Abstract
Previous studies show increased oxidative DNA and RNA damage and diminished 8-oxoguanine glycosylase (OGG1) mediated base excision repair in vulnerable brain regions of mild cognitive impairment and late-stage Alzheimer’s disease (LAD) subjects compared to normal control (NC) subjects. Recently, a preclinical stage of AD (PCAD) has been described in which subjects show no overt clinical manifestations of AD but demonstrate significant AD pathology at autopsy. To determine if DNA or RNA oxidation are significantly elevated in PCAD brain we quantified 8-OHG in sections of hippocampus/parahippocamapal gyri in PCAD and NC subjects using immunohistochemistry and confocal microscopy and in superior and middle temporal gyri (SMTG) using gas chromatography/mass spectrometry. To determine if increased DNA oxidation is associated with altered repair capacity, levels of OGG1 protein in HPG were measured by immunohistochemistry and levels of OGG1 mRNA were measured in SMTG using quantitative PCR. Results show significantly increased (p < 0.05) 8-OHG immunostaining in DNA and RNA of PCAD HPG and significantly increased 8-OHG in PCAD SMTG. Quantification of OGG1 showed significantly elevated mRNA in PCAD SMTG and a trend toward elevated immunostaining in PCAD HPG. Overall, the data suggest oxidative damage to nucleic acids and a compensatory increase in OGG1 expression occur early in the pathogenesis of AD.
Keywords: preclinical AD, DNA oxidation, RNA oxidation, base excision repair, oxidative stress
Introduction
Increasing evidence supports a role for oxidative damage in the pathogenesis of Alzheimer’s disease (AD). Multiple studies demonstrate increased lipid peroxidation (Markesbery et al., 1998; Lovell et al., 2001; McGarth, 2001; Reich, 2001; Markesbery et al., 2005; Williams et al., 2006), protein oxidation (reviewed in (Sultana et al., 2006; Sultana et al., 2009) and DNA (reviewed in (Lovell et al., 2007) and RNA oxidation (Nunomura et al., 2001; Shan et al., 2003; Ding et al., 2006; Shan et al., 2006) in vulnerable regions of brain in late-stage AD (LAD). In addition, more recent studies show increased levels of markers of oxidative damage in the brain and cerebrospinal fluid (CSF) of subjects with mild cognitive impairment (MCI), the earliest clinical manifestation of AD. Increased levels of lipid peroxidation markers including the α,β-unsaturated aldehydes acrolein, 4-hydroxynonenal (HNE) and 4-hydroxyhexenal (HHE) have been reported in vulnerable regions of MCI brain (Williams et al., 2006; Sultana and Butterfield, 2009; Bradley et al.). Other studies of levels of neuroprostanes and isoprostanes, non-enzymatic by products of lipid peroxidation demonstrate increased levels in CSF of MCI subjects (Markesbery et al., 2005). Global levels of protein oxidation as well as multiple specific oxidatively modified proteins are elevated in MCI brain (reviewed in (Sultana and Butterfield, 2009) and markers of DNA and RNA oxidation are also significantly elevated in MCI brain (Ding et al., 2005; Ding et al., 2006; Wang et al., 2006; Lovell et al., 2008; Shao et al., 2008). Although increased levels of oxidative stress present in MCI and LAD brain are thought to contribute to the accumulation of DNA oxidative damage, it is possible that decreased DNA repair capacity may also be important. Studies of the oxoguanine glycosylase 1 (OGG1), a critical member of the predominant DNA repair pathway in brain show significantly decreased OGG1 activity in LAD and MCI brain (Lovell et al., 2000; Weissman et al., 2007; Shao et al., 2008). Our studies of OGG1 in MCI brain showed no significant differences in OGG1 protein but a significant decrease in enzyme activity that was associated with increased post translational modification of the protein by 4-HNE (Shao et al., 2008).
Although considerable evidence suggests DNA and RNA oxidation is present in MCI and LAD brain as well as Parkinson’s disease (Zhang et al., 1999), and dementia with Lewy bodies (Nunomura et al., 2002) there have been no reported studies of DNA or RNA oxidation in subjects with preclinical AD (PCAD). Subjects with PCAD are characterized as those who demonstrate normal antemortem neuropsychological test scores but who exhibit significant AD pathology at autopsy. Recent studies of PCAD brain show by-products of lipid peroxidation are significantly elevated in PCAD HPG but not in superior and middle temporal gyrus (Bradley, 2010; Bradley et al.; Bradley et al.). In contrast, measures of protein oxidation did not show significant alterations in PCAD brain (Aluise et al.; Bradley, 2010; Bradley et al.; Bradley et al.). Because patterns of oxidative damage to lipids and proteins in PCAD brain were somewhat different compared to MCI and LAD brain the current study was carried out to determine if DNA and RNA oxidation occurs in vulnerable brain regions in PCAD using immunohistochemistry and confocal microscopy to quantify levels of 8-hydroxyguanine (8-OHG) in sections of hippocampus/ parahippocampal gyri (HPG) and gas chromatorgraphy/mass spectrometry (GC/MS) with stable labeled internal standards to quantify levels of 8-OHG in nuclear DNA from superior and middle temporal gyrus (SMTG). Levels of 8-OHG in RNA were quantified using sections pretreated with RNase free DNase whereas DNA associated 8-OHG was quantified in sections pretreated with DNase free RNase. To determine if decreased levels of OGG1 protein were associated with increased 8-OHG immunostaining, we also subjected sections of HPG to double labeling for total 8-OHG and OGG1. To verify our immunohistochemical data for OGG1 we also quantified OGG1 mRNA and protein levels in PCAD and NC SMTG using quantitative PCR (qPCR) and Western blot analysis.
