Abstract
The urokinase plasminogen activator receptor (uPAR) mediates cell motility and tissue remodeling. Although uPAR may be expressed transiently in many tissues during development and wound healing, its constitutive expression appears to be associated with several pathological conditions, including cancer. uPAR expression has been demonstrated in most solid tumors and several hematologic malignancies including multiple myeloma and acute leukemias. Unlike many tumor antigens, uPAR is present not only in tumor cells but also in a number of tumor-associated cells including angiogenic endothelial cells and macrophages. The expression of uPAR has been shown to be fairly high in tumor compared to normal, quiescent tissues, which has led to uPAR being proposed as a therapeutic target, as well as a targeting agent, for the treatment of cancer. The majority of therapeutic approaches that have been investigated to date have focused on inhibiting the urokinase plasminogen activator (uPA)-uPAR interaction but these have not led to the development of a viable uPAR targeted clinical candidate. Genetic knockdown approaches e.g. siRNA, shRNA focused on decreasing uPAR expression have demonstrated robust antitumor activity in pre-clinical studies but have been hampered by the obstacles of stability and drug delivery that have limited the field of RNA nucleic acid based therapeutics. More recently, novel approaches that target interactions of uPAR that are downstream of uPA binding e.g. with integrins or that exploit observations describing the biology of uPAR such as mediating uPA internalization and signaling have generated novel uPAR targeted candidates that are now advancing towards clinic evaluation. This review will discuss some of the pitfalls that have delayed progress on uPAR-targeted interventions and will summarize recent progress in the development of uPAR-targeted therapeutics.
Keywords: Urokinase plasminogen activator, uPAR targeting, cancer therapeutics, metastasis
INTRODUCTION
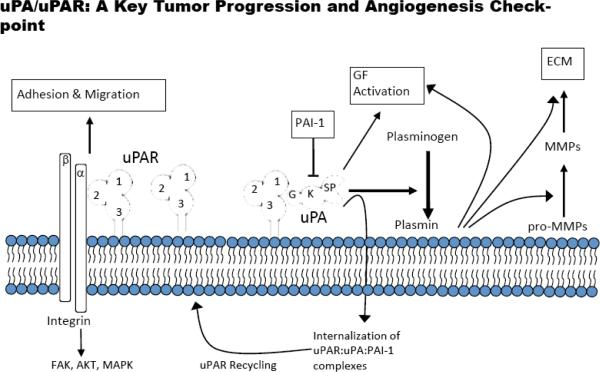
This urokinase plasminogen activator (uPA) system is comprised of a serine protease (uPA), its glycolipid (glycosylphosphatidylinositol) anchored receptor (uPAR), and several serine protease inhibitors (serpins) including plasminogen activator inhibitor 1 (PAI-1) [1]. uPA binds to uPAR, leading to the subsequent activation of plasminogen to plasmin [2] (Fig. 1). Plasmin is a promiscuous protease that initiates several extracellular proteolytic cascades and the binding of uPA to uPAR increases the efficiency of plasminogen activation [2]. It also serves to localize these proteolytic cascades to the migrating or invading edge of cells [3]. These proteolysis events are tightly controlled by PAI-1 and PAI-2, and the PAI-uPA-uPAR complex can be internalized with uPAR being recycled to the cell surface [4]. Extracellular proteolysis mediated by the uPA system is involved in extracellular matrix (ECM) remodeling, cell migration and invasion in normal processes such as wound healing and reproduction; however, the dysregulation of this system plays a major role in a number of pathological processes as well including tumor progression [1]. This observation has led to the numerous efforts attempting to intervene in the function of this system as a therapeutic approach for the treatment of cancer.
Fig. (1). uPAR-dependent pathways.
Extracellular proteolytic and intracellular signaling pathways are depicted. uPAR-domains 1, 2 and 3 are labeled; uPAG(GFD), K (kringle) and SP(serine protease domain); ECM-extracellular matrix; PAI-1-plasminogen activator inhibitor-1.
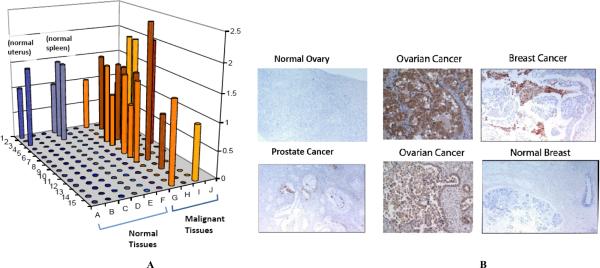
Although the uPA system is comprised of several constituents, uPAR is central to its activity. The uPAR protein is not expressed at detectable levels in most quiescent cells and tissues in adult humans (Fig. 2A) although it does appear to be expressed in a variety of different tumor tissues (Figs. 2A and B). The selective expression of uPAR in tumor tissue and its role in mediating cell motility has led to uPAR being proposed as a therapeutic target for the treatment of cancer. Numerous studies over the past 20 years have provided strong rationale for developing therapeutic agents that target uPAR [5–7]. However, despite significant activity in this regard, no uPAR targeted therapeutic agent has been advanced into clinical evaluation. A number of obstacles have retarded progress on the discovery and development of uPAR inhibitors for clinical evaluation in cancer patients including issues with species specificity, tumor model limitations and a rapidly evolving landscape on the relevant activities and epitopes of uPAR to target. First generation uPAR antagonists have, in general, been underwhelming with respect to their in vivo activity and this has led to pessimism as to the ability to target uPAR therapeutically. However, a deeper understanding of uPAR function has recently resulted in the identification of several novel therapeutic approaches that overcome some of these hurdles and show promise as clinical candidates. The evolution of uPAR targeted therapies and the status of their development for the clinic are the subject of this review.
Fig. (2). uPAR expression in normal and tumor tissues.
A) Tissue microarray (TMA)data of normal and randomly selected tumor tissue. TMA FDA801 (normal tissue) and FDA801-2 (normal and tumor) were purchased form Biomax (Rockville, MD) and analyzed for uPAR expression by immunohistochemistry (IHC) using ATN-658. IHC was performed by Phenopath Laboratories and immunostaining was reviewed and scored by a board certified pathologist. B) IHC using uPAR antibody on selected tumor sections. Random tumors and normal sections were analyzed for uPAR using ATN-658 immunostaining.
