Abstract
Tobacco smoking causes high rates of mortality and morbidity throughout the world. Despite the availability of smoking-cessation medications, maintenance of long-term abstinence is difficult, and most individuals who attempt to quit smoking relapse. Although tobacco smoke contains many substances, researchers and policymakers agree that nicotine is a major cause of tobacco dependence. Understanding the neural substrates of nicotine dependence is essential for the development of more effective antismoking medications than those currently available. This article focuses on the neural substrates, especially nicotinic acetylcholine receptors, that mediate the reinforcing effects of nicotine and the development of nicotine dependence. Neuroadaptations in the function of the neurotransmitters dopamine, glutamate, and gamma-aminobutyric acid (GABA), which have been shown to be critically involved in nicotine dependence, are also reviewed. Finally, the article discusses progress in the discovery and development of smoking-cessation medications.
Tobacco dependence is a major public health problem that results in significant morbidity, mortality, and health care costs for both smokers and society. The health benefits of smoking cessation are well-known, and nearly 40 percent of smokers in the United States try to quit each year. Nevertheless, only approximately 3 to 6 percent of those who attempt to quit succeed in avoiding smoking for 6 to 12 months, with the majority of quit attempts failing within the first 8 days (Hughes et al., 2004). Professionally administered smoking-cessation therapy improves the odds of a successful quit attempt, but its effectiveness is limited by a lack of highly effective medications. To date, the only smoking-cessation medications approved by the Food and Drug Administration (FDA) are nicotine replacement therapy (NRT), bupropion (Wellbutrin/Zyban), and varenicline (Chantix), along with the second-line agents nortriptyline and clonidine. Of these treatments, varenicline appears to be most effective, yielding abstinence rates of approximately 22 percent at the end of 1 year, compared with 9 percent with placebo (Gonzales et al., 2006). For a comprehensive review of current smoking-cessation medications, see Nides (2008).
An understanding of the neural substrates (structures and processes) that maintain nicotine dependence is essential for the development of effective smoking-cessation medications. Although people probably do not smoke solely to obtain nicotine—some of tobacco’s 4,000 other chemical ingredients, as well as sensory and conditioned effects, may also contribute to the habit—nicotine certainly plays a major role in tobacco dependence (Stolerman and Jarvis, 1995; Rose, 2006). This review discusses: (1) the interaction of nicotine with neuronal pathways in the brain that leads to the initiation and maintenance of the tobacco-smoking habit, (2) the adaptations in several neurotransmitter systems that result from chronic nicotine exposure and lead to continued smoking and the development of nicotine dependence, and (3) the implications of these interactions and neuroadaptations for the design and discovery of novel smoking-cessation treatments.
THREE PHASES OF NICOTINE DEPENDENCE
Nicotine dependence is characterized by three phases:
Acquisition and maintenance of nicotine-taking behavior:In humans, the administration of nicotine through tobacco smoking produces a mild pleasurable rush, mild euphoria, increased arousal, decreased fatigue, and relaxation (Henningfield et al., 1985). These reinforcing effects play an important role in the initiation and maintenance of tobacco smoking (Watkins et al., 2000; Markou, 2008). Several other species, such as rats and nonhuman primates, exhibit behavioral evidence of nicotine reinforcement by reliably self-administering intravenous nicotine (Rose and Corrigall, 1997).
Withdrawal symptoms upon cessation of nicotine intake: Chronic nicotine use induces neuroadaptations in the brain’s reward system that result in the development of nicotine dependence. Thus, nicotine-dependent smokers must continue nicotine intake to avoid distressing somatic and affective withdrawal symptoms. Newly abstinent smokers experience symptoms such as depressed mood, anxiety, irritability, difficulty concentrating, craving, bradycardia, insomnia, gastrointestinal discomfort, and weight gain (Shiffman and Jarvik, 1976; Hughes et al., 1991). Experimental animals, such as rats and mice, exhibit a nicotine withdrawal syndrome that, like the human syndrome, includes both somatic signs and a negative affective state (Watkins et al., 2000; Malin et al., 2006). The somatic signs of nicotine withdrawal include rearing, jumping, shakes, abdominal constrictions, chewing, scratching, and facial tremors. The negative affective state of nicotine withdrawal is characterized by decreased responsiveness to previously rewarding stimuli, a state called anhedonia.
Vulnerability to relapse: Abstinent smokers remain prone to relapse for weeks, months, or even years after cessation of tobacco smoking. Resumption of smoking, like relapse to other drugs of abuse, often occurs upon exposure to people, places, objects, or other stimuli that individuals have learned to associate with the positive rewarding effects of the drug (Hughes et al., 2004). Stress and cigarette smoking itself can also precipitate resumption of habitual smoking.
Comprehensive medication therapy for nicotine dependence will have to target all three phases of drug dependence. Whether by a single “magic bullet” or a sequential strategy using multiple medications, such therapy will need to attenuate the reinforcing effects of nicotine, alleviate the negative affective and somatic symptoms of withdrawal, strengthen abstinence behaviors, and block relapse to smoking. To do so, the medication or medications will need to act upon the neurobiological substrates that underlie each of those aspects of nicotine dependence. The remainder of this review focuses on our current knowledge of these substrates and their potential as targets for smoking-cessation medications.
Nicotine influences mood, cognition, and body function by activating nicotinic acetylcholine receptors located on neurons in the brain.
THE ROLE OF NICOTINIC RECEPTORS
Nicotine influences mood, cognition, and body function by binding to and activating nicotinic acetylcholine receptors (nAChRs) located on neurons in the brain (Figures 1 and 2). When activated by either nicotine or the endogenous neurotransmitter acetylcholine, the nAChR opens a channel that allows ions to pass through the neuron’s membrane from the exterior to the interior of the cell and trigger changes that activate the cell. In this section, we examine the interactions of nicotine with nAChRs and the possibility of treating nicotine dependence by altering these interactions.
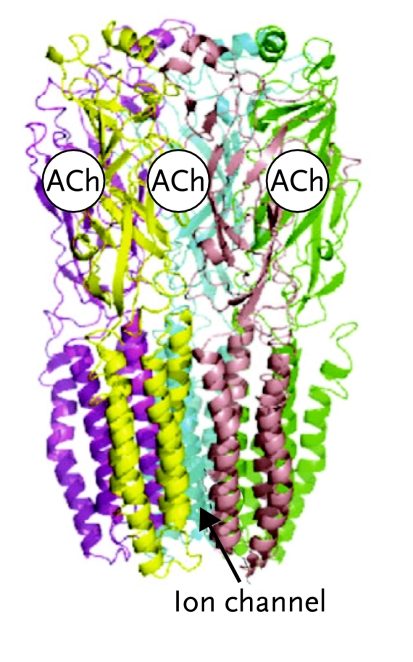
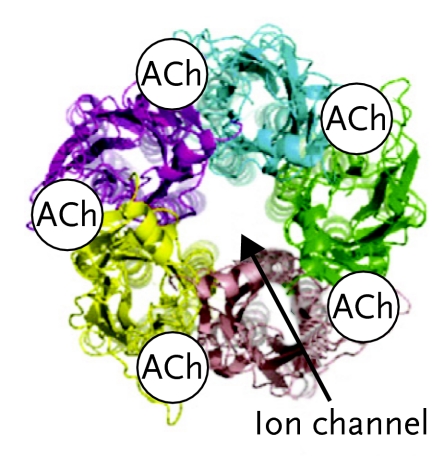
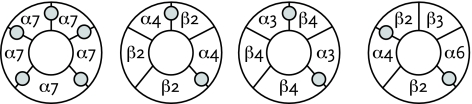
FIGURE 1.