1.Materials and Methods
2.1 Subject selection and neuropathologic examination
Sections (10 μm) of paraffin embedded HPG were obtained from short postmortem interval (PMI) autopsies of 10 subjects with PCAD (2 men, 8 women) and 8 age-matched normal control (NC) subjects (1 man, 7 women) through the Neuropathology Core of the University of Kentucky Alzheimer’s Disease Center (UK-ADC). All subjects were followed longitudinally in the UK-ADC where they underwent neuropsychological testing annually and physical examinations biannually. Both NC and PCAD subjects had antemortem Mini Mental Status Examination (MMSE) scores in the normal range (NC = 28.1 ± 0.5; PCAD = 28.5 ± 0.4) and showed no evidence of memory decline. These studies were carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans and were approved by the University of Kentucky Institutional Review Board.
Histopathologic examination of multiple sections of neocortex, hippocampus, entorhinal cortex, amygdala, basal ganglia, thalamus, nucleus basalis of Meynert, midbrain, pons, medulla, and cerebellum using hematoxylin and eosin and the modified Bielschowsky stains along with 10D-5 (for Aβ) and α-synuclein immunochemistry was carried out on all subjects. Braak staging scores were determined using the Gallyas stain on sections of entorhinal cortex, hippocampus, and amygdala, and the Bielschowsky stain on neocortex. Normal control subjects showed only age-associated changes and Braak staging scores of 0 to II and met the National Institute on Aging-Reagan Institute (NIA-RI Working Group, 1997) low likelihood criteria for the histopathologic diagnosis of AD. In contrast, subjects identified with PCAD had moderate or frequent neuritic plaque scores, Braak staging scores from III to V and met intermediate or high NIA-RI criteria for the histopathologic diagnosis of AD. Demographic data for all subjects in the study are shown in Table 1.
Table 1.
Subject demographic data.
| Mean ± SEM Age (years) | Sex | Mean ± SEM PMI (hours) | Median Braak Staging Score | Mean ± SEM MMSE Score | |
|---|---|---|---|---|---|
| NC | 83.9 ± 1.9 | 1M/7W | 2.4 ± 0.2 | I | 28.1 ± 0.5 |
| PCAD | 86.0 ± 2.1 | 2M/8W | 2.9 ± 0.2 | IV* | 28.5 ± 0.4 |
p < 0.05
To verify our immunohistochemical results we used gas chromatorgraphy mass spectrometry (GC/MS) with stable labeled internal standards to quantify 8-OHG in nuclear DNA and quantitative real time PCR (q-PCR) to quantify mRNA levels of OGG1 in the superior and middle temporal gyrus (SMTG) of a subset of the subjects analyzed by immunohistochemistry. The SMTG was chosen for these studies because of the limited availability of frozen HPG specimens and because SMTG shows considerable AD pathology.
2.2 Immunohistochemistry and antibodies
To visualize the cellular distribution of DNA and RNA oxidation, 10-μm sections of paraffin-embedded HPG from NC and PCAD subjects were cut using a Shandon Finesse microtome (Thermo Fisher Scientific, Waltham, MA), placed on Plus-slides, and rehydrated through xylene, descending alcohols, and water. Following rehydration, sections were digested with 10 μg/ml proteinase K (Boehringer Mannheim, Indianapolis, IN) in phosphate buffered saline (PBS, pH 7.4) for 40 min at 37ºC. Sections were then washed in Tris-buffered saline (TBS; 150 mM tris-HCl, 150 mM NaCl, pH 7.6) and pretreated with RNase free DNase-I (10 U/μl in PBS, Roche, Mannheim, Germany) or DNase-I free RNase (0.5 μg/μl PBS, Roche, Mannheim, Germany) for 2 h at 37ºC. Sections were then blocked for 2 hours in 1.5% normal goat serum in TBS at room temperature and incubated overnight in a 1:100 dilution of mouse anti-8-OHG (QED Biosciences, San Diego CA) in 1.5% goat serum/TBS at 4° C. Following thorough washing in TBS, sections were incubated in a 1:1000 dilution of Alexa-488 conjugated goat anti-mouse IgG (Molecular Probes, Eugene, OR) for 2 h at room temperature. After 5 rinses in TBS and distilled/deionized water, sections were coverslipped using fluorescent anti-fade (Molecular Probes) and imaged using a Leica DM IRBE confocal microscope equipped with argon, krypton and HeNe lasers and a 40X oil objective. Confocal images were captured from a single z plane without optical sectioning and were the average of 3 scans. Images were captured from 10 fields/section with an average of 10 to 20 cells per field without knowledge of subject diagnosis. All sections were analyzed on the same day with the same instrument settings to allow comparison of fluorescence intensities between sections. Sections were analyzed by subject number without knowledge of diagnoses. The first section analyzed was reimaged at the midpoint and end of the experiment to verify that instrumental parameters had not diminished. Fluorescence intensities of all cells in each field were quantified using Leica image analysis software. Mean fluorescence intensities were calculated for 8-OHG in DNA or RNA for each slide. Results are reported as mean ± SEM % control staining.
