Abstract
Glioblastoma multiforme (GBM) is a primary brain tumor with a median survival of 14.6 months postdiagnosis. The infiltrative nature of GBM prevents complete resection and residual brain tumor cells give rise to recurrent GBM, a hallmark of this disease. Recurrent GBMs are known to harbor numerous mutations/gene rearrangements when compared to the primary tumor, which leads to the potential expression of novel proteins that could serve as tumor neoantigens. We have developed a combined immune-based gene therapeutic approach for GBM using adenoviral (Ads) mediated gene delivery of Herpes Simplex Virus Type 1-thymidine kinase (TK) into the tumor mass to induce tumor cells' death combined with an adenovirus expressing fms-like tyrosine kinase 3 ligand (Flt3L) to recruit dendritic cells (DCs) into the tumor microenvironment. This leads to the induction of specific anti-brain tumor immunity and immunological memory. In a model of GBM recurrence, we demonstrate that Flt3L/TK mediated immunological memory is capable of recognizing brain tumor neoantigens absent from the original treated tumor. These data demonstrate that the Flt3L/TK gene therapeutic approach can induce systemic immunological memory capable of recognizing a brain tumor neoantigen in a model of recurrent GBM.
Introduction
Glioblastoma multiforme (GBM) is the most frequently diagnosed primary brain tumor in adults and is accompanied by a dismal prognosis. The current clinical management of GBM patients involves resection of the brain tumor mass, followed by radiotherapy and/or treatment with the chemotherapeutic agent temozolomide. The highly infiltrative nature of GBM into the normal brain parenchyma prevents its complete resection. Residual brain tumor cells invariably give rise to recurrent GBM that ultimately lead to the patient's death. With recent therapeutic advances, most notably the inclusion of temozolomide as standard of care for GBM, the current median survival has increased to 14.6 months (ranging 13.2–16.8 months) postdiagnosis while the 2 year survival rate remains at <30%.1,2
Immunotherapeutic strategies for GBM are currently under evaluation in preclinical animal models and in human clinical trials.3,4,5,6,7,8,9,10,11,12,13,14,15,16,17 The brain is an immune privileged organ with a paucity of dendritic cells (DCs), a population of powerful antigen presenting cells.18 The rationale underlying immunotherapeutic approaches is to elicit anti-brain tumor specific immune responses able to recognize and destroy the macroscopic tumor mass and infiltrating brain tumor cells, thus preventing or delaying tumor recurrences.19
Some examples of immunotherapeutic approaches currently under evaluation in clinical trials are direct vaccination with peptides derived from EGFRvIII,16,17 a tumor antigen which is expressed in ~30% of human GBM patients,20,21 vaccination with DCs pulsed with EGFRvIII,4,6,7,8 or vaccination with DCs pulsed ex vivo with autologous tumor lysates.3,10,12,15,22,23 These therapeutic approaches are designed to facilitate the presentation of specific tumor antigens to naive T cells, thereby inducing the proliferation of cytotoxic T cells specific for brain tumor antigens. However, the heterogeneity of malignant glioma may curtail the effectiveness of vaccination strategies that target a restricted set of tumor antigens.16
In addition to the highly infiltrative nature and the heterogeneity of GBM, another obstacle to the efficacious treatment of brain tumors is their high mutation rate. This phenomenon was recently highlighted in two studies that demonstrated an altered tumor phenotype following treatment with either bevacizumab,24 a monoclonal antibody to vascular endothelial growth factor, or with PEPvIII, an EGFRvIII-targeted peptide vaccine.16 Furthermore, there is evidence that the expression levels of numerous genes are altered in recurrent GBM tumors, i.e., the tumor suppressor gene TP53, the cellular oncogene MDM2, EGFR, and the mismatch repair gene MSH2.25 Finally, a recent analysis of GBM tissue from patient samples postchemotherapy revealed the presence of mutations in the mismatch repair gene MSH6, which are selected during temozolomide therapy and are causally associated with temozolomide resistance.26,27
In an attempt to address some of the therapeutic challenges imposed by GBM, we have developed an immunotherapeutic approach in which a combination gene therapy is delivered directly to the tumor mass to both kill brain tumor cells and concomitantly prime an immune response against brain tumor antigens.28,29,30,31,32,33 To do so, we engineered two, first generation adenoviral vectors (Ads) encoding either Herpes Simplex type-1 thymidine kinase (TK),30,34 a conditionally cytotoxic gene which kills proliferating tumor cells in the presence of the pro-drug ganciclovir (GCV), or human soluble fms-like tyrosine kinase 3 ligand (Flt3L),35 which results in increased levels of DCs in the brain tumor microenvironment in mice28,36 and rats.30,35,37 Using bone marrow chimeric mice bearing orthotopic syngeneic GL26 tumors, we demonstrated that Flt3L/TK gene therapy results in the infiltration of bone marrow-derived DCs into the brain tumor microenvironment.28
In this combined gene therapy approach, rather than vaccinating against specific tumor antigens, we aim to recruit DCs into the brain tumor mass where they will undergo in situ priming against brain tumor antigens. We have previously shown that codelivery of Ad-Flt3L and Ad-TK into a large brain tumor mass induces both cellular and humoral anti-GBM specific immune responses,28,29,32,33 leading to long-term survival of rats bearing intracranial CNS1, 9L, and F98 tumors,30,33 and in mice bearing intracranial GL26, GL261, and B16-F10 tumors.28,32 Importantly, Ad-Flt3L + Ad-TK induces GBM-specific immunological memory that eliminates distal, untreated brain tumor foci in an intracranial multifocal rat brain tumor model38 and protects against brain tumor rechallenge in orthotopic rat and mouse models of recurrent GBM.28,33,38,39
Herein, we wished to test the hypothesis that Flt3L/TK mediated gene therapy induces immunological memory capable of recognizing a novel antigen that was absent from the originally treated brain tumor. To do so, we engineered the rat brain tumor cell line CNS1 to stably express the influenza glycoprotein hemagglutinin (HA) as a surrogate tumor neoantigen.40 Rats bearing syngeneic orthotopic brain tumors derived from wild-type CNS1 cells were treated with Flt3L/TK gene therapy. Two months later, long-term survivors were rechallenged with CNS1-HA cells not treated in any way. Even though the primary brain tumors treated with Flt3L/TK gene therapy did not express HA, our data reveal the presence of HA specific T lymphocytes in the animals rechallenged with CNS1-HA tumor cells in the absence of treatment. These data demonstrate that Flt3L/TK induces brain tumor specific immunological memory which is also capable of detecting and mounting a specific immune response against a brain tumor neoantigen. These experiments highlight the effectiveness of the proposed approach to elicit regression of recurrent GBM.
