Abstract
Measles virus (MV) is a promising vector for cancer therapy and multivalent vaccination, but high prevalence of pre-existing neutralizing antibodies may reduce therapeutic efficacy, particularly following systemic administration. MV has only one serotype, but here we show that its envelope glycoproteins can be exchanged with those of the closely related canine distemper virus (CDV), generating a chimeric virus capable of escaping neutralization. To target its entry, we displayed on the CDV attachment protein a single-chain antibody specific for a designated receptor. To enhance oncolytic efficacy we armed the virus with a prodrug convertase gene capable of locally activating chemotherapeutic prodrugs. The new virus achieved high titers, was genetically stable, and was resistant to neutralization by sera from both MV-immunized mice and MV-immune humans. The new virus targeted syngeneic murine tumor cells expressing the designated receptor implanted in immunocompetent mice, and synergized with a chemotherapeutic prodrug in a model of oncolysis. Importantly, the chimeric MV remained oncolytic when administered systemically even in the presence of anti-MV antibodies capable of abrogating the therapeutic efficacy of the parental, nonshielded MV. This work shows that targeting, arming, and shielding can be combined to generate a tumor-specific, neutralization-resistant virus that can synergize with chemotherapeutics.
Introduction
Oncolytic virotherapy is a promising treatment paradigm that exploits the preferential replication of viruses within tumor cells, but not normal tissues, to achieve therapeutic responses.1,2,3 Viruses from many different families have been tested preclinically, and Adeno-, Pox-, Herpes-, Reo- and Paramyxoviridae are in clinical trials.1,4 Viruses are particularly promising anti-neoplastic agents because of their potential for cellular tropism restriction through multiple targeting mechanisms and their ability to deliver and express therapeutic genes within the tumor microenvironment for combinatorial therapies. However, the high prevalence of pre-existing antibodies in human populations against most oncolytic virotherapy vectors may reduce or eliminate efficacy.
In particular, measles virus (MV), an enveloped negative strand RNA virus in the genus Morbillivirus, is a promising vector both for oncolytic virotherapy4,5 and multivalent vaccination.6,7,8 Its cellular tropism can be efficiently retargeted to many different cell-surface molecules, including tumor-associated antigens such as the carcinoembryonic antigen (CEA),9 by expressing single-chain antibodies on the extracellular terminus of its attachment glycoprotein, hemagglutinin (H).10 MV can also express foreign transgenes from additional transcription units engineered into its genome,4 including the purine nucleoside phosphorylase (PNP) therapeutic transgene, which can activate the clinical chemotherapeutic fludarabine and the experimental purine analogue 6-methylpurine 2'-deoxyriboside (MeP-dR) into toxic, highly diffusible metabolites in vitro and in vivo.11,12 Engineered vaccine strain MV have shown efficacy in multiple preclinical disease models, and engineered MV therapeutics are being tested in phase 1 clinical trials against multiple myeloma, ovarian cancer, and glioblastoma multiforme.13,14 Most importantly, the vaccine strain of MV has a perfect safety record, with no pathogenic reversion in over 50 years of use.7
Despite the promise of MV as an oncolytic agent, one serious challenge facing clinical application is pre-existing immunity from vaccination or natural infection. The ultimate goal of oncolytic virotherapy is systemic delivery of vector to all foci of metastatic disease. In the case of MV, systemic delivery directly exposes therapeutic viruses to pre-existing neutralizing antibodies, greatly reducing the number of infectious particles reaching the sites of disease. Since MV has only one serotype, to overcome humoral immunity we aimed to generate the equivalent of a new serotype by exchanging its envelope glycoproteins, fusion (F) and H, with those of the closely related but not immunologically cross-reactive morbillivirus canine distemper virus (CDV).
Simultaneous characterization of the efficacy of virus shielding and oncolytic competence requires an immunocompetent host in which a syngeneic cancer cell line can be implanted. Since a syngeneic murine colon adenocarcinoma model, expressing human CEA as the designated receptor, has been previously used to test efficacy of a retargeted MV,12,15 we displayed a CEA-specificity domain (single-chain variable fragment) on the CDV H-protein, which allowed use of the same experimental system. However, murine cells not only lack MV receptors, but also do not sustain efficient virus replication. Thus we armed the new virus with the PNP prodrug convertase16 to enhance bystander killing of uninfected cells.
We show here that the new virus is genetically stable, reaches high titers, and can synergize with fludarabine to enhance tumor cell clearance by inducing bystander killing. Moreover, in contrast to a control virus with an unmodified MV envelope, the new virus remains oncolytic when given intravenously (i.v.) to immunocompetent mice with neutralizing anti-MV antibodies established by previous vaccination. Together, these data establish proof-of-principle not only for shielding MV-based vectors from neutralizing antibodies, but also for combining shielding with arming and targeting for oncolysis.
Results
Generation and characterization of envelope-chimeric MV
Since neutralizing antibodies against MV recognize epitopes on the ectodomains of both H and F glycoproteins, we sought to replace them with the homologous proteins from the closely related CDV, as diagrammed in the genome map of Figure 1a. Moreover, a single-chain antibody against human CEA was added to the CDV-H carboxy-terminus to promote targeted entry (Figure 1a, bottom), and the PNP gene was inserted in an additional transcription unit upstream of N to allow synergy with chemotherapeutics12 (Figure 1a, top left). This plasmid was transfected in helper cells17 and a replication-competent agent was rescued and named MVPNPCDVenvantiCEA. This new virus differs from the MV vaccine strain in three ways: targeted cell entry, neutralization resistance, and the ability to activate a chemotherapeutic. In parallel, the previously described nonchimeric control, MVPNPantiCEA, was also rescued.12
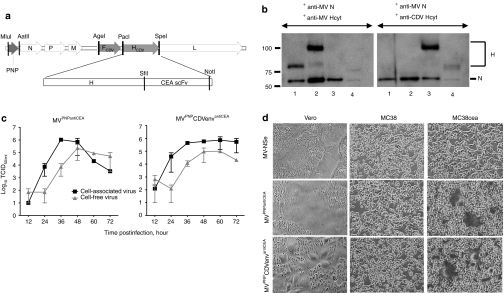
Figure 1.
