovirus infection; (ii) HUVEC/EGF-VEGFR1 cells grown in the absence of VEGF, given an EGF burst and subsequent reovirus infection; (iii) HUVEC/EGF-VEGFR2 cells grown in the absence of VEGF, given an EGF burst but no reovirus infection; (iv) HUVEC/EGF-VEGFR2 cells grown in the absence of VEGF, given an EGF burst and subsequent reovirus infection; (v) HUVEC transduced with both the EGF-VEGFR1 and EGF-VEGFR2 chimeric receptors (at the same levels as used individually) grown in the absence of VEGF, given an EGF burst and subsequent reovirus infection; (vi), HUVEC transduced with both the EGF-VEGFR1 and EGF-VEGFR2 chimeric receptors grown in the absence of VEGF, given an EGF burst but no reovirus infection; (vii), HUVEC-EGF-VEGFR1+EGF-VEGFR2 cells grown in the absence of VEGF, given an EGF burst and subsequent reovirus infection in the presence of kinase inhibitor at 400 nmol/l which inhibits both VEGFR1 and VEGFR2; (viii), HUVEC-EGF-VEGFR1+EGF-VEGFR2 cells grown in the absence of VEGF, given an EGF burst but no reovirus infection and kinase inhibitor at 400 nmol/l; IX, RL2 natural killer (NK) cells exposed to reovirus (no HUVEC) for 48 hours. Levels of TNF-α released into the culture supernatants are shown from triplicate wells per treatment. (d–h) Six hours after addition of RL2 human NK cells, which appear as small dark spherical cells, HUVEC cultures, which appear as elongated opaque adherent cells, were washed three times with phosphate-buffered saline (PBS) to remove nonadherent NK cells and wells photographed. (d/e) HUVEC/EGF-VEGFR1 cells grown in the absence of VEGF, given an (d) EGF burst or no (e) EGF and subsequent reovirus infection. (f) HUVEC/EGF-VEGFR2 cells grown in the absence of VEGF, given an EGF burst and subsequent reovirus infection. (g/h) HUVEC grown in the absence of VEGF, given a (g) VEGF burst or no (h) VEGF and subsequent reovirus infection.
Even though HUVEC/EGF-VEGFR1 cells did not support reovirus replication (Figure 1), they activated TNF-α secretion from NK cells upon EGF treatment and exposure to reovirus (Figure 2c, column 2). In contrast, HUVEC/EGF-VEGFR2 cells were unable to induce TNF-α secretion, either following EGF alone or with an EGF burst and exposure to reovirus (Figure 2c, columns 3 and 4). HUVEC/EGF-VEGFR1+EGF-VEGFR2 (which supported replication of reovirus with EGF in Figure 1) treated with an EGF burst and reovirus, activated NK cells (Figure 2c, column 5), and at significantly higher levels than HUVEC expressing similar levels of EGF-VEGFR1 alone (Figure 2c, columns 2 and 5) (P < 0.002). NK cell activation by HUVEC/EGF-VEGFR1+EGF-VEGFR2 was almost completely abolished by kinase inhibition at concentrations which inhibit VEGFR1 signaling (Figure 2c, columns 5 and 7) (P = 0.0001). In contrast to the results of Figure 2c, secretion of TNF-α by NK cells in response to EGF treatment of HUVEC transduced by the EGF-VEGFR1 chimeric receptor was abolished if the NK cells were separated from the EGF-stimulated HUVEC-E-VEGFR1 cells by a transwell. Similarly, NK cells could not be induced to secrete TNF-α by HUVEC treated by a VEGF burst unless they were directly cocultured with the HUVEC (Figure 2b) rather than separated by a transwell. These data suggest that NK cell activation by VEGFR1 signaling requires direct cell–cell contact between endothelial cells and NK cells—but they do not rule out the involvement of VEGFR1-induced, endothelial derived cytokines, or other factors, which may mediate direct NK cell activation upon cell–cell contact.
Expression of EGF/VEGFR1 also induced rapid adhesion of NK cells to reovirus-exposed HUVEC in the presence of EGF (Figure 2d). Neither EGF-VEGFR1 in the presence of virus, but absence of EGF (Figure 2e), nor in the presence of activated VEGFR1 but absence of virus (data not shown), induced NK adhesion to HUVEC. Expression of EGF-VEGFR2 did not activate NK cell adhesion to HUVEC, even with both EGF signaling and reovirus (Figure 2f). Activation of NK cell adhesion by a burst of VEGFR1 signaling in the presence of reovirus (Figure 2d) was very similar to that observed by treating untransduced HUVEC with VEGF burst and exposure to reovirus (Figure 2g), dependent upon both VEGF signaling (Figure 2h) and virus (data not shown).
Crosstalk between VEGFR1 and VEGFR2
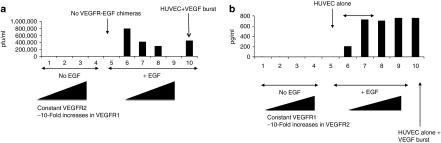
Although HUVEC expressing only EGF-VEGFR2 supported reovirus replication following a burst of EGF (Figure 3a, column 6), cotransduction with increasing levels of EGF/VEGFR1 progressively inhibited reovirus replication (Figure 3a, columns 6–9), confirming that VEGFR1 signaling inhibited the proviral state induced by VEGR2 (Figure 1c). In contrast, HUVEC-EGF/VEGFR1 treated with both EGF burst and reovirus activated NK cells significantly more effectively when cotransduced with EGF/VEGFR2 up to a threshold level of EGF/VEGFR2 expression (Figure 3b, columns 6–9).
Figure 3.
