Abstract
Embryonic stem cells (ESCs) harbor the potential to generate every cell type of the body by differentiation. The use of hESCs holds great promise for potential cell replacement therapies for degenerative diseases including diabetes mellitus. The recently discovered induced pluripotent stem cells (iPSCs) exhibit immense potential for regenerative medicine as they allow the generation of autologous cells tailored to the patients' immune system. Research for insulin-producing surrogate cells from ESCs has yielded highly controversial results, because many steps and factors in the differentiation process are currently still unknown. Thus, there is no consensus on common standard protocols. The protocols presently used established the differentiation from pluripotent cells toward pancreatic progenitor cells. However, none of the differentiation protocols reported to date have generated by exclusive in vitro differentiation sufficient numbers of insulin-producing cells meeting all essential criteria of a β-cell. The cells often lack the crucial function of regulated insulin secretion upon glucose stimulation. This review focuses on past and current approaches to the generation of insulin-producing cells from pluripotent sources, such as ESCs and iPSCs, and critically discusses the hurdles to be taken before insulin-secreting surrogate cells derived from these stem cells will be of clinical use in humans.
Introduction
Diabetes mellitus is a major health problem currently affecting around 280 million people worldwide and predicted to increase to 440 million adults by 2030.1 Diabetes imposes a heavy burden of morbidity and premature mortality2 and incurs a large and steadily increasing financial cost in the health system.3 Once lost, the function of the insulin-producing β-cells cannot be recovered, rendering the diabetic patient dependent on a life-long supplementation therapy with insulin. Transplantation of a human donor pancreas or pancreatic islets offers a cure. However, donor organs are very limited and transplantation is therefore possible only for a few severely ill type 1 diabetic patients. Therefore, much attention has been focused on the potential of bioengineered insulin-producing surrogate cells.4,5,6,7 Several sources have been considered for the in vitro generation of insulin-producing cells including ex vivo expanded β-cells,8 endocrine progenitor cells,9 transdifferentiated or transduced liver or intestinal cells,10,11 bone marrow mesenchymal stem cells,12 and pluripotent embryonic stem cells (ESCs).13,14 ESCs harbor great potential for future cell replacement therapy of diabetes (Figure 1) because they offer two unique features: availability in potentially unlimited numbers and the plasticity to generate any cell type of the body by in vitro differentiation.
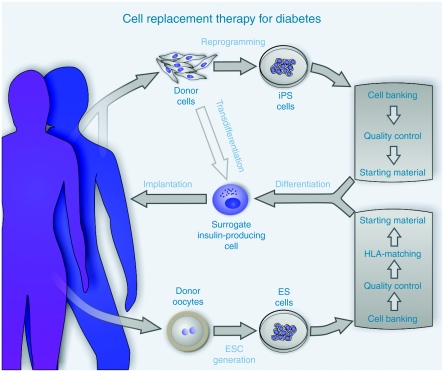
Figure 1.
Strategy to obtain insulin-producing surrogate cells from pluripotent cell sources. The cell replacement therapy of diabetes with differentiated pluripotent cells requires either human embryonic stem cells generated from fertilized donor oocytes or reprogrammed somatic cells, induced pluripotent stem cells (iPSCs), as starting material. The intended therapeutic use presupposes “clinical grade” cell lines as seed stocks for the in vitro generation of insulin-producing cells. Cell banking systems assure that a uniform population of pluripotent cells is preserved, stocked, and made available to facilitate high quality and standardized research and later clinical use. To improve the chances of a successful implantation the banked cell lines would be analyzed for human leukocyte antigen (HLA)-antigen presentation and minimum matching levels must be defined in order to choose appropriate starting material for the patient. This step can be circumvented when patient-specific iPSCs are used. The differentiation of pluripotent cells recapitulates the pancreas development and organogenesis of islets of Langerhans. A purification step would be needed to enrich the endocrine β-cell population which must be devoid of contaminating cells harboring teratogenic potential. Before implantation, rigorous in vitro testing would determine whether the cells meet the functional criteria such as glucose-induced insulin secretion and other parameters as mentioned in Table 1. Directed transdifferentiation of somatic cells into insulin-producing surrogate cells, as recently shown for neurons, blood progenitors, and cardiomyocytes, may render the pluripotent intermediate expendable.
What are the minimal requirements for an insulin-producing surrogate cell of ESC origin?
Ideally a surrogate cell should be sufficiently differentiated toward an insulin-producing phenotype to ensure expression of all structures and components required to synthesize and release insulin in response to changes in extracellular glucose over the physiological range, adequately meeting the insulin demands without the risk of hypoglycemia. Such a system should comprise a glucose transporter system to facilitate the uptake of glucose at physiological concentrations. A glucose sensor is required to translate changes in intracellular glucose into corresponding changes in metabolic fluxes to generate an adequate signal for both insulin biosynthesis and the regulated exocytosis of insulin stored in secretory granules.15 Table 1 addresses some of the desirable and unacceptable phenotypical characteristics of surrogate β-cells destined for β-cell replacement through implantation in patients with type 1 diabetes. The data summarized in Table 2 depict the deficiencies of present differentiation protocols which currently prevent their use in patients with diabetes for β-cell replacement therapy.
Table 1. Functional phenotype of surrogate β-cells for transplantation therapy of type 1 diabetes.
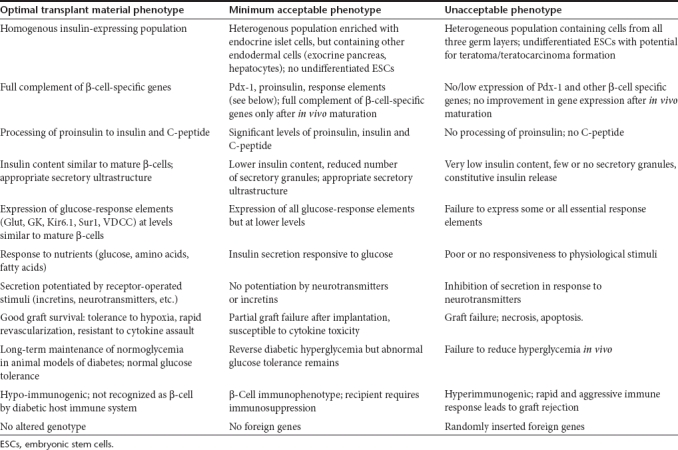
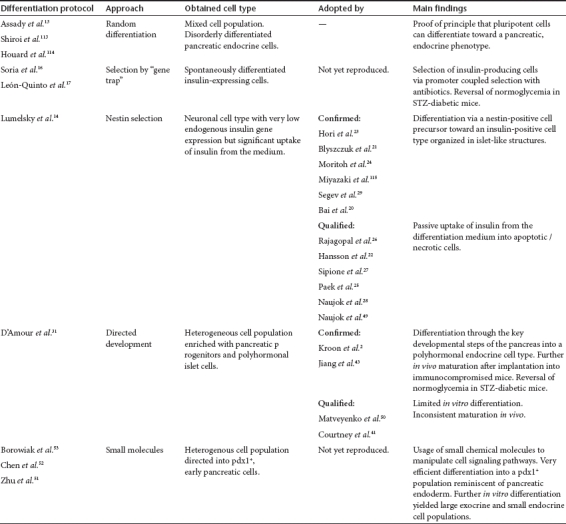
Table 2. Overview of the differentiation protocols used for mouse and human ESCs.
Current Status of ES Cell Research— How Close Are we to a β-Cell?