Generation and characterization of an armed and targeted MV-based vector with a foreign envelope. (a) Schematic diagram of the genome of a chimeric MV, with gray genome segments representing foreign genes (PNP or FCDV, HCDV). The CDV-H coding region is continuous with an antiCEA single-chain antibody (scFv) coding region. Restriction enzymes used for plasmid construction are indicated above the genome. CEA, carcinoembryonic antigen; F, fusion; H, hemagglutinin; L, large/polymerase; M, matrix; N, nucleocapsid; P, phosphoprotein; PNP, purine nucleoside phosphorylase; scFV, single-chain antibody. (b) Immunoblot analysis of protein incorporation in ~5,000 infectious particles of MV-NSe (lane 1), MVPNPantiCEA (lane 2), MVPNPCDVenvantiCEA (lane 3), and CDV (lane 4). The H-protein was detected using antibodies against the cytoplasmic tail of either MV-H (left panel) or CDV-H (right panel). CDV, canine distemper virus; Hcyt, hemagglutinin cytoplasmic tail; MV, measles virus. (c) Growth kinetics of MVPNPantiCEA (left panel) and MVPNPCDVenvantiCEA (right panel). Vero cells were infected at an multiplicity of infection (MOI) of 0.1 and cell-associated or released virus titrated on Vero cells to determine 50% tissue culture infective dose. Data points, mean of three independent experiments; bars, SD. (d) CEA-dependent cell fusion of MVPNPCDVenvantiCEA. Vero, MC38, or MC38CEA cells were infected at MOI 0.5 with vaccine-lineage MV (upper row), MVPNPantiCEA (middle row), or MVPNPCDVenvantiCEA (bottom row) and photographed 36 hours later.
To characterize the envelope-chimeric MV, particles were purified and analyzed using antibodies against MV-N, MV-H, and CDV-H. This analysis confirmed the ~ 25 kD shift in H corresponding to the appended CEA-single chain variable fragment, as well as the incorporation of the CDV-H and MV N proteins in MVPNPCDVenvantiCEA particles (Figure 1b, left panel, compare lanes 1 and 2). In addition, CDV-H was incorporated into the envelope of MVPNPCDVenvantiCEA as efficiently as MV-H into MVPNPantiCEA, as documented by their similar levels on immunoblot (Figure 1b, compare lane 2 in left panel with lane 3 in right panel).
We then assessed whether MVPNPCDVenvantiCEA had growth characteristics similar to MVPNPantiCEA. Indeed these two viruses displayed similar growth curves in Vero cells (Figure 1c, compare left and right panels). Moreover, both viruses showed tight cell entry specificity, infecting and fusing Vero cells through CD46 and MC38CEA cells through CEA, but not parental MC38 cells, which do no express natural or targeted MV receptors (Figure 1d). Finally, MVPNPCDVenvantiCEA maintained CDV-glycoprotein and PNP expression, as well as CEA-targeted cell entry, for up to five passages, the maximum number used in our experiments. Together, these data show that the shielded MVPNPCDVenvantiCEA retains the replication characteristics of MVPNPantiCEA, and that CDV-H is capable of supporting targeted entry through CEA.
Envelope-chimeric MV retains oncolytic function
We next sought to assess oncolytic efficacy of MVPNPCDVenvantiCEA in a syngeneic, immunocompetent mouse model using murine colon adenocarcinoma cells stably expressing human CEA. To measure efficiency of tumor cell killing, MC38CEA tumors were implanted subcutaneously in C57BL/6 mice. After growing to an average volume of 20–40 µl, each tumor was treated four times with either virus, or mock infected. Compared to controls, MVPNPCDVenvantiCEA and MVPNPantiCEA both significantly inhibited tumor growth at 16 days postimplantation, the last day on which all mice were surviving (Figure 2a,b; triangles/diamonds; P = 0.0049/0.0045, by Dunnett's test), with MVPNPCDVenvantiCEA achieving one complete response. Thus, MVPNPCDVenvantiCEA retains oncolytic efficacy similar to MVPNPantiCEA in an animal model.
Figure 2.
MVPNPCDVenvantiCEA is oncolytic after intratumoral administration. MC38CEA tumors were established using one million cells injected subcutaneously in C57BL/6 mice. (a) average tumor volume over time; (b) tumor volume for all mice on day 16. Circles: mock-infections with Opti-minimal essential medium. Triangles: MVPNPCDVenvantiCEA treatment. Diamonds: MVPNPantiCEA treatment. The defined endpoint was 1,500 µl tumor volume. CDV, canine distemper virus; CEA, carcinoembryonic antigen; MV, measles virus; PNP, purine nucleoside phosphorylase.
PNP-arming promotes oncolytic synergy with chemotherapeutics
To assess the function of the PNP gene in MVPNPCDVenvantiCEA, we initially measured PNP activity in cells infected with MVPNPCDVenvantiCEA, and PNP-mediated bystander killing in vitro. Figure 3a is an immunoblot documenting that in two cell lines, Vero and MC38CEA, MVPNPCDVenvantiCEA and MVPNPantiCEA expressed PNP at similar levels.
Figure 3.
Synergistic cytotoxicity between vector and prodrug. (a) Characterization of PNP expression in infected cells. Cell lysates were collected 36–48 hours postinfection at multiplicity of infection (MOI) of 0.5. Lane 1: mock infection; lane 2: MV-NSe; lane 3: MVPNPantiCEA; lane 4: MVPNPCDVenvantiCEA. PNP, purine nucleoside phosphorylase; N, nucleocapsid. (b) Synergistic cytotoxicity between vector and 6-methylpurine 2'-deoxyriboside (MeP-dR) in MC38CEA cells. MC38CEA cells were infected with MVPNPCDVenvantiCEA at MOI of 1 (squares) or 0.1 (circles), or no virus (triangles, dashed lines) and after 24 hours 100 µmol/l of MeP-dR was added (black lines) or not (gray lines) to the culture before determining cell viability at indicated time points using 3-(4, 5-dimethyldiazoyl-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Mock-treated cells are used to define 100% viability. (c) Bystander killing by activated prodrug in the supernatant of infected cultures. Cells were mock infected (open bar), treated with MeP-dR only (vertical hatching), infected with MVPNPCDVenvantiCEA only (MOI 1, horizontal hatching), or treated with both virus for 36 hours and MeP-dR for 12 hours (MOI 0.1, diagonal hatching; MOI 1, x-hatching). Supernatant was removed and heat-inactivated to kill free virus, and then incubated on fresh cultures of MC38CEA cells at 2× (left) or 10× (right) dilutions for 72 hours. Cell viability was determined by MTT assay. Columns, mean of two independent experiments; bars, SD. CDV, canine distemper virus; CEA, carcinoembryonic antigen; MV, measles virus; PNP, purine nucleoside phosphorylase.
