Abstract
Endothelial progenitor cells (EPCs) promote angiogenesis, and clinical trials have shown such cell therapy to be feasible for treating ischemic disease. However, clinical outcomes have been contradictory owing to the diverse range of EPC types used. We recently characterized two EPC subtypes, and identified outgrowth endothelial cells as the only EPC type with true progenitor and endothelial characteristics. By contrast, myeloid angiogenic cells (MACs) were shown to be monocytic cells without endothelial characteristics despite being widely described as “EPCs.” In the current study we demonstrated that although MACs do not become endothelial cells or directly incorporate into a microvascular network, they can significantly induce endothelial tube formation in vitro and vascular repair in vivo. MAC-derived interleukin-8 (IL-8) was identified as a key paracrine factor, and blockade of IL-8 but not vascular endothelial growth factor (VEGF) prevented MAC-induced angiogenesis. Extracellular IL-8 transactivates VEGFR2 and induces phosphorylation of extracellular signal-regulated kinases. Further transcriptomic and immunophenotypic analysis indicates that MACs represent alternative activated M2 macrophages. Our findings demonstrate an unequivocal role for MACs in angiogenesis, which is linked to paracrine release of cytokines such as IL-8. We also show, for the first time, the true identity of these cells as alternative M2 macrophages with proangiogenic, antiinflammatory and pro–tissue-repair properties.
INTRODUCTION
Endothelial progenitor cells (EPCs) have been shown to promote revascularization of ischemic tissues (1) and tumors (2). These cells, therefore, have the potential to be harnessed for cell-based therapies to induce vascular repair. Recent clinical trials have demonstrated that EPC delivery to ischemic tissue is feasible, safe and effective for the treatment of myocardial infarction (3) and peripheral arterial disease (4). EPCs have also been described as critical regulators of the angiogenic switch in metastatic progression (5), and there have been reported attempts to control tumor growth by targeting EPCs, either by using EPCs as vectors for gene delivery to tumors (6) or by blocking the recruitment of mobilized EPCs after chemotherapy (7).
Despite an exponential increase in EPC research, controversy still surrounds the definition of EPCs (8), and cell-therapy–based clinical studies in particular have often used ill-defined, heterogeneous cells. Currently, EPCs are regarded as blood circulating cells that contribute to angiogenesis, although the scope of this definition is extremely broad and includes a diverse range of cell types (9,10). Recently we have characterized two EPC subtypes in vitro: early EPCs (herein called myeloid angiogenic cells [MACs]) and outgrowth endothelial cells (OECs) (11) (also known as endothelial colony-forming cells). OECs possess well-defined endothelial progenitor characteristics such as commitment to an endothelial lineage, de novo tubulogenic potential and an ability to fully incorporate into a resident vascular network (12). In contrast, MACs show none of these EPC properties; however, MACs may still play a significant role in therapeutic angiogenesis (13,14). The proangiogenic, cytokine-mediated effects of various EPC subtypes have been described (15–20). However, the current study is the first investigation of the underlying molecular mechanisms whereby an important EPC subtype (MACs) can promote angiogenesis downstream of cytokine release. In particular, our data demonstrate that MAC-derived interleukin-8 (IL-8) is capable of transactivating vascular endothelial growth factor receptor 2 (VEGFR2) independently of extracellular VEGF.
EPCs are not the only cell population that influences angiogenesis; mounting evidence indicates that myeloid cells also regulate this process (21). Distinct myeloid cell populations, such as tumor-associated macrophages (22,23), Tie2- expressing monocytes (24) and myeloid-derived suppressor cells (25), have been implicated as key players in the angiogenic process. Although initially these monocytic subpopulations were described as having distinct phenotypes, it is now evident that they exhibit some overlapping markers (26) and share similar proangiogenic properties. In fact, they have been associated with alternative M2 macrophages, which are characterized as being antiinflammatory, proangiogenic cells involved in tissue repair and vascular remodeling (27). Because MACs have been previously shown to have monocytic features, in the current study we addressed the important question of whether these cells could, in fact, be linked to alternative M2 macrophages. It is crucial to assess MACs in relation to the M1/M2 polarization phenotypes because the macrophage contribution to angiogenesis is radically dictated by their polarization state. Such information has major therapeutic value because various hematopoietic/myeloid cells, including MACs, are already being used in the clinical setting.
MATERIALS AND METHODS
Cell Isolation and Culture
Circulating proangiogenic cells (hereafter referred to as MACs) were isolated from human peripheral blood according to previously established protocols (11). Fresh human peripheral blood (40 mL) was obtained with full ethics approval from healthy volunteers aged 25–35 years who were nonsmokers and were not receiving any medication. Briefly, mononuclear cells were obtained by density gradient centrifugation and resuspended in EGM-2-MV medium (Lonza, Slough, UK) supplemented with 10% fetal bovine serum (FBS) and plated on flasks coated with fibronectin (Sigma, Gillingham, UK) at a density of 2 × 106 cells/mL. MACs at days 7–9 were used for experiments.
Primary retinal capillary-derived microvascular endothelial cells (RMECs) were freshly prepared from bovine eyes according to standard isolation procedures previously described (28). RMECs were cultured on 1% gelatin-coated flasks, with DMEM (PAA Labs GmbH, Pasching, Austria) supplemented with 10% porcine serum (Sigma), 100 μg/mL insulin (Sigma), 1 mg/mL heparin (Sigma), and 500 mg/mL Primocin (Auto gen Bioclear, Wiltshire, UK). RMECs at passages 3 to 6 were used for experiments.
The human microvascular endothelial cell line (HMEC-1) was provided by the Centers for Disease Control and Prevention, Emory University School of Medicine, Atlanta, Georgia. HMEC-1 were grown in MCDB-131 medium (Invitrogen, Paisley, UK) supplemented with 10% FBS, 10 ng/mL epidermal growth factor (BD Biosciences Europe, Erembodegem, Belgium), 1 μg/mL hydrocortisone (Sigma) and 10 mmol/L L-glutamine (Sigma).
In Vitro Tubulogenesis Assay
RMECs and MACs were labeled with a PKH membrane-labeling kit (Sigma) prior to experiments. RMECs were mixed in a 4:1 ratio with MACs and suspended in growth factor reduced Matrigel (BD Biosciences), then 50-μL aliquots were spotted onto a 24-well plate. After polymerization, spots were covered with DMEM containing 5% porcine serum and respective treatments. After 72 h, wells were assessed for the presence of tubules, defined as an interconnected vascular network of elongated tubelike structures. In a separate set of experiments, conditioned medium obtained from MACs was used in this tubulogenesis model. To gain insight into the molecular mechanisms underlying the effect of MACs on RMECs, we neutralized IL-8 and VEGF by using 10 μg/mL of antihuman CXCL8/IL-8 (R&D Systems, Abingdon, UK) and 1 μg/mL of antihuman VEGF (R&D Systems). Respective IgG isotype controls were also used. Images were acquired by using a laser confocal microscope (Nikon, Tokyo, Japan), and vascular tube area and length were quantified by using Volocity (PerkinElmer, Waltham, MA, USA) and NIS elements (Nikon) software, respectively. All measurements were done on bidimensional images.
Flow Cytometry
We prepared 1 × 106 cells for staining by filtering through 35-μm nylon mesh (BD Biosciences, Bedford, UK) and resuspended them in 100 μL of flow cytometry staining buffer (eBioscience, San Diego, CA, USA). Cells were incubated with fluorophore-conjugated antibodies against CD45, CD14, CD68, CD163, CD83, CD209, CD16 (eBioscience), CD206 (BD Biosciences) and Tie2 (Bio-Legend, San Diego, CA, USA) for 45 min at 4°C. For CD68 staining, cell fixation and permeabilization were required because CD68 is predominantly an intra-cellular protein. After staining, cells were washed and resuspended in 500 μL staining buffer for analysis performed by using a FACSCalibur flow cytometer (BD Biosciences) with CellQuest (BD Biosciences) and FlowJo (Tree Star, Ashland, OR, USA) software. At least 15,000 events were acquired per sample, and respective immunoglobulin (Ig)G isotype controls were used to determine accurate settings for data analysis.