To verify 8-OHG immunostaining was uniquely associated with neurons and not astrocytes, representative sections of HPG were triple labeled for 8-OHG, neuron specific β-tubulin (β-tubulin-III; Tuj-1), and GFAP, an astrocyte specific marker. The sections were labeled for 8-OHG as described above, blocked 1 h in 1.5% normal goat serum/TBS followed by incubation overnight in a 1:100 dilution of monoclonal anti-tubulin-III (Tuj-1; Covance, Denver, PA ) and a 1:100 dilution of rabbit anti-GFAP (Dako, Capinteria, CA). The sections were washed three times in TBS and incubated in 1.5% normal goat serum/TBS containing Alexa-568 labeled goat anti-rabbit IgG (1:1000) and Alexa-633 labeled goat anti-mouse IgG (1:1000) for 1 h. Following three washes in TBS the sections were coverslipped using fluorescent anti-fade and imaged as described above using a 100X objective.
Oxoguanine glycosylase-1 immunostaining was carried out by subjecting rehydrated sections to antigen retrieval using 1 mM EDTA (pH 8.0) and an IHC Antigen Retireval system (Thermo Fisher, Fremont, CA). Following antigen retrieval, sections were blocked in 1.5% goat serum/TBS for 2 hours and total 8-OHG (DNA + RNA) immunolabeled by incubation overnight in a 1:100 dilution of anti-8-OHG. Treatment with proteinase K and DNase or RNase was omitted because if led to diminished OGG1 immunostaining. After thorough washing in TBS sections were incubated in a 1:1000 dilution of Alexa-488 labeled goat anti-mouse IgG as described above. The sections were then double labeled using a mouse antibody against OGG-1 by incubation overnight at 4°C in a 1:100 dilution of anti-OGG1 (Abcam; Cambridge, MA). Sections were washed three times in TBS and then incubated in a 1:1000 dilution of Alexa-565 labeled goat anti-mouse IgG for 2 h at room temperature. The sections were rinsed 5 times with TBS, twice with distilled/deionized water and coverslipped and imaged as described above.
2.3 GC/MS quantification of 8-OHG in DNA
Nuclear DNA was purified as described by Meccoci et al. (Mecocci, 1994) with minor modifications as described by Wang et al. (Wang, 2006). Briefly, nuclear isolates were resuspended in digestion buffer (DB) containing SDS (0.5%), Tris (0.05M), EDTA (0.1 M) with proteinase K (0.5 mg/mL) and incubated overnight at 55 °C for nDNA extraction. NaCl (160 μL/10 mL DB) was added and the solution extracted 3X with tris-buffered phenol contained 8-hydroxyquinoline (5.5 mM) and 3X with isoamyl alchohol/chloroform (4% (vol/vol)). nDNA was precipitated from the aqueous layer by addition of 800 μl NaCl (5M)/10ml DB and an equal volume of absolute ethanol overnight at −20°C. Pellets were washed 3X with 60% (vol/vol), air dried, and resuspended in autoclaved distilled/deionized water. The concentration and purity of DNA samples were determined by NanoDrop 1000 Spectrophotometey (NanoDrop, Wilmington, DE, USA). Samples were stored at −80 °C until analysis.
For GC/MS analyses nDNA (10 μg) samples were prepared placed in 5 ml conical glass tubes along with 10 nmol stable labeled 8-OHG (8-[8-13C,7,9-15N2] hydroxyguanine). The samples were lyophilized and the tubes evacuated. Samples were hydrolyzed in 250 μl 90% formic acid at 145 C for 30 min. The samples were re-lyophilized and derivatized with using 1:1 pyridine/BSTFA (200 μL) in evacuated sealed tubes for 2 hr at room temperature. Samples were dried under a stream of nitrogen in a water bath at 37 °C and resuspended in BSTFA (20 μL) before GC/MS analysis.
Derivitized samples were analyzed using an Agilent 7890A gas chromatograph on a HP- 5ms capillary column (0.25 mm internal diameter, 0.25 μm film thickness, and 30-m length; Hewlett Packard, Palo Alto, CA, USA). Chromatographic parameters were as follows: an initial temperature was held for 2 min at 100 C after sample injection with the following ramps: ramp 1, 100-178 C at 3 C/min; ramp 2 178-181 C at 0.3 C/min; ramp 3 81-208 at 3 C/min; and ramp 4 208-280 at 10 C/min. The final temperature was maintained for 2 min. The injector temperature was maintained at 250 C and the source temperature at 180 C. For analysis, 1 μL of derivatized samples was introduced using a pulsed splitless mode with helium carrier gas (99.999%) at an inlet pressure of 11.8 psi under a constant flow. Total analysis time was 56.2 min. Derivatized nitrogenous base spectra were acquired in selective ion monitoring mode at the at m/z ratios of 443 for stable labeled 8-OHG and 440 for 8-hydroxyguanine from subject DNA. Instrument response plots of integrated peak of stable labeled analyte signal added were determined over a range of 0.5 nmol to 100 nmol per stable labeled isotope analyte. The integrated area of each analyte signal was normalized with respect to the integrated area of the corresponding internal standards for all samples and corrected based on instrument response plots for a given internal standard.