To confirm PNP function, we used MeP-dR, a purine analogue prodrug that is a PNP substrate.18 We infected MC38CEA with MVPNPCDVenvantiCEA with or without addition of MeP-dR (Figure 3b). Virus alone achieved limited killing due to minimal permissivity of these murine cells (gray solid lines), and MeP-dR when added alone was nontoxic (dotted black line). However, when MeP-dR was added to infected cells, a pronounced oncolytic synergy was observed, with multiplicities of infection (MOI) of 1 (solid black line, squares) and 0.1 (solid black line, circles) sustaining complete killing of the culture.
To assess the efficacy of bystander killing, supernatant from infected cultures with or without prodrug was heat-inactivated and then added to fresh, uninfected cultures of MC38CEA cells. Supernatant from cultures infected with virus alone or receiving MeP-dR alone was not toxic when added to new cells. However, supernatant from cells infected with MVPNPCDVenvantiCEA in the presence of MeP-dR killed more than 90% of the cultured cells, even when diluted 1:10 (Figure 3c). In all assays, MVPNPCDVenvantiCEA achieved activity equivalent to MVPNPantiCEA (data not shown). Thus MVPNPCDVenvantiCEA expresses PNP and can synergize with chemotherapeutics in vitro. We recently showed that synergistic effects of virus and prodrug can also be observed in vivo, provided an appropriate sequence of administration.19
PNP-arming is effective in an animal model
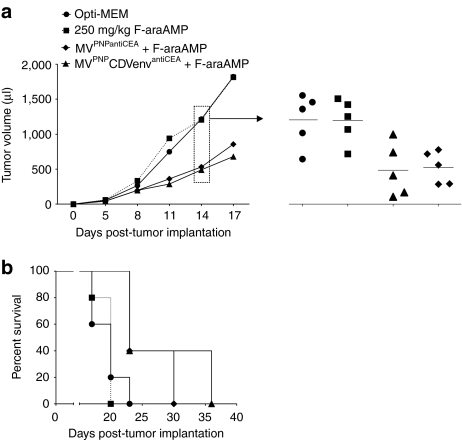
Next we sought to test arming in vivo by combining i.v. virus administration with fludarabine, a clinically relevant chemotherapeutic that is also activated by PNP to a highly diffusible toxic metabolite. MC38CEA tumors were implanted in immunocompetent C57BL/6 mice and grown to 20–40 µl. Mice were then injected i.v. with virus for four consecutive days, followed by 3 consecutive days of intraperitoneal fludarabine. This resulted in significant reduction in tumor volume compared to control and fludarabine-only groups: on days 11–17 post-tumor implantation, the average tumor volume in mice treated with MVPNPCDVenvantiCEA or MVPNPantiCEA was less than half of the volume of tumors in control mice (Figure 4a; triangles/diamonds; P = 0.009/0.013, day 14, by Dunnett's test). In addition, survival was prolonged to similar extents for both MVPNPCDVenvantiCEA and MVPNPantiCEA compared to control (Figure 4b; 23/23 versus 20 days median survival; P = 0.012/0.012, by Log-rank test). Thus the CDV-shielded virus is as efficient as MVPNPantiCEA at activating fludarabine and achieving oncolysis in vivo after i.v. administration in MV-naive mice.
Figure 4.
MVPNPCDVenvantiCEA synergizes with fludarabine in an animal model. Subcutaneous MC38CEA tumors were established in C57BL/6 mice with one million cells. Mice received four consecutive intravenous injections of MVPNPCDVenvantiCEA (triangles), MVPNPantiCEA (diamonds), or mock (squares), followed by 3 consecutive days of intraperitoneal injection of 250 mg/kg of fludarabine (F-araAMP). Control mice received neither virus nor F-araAMP (circles). (a) Left, average tumor volume over time; right, tumor volume for all mice on day 14. (b) Survival curves. The defined endpoint was 1,500 µl tumor volume. CDV, canine distemper virus; CEA, carcinoembryonic antigen; MV, measles virus; PNP, purine nucleoside phosphorylase.
Shielding protects from pre-existing neutralizing antibodies
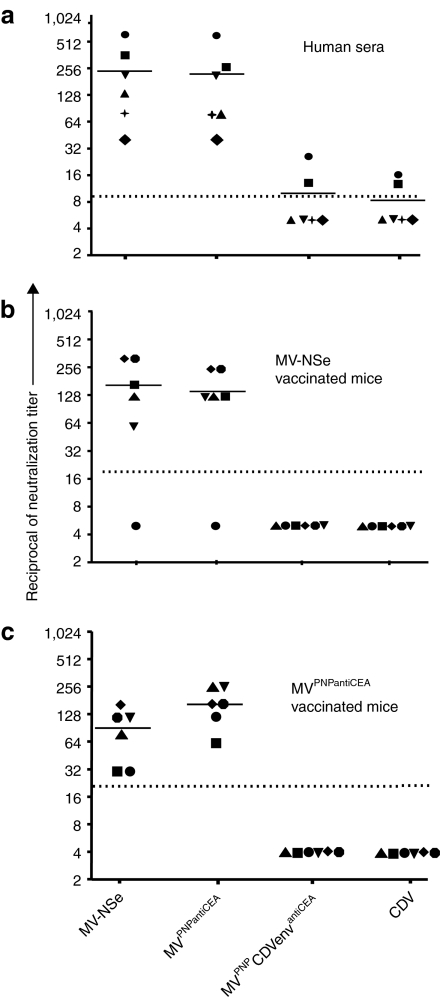
Since pre-existing neutralizing antibodies against MV interfere with efficient systemic therapy, we measured resistance of MVPNPCDVenvantiCEA to neutralization. We tested this initially in vitro, using serum obtained from healthy individuals. These sera completely neutralized the control vaccine-lineage strain MV-NSe and its targeted and armed derivative MVPNPantiCEA at dilutions of 1:32 or greater, whereas only two sera neutralized MVPNPCDVenvantiCEA above detection levels, and four sera had no neutralization capacity (Figure 5a).
Figure 5.