Human Angiogenesis Protein Array
Medium was obtained from coculture experiments and analyzed by using the Proteome Profiler human angiogenesis array (R&D Systems) in accordance with manufacturer guidelines. Briefly, samples were adjusted to a final volume of 1.5 mL with array buffer and mixed with a detection antibody cocktail for 1 h. After a membrane-blocking step, samples containing antibody cocktail were then added to membranes and incubated overnight at 4°C on a rocking platform. Next, membranes were given several washes and incubated with streptavidin–horseradish peroxidase secondary antibody (R&D Systems). Spots were detected by using chemiluminescence (Millipore, Billerica, MA, USA) and densitometry was quantified by using LabWorks software (PerkinElmer).
RNA Extraction and Microarray Analysis
Total RNA was extracted by using an RNAqueous kit (Ambion, Cambridgeshire, UK), and 1 μg of RNA from each cell sample was labeled and hybridized to an Illumina WG-6 v3.0 Expression Beadchip (Illumina, San Diego, CA, USA). Samples analyzed in the array were three biological replicates for peripheral blood (PB)-derived MACs and a single sample of PB-derived CD14+ monocytes. Gene expression data obtained from Illumina Beadstudio was normalized by using an “R” bioconductor with a “lumi” package. Data were processed and analyzed by using the National Institute on Aging (NIA) array (Bethesda, MD, USA), GenePattern (Broad Institute, Cambridge, MA, USA), and integrative visual analysis tool for biological networks and pathways (visANT) software (Boston University, Boston, MA, USA). Microarray data are available at the NCBI Gene Expression Omnibus (GEO) with accession number GSE20283.
Real-Time Reverse-Transcription Polymerase Chain Reaction
Total RNA was isolated from three different biological replicates for MACs and CD14+ monocytes, respectively, by using the RNAqueous kit (Ambion), including the DNAse digestion step. Complementary DNA (cDNA) was synthesized from 500 ng of RNA by using randon hexamers and Superscript II (Invitrogen). Quantitative real-time polymerase chain reaction (PCR) was performed with 2× Maxima SYBR Green qPCR Mastermix (Fermentas, York, UK) in 10-μL reactions containing 2 μL of 1:15 cDNA dilution and 0.5 μmol/L of gene-specific primers for 45 cycles, including denaturation at 94°C for 10 s, annealing at 55°C for 15 s, and extension at 72°C for 15 s in a LightCycler 480 (Roche, Welwyn Garden City, UK). Pairs of primers used for amplification of target genes were: CD163, AGGATGCTGGAGTGATTTGC (forward) and CCAGCCGTCATCACATATTG (reverse); CCL2, TCTGTGCCTGCTGCTCATAG (forward) and GTGACTGGGGCATTGATTG (reverse); MSR1, GGGAACATTCTCAGACCTTG (forward) and AATCCTCGTGGACCACTTTC (reverse); MMP-9, AGTGGCACCACCACAACATC (forward) and ACACGCGAGTGAAGGTGAG (reverse); IL-1β, CTGATGGCCCTAAACAGATG (forward) and GTCGGAGATTCGTAGCTGGAT (reverse); PTGS2, GGCTAGACAGCGTAAACTGC (forward) and TAGATGCTCAGGGACTTGAGG (reverse); TNFSF10, TCACAGTGCTCCTGCAGTCT (forward) and TGGGGTCCCAATAACTGTCA (reverse); VEGFB, CTGTCTCCCAGCCTGATG (forward) and AGTCAAGGGCACCACCAC (reverse); IL-10, GTCATCGATTTCTTCCCTGTG (forward) and ACTCATGGCTTTGTAGATGCCT (reverse); RPL11 (housekeeping gene), TTCAGCATCGCAGACAAGAAGC (forward) and TTTGCCAGGAAGGATGATCCCA (reverse). The relative gene expression was determined by using REST software (Qiagen, Dorking, Surrey, UK).
Protein Extraction, Immunoprecipitation and Western Blotting
Protein was extracted by lysing cells in 1× RIPA buffer (Thermo Scientific, Leicestershire, UK) containing protease and phosphatase inhibitors with EDTA (Thermo Scientific). Immunoprecipitation was performed for VEGFR2 experiments, and whole cell lysates were used for extracellular signal-regulated kinase (ERK) experiments. For immunoprecipitation experiments, whole cell lysates were diluted to 1 μg protein/μL of RIPA buffer and first precleared by using protein A/G PLUS-agarose immunoprecipitation beads (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Lysates were incubated with rabbit monoclonal VEGF2 (Cell Signaling Technology, Danvers, MA, USA) overnight at 4°C. Then, protein A/G PLUS-agarose immunoprecipitation beads were added to lysates and gently rocked for 4 h. After several washing steps to eliminate nonspecific binding, pellets were diluted in 2× LDS sample buffer (Invitrogen) and heated at 95°C for 5 min to detach beads. After electrophoresis, proteins were transferred to a polyvinylidene difluoride (PVDF) membrane. Membranes were blocked for 1 h in 3% Blotto, nonfat dry milk (Santa Cruz Biotechnology) dissolved in Tris-buffered saline with 0.1% Tween-20 (TBST). Total and phosphorylated FLK were detected with antibodies from Santa Cruz Biotechnology, and total and phosphorylated ERKs were detected (antibodies from Cell Signaling). After washing with TBST, respective horse radish peroxidise–conjugated secondary antibodies (Santa Cruz Biotechnology) were applied, and blots were developed by using a UVP bioimaging system (Ultra-Violet Products Ltd, Cambridge, UK). PVDF membranes were reprobed with a monoclonal antibody to β-actin (Cell Signaling) as a loading control.
Phagocytosis Assay
To evaluate if MACs could carry out phagocytosis, pHrodo™ Escherichia coli BioParticles® (Invitrogen) were added to the cell culture medium. After 2 h of incubation at 37°C, cells were washed twice with PBS and incubated in fresh medium. Live cell images were acquired by using a laser confocal scanning microscope (Nikon), and the phagocytic index was determined by measuring the fluorescence intensity per cell by using the Volocity software (PerkinElmer).
Endothelial Monolayer Permeability
Cell permeability was measured by using the xCELLigence Real Time Cell Analysis DP instrument (Roche). This assay is based on measuring electrical resistance between gold electrodes, which is impeded by the integrity of the cell monolayer. Briefly, RMECs were plated on an E-16 plate at a density of 20,000/well in 200 μL of DMEM. Once RMECs had formed a confluent monolayer, and at the time of greatest impedance, the experiment was paused while 100 μL of medium was removed and replaced with fresh DMEM alone or DMEM containing IL-8 (200 ng/mL) or VEGF (200 ng/mL) so that final concentrations are 100 ng/mL. Cell impedance was recorded for another 12 h, and data were exported and analyzed by using Microsoft Excel 2007 (Microsoft, Redmond, WA, USA).
Murine Model of Retinal Ischemia
C57BL/6 mice were purchased from Harlan Laboratories, UK. All experiments were performed in conformity to the Association for Research in Vision and Ophthalmology statement for the use of animals in ophthalmic and vision research and UK Home Office regulations. Ischemic retinopathy was induced in C57BL/6 mice by using the previously described oxygen-induced retinopathy model (OIR) (29). Briefly, mouse pups at postnatal day 7 (P7) and their nursing dams were exposed to 75% oxygen (Pro-Ox 110 Chamber Controller, Biospherix, Redfield, NY, USA) for 5 d to induce retinal vasodegeneration. At P12, mice were transferred back to room air to create overt central retinal ischemia and received an intravitreal injection into the right eye containing 1 μL of 1 × 105 MACs that had previously been labeled with Qtracker® 655 (Invitrogen). As a control, phenol red-free DMEM containing no growth factors or serum was injected into the left eye of each pup.
A separate group of mice received a daily intraperitoneal injection of CXC-chemokine receptor 2 (CXCR2) inhibitor SB225002 (Enzo Life Sciences, Exeter, UK) at a dose of 5μg/g from P12 until P15. Pups were then euthanized by using sodium pentobarbital, and the whole eyes were fixed in 4% parafor maldehyde. Retinal flat mounts were stained with isolectin B4 (Sigma) and streptavidin-Alexa 488 (Invitrogen). In a separate set of experiments, unlabeled MACs were injected and detected by using human-specific antibody CD68 (AbD Serotec). Stained retinas were visualized and imaged by using a Nikon confocal scanning microscope. Area quantification was done by using Image J software (National Institutes of Health, Washington, DC, USA). Analysis was also performed to quantify the number of retinal phagocytes by using NIS elements software (Nikon). Three-dimensional (3D) image reconstruction was performed by using Volocity software (PerkinElmer).