Results
Characterization of the CNS1-HA cell line and intracranial tumor model
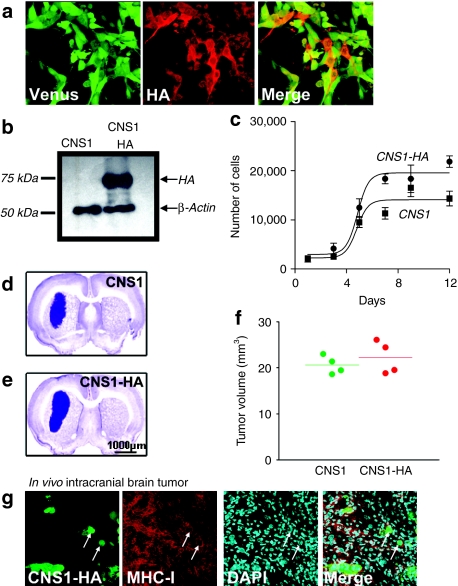
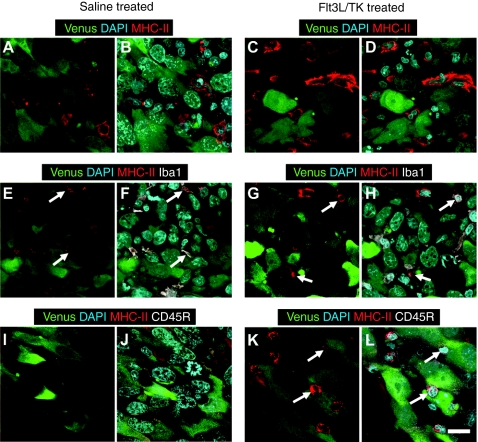
The CNS1-HA cell line was assessed for expression of the fluorescent protein Venus and the influenza protein HA by immunocytochemistry using a custom-made polyclonal primary antibody specific for HA (1:1,000) generated and described by us previously.41 Immunocytochemistry followed by immunofluoresence revealed that CNS1-HA cells express Venus (green) and HA (red) proteins (Figure 1a). Western blot analysis confirmed high levels of HA expression from CNS1-HA cells and a lack of HA expression from wild-type CNS1 cells (Figure 1b). In vitro (Figure 1c) analysis did not reveal a difference in the levels of cell proliferation of CNS1-HA cells compared to CNS1 cells. Also our in vivo results show that both intracranial brain tumors are approximately the same size at the time of treatment (Figure 1d–f). Intracranial tumors derived from CNS1-HA cells formed a large tumor mass that exhibited widespread expression of major histocompatibility complex (MHC)-I (Figure 1g). To assess whether MHC II expression can be upregulated in CNS1-HA tumor cells in response to the therapy, or is restricted to tumor infiltrating immune cells, Lewis rats bearing intracranial CNS1-HA tumors were treated with either Ad-Flt3L+Ad-TK, or saline. Immunofluorescence staining followed by confocal microscopy analysis revealed that MHC II expression did not colocalize with CNS1-HA cells in either saline or Ad-Flt3L+Ad-TK treated animals (Figure 2a–d). Furthermore double labeling immunofluoresence with markers for activated macrophages/microglia (Iba1, Figure 2e–h) or B cells and plasmacytoid DCs (CD45R, Figure 2i–l) demonstrates that MHC II immunoreactivity is colocalized with tumor infiltrating immune cells in both saline or Ad-Flt3L+Ad-TK treated animals. These data indicate that MHC II expression is not induced in brain tumor cells in response to the treatment.
Figure 1.
In vitro and in vivo characterization of CNS1-HA cells. (a) CNS1-HA cells were analyzed by immunocytochemistry with a primary antibody specific for hemagglutinin (HA) (red). Venus fluorescence (green) was used to identify the tumor cells. (b) Western blot analysis of CNS1 cells or CNS1-HA cells labeled with a primary antibody specific for HA, or β-actin as an internal loading control. (c) In vitro cell growth rates of CNS1 and CNS1-HA rat glioblastoma multiforme cells (n = 3 wells per group). Cells (2,000) were seeded on day 0, and cells were harvested and counted every third day for up to 12 days. (d–e). To assess in vivo growth characteristics, (d) CNS1 wild type or (e) CNS1-HA cells were implanted into the striatum of Lewis rats. Nine days later, animals were euthanized and brains were fixed, sectioned and stained with NissI to identify the intracranial brain tumor mass. (f) The volume of intracranial tumors was quantitated. (g) Immunofluoresence was used to analyze the co-localization of tumor cells (identified by Venus fluorescence) and MHC I (red). White arrows indicate tumors cells which colocalize with MHC I immunoreactivity. MHC, major histocompatibility complex.
Figure 2.
Intratumoral MHC II expression is restricted to tumor infiltrating immune cells and microglial cells. Brain sections from CNS1-HA tumor bearing rats treated with either saline or Ad-Flt3L+Ad-TK were analyzed by double labeling immunofluoresence followed by confocal microscopy. (a–d) MHC II immunoreactivity was visualized using a primary antibody specific for MHC II (red) and tumor cells were identified by Venus expression (green). 4′,6-diamidino-2-phenylindole (DAPI) was used to stain nuclei. (e–h) Brain sections were labeled for MHC II (red) and activated macrophages/microglia (Iba1, white). Tumor cells were identified by Venus expression (green). DAPI was used to stain nuclei. (i–l) Brain sections were labeled for MHC II (red) and B cells and plasmacytoid dendritic cells (CD45R, white). Tumor cells are identified by Venus expression (green). DAPI was used to stain nuclei. White arrows indicate MHC II immunoreactive cells that colocalize with either Iba1 or CD45R immunoreactivity. Bar = 18.75 µm. Flt3L, fms-like tyrosine kinase 3 ligand; MHC, major histocompatibility complex; TK, thymidine kinase.
Flt3L/TK gene therapy in the syngeneic CNS1-HA GBM model induces tumor regression and an HA specific immune response
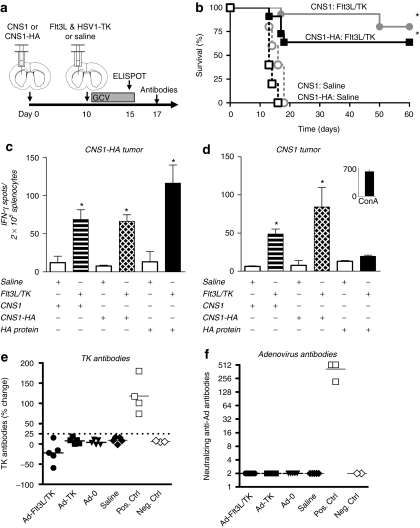
Given that we have previously shown that treatment of Lewis rats bearing intracranial syngeneic wild-type CNS1 tumors with Ad-Flt3L and Ad-TK results in brain tumor regression and long-term survival,29,30,33,38,39 we wished to assess whether Flt3L/TK gene therapy could elicit an immune response to a specific tumor antigen, i.e., HA used as a surrogate tumor antigen when expressed in CNS1 cells (Figure 3a). Figure 3b demonstrates that delivery of Ad-Flt3L and Ad-TK into CNS1-HA tumors leads to ~60% long-term survival. Five days after Flt3L/TK treatment of Lewis rats bearing either intracranial CNS1-HA tumors (Figure 3c), or wild-type CNS1 tumors (Figure 3d), splenocytes were isolated and used for an interferon (IFN)-γ ELISPOT to assess the specificity of T lymphocyte precursors in both tumor models. ELISPOT analysis revealed that both tumor models displayed an increase in the frequency of IFN-γ secreting T lymphocyte precursors when stimulated with either wild-type CNS1 or CNS1-HA cell lysates. Figure 3c,d reveals that only Flt3L/TK treated animals bearing CNS1-HA tumors exhibit evidence of an increase in the frequency of HA specific T lymphocyte precursors. Figure 3e,f reveal that treatment of intracranial brain tumors with Flt3L/TK gene therapy does not induce the production of circulating anti-TK antibodies (Figure 3e) or anti-adenovirus neutralizing antibodies (Figure 3f), thus suggesting tumor regression is a result of an anti-GBM immune response rather than by an immune response against Ad capsid proteins or transgene products.