MVPNPCDVenvantiCEA escapes neutralization. Virus infectivity after mixing with (a) human sera or (b) sera from mice inoculated with a vaccine-lineage MV, (c) or sera from mice inoculated with a retargeted MV. Mice were immunized twice (days 1 and 7) with 106 plaque forming units of either (b) MV-NSe or (c) MVPNPantiCEA. CDV, canine distemper virus; CEA, carcinoembryonic antigen; MV, measles virus; PNP, purine nucleoside phosphorylase. Different shapes represent independent samples.
We also tested serum from mice immunized with MV. The neutralization capacity of these murine sera was similar to immune human sera, with an average reciprocal of neutralization titer over 100 against both MV-NSe and MVPNPantiCEA, but no detectable neutralization capacity against MVPNPCDVenvantiCEA or CDV (Figure 5b). One murine serum had no neutralization capacity against any tested virus (Figure 5b), which we interpret as immunization failure.
A second group of mice was immunized with the targeted virus MVPNPantiCEA. Sera from these mice neutralized both MV-NSe and MVPNPantiCEA, but importantly no neutralization activity was observed against MVPNPCDVenvantiCEA or CDV (Figure 5c). Thus the displayed antiCEA single-chain antibody does not elicit neutralizing antibodies in immunocompetent mice.
Envelope-chimeric MV is oncolytic in MV-immunized hosts
Finally, we sought to determine whether MVPNPCDVenvantiCEA remained oncolytic when administered i.v. into MV-immune mice bearing MC38CEA tumors. Mice were inoculated twice with a vaccine-lineage MV strain on days 1 and 7. Tumors were implanted on day 35, and i.v. administration of virus was started when tumors reached an average volume of 20–40 µl, with each mouse receiving injections every other day for three total injections. Tumor volume was analyzed on day 14 after tumor implantation, the last day before tumors began reaching the predefined endpoint.
Figure 6a displays individual MV neutralization titers on day 34, showing seroconversion for immunized mice and no detectable anti-MV humoral immunity in naive mice. After virotherapy, both immunized and naive mice receiving MVPNPCDVenvantiCEA had significantly smaller tumors than control mice on day 14 after tumor implantation (Figure 6b; filled/open triangles; P = 0.0204/0.0142, by Dunnett's test). In contrast, the response of mice receiving MVPNPantiCEA was dependent on immunization status: naive mice had nearly significant tumor responses (Figure 6b; open diamonds; P = 0.0594, by Dunnett's test) whereas immunized mice had tumor volumes equivalent to controls (Figure 6b; filled diamonds; P = 0.626, by Dunnett's test). Thus, MVPNPCDVenvantiCEA remains oncolytic after i.v. therapy even in mice with high titers of MV-neutralizing antibodies.
Figure 6.
Envelope-chimeric virus remains oncolytic after systemic administration to MV-immune mice. Subcutaneous MC38CEA tumors were established in C57BL/6 mice with one million cells. Mice received intravenous injections every other day for three days of MVPNPCDVenvantiCEA (triangles), MVPNPantiCEA (diamonds), or mock (circles). (a) Neutralizing anti-MV titers on day 34 after immunization. All mice were seronegative for MV on day 0. (b) Tumor volume for all mice on day 14 post-tumor implantation. CDV, canine distemper virus; CEA, carcinoembryonic antigen; Imm, MV-immunized (filled symbols); Naive, MV-naive (open symbols); MV, measles virus; PNP, purine nucleoside phosphorylase.
Discussion
We established here proof-of-principle for shielding an oncolytic enveloped virus from neutralizing antibodies. The envelope of vaccine-lineage MV contains only two proteins against which all neutralizing antibodies are directed. However, because MV has only one serotype, it is not possible to replace immunogenic epitopes of one serotype with those of another, as has been done with vesicular stomatitis virus20 and adenovirus.21
Instead, we took advantage of the related animal morbillivirus CDV envelope glycoproteins, which differ from the MV glycoproteins in 34% (F) and 63% (H) of their amino acids. This level of glycoprotein variability is similar to that observed between G glycoproteins of different serotypes of other enveloped viruses, for example vesicular stomatitis virus,22 and viable exchange of H between CDV and MV has previously been shown.23 Thus we postulated, and indeed showed, that both CDV glycoproteins are structurally similar enough to MV glycoproteins to sustain efficient particle assembly and virus replication after exchange, but different enough to escape neutralization by MV antibodies. We note that HN and F glycoprotein exchange has been successful between closely related human parainfluenza viruses type 1 and 3,24 whereas only ectodomain exchange was possible between more distantly related human parainfluenza viruses types 2 and 3.25
Using a syngeneic, immunocompetent mouse model, we show that CDV-enveloped MV is resistant to pre-existing anti-MV immunity, remaining oncolytic even when injected i.v. into MV-vaccinated mice, whereas nonshielded MV was oncolytic only when injected into MV-naive mice. To our knowledge, this is the first demonstration of efficacy after systemic oncolytic therapy in fully immunocompetent, MV-immunized mice, illustrating the utility of glycoprotein exchange between closely related viruses that are not immunologically cross-reactive.
We also show here that CDV-H can be retargeted. CDV-H is only the third viral attachment protein shown to support single-chain antibody-mediated cell entry, after the MV9,26,27 and Tupaia paramyxovirus28 attachment proteins. Importantly, it was recently shown that the MV envelope glycoproteins can be used for lentiviral vector pseudotyping.29,30,31 Pseudotyping and retargeting lentiviral vectors with the CDV envelope glycoproteins has the potential to expand their utility in the context of pre-established MV immunity, a valuable property for targeted transduction in vivo.
An important extension of this proof-of-principle study is the generation of more MV chimeras to be used in sequential cycles of oncolytic virotherapy. Transfer of the Tupaia paramyxovirus glycoproteins to MV has proven successful, provided that their cytoplasmic tails are adequately trimmed (A.W. Hudacek and R. Cattaneo, unpublished results). Multiple shielded vectors, all supporting targeted cell entry, could be employed sequentially to ensure prolonged therapy with oncolytic viruses even as humoral immunity develops against a given vector, much in the same way that multiple serotypes of adenovirus have been exploited to maintain efficacy in immunocompetent mouse models.32 When combined with careful immunosuppression, a collection of shielded MVs should open a large therapeutic window for systemic oncolytic virotherapy in immunocompetent patients initiating therapy with pre-existing MV immunity.