A decade ago the first proof-of-concept studies describing differentiation of ESCs into insulin-producing cells were published. In an elegant approach, Soria and co-workers differentiated a mouse ES cell line in which an antibiotic resistance gene was driven by the human insulin promoter.16 Cells differentiated from this ES cell line corrected hyperglycemia when implanted into streptozotocin diabetic mice.16 In a later study, the insulin promoter was replaced by the β–cell-specific Nkx6.1 promoter yielding comparable results.17 Though in principle attractive, this approach has not been adopted in later studies for the generation of insulin-producing surrogate cells. The strategy of selecting insulin-expressing (and thus antibiotic resistant) cells after essentially spontaneous differentiation resulted in relatively low yields when compared to directed differentiation protocols, and the genetically altered phenotype would preclude their use in clinical studies (Table 1).
In a study that initially appeared ground breaking Lumelsky and co-workers14 published a five stage, directed differentiation protocol which differentiated mouse ESCs (mESCs) into islet-like clusters composed of glucagon-, somatostatin-, and insulin-positive cells releasing insulin in response to glucose. The concept underlying this work was primarily based on a previous study in which functional neurons were generated from mESCs18 and the suggestion that β-cells and neurons share a common progenitor cell expressing the neural stem cell-specific neurofilament nestin.19 Various groups reproduced this protocol in both mouse and human ESCs, with variable degrees of success.20,21,22,23,24,25,26,27,28,29 The origin of the protocol and the variable results obtained by other groups raised questions about the nature of the obtained cells with the Lumelsky protocol. It was suggested that these cells were primarily of a neuronal phenotype with a low level of insulin gene expression.27 It became clear that cells generated with this protocol were highly prone to apoptosis or necrosis and they adsorbed significant amounts of insulin from the differentiation medium supplemented with a very high concentration (25 µg × ml−1) of insulin.22,25,26
It became apparent that progress in the design of efficient protocols for generation of pancreatic β-cells from ESCs would depend on application of knowledge about the normal developmental mechanisms of the pancreas. Intensive study of the development of the endocrine pancreas, greatly assisted by the use of gene deletions in mice, has provided detailed information about the sequence of developmental events driving cells from foregut endoderm into fully differentiated β-cells.30 Some of the key stages are shown in Figures 2 and 3. Mapping these events onto ESCs maintained in culture should allow the recapitulation in vitro of the normal in vivo developmental process. However, it is worth noting that the in vitro directed differentiation protocols do not exactly map onto normal in vivo development for human β-cells (Figure 2). Thus, the most influential directed differentiation protocols for human ESCs (hESCs)31,32,33 allow ~20 days to differentiate into mature endocrine islet cells, although all available evidence suggests that insulin-expressing cells are first detected in the human fetal pancreas at around 8 weeks of development.34 It remains to be seen whether some of the problems with current in vitro protocols reflect this artificial compression of the developmental process. It would be interesting to determine whether extending the in vitro protocols to accurately reflect in vivo development would improve their efficiency and explain the current requirement for an in vivo maturation stage, as discussed below.
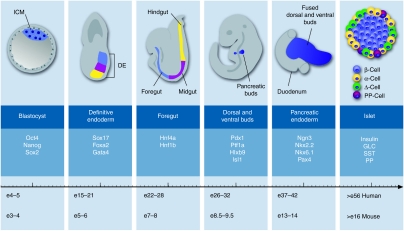
Figure 2.
Simplified schematic presentation of the pancreas development in mice. The inner cell mass (blue) of the blastocyst, sometimes referred as embryoblast, gives rise to the three germ layers in the process of gastrulation. The definitive endoderm is then formed by the recruitment of epiblast cells through the primitive streak via a mesendodermal progenitor with the latter cells of the foregut (blue), midgut (purple), and hindgut (yellow). Morphogenesis of the primitive gut is a result of an invagination movement by which the layered definitive endoderm becomes a tube structure. The pancreas formation begins with the independent budding of the dorsal and ventral buds at the posterior region of the foregut. These two buds grow into the surrounding mesenchyme, branch in a tree-like structure and eventually fuse after rotation of the gut to form the definitive pancreatic endoderm. This predifferentiated epithelium grows in size with distinct endocrine and exocrine differentiation. The endocrine cells are organized in islets which are embedded in exocrine tissue and are composed of four major hormone-secreting cells types. Insulin is secreted by β-cells (blue), glucagon by α-cells (yellow), somatostatin by Δ-cells (green), and pancreatic polypeptide by PP-cells (purple). The timeline plots these key events for mouse. For comparison only, comparable stages of human β-cell development have been mapped on the timeline. Several markers characteristic of each developmental step are listed. DE, definitive endoderm; GLC, glucagon; ICM, inner cell mass; PP, pancreatic polypeptide; SST, somatostatin.
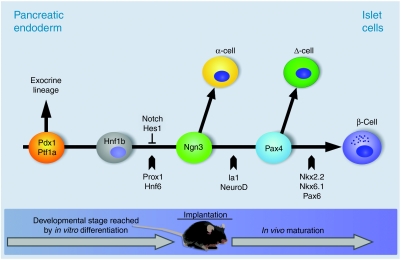
Figure 3.
Simplified schematic presentation of the transcriptional hierarchy of essential factors and signaling pathways during endocrine specification of the pancreatic endoderm. The pdx1+/ptf1a+ population (orange) is the earliest cell type defining the pancreatic lineage beginning with the evagination of the pancreatic buds. With the fusion of the dorsal and ventral buds pdx1 and other transcription factors are downregulated (though still detectable) and endocrine cells that are marked by expression of ngn3 (green) become visible in the epithelium of the ducts. An intermediate cell type, presumably an hnf1b+ precursor duct cell (gray) is considered to be an ngn3-precursor. Proliferation of ngn3+ cells is negatively regulated by the notch signaling pathway via the repressor Hes1. Pax4+ cells (pale blue) are an intermediate cell type directly regulated by ngn3 and function as a switch to specify between β-cells (blue) and Δ-cells (dark green). Arrows point to the developmental step at which indicated transcription factors play an essential role. The time line along the bottom of the diagram shows the differentiation stages which have been achieved by in vitro differentiation alone or by a combination of in vitro differentiation and in vivo maturation.