Statistical Analysis
All data are expressed ± SEM. Statistical significance of differences between groups was determined by using one-way ANOVA with the Student-Newman-Keuls posttest, with GraphPad InStat version 3.06 for Windows (Graphpad Software, San Diego, CA, USA).
All supplementary materials are available online at www.molmed.org.
RESULTS
MACs Induce Endothelial Tube Formation In Vitro in a Paracrine Manner
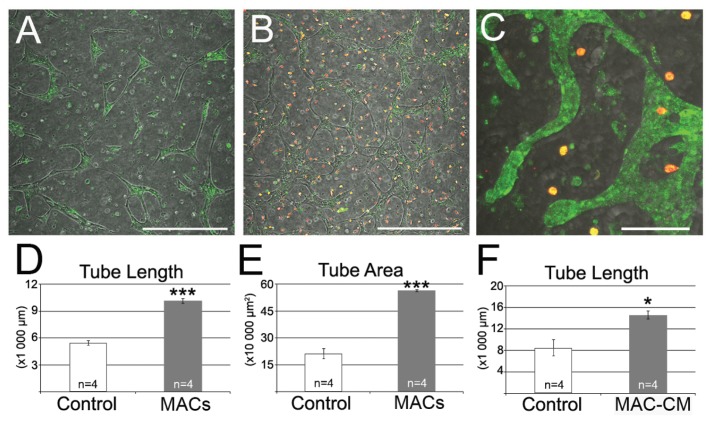
To investigate whether MACs have proangiogenic properties, we used the 3D matrigel tube formation assay, in which RMECs form tubelike structures (Figure 1A). A coculture approach, with prelabeled RMECs (green) and MACs (red), allowed unambiguous cell identification and accurate tubulogenesis quantification (Figure 1B). MACs did not incorporate into the microvascular network, and they remained remote as single spherical cells (Figure 1C). However, quantification of RMEC tube length (Figure 1D) and tube area (Figure 1E) indicated that MACs significantly increased RMEC tubulogenesis by approximately two-fold (P < 0.001). The lack of physical interaction between MACs and RMECs suggested a paracrine effect, which was tested by adding MAC-conditioned medium (MAC-CM) to the RMEC tube-formation assay. MAC-CM significantly enhanced RMEC tube formation (P < 0.05) (Figure 1F), confirming that MACs release proangiogenic factors into the medium.
Figure 1.
MACs induce retinal microvascular network formation. (A) Green-labeled RMECs formed tubes in Matrigel. (B) Red-labeled MACs promoted RMEC tube formation. Scale bars: 500 μm. (C) Higher magnification of coculture showing red-labeled MACs did not incorporate into the vascular network. Scale bar: 200 μm. (D) Quantification of tube length. (D) Quantification of tube area. (E) Addition of MAC-CM significantly increased RMEC tube formation as measured by tube length when compared with nonconditioned media. Data expressed as mean ± SEM. ***P < 0.001 versus control; *P < 0.05 versus control.
MACs Promote Vascular Repair in the Ischemic Retina In Vivo
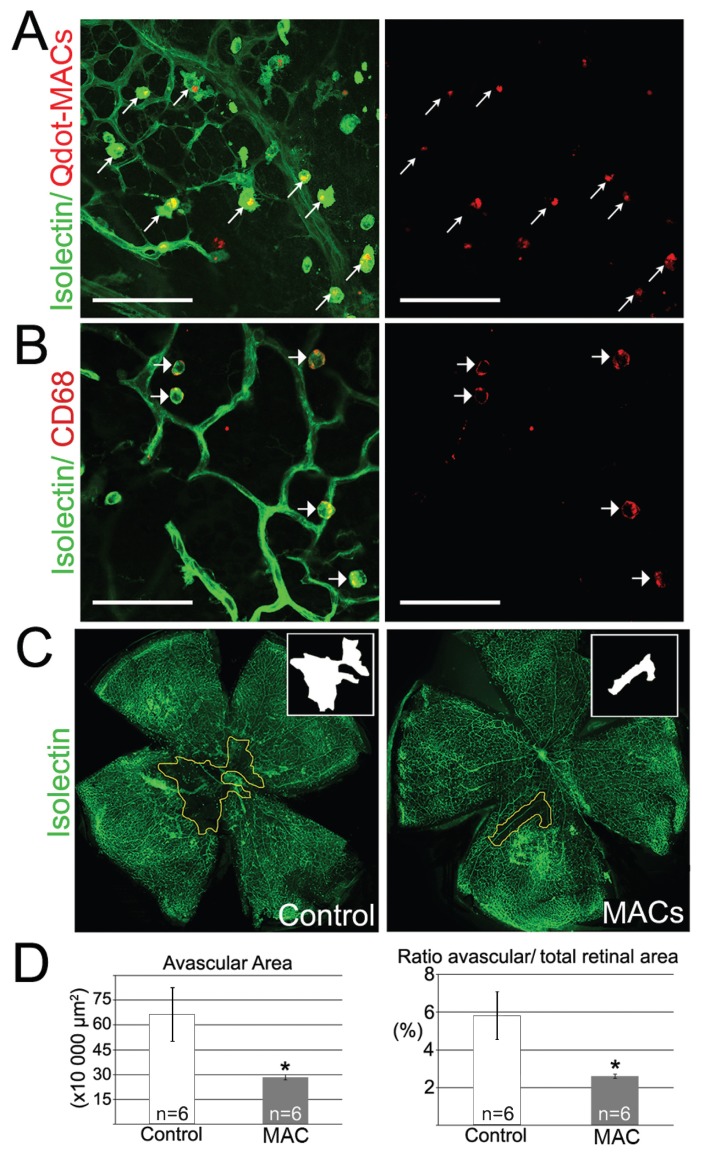
We next explored the potential of MACs to regulate ischemia-induced angiogenesis in vivo. The OIR model was chosen because it adequately and consistently reproduces ischemia-related retinopathy in mice and is an established model for assessing angiogenic responses (30). Quantum-dot (Qdot)-labeled MACs were delivered into the vitreous of each mouse at P12 at the time point in OIR during which the retina initially experiences hypoxic stimuli (30). After 72 h (P15), the retinal vasculature was evaluated. Injected MACs penetrated the retinal neuropile but did not integrate into the resident vascular network (Figure 2A). The MACs remained within the retina, but localized to the nerve fiber layer underneath the inner limiting membrane (Supplemental Video 1). MACs retained their myeloid features in the host retina, such as CD68 expression and amoeboid/spherical morphology (Figure 2B). None of the injected MACs adopted a ramified morphology, suggesting they did not become retinal microglia or dendritic cells. Interestingly, MACs delivered into the retina added to host phagocytes and increased the total amount of retinal amoeboid phagocytes by three-fold (Supplementary Figure 1). Despite the fact that MACs did not directly contribute to vascular formation by incorporation into the endothelium, they significantly induced intraretinal angiogenesis and reperfusion in the ischemic retina, reducing avascular areas by 67% compared with vehicle-injected retinas (P < 0.05) (Figures 2C, D). There was no difference in preretinal neovascular vessels between groups.
Figure 2.
MACs promote vascular repair in the ischaemic retina. (A) Injected MACs labeled with red Qdots did not incorporate into the resident vascular network labeled in green with isolectin. Scale bars: 100 μm. (B) Injected human MACs retained a myeloid phenotype as demonstrated by expression of human-specific CD68 and typical amoeboid morphology. Scale bars: 75 μm. (C) Representative flat mounted retinas injected with vehicle (Control) or MACs, respectively. Isolectin staining in green identifies retinal vasculature, and avascular regions are outlined in yellow. Insets show ischaemic areas in white. (D) Quantification of avascular areas and ratio of avascular/total retinal area shows that injection of MACs into the eye significantly induced vascular repair compared with injection of vehicle injected. *P < 0.05 versus control.
