Abstract
Accumulated evidence from animal studies implicates the ventral striatum in the processing of reward information. Recently, deep brain stimulation (DBS) surgery has enabled researchers to analyze neurophysiological recordings from humans engaged in reward tasks. We present data recorded from the human ventral striatum during DBS surgery as a participant played a video game coupled to the receipt of visual reward images. To our knowledge, we identify the first instances of reward-sensitive single unit activity in the human ventral striatum. Local field potential data suggests that alpha oscillations are sensitive to positive feedback, while beta oscillations exhibit significantly higher power during unrewarded trials. We report evidence of alpha–gamma cross-frequency coupling that differentiates between positive and negative feedback.
Keywords: ventral striatum, deep brain stimulation, nucleus accumbens, reward
Introduction
The role of the ventral striatum (VS) for processing reward information, mediating appetitive behavior, instigating addictive behaviors, and mediating reward learning have made the structure a fecund target for human and animal experimentation (for a review, see Haber et al. [1]). Animal studies have demonstrated that neuronal activity in the ventral striatum is elicited by a wide range of reward-related stimuli, including cue imagery, specific movements that lead to rewards, receipt of preferred vs non-preferred rewards, and reward prediction error [2, 3]. In humans, fMRI studies have demonstrated that the ventral striatum is sensitive to a wide array of reward features. It exhibits BOLD signal changes in response to monetary reward and loss even if no physical money is given to an individual and only abstract remuneration occurs [4]. The VS exhibits increased activity in response to images of food [5], to sexual imagery [6], to images of desirable material objects [7], and to cues related to alcohol consumption [8].
Alterations in reward circuitry (including the VS) are thought to contribute to the clinical manifestations of a variety of disorders including depression, obsessive-compulsive disorder, schizophrenia, and anxiety [9]. This insight has prompted clinicians to attempt deep brain stimulation (DBS) of the ventral striatum to treat this condition [10]; such procedures have afforded researchers the unique opportunity to obtain direct neurophysiological recordings from the VS.
In the neocortex, theta and gamma oscillations have been linked to memory encoding and retrieval [11]. The project of applying spectral analytic techniques to local field potential (LFP) data from subcortical structures (outside of the hippocampus) is in its infancy, but there is evidence that oscillatory changes in specific frequency bands are part of the response of reward circuitry to feedback stimuli. LFP activity recorded from the human ventral striatum suggests that alpha and theta oscillations are responsive to strategy-switching during reward learning [12, 13]. Knowledge of which frequency bands exhibit oscillatory changes during behavior may present an electrophysiological target for modulation via deep brain stimulation, akin to beta oscillations in the subthalamic nucleus [14].
To our knowledge, no evidence linking single unit activity in the human VS to reward phenomena have been reported. Using intraoperative recording techniques [15], we describe single unit activity and LFPs obtained as a patient underwent DBS implantation surgery. We present the first human data linking single unit activity in the VS with the processing of reward information, and our LFP findings extend the existing literature describing neurophysiological activity in the human ventral striatum by identifying both alpha and beta oscillatory activity associated with different feedback conditions [12, 13].
Methods
Participant data
The participant was a 42 year old female undergoing implantation of DBS electrodes in the ventral striatum for treatment-resistant depression. The patient participated voluntarily in our cognitive study after informed consent was obtained during pre-operative consultation for the surgery. Per protocol, the patient’s depression medications were continued prior to surgery. The patient had suffered clinical depression for over 10 years, failing pharmacological treatment, counseling, and electro-convulsive therapy. This investigation was carried out in accordance with a University of Pennsylvania IRB approved protocol.