Crosstalk between VEGFR1 and VEGR2 signaling in human umbilical vein endothelial cell (HUVEC). (a) HUVEC/EGF-VEGFR2 cells were grown in the absence of vascular endothelial growth factor (VEGF), with no endothelial growth factor (EGF) burst (columns 1–4), or with EGF burst (columns 5–10), and subsequent reovirus infection. The cells transduced with EGF-VEGFR2 were either mock infected (columns 1 and 6) or were infected with 10-fold increasing levels of EGF-VEGFR1 virus at levels of 1 µl (columns 2 and 7), 10 µl (columns 3 and 8) or 100 µl (columns 4 and 9) of virus supernatants. Column 5, HUVEC alone, not transduced with EGF-VEGFR1 or 2 chimeric receptors, grown in the absence of VEGF; column 10, HUVEC alone deprived of VEGF for 48 hours then given a VEGF burst and subsequent reovirus infection. Reoviral titers shown are the mean of duplicate wells per treatment and the data are representative of four different experiments. (b) HUVEC/EGF-VEGFR1 cells were grown in the absence of VEGF, with no EGF burst (columns 1–4), or with EGF burst (columns 5–10), subsequent reovirus infection and addition of RL2 NK cells as described in Figure 2a. HUVEC/EGF-VEGFR1 cultures were either mock infected (columns 1 and 6) or were infected with 10-fold increasing levels of EGF-VEGFR2 virus at levels of 1 µl (columns 2 and 7), 10 µl (columns 3 and 8) or 100 µl (columns 4 and 9) of virus supernatants. Column 5, HUVEC alone, not transduced with EGF-VEGFR1 or 2 chimeric receptors, grown in the absence of VEGF. Column 10, HUVEC alone deprived of VEGF for 48 hours then given a VEGF burst and subsequent reovirus/NK treatment. Reoviral titers shown are the mean of duplicate wells per treatment and the data are representative of three different experiments.
Rebound from paclitaxel, with VEGF, induces a proviral state
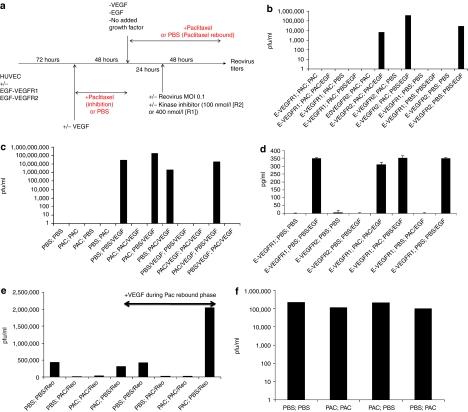
While testing modulation of VEGFR1/2 signaling by clinically relevant chemotherapies, we observed that, when VEGFR2 signaling was induced during a period of rebound from prior paclitaxel treatment, HUVEC/EGF-VEGFR2 cells became permissive for reovirus replication (Figure 4a,b, column 8) in a similar manner to that induced by VEGF burst (Figure 4c, column 5). However, this required VEGFR2 signaling (Figure 4b, column 7). Continuous paclitaxel treatment both before, and during, a burst of VEGFR2 signaling did not abolish, but did significantly inhibit, reovirus replication (Figure 4b, column 6). In contrast, HUVEC/EGF-VEGFR1 cells did not support reovirus replication either with, or without, rebound from paclitaxel and/or VEGFR1 signaling (Figure 4b, columns 1–4). Interestingly, reovirus replication in HUVEC/EGF-VEGFR2 cells following rebound from paclitaxel (Figure 4b, column 8) was consistently up to 1 log greater than that induced by EGF burst treatment of HUVEC/EGF-VEGFR2 cells alone without prior paclitaxel (Figure 4b, column 12).
Figure 4.
Rebound from paclitaxel-mediated inhibition of VEGFR2 signaling establishes a proviral state. (a) The protocol of Figure 1a was modified to include a period of paclitaxel inhibition for 48 hours before a period of rebound from paclitaxel treatment as shown. (b) HUVEC/EGF-VEGFR1 cells (columns 1–4) or HUVEC/EGF-VEGFR2 cells (columns 5–8) were treated for 48 hours with paclitaxel inhibition followed by continued paclitaxel, no EGF burst and reovirus infection (columns 1 and 5) or with continued paclitaxel, EGF burst, and reovirus infection (columns 2 and 6). Alternatively, the cells were treated for 48 hours with paclitaxel inhibition followed by phosphate-buffered saline (PBS) (paclitaxel rebound) no EGF burst and reovirus infection (columns 3 and 7) or with PBS (paclitaxel rebound), EGF burst and reovirus infection (columns 4 and 8). HUVEC/EGF-VEGFR1 (columns 9 and 10) or HUVEC/EGF-VEGFR2 (columns 11 and 12) cells were also grown in the absence of paclitaxel and given either no EGF burst (columns 9 and 11) or EGF burst (columns 10 and 12) along with reovirus infection. Reovirus titers shown are the means of duplicate wells per treatment and the data are representative of four separate experiments. (c) HUVEC (not transduced with chimeric receptors) were grown in the absence of VEGF either without (columns 1 and 4) or with (columns 2 and 3) 48 hours of paclitaxel inhibition, followed by removal of paclitaxel (paclitaxel rebound) (columns 1 and 3) or added paclitaxel (columns 2 and 4), followed by infection of reovirus as shown. Additional cultures of HUVEC were treated similarly but with the addition of a VEGF burst during the paclitaxel rebound phase (columns 5–8) or in the continuous presence of VEGF through both the first period of paclitaxel inhibition and the subsequent rebound phase (columns 9–12). Reovirus titers shown are the means of duplicate wells per treatment and the data are representative of two separate experiments. (d) The protocol of Figure 4a was modified with the addition of RL2 NK cells 24 hours following infection with reovirus as described in Figure 2a. Forty-eight hours following NK cell addition, supernatants were assayed for tumor necrosis factor-α (TNF-α) from HUVEC/EGF-VEGFR1 cells (1 and 2) or HUVEC/EGF-VEGFR2 cells (3 and 4) grown continuously in the absence of VEGF with no paclitaxel and either no EGF burst (1 and 3) or an EGF burst (2 and 4). In addition, HUVEC/EGF-VEGFR1 cells were treated either with a 48-hour period of paclitaxel inhibition (5 and 6) or without paclitaxel inhibition (7 and 8), followed by an EGF burst in the presence of paclitaxel (5 and 7), or an EGF burst without paclitaxel (paclitaxel rebound) (6 and 8). Levels of TNF-α released into the culture supernatants are shown from triplicate wells per treatment and are representative of two different experiments. (e) 2H11 murine endothelial cells were grown in the absence of VEGF and used in the protocol of Figure 4a and treated as shown with either PBS (1 and 2) or paclitaxel (3 and 4) during the paclitaxel inhibition phase, followed by either PBS (1 and 4) or paclitaxel (2 and 3) during the paclitaxel rebound phase. These treatments were repeated in the presence of a VEGF burst during the paclitaxel rebound phase (5–8). Reovirus titers are the means of duplicate wells per treatment and are representative of two separate experiments. (f) 104 B16 tumor cells were cultured in the protocol of Figure 4a (no HUVEC) in the absence of paclitaxel (i), in continuous paclitaxel (ii), for a 48-hour period of paclitaxel inhibition followed by its removal (paclitaxel rebound) (iii) or with no paclitaxel for 48 hours followed by paclitaxel.50 Forty-eight hours after infection with reovirus, viral titers were determined (values shown are means of duplicate wells). Results are representative of two different experiments.