Nicotine Acetylcholine Receptor
(A) Side view of the α7 nAChR showing binding sites for acetylcholine or nicotine. (B) Top view of the α7 nAChR showing binding sites. (C) Schematic top views of four nAChR subtypes, showing their subunit composition: α7 (homomeric); α4β2 (heteromeric); α3β4 (heteromeric); α4α6β2β3 (heteromeric). Reproduced with permission from Changeux and Taly, 2008.
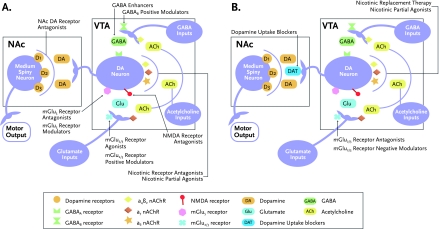
FIGURE 2.
Pharmacological Strategies to Attenuate Nicotine Reinforcement and Alleviate Withdrawal
A) Potential targets in the mesolimbic reward system can promote smoking cessation by attenuating the reinforcing effects of nicotine. The reinforcing effects of nicotine are partly mediated by the activation of dopamine neurons in the ventral tegmental area (VTA) and the release of dopamine (DA) in the nucleus accumbens (NAc). The activity of dopamine neurons in the VTA is regulated by glutamatergic and GABAergic inputs from different brain regions. Pharmacological strategies that attenuate the reinforcing effects of nicotine and cue-induced reinstatement of nicotine seeking in animals include compounds that block nicotine and acetylcholine from stimulating nACh receptors on glutamate- and dopamine-releasing neurons, such as nicotinic receptor antagonists and partial agonists; compounds that reduce excitatory glutamatergic neurotransmission in the VTA, such as the presynaptic mGlu2/3 receptor agonists/positive modulators, postsynaptic mGlu5 antagonists/negative modulators, and N-methyl-d-aspartate (NMDA) receptor antagonists; and compounds that increase the influence of the inhibitory neurotransmitter GABA at receptors on dopamine neurons such as GABA enhancers and GABAB positive modulators. Compounds that reduce dopaminergic neurotransmission, such as DA receptor antagonists, also attenuate nicotine reinforcement and reinstatement in animal models.
B) Potential targets in the mesolimbic reward system may help to alleviate the negative affective symptoms seen in smokers who quit smoking. Chronic nicotine exposure results in decreased dopamine and glutamate neurotransmission in the VTA and NAc. Pharmacological compounds that facilitate dopamine and/or glutamate release in the VTA and/or NAc alleviate the negative affective effects of nicotine withdrawal in animals. Such compounds/strategies include nicotine replacement therapy, nicotine receptor partial agonists, mGlu2/3 receptor antagonists/negative modulators, and dopamine uptake blockers.
Nicotine produces rewarding effects by interacting with nAChRs on neurons in the brain’s mesolimbic reward system. This system comprises dopaminergic neurons that originate in the ventral tegmental area (VTA) and release the neurotransmitter dopamine in regions involved in information processing, memory, and emotions, such as the nucleus accumbens (NAc), hippocampus, amygdala, and prefrontal cortex (PFC). Increases in dopamine levels within the mesolimbic system give rise to rewarding effects. Nicotine directly enhances dopamine levels in the mesolimbic system by interacting with nAChRs on the dopaminergic neurons and causing them to release more of the neurotransmitter (Balfour, 2009; Barrett et al., 2004; Koob and Volkow, 2010). Nicotine also modulates dopamine release indirectly by binding to nAChRs located on excitatory glutamatergic and inhibitory gamma aminobutyric acid (GABAergic) neurons in the VTA. These glutamatergic and GABAergic neurons originate from a number of brain areas, such as the NAc, hippocampus, PFC, amygdala, ventral pallidum, and pedunculopontine tegmental nucleus, and regulate the activity of dopaminergic neurons.
The mix of subunits in each nAChR gives the receptor its distinct pharmacological properties.
In contrast, binding of nicotine to nAChRs located on excitatory glutamatergic terminals results in glutamate release, which in turn stimulates dopaminergic neurons. Binding of nicotine to nAChRs located on inhibitory GABAergic projections leads to the release of gamma aminobutyric acid (GABA), which in turn inhibits dopaminergic neurons. Both glutamate and GABA neurotransmission play important roles in the development of nicotine dependence (for a review, see Markou, 2008). nAChRs are also present on neurons that release other neurotransmitters, including opioids, norepinephrine, serotonin, orexin, and cannabinoids, but the role of these neurotransmitter systems in nicotine dependence has not been extensively studied.
Nicotinic Receptor Subunits and Nicotine Reinforcement
The ability of nicotine to activate a particular nAChR depends on the subunits that make up the receptor. nAChR subunits exist in 12 isoforms (variant forms), labeled α2–α10 and β2–β4 (Figure 1; Dani and Bertrand, 2007). Every nAChR consists of five subunit molecules arranged in a ring around a central channel that opens to admit ions when the receptor is activated. In some nAChRs, called homomeric nAChRs, all five subunits are the same—for example, all are α7. Other nAChRs, called heteromeric, have a mix—for example, two α4 and three β2 subunits. The mix of subunits in each nAChR gives the receptor its distinct pharmacological properties, including its response to nicotine stimulation.
Extensive preclinical evidence suggests that β2-, α4-, α5-, α6-, and α7-containing nAChRs mediate the reinforcing and behavioral effects of nicotine (Fowler et al., 2008; Markou, 2008). Here we briefly summarize this evidence (Figure 2):
β2-containing nAChRs: Mice that lack the β2 nAChR subunit as a result of genetic manipulation, called β2 knockout mice, do not self-administer nicotine (Picciotto et al., 1998). However, β2 knockout mice begin to self-administer nicotine when the missing receptor subunit is introduced into their brains with another genetic manipulation. Furthermore, rats treated with a compound that blocks the action of nicotine at α4β2-containing nAChRs self-administer less nicotine than control animals given an inert vehicle like saline.