Experimental paradigm
We designed a paradigm in which the patient played a simple video game in order to receive reward stimuli. Ethical concerns prevented us from using a task with actual financial remuneration, and our experience in intraoperative DBS recordings suggested that participant motivation to achieve abstract points in a game would quickly wane without a primary pleasurable stimulus. Clinical concerns precluded using the delivery of pharmacological rewards. In a pre-operative session, the participant’s preferences for different categories of visual images were assessed. She voted her preference for images from 11 different categories, presented 2 at a time on a computer screen. The image archive consisted of 120 images in each category compiled from the International Affective Picture System (IAPS) database and supplemented from additional internet image archives [16], compiled to appeal to a range of aesthetic tastes. Using the results of this category voting procedure, preferences were ranked using the Tideman method of ranked pairs [17].
The experimental task consisted of a video game in which the subject had to click a button (either left or right) when an object moved into a target area on the screen. The speed of the moving object was adjusted to target 70% accuracy. Prior to each trial, the participant was cued as to the category from which the reward image would come if the game task was successfully executed. Successful game completion was rewarded by the full presentation of an example image from the category of images that had been cued accompanied by a reinforcing tone (positive feedback). Failed trials were followed by images of trash or unkempt urban streets and a buzz tone (negative feedback). The game portion also included neutral-reward trials in which the participant was cued that no positive or negative feedback was on offer for that trial. These trials required the participant to click a button when prompted after viewing a moving stimulus. (See pdf, Supplemental Digital Content 1 http://links.lww.com/WNR/A151, for a schematic of the game and a more detailed description of the preference voting procedure)
DBS procedure and spike acquisition
Intraoperative targeting for recording and then implantation was 7 mm lateral to the AC-PC line (x axis), 2 mm anterior to the posterior edge of the anterior commissure (y axis), and 4 mm below the AC-PC line (z axis), in line with those used during the initial trials of DBS of the ventral striatum [18]. As part of the surgical protocol, spiking activity for localization of the appropriate brain structure was recorded using 1μm tungsten tip microelectrodes as previously described [15]. Local field potential recordings for power analysis were made from the left DBS macroelectrode. The two inferior contacts were used for recording, referenced to a more proximal contact located in the ventral internal capsule in bipolar fashion [12]. Signals were sampled at 25 kHz for microelectrode data and 1.25 kHz for macroelectrode data (16 bit A/D converter) for data analysis. Spikes were identified and sorted using the WaveClus software package [19]. Response to stimuli was quantified by comparing post-stimulus spike rates to the average spike rate over the 2 second period that preceded the appearance of the cue stimulus via t-test.
Analysis of local field potentials
We used Morlet wavelet decomposition (wave number of 6) to compute the spectral power as a function of time for analysis of local field potentials as previously described [20]. For significance testing, we compared power between conditions using a Wilcoxon rank sum test with a permutation procedure applied to two time windows: 0–750 msec and 750–1500 msec after stimulus onset [20]. Power values at each frequency and for each event were averaged across these time periods and compared between positive, negative, and neutral feedback conditions.
We analyzed the effect of phase of theta (4–8 Hz), alpha (10–14 Hz), and beta (16–24 Hz) oscillations on gamma oscillatory power [12]. We extracted phase information using the Hilbert transform and divided phase values into 4 phase bins 0.4 radians in width. The gamma band was divided into low and high gamma frequencies at 70 Hz based on existing literature [21]. Based on the time-frequency spectrograms, we focused on the 600 msec time window from 100 to 700 msec after the onset of feedback. Power in each of these four bins was compared via a 1-way ANOVA. The F statistic threshold corresponding to a 5% false positive rate was determined using a permutation procedure. This was repeated 1000 times to create a distribution of F ratios from which the largest 5% values were determined. The p values are therefore derived from this distribution of F ratios. A post-hoc test compared gamma power during the peaks of positive versus negative feedback events using a rank-sum test with permutation procedure.
Results
We recorded single unit activity and local field potentials intraoperatively during bilateral DBS implantation. Playing a video game reward task, the participant completed 104 trials in total. She played the game successfully on 57 trials (31 failed trials). She also completed 16 neutral trials for which there was no competitive game portion and no feedback. The participant played the game during implantation in both the right and left ventral striatum. In total, two microelectrode and one macroelectrode recordings were analyzed from the experiment.