uPAR as a Cancer Target
One of the most important considerations that must be taken into account when designing a molecularly targeted therapeutic is target validation. Although the concept of validation has a different meaning and level of rigor depending on the stage of therapeutic development, in its earliest incarnation a potential therapeutic target must be shown to be present in and associated with disease. In later stages of development the target must have a demonstrated role in the disease processes and progression. Cell line data and analysis of tumor samples have demonstrated that uPAR plays a role in a number of different processes critical to tumor progression including angiogenesis [8], tumor growth [9], and metastasis [10]. uPAR is expressed in most solid cancers as well as in several hematologic malignancies including myeloproliferative disorders, acute leukemias (e.g. ALL, AML) and multiple myeloma [11–13]. uPAR is an attractive target for the treatment of cancer not only because it seems to have multiple functional roles associated with tumor progression but also because its expression is restricted quite tightly to tumor tissue and it is rarely expressed in adjacent normal quiescent tissue (Fig. 2) [14, 15]. uPAR expression is transiently up-regulated during several physiological processes, such as during wound healing and inflammatory response to infection [16–18]. However, in cancer, uPAR expression appears to be constitutive and associated with disease aggressiveness [19,20]. uPAR is expressed by multiple tumor-associated cell types found in tumors including tumor and endothelial cells and infiltrating inflammatory cells such as neutrophils and macrophages. Expression of uPAR is further up-regulated by hypoxia and may facilitate the epithelial-mesenchymal transition hypothesized to occur as a tumor acquires an invasive phenotype [21]. In general, the uPAR expression pattern in tumors falls into two categories: tumors in which uPAR is expressed by both tumor and tumor-associated cells (e.g., pancreatic cancer [22], bladder cancer [23], and renal cell carcinoma [15]) and tumors in which uPAR is expressed on tumor-associated cells but not on the tumor cells themselves e.g., colon cancer [24]. Targeting uPAR expressed on tumor-associated cells may be as important as targeting uPAR expressed on tumor cells and may lead to enhanced antitumor activity especially in those tumor types expressing uPAR on both types of cells. It is now well accepted that the tumor microenvironment and stroma is critical component in tumor progression [25]: angiogenic endothelium in a tumor is required for tumor growth and it is fueled by tumor infiltrating cells, such as macrophage, which promote tumor progression via growth factor and cytokine flux. This was reaffirmed in a recent study in breast cancer patients in which uPAR expression in tumor-associated stromal and inflammatory cells correlated with poor prognosis [26]. uPAR expression is up-regulated on macrophages associated with a tumor compared with monocytes circulating in the blood or their counterparts residing within normal tissue [27]. uPAR expression is also observed on angiogenic endothelium [28] within a tumor as well as on other tumor stromal cells [29]. A corollary of these observations is that uPAR expression appears to increase with grade or stage (i.e. aggressiveness) of the tumor and may be enriched in metastatic lesions [30]. These recent studies emphasize the importance of targeting tumor-associated cells in addition to the tumor cells themselves and provide a strong rationale for the discovery and development of uPAR targeted therapeutics.
Therapeutic Targeting of uPAR: A Historical Perspective
uPAR was initially identified as a regulatory molecule that controlles fibrinolysis by virtue of its role in regulating plasminogen activation, fibrin dissolution and therefore extracellular proteolysis (Fig. 1). Extracellular proteolysis is associated with ECM remodeling and can expose cryptic epitopes in ECM proteins that regulate pericellular biology. uPAR binds to the serine protease urokinase plasminogen activator (uPA), which is secreted as a single-chain zymogen, pro-uPA or single-chain uPA (scuPA), and requires binding to uPAR for activation [31]. uPA bound to uPAR activates cell-surface bound plasminogen (which also binds to several cell surface proteins) 50-fold more efficiently than if both molecules were free in solution. Thus, one of the roles of uPAR in this extracellular plasminogen activation cascade is to increase the efficiency of plasminogen activation [2]. Once plasminogen is activated to plasmin, a series of downstream reactions can be initiated both temporally and simultaneously (Fig. 1). Plasmin is able to directly proteolyze ECM components and release ECM-bound growth factors such as vascular endothelial growth factor and basic fibroblast growth factor either directly [32] or indirectly [33] through the activation of pro-matrix metalloproteinases, which when activated can also further degrade ECM. uPAR also imparts directionality to the proteolytic process. uPAR is a glycosylphosphatidylinositol (GPI) anchored receptor with no transmembrane domain and has been demonstrated to redistribute to the invasive or migrating front of a cell [34]. Since the GPI anchor is connected only to the outer leaf of the cell membrane, redistribution of uPAR in response to pro-migratory signals may occur rapidly. This focuses proteolysis in the direction of movement, creating a path through the ECM and generating a pro-migratory gradient for movement by exposing chemo-tactic ECM epitopes and fragments and freeing growth factors [3]. Many of these activities related to extracellular proteolysis have been implicated in tumor growth and metastasis and suggested reasonable targets for treating cancer. Thus, in an attempt to inhibit the extracellular proteolysis initiated by uPA, initial studies on how to therapeutically target uPAR focused on decreasing plasminogen activation by inhibiting the binding of uPA to uPAR. Unfortunately, the results from this strategy have been, for the most part, underwhelming with little effect observed on tumor growth. The modest effects observed on metastasis were only observed when treatment was started early in the study before seeded metastatic tumors had an opportunity to grow out. Peptide and small-molecule inhibitors of uPA binding to uPAR have been described and many of these focused on developing analogs based on the uPAR binding region of uPA, the growth factor domain (GFD). The GFD is comprised of approximately 48 amino acids and its structure is maintained by three disulfide bonds, one of which forms the so-called Ω-loop that contains the uPAR binding region (aa 22–28) [35]. Cyclic peptide inhibitors based on the Ω-loop that were cyclized using non-disulfide based linkers have been described to have anti-invasive activity in vitro [36,37]. Multifunctional inhibitors containing peptides derived from the GFD have also been shown to inhibit the growth and invasion of ovarian cancer cells in vivo [38]. A pegylated human GFD (amino acids 1–48 of uPA) had modest effects on survival when used as a monotherapy in a U87MG glioma xenograft model [39] although a pegylated mouse GFD, which would be expected to target uPA expressed on tumor associated cells, had a substantially greater effect on survival in this study, an effect that was enhanced when the human and mouse GFD were combined (emphasizing the importance of targeting multiple uPAR expressing cellular compartments within a tumor). Unfortunately, U87MG tumors are not invasive, in contrast to human glioma [40], and the effects observed in this study were primarily antiangiogenic. Several linear peptide approaches again based on the sequence of the GFD have also been described. Kobayashi et al. showed that a linear peptide derived from the GFD could inhibit the invasion and metastasis of 3LL cells [41]. Recently, peptides with some homology to the uPAR-binding region of uPA were discovered via phage display and further optimized using combinatorial approaches [42]. This resulted in a set of peptides containing D-isomer and non-natural amino acids that bound to human uPAR with high affinity (Kd < 1 nM). Several of these (AE120 and AE 152) were shown to inhibit HEp3 carcinoma cell intravasation in a chick chorioallantoic membrane model [42].
Several small-molecule inhibitors of the uPA-uPAR interaction have also been reported. Glycinamide inhibitors, which inhibit the binding of uPA to uPAR with IC50 values in the double-digit nM range have been described but these were never evaluated in functional assays (e.g. migration, invasion) in vitro or in animal tumor models [43]. Similarly, oligothiophene derivatives that inhibit uPA binding to uPAR with IC50 values in the μM range [44], O-substituted hydroxycumaranones, which are described as inhibiting uPA binding to uPAR with IC50 values of ~0.05 μg/mL [45], and substituted aminobiphenyl carboxylicacid derivatives, the most potent of which inhibits the binding of uPA to uPAR with an IC50 value of 0.8 nM, have also been described [46]. No additional data beyond characterization of their inhibitory potential is available for these compounds and to our knowledge, they have not advanced into preclinical development.