MACs Represent Alternative M2 Macrophages
Previously, we have reported that from the genotypic, proteomic and immuno phenotypic perspectives, MACs are monocytic in nature (11); however, it is unknown whether these cells acquire M1/M2 macrophage polarization states. To determine the MAC relationship to monocytes, transcriptomes from three biological replicates of MACs were compared with peripheral blood circulating monocytes sorted by their expression of CD14. Statistical assessment for genes enriched at least two-fold (false discovery rate of 0.01) showed 943 upregulated and 881 downregulated transcripts (Supplementary Figure 2). Upregulated genes included mRNAs encoding complement factors, matrix metalloproteinases (MMPs), VEGFB, CD163, and the monocyte to the macrophage differentiation associated gene MMD. Among the downregulated genes were S100A12, adhesion molecules ICAM3 and selectin L, interferon regulatory factor-1, and lysozyme. Furthermore, interactome analysis, performed by using visANT, demonstrated that upregulated MAC gene networks were linked to angiogenesis, endocytosis and antiinflammatory pathways. Downregulated gene networks were associated with inflammation, apoptosis and proteasomes (Supplementary Figure 3).
Further investigation, focusing specifically on M1/M2 markers and proangiogenic genes, demonstrated that MACs highly expressed M2 macrophage markers such as macrophage scavenger receptor 1 (MSR1), mannose receptor, C type 1 (MRC1), IL-10, TGFB2 (transforming growth factor, beta 2), and CD163 (Figure 3A). By contrast, expression of the proinflammatory M1 macrophage markers TNF (tumor necrosis factor), IL-1β, IL-6, IL-12, and COX2 (cytochrome c oxidase subunit II), was minimal. In addition, proangiogenic genes VEGFB, CTGF (connective tissue growth factor), PDGFB (platelet-derived growth factor beta polypeptide), NRP1 (neuropilin 1) and MMP-9 were also highly expressed. Normalized gene expression profiles comparing monocytes to MACs strongly corroborated previous findings (Figure 3B) and highlighted significant upregulation of proangiogenic genes and M2 macrophage markers, whereas M1 markers were downregulated. Furthermore, when we compared MACs to monocytes, gene microarray results were validated by the demonstration by real-time quantitative reverse-transcription PCR of significant upregulation of CD163, IL-10, MSR1, chemokine (C-C motif) ligand 2 (CCL2), MMP-9, and VEGFB, and downregulation of IL-1β and PTGS2 (prostaglandin-endoperoxide synthase 2) (Supplementary Figure 4).
Figure 3.
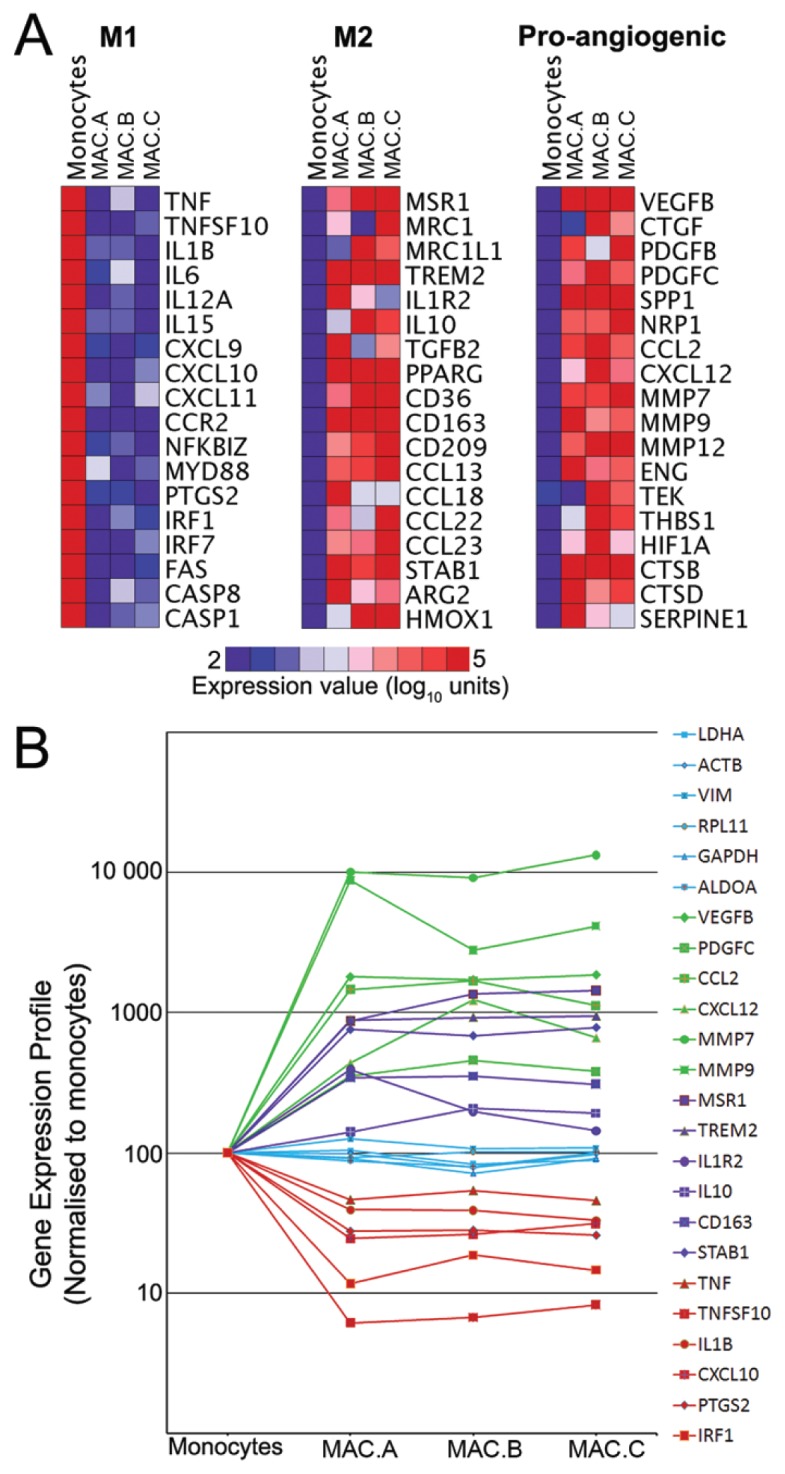
Transcriptome analysis revealed that MACs represent angiogenic M2-activated macrophages. (A) Heat maps of typical M1, M2 markers and proangiogenic genes demonstrated that the MAC gene signature is highly enriched for M2 markers, whereas expression of M1 macrophage markers was weak/low. Proangiogenic genes were also highly expressed. (B) Gene expression profiles of MACs were normalized to monocytes. Proangiogenic genes (green) and M2 markers (purple) were upregulated and M1 markers (red) were downregulated compared with housekeeping genes (blue).
To support transcriptomic data at the protein level, fluorescence-activated cell sorting (FACS) immunophenotyping was performed (Supplementary Figure 5). Corresponding with other analysis in the current investigation, MACs shared many characteristics with myeloid cells because they express CD45, CD14 and CD68. Expression of CD163 and CD206 suggested that MACs exhibit an M2 macrophage phenotype. Moreover, MACs are not dendritic cells, because they lack CD83 and CD209 expression. Similar FACS immunophenotyping was performed on colony-forming unit– endothelial cells (CFU-EC) CFU-Hill, a third subtype of EPCs grown in vitro. As expected, CFU-Hill represents a more heterogeneous population of cells; however, the main population (82% of cells) also express M2 markers CD163 and CD206 (Supplementary Figure 6), suggesting CFU-Hill and MACs are both M2 macrophages.
Alternative activated macrophages are frequently described as professional phagocytes involved in clearance of cellular debris and apoptotic bodies during tissue remodeling; therefore MACs phagocytic activity was evaluated by using fluorescent E. coli bioparticles. The phagocytic index of MACs was equivalent to M1 macrophages; however, their cellular morphology was clearly different (Supplementary Figure 7). Whereas MACs had a spindle, elongated shape, M1 macrophages showed distinctively flat, “fried-egg–like” morphology. These two contrasting macrophage morphologies have been described previously (31) and are related to M2 and M1 phenotypes, respectively.
MACs Secrete Angiogenic Factors
To identify some of the angiogenic cytokines and growth factors secreted by MACs, we characterized the media from previous coculture experiments described in Figure 1. For this characterization we used a human proteome array for profiling the expression of 55 angiogenesis-related proteins. Conditioned media from RMECs alone in the 3D Matrigel assay contained minimal levels of detectable cytokines, with only endothelin-1 protein being observed (Figure 4A). By contrast, eight different proteins were highly expressed in MAC-RMEC coculture media (Figure 4A), and these included established proangiogenic cytokines and enzymes such as IL-8, monocyte chemotactic protein 1 (MCP-1) and MMP-9.