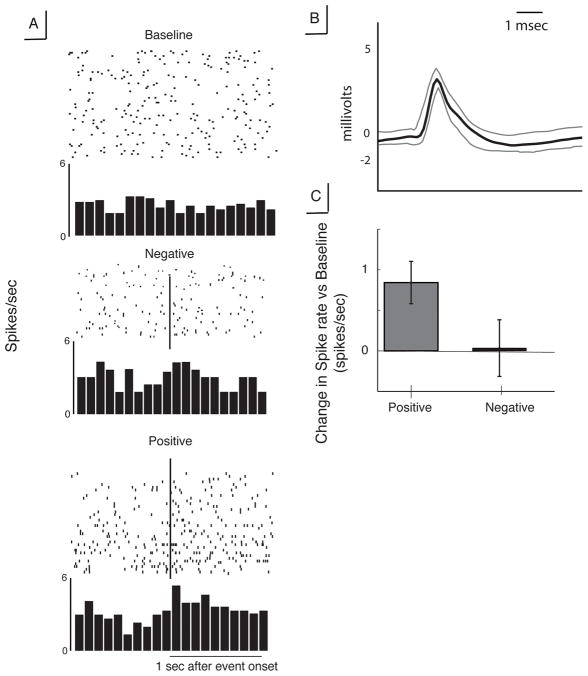
We extracted and sorted single-unit activity captured from the VS microelectrode recordings to find four uniquely identifiable spike clusters (using the WaveClus software package, [19]). We restricted our analysis to the two clusters (from here referred to as neurons) that had a spike rate >1 Hz (one each from the left and right VS). The recording from the right striatum showed a significant increase in spike rate after positive feedback as compared to baseline (t(29)=3.28, p=0.0027, Figure 1). There was no difference in firing rate in response to negative feedback compared to baseline. There were no significant changes in spiking activity relative to baseline for the reward-neutral feedback trials.
Figure 1. Characteristics of neuron identified in right ventral striatum.
A. Raster plots and peri-event histograms for spiking activity in response to positive (top) and negative (bottom) feedback. Vertical lines indicate time of feedback presentation B. Mean spike waveform for this neuron, showing broad depolarization and long after-spike hyperpolarization. Gray lines represent ±SEM. C. Change in spiking activity relative to baseline period for positive and negative feedback.
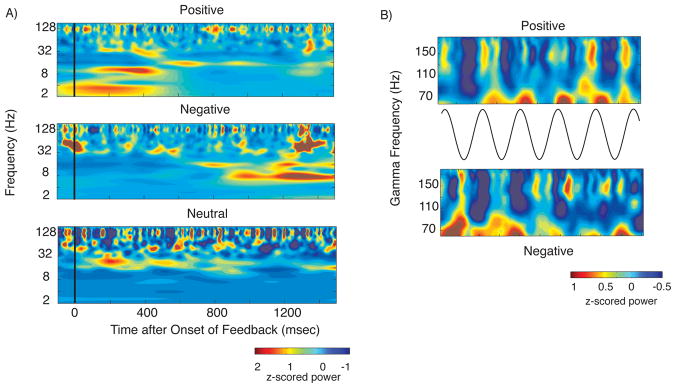
We examined local field potential data recorded from contacts 0 and 1 (most ventral) of the DBS macroelectrodes implanted in the left VS as the patient engaged in the same reward task. Normalized power values recorded from the ventral-most electrode (contact 0) are shown in Figure 2A for each of the three conditions: positive feedback, negative feedback, and reward-neutral feedback (see supplemental material for dorsal electrode data). In both electrodes, alpha band (10–14 Hz) oscillatory power significantly increased in the first 750 msec after positive feedback compared to negative feedback (p=0.002, rank-sum with permutation procedure, FDR corrected). The burst in alpha activity dissipated after 750 msec, at which time negative feedback-related activity showed a significant theta (4–8 Hz) and beta (16–24 Hz) power increase relative to positive feedback (p =0.002, rank sum with permutation procedure, FDR corrected, Figure 2A). Neutral feedback trials demonstrated a significant increase in beta activity compared to both positive and negative feedback during the initial 750 msec after feedback onset (p =0.001, rank sum test with permutation procedure, FDR corrected, Fig. 2A). This beta effect was robust; 90% of neutral feedback events exhibited average beta-band power that was higher than the median value for positive and negative feedback events.