The observation that mESCs can be forced into the germ layer of definitive endoderm (DE) by treatment with activin A in the absence of fetal bovine serum offered an important first stage in directed differentiation protocols.35 Activin A is a member of the tumor growth factor-β superfamily acting by Nodal signaling on the tumor growth factor-β pathway. Supplementation of the differentiation medium with high concentrations of activin A yielded 60% DE-cells from mESCs35 and up to 80% from hESCs.36 The effectiveness of this treatment can vary between different ES cell lines, consistent with reports that human ES cell lines differ in their differentiation propensities.37,38 D'Amour et al. at NovoCell subsequently developed an in vitro differentiation protocol that guided hESCs through the key developmental steps of the pancreas from a pluripotent cell into DE, foregut, pancreatic endoderm, endocrine progenitor, and finally into a pancreatic endocrine cell by treatment with a sequential cocktail of growth factors and bioactive small molecules.31 The cells produced with this differentiation protocol had an insulin content comparable to that of human islets and were positive for insulin and C-peptide as proof of de novo synthesis of insulin rather than passive uptake from the culture medium. The cells released insulin in response to various secretagogues, although not in response to glucose.31 Moreover, the cells were double-positive for insulin and glucagon or for insulin and somatostatin suggesting improper endocrine specification (Table 1, unacceptable phenotype). Cho and co-workers reproduced this differentiation protocol for hESCs but added β-cellulin and nicotinamide to the final differentiation medium.39 This allowed a sustained expression of PDX1, an important transactivator of the insulin promoter.40 However, the results were not fully comparable with those of D'Amour et al. and the reported effectiveness of the protocol was considerably lower.39 This may reflect differences in the differentiation potential of the initial hESC lines, emphasizing the requirement for a systematic evaluation of the cell lines used by different groups working in this area to identify the most suitable starting material from which to differentiate functional β-cells.41
Shim et al. used a shorter differentiation protocol and treated hESCs sequentially with fetal calf serum, activin A, and retinoic acid (RA).42 After activin A treatment they obtained a SOX17+ cell population which was committed to DE and by further treatment with RA they generated a PDX1+/FOXA2+ population. It was concluded that RA may convert gut tube endoderm into pancreatic endoderm, thereby confirming the results of D'Amour et al.31 Nonetheless, further in vitro differentiation did not yield a significant fraction of insulin-producing cells, suggesting that additional cues are necessary to drive in vitro generated pancreatic endoderm toward endocrine progeny. An alternative approach by Jiang et al. started with the derivation of DE by treatment with a combination of activin A and sodium butyrate.43 The developmental trigger using RA was replaced in this protocol by a combination of EGF, bFGF and Noggin, and endocrine specification was induced by the addition of nicotinamide and IGF-II to the culture medium. The differentiated cells displayed a glucose-responsive C-peptide release. However, the partially abnormal expression of pancreatic transcription factors during the differentiation and the detection of polyhormonal cells positive for both C-peptide and glucagon, or for C-peptide and somatostatin suggest that these cells were not typical mature β-cells. Another approach which comprised treatment with activin A, RA and finally bFGF and nicotinamide resulted in islet-like structures with distinct insulin-, glucagon-, and somatostatin-positive monohormonal cells.44 These cells showed low levels of glucose-stimulated release of insulin and C-peptide when cultured in adherence, but this was significantly enhanced when the differentiated cells were transferred to a suspension culture system.44 A similar effect has been reported for the immortal insulin-producing MIN6 cell line, where responsiveness to nutrients is greatly enhanced by culture as nonadherent islet-like structures.45,46.
In vitro vs. in vivo differentiation—maturation effects in vivo
Advances in the understanding of the differentiation potential of hESCs have raised expectations that large numbers of functional surrogate β-cells maybe produced by in vitro differentiation. Despite the promising results and the proof-of-principal studies demonstrating that mouse and human ESCs can give rise to a pancreatic endocrine progeny, most published protocols yielded populations of functionally restricted insulin-producing cells. These had either a polyhormonal phenotype,31,42,43 lacked appropriate glucose-induced insulin secretion,31 or were contaminated with other cell types, including undifferentiated ESCs that could give rise to teratomas after implantation (Table 1, unacceptable phenotype).21,32,47,48 Thus, it remains an unresolved question whether insulin-producing cells can be solely produced by in vitro differentiation.
The NovoCell group used hESCs to generate a population one step before terminally differentiated insulin-producing β-cells.32 These cells were characterized by expression of the transcription factors NKX6.1, NGN3, and NKX2.2, a phenotype comparable to fetal 6–9-week-old pancreatic epithelial cells. Upon implantation into immunocompromised mice, the expression of human C-peptide was detected after 30 days and human insulin was readily detected 94 days postimplantation, demonstrating that triggers in the in vivo environment facilitated the final maturation into islet cells of the in vitro generated endocrine progenitor cells (Table 1, minimum acceptable phenotype).32 The beneficial effect of the in vivo environment was also reported for mESCs49 and for hESCs differentiated using a protocol different from that of NovoCell.42 The basis of this effect remains unknown and seems to be independent from the implantation site being used, but the identification of the biochemical and physical factors involved in this process might provide the missing information required to generate fully matured β-cells by in vitro differentiation. In a recent study using implantation in nude rats Matveyenko et al.50 reported inconsistent maturation of insulin-producing cells generated by NovoCell from hESCs according to their differentiation protocol.32 These problems with reproducibility led Matveyenko and colleagues to conclude that “the extent of islet formation and its function is not yet sufficiently reproducible to be clinically useful.”50 Thus, it remains unclear, whether in vivo maturation alone can solve the problem of incomplete in vitro differentiation.
Role of small molecules during differentiation
Differentiation protocols for ESCs have so far been based on combinations of recombinant growth factors, supplements, and bioactive molecules. Nonetheless, the in vitro differentiation of ESCs is often poorly controlled probably because of differences in the activity of recombinant proteins, their half-lives, and their concentration dependency with respect to the particular stem cell line being used, all of which may affect the reproducibility of the differentiation protocols. To address this issue efforts have been made to identify membrane-permeable small molecules that can control cellular processes and induce endodermal and subsequently pancreatic differentiation.51,52,53 The use of these molecules should offer a more controllable and reproducible approach because they can be chemically synthesized in high purity allowing standardization between laboratories. Recently a number of small molecules have been reported to direct mESCs and hESCs into the DE lineage, including the compounds IDE1/253 and the staurosporine family member stauprimide.51 IDE1/2 induced a SOX17+/FOXA2+ cell population, reminiscent of DE, earlier and more effectively than the activin A/Wnt3a combination via the same tumor growth factor-β/Nodal signaling pathway. Stauprimide possibly interacts with NME2, a transcription factor controlling c-MYC expression, which is required by ESCs to maintain their pluripotent state. Upon binding of stauprimide to the NME2, its nuclear localization is inhibited and c-MYC expression is downregulated thereby priming ESCs for differentiation. In combination with small amounts of activin A, 60% of DE-cells were generated from mESCs and 80% of DE-cells using hESCs.51 ESCs committed to DE can be manipulated to further develop via the foregut stage into pancreatic endoderm by treatment with (−)-Indolactam V.52 This treatment generated populations with 20% pdx1+ cells from DE-committed ESCs, and this was further enhanced when (−)-Indolactam V was combined with FGF-10, resulting in up to 46% pdx1+ cells.52 (−)-Indolactam V is a broad spectrum agonist of novel and classical PKCs.54,55 But the novel PKC isoform Δ is a target of the RA-signaling pathway, providing evidence for an analogous effect of (−)-Indolactam V to that of RA on ESCs.56,57 On the other hand, neither knock out of classical PKCs58,59 nor knockout of novel PKCs 60,61 had a negative effect on mouse pancreatic, endocrine development as would be expected if PKC did play a critical role. Additional work is needed to fully understand the role of PKC-signaling and its isoforms during pancreas organogenesis.
However, these findings are a major contribution to the field, since the discovery of additional small molecules (reviewed in refs. 62,63) exerting their effects on this particular developmental stage would drive ESCs to the ngn3+ endocrine progenitor stage, the developmental step prior to the mature islet cell (Figure 3).
Patient-Specific Stem Cells
Human ESCs are derived from embryos and although it is beyond the scope of this review, the use of embryos for this purpose remains controversial. In addition to the obvious ethical concerns about the use of hESCs, one of the major barriers to their use for cellular replacement therapy for type 1 diabetes is the challenge of immune rejection of the transplanted cells. This issue has yet to be addressed using somatic cell nuclear transfer technology to derive patient-specific hESC lines and as a result, the search for an alternative source of autologous stem cells has continued to receive significant attention.