Figure 4.
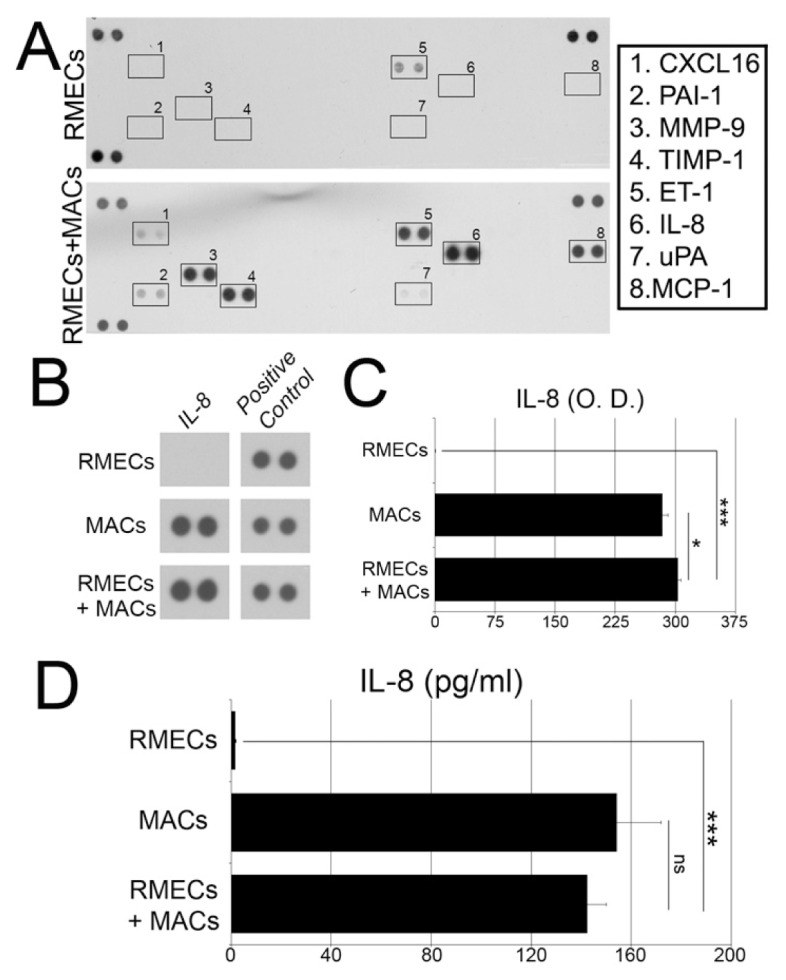
Angiogenic cytokines produced by MACs. (A) A human angiogenesis proteome profiler array was used to identify soluble factors released by cells to the medium. RMECs secrete minimal amounts of ET-1, and medium from a MAC and RMEC coculture system showed significant expression of proangiogenic proteins such as MMP-9, IL-8, and MCP-1. Three pairs of positive controls are included in the top corners and bottom left corner. A pair of negative control spots in the bottom right corner indicates very low background levels. (B) IL-8 proteome array assessment of conditioned media of RMECs, MACs and RMECs + MACs. (C) Quantification of proteome profiler shown in (B) by densitometry (O. D., optical density); N = 6. (D) ELISA quantification of IL-8 in conditioned media of RMECs, MACs and RMECs + MACs. N = 4. Data in (C) and (D) expressed as mean ± SEM. ***P < 0.001 versus RMECs; *P < 0.05 versus RMECs; ns, not significant.
Because IL-8 was the most abundant protein in the coculture system, its expression was further assessed when cells were cultured separately. Interestingly, IL-8 was found in the conditioned media of MAC cultures but not RMECs (Figure 4B). Results of densitometry analysis indicated that IL-8 protein expression in the conditioned media of MACs was comparable to that found in the cocultures (Figure 4C), suggesting that IL-8 comes from MACs and not from RMECs. To further corroborate this finding, we performed absolute quantification of IL-8 in the different conditioned media by enzyme-linked immunosorbent assay (ELISA) (Figure 4D). In agreement with previous findings, IL-8 in RMEC-conditioned media was minimal (1.7 pg/mL) compared with MAC-conditioned media (154.4 pg/mL). The amount of IL-8 in co-cultures (142.6 pg/mL) was similar to MAC-conditioned media. Taken together, proteome analysis and ELISA results indicated that IL-8 is being secreted by MACs and not RMECs.
IL-8 Is Essential for MAC Proangiogenic Effects
Continuing on from the identification of MAC-derived cytokines, we separately added neutralizing antibodies against VEGF or IL-8 to the coculture system (Figures 5A, B) to evaluate whether the MAC proangiogenic effect was dependent on release of these two soluble factors. Interestingly, addition of anti–IL-8 antibody (Figure 5D), but not anti-VEGF (Figure 5C), significantly decreased RMEC tube formation to baseline levels (Figure 5E). Respective IgG isotype controls did not have any effect. These results indicated that the MAC-mediated angiogenic response is highly dependent on secreted IL-8 but not VEGF. In addition, a neutralizing antibody against VEGFR2 inhibited RMEC tube formation induced by MACs (Supplementary Figure 8). This result suggests that although the MAC proangiogenic effect on RMECs is independent of extracellular VEGF, it still depends on activation of the VEGF signaling pathway downstream of VEGFR2.
Figure 5.
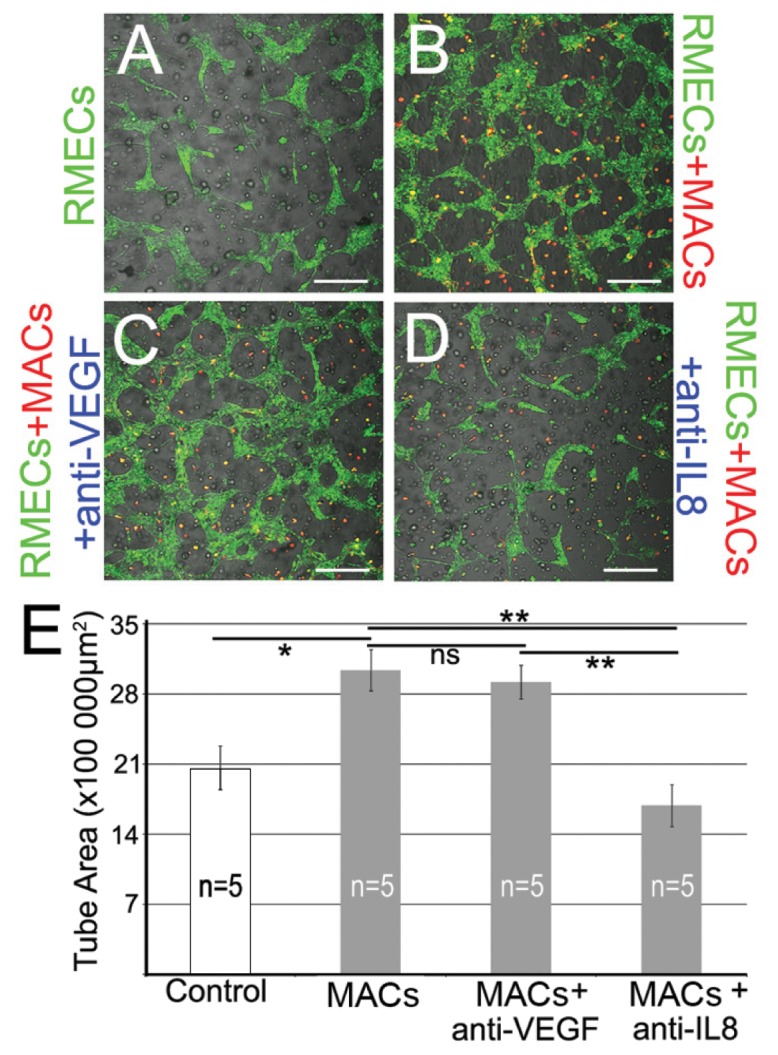
The MAC proangiogenic paracrine effect is dependent on secreted IL-8, but independent of extracellular VEGF. Using a PKH kit, we prelabeled RMECs in green and MACs in red before culturing them in the 3D Matrigel Tubulogenesis Assay. Representative images are shown of RMECs alone forming tubes (A); cocultures of RMECs and MACs (B); addition of IL-8 neutralizing antibody to a coculture system (C); and addition of neutralizing VEGF antibody to cocultures (D). Scale bars for A–D: 250 μm. (E) Quantification of tube areas in response to treatments. Anti–IL-8 significantly decreased RMEC tube formation; however, neutralizing VEGF had no significant effect on RMEC tubulogenesis. Data are expressed as mean ± SEM. **P < 0.01 versus control; *P < 0.05 versus control; ns: not significant.