One of the challenges that has presented an obstacle to developing therapeutics that target the binding of uPA to uPAR has been the species specificity of the uPA-uPAR interaction. Mouse uPA binds to human uPAR very poorly, with a Kd that is 100–1000× higher than that for the interaction of human uPA with human uPAR and vice versa. Thus, in a typical tumor xenograft model where tumor cells of human origin are grown in a mouse, only the effects of targeting the uPA-uPAR interaction directly on the tumor cell with an antagonist can be evaluated (assuming that the tumor cells also make uPA, which is often the case in tumor cell lines that express uPAR). The structural basis for this specificity has recently been described [47]. The development of a therapeutic targeting the human uPA-uPAR interaction would likely not bind to mouse uPAR, creating a challenge to the evaluation of these types of antagonists in mouse models and may explain some of the disappointing results obtained with the uPAR antagonists described above. Alternatively, the observation that uPAR interacts with many different ligands in addition to uPA suggests that perhaps targeting the uPA-uPAR interaction is not the most relevant target for inhibiting tumor progression. Recent data in the patent literature describing a panel of fully human monoclonal antibodies that block the uPA-uPAR interaction support this hypothesis. Pharmacologically, these antibodies would be expected to be far superior to peptides that inhibit uPA binding to uPAR yet their effects on tumor growth in xenografts are also modest at best [48]. In contrast, a number of genetic knockdown experiments that decrease uPAR expression in tumor cells have demonstrated robust antitumor effects, which is further evidence of the importance of uPAR as a cancer target if the correct “epitope” on uPAR can be identified and targeted. For example, antisense uPAR approaches in human glioma tumor cell lines showed that decreasing uPAR expression in these tumor cells could have profound effects on tumor cell growth, apoptosis, and invasion [49,50]. Thus, even targeting only the tumor cell population in a xenograft model by decreasing uPAR function could lead to attenuated tumor growth and metastasis. Further, these xenograft experiments likely under-predict the antitumor activity (and the potential for toxicity) of uPAR targeting that might be observed in humans since the effects of inhibiting uPAR function in tumor-associated cells such as angiogenic endothelium and macrophages were not evaluated in these studies. The disconnect between an all-encompassing effect on uPAR function using antisense and the data obtained using molecules that blocked the uPA-uPAR interaction or uPA enzymatic activity on a tumor cell suggests that, in addition to targeting the uPA-uPAR interaction, targeting other aspects of uPAR function might also have significant therapeutic implications.
Novel Approaches to Targeting uPAR and Their Translation Toward the Clinic
In addition to binding to uPA and regulating cell surface proteolysis, GPI anchored uPAR can interact with a number of other protein ligands including integrins [51], epidermal growth factor receptor (EGFR) [52], platelet-derived growth factor receptor (PDGFR) [53], the internalizing receptor lipoprotein receptor-related protein (LRP) [54, 55], caveolin [56], the G-protein-coupled receptor FPRL-1, a homologue to the fMLP receptor [57], and vitronectin [58]. The interaction of uPAR with these proteins in many cases regulates cell signaling and several uPAR ligands (integrins, EGFR, PDGFR) have independently been identified as cancer targets. The interaction of uPAR with these proteins may present novel epitopes for inhibiting uPAR function and evaluating the effect of perturbing these interactions in mouse tumor models may not face the same obstacles of species specificity as observed with uPA-uPAR binding antagonists. For example, a direct association of uPAR with α5β1integrin has been described and several regions of uPAR have been implicated in this interaction (amino acids 130–142 (D2A), 240–248, and amino acid 249) [59–61]. Mutation of a single amino acid (Ser245) within this region to alanine (S245A) in cell surface–expressed uPAR impaired its interaction with α5α1. Direct interactions of uPAR with α3β1(46), α4β1, α6β1, α9β1 αvβ3 (47), and Mac-1(CD11b/CD18; αMβ2) [62] have also been reported in a variety of cell types including tumor cells and monocytes/macrophages. Many of these uPAR-integrin interactions stimulate intracellular signaling via SRC [63–65]. SRC activates focal adhesion kinase leading to alterations in matrix assembly, proliferation and inhibition of integrin interactions [64–66]. uPAR can also have indirect effects on various signaling pathways. For example, uPAR is required in order for epidermal growth factor receptor to transmit a mitogenic signaling response to epidermal growth factor, a process that is also dependent on SRC [64]. In some processes, such as matrix assembly (50) or epidermal growth factor–mediated migration, ternary complexes of uPAR-integrin-epidermal growth factor receptor may also be involved [65].
As described above, uPAR knockdown experiments alter cell signaling and tumor cell phenotype, which may lead to the identification of other uPAR epitopes that can be targeted. For example, the down-regulation of uPA and uPAR expression using RNA interference in SNB19 human glioma cells inhibited both RAS- and MEK-mediated signaling, affecting the activation of several potential cancer targets including extracellular signal regulated kinase 1/2, phosphatidylinositol 3-kinase, p38 kinase, and c- Jun NH2-terminal kinase and promoted apoptosis in vitro [67]. Anti-uPAR antisense also inhibited tumor cell invasiveness and proliferation of melanoma cells in vitro and tumor growth and metastasis in vivo [68]. Many of the signaling effectors that have been described to mediate uPAR signaling are being pursued as cancer targets in their own rite. The fact that uPAR signaling is pleiotropic and occurs through several pathways leads to the hypothesis that targeting uPAR may capture multiple signaling pathways using a single antagonist. These observations suggest that alternative therapeutic strategies targeting uPAR may lead to more robust antitumor effects than simply targeting the uPA-uPAR interaction.
To that end, several novel uPAR targeted proof-of-principle approaches have recently been described and these can generally be divided into approaches that exploit the selective overexpression of uPAR and uPA in tumor compared to normal tissue and approaches that antagonize a uPAR dependent interaction/function other than the interaction of uPAR with uPA.
A. Approaches that Exploit the Selective Overexpression of uPAR and uPA in Tumor Compared to Normal Tissue
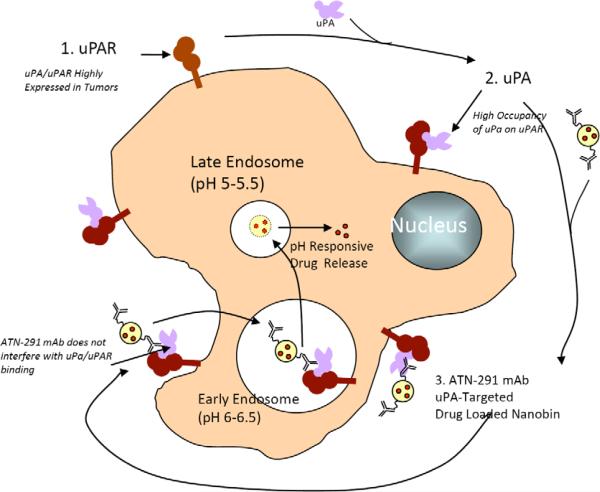
As described above, uPAR is selectively overexpressed (Fig. 1B) in most solid tumors and several hematological malignancies and there is a high correlation between tumors that express uPAR with those that express its ligand. The internalization of uPAR has been described and requires formation of the uPA-PAI-1-uPAR complex (Fig. 2) [4]. Thus, a number of recent studies have attempted to exploit the internalization of uPAR to deliver cytotoxic therapy to a tumor. For example, disulfide cyclized GFD-derived peptide (amino acids 19–31)-DOTA conjugates bound to 213Bi (an α emitter) were cytotoxic to OV-MZ-6 uPAR expressing ovarian cancer cells in vitro and furthermore could be localized to OV-MZ-6 tumors when they were injected intraperitoneally in mice [69]. It was not clear from these studies whether the conjugates required internalization to exert their cytotoxic effects since ionizing radiation has the ability to penetrate several layers of cells and induce cell damage and apoptosis without necessarily having to enter a cell, in contrast to cytotoxic small-molecules. Several groups have also focused on using the amino terminal fragment of uPA (ATF, which contains the GFD) to deliver their payload. Similar to the GFD, the ATF binds to uPAR with an affinity that is similar to full size uPA [70]. The advantage of the ATF is that it provides a larger scaffold for either conjugation or fusion, increasing the possibility of generating conjugates or fusion proteins that do not alter the binding affinity of the ATF to uPAR. In addition, the ATF also contains a kringle domain which may have endogenous antiangiogenic activity [71]. Several ATF-toxin fusions have been reported. For example, the ATF-PE (Psudomonas exotoxin) fusion protein has been described in the literature [72]. ATF-PE retained the binding activity of free ATF and was potently cytotoxic to a number of cell lines in vitro with IC50 values as low as 0.3 pM [72]. The fusions required internalization for their cytotoxic activity but this internalization was not mediated by uPAR but rather by the PE moiety. In general, the ATF is not internalized, consistent with these reports. ATF-diphteria toxin (DTAT) conjugates have also been described. DTAT also retained the binding activity of free ATF and was potently cytotoxic to U87 glioma cells in vitro with an IC50 similar to the Kd for binding [73]. DTAT was also evaluated in a sub-cutaneous U87 model in vivo where treatment with DTAT initiated on day 6 post-tumor cell inoculation significantly delayed tumor growth, more than doubling the time it took for tumors to achieve 2000 mm3 [73]. More recently, several groups have attempted to utilize ATF-mediated delivery via uPAR to target various nanoparticles to uPAR. Unfortunately, although these studies qualitatively show ATF-mediated targeting to tumor cells, there are a number of issues that remain unclear. Yang et al describe the conjugation of iron oxide (IO) nanoparticles to ATF for delivery of ATF-IO to uPAR expressing breast cancer cells [74]. ATF-IO does appear to be bound to tumor cells and localizes to tumor in vivo but the roles of uPAR and ATF in this process is unclear. The authors use a mouse ATF, which is expressed in E.coli, but the folding state and ability to bind to uPAR has not been described. Concentrations for in vitro studies and doses for in vivo studies were not described so it is difficult to assess the utility of these ATF conjugates as uPAR targeting agents. Abdalla et al have also described theragnostic ATF conjugates containing IO and noscapine, also using ATF expressed in E.coli [75]. The dose-response data suggests that these ATF conjugates are not folded properly. Cytotoxicity of these conjugates against prostate cancer cells is observed in the μM range, which is 1000–10,000× above the Kd for binding of native ATF to uPAR. Since native ATF is not generally thought to be internalized via uPAR, these studies raise issues with developing ATF as a uPAR targeting agent that remain to be resolved.