2.5 mRNA isolation and q-PCR
mRNA was isolated using Qiagen RNeasy Mini kits per manufacturer’s instructions and was converted to cDNA using an RT2 First Strand Kit (SABiosciences, Frederick MD) according to manufacturer’s instructions. Briefly, around 100 ng of RNA was incubated with genomic DNA elimination buffer at 42 °C for 5 minutes in order to eliminate genomic DNA contamination. The reaction mixture was then mixed with a reverse transcription cocktail provided in the kit and incubated at 42 °C for exactly 15 minutes. The reverse transcription reaction was quenched by heating the mixture at 95 °C for 5 minutes. The sample was diluted with DNAse/RNAse free H2O and stored at 20 °C overnight. Real time quantitative PCR (qPCR) was carried out with an aliquot of the reaction mixture on an ABI 7000 Sequence Detection System using TaqMan 2x PCR Master Mix (Applied Biosystems, Carlsbad, CA) and SYBR Green for detection. The thermal cycler was run according to the real-time thermal cycler program recommended by the manufacturer (Applied Biosystems, Carlsbad, CA). The 25 uL qPCR reaction mixture contained 1× TaqMan PCR Master Mix, 5 μM primer mix (forward and reverse), SYBR Green, ROX reference dye and 250 ng cDNA. The absolute concentrations of intact DNA in the template were calculated using a standard curve derived from 5-fold serial dilutions of genomic DNA with known concentration.
2.6 Statistical analysis
Age, PMI and MMSE scores were compared using analysis of variance (ANOVA) and ABSTAT software (AndersonBell, Arvada, CO). Results of 8-OHG and OOG1 immunostaining are reported as mean ± SEM% control immunostaining and were compared using ANOVA. Braak staging scores were compared using non-parametric testing and the Mann-Whitney U-test and results are expressed as the median. Results of 8-OHG in nuclear DNA and q-PCR data were compared using the Mann-Whitney U-test and are expressed as median [range] % of control to allow comparison to immunohistochemical data.
2. RESULTS
Subject demographic data are shown in Table 1 and demonstrate that age, PMI or MMSE scores were not significantly different between NC and PCAD subjects. Braak staging scores were significantly higher in PCAD subjects (median = IV) compared to NC subjects (median = I). Comparison of Braak staging scores of PCAD and MCI subjects in previous studies show no significant difference between the two subject groups.
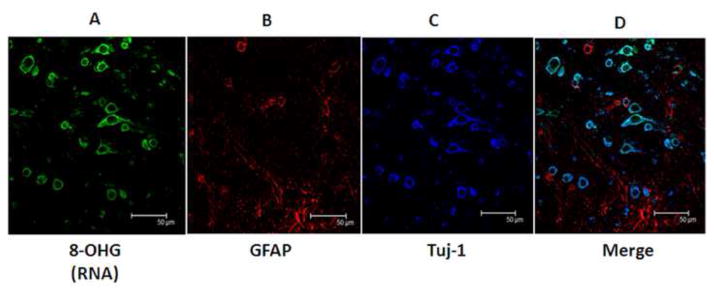
3.1 8-OHG is localized in Tuj-1 positive neurons
Figure 1 shows representative confocal micrographs of a section of PCAD HPG triple labeled for 8-OHG (1A; green), GFAP (1B; red) as a marker of glial cells and Tuj-1 (1C; blue) as a neuron-specific marker. The final image (1D) is a merged image. Immunostained neurons were typically flame-shaped, globular and hemispiked. The figure demonstrates that 8-OHG immunostaining was uniquely associated with Tuj-1 positive neurons and minimally associated with GFAP-positive astrocytes. Previous studies from our laboratory and others (Lovell and Markesbery, 2008) show pretreatment of sections with DNase or RNase effectively leads to immunostaining of RNA and DNA separately. In addition, we (Lovell and Markesbery, 2008) showed preincubation of the 8-OHG antibody with 8-OHG significantly diminished immunostaining suggesting the antibody is specific for 8-OHG.
Figure 1.
Representative confocal micrographs showing RNA associated 8-OHG staining (A) is associated with Tuj-1 positive neurons (C) but not GFAP-positive astrocytes (B). The last panel shows a merged image.
3.2 8-OHG is increased in DNA and RNA in PCAD
Figure 2 shows representative confocal micrographs of NC (Fig. 2A) and PCAD (Fig. 2B) HPG immunostained for total 8-OHG, RNA-associated 8-OHG and DNA-associated 8-OHG. Immunostaining of neurons for RNA associated 8-OHG showed pronounced cytoplasmic staining with minimal nuclear staining. 8-OHG immunostaining associated with DNA showed diffuse cytoplasmic staining in addition to nuclear staining, suggesting elevations of mitochondrial DNA labeling. Previous studies have shown pretreatment of sections with DNase free RNase significantly diminishes 8-OHG staining of RNA suggesting the diffuse cytoplasmic staining that accompanies nuclear staining may be associated with mitochondrial DNA. Comparison of levels of immunofluorescence showed a significant (p < 0.05) elevation of RNA-associated 8-OHG in PCAD subjects (170.9 ± 16.9% control) compared to NC subjects (100 ± 15.7% control) (Fig. 3). Similarly, levels of DNA associated 8-OHG were significantly (p < 0.05) elevated in PCAD brain (136.8 ± 12.2% control) compared to NC subjects (100 ± 2.9% control). To determine if oxidative damage to DNA is more widespread in PCAD brain and to verify our immunohistochemical results we used GC/MS with stable labeled internal standards to quantify levels of 8-OHG in nuclear DNA purified from SMTG of a subset of 5 NC and 4 PCAD subjects analyzed by immunohistochemistry. Specimens of SMTG were chosen for analysis because of the limited availability of frozen specimens of HPG and because SMTG demonstrates significant AD pathology. Comparison of levels of 8-OHG in PCAD nDNA showed a significant increase in PCAD brain (median = 1039.4% control; range = 123.4–2931.1% control) compared to NC subjects (median = 102.9; range = 50.9–171.7% control) (Table 2).