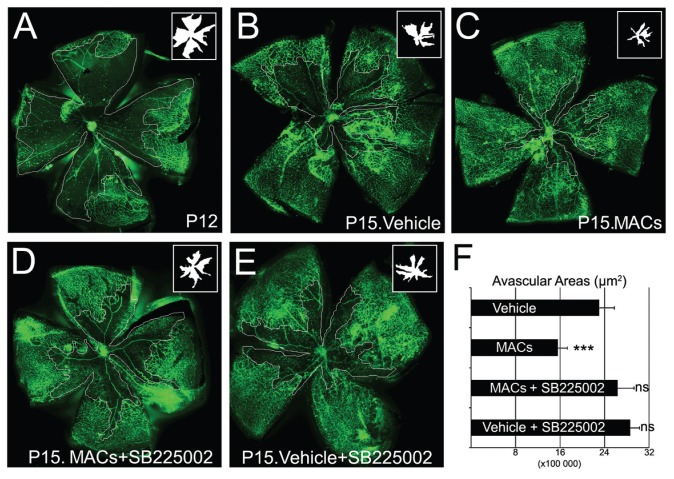
To assess the importance of MAC- mediated IL-8 signaling in vivo, the OIR mouse model was treated with the CXCR2 inhibitor SB225002 to block IL-8–receptor signal transduction. In contrast to non–drug-treated controls, SB225002-treated animals did not benefit from MAC intravitreal injections, as shown by the extent of avascular areas that were comparable to vehicle-injected control retinas (Figure 6). Only the mouse group that received MACs but no SB225005 showed a significant decrease in retinal ischemia (P < 0.001). This finding suggests that the proangiogenic paracrine effect of MACs in vivo can be significantly diminished by blocking CXCR2 receptor-mediated cytokine signaling.
Figure 6.
Vascular repair induced by MAC delivery was blocked by CXCR2 inhibitor SB225002. Flat mounted retina microvasculature was stained green with isolectin, and avascular areas (insets) are outlined in white. A representative retina per group is shown: (A) ischemic retina at P12; (B) vehicle-injected retina at P15; (C) MAC-injected retina at P15; (D) MAC-injected retina at P15 in mouse that received SB225005 intraperitoneal injections; (E) vehicle-injected retina at P15 in mouse that received SB225005 intraperitoneal injections; (F) quantification of avascular areas shows that SB225005 significantly inhibited MAC proangiogenic effects. N = 7; ***P < 0.001; ns: not significant.
IL-8 Activates VEGFR2 Independently of VEGF
In our in vitro experiments we determined that blocking the bioactivity of extracellular VEGF did not inhibit MAC proangiogenic effects (Figure 5). This finding was surprising because it is established that activation of VEGFR2 signaling is critical for angiogenesis (32,33). These data suggested that IL-8 could transactivate VEGFR2, independently of extracellular VEGF, as has been previously reported (34). To evaluate if this transactivation phenomenon is central to IL-8 angiogenic function, we treated endothelial cells with 100 ng/mL IL-8 for 15 min, and assessed consequent VEGFR2 phosphorylation. IL-8 induced significant VEGFR2 phosphorylation (Figure 7A), which indicated that IL-8 is capable of transactivating the VEGF signaling pathway. To test functionality of VEGFR2 signaling induced by IL-8, we assessed endothelial barrier permeability because the effects of VEGF on permeability are primarily mediated by VEGFR2 (35). Recombinant human IL-8 decreased RMEC monolayer impedance to the same level as VEGF (Supplementary Figure 9). This result provides indirect evidence that IL-8 is capable of inducing VEGFR2 signaling and downstream endothelial permeability.
Figure 7.
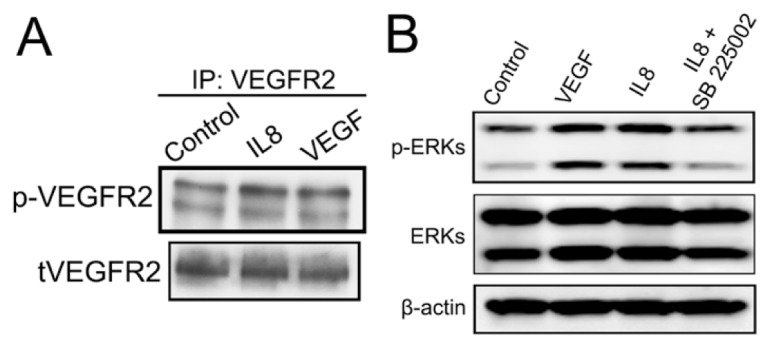
IL-8 transactivates VEGFR2 and induces ERK phosphorylation in endothelial cells. (A) HMEC-1 cells were treated with 100 ng/mL IL-8 for 15 min, followed by immunoblot analysis using a phospho-VEGFR2 antibody. IL-8 increased VEGFR2 phosphorylation levels compared with controls; 100 ng/mL VEGF treatment was used as a positive control. (B) RMECs were also treated with 100 ng/mL IL-8 for 15 min, and immunoblot analysis for phospho-ERKs was performed. IL-8 induced ERK phosphorylation in RMECs; this effect was completely abolished by the CXCR2 inhibitor SB225002.
ERK phosphorylation was also investigated to further evaluate IL-8 effects on endothelial cells downstream of both IL-8 and VEGF signaling. IL-8 significantly induced phosphorylation of ERKs in RMECs (Figure 7B). Addition of SB225002 completely abrogated ERK phosphorylation induced by IL-8 and indicated that binding to the cognate receptor CXCR2 is required for phosphorylation of ERKs.
DISCUSSION
EPCs represent a range of cell types of which only some have been definitively proven to differentiate into endothelium and incorporate into blood vessels (9,36). The OEC (also called ECFC) subtype is now widely regarded as the “true EPC,” although, surprisingly, these cells have yet to be used in the clinical context. Instead, in most patient-based studies to date, investigators have used cells sorted from the mononuclear fraction of peripheral blood with a narrow-range of markers, such as CD34. Such isolates contain a high proportion of circulating monocytic cells that we now refer to as “MACs.” These cells have also been referred to as proangiogenic hematopoietic progenitors (37). These marrow-derived cells, which have often been credited with the capacity of becoming endothelium (38), were first described as CD34+ colony-forming cells that acquired the expression of endothelial markers CD31, VEGFR2 and Tie2 (39). However, these “endothelial” markers are not specific, because they have also been found in monocytic cells (40). Indeed, it has been reported that platelet microparticle uptake by mononuclear cells in culture may explain the presence of the endothelial markers CD31 and von Willebrand factor in MACs (41). For these reasons, alternative names other than “EPC” have been proposed, such as MACs, proangiogenic monocytes and vascular accessory cells (9,10). Novel evidence provided in the current investigation strongly supports the assertion that MACs are myeloid cells with no potential to differentiate into endothelium.
One of the key findings of the present study was the recognition of MACs as alternatively activated M2 macrophages that support angiogenesis in a paracrine manner. While investigating molecular mechanisms to explain the proangiogenic effects of MACs, we identified striking immunophenotypic similarities between these cells and M2 macrophages. MACs express typical M2 markers such as the macrophage scavenger receptor MSR1 (CD204), hemoglobin scavenger receptor (CD163), mannose receptor MRC1 (CD206) and cytokine IL-10. This new evidence is important because M2 macrophages have been shown to function by dampening inflammatory responses, scavenging cellular debris, promoting angiogenesis, and enhancing tissue repair (27,42). Our data indicate that MACs represent alternative M2 macrophages and, as such, promote repair and limit tissue injury.