In 2006, Yamanaka and co-workers reported a significant advance in this area. From an initial pool of 24 candidate transcription factors they demonstrated that the ectopic expression of just four of these factors (Oct3/4, Klf4, Sox2, and c-Myc) was sufficient to reprogram mouse embryonic fibroblasts into pluripotent stem cells, which were termed induced pluripotent stem cells (iPSCs).64 These cells were shown to be morphologically similar to ESCs, to possess a normal karyotype, to express ESC-marker genes and to maintain the developmental potential to form teratomas of all three germ layers when injected into nude mice. Within a year, two independent groups had successfully reprogrammed adult human fibroblasts to produce human iPSCs.65,66 Besides fibroblasts, iPSCs have now been derived from a range of somatic cell types including neural progenitor cells,67 keratinocytes,68 peripheral blood cells,69 pancreatic β cells,70 and hepatocytes.71
In addition to the obvious benefits of an autologous cell population, these cells have the major advantage that their derivation does not require the use of human embryos or oocytes, making their use less controversial than hESCs, both ethically and politically. However, in spite of this, there remain significant barriers to the clinical use of iPSCs. Genes that are known or suspected to be oncogenes must be omitted from the reprogramming protocol. Thus, reports have demonstrated that iPSCs can be generated, albeit at lower efficiency, in the absence of the known oncogenes c-myc and Klf4.72 Furthermore, although the initial reports describing the derivation of iPSCs used lentivirus or retrovirus to introduce the exogenous reprogramming factors, the potential for insertional mutagenesis using this approach renders it unlikely to lead to the generation of clinically useful cell populations. As a result, a number of alternative nonintegrating reprogramming strategies have been described, including the use of expression plasmids,73 episomal vectors,74 “piggyBac” transposition,75 Cre- or Flp-recombinase-based excisable viruses,76,77 and most recently membrane-soluble protein-induced methods,78,79 although the efficiency of these protein-based methods is very low. The introduction of these more clinically acceptable reprogramming strategies meant that research into the generation of differentiated cell types for replacement therapy has gathered momentum. Indeed, this has included reports describing the differentiation of insulin-producing cells from human iPSCs generated from normal human fibroblasts33,80 and fibroblasts from patients with type 1 diabetes.81 These studies are based on differentiation protocols that begin with the induction of DE using activin A and then proceed with the subsequent stepwise differentiation into precursor populations similar to those found during in vivo pancreatic development. The authors demonstrated, using RT-PCR and immunofluorescence analysis, the appearance of markers of the pancreatic lineage. These include, amongst others, PDX1, HNF6, HNF4a, and NKX6.1 and the islet hormones insulin and glucagon. In addition, these iPSC-derived, insulin-expressing cells responded to elevations in glucose concentrations with either modest increases in C-peptide release33,80 or in the case of one study,81 increases that were at least fivefold over basal levels, suggesting the presence of functional glucose-sensing cells. However, although all three studies concluded that it was possible to derive insulin-expressing β-like cells from iPSCs, they universally added the caveat, that until differentiation protocols are improved, it will not be possible to directly compare iPSC-derived insulin-expressing cells to pancreatic β-cells. As discussed above, this is very similar to current thinking with respect to the differentiation of hESCs into pancreatic β-cells. Indeed, in reports where differentiation toward a β-cell phenotype has been compared between hESCs and iPSCs, much of the data are largely comparable.33,80,81
It is clear that the minimum requirements for insulin-producing cells derived from hESCs will also apply to cells derived from iPSCs. These cells will therefore have to exhibit absolutely no tumorigenic potential, be xenogen-free, have a high degree of cellular homogeneity and exhibit appropriate insulin content and secretion in response to physiological concentrations of glucose and, ideally, to other relevant stimuli. However, there are additional considerations when deriving insulin-producing cells from iPSCs, because full concordance of the iPSC-genome, transcriptome, and methylome with ESCs has yet to be demonstrated. Epigenetic markers, such as DNA-methylation, have been identified as a barrier to full reprogramming82 and some somatic cell types, particularly somatic stem cell populations such as neural and myeloid stem cells, may be more amenable to reprogramming. Guenther et al. reported minimal differences in the chromatin structure and gene expression of human iPSCs compared to hESCs,83 but there is now evidence that iPSCs may retain the epigenetic signature associated with their somatic cell type of origin.84,85 Several studies revealed substantial epigenetic differences, reprogamming variability and somatic memory,86,87,88,89 which are transmitted to iPSCs derived from somatic cells,89 and which may increase variability and affect the differentiation potential of the iPSCs.90,91 Recently gene copy number variations in early passage iPSCs92,93 have been reported.85,86 Some iPSC lines cumulated somatic coding mutations94 and chromosomal aberrations,95 raising concerns about an increased disease risk. Additionally, the rate of teratoma formation after implantation into immunodeficient mice has been shown to be increased in iPSCs generated from adult cell types compared to hESCs or iPSCs generated from mouse embryonic fibroblasts.96 The therapeutic benefit of iPSCs compared to ESCs as a renewable, autologous cell population has been questioned by a recent report where T-cell-mediated immune-rejection of mouse iPSCs was observed in syngenic recipients.97 These results encourage to classify iPSCs as overall similar, but not identical to ESCs and, thus, it will be important to identify the most appropriate starting population for iPSC-derivation and to carefully analyze the genetic, epigenetic, and immunogenic status of any derived iPSC-line.
Although pluripotent cells hold enormous promise for cell therapy of type 1 diabetes, both iPSCs and ESCs share the adverse capability of uncontrolled cellular proliferation and formation of teratomas upon implantation into a host organism.98,99 The crucial issue of tumourigenicity of ESCs and iPSCs has been reviewed recently.100,101 Though principally benign, teratomas not only pose a risk to the graft47 but also a serious health risk to the patient. The exclusion of such potentially dangerous cells from transplant material is a prerequisite before pluripotent cells will ever become acceptable as a source for cell replacement therapy in regenerative medicine (Table 1, unacceptable phenotype). To achieve this several options have been considered: implantation of mature cells without contaminating residual undifferentiated cells, sorting techniques, positive selection utilizing resistance genes16,17,102,103 and selective ablation of undifferentiated cells.104,105,106 Thus, effective techniques to remove potentially teratogenic cells from mixed populations have been reported, but they have not yet been combined with protocols for differentiation of pluripotent cells into insulin-producing β-cells. This prevents pluripotent cells from clinical use at present.
Finally, recent work describing the directed conversion of fibroblasts to functional neural cells,107 similar reports for directed conversion of human fibroblasts into multilineage blood progenitors,108 mouse fibroblasts into cardiomyocytes109 and mouse fibroblasts into functional hepatocyte-like cells110 may render both hESCs and iPSCs redundant for regenerative medicine applications. Indeed, similar studies investigating the direct in vivo reprogramming of fully differentiated pancreatic exocrine cells into cells that closely resemble pancreatic β-cells by transient adenoviral overexpression of the three transcription factors Ngn3, Pdx1, and MafA, suggest that this direct reprogramming approach may offer an attractive alternative therapeutic strategy.111
Conclusion
The derivation of β-cells from human pluripotent cells for cell replacement therapy of diabetes remains an unresolved issue. While possible in principle, current differentiation protocols yield insufficient numbers of insulin-producing cells which do not yet meet the functional criteria of genuine β-cells. The cells obtained so far were heterogeneous populations of mixed phenotypes and harbored teratogenic potential (Table 1, unacceptable phenotype). Progress has been made by applying development principles to in vitro differentiation protocols. However, much of that what is known today about the development of the endocrine pancreas has been obtained by studying gene functions in knockout mice. One of the key challenges will be to address the question whether the transcriptional networks driving pancreas development in mice also play a crucial role in human pancreas development.