α5-containing nAChRs: Mice bred to lack the α5 subunit (i.e., α5 knockout mice) had fewer nicotine-induced seizures and attenuated nicotine-induced locomotor activity compared with wildtype mice. Importantly, α5 knockout mice showed increased nicotine self-administration compared with wildtype mice. This work by Fowler and colleagues (2011) suggests that activity of the α5 subunit in the medial habenula-interpeduncular nucleus pathway reduces the aversive effects of high doses of nicotine. Humans who have a single-nucleotide polymorphism (SNP) in the gene for the α5 subunit that decreases the expression of the α5-containing nAChRs are twice as likely as those without this SNP to develop nicotine dependence. Taken together with the animal findings, this pattern of results suggests that agonists of α5-containing nAChRs or other compounds that increase the activity of α5-containing nAChRs may promote smoking cessation by enhancing the aversive effects of nicotine.
α6-containing nAChRs: Compounds that selectively reduce the activity of α6-containing nAChRs dose-dependently decrease nicotine self-administration in rats, suggesting these nAChRs are important in nicotine’s reinforcing effects (Dwoskin et al., 2009).
α7-containing nAChRs: Rats self-administered less nicotine after systemic administration of a compound that prevents nicotine from interacting with predominantly homomeric α7-containing nAChRs (Markou and Paterson, 2001). These results suggest a role for α7-containing nAChRs in the reinforcing effects of nicotine.
In summary, activation of nAChRs that contain β2, α4, α6, or α7 subunits appears to promote the reinforcing effects of nicotine. By contrast, α5-containing nAChRs appear to limit nicotine reinforcement, possibly by mediating the drug’s aversive effects. An important unanswered question is whether every nAChR that contains one of these subunits contributes to nicotine reinforcement, or whether only subsets of these nAChRs are involved. Ultimately, the complete subunit composition and stoichiometry of nAChRs containing these five subunits needs to be fully understood to develop medications that will block the reinforcing effects of nicotine and promote smoking cessation.
Nicotinic Receptor Subunits and Nicotine Withdrawal
Evidence from rodent models and genetically modified mice suggests that nAChRs and their various subunits mediate the aversive somatic and negative affective signs of nicotine withdrawal (for reviews, see Kenny and Markou, 2001; Fowler et al., 2008). For example, nicotine-dependent rats treated with a compound that blocks α4β2-containing nAChRs exhibited a negative affective or depression-like state, during which they were less responsive to electrical stimulation of their reward system but showed no somatic signs of nicotine withdrawal (Epping-Jordan et al., 1998).
Varenicline’s dual actions attenuate nicotine’s reinforcing effects and reduce withdrawal symptoms.
By contrast, nicotine-dependent rats treated with a compound that blocks multiple nAChR subtypes, including β4-containing nAChRs, exhibited both a depression-like state and somatic signs of nicotine withdrawal. Importantly, experiments with knockout mice suggest that nAChRs containing α5, α7, and β4 subunits are instrumental in the expression of somatic signs of nicotine withdrawal, whereas β2-containing nAChRs contribute to the nicotine withdrawal-induced negative affective state (Fowler et al., 2008).
Nicotinic Receptor–Based Treatment Strategies
Developers of antismoking medications have long focused on compounds that interact with nAChRs (see Table 1). Varenicline, for example, is a partial agonist at α4β2-containing nAChRs. Varenicline attenuates the reinforcing effects of nicotine by occupying the binding sites on these receptors and blocking nicotine from binding to them. Varenicline and other partial agonists also weakly stimulate α4β2-containing nAChRs, thereby reducing withdrawal symptoms.
TABLE 1.
FDA-Approved and Investigational Smoking-Cessation Medications Targeting Nicotinic Acetylcholine Receptors
| MEDICATIONS/COMPOUNDS | MECHANISM | STATUS | REFERENCE |
|---|---|---|---|
| Nicotine replacement therapies* | Replace nicotine obtained from tobacco smoke through the use of safer options | FDA approved | Nides, 2008 |
| Varenicline | Partial α4β2 nAChR agonist Acts as an antagonist in the presence of nicotine to decrease the reinforcing effects of nicotine Activates nicotinic receptors during abstinence and limits the aversive effects associated with nicotine withdrawal |
FDA approved | Gonzales et al., 2006 |
| Mecamylamine | Nonselective nicotinic receptor antagonist | Utility limited due to non-selective action | Schnoll and Lerman, 2006 |
Nicotine patch, gum, inhaler, microtab, nasal spray, and lozenge.
On the basis of the preclinical literature discussed above, compounds that act at nAChRs that contain the β2, α5, α7, and β4 subunits have the potential to reduce the negative affective and aversive somatic symptoms of nicotine withdrawal.
Researchers are also exploring the potential for a new generation of smoking-cessation therapies based on allosteric modulation of nAChRs (Yoshimura et al., 2007). Allosteric modulators are compounds that bind to sites on the receptor that are different from the sites where agonists or antagonists bind. Allosteric modulators do not directly stimulate nAChRs, but instead increase or decrease their responsiveness to agonists like nicotine or natural neurotransmitters like acetylcholine. Researchers have identified several allosteric binding sites on nAChRs. Allosteric compounds acting at these sites may either help to reduce the reinforcing effects of nicotine or may alleviate the aversive effects of nicotine withdrawal, or both, and thus may help prevent relapse (Taly et al., 2009).
Other promising strategies currently under investigation aim to inhibit stimulation of nAChRs by reducing the amount of nicotine that reaches the brain. These include immune-based approaches, such as nicotine vaccines and pharmacokinetic treatments that alter the availability of nicotine by enhancing its peripheral metabolism and clearance (Xi et al., 2009).
NEUROTRANSMITTER SYSTEMS IN NICOTINE ADDICTION
As described above, nicotine initiates the processes leading to nicotine dependence by interacting with nAChRs on dopaminergic, glutamatergic, and GABAergic neurons. Accordingly, as discussed, one set of potential medication strategies for treating nicotine dependence focuses on modulating the nicotine-nAChR interaction. A second group of potential strategies targets the receptors and transporters that mediate the effects of dopamine, glutamate, and GABA after exposure to nicotine.
Dopamine
Dopamine has been strongly implicated in the reinforcing and withdrawal effects of nicotine. The key evidence includes experiments in laboratory animals that show:
administering nicotine increases dopamine transmission within the mesolimbic reward system; and
administering compounds that block dopamine binding to its receptors (D1, D2, D3, D4, and D5 receptors) decreases the reinforcing effects of nicotine.
