Over the fve decades since their frst defnitive identifcation,1,2 hemato-poietic stem cells (HSCs) have emerged as the most clinically exploited somatic stem cell population, with more than 55,000 bone marrow transplants (au-tologous and allogeneic combined) performed worldwide in 2009, including about 20,000 in the United States alone.3 Our inability to directly identify human HSCs among progenitors of more limited potential has hampered high-resolution molecular analysis of human long-term HSCs (LT-HSCs), which is the key to unlocking their clinical and therapeutic potential and bridging the gap between suitable stem cell supply and demand. A recent xenograf study reported by Notta et al. in Science4 has brought one step closer the possibility of modulating human LT-HSCs ex vivo for clinical therapies.
Bone marrow transplantation has become the standard of care for many malignant and nonmalignant hematopoietic diseases, including Hodgkin's disease, non-Hodgkin's lymphoma, multiple myeloma, acute leukemia, chronic leukemia, aplastic anemia, and myelodysplastic syndromes. Despite this clinical success, the demand for compatible transplant marrow far outweighs the supply of suitable donor material. Eforts to bridge this disparity have led to experimental studies to better identify and expand the most important human HSC subsets. The mouse has served as the most widely used experimental model system for studying HSC biology. A vast array of markers have been described that can be used in fow-cytometric sorting to obtain populations of mouse bone marrow cells that are highly enriched for HSCs.5,6,7,8,9,10,11 Tis has led to many elegant molecular studies of highly purifed mouse HSCs, yielding tremendous insight into the mechanisms that empower their unique characteristics. However, the same cannot be said for human HSC research. The markers used for segregation of true murine LT-HSCs from short-lived or lineage-restricted progenitors are not necessarily conserved between mice and humans.
Notta et al.4 used a mouse model to identify a population of human cells with the phenotype of CD34+CD38−CD45RA− Ty1+RholoCD49f+, which was highly enriched for long-term in vivo HSC activity at the single-cell level. One of the major milestones in this paper was the delineation of true human stem cell activity from that of multipotent progenitors that are able to give rise to multilineage diferentiation in vivo, albeit only transiently (most activity gone by 10 weeks post-transplant). Discrimination of human cell populations with diferent in vivo functional potential using their new markers and this sensitive transplantation assay will allow for powerful molecular analysis of highly enriched cell populations (Figure 1).
Figure 1.
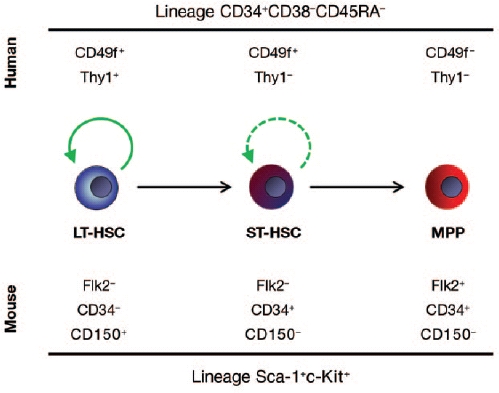
Cell surface markers used for segregation of human and mouse LT-HSCs from ST-HSCs and multipotent progenitors. Green arrows represent self-renewal potential, with the long-term hematopoietic stem cells (LT-HSCs) possessing durable self-renewal potential and the short-term HSCs (ST-HSCs) showing more limited capacity. Multipotent progenitors (MPPs) have no long-term self-renewal in vivo.
Ultimately, HSCs are defned by function, not by phenotype, and the gold standard for in vivo HSC activity is bone marrow transplantation. The operational defnition of an HSC in general terms is the ability of a cell to repopulate a recipient mouse with long-term (>4 months) multilineage reconstitution, with a single clone contributing to my-eloid, B-, and T-cell lineages. In the past, clonality was examined by specifc chromosomal translocations, then by unique retroviral integration sites. More recently, the “platinum standard” has been to transplant mice with a single cell. With the highest-purity murine stem cell populations, around one in three to one in fve of transplanted mice will show mul-tilineage blood contribution with a single HSC.,6,9,12,13,14,15
Although the studies described above have allowed refnement of the phenotyp-ic defnition of HSCs and enabled markers to be identifed that allow separation of HSC subtypes,11,14,15 similar progress in human HSC research has lagged behind. Human HSCs have been defned by either in vitro activity or transplantation into mice. Over the past 20 years, several mouse strains have been developed and tested for their ability to accept human hematopoietic grafs. Although many immunocompromised mouse strains will support some human hematopoietic development, the various models have supported some lineages better than others, making it difcult to discern true HSC quality diferences. Over time, the use of the various strains has become more refned with the use of severely immuno-compromised recipient mice such as NOD-scid-IL2Rγ−/− (NSG). NSG mice lack mature T cells, B cells, and functional natural killer cells and are defcient in cytokine signaling, providing the most permissive environment for engrafment of human donor cells. Although these refnements have markedly improved sensitivity of engrafment into mice, they restrict the techniques almost exclusively to the hands of the most expert labs because the immunocompromised mice are expensive to maintain and the experiments technically demanding. Nevertheless, these studies are essential, for even though the mouse HSC has served as the model of choice for experimental hema-tology, many of the fndings cannot be extrapolated to human HSCs, including, notably, the cell surface markers used for HSC purifcation (Figure 1).