Figure 3.
Intratumoral administration of Ad-Flt3L and Ad-TK induces an expansion of IFN-γ secreting T lymphocyte precursors specific for both tumor cells and also a neo-brain tumor antigen. (a). CNS1-HA cells or CNS1 wild type cells were implanted into the striatum of Lewis rats. Ten days later, animals received an intratumoral injection of Ad-Flt3L + Ad-TK, or saline (n = 4–5). Ganciclovir (GCV) was administered via intraperitoneal injection for 7 days and animals were assessed for survival, or euthanized 5 days later for ELISPOT analysis or 7 days later for antibody analysis. (b) Kaplan–Meier curve depicting survival of tumor bearing rats treated with Ad-Flt3L and Ad-TK, or saline. *P < 0.05 versus saline, Mantel log-rank test. A subset of animals bearing (c) CNS1-HA or (d) CNS1 wild type tumors (n = 4/cohort) were euthanized 5 days post-treatment and splenocytes were isolated to quantitate the frequency of IFN-γ-secreting T lymphocyte precursors specific for CNS1 or CNS1-HA brain tumor cells, or HA by IFN-γ ELISPOT. Splenocytes from each experimental group were stimulated with either CNS1 cell lysates, CNS1-HA cell lysates, or HA pure protein. Concanavalin A stimulation of splenocytes was used as a control in lieu of stimulation with tumor cells extracts or hemagglutinin (HA) protein. *P < 0.05 versus saline from same stimuli group, Student's t-test. (e, f) Animals bearing CNS1 wild type brain tumors were euthanized 7 days post gene therapy treatment to assess for circulating anti-TK antibodies and anti-adenovirus neutralizing antibodies. As controls animals were also treated with an intratumoral injection with either Ad-TK alone, Ad-0, or saline. (e) The incidence of anti-TK antibodies are shown. The titer of Anti-TK antibodies is represented as the % change in signal intensity from sera incubated with cell lysates from Ad-TK infected cells when compared to mock infected cells. Samples were considered positive when the % change was ≥25%; this threshold is indicated by the dashed line. Positive and negative assay controls are also shown, (Pos. Ctrl. and Neg. Ctrl, respectively).(f) The prevalence of anti-adenovrius neutralizing antibodies are shown. Neutralizing antibody titer for each animal is reported as the reciprocal of the highest dilution of serum at which 50% of Ad-hCMV-lacZ transduction is inhibited (Pos. Ctrl. and Neg. Ctrl, respectively). Flt3L, fms-like tyrosine kinase 3 ligand; IFN, interferon; TK, thymidine kinase.
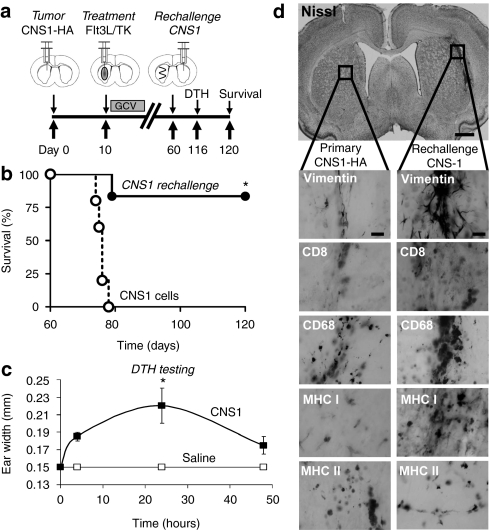
Anti-GBM immunological memory protects animals from rechallenge with GBM cells expressing a surrogate neoantigen
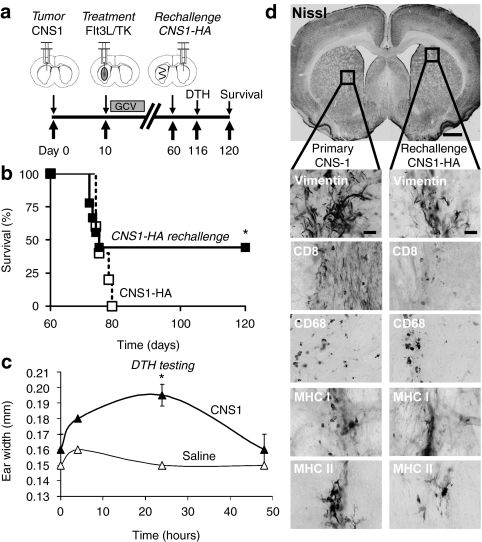
We have previously shown that rats bearing intracranial CNS1 tumors survive long-term when treated with Flt3L/TK gene therapy; importantly long-term survivors exhibit immunological memory that confers protection from rechallenge with CNS1 tumor cells implanted in the contralateral hemisphere,39 or in the periphery.33 To determine whether Flt3L/TK mediated immunological memory would also be capable of recognizing a brain tumor neoantigen, CNS1-wild type tumor bearing animals treated with Ad-Flt3L and Ad-TK that survived long-term were rechallenged with CNS1-HA cells in the contralateral hemisphere (Figure 4a). Long-term survivors rechallenged with CNS1-HA cells displayed long-term survival in 40% of the rechallenged animals (Figure 4b). Cellular immunity against brain tumor cells was also assessed using the delayed type hypersensitivity (DTH) reaction in the rechallenged long-term survivors (Figure 4c). DTH testing was performed ~2 months after CNS1-HA rechallenge of long-term survivors bearing wild-type CNS1 primary brain tumors. Rechallenged animals developed a strong DTH reaction, detected at 48 hour after injection of irradiated CNS1 cell lysates in the ear (Figure 4c) indicating the presence of brain tumor specific memory T cells. Animals surviving 60 days after the rechallenge with CNS1-HA cells were euthanized and their brains were analyzed by immunohistochemistry for tumor cells and markers of immune cells. A lack of gross neurotoxicity in either brain hemisphere with minimal levels of immune cells was observed in long-term survivors rechallenged with CNS1-HA tumor cells (Figure 4d).
Figure 4.
Rats bearing intracranial CNS1 brain tumors treated with Ad-Flt3L and Ad-TK survive rechallenge with CNS1-HA cells. (a) CNS1 cells were implanted into the striatum of Lewis rats. Ten days later, animals received an intratumoral injection of Ad-Flt3L + Ad-TK. Ganciclovir (GCV) was administered via intraperitoneal injection for 7 days. Sixty days later, animals surviving long-term were rechallenged with CNS1-HA cells in the contralateral hemisphere. No further treatment was administered. Naive animals were implanted with CNS1-HA cells to control for cell viability (n = 4–5). Animals were monitored for survival and underwent delayed type hypersensitivity (DTH) testing 4 days before euthanasia. (b) Kaplan–Meier curve depicting survival of tumor bearing rats rechallenged with CNS1-HA cells or naive rats implanted with CNS1-HA cell as a control. *P < 0.05, Mantel log-rank test. (c) DTH testing was performed in long-term survivors 4 days before euthanasia. Irradiated CNS1 cells were injected intradermally into the pinna of the right ear, and the left pinna received saline. The thickness of the pinna was recorded with slide calipers after 4, 24, and 48 h. *P < 0.05 versus saline ear (randomization test). (d) Neuropathological analysis of the brain at 60 days after rechallenge. CD8, CD8α+ cytotoxic T cells; CD68, macrophages/activated microglia; Flt3L, fms-like tyrosine kinase 3 ligand; MHC, major histocompatibility complex; TK, thymidine kinase; vimentin, tumor cells and reactive astrocytes.