Figure 2. LFP results.
A. Normalized power across feedback conditions Top Panel: Positive feedback trials demonstrates a significant burst of alpha activity between 0 and 1 second. Middle Panel: Negative feedback trials demonstrate a significant theta and beta band power increase from 1 to 2 seconds after feedback onset. Bottom Panel: Neutral feedback trials, characterized by significantly higher beta oscillatory power than in positive or negative feedback trials 0–1 seconds after feedback onset. B. Alpha-gamma cross frequency coupling. Normalized high gamma power over 5 cycles of the alpha oscillation centered upon all the troughs of the oscillation between 100 and 700 msec after feedback onset. Plot is average of all of these 5-cycle windows. Top panel shows positive feedback events in which gamma power is elevated at the peak of the alpha cycle, while the bottom row shows negative feedback events with power elevated at the trough of the cycle.
We identified evidence of alpha oscillatory (10–14 Hz) modulation of gamma band activity that was present during both positive and negative feedback trials for the ventral-most electrode (p=0.002, and p=0.02 for positive and negative respectively, ANOVA with p derived from permutation procedure across 4 phase bins). This was specific for high gamma power; results were not significant for the low gamma band for positive or negative feedback (35–70 Hz, p=0.22 and p=0.35 respectively). Results were not significant for the dorsal electrode (see Supplemental Digital Content). Figure 2B shows gamma power plotted over 5 alpha cycles locked to each alpha trough occurring in the 600 msec that follow feedback onset. It suggests that the significant phase locking identified for positive feedback events is driven by increased high gamma power during the peaks of the alpha oscillation, consistent with existing evidence [12]. By contrast, gamma power is elevated during the trough of the alpha cycle during negative feedback. We confirmed this difference by comparing high gamma power during alpha peaks directly between positive and negative feedback conditions, revealing significantly higher power during positive feedback (p=0.013, rank sum test with permutation procedure, uncorrected). We confirmed this analysis by checking for cross-frequency coupling using the Modulation Index [12]. (See pdf, Supplemental Digital Content 1, for the methods and results of this analysis as well as the results of an analysis of phase reset).
Discussion
Our results are derived from the study of a single participant who suffered from major depression. As such, both neuronal and LFP results must be interpreted with caution. Nonetheless, the paucity of human data from the ventral striatum and use of the ventral striatum as a therapeutic target make our findings worthy of interest. To our knowledge, we identify the first examples in the literature of reward-sensitive neurons in the human ventral striatum. The cells we identified appeared to have firing rates and morphologies most suggestive of tonically active neurons (TANs) [22, 23, 24]. Such neurons are a small percentage of striatal cells, although their large size makes them readily identifiable during in vivo recording and they may represent a higher proportion of neurons in humans as compared to rodents or non-human primates [24]. The firing rate for both cells we observed, ~3 Hz, along with the broad spike waveform and long after-hyperpolarization are consistent with this conclusion. We did not observe the characteristic pause in firing rate that TANs typically exhibit in primate studies of striatal reward processing [24]. This may be attributable to the limited number of cells from which we were able to sample, preventing us from searching for a highly-tuned reward-responsive cell for which dramatic pauses would have been visible.