In addition, CDV-enveloped MV could have utility in multivalent immunization against pathogens for which a vaccine is not available. MV is one of the most successful and cost-effective viral vaccines in history, and is being developed as a divalent vaccine against multiple pathogens.7 Even if vaccination with MV-based vectors can succeed in the presence of pre-existing immunity,33 multivalent vaccines based on envelope-chimeric MV may be more effective and reliable, fully harnessing the efficacy of the live MV vaccine platform to achieve long term immunization against additional pathogens.
While pre-existing immunity to MV does limit efficacy of therapeutic virus, it does function as an important component of safety when replication-competent virus is used in human patients. However, MVPNPCDVenvantiCEA may not pose a greater pathogenic risk than vaccine strain MV used in childhood immunization. Its replicative unit is that of the vaccine strain MV, and the CDV envelope, derived from a nonpathogenic CDV vaccine strain, is unlikely to increase pathogenicity because it has lost signaling lymphocyte activation molecule (SLAM)-dependent targeting to immune cells, analogous to the MV vaccine strain.
Remarkably, in human MV infections, the early antibody response is directed against the nucleoprotein (N).34 While it was suggested that N can be secreted,35 it is possible that release of helical ribonucleocapsids after cell lysis primes this antibody response. Since MV replication is limited in mouse cells, lysis may not occur and thus we do not expect our animal system to fully recapitulate this aspect of the human immune response. We note that anti-N immunity can protect Lewis rats from encephalitis caused by intracerebral inoculation of neurotropic MV.36 However, anti-N immunity is not protective in a mouse model of measles encephalitis37 or a cotton rat model of MV respiratory infection.38 Since neutralizing antibodies are never observed after MV-N immunization, our model is appropriate for testing the resistance of oncolytic virus to neutralization.
An alternative approach to circumventing established MV immunity is infected cell carriers, which serve as “Trojan Horses” for viral particles.39,40,41 This approach relies on viral glycoprotein expression on the surface of infected cell carriers to achieve heterofusion between infected cell carrier and target tumor cell. While infected cell carriers are largely resistant to passively transferred MV immunity,41 pre-existing MV antibodies recognizing the exposed ectodomains of F and H may still reduce the efficacy of this virus delivery system. Cell carrier delivery and CDV-glycoprotein exchange are complementary technologies for avoiding pre-established anti-MV immunity and may be combined.
In conclusion, we have generated an oncolytic MV able to synergize with chemotherapeutics and enter tumor cells through a targeted receptor, while escaping pre-established MV-neutralizing antibodies via shielding with CDV glycoproteins. Proof-of-principle was achieved in an immunocompetent murine model using murine tumor cells that are poorly supportive of MV replication. Success in this demanding experimental system motivates additional preclinical studies of biodistribution and toxicity42 to facilitate progression toward clinical trials.
Materials and Methods
Cell culture. Vero African green monkey kidney cells were purchased from American Type Tissue Culture Collection (Manassas, VA). MC38 and MC38CEA (clone MC38-CEA 2) were both kind gifts from Dr Jeffrey Schlom.15 The Vero, MC38, and MC38CEA were all grown in 10% fetal calf serum with 5 ml of penicillin (10,000 IU/ml) /streptomycin (10,000 µg/ml) (Mediatech, Manassas, VA) in Dulbecco's modified Eagle's medium (Mediatech) at 37 °C, 5% CO2 humidified incubator. Geneticin, G-418 (Invitrogen, Grand Island, NY) was added to a final concentration of 0.5 mg/ml in the growth medium for the MC38CEA cells.
Generation of MVPNPCDVenvantiCEA. To generate MVPNPCDVenvantiCEA, we first constructed pCG-HCDVantiCEA. We started with the CDV Onderstepoort vaccine strain H gene (pCG-HOL),23 and removed the SpeI site from the open reading frame by introducing a silent mutation by site-directed mutagenesis (QuikChange site-directed mutagenesis kit, Stratagene). The HOL open reading frame was then amplified from this plasmid with Pfu Turbo polymerase (Stratagene, La Jolla, CA) using forward primer 5′-tccgttaattaaaacttagggtgcaagatcatccccaacaatgctcccc-3′ and reverse primer 5′-ttcactagttaaataaggccggctgggcccgacggttacatgagaatc-3′. The primers provided the PacI, SpeI, and SfiI restriction sites (underlined, respectively) needed for the following cloning steps. To increase fusogenicity, we replaced the amino-terminal untranslated region of CDV, previously shown to have negative regulatory function,43 with the appropriate sequence from MV (forward primer, bold sequence).
The PacI/SpeI digested PCR product was cloned into pCG to generate pCG-HCDV. To target the CDV-H to human CEA, the SfiI/SpeI fragment from pCG-HXL9 was cloned into pCG-HCDV, generating pCG-HCDVantiCEA. The PacI/SpeI fragment from pCG-HCDVantiCEA and the AgeI/PacI fragment from Onderstepoort vaccine strain CDV,44 which contains CDV-F (FOL), were cloned into the full-length p(+)MV-NSe,45 generating p(+)MV-NSe-FCDVHCDVantiCEA. We then cloned the NarI/SpeI-digested FCDVHCDVantiCEA fragment into our previously constructed full-length plasmid p(+)MVPNPantiCEA,12 generating p(+)MVPNPCDVenvantiCEA. The correct sequences of all constructs were confirmed by sequencing (ABI Prism 377 DNA sequencer; Perkin-Elmer Applied Biosystem, Foster City, CA).
The recombinant virus was rescued using the MV reverse genetics system.17 The virus stock was propagated by infecting Vero cells at the MOI of 0.03 and incubating for 48 hours at 37 °C, 5% CO2 humidified incubator. Cells were scraped in Opti-MEM, subjected to two freeze-thaw cycles in liquid nitrogen, and centrifuged at 1,950g for 15 minutes at 4 °C. The virus titer was determined by 50% tissue culture infective dose endpoint method according to Spearman–Karber on Vero cells.46,47
Virus growth kinetics. Vero cells (5 × 105 Vero cells/six-well plate) were infected with MVPNPantiCEA or MVPNPCDVenvantiCEA at an MOI of 0.1 plaque forming unit (pfu)/cell in Opti-MEM (Gibco, Invitrogen, Carlsbad, CA) for 2.5 hours at 37 °C, after which free virus was removed and fresh growth media added. At the indicated time points (12-hour interval for 3 days), supernatants were cleared by centrifugation at 2,500g for 10 minutes at 4 °C and cells were scraped with 1 ml of Opti-MEM and subjected to one freeze-thaw cycle. The titers of released and cell-associated viruses were then determined by 50% tissue culture infective dose titration on Vero cells.