As a control, we also performed the converse experiment, i.e., Flt3L/TK gene therapy treatment of primary brain tumors derived from CNS1-HA cells followed by rechallenging the long-term survivors with CNS1 wild type cells in the contralatereral hemisphere (Figure 5a). Long-term survivors rechallenged with CNS1 wild type cells displayed high levels of long-term survival (Figure 5b). These data demonstrate that Flt3L/TK treatment of CNS1-HA tumors results in not only an HA specific immune response as demonstrated in (Figure 3c), but also a brain tumor specific immune response. These data suggest that while highly immunogenic, expression of HA in the primary brain tumor does not prevent the immune system from establishing an immune response against less immunodominant epitopes contained in the brain tumor. DTH testing of rechallenged animals revealed the presence of brain tumor specific memory T cells (Figure 5c). Animals surviving 60 days after the rechallenge with CNS1 wild type cells were euthanized and their brains were analyzed by immunohistochemistry for the presence of residual tumor cells and markers of immune cells. Figure 5d reveals a lack of gross neurotoxicity with minimal levels of immune cells in either brain hemisphere. Minor immunoreactivity for vimentin was noted in the contralateral hemisphere; however, a detailed analysis of the cell morphology suggests these cells are reactive astrocytes, rather than tumor cells.
Figure 5.
Rats bearing intracranial CNS1-HA brain tumors treated with Ad-Flt3L and Ad-TK survive rechallenge with CNS1 cells. (a) CNS1-HA cells were implanted into the striatum of Lewis rats. Ten days later, animals received an intratumoral injection of Ad-Flt3L + Ad-TK. Ganciclovir (GCV) was administered via intraperitoneal injection for 7 days. Sixty days later, animals surviving long-term were rechallenged with CNS1 cells in the contralateral hemisphere. No further treatment was administered. Naive animals were implanted with CNS1 cells to control for CNS1 cell viability (n = 4–5). Animals were monitored for survival and underwent delayed type hypersensitivity (DTH) testing 4 days before euthanasia. (b) Kaplan–Meier curve depicting survival of tumor bearing rats rechallenged with CNS1 cells or naïve rats implanted with CNS1 cell as a control. *P < 0.05, Mantel log-rank test. (c) DTH testing was performed in long-term survivors 4 days before euthanasia. Irradiated CNS1 cells were injected intradermally into the pinna of the right ear, and the left pinna received saline. The thickness of the pinna was recorded with slide calipers after 4, 24, and 48 h. *P < 0.05 versus saline ear (randomization test). (d) Neuropathological analysis of the brain at 60 days after rechallenge. CD8, CD8α+ cytotoxic T cells; CD68, macrophages/activated microglia; Flt3L, fms-like tyrosine kinase 3 ligand; HA, hemagglutinin; MHC, major histocompatibility complex; TK, thymidine kinase; vimentin, tumor cells and reactive astrocytes.
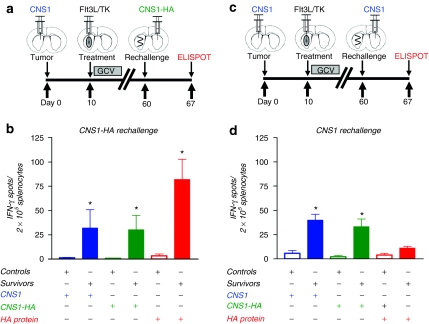
Flt3L/TK gene therapy-mediated anti-brain tumor immunological memory induces memory T cells which release IFN-γ in response to a surrogate tumor neoantigen
We have previously demonstrated that Flt3L/TK treatment of primary CNS1 brain tumors in rats39 and GL26 brain tumors in mice28 leads to immunological memory that protects long-term survivors from GBM rechallenge. To assess whether Flt3L/TK mediated immunological memory is able to adapt and detect a brain-tumor neoantigen, we treated primary brain tumors derived from CNS1 wild type cells with Flt3L/TK gene therapy followed by rechallenging the long-term survivors with either CNS1-HA (Figure 6a) or CNS1 wild type cells (Figure 6c) in the contralatereral hemisphere. Animals were euthanized 7 days after rechallenge and splenocytes were used to quantitate the levels of T lymphocyte precursors specific for either tumor cells, or for the surrogate brain tumor neoantigen HA. When animals bearing primary CNS1 wild type tumors were treated with Flt3L/TK gene therapy and then rechallenged with CNS1-HA tumor cells, we detected an increase in IFN-γ-secreting T lymphocyte precursors specific for either CNS1 cells or CNS1-HA cells, and also specific for HA (Figure 6b). Since the primary tumor treated with Flt3L/TK was not derived from brain tumor cells expressing HA, these data demonstrate that Flt3L/TK mediated immunological memory is able to detect and mount a specific immune response against a surrogate brain tumor neoantigen. As a control, when long-term survivors were rechallenged with CNS1 wild-type cells without further treatment, we failed to detect an increase in IFN-γ-secreting T lymphocyte precursors specific HA (Figure 6d).
Figure 6.
Ad-Flt3L and Ad-TK treated long-term survivors rechallenged with CNS1-HA tumor cells exhibit an expansion of IFN-γ secreting T lymphocyte precursors specific for hemagglutinin (HA). CNS1 cells were implanted into the striatum of Lewis rats. Ten days later, animals received an intratumoral injection of Ad-Flt3L + Ad-TK, or saline. Ganciclovir (GCV) was administered via intraperitoneal injection for seven days and animals were assessed for survival. Sixty days later, animals surviving long-term were rechallenged with either (a) CNS1-HA cells or (c) CNS1 wild type cells in the contralateral hemisphere. No further treatment was administered. Naive rats were injected with tumor cells as controls. Animals were euthanized 7 days after rechallenge and splenocytes were used for an IFN-γ ELISPOT. (b) Splenocytes from animals rechallenged with CNS1-HA or (d) CNS1-wild type tumors (n = 4/cohort) were isolated to quantitate the frequency of IFN-γ-secreting T lymphocyte precursors specific for CNS1 or CNS1-HA brain tumor cells, or HA protein by IFN-γ ELISPOT. Splenocytes from each experimental group were stimulated with either CNS1 cell lysates, CNS1-HA cell lysates, or HA pure protein. *P < 0.05 versus saline from same stimuli group, Student's t-test. IFN, interferon; Flt3L, fms-like tyrosine kinase 3 ligand; TK, thymidine kinase.