The results of our LFP analysis complement and extend the existing human literature for reward-related LFP activity in the striatum [12]. We add the identification of an alpha band power difference to evidence of alpha-gamma cross-frequency coupling during reward processing. The alpha power changes we identify may reflect synchrony with other brain areas involved in reward processing, such as the orbitofrontal cortex. Examining orbitofrontal sites for alpha band effects during reward processing may prove insightful. The specificity of cross-frequency coupling for high-gamma band activity reinforces existing literature suggesting that low and high gamma oscillations are functionally distinct: the former represents a localized oscillation while the latter is indicative of multi-unit activity [21].
The inclusion of non-reward trials in our experiment resulted in identification of a striking beta band effect. Beta oscillations in the basal ganglia of humans have been studied most carefully for their role in Parkinson’s disease, in which beta oscillatory activity in the globus pallidus is abolished immediately prior to movement, and abnormal STN beta synchrony is associated with symptoms of Parkinson’s disease [14]. The transition from a non-reward cue to the non-reward feedback period required the patient to perform movement (left and right, randomized for each trial), suggesting the beta oscillation was not attributable to a lack of movement present only in the non-reward trials. Beta oscillations in different basal ganglia structures (e.g. striatum and pallidum) may be functionally similar, by which they hold a brain structure in a default, inactive state that must be abolished before information processing or action may commence [25]. If this is the case, it raises the fascinating possibility that overactive beta synchrony may underlie the anhedonic effects of depression which may offer a novel target for therapeutic manipulation.
Conclusion
We recorded neuronal activity and local field potentials from the human ventral striatum during a reward processing task. We identify the first evidence of reward-responsive spiking in human striatal neurons. Alpha oscillations distinguish positive from negative feedback, while we also uncover evidence of beta oscillations sensitive to reward-neutral conditions. The phase of alpha-gamma cross-frequency coupling also distinguishes feedback types. These observations provide data for the design of closed-loop DBS devices to treat depression.
Supplementary Material
Acknowledgments
Work supported by a grant from the Dana Foundation. The authors would also like to acknowledge the contributions of Marie Kerr for her assistance in maintaining IRB documents.
References
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2009;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromwell HC, Hassani OK, Schultz W. Relative reward processing in primate striatum. Experimental Brain Research. 2005;162(4):520–525. doi: 10.1007/s00221-005-2223-z. [DOI] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cerebral Cortex. 2000;10(3):272. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- Cooper JC, Knutson B. Valence and salience contribute to nucleus accumbens activation. Neuroimage. 2008;39(1):538–547. doi: 10.1016/j.neuroimage.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver JD, Lawrence AD, van Ditzhuijzen J, Davis MH, Woods A, Calder AJ. Individual differences in reward drive predict neural responses to images of food. Journal of Neuroscience. 2006;26(19):5160. doi: 10.1523/JNEUROSCI.0350-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M, Bermpohl F, Mouras H, Schiltz K, Tempelmann C, Rotte M, Heinze HJ, Bogerts B, Northoff G. Distinguishing specific sexual and general emotional effects in fMRI Subcortical and cortical arousal during erotic picture viewing. Neuroimage. 2008;40(4):1482–1494. doi: 10.1016/j.neuroimage.2008.01.040. [DOI] [PubMed] [Google Scholar]
- Erk S, Spitzer M, Wunderlich AP, Galley L, Walter H. Cultural objects modulate reward circuitry. Neuroreport. 2002;13(18):2499. doi: 10.1097/00001756-200212200-00024. [DOI] [PubMed] [Google Scholar]