ESCs can be forced via the DE-stage into a pdx1+ cell population reminiscent of fetal pancreatic endoderm, but further in vitro differentiation toward authentic β-cells has been hampered by low efficiencies and a high exocrine to endocrine ratio of the generated cells.50 This can be overcome, at least in part, through an in vivo incubation period after implantation of the cells into small laboratory animals, where extensive maturation into islet-like structures with a robust insulin and C-peptide release has been reported. Transplant material generated by this in vivo approach is obviously not suitable for therapeutic use in humans, so alternative approaches must be devised for driving ESCs from pdx1+ progenitor cells toward fully functional β-cells. In future studies additional parameters to insulin content or release will have to be taken into account. Functional criteria for an authentic β-cell phenotype must be assessed, e.g., whether the differentiated cells express the full complement of β-cell-specific transcription factors, amongst them NEUROD1, MAFA, ISL1, the expression of structural genes necessary for glucose-responsive insulin secretion at similar levels to mature β-cells, including glucose transporters, glucokinase, KIR6.1/SUR1, and voltage-dependent calcium channels (Table 1, optimal transplant material phenotype). Moreover, long-term studies in large animal models are required to demonstrate that implanted insulin-producing cells generated from ESCs or iPSCs engraft successfully and maintain their function over a time-span of years. Whether implanted insulin-producing surrogate cells of ESC/iPSC origins will present a target for autoimmune cell destruction in patients with type 1 diabetes remains unknown. If so, patient-specific, pluripotent cell lines (such as iPSCs) may not be the most appropriate starting material for differentiation and later implantation. Complete identity between donor and recipient, as well as complete discordance between them may cause subsequent problems,112 so a more effective therapeutic option to ensure long-term function might be to use insulin-producing cells generated from an human leukocyte antigen-matched hESC-line with mild immunosuppression after implantation. Alternatively, it may be possible to engineer cells to resist the potential autoimmune attack, but this would require genetic manipulation, thereby raising concerns about insertional mutagenesis. Finally, implantation of cells derived from pluripotent stem cells entails the risk of tumor formation and this safety issue will need to be addressed to enable the translation of pluripotent stem cell-based therapies into clinical treatment of diabetes.
In summary, this is an exciting and fast-moving area of research and recent experimental studies have provided proof-of-concept that pluripotent stem cells can be driven to differentiate into insulin-expressing cells. There remain several obstacles to translating these observations into clinical treatments of diabetes, but none of these appears to be insurmountable in the future. These include (i) assessment of the differentiation potential of pluripotent stem cell populations to select the most appropriate starting material; (ii) refinements of current protocols to enable the generation of functionally competent β cells entirely in vitro under defined culture conditions; (iii) validation of purification methods of sufficient stringency to ensure the absolute exclusion of potentially teratogenic, pluripotent cells; (iv) development of techniques to scale-up laboratory based protocols to generate the large numbers of cells required for clinical use.
Finally, the wide-spread adoption of any new therapy will depend on it being shown to be at least as effective and as safe as the well-tested current choice of administration of exogenous insulin. It remains to be seen how the current obstacles to therapeutic translation can be overcome.
Acknowledgments
Own work cited in this article has been supported by the Deutsche Forschungsgemeinschaft (German Research Foundation) within the framework of the Excellence Cluster REBIRTH, and by Diabetes UK (P.M.J., C.B., grant BDA:RD 05 /0003111). The authors declared no conflict of interest.
REFERENCES
- Shaw JE, Sicree RA., and, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Feltbower RG, Bodansky HJ, Patterson CC, Parslow RC, Stephenson CR, Reynolds C.et al. (2008Acute complications and drug misuse are important causes of death for children and young adults with type 1 diabetes: results from the Yorkshire Register of diabetes in children and young adults Diabetes Care 31922–926. [DOI] [PubMed] [Google Scholar]
- Huang ES, Basu A, O'Grady M., and, Capretta JC. Projecting the future diabetes population size and related costs for the U.S. Diabetes Care. 2009;32:2225–2229. doi: 10.2337/dc09-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowiak M., and, Melton DA. How to make β cells. Curr Opin Cell Biol. 2009;21:727–732. doi: 10.1016/j.ceb.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T., and, Hebrok M. Stem cells to pancreatic β-cells: new sources for diabetes cell therapy. Endocr Rev. 2009;30:214–227. doi: 10.1210/er.2009-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguayo-Mazzucato C., and, Bonner-Weir S. Stem cell therapy for type 1 diabetes mellitus. Nat Rev Endocrinol. 2010;6:139–148. doi: 10.1038/nrendo.2009.274. [DOI] [PubMed] [Google Scholar]
- Halban PA, German MS, Kahn SE., and, Weir GC. Current status of islet cell replacement and regeneration therapy. J Clin Endocrinol Metab. 2010;95:1034–1043. doi: 10.1210/jc.2009-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner A, Nolan AL, Blacken RA., and, Habener JF. Redifferentiation of insulin-secreting cells after in vitro expansion of adult human pancreatic islet tissue. Biochem Biophys Res Commun. 2005;327:581–588. doi: 10.1016/j.bbrc.2004.12.043. [DOI] [PubMed] [Google Scholar]
- Yatoh S, Dodge R, Akashi T, Omer A, Sharma A, Weir GC.et al. (2007Differentiation of affinity-purified human pancreatic duct cells to β-cells Diabetes 561802–1809. [DOI] [PubMed] [Google Scholar]
- Ferber S, Halkin A, Cohen H, Ber I, Einav Y, Goldberg I.et al. (2000Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia Nat Med 6568–572. [DOI] [PubMed] [Google Scholar]
- Elsner M, Jörns A., and, Lenzen S. Diabetes therapy by lentiviral hepatic insulin gene expression without transformation of liver. Diabetologia. 2008;51:694–5; author reply 696. doi: 10.1007/s00125-008-0931-1. [DOI] [PubMed] [Google Scholar]
- Ianus A, Holz GG, Theise ND., and, Hussain MA. In vivo derivation of glucose-competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. J Clin Invest. 2003;111:843–850. doi: 10.1172/JCI16502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assady S, Maor G, Amit M, Itskovitz-Eldor J, Skorecki KL., and, Tzukerman M. Insulin production by human embryonic stem cells. Diabetes. 2001;50:1691–1697. doi: 10.2337/diabetes.50.8.1691. [DOI] [PubMed] [Google Scholar]
- Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R., and, McKay R. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science. 2001;292:1389–1394. doi: 10.1126/science.1058866. [DOI] [PubMed] [Google Scholar]
- Lenzen S.1992Glucokinase: signal recognition enzyme for glucose-induced insulin secretionIn Flatt PR (ed.). Nutrient Regulation of Insulin Secretion Portland Press Ltd: Chapel Hill; 101–124. [Google Scholar]