Dopaminergic neurotransmission is decreased during nicotine withdrawal. For example, in nicotine-dependent rats, the induction of nicotine withdrawal by administration of the nAChR antagonist mecamylamine resulted in decreased dopamine levels in the NAc compared with administration of an insert substance (Hildebrand et al., 1998). In addition, the decrease in NAc dopamine correlated well with the somatic and affective signs of nicotine withdrawal. The decrease in NAc dopamine was greater in adult rats compared with adolescent rats (Natividad et al., 2010), possibly indicating a more important role for mesolimbic dopamine in nicotine withdrawal in adult rats compared with adolescent rats. Dopamine-based smoking-cessation medications that are currently available or under development are directed toward either alleviating nicotine withdrawal symptoms, blocking nicotine reinforcement, or both (see box, Treatment Strategies for Nicotine Dependence Based on Dopaminergic Neurotransmission).
TREATMENT STRATEGIES FOR NICOTINE DEPENDENCE BASED ON DOPAMINERGIC NEUROTRANSMISSION.
Alleviation of withdrawal symptoms by blockade of dopamine uptake transporter
Bupropion is approved by the Food and Drug Administration (FDA) as a smoking cessation medication. It acts by blocking the uptake of synaptic dopamine via the dopamine transporter (DAT). In addition to blocking dopamine uptake, bupropion has other potentially therapeutic actions, such as blockade of nicotinic acetylcholine receptors and norepinephrine uptake (Paterson, 2009). Clinically, bupropion alleviates negative affective symptoms associated with smoking cessation, such as depression, difficulty in concentrating, and irritability.
Attenuation of the reinforcing effects of nicotine via blockade of dopamine receptors
Blocking the reinforcing effects of nicotine using a dopamine receptor antagonist is another dopamine-based strategy that can be developed for smoking cessation (Figure 2, page 7). Blockade of D1, D2, and D3 dopaminergic receptors decreases nicotine self-administration. There is considerable interest in D3 receptor antagonists because this subtype of dopamine receptors has high affinity for dopamine and is extensively expressed in the mesolimbic dopamine system (Diaz et al., 2000). Another reason for the interest in D3 dopamine receptors is that D1 and D2 receptor antagonists tend to produce adverse effects in humans.
Glutamate
Glutamate, the brain’s primary excitatory neurotransmitter, also plays a critical role in the development of nicotine dependence (Liechti and Markou, 2008). Nicotine increases the release of glutamate by binding to excitatory α7-containing nAChRs located on presynaptic terminals of glutamatergic neurons in the VTA, NAc, amygdala, hippocampus, and PFC (Mansvelder and McGehee, 2002).The released glutamate binds to ionotropic and metabotropic glutamate receptors located on neurons in these areas (Figure 2).
Decreases in dopamine in the nucleus accumbens correlate well with symptoms of withdrawal.
Glutamate and nicotine reinforcement
Glutamate released into the VTA after nicotine administration binds to glutamate receptors on dopaminergic neurons. The resulting increased firing of VTA dopaminergic neurons leads to dopamine release in the NAc and, consequently, nicotine reward (Grillner and Svensson, 2000). The rewarding effect of nicotine can be attenuated by administering compounds that reduce glutamate transmission. For example, animals self-administered less nicotine than control animals when treated with compounds that:
prevent glutamate from binding to postsynaptic ionotropic or metabotropic glutamate receptors, such as antagonists at postsynaptic N-methyl-d-aspartate (NMDA), AMPA receptors, or mGlu5 receptors (Liechti and Markou, 2008).
activate presynaptic inhibitory mGlu2/3 receptors.
Furthermore, rats treated with glutamate receptor antagonists showed reduced nicotine-induced dopamine release compared with rats receiving an inert substance (Fu et al., 2000). These findings suggest that decreasing glutamate transmission can facilitate smoking cessation by decreasing the reinforcing effects of nicotine.
Glutamate and nicotine withdrawal
Withdrawal after chronic nicotine exposure is characterized by decreased glutamate transmission and compensatory changes in glutamate receptors (Mansvelder et al., 2002). For example, rats exhibited decreased expression of NMDA ionotropic glutamate receptor subunits, as well as decreased functioning of mGlu2/3 receptors in the PFC, during early withdrawal following chronic nicotine self-administration (Kenny et al., 2009; Liechti et al., 2007). Because presynaptic mGlu2/3 receptors reduce glutamate release when activated, the decreased functioning of mGlu2/3 receptors appears to be a compensatory mechanism to offset the reduction in synaptic glutamate levels that occurs during early withdrawal.
One approach to help smokers maintain abstinence is to weaken and overwrite memories that link stimuli to smoking and the rewarding effects of nicotine.
Similarly, rats in early withdrawal after chronic nicotine self-administration exhibited downregulation of the glutamate transporter (GLT1) in the NAc and cystine-glutamate transporter (xCT) in the VTA and NAc (Knackstedt et al., 2009). These transport proteins are located on glial cells and have opposite roles in the regulation of synaptic glutamate levels. GLT1 decreases synaptic glutamate by drawing glutamate out of the synapse into the glia; by contrast, xCT extrudes vesicular glutamate from glia in exchange for synaptic cystine. The downregulation of GLT1 and xCT during nicotine withdrawal are therefore examples of compensatory mechanisms in response to synaptic glutamate depletion that occurs after chronic nicotine exposure.
In summary, preclinical data suggest that withdrawal from chronic nicotine exposure is characterized by decreased glutamatergic transmission. Thus, medications that increase synaptic glutamate levels may help to alleviate withdrawal symptoms in abstinent smokers.
Glutamate and cue-induced relapse
As described above, memory associations that link certain people, places, and things with the rewarding effects of smoking can trigger intense cravings that lead to relapse in abstinent smokers. In animals, exposure to cues previously associated with nicotine can enhance mesolimbic glutamatergic neurotransmission, resulting in the resumption of drug-seeking behavior (Kalivas and O’Brien, 2008). Treating animals with compounds that block glutamatergic neurotransmission suppresses this effect (Liechti and Markou, 2008). Accordingly, one strategy to prevent relapse in humans is to administer compounds that block glutamatergic neurotransmission.
Unfortunately, learned associations that induce craving cannot be easily erased. Therefore one approach to help smokers maintain abstinence is to replace memories that link stimuli to smoking and the reward effects of nicotine with new memories that will not induce craving or raise the risk of relapse (Taylor et al., 2009). This type of learning, which decreases the reward value of stimuli previously associated with smoking, is called extinction learning (Myers et al., 2011). Interestingly, increasing glutamate transmission facilitates extinction learning. Therefore, another strategy to prevent relapse in abstinent smokers is to aid extinction learning via administration of compounds that increase glutamatergic neurotransmission.
Glutamate-based treatment strategies
Table 2 lists specific glutamate-based strategies that target the different glutamatergic receptors and transporters for the treatment of nicotine dependence (see also Figure 2). Major preclinical findings that involve glutamatergic substrates are described below (for a more detailed review, see Liechti and Markou, 2008):
TABLE 2.