VEGF burst induced reovirus replication in HUVEC (Figure 4c, column 5) was completely inhibited by continuous paclitaxel or, incompletely, by paclitaxel during the VEGF burst (Figure 4c, columns 6 and 8). However, HUVEC recovering from paclitaxel, exposed to reovirus + VEGF, supported reovirus replication at levels up to 1 log higher than with VEGF burst alone (Figure 4c, columns 5 and 7). As before, if VEGF was present throughout the culture, no proviral state was induced (Figure 4c, column 9). However, paclitaxel conditioned VEGFR signaling sufficiently such that HUVEC rebounding from paclitaxel still acquired reduced permissivity for reovirus replication with VEGF signaling continuously present (Figure 4c, columns 11 and 7).
VEGFR1-mediated signaling of NK cell activation by reovirus was neither significantly inhibited by continuous paclitaxel, nor enhanced in cells rebounding from paclitaxel (Figure 4d, columns 5–8).
Before translating these findings into a murine in vivo model of tumor therapy, we confirmed that murine endothelial cells had similar responses. 2H11 murine endothelial cells supported modest levels of reovirus replication without a VEGF burst, consistent with the fact that they are fully transformed37 (Figure 4e, column 1). However, paclitaxel almost completely inhibited reovirus replication, when present continuously or just during virus exposure (Figure 4e, columns 2 and 3) but had little effect if it was withdrawn just before virus exposure in the absence of exogenous VEGF signaling (Figure 5a, column 4). In contrast, 2H11 cells exposed to reovirus during rebound from prior paclitaxel, in the presence of a VEGF burst, produced between four- to fivefold more virus than with VEGF burst alone (Figure 4e, columns 5 and 8) or paclitaxel rebound alone (Figure 4e, columns 4 and 8).
Figure 5.
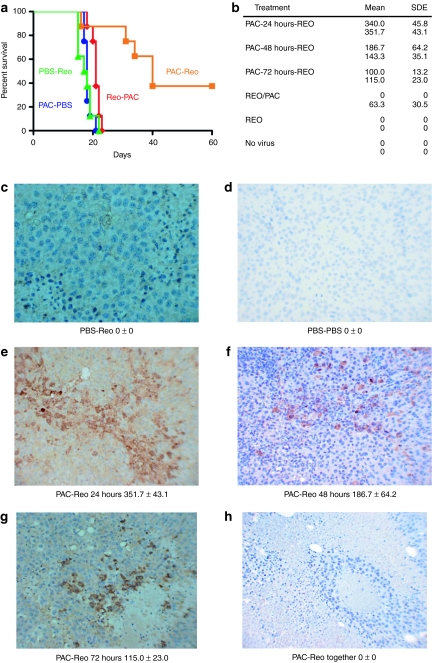
Careful scheduling of paclitaxel chemotherapy and oncolytic virotherapy leads to significant tumor therapy. (a) C57BL/6 mice (7/8 per group) seeded 7 days previously with B16-VEGF tumors were treated intraperitoneally with an injection of either phosphate-buffered saline (PBS) (PBS-Reo) or paclitaxel (Pac-PBS; Pac-Reo) for three consecutive days followed by intravenous injections of either reovirus (PBS-Reo; Pac-Reo) or PBS (Pac-PBS) for the following two consecutive days. A fourth group received two daily injections of Reovirus followed by three daily injections of paclitaxel (Reo-Pac). This cycle of five injections, with 2 days rest, was repeated three times, so that the whole treatment schedule was completed in 3 weeks. Survival with time (tumors reaching a diameter of >1.0 cm in any diameter) is shown. Results are representative of three different experiments. (b) Correlation of in vivo transduction of B16 tumors by systemically delivered reovirus with paclitaxel treatment. C57BL/6 mice bearing 14 day established B16 tumors were given daily injections of paclitaxel followed 24 hours, 48 hours or 72 hours later by a single intravenous injection of reovirus. Alternatively tumor-bearing mice were treated with reovirus and paclitaxel on the same days, with just reovirus or with no virus. Forty-eight hours following the injection of virus, tumors were excised and prepared for immunohistochemistry. Data shown are the mean number of viral infected cells/1 cm area (n = 3) and the s.d. of the mean. *Mean number of viral infected cancer cells per 1 cm area; **Standard deviation of the mean. Representative results from two mice per group (n = 3–5) are shown. (c–h) In separate experiments, C57BL/6 mice bearing 14 day established B16 tumors were given daily injections of PBS followed 24 hours later by a single injection of (c) reovirus or (d) PBS; or mice were given daily injections of paclitaxel followed (e) 24 hours, (f) 48 hours, or (g) 72 hours later by a single intravenous injection of reovirus. (h) B16 tumors from mice treated with daily injections of paclitaxel and reovirus. Forty-eight hours following the injection of virus, tumors were excised and prepared for immunohistochemistry. Virally infected cells are brown while negative tumor cells are blue. Cancer cells infected with reovirus were very commonly found around necrotic/degenerated cancer cells.