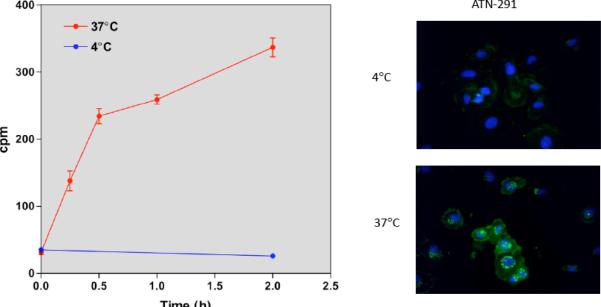
We have identified a monoclonal antibody, ATN-291, that is specific for human uPA and binds to the kringle domain. ATN-291 binds to uPA with a Kd ~0.5 nM and is internalized in a uPA specific manner that does not appear to require uPA to be activated or to form a uPA-PAI-1 complex (Figs. 3, 4). Our group has recently described novel stealth liposomes termed nanobins (NB) that exhibit robust antitumor activity in a xenograft model of triple negative breast cancer [76]. The nanobin formulation of arsenic trioxide is able to inhibit mammary tumor growth, while free arsenic trioxide does not affect tumor growth. The cytotoxicity of the NB can be greatly potentiated when targeted for endosome processing with the folate receptor, and demonstrates the advantage of targeting the nanobin [77,78]. We have recently prepared ATN-291-NB conjugates and have observed that these retain the binding affinity of free ATN-291 and are taken up by uPAR expressing but not uPAR null cells (data not shown). ATN-291-NB have been extensively characterized with regard to synthesis, scale-up, affinity, antibody and lipid content. Studies are presently underway to characterize the in vitro and in vivo anti-tumor activity of ATN-291-NB and to confirm that the specificity of any observed effects is dependent on uPAR expression.
Fig. (3). ATN-291 is internalized by the breast cancer cell line, MDA-MB-231.
A) Endocytosis is a temperature-dependent process and occurs at 37 °C but not 4°C. The internalization of [125I]ATN-291 was followed at 4° and 37°C as previously described [4]. MDA-MB-231 cells express both uPA and uPAR and ATN-291 specifically binds to endogenously expressed uPA on MDA-MB-231 cells (data not shown). B) Oregon green-labeled ATN-291 (OG-ATN-291) was visualized in MDA-MB-231 cells after internalization by fluorescence microscopy. Cells were treated with 25 nM OG-ATN-291 for 1 hr. at 4°C. Cells were washed and transferred to buffer kept at 4 °C or warmed to 37 °C to initiate internalization. After 1 hour, cells were fixed and visualized.
Fig. (4).
Proposed scheme for uPA targeted delivery of nanobin formulated drugs to tumor.
B. Approaches that Inhibit Interactions of uPAR that are Downstream of uPA Binding
Several proof-of-concept studies support the targeting of uPAR interactions with ligands other than uPA. Peptides derived from integrins that block uPAR-integrin interactions have been described. M25, a peptide derived from the β2 subunit of Mac-1 inhibited leukocyte adhesion to fibrinogen, vitronectin, and cytokine-stimulated endothelial cells. M25 also blocked the association of uPAR with β1 and impaired β1-integrin-dependent spreading and migration of human vascular smooth muscle cells on fibronectin and collagen [79]. A second peptide that inhibits uPAR-integrin complex formation, P25, was able to decrease tumor cell attachment to vitronectin and increase tumor cell attachment to fibronectin [80]. P25 administered using osmotic mini-pumps also inhibited the experimental metastasis of MDA-MB-231 cells to bone [81]. Peptides derived from uPAR are also able to inhibit integrin interactions with uPAR. The D2A peptide (amino acids 130–142 of mature human uPAR) and a truncated version of this peptide (GEEG; amino acids 133–136) stimulate integrin-dependent signaling and migration of smooth muscle cells whereas antagonist versions of these peptides (D2A-Ala and GAAG, respectively) inhibit these activities (59). In addition, a mutated peptide (S245A) derived from a sequence in DIII of uPAR (aa 240–248) also inhibits the uPAR-α5β1 interaction and down-regulates ERK signaling. However, despite these initial results implicating uPA-integrin interactions as potential targets for uPAR targeted therapy, there has been little progress in developing therapeutic agents that inhibits these interactions.
Several groups have also focused on using antibodies to target uPAR. Interest in antibody therapeutics in general has been increasing over the past decade due to a number of approved agents for the treatment of cancer and other diseases validating this therapeutic strategy and this trend in pharmaceutical development is now spilling over into the uPAR field. In a proof-of-principle study, Rabbani and Gladu showed that a polyclonal rat antibody targeting the NH2 terminus of rat uPAR could inhibit tumor growth and lead to regressions in a syngeneic rat breast cancer model using Mat BIII cells [81]. In that study, significant apoptosis as well as inhibition of invasion were noted in the tumors. Because these were immunocompetent animals and the antibody was also of rat origin, it was possible that antibody-dependent cell-mediated cytotoxicity could have contributed to the observed antitumor effects. Since the antibody used in these studies was a polyclonal, albeit only targeting the first ~100 amino acids of uPAR, it could also interfere with multiple epitopes and activities of uPAR. Despite this promising result, there has been very little activity in developing an antibody therapeutic targeting uPAR until recently. Fully human monoclonal antibodies that block uPA binding to uPAR were described above [4]. Consistent with other approaches that target the uPA-uPAR interaction, these antibodies exhibit modest antitumor effects inhibit tumor cell invasion in vitro and tumor growth by 25% to 50% in vivo and affect uPAR-dependent signaling by down-regulating extracellular signal-regulated kinase and focal adhesion kinase phosphorylation in tumors grown in vivo.