Discussion
Brain tumor immunotherapy clinical trials using direct vaccination with peptides derived from a GBM-specific antigen, i.e., EGFRvIII, vaccination with autologous DCs pulsed with either brain tumor restricted epitopes (i.e., EGFRvIII) or the patient's tumor cell lysates have shown them to be clinically safe and well tolerated. Experimental evidence from several clinical trials has shown that brain tumor immunotherapies elicit antitumor cellular and humoral immune responses.3,6,7,10,12,15,16,22,23
We have previously shown that intratumoral delivery of Flt3L/TK gene therapy induces brain tumor specific cellular immune responses in syngeneic orthotopic mouse28,32 and rat models of GBM,33 and both cellular and humoral immune responses in a syngeneic ectopic rat model of GBM.33 We demonstrated that intratumoral delivery of Ad vectors encoding Flt3L and TK mediates in situ vaccination against brain tumor antigens which leads to long-term survival28,29,30,33 and immunological memory28,33,38,39 in tumor bearing animals. Rats and mice bearing orthotopic brain tumors treated with Ad-TK alone, Ad-Flt3L alone, or Ad-0 did not exhibit significantly improved survival;28,30 these animals succumbed to tumor burden at the same time as the saline treated animals supporting that treatment with Ad-TK alone does not elicit antitumor immune responses in our model. Tumor regression in the Ad-TK treated animals is elicited mainly by the tumor killing activity of this arm of the therapy. We previously demonstrated that inducing anti-GBM immune responses in this fashion elicits a specific response against specific brain tumor antigens, i.e., Trp2.28,32 Furthermore, we have shown that Flt3L/TK gene therapy induces immunological memory capable of recognizing and eliminating brain tumor cells implanted in the contralateral hemisphere.39
We now wished to test the hypothesis that intratumoral delivery of Flt3L/TK could provide surveillance against potential novel brain tumor neoantigens months after the initial gene therapy. To assess this, we used HA as a surrogate brain tumor neoantigen expressed in CNS1 cells, which are syngeneic in Lewis rats. Results from experiments in rats bearing wild-type CNS1 primary tumors treated with Flt3L/TK in which long-term survivors were rechallenged with CNS1-HA cells, and received no further treatment, show that the rechallenged animals survived the second tumor expressing the neoantigen, i.e., HA. Analysis of the frequency of IFN-γ secreting T lymphocytes in these animals reveals a high frequency of T cells specific for HA, thus providing experimental evidence that Flt3L/TK gene therapy induces immunological memory that can detect a brain tumor neoantigen that was not present in the primary, treated tumor.
CNS1-HA orthotopic brain tumors are not rejected in rats treated with saline, indicating that HA lacks sufficient immunogenicity to induce an effective antitumor immune response. HA is one of the most well characterized proteins used extensively as a surrogate antigen and thus, serves as an established and effective tool to study antigen specific immune responses. While many brain tumor antigens are not highly immunogenic, this has not been demonstrated for all known GBM neoantigens. Known brain tumor antigens, such as EGFRvIII, have previously been shown to display sufficient immunogenicity to justify its use in human phase 2 clinical trials eliciting antigen specific humoral and DTH immune responses and encouraging therapeutic efficacy.16,42 Furthermore two novel glioma associated tumor antigens were recently identified, i.e., transthyretin and calgranulin B; both brain tumor antigens were determined to be sufficiently immunogenic to establish significant CD4+ and CD8+ T cell responses in human patients.43 Finally, another study has demonstrated that nucleic acids and proteins from human cytomegalovirus infections were associated with malignant glioma in human patients,44 thus representing another potential source of neoantigens, which are likely to be immunogenic since they are viral derived antigens.
DTH testing was used in patients enrolled in two recent clinical trials studying the safety and efficacy of autologous DCs pulsed with either EGFRvIII peptide13 or autologous tumor cell lysates.11 Positive DTH responses are characterized by erythema and induration at the site of antigen injection and the presence of antigen specific memory T cells are required to initiate positive DTH responses.45 In both clinical trials, DTH testing using either the EGFRvIII peptide or autologous tumor cells as inoculum revealed that both vaccination strategies induced memory T cell immune responses specific for the brain tumor (i.e., displayed DTH in response to tumor antigens). In this study, we used the DTH reaction to demonstrate that Flt3L/TK gene therapy induces memory T cells 60 days post-treatment that are specific for CNS1 brain tumor cells.
A major concern of using intratumoral expression of Flt3L to prime an in situ vaccination against brain tumors is an increased potential of immune responses targeting brain protein self-epitopes. We have recently demonstrated that delivery of Ad-Flt3L alone, or codelivered with an Ad vector encoding HA, into the normal brain induced a mild, yet not pathologic, immune response against two self brain antigens, myelin basic protein and proteolipid protein.41 These immune responses, however, failed to induce overt demyelination or behavioral abnormalities as would be expected in an experimental autoimmune encephalomyelitis model and indicate that Ad-Flt3L can be safely delivered into the brain.41 Our data herein further support this finding using a recurrent intracranial rat brain tumor model expressing a surrogate brain tumor neoantigen. Neuropathological analysis did not reveal a strong increase in T cells infiltrating into the brain parenchyma in long-term survivors, while ELISPOT analysis revealed an increase in T lymphocyte precursors specific for tumor cells and for HA antigen.
Recent integrated genomic analysis of over 200 human GBM tumors revealed numerous point mutations and frame-shift mutations in genes such as TP53, RB1, EGFR, PTEN, NF1, IDH1, PIK3Ca, PIK3R1, and ERBB2.46,47 Furthermore, studies have shown that some patients with recurrent GBM tumors previously treated with temozolomide display somatic mutations in the mismatch repair gene MSH6, which has been causally associated with temozolomide resistance.26,27 While these mutations in DNA coding sequences may alter the physiological function of the translated protein, these mutated proteins could also serve as a potential source of novel epitopes and neoantigens in recurrent brain tumors. Since recurrent brain tumors are a hallmark of GBM and they express novel antigens, the development of brain tumor immunotherapies that can mount an immune response against novel brain tumor antigens likely constitutes a critical milestone in the development of novel therapeutic strategies for GBM.
One of the main obstacles of adoptive T cell therapy is that tumor cells have evolved a range of passive and active immune evasion strategies such as failure to present tumor antigens or selection of tumor variants that have lost their tumor antigens targeted in the initial adoptive T cell therapy.48 While other immunotherapy approaches currently under investigation could also potentially elicit immunological memory with sufficient flexibility to recognize brain tumor neoantigens, the data reported herein represent the first experimental evidence that Flt3L/TK mediated gene therapy is able to induce long-lasting immunological memory, capable of recognizing a brain tumor neoantigen that was absent from the primary brain tumor. These data highlight the effectiveness of the proposed approach to elicit regression of recurrent GBM expressing tumor antigenic epitopes not present in the primary lesion.
Materials and Methods
Ad vectors and cell lines. The Ad vectors used in this study are first generation, replication-deficient, recombinant Ad type 5 vectors encoding transgenes driven by the human cytomegalovirus intermediate early promoter situated within the E1 region. Ad-TK expressing HSV-1 TK30,34 and Ad-Flt3L expressing human soluble Flt3L35,37 were generated and characterized by us as previously described.49 All viral preparations were tested to be devoid of replication-competent adenovirus and lipopolysaccharide contamination using methodologies described previously.49
CNS1-HA cells were derived by stable transfection of a mammalian expression plasmid pHA-IRES-Venus, which encodes influenza HA and the fluorescent protein Venus. Primers (5′ CCAACGCGTGCCACCATGAAGGCAAACCTACTGGTCCTG3′ and 5′ CCCAACGCGTCAGATGCATATTCTGCACTGCAAAG 3′) were used to PCR amplify the HA coding sequence (HA cDNA provided by A. Caton, Wistar Institute, Philadelphia, PA).40,41 Primers (5′ CCGTCGACGCCACCATGGTGAGCAAGGGCGAGGAG 3′ and 5′ GGGCGGCCGCTTACTTGTACAGCTCGTCCATGCC 3′) were likewise used to PCR amplify the Venus coding sequence (Venus cDNA provided by Atsushi Miyawaki, Riken Brain Science Institute, Japan). Stably transfected clones were selected with G418 for 4 weeks before three rounds of fluorescence activated cell sorting (FACS) for Venus expression. Expression of HA was confirmed by western blot analysis using a primary antibody specific for HA as described by us previously.41 Cell proliferation assay was performed as described by us previously.33 Briefly, 2,000 cells were plated and individual wells were counted at 1, 3, 5, 7, 9, and 12 days later by Trypan blue dye exclusion.