Our data demonstrate that MACs have the ability to mediate angiogenesis in vitro and in vivo. They achieve this without ever incorporating into the lumen of vessels, and they often remain completely remote from the vasculature. Therefore, MACs must influence angiogenic behavior in a paracrine manner through the release of cytokines or growth factors and creation of a concentration gradient to which endothelial cells respond. Although this paracrine effect has previously been described (15,18), respective molecular mechanisms have not been addressed in sufficient detail. Our data provide the first evidence directly linking IL-8 secretion by MACS to downstream VEGFR2 transactivation and consequently ERKs phosphorylation in mature endothelial cells. Although not investigated in the current study, there is recent evidence that some circulating blood-derived progenitor cells may form transient, nanotubelike intercellular connections to other distinct cell types that act as conduits for transfer of proteins and other macromolecules and thereby alter function (43). Whether this is a feature of MAC communication with resident endothelium is uncertain. Furthermore, endothelium-derived cytokines can also influence MAC phenotype and behavior. Hence endothelial cell–MAC interactions are highly complex, and we are currently investigating key aspects of this cellular cross-talk. In the current study, however, our data consistently demonstrated the existence of at least one key component of intercellular communication: MACs secrete IL-8, which induces phosphorylation of VEGFR2 in mature endothelial cells.
In the context of ischemic injury, we have now shown that intravitreal delivery of MACs into the ischemic eye can effectively promote retinal vascular repair and reperfusion. Although this effect has never been shown before in the eye, the findings are consistent with reports of the use of “MAC-like” cells to enhance neovascularization of other ischemic tissues in models of myocardial infarction (13) and hind limb ischemia (14). These data, in addition to ours, indicate that MACs have similar vasoreparative properties in at least three different vascular beds. Taken together, this evidence supports some of the preclinical and patient-based evidence that MACs have the potential to induce therapeutic angiogenesis. However, it should be noted that we have used a relatively homogenous population of cells selected according to immunophenotype and cell morphology, cultured in vitro, and that such potent proangiogenic properties are not common to all monocytic cells. Indeed, transplanting freshly isolated CD14+ cells, cultured dendritic cells, or cultured macrophages (M1) in a murine hind-limb ischemic model did not improve neovascularization; only MAC transplantation significantly increased ischemic tissue revascularization (14). In agreement with the results of these other published studies, our microarray transcriptional profiling results corroborated other evidence that MACs are distinct from freshly isolated monocytes, and suggest that ex vivo culture conditions are partly responsible for the acquisition of a proangiogenic and antiinflammatory phenotype. The in vivo cell type that correlates with MACs is the alternative M2 macrophage. The role of macrophages in tissue repair has recently been highlighted by work in wound healing, in which the switch from M1 to M2 macrophages is required for resolution of inflammation and physiologic wound healing (44).
Other investigators have shown that MACs have the potential to release a range of cytokines (16,18,20). This finding is consistent with the results of our analysis, in which we identified IL-8, MCP-1 and MMP-9 as being released by MACs in relatively high concentrations. In addition, our results have revealed that IL-8 plays a critical role, as demonstrated by the ability to abrogate the proangiogenic effects of MACs by blockade of IL-8, a response that is independent of extracellular VEGF. We have also demonstrated that IL-8 transactivates VEGFR2 and induces proangiogenic signaling cascades that control processes such as endothelial proliferation, migration and tube formation. Although we focused on IL-8/VEGFR2/ERK signaling pathways, other mechanisms to explain IL-8–induced angiogenesis are also possible. For example, nuclear factor κ B (NFκB) transcription, activation of SREBPs (sterol regulatory element-binding proteins) and RhoA, and stimulation of IP3 (inositol-1,4,5-trisphosphate) production through activation of phospholipase C, may also be involved. Delineation of other mechanisms by which IL-8 regulates angio genesis should be the focus of future research.
Despite in vitro evidence indicating that MAC-secreted IL-8 is necessary and sufficient to induce RMEC angiogenesis, the significant beneficial effect of injecting MACs into an ischemic retina is unlikely to be caused by IL-8 release alone. As stated previously, MACs, acting as alternative activated M2 macrophages, can modulate inflammatory and immune responses to ischemia by secreting a range of soluble factors (including IL-8) that will modify tissue cytokine profiles and thereby reduce tissue damage. A similar mechanism has been reported to explain neuroprotective effects of bone-marrow–derived progenitor cells injected into a murine model of ischemic brain injury (45). Paracrine effects play a significant role in tissue repair by modulating angiogenesis. The “secretory” nonendothelial phenotype of MACs contrasts with the “structural” endothelial phenotype of OECs; therefore the combined use of both EPC cell types may be an option for cellular therapies (46). Although considerably more work on such combinations is required, in a murine hind-limb ischemia model, it has been demonstrated that a mixed transplantation of MACs and OECs results in superior neovascularization in vivo compared with transplantation of either cell alone (18).
To dissect molecular mechanisms, we focused on IL-8 because this cytokine is classically known as a macrophage-derived mediator of angiogenesis (47). Pathological neovascular states in the eye such as proliferative diabetic retinopathy and Eales disease were found to be associated with high IL-8 levels in the vitreous of these patients (unpublished data). In addition, it was reported that the angiogenic effects of IL-8 in microvascular endothelial cells are mediated by CXCR2 (48), and that IL-8 transactivates VEGFR2 (34). In the study we report here, we demonstrated that the provascular repair effect of MACs in vivo was abrogated when CXCR2 signaling was inhibited, and this result was consistent with in vitro findings showing that MACs secrete IL-8, which induces phosphorylation of VEGFR2 and ERKs. This finding provides a mechanistic explanation for the proangiogenic effect of MACs, although it should be appreciated that CXCR2 has a complex ligand-binding pattern that includes growth-related oncogene (GRO)1, GRO3, GCP2 (granulocyte chemotactic protein-2) and MIP2 (macrophage inflammatory protein-2) (49). Therefore we cannot exclude a possible role for other CXCR2 ligands acting alongside IL-8 in cytokine-mediated angiogenesis. Nevertheless, we provide evidence demonstrating that IL-8 is capable of inducing VEGFR2 and ERK phosphorylation, a response that is dependent on CXCR2 binding but is independent of VEGF. This evidence is in agreement with recent findings that indicate physical interactions between VEGFR2 and the IL-8 receptors, and that Src kinases are involved upstream of receptor complex formation and VEGFR2 transactivation (34). Therefore, the VEGF-signaling pathway can be added to the many downstream targets of IL-8–receptor binding (50).
In the current study we demonstrated that MACs have the ability to enhance angiogenesis; however, the concept of these cells as solely proangiogenic with positive reparative potential may be an oversimplification and could be misleading. Macrophages are known to retain considerable plasticity (42) and can respond to environmental signals by changing between their M1 and M2 phenotypes. Although we have now shown MACs to be M2-macrophage like, it is possible that certain environmental conditions, such as chronic hypoxia, extensive tissue necrosis/apoptosis, inflammation, and/or a diabetic milieu, could trigger a phenotypic switch that is associated with pathology rather than repair. As an example of this, it has been previously reported that diabetic EPCs (in this case MAC-like cells) did not efficiently repair ischemic vascular damage (51). Indeed, such cells converted to an anti angiogenic phenotype in obese diabetic mice (52). This finding suggests that clinicians who employ any future therapy based on delivering MACs should carefully take into account both the source of the cells and the environment into which they will be injected, because these features will determine the M1/M2 phenotype and the potential for reparative angiogenesis.