Our laboratory has focused on identifying uPAR antibodies that do not inhibit the uPA-uPAR interaction but that can alter other partner interactions. We developed a panel of uPAR monoclonal antibodies (MAbs) raised against the DIIDIII fragment of uPAR. These antibodies bind to intact uPAR as well as the DIIDIII fragment of uPAR with similar affinity but do not inhibit uPA binding to uPAR [82]. None of the antibodies raised against the DIIDIII fragment of uPAR were internalized and were therefore not amenable to drug conjugation and delivery; however they do show interesting therapeutic potential. These antibodies were screened in a panel of in vitro assays which led to the identification of several lead uPAR MAb and these were evaluated in subcutaneous A549 tumors in SCID-beige mice. Based on initial studies, we identified a candidate uPAR MAb, ATN-658, that has now shown significant antitumor activity in several orthotopic animal tumor models. ATN-658 inhibited the growth, invasion, and metastasis of the pancreatic carcinoma cell line L3.6pl and was able to enhance the antitumor activity of the positive control, gemcitabine [83]. In that study, ATN-658 monotherapy was able to significantly inhibit (~70%) tumor cell proliferation in vivo, an unexpected observation for a uPAR targeted therapy. We also determined that ATN-658 is absolutely specific for human uPAR and does not cross-react with nonhuman uPAR(except for chimpanzee and gorilla uPAR). Thus, the observed antiproliferative activity was likely due to a direct antagonistic effect on the xenografted tumor cell uPAR because this antibody is not able to mediate antibody-dependent cell-mediated cytotoxicity in mice and does not bind to host uPAR and tumor vasculature. The lack of cross-reactivity of ATN-658 for nonhuman uPAR also suggests that xenograft tumor models may underestimate the antitumor activity of this antibody in man. In a model of colon cancer metastasis, where the human colon carcinoma cell line HCT-116 is inoculated directly into the liver, ATN-658 inhibited tumor growth regardless of whether treatment was started when tumor burden was low (day 4) or high (day 11, average tumor volume in a satellite group of animals was >400 mm3) [84]. The antitumor effect was more pronounced in the animals with high tumor burden where ATN-658 inhibited tumor growth by ~85%. Similar to the pancreatic cancer studies, a profound inhibition of tumor cell proliferation was also observed in these studies.
The mechanistic aspects of the antitumor effects of ATN-658 were evaluated at the molecular level in several additional tumor model studies. ATN-658 inhibited PC-3 cell growth subcutaneously as well as when cells were inoculated in the tibia in a model of prostate cancer metastasis [82]. Qualitatively, the inhibition of tumor growth in the tibia was greater than when the tumors were grown SC. In this study, ATN-658 inhibited activation of FAK, MAPK, and AKT in PC-3 cells cultured in vitro and grown intratibially in vivo. More recently, ATN-658 was demonstrated to inhibit the growth and dissemination of several different ovarian cancer cell lines (CaOV3, SKOV3ip, Hey8) inoculated intraperitoneally in vivo [85]. Similar to the pancreatic cancer study described above [85], the magnitude of the antitumor activity of ATN-658 monotherapy was similar to the chemotherapy positive control (in this study, paclitaxel), and an enhancement of antitumor activity was observed when the two agents were combined [85]. Tumors from ATN-658 and vehicle treated mice were analyzed using Affymatrix gene chips and a number of changes in gene expression were observed in the ATN-658 treated cohort including the down regulation of FGFR1, β3, c-Met, HGF, CD44, RHO, CDK8, JAK1, and JAK3 expression. Many of these genes have been implicated as being important to tumor progression and as therapeutic targets. To confirm the gene chip analysis results, the down-regulation of expression of FGFR1 and β3 were further confirmed by qRT-PCR and western blotting [85]. Treatment of ovarian carcinoma cell lines with ATN-658 also resulted in downregulation of expression of FGFR1 and β3 and these results were consistent across several different ovarian carcinoma cell lines (CaOV3, SKOV3ip) in vitro and in vivo. In these studies, ATN-658 also down-regulated uPA and uPAR expression as well as the co-localization with and expression of α5 β1, which resulted in increased tumor cell apoptosis. Again, these effects could be observed both in vitro and in vivo and were consistent across several different ovarian cancer cell lines tested.
ATN-658 has been now been humanized (huATN-658) and is currently in preclinical development with an Investigational New Drug filing anticipated in late 2011. Assuming that huATN-658 is advanced into human cancer clinical trials, it will be the first uPAR targeted therapy to be evaluated in patients.
FUTURE DIRECTIONS
uPAR targeted therapeutic approaches have finally reached a stage where targeting uPAR has been validated as a promising therapeutic intervention in cancer and the first uPAR targeted molecule is expected to advance into the clinic in the near future. The pleiotropic nature of uPAR interactions and function has presented both a challenge and an opportunity for targeting this receptor. Through the mechanistic study of uPAR and its ligands, multiple therapeutic approaches have emerged. Unfortunately, it has taken some time to sort through the catalog of approaches to identify those that might be most viable. The current level of understanding of uPAR function has just reached a level where several “druggable” functions are now being pursued. uPAR dependent internalization may represent one way of delivering targeted therapy to cells and is especially attractive because it appears to be very selectively expressed in tumor but not in normal tissue. However, direct targeting of uPAR using an antibody raised against DIIDIII of uPAR (i.e. ATN-658) does not mediate internalization, which is however elicited in our studies using an antibody raised against uPA (ATN-291). Antitumor effects observed from the direct targeting of uPAR using ATN-658 are likely due to direct antagonistic effects of this antibody on uPAR function as this antibody is an IgG1 isotype and has been confirmed not to mediate ADCC in the studies described above. ATN-658 affects gene expression and signaling relevant to tumor growth and down-regulates uPA and uPAR expression as well as uPAR-α5β1 co-localization, providing strong rationale for advancing the humanized version of ATN-658 (huATN-658) into clinical evaluation. Thus, the hypothesis that interfering with uPAR function may have therapeutic relevance for the treatment of cancer will hopefully become a testable reality in the near future.
ACKNOWLEDGEMENTS
We thank Phenopath, Mosaic and Charles River Laboratories for IHC analysis of TMA and tissue sections for uPAR expression. Parts of the work described herein were supported by the NCI Cancer Nanotechnology Platform Partnership U01CA151461 (TVO, RWA). RWA is also supported by CDMRP Breast Cancer Research Program Predoctoral Fellow grant (Grant No. BC073413).