Finally, paclitaxel treatment of B16 tumor cells had no significant impact on reovirus replication in vitro, either when it was continuously present, or when it was withdrawn before exposure to virus (Figure 4f).
Chemotherapy and systemic reovirus cures established tumors
Therefore, we hypothesized that it would be possible to use these effects to prime tumor endothelium in vivo for reovirus replication leading to vascular destruction and antitumor therapy.
In mice bearing B16-VEGF tumors, neither intravenous reovirus, nor paclitaxel chemotherapy alone, had any therapeutic effect (Figure 5a). In addition, coadministration of reovirus and paclitaxel for three consecutive days (data not shown) or treatment with reovirus followed by paclitaxel were completely ineffective (Figure 5a). In contrast, sequential treatment with paclitaxel for three consecutive days, followed by two daily intravenous injections of reovirus, with this cycle repeated for 3 weeks, induced highly significant (P < 0.02, PAC-Reo compared to all other groups) improvements in survival (Figure 5a). In over three separate experiments, treatment with this paclitaxel/Reo regimen cured 48% (10 of 21) of treated mice, which was significantly better (P < 0.01) than treatment with either reovirus alone (0%, [0/20) long-term cures], or paclitaxel alone [5% (1/20) long-term cures]. If the last paclitaxel treatment was separated from the first reovirus treatment by 48 hours (instead of 24 hours), although there was still a trend to improved survival in two experiments, this did not reach significance (data not shown). Further temporal separation of paclitaxel withdrawal from reovirus treatment by > 48 hours led to a complete loss of therapy.
Consistent with Figure 5a, no virus could be detected histologically in tumors from mice treated with intravenous injections alone (Figure 5b–d). In contrast, highest levels of virus were detected in tumors of mice treated with the optimal paclitaxel/Reo regimen that generated therapy in Figure 5a (Figure 5b,e–g). No virus was detected in tumors when virus and paclitaxel were delivered simultaneously (Figure 5b,h).
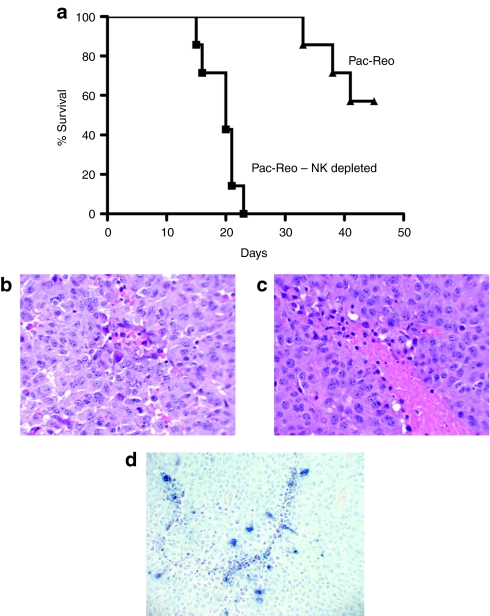
Consistent with our studies using VEGF burst to enhance reovirus therapy of B16 tumors,31 antitumor therapy induced by paclitaxel and systemic reovirus was also dependent upon NK cells (P < 0.001 for NK depleted compared to nondepleted groups) (Figure 6a). In a single additional experiment, pretreatment of tumor-bearing mice with a control, nondepleting immunoglobulin G isotype, followed by the optimal paclitaxel/Reo regimen, cured 75% (6/8 mice) of the animals. However, depletion of neither CD4+, nor of CD8+, T cells significantly inhibited this therapy, with long-term (>60 days post-tumor seeding) cures of 71% (5/7 mice) or 86% (6/7 mice), respectively. Consistent with an immune mediated mechanism of vascular collapse, virus proliferation, and tumor degeneration showed a striking perivascular distribution (Figure 5c–h). In addition, hematoxylin and eosin staining showed perivascular degenerative changes, associated with immune infiltrates, in tumors from mice treated with the optimal paclitaxel/Reo regimen (Figure 6b,c), which were not present in tumors treated with either virus, or paclitaxel, alone. Similarly, paclitaxel/Reo treated tumors showed striking perivascular apoptosis by TUNEL assay (Figure 6d).
Figure 6.
Systemic delivery of reovirus to paclitaxel preconditioned tumors is natural killer (NK) cell dependent and induces perivascular degeneration. (a) C57BL/6 mice (7 per group) seeded 7 days previously with B16-VEGF tumors were treated intraperitoneally with an injection of paclitaxel for three consecutive days followed by two daily intravenous injections of reovirus. This cycle of five injections was repeated three times. For NK cell depletion, one group of mice received ~0.75 mg/mouse anti-asialo-GM1 intraperitoneally on the first day of each treatment cycle (Pac-Reo-NK Depleted), whereas the other group received rabbit immunoglobulin G (IgG) isotype control (Pac-Reo). Survival with time (tumors reaching a diameter of >1.0 cm in any diameter) is shown. Results are representative of three different experiments. (b–d). H+E sections of 14 day established B16 tumors from C57BL/6 mice treated with daily injections of paclitaxel followed 24 hours later by a single intravenous injection of reovirus and excised 48 hours following the virus injection showing very early (b) and more advanced (c) signs of perivascular degeneration which were not observed in sections from tumors taken from mice treated with only paclitaxel or virus alone. (d) TUNEL staining for apoptosis from a tumor treated as in b and c above.