Figure 2.
Representative confocal micrographs showing total, RNA-associated, and DNA associated 8-OHG in NC (A) and PCAD (B) hippocampus/parahippocampal gyri. Note elevated immunofluorescence associated with DNA and RNA associated 8-OHG.
Figure 3.
Quantification of 8-OHG and OGG1 immunostaining. 8-OHG was significantly (p < 0.05) elevated in RNA and DNA in PCAD subjects compared to age-matched NC subjects. Levels of OGG1 were elevated in PCAD, although the difference was not statistically significant.
Table 2.
Levels of 8-hydroxyguanine in nuclear DNA were significantly elevated in SMTG from PCAD subjects compared to age matched NC subjects. Median levels of OGG1 mRNA were significantly elevated in PCAD SMTG.
| Median [Range] % Control 8-OHG in SMTG | Median [Range] % Control OGG1 mRNA in SMTG | |
|---|---|---|
| NC | 102.9 [50.9–171.7] | 120.7 [43.1–126.4] |
| PCAD | 1039.4 [123.4–2931.1]* | 145.1 [127.9–367.8]* |
p < 0.05
3.3 OGG1in PCAD HPG and SMTG
Double labeling of a subset of sections (5 PCAD and 5 NC subjects) for total 8-OHG and OGG1 showed an increase in OGG1 immunofluorescence in PCAD neurons (Fig. 3 and 4A; 130.3 ± 18.2% control) compared to NC subjects (Fig. 3 and 4B; 100 ± 18.2% control), although the difference was not statistically significant. Correlation analyses showed a positive but non-significant relationship between OGG1 and 8-OHG levels. Quantification of levels of OGG1 mRNA from frozen specimens of SMTG showed a significant increase in PCAD subjects (median = 145.1% control; range = 127.9–367.8% control) compared to NC subjects (median = 120.7% control; range = 43.1–126.4% control) (Table 2).
Figure 4.

Representative confocal micrographs of sections of NC (A) and PCAD (B) hippocampus/parahippocampal gyri immunostained for OGG-1. Note elevated OGG1 immunostaining in PCAD.
4. DISCUSSION
Considerable evidence suggests an accumulation of oxidative damage to lipids, proteins and nucleic acids in the progression of AD, although it has remained unclear whether the damage is a primary or secondary event in the pathogenesis of neurodegeneration. With the characterization of subjects with PCAD, the potential exists to investigate the relationship between markers of oxidative damage and the neuropathologic lesions of AD prior to the onset of clinical symptoms.
Previous studies of by-products of lipid peroxidation in PCAD brain show significant elevations of 4-HNE and acrolein in the HPG of PCAD subjects but not in other less vulnerable areas including the superior and middle temporal gyri and inferior parietal lobule (Aluise et al.; Bradley, 2010; Bradley et al.). In contrast, levels of protein oxidation were not significantly altered in any brain region studied. These results differ from studies of MCI which showed significantly increased lipid peroxidation in SMTG and increased protein oxidation in IPL compared to NC subjects. These differences suggest that despite similar levels of pathology PCAD subjects may be following a different oxidative stress cascade. Based on the subtle differences in oxidative stress profiles between MCI and PCAD subjects we carried out the current study to determine if there are significant alterations in DNA and RNA oxidation in PCAD brain. Our data show a significant increase in levels of 8-OHG, the predominant marker of oxidative modification of nucleic acids in both RNA and DNA in the HPG of PCAD subjects compared to NC subjects and a significant increase in 8-OHG in isolated nuclear DNA from the SMTG of PCAD subjects. Immunolabeling of DNA associated 8-OHG in RNase treated sections showed diffuse cytoplasmic staining in addition to the expected nuclear staining suggesting damage to mitochondrial DNA is occurring in PCAD brain. Results of the triple labeling experiments show 8-OHG immunostaining is predominately associated with Tuj-1 positive neurons and not GFAP-positive astrocytes. It remains unclear how PCAD subjects with significant AD pathology and evidence of pronounced oxidative damage remain cognitively normal. It’s possible that subjects in the progression of AD have critical thresholds of pathology and oxidative damage that define when clinical evidence of cognitive decline becomes evident and PCAD subjects have an unusually high threshold.