Virus purification. A discontinuous sucrose gradient was used to purify recombinant viruses according to our previously described method.27 Briefly, supernatants were harvested when 80–90% of infected Vero cells were in syncytia and centrifuged at 8,000 rpm for 30 minutes. Clarified supernatant was centrifuged at 28,000 rpm (Optima L-90K Ultracentrifuge, Beckman Coulter, Brea, CA) through 20% sucrose and onto a 60% sucrose cushion. The virus fraction was harvested, diluted in TNE buffer [10 mmol/l Tris (pH 7.8), 100 mmol/l sodium chloride, 1 mmol/l EDTA], and pelleted by an additional 2-hour centrifugation at 28,000 rpm. The viruses were dissolved in 200 µl of phosphate-buffered saline and titrated for 50% tissue culture infective dose before being subjected to immunoblot analysis.
Immunoblot analysis. For virus characterization, ~5,000 infectious particles were analyzed using rabbit anti-MV N protein (1:5,000)48 and either with rabbit anti-MV Hcyt (1:5,000)49 or anti-CDV Hcyt (MC712; 1:1,500)23 as previously described.12 PNP expression was detected using rabbit anti-PNP (1:10,000; a generous gift from Dr Jeong S. Hong) as described previously.12
Cell viability assay. The cell viability assay was based on 3-(4, 5-dimethyldiazoyl-2-yl)-2,5-diphenyltetrazolium bromide assay performed in 96-well microtiter plates using the Cell Proliferation kit I (Roche, Indianapolis, IN). Cell viability was calculated as the mean of quadruplicate optical density values, divided by the mean quadruplicate optical density values of identical cultures without prodrug or virus (mock control cells) and expressed as a percentage of control, as previously described.12
Measurement of bystander effect in vitro. The bystander effects were determined for both MVPNPantiCEA and MVPNPCDVenvantiCEA in MC38CEA cells 36 and 48 hours after infection, respectively, as previously described.12 Mock-infected supernatant without prodrug was used as the reference for calculating percent viability.
Neutralization assay. C57BL/6 mice were immunized (day 1) and boosted (day 7) by intraperitoneal injection with 106 pfu of either MV-NSe or MVPNPantiCEA, and allowed to seroconvert for 35 days. To obtain mouse plasma, whole blood was withdrawn from the submandibular vein into heparin/EDTA tubes and centrifuged at 8,600g. Mouse serum was obtained from the submandibular vein, clotted at 37 °C for an hour, and centrifuged at 10,000g for 10 minutes. To obtain human plasma, whole blood was withdrawn into heparin/EDTA tubes and centrifuged for 10 minutes at 2,600g. All plasma/serum was heat-inactivated at 56 °C for 30 minutes prior the neutralization assay. The neutralizing antibody titer was determined as previously described,50 with minor modifications. A serial twofold dilution of 50 µl of plasma or serum samples was titrated in triplicates, starting from 1:2 or 1:10 dilutions, respectively, by incubating with 50 pfu MV-NSe in 50 µl at 37 °C for an hour. After incubation, Vero cells were added directly to each well (1 × 104 cells/100 µl) and incubated at 37 °C for 5 days. The neutralization capacity of each plasma or serum sample was determined by the presence or absence of syncytia, and reported as the highest dilution to completely neutralize the virus.
Assessment of oncolytic efficacy. C57BL/6 mice were purchased from Harlan Laboratories and housed in the Mayo animal facility. All studies were approved by the Mayo Institutional Animal Care and Use Committee. For intratumoral efficacy studies, 6–8 week old female C57BL/6 mice received one million MC38CEA cells injected subcutaneously in the rear right flank. Tumors were allowed to grow to an average volume of 20–40 µl, at which time virus (2 × 106 pfu/100 µl Opti-MEM) was injected directly into the tumor for four consecutive days. Tumor volume was monitored every 3 days.
For systemic virus plus fludarabine experiments, tumor implantation was conducted as described above. When tumors reached 20–40 µl in volume, each mouse received 4 consecutive days of i.v. injection of virus (1.2 × 106 pfu in 100 µl Opti-MEM) or mock treatment with 100 µl of Opti-MEM followed by three consecutive days of intraperitoneal injection of 250 mg/kg of fludarabine (F-araAMP). Tumor volume was monitored every 3 days.
For systemic virus therapy in pre-immunized mice, 4–6 week old female C57BL/6 mice were immunized with two doses of 106 pfu of MV-NSe on days 1 and 7. On day 0 and 34, plasma was obtained from each mouse via cheek vein, and tested for MV-neutralizing antibody titer as described above. MC38CEA tumors were then established with 1 × 106 cells/100 µl as described above. Virus/mock treatments were initiated when the tumor reached 20–40 µl, with each mouse receiving three injections of ~1.7 × 106 pfu in 200 µl Opti-MEM every other day (~5 × 106 pfu total per mouse). Tumor volume was monitored every 3 days. In all in vivo studies, tumor burden was measured according to the formula V = a2b/2, where a is the shortest and b is the longest diameter. The defined tumor burden endpoint is 1,500 µl (~10% of body weight).
Statistical analyses. Tumor volume data were analyzed using one-way ANOVA. Dunnett's test was used for multiple pairwise comparisons of tumor volume between treatment groups and a defined control group. The survival data was analyzed using the Kaplan–Meier method and the log-rank test was used to determine the significance between the groups as described previously.12 Two-tailed P values <0.05 are considered statistically significant; the JMP program version 9 was used for all statistical analysis.
Acknowledgments
This work was supported by a grant of the Alliance for Cancer Gene Therapy, NIH Grant RO1 CA139389, and the Mayo Clinic Cancer Center. We thank William B. Parker and Eric J. Sorscher for MeP-dR, and Jeong Hong for providing the PNP-antibody. Patent applications for which R.C. is an inventor have been licensed to NISCO. Mayo has an equity position in NISCO. Mayo has not yet received royalties from products developed by the company, but may receive these in future.