- Soria B, Roche E, Berná G, León-Quinto T, Reig JA., and, Martín F. Insulin-secreting cells derived from embryonic stem cells normalize glycemia in streptozotocin-induced diabetic mice. Diabetes. 2000;49:157–162. doi: 10.2337/diabetes.49.2.157. [DOI] [PubMed] [Google Scholar]
- León-Quinto T, Jones J, Skoudy A, Burcin M., and, Soria B. In vitro directed differentiation of mouse embryonic stem cells into insulin-producing cells. Diabetologia. 2004;47:1442–1451. doi: 10.1007/s00125-004-1458-8. [DOI] [PubMed] [Google Scholar]
- Lee SH, Lumelsky N, Studer L, Auerbach JM., and, McKay RD. Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat Biotechnol. 2000;18:675–679. doi: 10.1038/76536. [DOI] [PubMed] [Google Scholar]
- Zulewski H, Abraham EJ, Gerlach MJ, Daniel PB, Moritz W, Müller B.et al. (2001Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes Diabetes 50521–533. [DOI] [PubMed] [Google Scholar]
- Bai L, Meredith G., and, Tuch BE. Glucagon-like peptide-1 enhances production of insulin in insulin-producing cells derived from mouse embryonic stem cells. J Endocrinol. 2005;186:343–352. doi: 10.1677/joe.1.06078. [DOI] [PubMed] [Google Scholar]
- Blyszczuk P, Czyz J, Kania G, Wagner M, Roll U, St-Onge L.et al. (2003Expression of Pax4 in embryonic stem cells promotes differentiation of nestin-positive progenitor and insulin-producing cells Proc Natl Acad Sci USA 100998–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson M, Tonning A, Frandsen U, Petri A, Rajagopal J, Englund MC.et al. (2004Artifactual insulin release from differentiated embryonic stem cells Diabetes 532603–2609. [DOI] [PubMed] [Google Scholar]
- Hori Y, Rulifson IC, Tsai BC, Heit JJ, Cahoy JD., and, Kim SK. Growth inhibitors promote differentiation of insulin-producing tissue from embryonic stem cells. Proc Natl Acad Sci USA. 2002;99:16105–16110. doi: 10.1073/pnas.252618999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritoh Y, Yamato E, Yasui Y, Miyazaki S., and, Miyazaki J. Analysis of insulin-producing cells during in vitro differentiation from feeder-free embryonic stem cells. Diabetes. 2003;52:1163–1168. doi: 10.2337/diabetes.52.5.1163. [DOI] [PubMed] [Google Scholar]
- Paek HJ, Morgan JR., and, Lysaght MJ. Sequestration and synthesis: the source of insulin in cell clusters differentiated from murine embryonic stem cells. Stem Cells. 2005;23:862–867. doi: 10.1634/stemcells.2004-0288. [DOI] [PubMed] [Google Scholar]
- Rajagopal J, Anderson WJ, Kume S, Martinez OI., and, Melton DA. Insulin staining of ES cell progeny from insulin uptake. Science. 2003;299:363. doi: 10.1126/science.1077838. [DOI] [PubMed] [Google Scholar]
- Sipione S, Eshpeter A, Lyon JG, Korbutt GS., and, Bleackley RC. Insulin expressing cells from differentiated embryonic stem cells are not β cells. Diabetologia. 2004;47:499–508. doi: 10.1007/s00125-004-1349-z. [DOI] [PubMed] [Google Scholar]
- Naujok O, Francini F, Picton S, Jörns A, Bailey CJ., and, Lenzen S. A new experimental protocol for preferential differentiation of mouse embryonic stem cells into insulin-producing cells. Cell Transplant. 2008;17:1231–1242. doi: 10.3727/096368908787236549. [DOI] [PubMed] [Google Scholar]
- Segev H, Fishman B, Ziskind A, Shulman M., and, Itskovitz-Eldor J. Differentiation of human embryonic stem cells into insulin-producing clusters. Stem Cells. 2004;22:265–274. doi: 10.1634/stemcells.22-3-265. [DOI] [PubMed] [Google Scholar]
- Bhushan A, Itoh N, Kato S, Thiery JP, Czernichow P, Bellusci S.et al. (2001Fgf10 is essential for maintaining the proliferative capacity of epithelial progenitor cells during early pancreatic organogenesis Development 1285109–5117. [DOI] [PubMed] [Google Scholar]
- D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG.et al. (2006Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells Nat Biotechnol 241392–1401. [DOI] [PubMed] [Google Scholar]
- Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S.et al. (2008Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo Nat Biotechnol 26443–452. [DOI] [PubMed] [Google Scholar]
- Zhang D, Jiang W, Liu M, Sui X, Yin X, Chen S.et al. (2009Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells Cell Res 19429–438. [DOI] [PubMed] [Google Scholar]
- Jeon J, Correa-Medina M, Ricordi C, Edlund H., and, Diez JA. Endocrine cell clustering during human pancreas development. J Histochem Cytochem. 2009;57:811–824. doi: 10.1369/jhc.2009.953307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo A, Shinozaki K, Shannon JM, Kouskoff V, Kennedy M, Woo S.et al. (2004Development of definitive endoderm from embryonic stem cells in culture Development 1311651–1662. [DOI] [PubMed] [Google Scholar]
- D'Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E., and, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- Adewumi O, Aflatoonian B, Ahrlund-Richter L, Amit M, Andrews PW, Beighton G, International Stem Cell Initiative et al. Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nat Biotechnol. 2007;25:803–816. doi: 10.1038/nbt1318. [DOI] [PubMed] [Google Scholar]
- Osafune K, Caron L, Borowiak M, Martinez RJ, Fitz-Gerald CS, Sato Y.et al. (2008Marked differences in differentiation propensity among human embryonic stem cell lines Nat Biotechnol 26313–315. [DOI] [PubMed] [Google Scholar]
- Cho YM, Lim JM, Yoo DH, Kim JH, Chung SS, Park SG.et al. (2008β Cellulin and nicotinamide sustain PDX1 expression and induce pancreatic β-cell differentiation in human embryonic stem cells Biochem Biophys Res Commun 366129–134. [DOI] [PubMed] [Google Scholar]
- Ahlgren U, Jonsson J, Jonsson L, Simu K., and, Edlund H. β-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the β-cell phenotype and maturity onset diabetes. Genes Dev. 1998;12:1763–1768. doi: 10.1101/gad.12.12.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney ML, Jones PM., and, Burns CJ. Importance of quantitative analysis in the generation of insulin-expressing cells from human embryonic stem cells. Pancreas. 2010;39:105–107. doi: 10.1097/MPA.0b013e3181b79d3c. [DOI] [PubMed] [Google Scholar]
- Shim JH, Kim SE, Woo DH, Kim SK, Oh CH, McKay R.et al. (2007Directed differentiation of human embryonic stem cells towards a pancreatic cell fate Diabetologia 501228–1238. [DOI] [PubMed] [Google Scholar]
- Jiang J, Au M, Lu K, Eshpeter A, Korbutt G, Fisk G.et al. (2007Generation of insulin-producing islet-like clusters from human embryonic stem cells Stem Cells 251940–1953. [DOI] [PubMed] [Google Scholar]
- Jiang W, Shi Y, Zhao D, Chen S, Yong J, Zhang J.et al. (2007In vitro derivation of functional insulin-producing cells from human embryonic stem cells Cell Res 17333–344. [DOI] [PubMed] [Google Scholar]
- Luther MJ, Hauge-Evans A, Souza KL, Jörns A, Lenzen S, Persaud SJ.et al. (2006MIN6 β-cell-β-cell interactions influence insulin secretory responses to nutrients and non-nutrients Biochem Biophys Res Commun 34399–104. [DOI] [PubMed] [Google Scholar]
- Barrientos R, Baltrusch S, Sigrist S, Legeay G, Belcourt A., and, Lenzen S. Kinetics of insulin secretion from MIN6 pseudoislets after encapsulation in a prototype device of a bioartificial pancreas. Horm Metab Res. 2009;41:5–9. doi: 10.1055/s-0028-1087185. [DOI] [PubMed] [Google Scholar]