Potential Therapeutic Utility of Glutamatergic Drugs Based on Preclinical Evidence
| POTENTIAL THERAPEUTIC UTILITY | GLUTAMATE RECEPTOR | COMPOUND(S)/ LIGAND(S) | |
|---|---|---|---|
| 1 | Promote smoking cessation by blocking the reinforcing effects of nicotine | mGlu2/3 receptor | mGlu2/3 receptor agonists mGlu2/3 receptor positive allosteric modulators |
| mGlu5 receptor | mGlu5 receptor antagonists mGlu5 receptor negative allosteric modulators |
||
| NMDA receptor | Glycine-site partial agonists | ||
| Cystine-glutamate exchanger | Cystine-glutamate activators, such as N-acetylcysteine | ||
| 2 | Prevent relapse by decreasing the aversive effects associated with abstinence from nicotine | AMPA receptor | AMPA receptor positive modulators |
| mGlu2/3 receptor | mGlu2/3 receptor antagonists mGlu2/3 receptor negative modulators |
||
| 3 | Prevent relapse by enhancing extinction of nicotine-seeking behavior | NMDA receptor | NMDA receptor co-agonists, such as D-cycloserine |
| mGlu5 receptor | mGlu5 receptor agonists mGlu5 receptor allosteric positive modulators |
||
| 4 | Prevent relapse by blocking reinstatement of nicotine-seeking behavior | mGlu2/3 receptor | mGlu2/3 receptor agonists mGlu2/3 receptor allosteric positive modulators |
| mGlu5 receptor | mGlu5 receptor antagonists mGlu5 receptor allosteric negative modulators |
||
| Cystine-glutamate exchanger | Cystine-glutamate activator, such as N-acetylcysteine | ||
| Glutamate transporter | Inhibitors of glutamate transporters, such as ceftriaxone |
mGlu2/3 receptors
Administration of an mGlu2/3 receptor agonist in rats decreases nicotine self-administration. Furthermore, such administration decreased reinstatement of nicotine-seeking behavior upon exposure to cues previously associated with the effects of nicotine (Liechti et al., 2007). These findings suggest that mGluR2/3 receptor agonists may help promote smoking cessation and prevent relapse in humans.
However, the attenuating effects of mGlu2/3 receptor agonists on nicotine self-administration in rats waned after repeated administration. This loss of effectiveness suggests the development of tolerance that may limit the therapeutic utility of full agonists at mGlu2/3 receptors. Currently, positive allosteric modulators for mGlu2/3 receptors are available and are being evaluated clinically for other indications, including treatment of schizophrenia. mGlu2/3 receptor positive allosteric modulators may prove to be more suitable than the full mGlu2/3 receptor agonists. However, the utility of these compounds as potential antismoking medications still needs to be determined both preclinically and clinically.
As described above, animals exhibit a depression-like state upon withdrawal from chronic nicotine exposure. That state is attributed to a decrease in glutamatergic neurotransmission. In animal studies, administration of compounds that increase glutamatergic neurotransmission by blocking presynaptically located inhibitory mGlu2/3 receptors reversed the depression-like state associated with nicotine withdrawal. Thus, mGluR2/3 antagonists may be useful for treating the negative affective symptoms resulting from smoking abstinence in humans.
mGlu5 receptors
Administration of compounds that block the mGlu5 receptors decrease the self-administration of nicotine and attenuate the reward-enhancing effects of nicotine in rats (Kenny et al., 2003; Paterson et al., 2003), suggesting that mGlu5 receptor antagonists decrease the reinforcing effects of nicotine. Administration of mGlu5 receptor antagonists in rats also blocked the reinstatement of nicotine-seeking behavior in response to cues previously associated with nicotine, suggesting that these compounds may help prevent relapse in humans (Markou, 2008).
However, administration of mGlu5 receptor antagonists aggravated the depression-like state and somatic signs associated with nicotine withdrawal in rats. Furthermore, at the highest tested dose, mGlu5 receptor antagonists on their own induced a depression-like state in nicotine-naïve rats. Thus, these compounds may have negative affective effects in humans. Taken together, these data suggest that, in humans, mGlu5 receptor antagonists may promote smoking cessation and prevent relapse after a period of abstinence but may worsen the symptoms of early nicotine withdrawal.
Smokers given D-cycloserine had fewer cravings on exposure to smoking-related cues.
Negative allosteric modulators of the mGlu5 receptor may prove to be better smoking-cessation aids than full antagonists. Compounds of this type are currently being clinically evaluated for indications including gastroesophageal reflux disease, migraine, and pain. Evaluation for potential use as a treatment for nicotine dependence is highly warranted.
NMDA receptors
In animals, administration of compounds that reduce glutamate neurotransmission by blocking the NMDA receptors decreased nicotine self-administration, suggesting that NMDA receptor antagonists can decrease the reinforcing effects of nicotine (Kenny et al., 2009). In a small study, memantine, a low-affinity NMDA receptor antagonist that also blocks some nAChRs, did not reduce cigarette smoking or craving in smokers. Unfortunately, high-affinity NMDA receptor antagonists are known to produce severe adverse neurotoxic and psychogenic effects in humans and therefore are unlikely to be developed into medications for human use.
The structure of the NMDA receptor is quite complex, and its activation requires the binding of both glutamate and a co-agonist, such as glycine. Therefore glutamate neurotransmission can also be decreased with compounds that block the binding of glycine to the NMDA receptor. In mice, administration of such a compound blocked both the acquisition and expression of preference for a nicotine-associated environment in a conditioned place preference (CPP) study. This finding suggests that compounds that occupy the glycine binding site on the NMDA receptor have the potential to decrease the reinforcing effects of nicotine and thus may help people quit smoking.
Interestingly, activation of the NMDA receptor facilitates extinction learning. There is strong preclinical evidence to suggest that administration of D-cycloserine, an NMDA receptor co-agonist that subtly activates the NMDA receptor, facilitates extinction of learned fear responses in animals (Woods and Bouton, 2006). D-cycloserine is currently being evaluated as a treatment for people who suffer from extreme anxiety or fear (phobias) of certain places and situations in their environment (Hofmann et al., 2006). It is hypothesized that D-cycloserine will help these people establish new learned associations that will eventually alleviate their phobias. In rats, administration of D-cycloserine facilitates extinction of cocaine seeking and cocaine-induced CPP behavior (Myers et al., 2011). Importantly, in a small preliminary study, smokers who were given D-cycloserine had fewer cravings when exposed to smoking-related cues than smokers given a placebo (Santa Ana et al., 2009). However, these data are very preliminary and further investigations are required to examine whether D-cycloserine facilitates extinction of nicotine-seeking behavior.