REFERENCES
- Liu TC, Galanis E., and, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Clin Pract Oncol. 2007;4:101–117. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- Kumar S, Gao L, Yeagy B., and, Reid T. Virus combinations and chemotherapy for the treatment of human cancers. Curr Opin Mol Ther. 2008;10:371–379. [PubMed] [Google Scholar]
- Ottolino-Perry K, Diallo JS, Lichty BD, Bell JC., and, McCart JA. Intelligent design: combination therapy with oncolytic viruses. Mol Ther. 2010;18:251–263. doi: 10.1038/mt.2009.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R, Miest T, Shashkova EV., and, Barry MA. Reprogrammed viruses as cancer therapeutics: targeted, armed and shielded. Nat Rev Microbiol. 2008;6:529–540. doi: 10.1038/nrmicro1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SJ., and, Peng KW. Measles virus for cancer therapy. Curr Top Microbiol Immunol. 2009;330:213–241. doi: 10.1007/978-3-540-70617-5_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Valle JR, Devaux P, Hodge G, Wegner NJ, McChesney MB., and, Cattaneo R. A vectored measles virus induces hepatitis B surface antigen antibodies while protecting macaques against measles virus challenge. J Virol. 2007;81:10597–10605. doi: 10.1128/JVI.00923-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeter MA, Naim HY., and, Udem SA. Reverse genetics of measles virus and resulting multivalent recombinant vaccines: applications of recombinant measles viruses. Curr Top Microbiol Immunol. 2009;329:129–162. doi: 10.1007/978-3-540-70523-9_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandler S, Ruffie C, Najburg V, Frenkiel MP, Bedouelle H, Desprès P.et al. (2010Pediatric measles vaccine expressing a dengue tetravalent antigen elicits neutralizing antibodies against all four dengue viruses Vaccine 286730–6739. [DOI] [PubMed] [Google Scholar]
- Hammond AL, Plemper RK, Zhang J, Schneider U, Russell SJ., and, Cattaneo R. Single-chain antibody displayed on a recombinant measles virus confers entry through the tumor-associated carcinoembryonic antigen. J Virol. 2001;75:2087–2096. doi: 10.1128/JVI.75.5.2087-2096.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Peng KW, Harvey M, Greiner S, Lorimer IA, James CD.et al. (2005Rescue and propagation of fully retargeted oncolytic measles viruses Nat Biotechnol 23209–214. [DOI] [PubMed] [Google Scholar]
- Ungerechts G, Springfeld C, Frenzke ME, Lampe J, Johnston PB, Parker WB.et al. (2007Lymphoma chemovirotherapy: CD20-targeted and convertase-armed measles virus can synergize with fludarabine Cancer Res 6710939–10947. [DOI] [PubMed] [Google Scholar]
- Ungerechts G, Springfeld C, Frenzke ME, Lampe J, Parker WB, Sorscher EJ.et al. (2007An immunocompetent murine model for oncolysis with an armed and targeted measles virus Mol Ther 151991–1997. [DOI] [PubMed] [Google Scholar]
- Msaouel P, Dispenzieri A., and, Galanis E. Clinical testing of engineered oncolytic measles virus strains in the treatment of cancer: an overview. Curr Opin Mol Ther. 2009;11:43–53. [PMC free article] [PubMed] [Google Scholar]
- Galanis E, Hartmann LC, Cliby WA, Long HJ, Peethambaram PP, Barrette BA.et al. (2010Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer Cancer Res 70875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins PF, Kantor JA, Salgaller M, Hand PH, Fernsten PD., and, Schlom J. Transduction and expression of the human carcinoembryonic antigen gene in a murine colon carcinoma cell line. Cancer Res. 1991;51:3657–3662. [PubMed] [Google Scholar]
- Williams SR, Goddard JM., and, Martin DW., Jr Human purine nucleoside phosphorylase cDNA sequence and genomic clone characterization. Nucleic Acids Res. 1984;12:5779–5787. doi: 10.1093/nar/12.14.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radecke F, Spielhofer P, Schneider H, Kaelin K, Huber M, Dötsch C.et al. (1995Rescue of measles viruses from cloned DNA EMBO J 145773–5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker WB, Allan PW, Shaddix SC, Rose LM, Speegle HF, Gillespie GY.et al. (1998Metabolism and metabolic actions of 6-methylpurine and 2-fluoroadenine in human cells Biochem Pharmacol 551673–1681. [DOI] [PubMed] [Google Scholar]
- Ungerechts G, Frenzke ME, Yaiw KC, Miest T, Johnston PB., and, Cattaneo R. Mantle cell lymphoma salvage regimen: synergy between a reprogrammed oncolytic virus and two chemotherapeutics. Gene Ther. 2010;17:1506–1516. doi: 10.1038/gt.2010.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose NF, Marx PA, Luckay A, Nixon DF, Moretto WJ, Donahoe SM.et al. (2001An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants Cell 106539–549. [DOI] [PubMed] [Google Scholar]
- Roberts DM, Nanda A, Havenga MJ, Abbink P, Lynch DM, Ewald BA.et al. (2006Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity Nature 441239–243. [DOI] [PubMed] [Google Scholar]
- Gallione CJ., and, Rose JK. Nucleotide sequence of a cDNA clone encoding the entire glycoprotein from the New Jersey serotype of vesicular stomatitis virus. J Virol. 1983;46:162–169. doi: 10.1128/jvi.46.1.162-169.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Messling V, Zimmer G, Herrler G, Haas L., and, Cattaneo R. The hemagglutinin of canine distemper virus determines tropism and cytopathogenicity. J Virol. 2001;75:6418–6427. doi: 10.1128/JVI.75.14.6418-6427.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao T, Durbin AP, Whitehead SS, Davoodi F, Collins PL., and, Murphy BR. Recovery of a fully viable chimeric human parainfluenza virus (PIV) type 3 in which the hemagglutinin-neuraminidase and fusion glycoproteins have been replaced by those of PIV type 1. J Virol. 1998;72:2955–2961. doi: 10.1128/jvi.72.4.2955-2961.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao T, Skiadopoulos MH, Davoodi F, Riggs JM, Collins PL., and, Murphy BR. Replacement of the ectodomains of the hemagglutinin-neuraminidase and fusion glycoproteins of recombinant parainfluenza virus type 3 (PIV3) with their counterparts from PIV2 yields attenuated PIV2 vaccine candidates. J Virol. 2000;74:6448–6458. doi: 10.1128/jvi.74.14.6448-6458.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucheit AD, Kumar S, Grote DM, Lin Y, von Messling V, Cattaneo RB.et al. (2003An oncolytic measles virus engineered to enter cells through the CD20 antigen Mol Ther 762–72. [DOI] [PubMed] [Google Scholar]