Studies of oxidative damage to DNA show significant elevations of 8-OHG, 8-hydroxyadenine, 5-hydroxycytosine in neocortical regions of both MCI (Wang et al., 2006) and LAD brain (reviewed in (Lovell and Markesbery, 2007). Although several studies clearly show an accumulation of oxidative damage in DNA during the progression of AD, several questions remain unanswered. For example, it is unclear if accumulated DNA oxidation is due to increased levels of oxidative stress, diminished capacity for repair of oxidative modifications by base excision or nucleotide excision repair or a combination of the two. Our data show a trend toward elevated levels of OGG1 the major base excision repair enzyme responsible for repair of oxidatively modified guanine, although the elevations were not statistically significant and a significant elevation of OGG1 mRNA in PCAD SMTG. Previous studies of OGG1 protein levels and base excision repair capacities in MCI and LAD brain show significantly decreased BER activity in MCI and LAD brain (Lovell et al., 2000; Mao et al., 2007; Weissman et al., 2007; Shao et al., 2008). In contrast to our current data, our Western blot studies of OGG1 protein levels in MCI showed no significant changes in protein level despite significant decreases in enzyme activities (Shao et al., 2008). Further immunoprecipitation studies of OGG1 from MCI brain showed that it was post-translationally modified by 4-HNE and that 4-HNE effectively inactivated OGG-1 activity in vivo. In the current study we did not measure OGG-1 mediated BER activities but it is possible that the observed elevation of OGG1 immunostaining and mRNA represents a compensatory increase in protein expression to mitigate loss of activity due to post translation modifications. Whether the oxidative damage observed in PCAD is widespread or localized to specific segments of DNA, particularly those that code for proteins altered in disease progression, is unknown. It is possible that increased oxidative modification of DNA at binding sites for transcription factors may lead to increased or decreased binding and altered action. Although the current study was not designed to address these issues, it does demonstrate that oxidative damage of DNA is significantly elevated in neurons of the HPG from subjects with PCAD suggesting oxidative damage to DNA is an early event in the progression of AD and that these alterations precede the onset of clinical symptoms.
In general RNA oxidation in the progression of AD has been studied less than DNA oxidation. Previous immunohistochemical studies demonstrate significantly increased neuronal RNA oxidation in LAD (Nunomura et al., 1999; Nunomura et al., 2004), dementia with Lewy bodies (Nunomura et al., 2002), Parkinson’s disease (Zhang et al., 1999) and MCI (Lovell and Markesbery, 2008). Although these studies demonstrate elevated RNA oxidative modification, they are measures of total RNA and do not provide delineation of oxidative damage to specific RNAs. Shan et al. (Shan et al., 2003) described several specific oxidized mRNA species in LAD brain that when expressed in cell lines led to abnormal translation of protein. Subsequent studies by Shan and Lin (Shan and Lin, 2006) and by Ding et al. (Ding et al., 2006) showed a significant fraction of mRNAs in LAD frontal cortex were oxidatively modified. Although the dominant marker of RNA oxidation is 8-OHG, other possible modifications include introduction of strand breaks (Rinke et al., 1978; Jezowska-Bojczuk et al., 2002; Martinet et al., 2004; Singh et al., 2004), RNA crosslinking and additional base modifications including formation of thymine glycol and hydroxymethyl uracil (Jezowska-Bojczuk et. al, 2002 ). Although our current data are consistent with these earlier studies, it remains unclear whether RNA oxidation occurs in nucleus or cytoplasm.
The location of RNA oxidative damage, reasons for accumulation of oxidative damage to RNA and the functional effect of accumulated damage in PCAD brain remain unclear. RNA is normally thought to undergo rapid turnover that would limit accumulation of oxidative damage. Degradation of RNA, particularly mRNA, is essential for regulation of gene expression and is controlled by endogenous or exogenous signals by a gradual shortening of the poly (A) tail (Beelman et al., 1995; van Hoof et al., 1999; Tucker et al., 2000; Mitchell et al., 2001). Following poly A shortening, recent data suggest mRNA degradation occurs through action of a complex of endosomal proteins (van Hoof et al., 2002). Although genes for components of the endosomal complex have been identified in yeast and are highly conserved among eukaryotes, it is unclear if they are involved in the degradation of mRNA in humans or if they are altered in AD. As is the case for DNA, it is unclear if RNA oxidation in PCAD is widespread or is specific to select mRNAs.
3. CONCLUSIONS
Overall, the current study demonstrates significant elevations of 8-OHG in both RNA and DNA in the HPG and in nuclear DNA from SMTG in subjects with PCAD compared to NC subjects. The data also demonstrate that levels of OGG1 mRNA and protein are increased perhaps as a compensatory response to increased DNA oxidative damage. Studies are currently underway to determine if increased OGG1 mRNA and protein corresponds to increased enzyme activity. The presence of markers of DNA and RNA oxidation in brain regions vulnerable to AD pathology prior to the onset of clinical symptoms suggest a role for oxidative damage to nucleic acids in the pathogenesis of neurodegeneration in AD.
Highlights.
RNA associated 8-OHG is increased in PCAD hippocampus/parahippocampal gyri (HPG) compared to NC HPG
DNA associated 8-OHG is increased in PCAD HPG compared to NC HPG
Oxoguanine gylcosylase 1 is increased in PCAD although the difference was not statistically significant.