AMPA receptors
As described previously, cessation of smoking in humans can result in negative affective symptoms, including irritability, changes in mood, and depression. These effects cause many abstinent smokers to restart the smoking habit. In nicotine-dependent rats, inhibition of glutamatergic transmission by a compound that blocks AMPA receptors decreased responsiveness to previously rewarding stimuli, resulting in the development of a depression-like state resembling nicotine withdrawal (Kenny, Gasparini, and Markou, 2003). This finding suggests that antagonists at the AMPA receptor will worsen the negative affective symptoms that result from smoking cessation in humans. Thus, conversely, medications that stimulate AMPA receptors, such as AMPA receptor agonists or AMPA receptor positive modulators, may alleviate these negative symptoms. Interestingly, AMPA receptor positive modulators have produced antidepressant-like effects in animal models of depression (Paul and Skolnick, 2003). Such compounds need to be evaluated clinically before conclusions can be reached about their potential utility as treatments for nicotine dependence.
Glutamate transporters
On the basis of preclinical evidence, it is hypothesized that the hypoglutamatergic state resulting from the cessation of tobacco smoking promotes nicotine-seeking behavior (Markou, 2007). Therefore, restoring normal glutamatergic neurotransmission through manipulation of glutamate transporters, such as xCT and GLT1, may help smokers maintain abstinence. Consistent with these hypotheses, smokers in a small pilot study smoked fewer cigarettes when given a compound such as N-acetylcysteine compared with smokers who received a placebo; alcohol consumption was taken into consideration. N-acetylcysteine binds to xCT and releases glutamate into the synapse from intracellular stores in exchange for synaptic cystine (Knackstedt et al., 2009). However, further clinical work is required to determine if this strategy will be effective in promoting smoking cessation and preventing relapse in all smokers.
In summary, the function of the neurotransmitter glutamate has considerable promise as a target for smoking-cessation medications. In addition, compared with ionotropic glutamate receptors, metabotropic glutamate receptors are potentially excellent targets for medication development because they are slow-acting, populate brain areas involved in reward and emotion, subtly modulate glutamate transmission, and are less likely to produce undesirable side effects seen with manipulation of fast-acting ionotropic receptors (Markou, 2007). Furthermore, allosteric modulators that subtly modulate the endogenous glutamatergic system, and thus produce fewer undesirable effects compared with full glutamate receptor agonists and antagonists, appear to have a good chance of proving useful in humans (Rudd and McCauley, 2005). Although currently there are no FDA-approved glutamate-based smoking-cessation agents, several of these compounds are being evaluated clinically for treatment of psychiatric disorders that are associated with high rates of smoking, such as depression and schizophrenia (Conn and Jones, 2009). These studies may shed light on whether these compounds may also have effects on cigarette smoking.
Gamma-Aminobutyric Acid
GABA is the major inhibitory neurotransmitter in the mammalian nervous system. An increase in GABA levels in the VTA limits reward and reinforcement by reducing the activity of mesolimbic dopaminergic neurons. GABA is released in the VTA by neurons that originate in several brain areas, such as the pedunculopontine tegmental nucleus, ventral pallidum, and NAc, as well as interneurons located within the VTA itself (Kalivas and O’Brien, 2008). Endogenous GABA acts via ionotropic GABAA and GABAC receptors and metabotropic GABAB receptors.
Role of GABA in nicotine reinforcement, withdrawal, and relapse
In nicotine-naïve animals, acute nicotine exposure increases GABA release by activating excitatory 4 2-containing nAChRs that are located on GABAergic neurons in the VTA. Thus initially, nicotine-induced GABA release limits the rewarding effects of nicotine. By contrast, chronic nicotine exposure desensitizes 4 2-containing nAChRs on GABAergic receptors (Mansvelder and McGehee, 2002). Hypothetically, this desensitization will decrease nicotine-induced GABA release, leading to decreased inhibition of VTA dopaminergic neurons and increased dopamine release in the NAc—and so facilitate the reinforcing effects of nicotine. Administration of compounds that increase GABAergic neurotransmission decreases both the reinforcing effects of nicotine and reinstatement of cue-induced nicotine-seeking behavior in rats (Markou, 2008; Vlachou et al., 2011). Thus, treatments that enhance GABA transmission may prevent relapse to tobacco smoking.
Restoring normal glutamate transmission may help smokers maintain abstinence.
GABA-based treatment strategies
GABA-based drug discovery for smoking cessation has focused on increasing GABAergic transmission, either by inhibiting the breakdown of GABA or by activating GABAergic receptors (Markou, 2008; Figure 2).
Inhibition of GABA breakdown
The levels of GABA can be increased irreversibly by inhibiting GABA transaminase, the primary enzyme involved in GABA metabolism. In animal studies, administration of gamma-vinyl GABA (GVG; also referred to as vigabatrin), a compound that inhibits GABA transaminase, decreased nicotine self-administration and blocked both the acquisition and expression of nicotine-induced CPP (Dewey et al., 1999). These findings suggest that GVG reduces the reinforcing effects of nicotine. In addition, GVG dose-dependently lowered nicotine-induced increases in NAc dopamine in both nicotine-naïve and nicotine-treated rats. On the basis of these findings, it appears that GVG may help smokers quit. GVG is approved by the FDA for the treatment of infantile spasms and is currently used to treat epilepsy in many countries. However, its use is associated with serious adverse events, such as visual field defects, that may limit its utility as a smoking-cessation aid.
GABAB receptor activation
Administration of compounds that activate GABAB receptors, such as GABAB receptor agonists, decreased nicotine self-administration in rats. Importantly, the reduction in nicotine self-administration persisted even after repeated administration of the GABAB agonist, indicating that little tolerance to its effectiveness had developed (Paterson et al., 2005). In the same study, GABAB receptor agonists also blocked the reinstatement of nicotine-seeking behavior in rats upon re-exposure to nicotine-associated cues. Together these results suggest that GABAB receptor agonists may promote smoking cessation. Consistent with the above preclinical evidence, in a small double-blind clinical study, the GABAB receptor agonist baclofen reduced the number of cigarettes smoked per day as well as craving associated with abstinence (Franklin et al., 2009). However, GABAB receptor agonist administration in animals also resulted in nonspecific effects, such as decreased responding for nondrug rewards, including food and pleasurable electrical brain stimulation, and undesirable effects such as severe motor impairment. These preclinical findings suggest that GABAB receptor agonists may have limited utility for use in humans.
Treatments that enhance GABA transmission may prevent relapse to tobacco smoking.
GABAB receptor positive allosteric modulators
Researchers are investigating the effects of modulators that bind to allosteric sites on the GABAB receptor and enhance the receptor’s responsiveness to GABA. Importantly, these positive allosteric modulators tend to have more subtle effects than full agonists, which bind to the same GABAB site as GABA (Guery et al., 2007). For example, positive allosteric modulators of GABAB receptors do not cause the severe motor impairment seen with GABAB receptor agonists.
Administration of positive allosteric modulators of GABAB receptors in rats decreased nicotine self-administration at doses that did not affect responding for nondrug rewards such as food (Paterson et al., 2008). Furthermore, positive allosteric modulators of GABAB receptors also blocked the cue-induced reinstatement of nicotine-seeking behavior in rats (Vlachou et al., 2011). These findings strongly suggest that GABAB receptor positive allosteric modulators may help promote smoking cessation and prevent relapse in humans. In addition, these allosteric modulators may have better side-effect profiles than full GABAB receptor agonists.