- Schneider U, Bullough F, Vongpunsawad S, Russell SJ., and, Cattaneo R. Recombinant measles viruses efficiently entering cells through targeted receptors. J Virol. 2000;74:9928–9936. doi: 10.1128/jvi.74.21.9928-9936.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springfeld C, von Messling V, Tidona CA, Darai G., and, Cattaneo R. Envelope targeting: hemagglutinin attachment specificity rather than fusion protein cleavage-activation restricts Tupaia paramyxovirus tropism. J Virol. 2005;79:10155–10163. doi: 10.1128/JVI.79.16.10155-10163.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funke S, Maisner A, Mühlebach MD, Koehl U, Grez M, Cattaneo R.et al. (2008Targeted cell entry of lentiviral vectors Mol Ther 161427–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funke S, Schneider IC, Glaser S, Mühlebach MD, Moritz T, Cattaneo R.et al. (2009Pseudotyping lentiviral vectors with the wild-type measles virus glycoproteins improves titer and selectivity Gene Ther 16700–705. [DOI] [PubMed] [Google Scholar]
- Anliker B, Abel T, Kneissl S, Hlavaty J, Caputi A, Brynza J.et al. (2010Specific gene transfer to neurons, endothelial cells and hematopoietic progenitors with lentiviral vectors Nat Methods 7929–935. [DOI] [PubMed] [Google Scholar]
- Parks R, Evelegh C., and, Graham F. Use of helper-dependent adenoviral vectors of alternative serotypes permits repeat vector administration. Gene Ther. 1999;6:1565–1573. doi: 10.1038/sj.gt.3300995. [DOI] [PubMed] [Google Scholar]
- Lorin C, Mollet L, Delebecque F, Combredet C, Hurtrel B, Charneau P.et al. (2004A single injection of recombinant measles virus vaccines expressing human immunodeficiency virus (HIV) type 1 clade B envelope glycoproteins induces neutralizing antibodies and cellular immune responses to HIV J Virol 78146–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves M, Griffin DE, Johnson RT, Hirsch RL, de Soriano IL, Roedenbeck S.et al. (1984Development of antibody to measles virus polypeptides during complicated and uncomplicated measles virus infections J Virol 49409–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie JC, Saltel F, Escola JM, Jurdic P, Wild TF., and, Horvat B. Cell surface delivery of the measles virus nucleoprotein: a viral strategy to induce immunosuppression. J Virol. 2004;78:11952–11961. doi: 10.1128/JVI.78.21.11952-11961.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankamp B, Brinckmann UG, Reich A, Niewiesk S, ter Meulen V., and, Liebert UG. Measles virus nucleocapsid protein protects rats from encephalitis. J Virol. 1991;65:1695–1700. doi: 10.1128/jvi.65.4.1695-1700.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild TF, Bernard A, Spehner D., and, Drillien R. Construction of vaccinia virus recombinants expressing several measles virus proteins and analysis of their efficacy in vaccination of mice. J Gen Virol. 1992;73 (Pt 2):359–367. doi: 10.1099/0022-1317-73-2-359. [DOI] [PubMed] [Google Scholar]
- Schlereth B, Germann PG, ter Meulen V., and, Niewiesk S. DNA vaccination with both the haemagglutinin and fusion proteins but not the nucleocapsid protein protects against experimental measles virus infection. J Gen Virol. 2000;81 Pt 5:1321–1325. doi: 10.1099/0022-1317-81-5-1321. [DOI] [PubMed] [Google Scholar]
- Munguia A, Ota T, Miest T., and, Russell SJ. Cell carriers to deliver oncolytic viruses to sites of myeloma tumor growth. Gene Ther. 2008;15:797–806. doi: 10.1038/gt.2008.45. [DOI] [PubMed] [Google Scholar]
- Mader EK, Maeyama Y, Lin Y, Butler GW, Russell HM, Galanis E.et al. (2009Mesenchymal stem cell carriers protect oncolytic measles viruses from antibody neutralization in an orthotopic ovarian cancer therapy model Clin Cancer Res 157246–7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Russell SJ., and, Peng KW. Systemic therapy of disseminated myeloma in passively immunized mice using measles virus-infected cell carriers. Mol Ther. 2010;18:1155–1164. doi: 10.1038/mt.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers RM, Greiner SM, Harvey ME, Griesmann G, Kuffel MJ, Buhrow SA.et al. (2007Preclinical pharmacology and toxicology of intravenous MV-NIS, an oncolytic measles virus administered with or without cyclophosphamide Clin Pharmacol Ther 82700–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Messling V., and, Cattaneo R. Amino-terminal precursor sequence modulates canine distemper virus fusion protein function. J Virol. 2002;76:4172–4180. doi: 10.1128/JVI.76.9.4172-4180.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu MS, Husar W, Cook SD, Dowling PC., and, Udem SA. Canine distemper terminal and intergenic non-protein coding nucleotide sequences: completion of the entire CDV genome sequence. Virology. 1993;193:66–72. doi: 10.1006/viro.1993.1103. [DOI] [PubMed] [Google Scholar]
- Singh M, Cattaneo R., and, Billeter MA. A recombinant measles virus expressing hepatitis B virus surface antigen induces humoral immune responses in genetically modified mice. J Virol. 1999;73:4823–4828. doi: 10.1128/jvi.73.6.4823-4828.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spearman C. The method of right and wrong cases (constant stimuli) without Gauss's formulae. Br J Psychol. 1908;2:227–242. [Google Scholar]
- Kärber G. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Arch Exp Pathol Pharmakol. 1931;162:480–483. [Google Scholar]
- Toth AM, Devaux P, Cattaneo R., and, Samuel CE. Protein kinase PKR mediates the apoptosis induction and growth restriction phenotypes of C protein-deficient measles virus. J Virol. 2009;83:961–968. doi: 10.1128/JVI.01669-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathomen T, Naim HY., and, Cattaneo R. Measles viruses with altered envelope protein cytoplasmic tails gain cell fusion competence. J Virol. 1998;72:1224–1234. doi: 10.1128/jvi.72.2.1224-1234.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YD, Heath J, Collins J, Greene T, Antipa L, Rota P.et al. (1997Experimental measles. II. Infection and immunity in the rhesus macaque Virology 23385–92. [DOI] [PubMed] [Google Scholar]