CNS1 and CNS1-HA cells were maintained in culture in Dulbecco's modified Eagle's medium (CellGro, San Diego, CA) supplemented with 10% fetal bovine serum (Omega Scientific, Tarzana, CA), and 1% penicillin/streptomycin (CellGro). CNS1-HA cells were also cultured in the presence of 600 µg/ml of G418. The day of injection, cells were trypsinized, counted using Trypan blue to exclude dead cells, and resuspended in phosphate buffered saline (PBS) at a final concentration of 5,000 cells in 3 µl. Cells were stored on ice until injection.
Syngeneic intracranial rat tumor models and controls. Male Lewis rats (220–250 g; Harlan, Indianapolis, IN) were unilaterally injected in the striatum (from bregma: +1 mm anterior, +3 mm lateral, and −5 mm from the dura) with 3 µl of PBS containing 5,000 tumor cells.30,50 Ten days later, 5 × 107 i.u. of each Ad-Flt3L and Ad-TK were mixed together and resuspended in a final volume of 3 µl of saline. Utilizing the same drill hole, the vectors were delivered in three locations (−5.5, −5.0, −4.5 mm from dura, 1 µl/injection site) within the tumor mass or striatum.50 Control animals received an intracranial injection of saline alone. On the day after vector injection, 25 mg/kg GCV (Roche Laboratories, Nutley, NJ) was injected intraperitoneally twice daily for seven days. Tumor volume (n = 4 per cell line) was estimated using unbiased stereological techniques as described by us previously.33
Sixty days following initial tumor implantation, long-term survivors were rechallenged with an intracranial injection of 5,000 tumor cells into the contralateral striatum (from bregma: +1 mm anterior, −3 mm from lateral, −5 mm from dura). No further treatment was administered. Tumor cells were also intracranially injected into naïve rats.
Animals were monitored daily and euthanized under deep anesthesia at the first signs of moribund behavior, or at the end of the experiment, via terminal perfusion–fixation with oxygenated, heparinized Tyrode's solution followed by 4% paraformaldehyde (PFA) in PBS. Brains were removed for histopathological analysis. Animals were housed in a humidity- and temperature-controlled vivarium on a 12:12 hour light/dark cycle (lights on 07:00 ) with free access to food and water. All experimental procedures were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by Cedars-Sinai Medical Center Institutional Animal Care and Use Committee (CSMC IACUC).
IFN-γ ELISPOT. Splenocytes were assessed for the frequency of IFN-γ secreting T lymphocytes by ELISPOT using the rat IFN-γ development module (R&D Systems, Minneapolis, MN) as described by us previously.33 The following stimuli were used: 100 µg/ml protein equivalent of cell lysate prepared from CNS1 cells, CNS1-HA cells or 5 µg/ml His-HA protein.41
Anti-TK and neutralizing anti-adenovirus antibody assays. We established a custom-made enzyme linked immunosorbent assay to assess the titer of anti-TK antibodies in the sera of experimental animals. CNS1 cells were infected with Ad-TK at an multiplicity of infection of 200 i.u./cell, or mock infected as a control; 72 hours later, cells were harvested in PBS containing Halt Protease Inhibitor Cocktail EDTA-Free (Thermo Scientific, Waltham, MA) and freeze/thawed for four cycles. Cell lysates were diluted in 50 mmol/l carbonate buffer and then 10 µg of protein equivalent per well of either cell lysate were coated onto a 96-well plate (cat no. 442404; NUNC, Rochester, NY) by incubation overnight at 4 °C followed by washing. The following day, all serum samples were diluted 1:4 to a final volume of 100 µl of Reagent Diluent (cat no. DY 995; R&D Systems). Dilutions were performed in duplicate as each sample was incubated with both TK and mock cell lysates. As a negative control, sera from Lewis rats systemically immunized with an adenovirus encoding an unrelated transgene were used. As a positive control, we used sera from rabbits systemically immunized with TK previously described by us.34 Sera were added to wells coated with cell lysates from Ad-TK infected cells, or from mock infected cells and incubated for 2 hours at room temperature. The enzyme linked immunosorbent assay plate was then washed and all wells were incubated for 1 hour with a rabbit anti-rat IgG/biotinylated secondary antibody (1:10,000; Dako, Carpinteria, CA), except for wells containing positive control rabbit sera, which were incubated with a goat anti-rabbit IgG/biotinylated secondary antibody (1:10,000; Dako). Plates were washed and then incubated with streptavidin–HRP (R&D Systems) followed by visualization with Substrate Solution (R&D Systems). The optical density of each well was measured at a 500 nm wavelength. The % change of optical density was calculated for each sample incubated with TK cell lysates compared to mock lysates. The neutralizing anti-adenovirus antibody assay was performed as described by us previously.50
Delayed-type hypersensitivity (DTH) tests. DTH was done in long-term survivors ~2 months after rechallenge. CNS1 cell suspensions were prepared in PBS and then irradiated (30 Gy). Irradiated CNS1 cells (1 × 106; 50 µl) were injected intradermally into the pinna of right ear of each rat, and the left pinna received 50 µl of saline. Baseline measurements of the thickness of pinna were recorded with slide calipers, and the measurement was repeated after 4, 24, and 48 h.
Immunohistochemistry. Immunolabeling of PFA fixed brain sections was performed as described previously.29,33 Briefly, following perfusion with Tyrode's solution and 4% PFA, serial coronal sections (50 µm) were prepared and free-floating immunohistochemistry was performed using either immunofluoresence or immunoperoxidase.
Immunofluoresence was performed using the following primary antibodies: anti-rat major histocompatibility complex I (to label tumor cells and immune cells; MHC I, 1:1,000, MCA51G; AbD Serotec, Kidlington, Oxford), anti-rat major histocompatibility complex II (MHC II, 1:1,000, MCA46GA; AbD Serotec), isotype specific anti-rat major histocompatibility complex II (MHC II, 1:100, MCA 826R; AbD Serotec), anti-rat Iba1 (to label activated macrophages/microglia, 019-19741; Wako, Richmond, VA), and anti-rat CD45R (to label B cells and plasmacytoid DCs, 554879; BD Pharmingen, Franklin Lakes, NJ). AlexaFluor conjugated secondary antibodies used were: goat anti-mouse IgG1-594, A21125; goat anti-rabbit IgG1-647, A21245; goat anti-mouse IgG2b-647, A21242; goat anti-mouse IgG2a-594, A21135; and goat anti-mouse IgG1-647, A21240, all used at a 1:1,000 dilution (Invitrogen, Carlsbad, CA). Nuclei were stained with 4′, 6-diamidino-2-phenylindole (DAPI) (5 µg/ml, Invitrogen).