CONCLUSIONS
Tobacco smoking is a harmful habit that can be targeted to bring down both morbidity and future health care costs. Significant progress has been made over the last 2 decades in understanding the neural substrates involved in nicotine dependence. This article has reviewed our current understanding of nAChRs and the role of nicotinic receptor subtypes in nicotine reinforcement and dependence. Preclinical work that evaluates the role of non-nAChR substrates, such as dopamine, glutamate, and GABA, in nicotine reinforcement and dependence was also reviewed. Both nAChR-based and non-nAChR-based smoking-cessation strategies are under various stages of development. Although several of these strategies need to be clinically validated, there is much ground for hope that the next generation of smoking-cessation agents will provide better medications than those currently available.
Acknowledgments
This work was supported by National Institutes of Health research grants R01DA11946, R01DA232090, and 2U19DA026838 to Dr. Athina Markou. Dr. Manoranjan S. D’Souza was supported by fellowship 19FT-0045 from the Tobacco-Related Disease Research Program of the State of California. The authors would like to thank Michael Arends for editorial assistance.
REFERENCES
- Balfour DJK. The neuronal pathways mediating the behavioral and addictive properties of nicotine. Handbook of Experimental Pharmacology. 2009;192:209–233. doi: 10.1007/978-3-540-69248-5_8. [DOI] [PubMed] [Google Scholar]
- Barrett SP, et al. The hedonic response to cigarette smoking is proportional to dopamine release in the human striatum as measured by positron emission tomography and [11C]raclopride. Synapse. 2004;54(2):65–71. doi: 10.1002/syn.20066. [DOI] [PubMed] [Google Scholar]
- Changeux JP, Taly A. Nicotinic receptors, allosteric proteins and medicine. Trends in Molecular Medicine. 2008;14(3):93–102. doi: 10.1016/j.molmed.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Jones CK. Promise of mGluR2/3 activators in psychiatry. Neuropsychopharmacology. 2009;34(1):248–249. doi: 10.1038/npp.2008.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annual Review of Pharmacology and Toxicology. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- Dewey SL, et al. A pharmacologic strategy for the treatment of nicotine addiction. Synapse. 1999;31(1):76–86. doi: 10.1002/(SICI)1098-2396(199901)31:1<76::AID-SYN10>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Diaz J, et al. Dopamine D3 receptors expressed by all mesencephalic dopamine neurons. The Journal of Neuroscience. 2000;20(23):8677–8684. doi: 10.1523/JNEUROSCI.20-23-08677.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwoskin LP, et al. Nicotinic receptor-based therapeutics and candidates for smoking cessation. Biochemical Pharmacology. 2009;78(7):732–743. doi: 10.1016/j.bcp.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epping-Jordan MP, et al. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393(6680):76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Fowler CD, et al. Habenular α5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;471(7340):597–601. doi: 10.1038/nature09797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Arends MA, Kenny PJ. Subtypes of nicotinic acetylcholine receptors in nicotine reward, dependence, and withdrawal: Evidence from genetically modified mice. Behavioral Pharmacology. 2008;19(5–6):461–484. doi: 10.1097/FBP.0b013e32830c360e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, et al. The GABA B agonist baclofen reduces cigarette consumption in a preliminary double-blind placebo-controlled smoking reduction study. Drug and Alcohol Dependence. 2009;103(1–2):30–36. doi: 10.1016/j.drugalcdep.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, et al. Systemic nicotine stimulates dopamine release in nucleus accumbens: Re-evaluation of the role of N-methyl-D-aspartate receptors in the ventral tegmental area. Journal of Pharmacology and Experimental Therapeutics. 2000;294(2):458–465. [PubMed] [Google Scholar]
- Gonzales D, et al. Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: A randomized controlled trial. Journal of the American Medical Association. 2006;296(1):47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Grillner P, Svensson TH. Nicotine-induced excitation of midbrain dopamine neurons in vitro involves ionotropic glutamate receptor activation. Synapse. 2000;38(1):1–9. doi: 10.1002/1098-2396(200010)38:1<1::AID-SYN1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Guery S, et al. Syntheses and optimization of new GS39783 analogues as positive allosteric modulators of GABA B receptors. Bioorganic & Medicinal Chemistry Letters. 2007;17(22):6206–6211. doi: 10.1016/j.bmcl.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield JE, et al. Abuse liability and pharmacodynamic characteristics of intravenous and inhaled nicotine. Journal of Pharmacology and Experimental Therapeutics. 1985;234(1):1–12. [PubMed] [Google Scholar]
- Hildebrand BE, et al. Reduced dopamine output in the nucleus accumbens but not in the medial prefrontal cortex in rats displaying a mecamylamine-precipitated nicotine withdrawal syndrome. Brain Research. 1998;779(1–2):214–225. doi: 10.1016/s0006-8993(97)01135-9. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, et al. Augmentation treatment of psychotherapy for anxiety disorders with D-cycloserine. CNS Drug Reviews. 2006;12(3–4):208–217. doi: 10.1111/j.1527-3458.2006.00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, et al. Symptoms of tobacco withdrawal: A replication and extension. Archives of General Psychiatry. 1991;48(1):52–59. doi: 10.1001/archpsyc.1991.01810250054007. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99(1):29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33(1):166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, et al. Metabotropic glutamate 5 receptor antagonist MPEP decreased nicotine and cocaine self-administration but not nicotine and cocaine-induced facilitation of brain reward function in rats. Annals of the New York Academy of Sciences. 2003;1003:415–418. doi: 10.1196/annals.1300.040. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, et al. NMDA receptors regulate nicotine-enhanced brain reward function and intravenous nicotine self-administration: Role of the ventral tegmental area and central nucleus of the amygdala. Neuropsychopharmacology. 2009;34(2):266–281. doi: 10.1038/npp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Gasparini G, Markou A. Group II metabotropic and α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA)/kainate glutamate receptors regulate the deficit in brain reward function associated with nicotine withdrawal in rats. Journal of Pharmacology and Experimental Therapeutics. 2003;306(3):1068–1076. doi: 10.1124/jpet.103.052027. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Neurobiology of the nicotine withdrawal syndrome. Pharmacology Biochemistry and Behavior. 2001;70(4):531–549. doi: 10.1016/s0091-3057(01)00651-7. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, et al. Metabotropic glutamate 5 receptor antagonist MPEP decreased nicotine and cocaine self-administration but not nicotine and cocaine-induced facilitation of brain reward function in rats. Annals of the New York Academy of Sciences. 2003;1003:415–418. doi: 10.1196/annals.1300.040. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, et al. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biological Psychiatry. 2009;65(10):841–845. doi: 10.1016/j.biopsych.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME, et al. Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. Journal of Neuroscience. 2007;27(34):9077–9085. doi: 10.1523/JNEUROSCI.1766-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME, Markou A. Role of the glutamatergic system in nicotine dependence: Implications for the discovery and development of new pharmacological smoking cessation therapies. CNS Drugs. 2008;22(9):705–724. doi: 10.2165/00023210-200822090-00001. [DOI] [PubMed] [Google Scholar]