Acknowledgments
This research was supported by NIH grants 5P01-AG05119 and P30-AG028383. The authors thank the UK-ADC Clinical, Neuropathology and Biostatistics Cores for tissue procurement, neuropathologic and neuropsychological data. The authors also thank Ms. Sonya Anderson for subject demographic data and Ms. Paula Thomason for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aluise CD, Robinson RA, Beckett TL, Murphy MP, Cai J, Pierce WM, Markesbery WR, Butterfield DA. Preclinical Alzheimer disease: brain oxidative stress, Abeta peptide and proteomics. Neurobiol Dis. 2010;39:221–228. doi: 10.1016/j.nbd.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beelman CA, Parker R. Degradation of mRNA in eukaryotes. Cell. 1995;81:179–183. doi: 10.1016/0092-8674(95)90326-7. [DOI] [PubMed] [Google Scholar]
- Bradley MA, Markesbery WR, Lovell MA. Increased levels of 4-hydroxynonenal and acrolein in the brain in preclinical Alzheimer disease. Free Radic Biol Med. 2010a;48:1570–1576. doi: 10.1016/j.freeradbiomed.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MA, Xiong-Fister S, Markesbery WR, Lovell MA. Elevated 4-hydroxyhexenal in Alzheimer's disease (AD) progression. Neurobiol Aging. 2010b doi: 10.1016/j.neurobiolaging.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MA, Markesbery WR, Lovell MA. Increase levels of 4-hydroxynonenal and acrolein in the brain in preclinical Alzheimer's disease (PCAD) Free Radic Biol Med. 2010;48:1570–1576. doi: 10.1016/j.freeradbiomed.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, Markesbery WR, Cecarini V, Keller JN. Decreased RNA, and increased RNA oxidation, in ribosomes from early Alzheimer's disease. Neurochem Res. 2006;31:705–710. doi: 10.1007/s11064-006-9071-5. [DOI] [PubMed] [Google Scholar]
- Ding Q, Markesbery WR, Chen Q, Li F, Keller JN. Ribosome dysfunction is an early event in Alzheimer's disease. J Neurosci. 2005;25:9171–9175. doi: 10.1523/JNEUROSCI.3040-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezowska-Bojczuk M, Szczepanik W, Lesniak W, Ciesiolka J, Wrzesinski J, Bal W. DNA and RNA damage by Cu(II)-amikacin complex. Eur J Biochem. 2002;269:5547–5556. doi: 10.1046/j.1432-1033.2002.03260.x. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Markesbery WR. Oxidative DNA damage in mild cognitive impairment and late-stage Alzheimer's disease. Nucleic Acids Res. 2007;35:7497–7504. doi: 10.1093/nar/gkm821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell MA, Markesbery WR. Oxidatively modified RNA in mild cognitive impairment. Neurobiol Dis. 2008;29:169–175. doi: 10.1016/j.nbd.2007.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell MA, Xie C, Makresbery WR. Decreased base excision repair and increased helicase activity in Alzheimer's disease brain. Brain Research. 2000;855:166–173. doi: 10.1016/s0006-8993(99)02335-5. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Xie C, Markesbery WR. Acrolein is increased in Alzheimer's disease brain and is toxic to primary hippocampal cultures. Neurobiol Aging. 2001;22:187–194. doi: 10.1016/s0197-4580(00)00235-9. [DOI] [PubMed] [Google Scholar]
- Mao G, Pan X, Zhu BB, Zhang Y, Yuan F, Huang J, Lovell MA, Lee MP, Markesbery WR, Li GM, Gu L. Identification and characterization of OGG1 mutations in patients with Alzheimer's disease. Nucleic Acids Res. 2007;35:2759–2766. doi: 10.1093/nar/gkm189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markesbery WR, Lovell MA. Four-hydroxynonenal, a product of lipid peroxidation, is increased in the brain in Alzheimer's disease. Neurobiol Aging. 1998;19:33–36. doi: 10.1016/s0197-4580(98)00009-8. [DOI] [PubMed] [Google Scholar]
- Markesbery WR, Kryscio RJ, Lovell MA, Morrow JD. Lipid peroxidation is an early event in the brain in amnestic mild cognitive impairment. Ann Neurol. 2005;58:730–735. doi: 10.1002/ana.20629. [DOI] [PubMed] [Google Scholar]
- Martinet W, de Meyer GR, Herman AG, Kockx MM. Reactive oxygen species induce RNA damage in human atherosclerosis. Eur J Clin Invest. 2004;34:323–327. doi: 10.1111/j.1365-2362.2004.01343.x. [DOI] [PubMed] [Google Scholar]
- McGarth LT, McGleenon BM, Brennan S, McColl D, McIlroy S, Passmore AP. Increased oxidative stress in Alzheimer's disease as assessed with 4-hydroxynonenal but not malondialdehyde. Q J Med. 2001;94:385–490. doi: 10.1093/qjmed/94.9.485. [DOI] [PubMed] [Google Scholar]
- Mecocci P, MacGarvey U, Beal MF. Oxidative damage to mitochondrial DNA is increased in Alzheimer's disease. American Neurological Association. 1994;36:747–751. doi: 10.1002/ana.410360510. [DOI] [PubMed] [Google Scholar]
- Mitchell P, Tollervey D. mRNA turnover. Curr Opin Cell Biol. 2001;13:320–325. doi: 10.1016/s0955-0674(00)00214-3. [DOI] [PubMed] [Google Scholar]