Discussion
Using chimeric receptors in which VEGFR1 and VEGFR2 signaling could be clearly dissociated, we show that VEGFR2 signaling to endothelial cells conferred susceptibility to reovirus replication (Figure 1). Thus, HUVEC expressing EGF-VEGFR2, but not EGF-VEGFR1, supported ongoing replication of reovirus, but only in the presence of EGF, which mimics the VEGF burst (Figure 1). In contrast, VEGFR1 signaling mediated reovirus-induced NK cell activation (adhesion and cytokine secretion) (Figure 2), dependent upon both virus exposure/infection and VEGFR1 signaling, but occurred in the absence of VEGFR2-mediated viral replication. In addition, whereas increasing levels of VEGFR1 inhibited the ability of EGF to condition HUVEC expressing VEGFR2 to support reovirus replication (Figure 3b), a combination of VEGFR1 and VEGFR2 signaling significantly enhanced NK cell activation by reovirus exposure/infection (Figure 3c)—possibly because the increased viral replication induced by VEGFR2 enhanced the activation of the innate immune effectors.
Taken together, these data are consistent with a model in which VEGFR2 signaling confers a proviral state upon the cell, as a result of activation of signal transduction pathways involved in cell proliferation.28,29,30 The transient activation of such pathways could be exploited by reovirus, replication of which depends upon activation of proto-oncogene-associated proliferative signals,10,11 if infection occurs within the appropriate window of cell activation. In contrast, consistent with findings that VEGFR1 does not transmit significant mitogenic signals,29,38 VEGFR1 signaling leads to upregulation of a variety of immune activating molecules, involved in antigen presentation, NK activation and cell adhesion, but only in the presence of virus infection.
We used the studies of Figures 1–3 to screen clinically useful chemotherapeutic agents for properties which might mimic the conditioning of endothelial cells for either increased viral replication and/or viral-dependent immune stimulating activity. In this respect, reovirus/taxane combinations have been shown to be widely synergistic, even in cells not susceptible to either agent alone.39 Consistent also with the antiangiogenic properties of paclitaxel, mediated, at least in part, through VEGF signaling,32,40,41,42 continuous exposure to paclitaxel prevented VEGFR2-mediated signaling of a proviral state within HUVEC (Figure 4). However, within 24–48 hours of removal of paclitaxel inhibition, reovirus replication was facilitated in HUVEC in the presence of a VEGF burst (Figure 4), and at higher levels than that induced by a VEGF burst alone (Figure 4). Moreover, whereas continual VEGF prevented reovirus replication, treatment with paclitaxel inhibited this VEGF conditioning such that, upon withdrawal of the chemotherapy, HUVEC cells rebounding from paclitaxel, in the presence of VEGFR2 signaling, acquired sensitivity to reovirus replication. Finally, paclitaxel had no significant inhibitory, or stimulatory, effect on the ability of VEGFR1 signaling to induce NK cell activation by reovirus-exposed endothelial cells (Figure 4).
The data of Figure 4 suggested a therapeutic opportunity for a combination of chemotherapy with systemic virotherapy. Consistent with Figure 4, we identified a schedule in which preconditioning with paclitaxel opened a therapeutic window during which intravenous reovirus, within 24 hours of the last paclitaxel treatment, resulted in highly significant therapy and multiple long-term cures (Figure 5a). The therapeutic window closed relatively rapidly, because increasing the delay of virus administration from cessation of paclitaxel chemotherapy to 48 hours, or longer, led to a loss of therapy against the B16-VEGF tumors. Our ability to detect reovirus in tumors correlated closely with therapy (Figure 5). Moreover, the histological findings of perivascular virus, vascular degeneration and perivascular apoptosis (Figure 6) were highly suggestive of virus being released from infected endothelial sites, leading to viral replication and associated endothelial damage. This perivascular pattern is consistent with reports that induction of cancer cell apoptosis before viral injection enhanced delivery and penetration43 and that taxane treatment resulted in decreased tumor cell density (by apoptotic cell shrinkage) leading to decompression of blood vessels and improved transvascular transport of large macromolecules.44
Immune mediated recognition of virally infected cells played a critical part in the overall therapy since it was dependent upon NK cells (Figure 6a), consistent with our previous results with VEGF-mediated reovirus therapy of B16 tumors31 and the in vitro experiments of Figures 2–4.
Our data show that paclitaxel-rebound mediated sensitization to systemic reovirus therapy will be optimally effective clinically against VEGF-expressing tumors and that this VEGF-dependence for therapy provides a critical element of tumor specificity. Because normal endothelium will not normally be exposed to VEGF, only tumor-associated endothelium would be susceptible to de novo reovirus replication upon withdrawal of paclitaxel, and systemic delivery of reovirus, in vivo. This is consistent with our inability to detect any systemic toxicity associated with normal tissue and organs in mice treated with the carefully scheduled paclitaxel/Reo combination.
Our results are highly significant for clinical translation by showing that it will be possible to develop clinical protocols in which oncolytic viruses can be delivered systemically in combination with clinically approved chemotherapies. However, the therapeutic windows for effectively timed combinations may be relatively small.45 In addition, chemotherapies which have no documented activity against a certain histological type of tumor may still have considerable clinical value when used in combination with systemic delivery of oncolytic viruses, because of their activity against, or upon, endothelial, as opposed to tumor, cells. This approach may be an important step toward the development of treatment protocols which are effective against many different types of tumor which all share a common property—such as VEGF-driven endothelial expansion.
In summary, we show here that careful scheduling of a combination of a chemotherapy with systemic virotherapy, neither of which alone have therapeutic effects, can generate highly significant antitumor efficacy against established tumors. Therefore, even where conventional in vitro and in vivo preclinical studies indicate a lack of efficacy against any given tumor type, a range of effective novel combinations may be possible through targeting the tumor endothelium in association with truly systemic delivery of oncolytic viruses.
Materials and Methods
Cells and viruses. B16 murine melanoma cells (H2-Kb) have been described previously.46 B16-VEGF cells are B16 cells engineered to secrete VEGF165.31,47 2H11 cells, a transformed murine endothelial cell line,37 were purchased from the ATCC (Manassas, VA). HUVEC were purchased from ATCC. Cell lines were monitored routinely and were free of Mycoplasma infection.