For immunoperoxidase histochemistry, brain sections were first pretreated with 0.3% hydrogen peroxide to inactivate endogeneous peroxidase and then blocked with 10% horse serum. Brain sections were then incubated with the primary antibodies diluted in Tris buffered saline (pH 7.4) containing 1% horse serum and 0.5% Triton X-100 for 48 h. The following antibodies were used: anti-vimentin (to identify tumor cells and activated astrocytes, 1:1,000, Sigma V6630; Sigma, St Louis, MO), anti-rat CD8α (to identify cytotoxic T cells; 1:1,000, MCA48G; Abd Serotec), anti-rat CD68 (clone ED1 to identify macrophages/activated microglia; 1:1,000, MCA341R; Abd Serotec), anti-rat major histocompatibility complex I (to label tumor cells and immune cells; MHC I, 1:1,000, MCA51G; Abd Serotec), and anti-rat major histocompatibility complex II (MHC II, 1:1,000, MCA46GA; Abd Serotec). Then, the sections were incubated for 4 hours with biotin-conjugated anti-rabbit goat IgG or rabbit anti-mouse IgG (1:800, Dako E0432 and E0464, respectively), followed by 4 hours additional incubation with avidin-biotin complex (Vectastain Elite ABC Kit; Vector laboratories, Burlingame, CA). Nickel-enhanced 0.02% 3,3′-diaminobenzidine in sodium acetate was used as the chromogen.
Statistical analysis. Kaplan–Meier survival curves were analyzed using the Mantel-log Rank (GraphPad Prism version 3.00, GraphPad Software, San Diego CA). Randomization test was used to analyze cell growth properties in vitro and DTH data (NCSS). The ELISPOT data were analyzed by Students t-test. Differences between groups were considered significant at P < 0.05.
Acknowledgments
We thank Dr Young and his staff from the Department of Comparative Medicine Department, at Cedars-Sinai Medical Center for their excellent animal care and husbandry. We also thank John Ohlfest, University of Minnesota for insightful discussions. Our work was supported by National Institutes of Health/National Institute of Neurological Disorders & Stroke (NIH/NINDS) Grant 1R21-NS054143; 1UO1 NS052465 and 1RO1-NS057711 to M.G.C.; NIH/NINDS Grants 1RO1-NS 054193; and 1RO1-NS061107 to P.R.L; F32NS0503034 to G.D.K. The Bram and Elaine Goldsmith and the Medallions Group Endowed Chairs in Gene Therapeutics to P.R.L. and M.G.C., respectively, The Linda Tallen & David Paul Kane Foundation Annual Fellowship, The Drown Foundation and the Board of Governors at CSMC.
REFERENCES
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- Grossman SA, Ye X, Piantadosi S, Desideri S, Nabors LB, Rosenfeld M, NABTT CNS Consortium et al. Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin Cancer Res. 2010;16:2443–2449. doi: 10.1158/1078-0432.CCR-09-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DG, Laherty R, Tomlinson FH, Chuah T., and, Schmidt C. Results of a phase I dendritic cell vaccine trial for malignant astrocytoma: potential interaction with adjuvant chemotherapy. J Clin Neurosci. 2008;15:114–121. doi: 10.1016/j.jocn.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Heimberger AB., and, Sampson JH. The PEPvIII-KLH (CDX-110) vaccine in glioblastoma multiforme patients. Expert Opin Biol Ther. 2009;9:1087–1098. doi: 10.1517/14712590903124346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler CJ., and, Black KL. DCVax-Brain and DC vaccines in the treatment of GBM. Expert Opin Investig Drugs. 2009;18:509–519. doi: 10.1517/13543780902841951. [DOI] [PubMed] [Google Scholar]
- Schmittling RJ, Archer GE, Mitchell DA, Heimberger A, Pegram C, Herndon JE., 2ndet al. (2008Detection of humoral response in patients with glioblastoma receiving EGFRvIII-KLH vaccines J Immunol Methods 33974–81. [DOI] [PubMed] [Google Scholar]
- Heimberger AB, Sun W, Hussain SF, Dey M, Crutcher L, Aldape K.et al. (2008Immunological responses in a patient with glioblastoma multiforme treated with sequential courses of temozolomide and immunotherapy: case study Neuro-oncology 1098–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson JH, Archer GE, Mitchell DA, Heimberger AB., and, Bigner DD. Tumor-specific immunotherapy targeting the EGFRvIII mutation in patients with malignant glioma. Semin Immunol. 2008;20:267–275. doi: 10.1016/j.smim.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins RM, Shu CJ, Radu CG, Vo DD, Khan-Farooqi H, Soto H.et al. (2008Anti-tumor activity and trafficking of self, tumor-specific T cells against tumors located in the brain Cancer Immunol Immunother 571279–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liau LM, Prins RM, Kiertscher SM, Odesa SK, Kremen TJ, Giovannone AJ.et al. (2005Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment Clin Cancer Res 115515–5525. [DOI] [PubMed] [Google Scholar]
- Wheeler CJ, Black KL, Liu G, Mazer M, Zhang XX, Pepkowitz S.et al. (2008Vaccination elicits correlated immune and clinical responses in glioblastoma multiforme patients Cancer Res 685955–5964. [DOI] [PubMed] [Google Scholar]
- Yu JS, Liu G, Ying H, Yong WH, Black KL., and, Wheeler CJ. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64:4973–4979. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]
- Sampson JH, Archer GE, Mitchell DA, Heimberger AB, Herndon JE, 2nd, Lally-Goss D.et al. (2009An epidermal growth factor receptor variant III-targeted vaccine is safe and immunogenic in patients with glioblastoma multiforme Mol Cancer Ther 82773–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vleeschouwer S, Fieuws S, Rutkowski S, Van Calenbergh F, Van Loon J, Goffin J.et al. (2008Postoperative adjuvant dendritic cell-based immunotherapy in patients with relapsed glioblastoma multiforme Clin Cancer Res 143098–3104. [DOI] [PubMed] [Google Scholar]
- Yamanaka R, Homma J, Yajima N, Tsuchiya N, Sano M, Kobayashi T.et al. (2005Clinical evaluation of dendritic cell vaccination for patients with recurrent glioma: results of a clinical phase I/II trial Clin Cancer Res 114160–4167. [DOI] [PubMed] [Google Scholar]
- Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS.et al. (2010Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma J Clin Oncol 284722–4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein PR. Cancer vaccines in glioma: how to balance the challenges of small trials, efficiency, and potential adverse events. J Clin Oncol. 2010;28:4670–4673. doi: 10.1200/JCO.2010.32.1117. [DOI] [PubMed] [Google Scholar]
- Lowenstein PR. Immunology of viral-vector-mediated gene transfer into the brain: an evolutionary and developmental perspective. Trends Immunol. 2002;23:23–30. doi: 10.1016/s1471-4906(01)02063-4. [DOI] [PubMed] [Google Scholar]
- Walker PR, Calzascia T, de Tribolet N., and, Dietrich PY. T-cell immune responses in the brain and their relevance for cerebral malignancies. Brain Res Brain Res Rev. 2003;42:97–122. doi: 10.1016/s0165-0173(03)00141-3. [DOI] [PubMed] [Google Scholar]
- Kurpad SN, Zhao XG, Wikstrand CJ, Batra SK, McLendon RE., and, Bigner DD. Tumor antigens in astrocytic gliomas. Glia. 1995;15:244–256. doi: 10.1002/glia.440150306. [DOI] [PubMed] [Google Scholar]
- Saikali S, Avril T, Collet B, Hamlat A, Bansard JY, Drenou B.et al. (2007Expression of nine tumour antigens in a series of human glioblastoma multiforme: interest of EGFRvIII, IL-13Rα2, gp100 and TRP-2 for immunotherapy J Neurooncol 81139–148. [DOI] [PubMed] [Google Scholar]