- Nunomura A, Perry G, Hirai K, Aliev G, Takeda A, Chiba S, Smith MA. Neuronal RNA oxidation in Alzheimer's disease and Down's syndrome. Ann N Y Acad Sci. 1999;893:362–364. doi: 10.1111/j.1749-6632.1999.tb07855.x. [DOI] [PubMed] [Google Scholar]
- Nunomura A, Chiba S, Kosaka K, Takeda A, Castellani RJ, Smith MA, Perry G. Neuronal RNA oxidation is a prominent feature of dementia with Lewy bodies. Neuroreport. 2002;13:2035–2039. doi: 10.1097/00001756-200211150-00009. [DOI] [PubMed] [Google Scholar]
- Nunomura A, Chiba S, Lippa CF, Cras P, Kalaria RN, Takeda A, Honda K, Smith MA, Perry G. Neuronal RNA oxidation is a prominent feature of familial Alzheimer's disease. Neurobiol Dis. 2004;17:108–113. doi: 10.1016/j.nbd.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Smith MA. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- Reich EE, Markesbery WR, Roberts LJ, III, Swifte LL, Morrow JD, Montine TJ. Brain Regional Quantification of F-Ring and D-/E-Ring Isoprostanes and Neuroprostanes in Alzheimer's disease. Am J Pathol. 2001;158:293–297. doi: 10.1016/S0002-9440(10)63968-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinke J, Brimacombe R. 30S ribosomal proteins cross-linked to 16S RNA by periodate oxidation followed by borohydride reduction. Mol Biol Rep. 1978;4:153–156. doi: 10.1007/BF00777516. [DOI] [PubMed] [Google Scholar]
- Shan X, Lin CL. Quantification of oxidized RNAs in Alzheimer's disease. Neurobiol Aging. 2006;27:657–662. doi: 10.1016/j.neurobiolaging.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Shan X, Tashiro H, Lin CL. The identification and characterization of oxidized RNAs in Alzheimer's disease. J Neurosci. 2003;23:4913–4921. doi: 10.1523/JNEUROSCI.23-12-04913.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao C, Xiong S, Li GM, Gu L, Mao G, Markesbery WR, Lovell MA. Altered 8-oxoguanine glycosylase in mild cognitive impairment and late-stage Alzheimer's disease brain. Free Radic Biol Med. 2008;45:813–819. doi: 10.1016/j.freeradbiomed.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Dwarakanath BS, Mathew TL. DNA ligand Hoechst-33342 enhances UV induced cytotoxicity in human glioma cell lines. J Photochem Photobiol B. 2004;77:45–54. doi: 10.1016/j.jphotobiol.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Sultana R, Butterfield DA. Role of Oxidative Stress in the Progression of Alzheimer's Disease. J Alzheimers Dis. 2009 doi: 10.3233/JAD-2010-1222. [DOI] [PubMed] [Google Scholar]
- Sultana R, Perluigi M, Butterfield DA. Protein oxidation and lipid peroxidation in brain of subjects with Alzheimer's disease: insights into mechanism of neurodegeneration from redox proteomics. Antioxid Redox Signal. 2006;8:2021–2037. doi: 10.1089/ars.2006.8.2021. [DOI] [PubMed] [Google Scholar]
- Tucker M, Parker R. Mechanisms and control of mRNA decapping in Saccharomyces cerevisiae. Annu Rev Biochem. 2000;69:571–595. doi: 10.1146/annurev.biochem.69.1.571. [DOI] [PubMed] [Google Scholar]
- van Hoof A, Parker R. The exosome: a proteasome for RNA? Cell. 1999;99:347–350. doi: 10.1016/s0092-8674(00)81520-2. [DOI] [PubMed] [Google Scholar]
- van Hoof A, Parker R. Messenger RNA degradation: beginning at the end. Curr Biol. 2002;12:R285–287. doi: 10.1016/s0960-9822(02)00802-3. [DOI] [PubMed] [Google Scholar]
- Wang J, Markesbery WR, Lovell MA. Increased oxidative damage in nuclear and mitochondrial DNA in mild cognitive impairment. J Neurochem. 2006;96:825–832. doi: 10.1111/j.1471-4159.2005.03615.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Markesbery WR, Lovell MA. Increase oxidative damage in nuclear and mitochondrial DNA in mild cognitive impairment. Journal of Neurochemistry. 2006;96:825–832. doi: 10.1111/j.1471-4159.2005.03615.x. [DOI] [PubMed] [Google Scholar]
- Weissman L, Jo DG, Sorensen MM, de Souza-Pinto NC, Markesbery WR, Mattson MP, Bohr VA. Defective DNA base excision repair in brain from individuals with Alzheimer's disease and amnestic mild cognitive impairment. Nucleic Acids Res. 2007;35:5545–5555. doi: 10.1093/nar/gkm605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TI, Lynn BC, Markesbery WR, Lovell MA. Increased levels of 4-hydroxynonenal and acrolein, neurotoxic markers of lipid peroxidation, in the brain in Mild Cognitive Impairment and early Alzheimer's disease. Neurobiol Aging. 2006;27:1094–1099. doi: 10.1016/j.neurobiolaging.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Zhang J, Perry G, Smith MA, Robertson D, Olson SJ, Graham DG, Montine TJ. Parkinson's disease is associated with oxidative damage to cytoplasmic DNA and RNA in substantia nigra neurons. Am J Pathol. 1999;154:1423–1429. doi: 10.1016/S0002-9440(10)65396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]