Wild-type Reovirus type 3 (Dearing strain) is a unique isolate acquired from Oncolytics Biotech (Calgary, Alberta, Canada). Stock titers were measured by plaque assays on L929 cells.25
Transduction with EGR-VEGFR chimeric receptors. Retroviral vectors encoding chimeric receptors were constructed in which the extracellular, EGF-binding domain of the EGF receptor was fused to the intracellular signaling domain of either VEGFR1 or VEGFR2 as described.34,35 Expression of the chimeric receptors in HUVEC transduced with the appropriate viral stocks was confirmed by immunoblotting with antibodies specific for the C-terminus of VEGFR1 or VEGFR2 with prior immunoprecipitation of infected cell extracts with an antibody against the EGF receptor N terminus.34 Functional signaling through the EGFR-VEGFR1/2 chimeric receptors was confirmed by immunoblotting for the induction of tyrosine phosphorylation on the C-terminus of either VEGFR1 or VEGFR2 upon treatment with EGF, as described in ref. 34.
Reovirus replication from HUVEC cells treated with VEGF burst, EGF, or paclitaxel. 104 exponentially growing HUVEC cells were left untreated, or exposed to 0.5 ml of undiluted virus-containing supernatant (multiplicity of infection of ~10), in the presence of 4 µg/ml polybrene, to generate HUVEC/EGF-VEGFR1 or HUVEC/EGF-VEGFR2 transductants. Seventy-two hours later, cells were washed and replenished with media either lacking VEGF (VEGF deprivation) or supplemented with VEGF165 (6 ng/ml) (PROSPEC, Rehovot, Israel).31 Forty-eight hours later, media were changed again and, depending upon the experimental treatment, HUVEC, HUVEC/EGF-VEGFR1, or HUVEC/EGF-VEGFR2 cells were kept in the absence of VEGF, or were exposed either to VEGF165 (6 ng/ml) (HUVEC) or to EGF (10 ng/ml) (HUVEC/EGF-VEGFR1 or HUVEC/EGF-VEGFR2) to stimulate signaling through either VEGFR1 or VEGFR2, respectively (VEGF or EGF burst). Twenty-four hours later, cells were exposed to reovirus (multiplicity of infection 0.1, 1 or 10) either in the continued presence, or absence, of VEGF or EGF. In the appropriate experiments, reovirus infection was performed in the presence of Kinase IV inhibitor (Calbiochem, Cat# 676489) either at 100 nmol/l to inhibit VEGFR2 signaling, or at 400 nmol/l to inhibit both VEGFR1 and VEGFR2 signaling. 48 hours later, viral titers were determined by plaque assay (Figure 1a).
For experiments using paclitaxel,40,41 drug was added to the cultures (10 nmol/l) either 72 hours following viral transduction (paclitaxel inhibition) and/or 48 hours later at the time of the VEGF or EGF burst and through the period of reovirus infection (rebound phase).
NK cell activation by reovirus-exposed endothelial cells. The protocol above was repeated with the addition of the human NK cell line RL12, a derivative of the NK cell line NKL.48 Twenty-four hours following infection of HUVEC, HUVEC/EGF-VEGFR1, or HUVEC/EGF-VEGFR2 cells with reovirus at an effector:target ratio of 50:1. Forty-eight hours following addition of NK cells, supernatants were assayed for TNF-α (Figure 2a).
In vivo studies. Procedures were approved by the Mayo Foundation Institutional Animal Care and Use Committee. C57BL/6 mice (6–8 weeks old) (Jackson Laboratories, Bar Harbor, ME) were injected subcutaneously with 2 × 105 B16 or B16-VEGF cells (100 µl phosphate-buffered saline). Bidimensional tumor diameters were measured thrice-weekly using calipers and mice were killed when actively progressing tumors exceeded 1.0 × 1.0 cm. Immune cell depletions involved intraperitoneal injections (0.1 mg/mouse) of anti-CD8 (Lyt 2.43), anti-CD4 (GK1.5) (Monoclonal Antibody Core Facility; Mayo Clinic, Rochester, MN) and immunoglobulin G control (ChromPure Rat IgG; Jackson ImmunoResearch Laboratories, West Grove, DA). For NK depletion, ~0.75 mg/mouse anti-asialo-GM1 (Cedarlane) or rabbit IgG isotype control (Jackson ImmunoResearch Laboratories, Burlington, NC) was injected intraperitoneally. Fluorescence-activated cell sorting of spleens and/or lymph nodes confirmed subset-specific depletions.
Paclitaxel (Mayo Clinic Pharmacy, Rochester, MN) was injected intraperitoneally at 10 mg/kg per injection.40,41 For in vivo studies, reovirus was administered intravenously at 108 TCID50 per injection.
Virus titration. Cells infected with reovirus were harvested and lysed (three freeze-thaw cycles within 2 hours of removal). Virus in lysates was titered on L929 cells.25
Histopathology of tumor sections. Tumors were harvested, fixed in 10% formalin, paraffin-embedded and sectioned. For detection of reovirus, immunohistochemical analysis was done using a previously published protocol49 where DAB chromogen was used for detection of reovirus (brown is positive) with a counterstain of hematoxylin (blue is negative).
Statistics. Survival data from the animal studies was analyzed by log-rank test. Two-sample unequal variance Student's t-test analysis was applied for in vitro assays. Statistical significance was determined at P < 0.05.
Acknowledgments
We thank Toni Higgins for expert secretarial assistance. This work was supported by the Paul Family Gift, the Richard Schulze Family Foundation, the Mayo Foundation, Cancer Research UK and by NIH Grants CA107082, CA132734, and CA130878.