Supplemental Data
ACKNOWLEDGMENTS
This work was funded by grants from the Juvenile Diabetes Research Foundation, Fight for Sight UK, The Sir Jules Thorn Trust, the Department of Employment & Learnng (DEL) (Northern Ireland), and the Medical Research Council UK. AW Stitt holds a Royal Society Wolfson Merit Award. The authors thank Sharon Alexander for constant support and assistance in taking samples. We also thank Jessica Neisen and David Waugh for assistance with ELISA for IL-8.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–53. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 2.Nolan DJ, et al. Bone marrow-derived endothelial progenitor cells are a major determinant of nascent tumor neovascularization. Genes Dev. 2007;21:1546–58. doi: 10.1101/gad.436307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sekiguchi H, Ii M, Losordo DW. The relative potency and safety of endothelial progenitor cells and unselected mononuclear cells for recovery from myocardial infarction and ischemia. J Cell Physiol. 2009;219:235–42. doi: 10.1002/jcp.21672. [DOI] [PubMed] [Google Scholar]
- 4.Fadini GP, Agostini C, Avogaro A. Autologous stem cell therapy for peripheral arterial disease meta-analysis and systematic review of the literature. Atherosclerosis. 2010;209:10–7. doi: 10.1016/j.atherosclerosis.2009.08.033. [DOI] [PubMed] [Google Scholar]
- 5.Gao D, et al. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319:195–8. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- 6.Jevremovic D, et al. Use of blood outgrowth endothelial cells as virus-producing vectors for gene delivery to tumors. Am J Heart Circ Physiol. 2004;287:H494–500. doi: 10.1152/ajpheart.00064.2004. [DOI] [PubMed] [Google Scholar]
- 7.Murakami J, et al. Inhibition of accelerated tumor growth by blocking the recruitment of mobilized endothelial progenitor cells after chemotherapy. Int J Cancer. 2009;124:1685–92. doi: 10.1002/ijc.24085. [DOI] [PubMed] [Google Scholar]
- 8.Yoder MC. Defining human endothelial progenitor cells. J. Thromb. Haemost. 2009;7(Suppl 1):49–52. doi: 10.1111/j.1538-7836.2009.03407.x. [DOI] [PubMed] [Google Scholar]
- 9.Steinmetz M, Nickenig G, Werner N. Endothelial-regenerating cells: an expanding universe. Hypertension. 55:593–9. doi: 10.1161/HYPERTENSIONAHA.109.134213. [DOI] [PubMed] [Google Scholar]
- 10.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28:1584–95. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medina RJ, et al. Molecular analysis of endothelial progenitor cell (EPC) subtypes reveals two distinct cell populations with different identities. B. M. C. Med. Genomics. 2010;3:18. doi: 10.1186/1755-8794-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medina R, et al. Outgrowth endothelial cells: characterization and their potential for reversing ischemic retinopathy. Invest Ophthalmol Vis Sci. 2010;51:5906–13. doi: 10.1167/iovs.09-4951. [DOI] [PubMed] [Google Scholar]
- 13.Kawamoto A, et al. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103:634–37. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- 14.Urbich C, et al. Relevance of monocytic features for neovascularization capacity of circulating endothelial progenitor cells. Circulation. 2003;108:2511–6. doi: 10.1161/01.CIR.0000096483.29777.50. [DOI] [PubMed] [Google Scholar]
- 15.Sieveking DP, Buckle A, Celermajer DS, Ng MK. Strikingly different angiogenic properties of endothelial progenitor cell subpopulations: insights from a novel human angiogenesis assay. J Am Coll Cardiol. 2008;51:660–8. doi: 10.1016/j.jacc.2007.09.059. [DOI] [PubMed] [Google Scholar]
- 16.Urbich C, et al. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39:733–42. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–9. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 18.Yoon CH, et al. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112:1618–27. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- 19.Leifheit-Nestler M, et al. Overexpression of integrin beta 5 enhances the paracrine properties of circulating angiogenic cells via Src kinase-mediated activation of STAT3. Arterioscler Thromb Vasc Biol. 2010;30:1398–406. doi: 10.1161/ATVBAHA.110.206086. [DOI] [PubMed] [Google Scholar]
- 20.Urbich C, et al. Proteomic characterization of human early pro-angiogenic cells. J Mol Cell Cardiol. 2011;50:333–6. doi: 10.1016/j.yjmcc.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 21.Noonan DM, et al. Inflammation, inflammatory cells and angiogenesis: decisions and indecisions. Cancer Metastasis Rev. 2008;27:31–40. doi: 10.1007/s10555-007-9108-5. [DOI] [PubMed] [Google Scholar]
- 22.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–7. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–12. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 24.Venneri MA, et al. Identification of proangiogenic TIE2-expressing monocytes (TEMs) in human peripheral blood and cancer. Blood. 2007;109:5276–85. doi: 10.1182/blood-2006-10-053504. [DOI] [PubMed] [Google Scholar]
- 25.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coffelt SB, et al. Elusive identities and overlapping phenotypes of proangiogenic myeloid cells in tumors. Am J Pathol. 2010;176:1564–76. doi: 10.2353/ajpath.2010.090786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 28.McDonald DM, et al. Advanced glycation of the Arg-Gly-Asp (RGD) tripeptide motif modulates retinal microvascular endothelial cell dysfunction. Mol Vis. 2009;15:1509–20. [PMC free article] [PubMed] [Google Scholar]
- 29.Medina RJ, et al. The pleiotropic effects of simvastatin on retinal microvascular endothelium has important implications for ischaemic retinopathies. PloS. One. 2008;3:e2584. doi: 10.1371/journal.pone.0002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith LE, et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–11. [PubMed] [Google Scholar]
- 31.van der Plas MJ, van Dissel JT, Nibbering PH. Maggot secretions skew monocyte-macrophage differentiation away from a pro-inflammatory to a proangiogenic type. PloS One. 2009;4:e8071. doi: 10.1371/journal.pone.0008071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 33.Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312:549–60. doi: 10.1016/j.yexcr.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 34.Petreaca ML, et al. Transactivation of vascular endothelial growth factor receptor-2 by in-terleukin-8 (IL-8/CXCL8) is required for IL-8/CXCL8-induced endothelial permeability. Mol Biol Cell. 2007;18:5014–23. doi: 10.1091/mbc.E07-01-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gille H, et al. Analysis of biological effects and signaling properties of Flt-1 (VEGFR-1) and KDR (VEGFR-2). A reassessment using novel receptor-specific vascular endothelial growth factor mutants. J Biol Chem. 2001;276:3222–30. doi: 10.1074/jbc.M002016200. [DOI] [PubMed] [Google Scholar]
- 36.Watt SM, Athanassopoulos A, Harris AL, Tsaknakis G. Human endothelial stem/progenitor cells, angiogenic factors and vascular repair. J. R. Soc. Interface. 2010;7(Suppl 6):S731–51. doi: 10.1098/rsif.2010.0377.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Case J, et al. Human CD34+AC133+ VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol. 2007;35:1109–18. doi: 10.1016/j.exphem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 38.Krenning G, et al. Efficient differentiation of CD14+ monocytic cells into endothelial cells on degradable biomaterials. Biomaterials. 2007;28:1470–9. doi: 10.1016/j.biomaterials.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 39.Asahara T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 40.Kim SJ, et al. Circulating monocytes expressing CD31: implications for acute and chronic angiogenesis. Am J Pathol. 2009;174:1972–80. doi: 10.2353/ajpath.2009.080819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prokopi M, et al. Proteomic analysis reveals presence of platelet microparticles in endothelial progenitor cell cultures. Blood. 2009;114:723–32. doi: 10.1182/blood-2009-02-205930. [DOI] [PubMed] [Google Scholar]
- 42.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koyanagi M, et al. Cell-to-cell connection of endothelial progenitor cells with cardiac myocytes by nanotubes: a novel mechanism for cell fate changes. Circ Res. 2005;96:1039–41. doi: 10.1161/01.RES.0000168650.23479.0c. [DOI] [PubMed] [Google Scholar]
- 44.Sindrilaru A, et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest. 2011;121:985–97. doi: 10.1172/JCI44490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohtaki H, et al. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc Natl Acad Sci U S A. 2008;105:14638–43. doi: 10.1073/pnas.0803670105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stitt AW, et al. Vascular stem cells and ischaemic retinopathies. Prog Retin Eye Res. 2011;30:149–66. doi: 10.1016/j.preteyeres.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Koch AE, et al. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 48.Heidemann J, et al. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem. 2003;278:8508–15. doi: 10.1074/jbc.M208231200. [DOI] [PubMed] [Google Scholar]
- 49.Keeley EC, Mehrad B, Strieter RM. Chemokines as mediators of neovascularization. Arterioscler Thromb Vasc Biol. 2008;28:1928–36. doi: 10.1161/ATVBAHA.108.162925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–41. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 51.Caballero S, et al. Ischemic vascular damage can be repaired by healthy, but not diabetic, endothelial progenitor cells. Diabetes. 2007;56:960–7. doi: 10.2337/db06-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Awad O, et al. Obese diabetic mouse environment differentially affects primitive and monocytic endothelial cell progenitors. Stem Cells. 2005;23:575–83. doi: 10.1634/stemcells.2004-0185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.