- Wheeler CJ, Das A, Liu G, Yu JS., and, Black KL. Clinical responsiveness of glioblastoma multiforme to chemotherapy after vaccination. Clin Cancer Res. 2004;10:5316–5326. doi: 10.1158/1078-0432.CCR-04-0497. [DOI] [PubMed] [Google Scholar]
- Prins RM, Soto H, Konkankit V, Odesa SK, Eskin A, Yong WH.et al. (2011Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy Clin Cancer Res 171603–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot JF, Fuller G, Kumar AJ, Piao Y, Eterovic K, Ji Y.et al. (2010Tumor invasion after treatment of glioblastoma with bevacizumab: radiographic and pathologic correlation in humans and mice Neuro-oncology 12233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark AM, Witzel P, Strege RJ, Hugo HH., and, Mehdorn HM. p53, mdm2, EGFR, and msh2 expression in paired initial and recurrent glioblastoma multiforme. J Neurol Neurosurg Psychiatr. 2003;74:779–783. doi: 10.1136/jnnp.74.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter C, Smith R, Cahill DP, Stephens P, Stevens C, Teague J.et al. (2006A hypermutation phenotype and somatic MSH6 mutations in recurrent human malignant gliomas after alkylator chemotherapy Cancer Res 663987–3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip S, Miao J, Cahill DP, Iafrate AJ, Aldape K, Nutt CL.et al. (2009MSH6 mutations arise in glioblastomas during temozolomide therapy and mediate temozolomide resistance Clin Cancer Res 154622–4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin JF, Liu N, Candolfi M, Xiong W, Assi H, Yagiz K.et al. (2009HMGB1 mediates endogenous TLR2 activation and brain tumor regression PLoS Med 6e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candolfi M, Yagiz K, Foulad D, Alzadeh GE, Tesarfreund M, Muhammad AK.et al. (2009Release of HMGB1 in response to proapoptotic glioma killing strategies: efficacy and neurotoxicity Clin Cancer Res 154401–4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S, King GD, Curtin JF, Candolfi M, Xiong W, Liu C.et al. (2005Combined immunostimulation and conditional cytotoxic gene therapy provide long-term survival in a large glioma model Cancer Res 657194–7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candolfi M, Kroeger KM, Muhammad AK, Yagiz K, Farrokhi C, Pechnick RN.et al. (2009Gene therapy for brain cancer: combination therapies provide enhanced efficacy and safety Curr Gene Ther 9409–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Sanderson NS, Wawrowsky K, Puntel M, Castro MG., and, Lowenstein PR. Kupfer-type immunological synapse characteristics do not predict anti-brain tumor cytolytic T-cell function in vivo. Proc Natl Acad Sci USA. 2010;107:4716–4721. doi: 10.1073/pnas.0911587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghulam Muhammad AK, Candolfi M, King GD, Yagiz K, Foulad D, Mineharu Y.et al. (2009Antiglioma immunological memory in response to conditional cytotoxic/immune-stimulatory gene therapy: humoral and cellular immunity lead to tumor regression Clin Cancer Res 156113–6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey RA, Morrissey G, Cowsill CM, Stone D, Bolognani F, Dodd NJ.et al. (1999Chronic brain inflammation and persistent herpes simplex virus 1 thymidine kinase expression in survivors of syngeneic glioma treated by adenovirus-mediated gene therapy: implications for clinical trials Nat Med 51256–1263. [DOI] [PubMed] [Google Scholar]
- Ali S, Curtin JF, Zirger JM, Xiong W, King GD, Barcia C.et al. (2004Inflammatory and anti-glioma effects of an adenovirus expressing human soluble Fms-like tyrosine kinase 3 ligand (hsFlt3L): treatment with hsFlt3L inhibits intracranial glioma progression Mol Ther 101071–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin JF, Candolfi M, Fakhouri TM, Liu C, Alden A, Edwards M.et al. (2008Treg depletion inhibits efficacy of cancer immunotherapy: implications for clinical trials PLoS ONE 3e1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin JF, King GD, Barcia C, Liu C, Hubert FX, Guillonneau C.et al. (2006Fms-like tyrosine kinase 3 ligand recruits plasmacytoid dendritic cells to the brain J Immunol 1763566–3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King GD, Muhammad AK, Curtin JF, Barcia C, Puntel M, Liu C.et al. (2008Flt3L and TK gene therapy eradicate multifocal glioma in a syngeneic glioblastoma model Neuro-oncology 1019–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King GD, Kroeger KM, Bresee CJ, Candolfi M, Liu C, Manalo CM.et al. (2008Flt3L in combination with HSV1-TK-mediated gene therapy reverses brain tumor-induced behavioral deficits Mol Ther 16682–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caton AJ, Cerasoli DM., and, Shih FF. Immune recognition of influenza hemagglutinin as a viral and a neo-self-antigen. Immunol Res. 1998;17:23–32. doi: 10.1007/BF02786427. [DOI] [PubMed] [Google Scholar]
- Larocque D, Sanderson NS, Bergeron J, Curtin JF, Girton J, Wibowo M.et al. (2010Exogenous fms-like tyrosine kinase 3 ligand overrides brain immune privilege and facilitates recognition of a neo-antigen without causing autoimmune neuropathology Proc Natl Acad Sci USA 10714443–14448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson JH, Aldape KD, Archer GE, Coan A, Desjardins A, Friedman AH.et al. (2011Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma Neuro-oncology 13324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckhove P, Warta R, Lemke B, Stoycheva D, Momburg F, Schnölzer M.et al. (2010Rapid T cell-based identification of human tumor tissue antigens by automated two-dimensional protein fractionation J Clin Invest 1202230–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DA, Xie W, Schmittling R, Learn C, Friedman A, McLendon RE.et al. (2008Sensitive detection of human cytomegalovirus in tumors and peripheral blood of patients diagnosed with glioblastoma Neuro-oncology 1010–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black CA. Delayed type hypersensitivity: current theories with an historic perspective. Dermatol Online J. 1999;5:7. [PubMed] [Google Scholar]
- Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Cancer Genome Atlas Research Network et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P.et al. (2008An integrated genomic analysis of human glioblastoma multiforme Science 3211807–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner MK., and, Heslop HE. Adoptive T cell therapy of cancer. Curr Opin Immunol. 2010;22:251–257. doi: 10.1016/j.coi.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southgate T, Kroeger KM, Liu C, Lowenstein PR., and, Castro MG. Gene transfer into neural cells in vitro using adenoviral vectors. Curr Protoc Neurosci. 2008;Chapter 4:Unit 4.23. doi: 10.1002/0471142301.ns0423s45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puntel M, Kroeger KM, Sanderson NS, Thomas CE, Castro MG., and, Lowenstein PR. Gene transfer into rat brain using adenoviral vectors. Curr Protoc Neurosci. 2010;Chapter 4:Unit 4.24. doi: 10.1002/0471142301.ns0424s50. [DOI] [PMC free article] [PubMed] [Google Scholar]