REFERENCES
- Cattaneo R, Miest T, Shashkova EV., and, Barry MA. Reprogrammed viruses as cancer therapeutics: targeted, armed and shielded. Nat Rev Microbiol. 2008;6:529–540. doi: 10.1038/nrmicro1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martuza RL., and, Zwiebel J. Replication-selective virotherapy for cancer: Biological principles, risk management and future directions. Nat Med. 2001;7:781–787. doi: 10.1038/89901. [DOI] [PubMed] [Google Scholar]
- Parato KA, Senger D, Forsyth PA., and, Bell JC. Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer. 2005;5:965–976. doi: 10.1038/nrc1750. [DOI] [PubMed] [Google Scholar]
- Parato KA, Lichty BD., and, Bell JC. Diplomatic immunity: turning a foe into an ally. Curr Opin Mol Ther. 2009;11:13–21. [PubMed] [Google Scholar]
- Prestwich RJ, Errington F, Diaz RM, Pandha HS, Harrington KJ, Melcher AA.et al. (2009The case of oncolytic viruses versus the immune system: waiting on the judgment of Solomon Hum Gene Ther 201119–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JC, Coffin RS, Davis CJ, Graham NJ, Groves N, Guest PJ.et al. (2006A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor Clin Cancer Res 126737–6747. [DOI] [PubMed] [Google Scholar]
- Senzer NN, Kaufman HL, Amatruda T, Nemunaitis M, Reid T, Daniels G.et al. (2009Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma J Clin Oncol 275763–5771. [DOI] [PubMed] [Google Scholar]
- Harrington KJ, Vile RG, Melcher A, Chester J., and, Pandha HS. Clinical trials with oncolytic reovirus: moving beyond phase I into combinations with standard therapeutics. Cytokine Growth Factor Rev. 2010;21:91–98. doi: 10.1016/j.cytogfr.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap TA, Brunetto A, Pandha H, Harrington K., and, Debono JS. Reovirus therapy in cancer: has the orphan virus found a home. Expert Opin Investig Drugs. 2008;17:1925–1935. doi: 10.1517/13543780802533401. [DOI] [PubMed] [Google Scholar]
- Coffey MC, Strong JE, Forsyth PA., and, Lee PW. Reovirus therapy of tumors with activated Ras pathway. Science. 1998;282:1332–1334. doi: 10.1126/science.282.5392.1332. [DOI] [PubMed] [Google Scholar]
- Hirasawa K, Nishikawa SG, Norman KL, Coffey MC, Thompson BG, Yoon CS.et al. (2003Systemic reovirus therapy of metastatic cancer in immune-competent mice Cancer Res 63348–353. [PubMed] [Google Scholar]
- Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- Prestwich RJ, Errington F, Ilett EJ, Morgan RS, Scott KJ, Kottke T.et al. (2008Tumor infection by oncolytic reovirus primes adaptive antitumor immunity Clin Cancer Res 147358–7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestwich RJ, Errington F, Steele LP, Ilett EJ, Morgan RS, Harrington KJ.et al. (2009Reciprocal human dendritic cell-natural killer cell interactions induce antitumor activity following tumor cell infection by oncolytic reovirus J Immunol 1834312–4321. [DOI] [PubMed] [Google Scholar]
- Prestwich RJ, Ilett EJ, Errington F, Diaz RM, Steele LP, Kottke T.et al. (2009Immune-mediated antitumor activity of reovirus is required for therapy and is independent of direct viral oncolysis and replication Clin Cancer Res 154374–4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth P, Roldán G, George D, Wallace C, Palmer CA, Morris D.et al. (2008A phase I trial of intratumoral administration of reovirus in patients with histologically confirmed recurrent malignant gliomas Mol Ther 16627–632. [DOI] [PubMed] [Google Scholar]
- Vidal L, Pandha HS, Yap TA, White CL, Twigger K, Vile RG.et al. (2008A phase I study of intravenous oncolytic reovirus type 3 Dearing in patients with advanced cancer Clin Cancer Res 147127–7137. [DOI] [PubMed] [Google Scholar]
- Harrington KJ, Karapanagiotou EM, Roulstone V, Twigger KR, White CL, Vidal L.et al. (2010Two-stage phase I dose-escalation study of intratumoral reovirus type 3 dearing and palliative radiotherapy in patients with advanced cancers Clin Cancer Res 163067–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comins C, Spicer J, Protheroe A, Roulstone V, Twigger K, White CM.et al. (2010REO-10: a phase I study of intravenous reovirus and docetaxel in patients with advanced cancer Clin Cancer Res 165564–5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima H, Kaur B., and, Chiocca EA. Directing systemic oncolytic viral delivery to tumors via carrier cells. Cytokine Growth Factor Rev. 2010;21:119–126. doi: 10.1016/j.cytogfr.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmon C, Harrington K, Kottke T, Prestwich R, Melcher A., and, Vile R. Cell carriers for oncolytic viruses: Fed Ex for cancer therapy. Mol Ther. 2009;17:1667–1676. doi: 10.1038/mt.2009.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J, Wang H, Kottke T, White C, Twigger K, Diaz RM.et al. (2008Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus Clin Cancer Res 14259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power AT., and, Bell JC. Cell-based delivery of oncolytic viruses: a new strategic alliance for a biological strike against cancer. Mol Ther. 2007;15:660–665. doi: 10.1038/sj.mt.6300098. [DOI] [PubMed] [Google Scholar]
- Qiao J, Kottke T, Willmon C, Galivo F, Wongthida P, Diaz RM.et al. (2008Purging metastases in lymphoid organs using a combination of antigen-nonspecific adoptive T cell therapy, oncolytic virotherapy and immunotherapy Nat Med 1437–44. [DOI] [PubMed] [Google Scholar]
- White CL, Twigger KR, Vidal L, De Bono JS, Coffey M, Heinemann L.et al. (2008Characterization of the adaptive and innate immune response to intravenous oncolytic reovirus (Dearing type 3) during a phase I clinical trial Gene Ther 15911–920. [DOI] [PubMed] [Google Scholar]