- Braus DF, Wrase J, Grusser S, Hermann D, Ruf M, Flor H, Mann K, Heinz A. Alcohol-associated stimuli activate the ventral striatum in abstinent alcoholics. Journal of Neural transmission. 2001;108(7):887–894. doi: 10.1007/s007020170038. [DOI] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR. Deep brain stimulation for neurologic and neuropsychiatric disorders. Neuron. 2006;52(1):197–204. doi: 10.1016/j.neuron.2006.09.022. [DOI] [PubMed] [Google Scholar]
- Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, Joe AY, Kreft M, Lenartz D, Sturm V. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2007;33(2):368–377. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- Kahana Michael J. The cognitive correlates of human brain oscillations. Journal of Neuroscience. 2006;26(6):1669–1672. doi: 10.1523/JNEUROSCI.3737-05c.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Axmacher N, Lenartz D, Elger CE, Sturm V, Schlaepfer TE. Nuclei Accumbens Phase Synchrony Predicts Decision-Making Reversals Following Negative Feedback. Journal of Neuroscience. 2009;29(23):7591. doi: 10.1523/JNEUROSCI.5335-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Michael X, Axmacher N, Lenartz D, Elger CE, Sturm V, Schlaepfer TE. Good vibrations: Cross-frequency coupling in the human nucleus accumbens during reward processing. Journal of Cognitive Neuroscience. 2008 doi: 10.1162/jocn.2009.21062. [DOI] [PubMed] [Google Scholar]
- Kuhn AA, Williams D, Kupsch A, Limousin P, Hariz M, Schneider GH, Yarrow K, Brown P. Event-related beta desynchronization in human subthalamic nucleus correlates with motor performance. Brain. 2004;127(4):735. doi: 10.1093/brain/awh106. [DOI] [PubMed] [Google Scholar]
- Zaghloul Kareem A, Blanco Justin A, Weidemann Christoph T, McGill Kathryn, Jaggi Jurg L, Baltuch Gordon H, Kahana Michael J. Human substantia nigra neurons encode unexpected financial rewards. Science. 2009;323:1496–1499. doi: 10.1126/science.1167342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (iaps): Technical manual and affective ratings. Gainesville, FL: NIMH Center for the study of emotion and attention, University of Florida; 1999. [Google Scholar]
- Tideman TN, Tullock G. A new and superior process for making social choices. The Journal of Political Economy. 1976:1145–1159. [Google Scholar]
- Malone DA, Dougherty DD, Rezai AR, Carpenter LL, Friehs GM, Eskandar EN, Rauch SL, Rasmussen SA, Machado AG, Kubu CS, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biological psychiatry. 2009;65(4):267–275. doi: 10.1016/j.biopsych.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroga RQ, Nadasdy Z, Ben-Shaul Y. Unsupervised spike detection and sorting with wavelets and superparamagnetic clustering. Neural Computation. 2004;16:1661–1687. doi: 10.1162/089976604774201631. [DOI] [PubMed] [Google Scholar]
- Sederberg PB, Kahana MJ, Howard MW, Donner Elizabeth J, Madsen Joseph R. Theta and gamma oscillations during encoding predict subsequent recall. Journal of Neuroscience. 2003;23(34):10809–10814. doi: 10.1523/JNEUROSCI.23-34-10809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Maunsell JHR. Different Origins of Gamma Rhythm and High-Gamma Activity in Macaque Visual Cortex. PLoS Biology. 2011;9(4):e1000610. doi: 10.1371/journal.pbio.1000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graveland GA, Williams RS, DiFiglia M. Evidence for degenerative and regenerative changes in neostriatal spiny neurons in Huntington’s disease. Science. 1985;227(4688):770. doi: 10.1126/science.3155875. [DOI] [PubMed] [Google Scholar]
- Sharott A, Moll CKE, Engler G, Denker M, Grun S, Engel AK. Different subtypes of striatal neurons are selectively modulated by cortical oscillations. Journal of Neuroscience. 2009;29(14):4571. doi: 10.1523/JNEUROSCI.5097-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Bolam JP. Functional diversity and specificity of neostriatal interneurons. Current opinion in neurobiology. 2004;14(6):685–692. doi: 10.1016/j.conb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Courtemanche R, Fujii N, Graybiel AM. Synchronous, focally modulated {beta}-band oscillations characterize local field potential activity in the striatum of awake behaving monkeys. Journal of Neuroscience. 2003;23(37):11741. doi: 10.1523/JNEUROSCI.23-37-11741.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.