REFERENCES
- [1].Dass K, Ahmad A, Azmi AS, Sarkar SH, Sarkar FH. Evolving role of uPA/uPAR system in human cancers. Cancer Treat Rev. 2008;34:122–36. doi: 10.1016/j.ctrv.2007.10.005. [DOI] [PubMed] [Google Scholar]
- [2].Ellis V, Behrendt N, DanØ K. Plasminogen activation by receptor-bound urokinase. A kinetic study with both cell-associated and isolated receptor. J Biol Chem. 1991;266:12752–8. [PubMed] [Google Scholar]
- [3].Waltz DA, Natkin LR, Fujita RM, Wei Y, Chapman HA. Plasmin and plasminogen activator inhibitor type 1 promote cellular motility by regulating the interaction between the urokinase receptor and vitronectin. J Clin Invest. 1997;100:58–67. doi: 10.1172/JCI119521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nykjaer A, Conese M, Christensen EI, et al. Recycling of the urokinase receptor upon internalization of the uPA: serpin complexes. EMBO J. 1997;16:2610–20. doi: 10.1093/emboj/16.10.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].D'Alessio S, Blasi F. The urokinase receptor as an entertainer of signal transduction. Front Biosci. 2009;14:4575–87. doi: 10.2741/3550. [DOI] [PubMed] [Google Scholar]
- [6].Hildenbrand R, Allgayer H, Marx A, Stroebel P. Modulators of the urokinase-type plasminogen activation system for cancer. Expert Opin Investig Drugs. 2010;19:641–52. doi: 10.1517/13543781003767400. [DOI] [PubMed] [Google Scholar]
- [7].Mekkawy AH, Morris DL, Pourgholami MH. Urokinase plasminogen activator system as a potential target for cancer therapy. Future Oncol. 2009;5:1487–99. doi: 10.2217/fon.09.108. [DOI] [PubMed] [Google Scholar]
- [8].Binder BR, Mihaly J, Prager GW. uPAR-uPA-PAI-1 interactions and signaling: a vascular biologist's view. Thromb Haemost. 2007;97:336–42. [PubMed] [Google Scholar]
- [9].Hildenbrand R, Gandhari M, Stroebel P, et al. The urokinase-system-role of cell proliferation and apoptosis. Histol Histopathol. 2008;23:227–36. doi: 10.14670/HH-23.227. [DOI] [PubMed] [Google Scholar]
- [10].Sidenius N, Blasi F. The urokinase plasminogen activator system in cancer: recent advances and implication for prognosis and therapy. Cancer Metastasis Rev. 2003;22:205–22. doi: 10.1023/a:1023099415940. [DOI] [PubMed] [Google Scholar]
- [11].Mustjoki S, Alitalo R, Stephens RW, Vaheri A. Blast cell-surface and plasma soluble urokinase receptor in acute leukemia patients: relationship to classification and response to therapy. Thromb Haemost. 1999 May;81:705–10. [PubMed] [Google Scholar]
- [12].Béné MC, Castoldi G, Knapp W, et al. EGIL, European Group on Immunological Classification of Leukemias. CD87 (urokinase-type plasminogen activator receptor), function and pathology in hematological disorders: a review. Leukemia. 2004;18:394–400. doi: 10.1038/sj.leu.2403250. [DOI] [PubMed] [Google Scholar]
- [13].Hjertner O, Qvigstad G, Hjorth-Hansen H, et al. Expression of urokinase plasminogen activator and the urokinase plasminogen activator receptor in myeloma cells. Br J Haematol. 2000 Jun;109(4):815–22. doi: 10.1046/j.1365-2141.2000.02089.x. [DOI] [PubMed] [Google Scholar]
- [14].Li Y, Cozzi PJ. TargetinguPA/uPAR in prostate cancer. Cancer-Treat Rev. 2007;33:521–7. doi: 10.1016/j.ctrv.2007.06.003. [DOI] [PubMed] [Google Scholar]
- [15].Bhuvarahamurthy V, Schroeder J, Kristiansen G, et al. Differential gene expression of urokinase-type plasminogen activator and its receptor in human renal cell carcinoma. Oncol Rep. 2005;14:777–82. [PubMed] [Google Scholar]
- [16].Xia W, deBock C, Murrell GA, Wang Y. Expression of urokinase-type plasminogen activator and its receptor is up-regulated during tendon healing. J Orthop Res. 2003;21:819–25. doi: 10.1016/S0736-0266(03)00058-5. [DOI] [PubMed] [Google Scholar]
- [17].Gyetko MR, Sud S, Kendall T, et al. Urokinase receptor- deficient mice have impaired neutrophil recruitment in response to pulmonary Pseudomonas aeruginosa infection. J Immunol. 2000;165:1513–9. doi: 10.4049/jimmunol.165.3.1513. [DOI] [PubMed] [Google Scholar]
- [18].Schnaper HW, Barnathan ES, Mazar A, et al. Plasminogen activators augment endothelial cell organization in vitro by two distinct pathways. J Cell Physiol. 1995;165:107–18. doi: 10.1002/jcp.1041650114. [DOI] [PubMed] [Google Scholar]
- [19].Aguirre Ghiso JA, Alonso DF, Farías EF, Gomez DE, de Kier Joffè EB. Deregulation of the signaling pathways controlling urokinase production. Its relationship with the invasive phenotype. Eur J Biochem. 1999;263:295–304. doi: 10.1046/j.1432-1327.1999.00507.x. [DOI] [PubMed] [Google Scholar]
- 20.Forbes K, Gillette K, Kelley LA, Sehgal I. Increased levels of urokinase plasminogen activator receptor in prostate cancer cells derived from repeated metastasis. World J Urol. Apr;22:67–71. doi: 10.1007/s00345-003-0395-3. [DOI] [PubMed] [Google Scholar]
- [21].Lester RD, Jo M, Montel V, Takimoto S, Gonias SL. uPAR induces epithelial-mesenchymal transition in hypoxic breast cancer cells. J Cell Biol. 2007;178:425–36. doi: 10.1083/jcb.200701092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cantero D, Friess H, Deflorin J, et al. Enhanced expression of urokinase plasminogen activator and its receptor in pancreatic carcinoma. Br J Cancer. 1997;75:388–95. doi: 10.1038/bjc.1997.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bhuvarahamurthy V, Schroeder J, Denkert C, et al. situ gene expression of urokinase-type plasminogen activator and its receptor in transitional cell carcinoma of the human bladder. Oncol Rep. 2004;12:909–13. [PubMed] [Google Scholar]
- [24].Pyke C, Ralfkiaer E, RØnne E, et al. Immunohistochemical detection of the receptor for urokinase plasminogen activator in human colon cancer. Histopathology. 1994;24:131–8. doi: 10.1111/j.1365-2559.1994.tb01291.x. [DOI] [PubMed] [Google Scholar]
- [25].Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001 May 17;411(6835):375–9. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- [26].Giannopoulou I, Mylona E, Kapranou A, et al. The prognostic value of the topographic distribution of uPAR expression in invasive breast carcinomas. Cancer Lett. 2007;246:262–7. doi: 10.1016/j.canlet.2006.03.003. [DOI] [PubMed] [Google Scholar]
- [27].DanØ K, RØmer J, Nielsen BS, et al. Urokinase plasminogen activator receptor (CD87) expression of tumor-associated macrophages in ductal carcinoma in situ, breast cancer, and resident macrophages of normal breast tissue. J Leukoc Biol. 1999;66:40–9. doi: 10.1002/jlb.66.1.40. [DOI] [PubMed] [Google Scholar]
- [28].Yamamoto M, Sawaya R, Mohanam S, et al. Expression and localization of urokinase-type plasminogen activator receptor in human gliomas. Cancer Res. 1994;54:5016–20. [PubMed] [Google Scholar]