- Ellis LM., and, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8:579–591. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- Thorpe PE. Vascular targeting agents as cancer therapeutics. Clin Cancer Res. 2004;10:415–427. doi: 10.1158/1078-0432.ccr-0642-03. [DOI] [PubMed] [Google Scholar]
- Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20:4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- Hicklin DJ., and, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- Kowanetz M., and, Ferrara N. Vascular endothelial growth factor signaling pathways: therapeutic perspective. Clin Cancer Res. 2006;12:5018–5022. doi: 10.1158/1078-0432.CCR-06-1520. [DOI] [PubMed] [Google Scholar]
- Kottke T, Hall G, Pulido J, Diaz RM, Thompson J, Chong H.et al. (2010Antiangiogenic cancer therapy combined with oncolytic virotherapy leads to regression of established tumors in mice J Clin Invest 1201551–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavallaris M. Microtubules and resistance to tubulin-binding agents. Nat Rev Cancer. 2010;10:194–204. doi: 10.1038/nrc2803. [DOI] [PubMed] [Google Scholar]
- Lolkema MP, Arkenau HT, Harrington K, Roxburgh P, Morrison R, Roulstone V.et al. (2011A phase I study of the combination of intravenous reovirus type 3 Dearing and gemcitabine in patients with advanced cancer Clin Cancer Res 17581–588. [DOI] [PubMed] [Google Scholar]
- Zeng H, Dvorak HF., and, Mukhopadhyay D. Vascular permeability factor (VPF)/vascular endothelial growth factor (VEGF) peceptor-1 down-modulates VPF/VEGF receptor-2-mediated endothelial cell proliferation, but not migration, through phosphatidylinositol 3-kinase-dependent pathways. J Biol Chem. 2001;276:26969–26979. doi: 10.1074/jbc.M103213200. [DOI] [PubMed] [Google Scholar]
- Zeng H, Sanyal S., and, Mukhopadhyay D. Tyrosine residues 951 and 1059 of vascular endothelial growth factor receptor-2 (KDR) are essential for vascular permeability factor/vascular endothelial growth factor-induced endothelium migration and proliferation, respectively. J Biol Chem. 2001;276:32714–32719. doi: 10.1074/jbc.M103130200. [DOI] [PubMed] [Google Scholar]
- Cools J, Mentens N, Furet P, Fabbro D, Clark JJ, Griffin JD.et al. (2004Prediction of resistance to small molecule FLT3 inhibitors: implications for molecularly targeted therapy of acute leukemia Cancer Res 646385–6389. [DOI] [PubMed] [Google Scholar]
- Walter-Yohrling J, Morgenbesser S, Rouleau C, Bagley R, Callahan M, Weber W.et al. (2004Murine endothelial cell lines as models of tumor endothelial cells Clin Cancer Res 102179–2189. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M.et al. (2001Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions Nat Med 7575–583. [DOI] [PubMed] [Google Scholar]
- Sei S, Mussio JK, Yang QE, Nagashima K, Parchment RE, Coffey MC.et al. (2009Synergistic antitumor activity of oncolytic reovirus and chemotherapeutic agents in non-small cell lung cancer cells Mol Cancer 847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klosowska-Wardega A, Hasumi Y, Ahgren A, Heldin CH., and, Hellberg C. Combination therapy using imatinib and vatalanib improves the therapeutic efficiency of paclitaxel towards a mouse melanoma tumor. Melanoma Res. 2010. [DOI] [PubMed]
- Shaked Y, Henke E, Roodhart JM, Mancuso P, Langenberg MH, Colleoni M.et al. (2008Rapid chemotherapy-induced acute endothelial progenitor cell mobilization: implications for antiangiogenic drugs as chemosensitizing agents Cancer Cell 14263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strieth S, Eichhorn ME, Werner A, Sauer B, Teifel M, Michaelis U.et al. (2008Paclitaxel encapsulated in cationic liposomes increases tumor microvessel leakiness and improves therapeutic efficacy in combination with Cisplatin Clin Cancer Res 144603–4611. [DOI] [PubMed] [Google Scholar]
- Nagano S, Perentes JY, Jain RK., and, Boucher Y. Cancer cell death enhances the penetration and efficacy of oncolytic herpes simplex virus in tumors. Cancer Res. 2008;68:3795–3802. doi: 10.1158/0008-5472.CAN-07-6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffon-Etienne G, Boucher Y, Brekken C, Suit HD., and, Jain RK. Taxane-induced apoptosis decompresses blood vessels and lowers interstitial fluid pressure in solid tumors: clinical implications. Cancer Res. 1999;59:3776–3782. [PubMed] [Google Scholar]
- Ottolino-Perry K, Diallo JS, Lichty BD, Bell JC., and, McCart JA. Intelligent design: combination therapy with oncolytic viruses. Mol Ther. 2010;18:251–263. doi: 10.1038/mt.2009.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linardakis E, Bateman A, Phan V, Ahmed A, Gough M, Olivier K.et al. (2002Enhancing the efficacy of a weak allogeneic melanoma vaccine by viral fusogenic membrane glycoprotein-mediated tumor cell-tumor cell fusion Cancer Res 625495–5504. [PubMed] [Google Scholar]
- Jevremovic D, Gulati R, Hennig I, Diaz RM, Cole C, Kleppe L.et al. (2004Use of blood outgrowth endothelial cells as virus-producing vectors for gene delivery to tumors Am J Physiol Heart Circ Physiol 287H494–H500. [DOI] [PubMed] [Google Scholar]
- Robertson MJ, Cochran KJ, Cameron C, Le JM, Tantravahi R., and, Ritz J. Characterization of a cell line, NKL, derived from an aggressive human natural killer cell leukemia. Exp Hematol. 1996;24:406–415. [PubMed] [Google Scholar]
- Nuovo GJ. In situ detection of microRNAs in paraffin embedded, formalin fixed tissues and the co-localization of their putative targets. Methods. 2010;52:307–315. doi: 10.1016/j.ymeth.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Iwai M, Harada Y, Morikawa T, Muramatsu A, Mori T.et al. (2000Targeted killing of carcinoembryonic antigen (CEA)-producing cholangiocarcinoma cells by polyamidoamine dendrimer-mediated transfer of an Epstein-Barr virus (EBV)-based plasmid vector carrying the CEA promoter Cancer Gene Ther 71241–1250. [DOI] [PubMed] [Google Scholar]