The study by Notta et al.4 represents a landmark because it combined the latest and best xenotransplantation strategies with the newest human HSC markers to achieve remarkably robust long-term, multilineage engrafment from transplantation of highly purifed HSCs. The authors performed intrafemoral injections of purifed cell populations into NSG mice,4 thus avoiding the potential complications due to HSC homing that arise from the typical injection of donor cells into the circulatory system either retro-orbitally or via the tail vein. By doing this, they could demonstrate robust chimerism of human cells in the hema-topoietic system (blood, bone marrow, spleen, thymus) at 20 weeks post-transplant with contribution to erythroid, B-lymphoid, myeloid, and T-lymphoid lineages. Tey ultimately applied the platinum standard and transplanted single human HSCs into the mice, observing multilineage engrafment from 14 to 28% of individual human HSCs. Tese cells were also capable of repopulating secondary hosts, indicating extensive self-renewal ability.
The authors did note some variability in HSC frequency between experiments that they attribute to the genetic heterogeneity of diferent cord blood donors.4 Although this study did not demonstrate the level of HSC frequency seen in clonal transplantation of highly purifed mouse HSC fractions, it represents a remarkable technical feat achieved through years of refning techniques. Tetechnical challenges associated with single-cell transplantation (maintenance of cell viability during sorting, ensuring that the test cell is actually contained within the injection bolus, correct placement of injection site) probably result in underestimation of the HSC frequency in such studies. In addition, the technical challenges associated with this system may prevent most labs from using it as a routine assay. Tus, although this study sets a new standard, most other human HSC work should not be required to match this, and, indeed, a test of function on a clonal level is not needed in most experimental settings.
A devil's advocate could argue that xenograf transplantation of human cells into mice may not refect the true properties of human HSCs; the assay may best identify cells that can survive and proliferate in response to murine cyto-kines in a foreign environment rather than native human HSCs. Although the general bias of the feld is that an in vivo transplantation experiment, however foreign, is better than an in vitro ass ay, we cannot really know how well this simulates transplantation of marrow and cord blood into human patients aficted with hematological diseases. The dearth of reliable assays to test the activity of human cells remains a major impediment to much of cell therapy (and stem cell) research. Although more work is needed to determine whether the cells identifed by Notta et al. represent the real HSCs responsible for repopulating transplant patients, the study nonetheless represents a remarkable technical achievement and enables us to further investigate an important candidate stem cell. The challenge will now be to direct these cells into therapeutically useful applications such as ex vivo expansion and gene therapy.
REFERENCES
- Till JE., and, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14:213–222. [PubMed] [Google Scholar]
- Becker AJ, McCulloch EA., and, Till JE. Cyto-logical demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963;197:452–454. doi: 10.1038/197452a0. [DOI] [PubMed] [Google Scholar]
- Pasquini MC., and, Wang Z.2010Current use and outcome of hematopoietic stem cell transplantation: CIBMTR Summary Slides, 2010. Available at http://www.cibmtr.org
- Notta F, Doulatov S, Laurenti E, Poeppl A, Jurisica I., and, Dick JE. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science. 2011;333:218–221. doi: 10.1126/science.1201219. [DOI] [PubMed] [Google Scholar]
- Spangrude GJ, Heimfeld S., and, Weissman IL. Purifcation and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Osawa M, Hanada K, Hamada H., and, Nakauchi H. Long-term lymphohematopoietic reconsti-tution by a single CD34-low/negative hematopoi-etic stem cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- Goodell MA, Brose K, Paradis G, Conner AS., and, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen JL., and, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc Natl Acad Sci USA. 2001;98:14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C., and, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Balazs AB, Fabian AJ, Esmon CT., and, Mulligan RC. Endothelial protein C receptor (CD201) explicitly identifes hematopoietic stem cells in murine bone marrow. Blood. 2006;107:2317–2321. doi: 10.1182/blood-2005-06-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste P.et al. (2010Intermediate-term hematopoietic stem cells with extended but time-limited reconstitution potential. Cell Stem Cell 648–58. [DOI] [PubMed] [Google Scholar]
- Camargo FD, Chambers SM, Drew E, McNagny KM., and, Goodell MA. Hematopoietic stem cells do not engraft with absolute effciencies. Blood. 2006;107:501–507. doi: 10.1182/blood-2005-02-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykstra B.et al. (2007Long-term propagation of distinct hematopoietic differentiation programs in vivo. Cell Stem Cell 1218–229. [DOI] [PubMed] [Google Scholar]
- Challen GA, Boles NC, Chambers SM., and, Goodell MA. Distinct hematopoietic stem cell subtypes are differentially regulated by TGF-beta1. Cell Stem Cell. 2010;6:265–278. doi: 10.1016/j.stem.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita Y, Ema H., and, Nakauchi H. Heterogeneity and hierarchy within the most primitive hematopoietic stem cell compartment. J Exp Med. 2010;207:1173–1182. doi: 10.1084/jem.20091318. [DOI] [PMC free article] [PubMed] [Google Scholar]