- Fujikawa T, Oh SH, Pi L, Hatch HM, Shupe T., and, Petersen BE. Teratoma formation leads to failure of treatment for type I diabetes using embryonic stem cell-derived insulin-producing cells. Am J Pathol. 2005;166:1781–1791. doi: 10.1016/S0002-9440(10)62488-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshpeter A, Jiang J, Au M, Rajotte RV, Lu K, Lebkowski JS.et al. (2008In vivo characterization of transplanted human embryonic stem cell-derived pancreatic endocrine islet cells Cell Prolif 41843–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naujok O, Francini F, Picton S, Bailey CJ, Lenzen S., and, Jörns A. Changes in gene expression and morphology of mouse embryonic stem cells on differentiation into insulin-producing cells in vitro and in vivo. Diabetes Metab Res Rev. 2009;25:464–476. doi: 10.1002/dmrr.965. [DOI] [PubMed] [Google Scholar]
- Matveyenko AV, Georgia S, Bhushan A., and, Butler PC. Inconsistent formation and nonfunction of insulin-positive cells from pancreatic endoderm derived from human embryonic stem cells in athymic nude rats. Am J Physiol Endocrinol Metab. 2010;299:E713–E720. doi: 10.1152/ajpendo.00279.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Wurdak H, Wang J, Lyssiotis CA, Peters EC, Cho CY.et al. (2009A small molecule primes embryonic stem cells for differentiation Cell Stem Cell 4416–426. [DOI] [PubMed] [Google Scholar]
- Chen S, Borowiak M, Fox JL, Maehr R, Osafune K, Davidow L.et al. (2009A small molecule that directs differentiation of human ESCs into the pancreatic lineage Nat Chem Biol 5258–265. [DOI] [PubMed] [Google Scholar]
- Borowiak M, Maehr R, Chen S, Chen AE, Tang W, Fox JL.et al. (2009Small molecules efficiently direct endodermal differentiation of mouse and human embryonic stem cells Cell Stem Cell 4348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagita RC, Nakagawa Y, Yamanaka N, Kashiwagi K, Saito N., and, Irie K. Synthesis, conformational analysis, and biological evaluation of 1-hexylindolactam-V10 as a selective activator for novel protein kinase C isozymes. J Med Chem. 2008;51:46–56. doi: 10.1021/jm0706719. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Irie K, Yanagita RC, Ohigashi H., and, Tsuda K. Indolactam-V is involved in the CH/pi interaction with Pro-11 of the PKCΔ C1B domain: application for the structural optimization of the PKCΔ ligand. J Am Chem Soc. 2005;127:5746–5747. doi: 10.1021/ja050447d. [DOI] [PubMed] [Google Scholar]
- Nitti M, Furfaro AL, Cevasco C, Traverso N, Marinari UM, Pronzato MA.et al. (2010PKC Δ and NADPH oxidase in retinoic acid-induced neuroblastoma cell differentiation Cell Signal 22828–835. [DOI] [PubMed] [Google Scholar]
- Kambhampati S, Li Y, Verma A, Sassano A, Majchrzak B, Deb DK.et al. (2003Activation of protein kinase C Δ by all-trans-retinoic acid J Biol Chem 27832544–32551. [DOI] [PubMed] [Google Scholar]
- Leitges M, Plomann M, Standaert ML, Bandyopadhyay G, Sajan MP, Kanoh Y.et al. (2002Knockout of PKC α enhances insulin signaling through PI3K Mol Endocrinol 16847–858. [DOI] [PubMed] [Google Scholar]
- Standaert ML, Bandyopadhyay G, Galloway L, Soto J, Ono Y, Kikkawa U.et al. (1999Effects of knockout of the protein kinase C β gene on glucose transport and glucose homeostasis Endocrinology 1404470–4477. [DOI] [PubMed] [Google Scholar]
- Hennige AM, Ranta F, Heinzelmann I, Düfer M, Michael D, Braumüller H.et al. (2010Overexpression of kinase-negative protein kinase CΔ in pancreatic β-cells protects mice from diet-induced glucose intolerance and β-cell dysfunction Diabetes 59119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida T, Iwashita N, Ohara-Imaizumi M, Ogihara T, Nagai S, Choi JB.et al. (2007Protein kinase CΔ plays a non-redundant role in insulin secretion in pancreatic β cells J Biol Chem 2822707–2716. [DOI] [PubMed] [Google Scholar]
- Champeris Tsaniras S., and, Jones PM. Generating pancreatic β-cells from embryonic stem cells by manipulating signaling pathways. J Endocrinol. 2010;206:13–26. doi: 10.1677/JOE-10-0073. [DOI] [PubMed] [Google Scholar]
- Mfopou JK, Chen B, Sui L, Sermon K., and, Bouwens L. Recent advances and prospects in the differentiation of pancreatic cells from human embryonic stem cells. Diabetes. 2010;59:2094–2101. doi: 10.2337/db10-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., and, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K.et al. (2007Induction of pluripotent stem cells from adult human fibroblasts by defined factors Cell 131861–872. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S.et al. (2007Induced pluripotent stem cell lines derived from human somatic cells Science 3181917–1920. [DOI] [PubMed] [Google Scholar]
- Shi Y, Do JT, Desponts C, Hahm HS, Schöler HR., and, Ding S. A combined chemical and genetic approach for the generation of induced pluripotent stem cells. Cell Stem Cell. 2008;2:525–528. doi: 10.1016/j.stem.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Aasen T, Raya A, Barrero MJ, Garreta E, Consiglio A, Gonzalez F.et al. (2008Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes Nat Biotechnol 261276–1284. [DOI] [PubMed] [Google Scholar]
- Loh YH, Agarwal S, Park IH, Urbach A, Huo H, Heffner GC.et al. (2009Generation of induced pluripotent stem cells from human blood Blood 1135476–5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtfeld M, Brennand K., and, Hochedlinger K. Reprogramming of pancreatic β cells into induced pluripotent stem cells. Curr Biol. 2008;18:890–894. doi: 10.1016/j.cub.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoi T, Yae K, Nakagawa M, Ichisaka T, Okita K, Takahashi K.et al. (2008Generation of pluripotent stem cells from adult mouse liver and stomach cells Science 321699–702. [DOI] [PubMed] [Google Scholar]
- Huangfu D, Maehr R, Guo W, Eijkelenboom A, Snitow M, Chen AE.et al. (2008Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds Nat Biotechnol 26795–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita K, Ichisaka T., and, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II.et al. (2009Human induced pluripotent stem cells free of vector and transgene sequences Science 324797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woltjen K, Michael IP, Mohseni P, Desai R, Mileikovsky M, Hämäläinen R.et al. (2009piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells Nature 458766–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldner F, Hockemeyer D, Beard C, Gao Q, Bell GW, Cook EG.et al. (2009Parkinson's disease patient-derived induced pluripotent stem cells free of viral reprogramming factors Cell 136964–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelkel C, Galla M, Maetzig T, Warlich E, Kuehle J, Zychlinski D.et al. (2010Protein transduction from retroviral Gag precursors Proc Natl Acad Sci USA 1077805–7810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Wu S, Joo JY, Zhu S, Han DW, Lin T.et al. (2009Generation of induced pluripotent stem cells using recombinant proteins Cell Stem Cell 4381–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS.et al. (2009Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins Cell Stem Cell 4472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateishi K, He J, Taranova O, Liang G, D'Alessio AC., and, Zhang Y. Generation of insulin-secreting islet-like clusters from human skin fibroblasts. J Biol Chem. 2008;283:31601–31607. doi: 10.1074/jbc.M806597200. [DOI] [PubMed] [Google Scholar]