- [29].Dublin E, Hanby A, Patel NK, Liebman R, Barnes D. Immunohistochemical expression of uPA, uPAR, and PAI-1 in breast carcinoma. Fibroblastic expression has strong associations with tumor pathology. Am J Pathol. 2000;157:1219–27. doi: 10.1016/S0002-9440(10)64637-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Suzuki S, Hayashi Y, Wang Y, et al. Urokinase type plasminogen activator receptor expression in colorectal neoplasms. Gut. 1998;43:798–805. doi: 10.1136/gut.43.6.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Behrendt N, RØnne E, DanØ K. The structure and function of the urokinase receptor, a membrane protein governing plasminogen activation on the cell surface. Biol Chem Hoppe-Seyler. 1995;376:269–79. [PubMed] [Google Scholar]
- [32].Roth D, Piekarek M, Paulsson M, et al. Plasmin modulates vascular endothelial growth factor-A-mediated angiogenesis during wound repair. Am J Pathol. 2006;168:670–84. doi: 10.2353/ajpath.2006.050372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kim MH. Flavonoids inhibit VEGF/bFGF-induced angiogenesis in vitro by inhibiting the matrix-degrading proteases. JCell Biochem. 2003;89:529–38. doi: 10.1002/jcb.10543. [DOI] [PubMed] [Google Scholar]
- [34].Sullivan R, Graham CH. Hypoxia-driven selection of the metastatic phenotype. Cancer Metastasis Rev. 2007;26:319–31. doi: 10.1007/s10555-007-9062-2. [DOI] [PubMed] [Google Scholar]
- [35].Hansen AP, Petros AM, Meadows RP, Fesik SW. Backbone dynamics of a two-domain protein: 15N relaxation studies of the amino-terminal fragment of urokinase-type plasminogen activator. Biochemistry. 1994;33:15418–24. doi: 10.1021/bi00255a023. [DOI] [PubMed] [Google Scholar]
- [36].Mazar AP, Jones TR. Cyclic peptides targeting the urokinase receptor. US6277818. 2001 [Google Scholar]
- [37].Hall CL, Tsan R, Mugnai G, et al. Enhanced invasion of hormone refractory prostate cancer cells through hepatocyte growth factor (HGF) induction of urokinase-type plasminogen activator (u-PA) Prostate. 2004;59:167–76. doi: 10.1002/pros.20009. [DOI] [PubMed] [Google Scholar]
- [38].Krol J, Kopitz C, Kirschenhofer A, et al. Inhibition of intraperito-neal tumor growth of human ovarian cancer cells by bi- and tri-functional inhibitors of tumor-associated proteolytic systems. Biol Chem. 2003;384:1097–102. doi: 10.1515/BC.2003.122. [DOI] [PubMed] [Google Scholar]
- [39].Bu X, Khankaldyyan V, Gonzales-Gomez I, et al. Species-specific urokinase receptor ligands reduce glioma growth and increase survival primarily by an antiangiogenesis mechanism. Lab Invest. 2004;84:667–78. doi: 10.1038/labinvest.3700089. [DOI] [PubMed] [Google Scholar]
- [40].Wong K, Young GS, Makale M, Hu X, Yildirim N, Cui K, Wong ST, Kesari S. Characterization of a human tumor sphere glioma orthotopic model using magnetic resonance imaging. J Neurooncol. 2011 doi: 10.1007/s11060-010-0517-x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kobayashi H, Gotoh J, Fujie M, et al. Inhibition of metastasis of Lewis lung carcinoma by a synthetic peptide within growth factor-like domain of urokinase in the experimental and spontaneous metastasis model. Int J Cancer. 1994;57:727–33. doi: 10.1002/ijc.2910570520. [DOI] [PubMed] [Google Scholar]
- [42].Ploug M, Østergaard S, Gårdsvoll H, et al. Peptide derived antagonists of the urokinase receptor. Affinity maturation by combinatorial chemistry, identification of functional epitopes, and inhibitory effect on cancer cell intravasation. Biochemistry. 2001;40:12157–68. doi: 10.1021/bi010662g. [DOI] [PubMed] [Google Scholar]
- [43].Rosenberg S, Spear KL, Martin EJ. Urokinase receptor ligands. US6121240. 2000 [Google Scholar]
- [44].KÖnig B, Zimmermann G, De Cillis G, di Domenico R. Oligothiophenes useful as antimetastatic agents, a preparation thereof and pharmaceutical compositions containing them. WO 9906393. 1999 [Google Scholar]
- [45].De Cillis G, di Domenico R, KÖnig B, Oliva A. O-substituted hydroxycumaranone derivatives as antitumor and antimetastatic agents. WO 9906387. 1999 [Google Scholar]
- [46].Blood CH, Neustadt BR, Smith EM. Derivatives of aminobenzoic and aminobiphenylcarboxylic acids useful as anti-cancer agents. US6228985. 2001 [Google Scholar]
- [47].Lin L, Gårdsvoll H, Huai Q, Huang M, Ploug M. Structure-based engineering of species selectivity in the interaction between urokinase and its receptor: implication for preclinical cancer therapy. J Biol Chem. 2010;285:10982–92. doi: 10.1074/jbc.M109.093492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Zhao Q, Emery SC, Elvin P. Binding agents directed to uPAR and uses thereof. WO 07120693. 2006 [Google Scholar]
- [49].Yanamandra N, Konduri SD, Mohanam S, et al. Downregulation of urokinase-type plasminogen activator receptor (uPAR) induces caspase-mediated cell death in human glioblastoma cells. Clin Exp Metastasis. 2000;18:611–5. doi: 10.1023/a:1011941114862. [DOI] [PubMed] [Google Scholar]
- [50].Mohanam S, Jasti SL, Kondraganti SR, et al. Stable transfection of urokinase-type plasminogen activator antisense construct modulates invasion of human glioblastoma cells. Clin Cancer Res. 2001;7:2519–26. [PubMed] [Google Scholar]
- [51].Kugler MC, Wei Y, Chapman HA. Urokinase receptor and integrin interactions. Curr Pharm Des. 2003;9:1565–74. doi: 10.2174/1381612033454658. [DOI] [PubMed] [Google Scholar]
- [52].Jo M, Thomas KS, Marozkina N, et al. Dynamic assembly of the urokinase-type plasminogen activator signaling receptor complex determines the mitogenic activity of urokinase-type plasminogen activator. J Biol Chem. 2005;280:17449–57. doi: 10.1074/jbc.M413141200. [DOI] [PubMed] [Google Scholar]
- [53].Kiyan J, Kiyan R, Haller H, Dumler I. Urokinase induced signaling in human vascular smooth muscle cells is mediated by PDGFR-β. EMBO J. 2005;24:1787–97. doi: 10.1038/sj.emboj.7600669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Webb DJ, Nguyen DH, Gonias SL. Extracellular signal-regulated kinase functions in the urokinase receptor-dependent pathway by which neutralization of low density lipoprotein receptor-related protein promotes fibrosarcoma cell migration and Matrigel invasion. J Cell Sci. 2000;113:123–34. doi: 10.1242/jcs.113.1.123. [DOI] [PubMed] [Google Scholar]
- [55].Aguirre Ghiso JA. Inhibition of FAK signaling activated by urokinase receptor induces dormancy in human carcinoma cells in vivo. Oncogene. 2002;21:2513–24. doi: 10.1038/sj.onc.1205342. [DOI] [PubMed] [Google Scholar]
- [56].Stahl A, Mueller BM. The urokinase-type plasminogen activator receptor, a GPI-linked protein, is localized in caveolae. J Cell Biol. 1995;129:335–44. doi: 10.1083/jcb.129.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Furlan F, Orlando S, Laudanna C, et al. The soluble D2D3(88–274) fragment of the urokinase receptor inhibits monocyte chemotaxis and integrin-dependent cell adhesion. J Cell Sci. 2004;117:2909–16. doi: 10.1242/jcs.01149. [DOI] [PubMed] [Google Scholar]
- [58].Madsen CD, Sidenius N. The interaction between urokinase receptor and vitronectin in cell adhesion and signaling. Eur J Cell Biol. 2008;87:617–29. doi: 10.1016/j.ejcb.2008.02.003. [DOI] [PubMed] [Google Scholar]
- [59].Degryse B, Resnati M, Czekay RP, Loskutoff DJ, Blasi F. Domain 2 of the urokinase receptor contains an integrin-interacting epitope with intrinsic signaling activity: generation of a new integrin inhibitor. J Biol Chem. 2005;280:24792–803. doi: 10.1074/jbc.M413954200. [DOI] [PubMed] [Google Scholar]