- Malin DH, et al. Bupropion attenuates nicotine abstinence syndrome in the rat. Psychopharmacology. 2006;184(3–4):494–503. doi: 10.1007/s00213-005-0135-z. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33(6):905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Cellular and synaptic mechanisms of nicotine addiction. Journal of Neurobiology. 2002;53(4):606–617. doi: 10.1002/neu.10148. [DOI] [PubMed] [Google Scholar]
- Markou A. Metabotropic glutamate receptor antagonists: Novel therapeutics for nicotine dependence and depression? Biological Psychiatry. 2007;61(1):17–22. doi: 10.1016/j.biopsych.2006.03.053. [DOI] [PubMed] [Google Scholar]
- Markou A. Neurobiology of nicotine dependence. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2008;363(1507):3159–3168. doi: 10.1098/rstb.2008.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Paterson NE. The nicotinic antagonist methyllycaconitine has differential effects on nicotine self-administration and nicotine withdrawal in the rat. Nicotine & Tobacco Research. 2001;3(4):361–373. doi: 10.1080/14622200110073380. [DOI] [PubMed] [Google Scholar]
- Myers KM, Carlezon WA, Jr, Davis M. Glutamate receptors in extinction and extinction-based therapies for psychiatric illness. Neuropsychopharmacology. 2011;36(1):274–293. doi: 10.1038/npp.2010.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natividad LA, et al. Nicotine withdrawal produces a decrease in extracellular levels of dopamine in the nucleus accumbens that is lower in adolescent versus adult male rats. Synapse. 2010;64(2):136–145. doi: 10.1002/syn.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nides M. Update on pharmacologic options for smoking cessation treatment. American Journal of Medicine. 2008;121(4):S20–S31. doi: 10.1016/j.amjmed.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Paterson NE, et al. The mGluR5 antagonist MPEP decreased nicotine self-administration in rats and mice. Psychopharmacology. 2003;167(3):257–264. doi: 10.1007/s00213-003-1432-z. [DOI] [PubMed] [Google Scholar]
- Paterson NE, et al. Positive modulation of GABAB receptors decreased nicotine self-administration and counteracted nicotine-induced enhancement of brain reward function in rats. Journal of Pharmacology and Experimental Therapeutics. 2008;326(1):306–314. doi: 10.1124/jpet.108.139204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE. Behavioural and pharmacological mechanisms of bupropion’s anti-smoking effects: Recent preclinical and clinical insights. European Journal of Pharmacology. 2009;603(1–3):1–11. doi: 10.1016/j.ejphar.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Froestl W, Markou A. Repeated administration of the GABAB receptor agonist CGP44532 decreased nicotine self-administration, and acute administration decreased cue-induced reinstatement of nicotine-seeking in rats. Neuropsychopharmacology. 2005;30(1):119–128. doi: 10.1038/sj.npp.1300524. [DOI] [PubMed] [Google Scholar]
- Paul IA, Skolnick P. Glutamate and depression: Clinical and preclinical studies. Annals of the New York Academy of Sciences. 2003;1003:250–272. doi: 10.1196/annals.1300.016. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, et al. Acetylcholine receptors containing the β2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391(6663):173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Rose JE. Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology. 2006;184(3–4):274–285. doi: 10.1007/s00213-005-0250-x. [DOI] [PubMed] [Google Scholar]
- Rose JE, Corrigall WA. Nicotine self-administration in animals and humans: Similarities and differences. Psychopharmacology. 1997;130(1):28–40. doi: 10.1007/s002130050209. [DOI] [PubMed] [Google Scholar]
- Rudd MT, McCauley JA. Positive allosteric modulators of the metabotropic glutamate receptor subtype 2 (mGluR2) Current Topics in Medicinal Chemistry. 2005;5(9):869–884. doi: 10.2174/1568026054750281. [DOI] [PubMed] [Google Scholar]
- Santa Ana EJ, et al. D-cycloserine attenuates reactivity to smoking cues in nicotine dependent smokers: A pilot investigation. Drug and Alcohol Dependence. 2009;104(3):220–227. doi: 10.1016/j.drugalcdep.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA, Lerman C. Current and emerging pharmacotherapies for treating tobacco dependence. Expert Opinion on Emerging Drugs. 2006;11(3):429–444. doi: 10.1517/14728214.11.3.429. [DOI] [PubMed] [Google Scholar]
- Shiffman SM, Jarvik ME. Smoking withdrawal symptoms in two weeks of abstinence. Psychopharmacology. 1976;50(1):35–39. doi: 10.1007/BF00634151. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology. 1995;117(1):2–10. doi: 10.1007/BF02245088. [DOI] [PubMed] [Google Scholar]
- Taly A, et al. Nicotinic receptors: Allosteric transitions and therapeutic targets in the nervous system. Nature Reviews. Drug Discovery. 2009;8(9):733–750. doi: 10.1038/nrd2927. [DOI] [PubMed] [Google Scholar]
- Taylor JR, et al. Targeting extinction and reconsolidation mechanisms to combat the impact of drug cues on addiction. Neuropharmacology. 2009;56(S1):186–195. doi: 10.1016/j.neuropharm.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachou S, et al. Repeated administration of the GABAB receptor positive modulator BHF177 decreased nicotine self-administration, and acute administration decreased cue-induced reinstatement of nicotine seeking in rats. Psychopharmacology. 2011;215:117–128. doi: 10.1007/s00213-010-2119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins SS, et al. Neural mechanisms underlying nicotine addiction: Acute positive reinforcement and withdrawal. Nicotine & Tobacco Research. 2000;2(1):19–37. doi: 10.1080/14622200050011277. [DOI] [PubMed] [Google Scholar]
- Woods AM, Bouton ME. D-cycloserine facilitates extinction but does not eliminate renewal of the conditioned emotional response. Behavioral Neuroscience. 2006;120(5):1159–1162. doi: 10.1037/0735-7044.120.5.1159. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Spiller K, Gardner EL. Mechanism-based medication development for the treatment of nicotine dependence. Acta Pharmacologica Sinica. 2009;30(6):723–739. doi: 10.1038/aps.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura RF, et al. Negative allosteric modulation of nicotinic acetylcholine receptors blocks nicotine self-administration in rats. Journal of Pharmacology and Experimental Therapeutics. 2007;323(3):907–915. doi: 10.1124/jpet.107.128751. [DOI] [PubMed] [Google Scholar]