- Maehr R, Chen S, Snitow M, Ludwig T, Yagasaki L, Goland R.et al. (2009Generation of pluripotent stem cells from patients with type 1 diabetes Proc Natl Acad Sci USA 10615768–15773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka S. Elite and stochastic models for induced pluripotent stem cell generation. Nature. 2009;460:49–52. doi: 10.1038/nature08180. [DOI] [PubMed] [Google Scholar]
- Guenther MG, Frampton GM, Soldner F, Hockemeyer D, Mitalipova M, Jaenisch R.et al. (2010Chromatin structure and gene expression programs of human embryonic and induced pluripotent stem cells Cell Stem Cell 7249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo JM, Liu S, Figueroa ME, Kulalert W, Eminli S, Tan KY.et al. (2010Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells Nat Biotechnol 28848–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P.et al. (2010Epigenetic memory in induced pluripotent stem cells Nature 467285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi A, Park IH, Wen B, Murakami P, Aryee MJ, Irizarry R.et al. (2009Differential methylation of tissue- and cancer-specific CpG island shores distinguishes human induced pluripotent stem cells, embryonic stem cells and fibroblasts Nat Genet 411350–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, Shoemaker R, Xie B, Gore A, LeProust EM, Antosiewicz-Bourget J.et al. (2009Targeted bisulfite sequencing reveals changes in DNA methylation associated with nuclear reprogramming Nat Biotechnol 27353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtfeld M, Apostolou E, Akutsu H, Fukuda A, Follett P, Natesan S.et al. (2010Aberrant silencing of imprinted genes on chromosome 12qF1 in mouse induced pluripotent stem cells Nature 465175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister R, Pelizzola M, Kida YS, Hawkins RD, Nery JR, Hon G.et al. (2011Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells Nature 47168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu BY, Weick JP, Yu J, Ma LX, Zhang XQ, Thomson JA.et al. (2010Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency Proc Natl Acad Sci USA 1074335–4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jozefczuk J, Prigione A, Chavez L., and, Adjaye J. Comparative analysis of human embryonic stem cell and induced pluripotent stem cell-derived hepatocyte-like cells reveals current drawbacks and possible strategies for improved differentiation. Stem Cells Dev. 2011;20:1259–1275. doi: 10.1089/scd.2010.0361. [DOI] [PubMed] [Google Scholar]
- Laurent LC, Ulitsky I, Slavin I, Tran H, Schork A, Morey R.et al. (2011Dynamic changes in the copy number of pluripotency and cell proliferation genes in human ESCs and iPSCs during reprogramming and time in culture Cell Stem Cell 8106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein SM, Batada NN, Vuoristo S, Ching RW, Autio R, Närvä E.et al. (2011Copy number variation and selection during reprogramming to pluripotency Nature 47158–62. [DOI] [PubMed] [Google Scholar]
- Gore A, Li Z, Fung HL, Young JE, Agarwal S, Antosiewicz-Bourget J.et al. (2011Somatic coding mutations in human induced pluripotent stem cells Nature 47163–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayshar Y, Ben-David U, Lavon N, Biancotti JC, Yakir B, Clark AT.et al. (2010Identification and classification of chromosomal aberrations in human induced pluripotent stem cells Cell Stem Cell 7521–531. [DOI] [PubMed] [Google Scholar]
- Miura K, Okada Y, Aoi T, Okada A, Takahashi K, Okita K.et al. (2009Variation in the safety of induced pluripotent stem cell lines Nat Biotechnol 27743–745. [DOI] [PubMed] [Google Scholar]
- Zhao T, Zhang ZN, Rong Z., and, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–215. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- Evans MJ., and, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS.et al. (1998Embryonic stem cell lines derived from human blastocysts Science 2821145–1147. [DOI] [PubMed] [Google Scholar]
- Blum B., and, Benvenisty N. The tumorigenicity of human embryonic stem cells. Adv Cancer Res. 2008;100:133–158. doi: 10.1016/S0065-230X(08)00005-5. [DOI] [PubMed] [Google Scholar]
- Knoepfler PS. Deconstructing stem cell tumorigenicity: a roadmap to safe regenerative medicine. Stem Cells. 2009;27:1050–1056. doi: 10.1002/stem.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug MG, Soonpaa MH, Koh GY., and, Field LJ. Genetically selected cardiomyocytes from differentiating embronic stem cells form stable intracardiac grafts. J Clin Invest. 1996;98:216–224. doi: 10.1172/JCI118769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobinskaya I, Linn T, Saric T, Bretzel RG, Bohlen H, Hescheler J.et al. (2008Scalable selection of hepatocyte- and hepatocyte precursor-like cells from culture of differentiating transgenically modified murine embryonic stem cells Stem Cells 262245–2256. [DOI] [PubMed] [Google Scholar]
- Naujok O, Kaldrack J, Taivankhuu T, Jörns A., and, Lenzen S. Selective removal of undifferentiated embryonic stem cells from differentiation cultures through HSV1 thymidine kinase and ganciclovir treatment. Stem Cell Rev. 2010;6:450–461. doi: 10.1007/s12015-010-9148-z. [DOI] [PubMed] [Google Scholar]
- Choo AB, Tan HL, Ang SN, Fong WJ, Chin A, Lo J.et al. (2008Selection against undifferentiated human embryonic stem cells by a cytotoxic antibody recognizing podocalyxin-like protein-1 Stem Cells 261454–1463. [DOI] [PubMed] [Google Scholar]
- Tan HL, Fong WJ, Lee EH, Yap M., and, Choo A. mAb 84, a cytotoxic antibody that kills undifferentiated human embryonic stem cells via oncosis. Stem Cells. 2009;27:1792–1801. doi: 10.1002/stem.109. [DOI] [PubMed] [Google Scholar]
- Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Südhof TC., and, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo E, Rampalli S, Risueño RM, Schnerch A, Mitchell R, Fiebig-Comyn A.et al. (2010Direct conversion of human fibroblasts to multilineage blood progenitors Nature 468521–526. [DOI] [PubMed] [Google Scholar]
- Efe JA, Hilcove S, Kim J, Zhou H, Ouyang K, Wang G.et al. (2011Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy Nat Cell Biol 13215–222. [DOI] [PubMed] [Google Scholar]
- Huang P, He Z, Ji S, Sun H, Xiang D, Liu C.et al. (2011Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors Nature [DOI] [PubMed]
- Zhou Q, Brown J, Kanarek A, Rajagopal J., and, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to β-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley RK, Sutherland DE, Goetz F., and, Michael AF. Recurrent diabetes mellitus in the pancreas iso- and allograft. A light and electron microscopic and immunohistochemical analysis of four cases. Lab Invest. 1985;53:132–144. [PubMed] [Google Scholar]
- Shiroi A, Yoshikawa M, Yokota H, Fukui H, Ishizaka S, Tatsumi K.et al. (2002Identification of insulin-producing cells derived from embryonic stem cells by zinc-chelating dithizone Stem Cells 20284–292. [DOI] [PubMed] [Google Scholar]
- Houard N, Rousseau GG., and, Lemaigre FP. HNF-6-independent differentiation of mouse embryonic stem cells into insulin-producing cells. Diabetologia. 2003;46:378–385. doi: 10.1007/s00125-003-1041-8. [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Yamato E., and, Miyazaki J. Regulated expression of pdx-1 promotes in vitro differentiation of insulin-producing cells from embryonic stem cells. Diabetes. 2004;53:1030–1037. doi: 10.2337/diabetes.53.4.1030. [DOI] [PubMed] [Google Scholar]