- [60].Chaurasia P, Aguirre-Ghiso JA, Liang OD, et al. A region in urokinase plasminogen receptor domain III controlling a functional association with 5 1 integrin and tumor growth. J Biol Chem. 2006;281:14852–63. doi: 10.1074/jbc.M512311200. [DOI] [PubMed] [Google Scholar]
- [61].Wei Y, Tang CH, Kim Y, et al. Urokinase receptors are required for alpha 5 beta 1 integrin-mediated signaling in tumor cells. J Biol Chem. 2007;282:3929–39. doi: 10.1074/jbc.M607989200. [DOI] [PubMed] [Google Scholar]
- [62].Wei Y, Lukashev M, Simon DI, et al. Regulation of integrin function by the urokinase receptor. Science. 1996;273:1551–5. doi: 10.1126/science.273.5281.1551. [DOI] [PubMed] [Google Scholar]
- [63].Degryse B, Resnati M, Rabbani SA, Villa A, Fazioli F, Blasi F. Src-dependence and pertussis-toxin sensitivity of urokinase receptor-dependent chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. Blood. 1999;94:649–62. [PubMed] [Google Scholar]
- [64].Jo M, Thomas KS, Takimoto S, et al. Urokinase receptor primes cells to proliferate in response to epidermal growth factor. Onco-gene. 2007;26:2585–94. doi: 10.1038/sj.onc.1210066. [DOI] [PubMed] [Google Scholar]
- [65].Monaghan-Benson E, McKeown-Longo PJ. Urokinase-type plasminogen activator receptor regulates a novel pathway of fibronectin matrix assembly requiring Src-dependent transactivation of epidermal growth factor receptor. J Biol Chem. 2006;281:9450–9. doi: 10.1074/jbc.M501901200. [DOI] [PubMed] [Google Scholar]
- [66].Aguirre-Ghiso JA. Inhibition of FAK signaling activated by urokinase receptor induces dormancy in human carcinoma cells in vivo. Oncogene. 2002;21:2513–24. doi: 10.1038/sj.onc.1205342. [DOI] [PubMed] [Google Scholar]
- [67].Gondi CS, Kandhukuri N, Dinh DH, Gujrati M, Rao JS. Down-regulation of uPAR and uPA activates caspase-mediated apoptosis and inhibits the PI3K/AKT pathway. Int J Oncol. 2007;31:19–27. [PMC free article] [PubMed] [Google Scholar]
- [68].D'Alessio S, Margheri F, Pucci M, et al. Antisense oligodeoxynucleotides for urokinase-plasminogen activator receptor have anti-invasive and anti-proliferative effects in vitro and inhibit spontaneous metastases of human melanoma in mice. Int J Cancer. 2004;110:125–33. doi: 10.1002/ijc.20077. [DOI] [PubMed] [Google Scholar]
- [69].KnÖr S, Sato S, Huber T, et al. Development and evaluation of peptidic ligands targeting tumour-associated urokinase plasminogen activator receptor (uPAR) for use in -emitter therapy for disseminated ovarian cancer. Eur J Nucl Med Mol Imaging. 2008;35:53–64. doi: 10.1007/s00259-007-0582-3. [DOI] [PubMed] [Google Scholar]
- [70].Mazar AP, Buko A, Petros AM, Barnathan ES, Henkin J. Domain analysis of urokinase plasminogen activator (uPA): Preparation and characterization of intact A-chain molecules. Fibrinolysis. 2006;6(suppl.1):49–55. [Google Scholar]
- [71].E, Kim HK, Hong SH, Kim CK, Hong YK, Joe YA. Integrin alphavbeta3 is not significantly implicated in the anti-migratory effect of anti-angiogenic urokinase kringle domain. Oncol Rep. 2008;20:631–6. [PubMed] [Google Scholar]
- [72].Rajagopal V, Kreitman RJ. Recombinant toxins that bind to the urokinase receptor are cytotoxic without requiring binding to the alpha(2)-macroglobulin receptor. J Biol Chem. 2000 Mar 17;275(11):7566–73. doi: 10.1074/jbc.275.11.7566. [DOI] [PubMed] [Google Scholar]
- [73].Hall WA, Vallera DA. Efficacy of antiangiogenic targeted toxins against glioblastoma multiforme. Neurosurg Focus. 2006;20:E23. doi: 10.3171/foc.2006.20.4.15. [DOI] [PubMed] [Google Scholar]
- [74].Yang L, Peng XH, Wang YA, et al. Receptor-targeted nanoparticles for in vivo imaging of breast cancer. Clin Cancer Res. 2009;15:4722–32. doi: 10.1158/1078-0432.CCR-08-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Abdalla MO, Karna P, Sajja HK, Mao H, Yates C, Turner T, Aneja R. Enhanced noscapine delivery using uPAR-targeted optical-MR imaging trackable nanoparticles for prostate cancer therapy. J Control Release. 2010 doi: 10.1016/j.jconrel.2010.10.030. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Ahn RW, Chen F, Chen H, et al. A novel nanoparticulate formulation of arsenic trioxide with enhanced therapeutic efficacy in a murine model of breast cancer. Clin Cancer Res. 2010;16:3607–17. doi: 10.1158/1078-0432.CCR-10-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen H, Ahn R, Van den Bossche J, Thompson DH, O'Halloran TV. Folate-mediated intracellular drug delivery increases the anticancer efficacy of nanoparticulate formulation of arsenic trioxide. Mol Cancer Ther. 2009;8:1955–63. doi: 10.1158/1535-7163.MCT-09-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Chen H, Pazicni S, Krett NL, Ahn RW, Penner-Hahn JE, Rosen ST, O'Halloran TV. Angew Chem Int Ed. 2009;121:9295–9. doi: 10.1002/anie.200903655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Simon DI, Wei Y, Zhang L, et al. Identification of a urokinase receptor-integrin interaction site. Promiscuous regulator of integrin function. J Biol Chem. 2000;275:10228–34. doi: 10.1074/jbc.275.14.10228. [DOI] [PubMed] [Google Scholar]
- [80].van der Pluijm G, Sijmons B, Vloedgraven H, et al. Urokinase-receptor/integrin complexes are functionally involved in adhesion and progression of human breast cancer in vivo. Am J Pathol. 2001;159:971–82. doi: 10.1016/S0002-9440(10)61773-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Rabbani SA, Gladu J. Urokinase receptor antibody can reduce tumor volume and detect the presence of occult tumor metastases in vivo. Cancer Res. 2002;62:2390–7. [PubMed] [Google Scholar]
- [82].Rabbani SA, Ateeq B, Arakelian A, et al. An anti-urokinase plasminogen activator receptor antibody (ATN-658) blocks prostate cancer invasion, migration, growth, and experimental skeletal metastasis in vitro and in vivo. Neoplasia. 2010;12:778–88. doi: 10.1593/neo.10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Bauer TW, Liu W, Fan F, et al. Targeting of urokinase plasminogen activator receptor in human pancreatic carcinoma cells inhibits c-Met- and insulin-like growth factor-I receptor-mediated migration and invasion and orthotopic tumor growth in mice. Cancer Res. 2005;65:7775–81. doi: 10.1158/0008-5472.CAN-05-0946. [DOI] [PubMed] [Google Scholar]
- [84].Van Buren G, Gray M, Dallas NA, et al. A monoclonal antibody targeting the human urokinase plasminogen activator receptor (uPAR) combined with bevacizumab inhibits the growth of colon cancer metastases in the liver: differential effects mediated by tumor burden. Cancer. 2009;115:3360–8. doi: 10.1002/cncr.24371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Kenny HA, Leonhardt P, Ladanyi A, et al. Targeting the urokinase plasminogen activator receptor inhibits ovarian cancer metastasis. Clin Cancer Res. 2011;17:459–71. doi: 10.1158/1078-0432.CCR-10-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]