Abstract
Dendritic cell (DC)-based adoptive tumor immunotherapy approaches have shown promising results, but the incidence of tumor regression is low and there is an evident call for identifying culture conditions that produce DCs with a more potent Th1 potential. Routinely, DCs are differentiated in CO2 incubators under atmospheric oxygen conditions (21% O2), which differ from physiological oxygen levels of only 3–5% in tissue, where most DCs reside. We investigated whether differentiation and maturation of DCs under physiological oxygen levels could produce more potent T-cell stimulatory DCs for use in adoptive immunotherapy. We found that immature DCs differentiated under physiological oxygen levels showed a small but significant reduction in their endocytic capacity. The different oxygen levels did not influence their stimuli-induced upregulation of cluster of differentiation 54 (CD54), CD40, CD83, CD86, C-C chemokine receptor type 7 (CCR7), C-X-C chemokine receptor type 4 (CXCR4) and human leukocyte antigen (HLA)-DR or the secretion of interleukin (IL)-6, tumor necrosis factor (TNF)-α and IL-10 in response to lipopolysaccharide (LPS) or a cytokine cocktail. However, DCs differentiated under physiological oxygen level secreted higher levels of IL-12(p70) after exposure to LPS or CD40 ligand. Immature DCs differentiated at physiological oxygen levels caused increased T-cell proliferation, but no differences were observed for mature DCs with regard to T-cell activation. In conclusion, we show that although DCs generated under atmospheric or physiological oxygen conditions are mostly similar in function and phenotype, DCs differentiated under physiological oxygen secrete larger amounts of IL-12(p70). This result could have implications for the use of ex vivo–generated DCs for clinical studies, since DCs differentiated at physiological oxygen could induce increased Th1 responses in vivo.
INTRODUCTION
Dendritic cells (DCs) are the most potent antigen-presenting cells (1,2) and are critical for the induction of immune responses to pathogens and cancer (1,3). Because of these properties, DCs are being widely used for vaccines and immunotherapeutic strategies (3–10). The most common approaches for tumor immunotherapy involve the use of DCs generated from the progenitors CD34+ (11–13) or CD14+ in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) and inter-leukin (IL)-4 ex vivo (14). Tumor antigens are delivered to DCs using many different systems including whole tumor cells or lysates (15–17), RNA (18–21), peptides or viral vectors (22). Exposure to antigen is followed by the addition of a maturation stimulus in vitro, since mature DCs induce more potent immune responses than immature DCs, which can induce T-cell tolerance (23,24). One of the originally used maturation stimuli was monocyte-conditioned media (14), which was subsequently refined to a cocktail containing four components including prostaglandin E2 (PGE2), tumor necrosis factor (TNF)-α, IL-1β and IL-6 (8,25–27).
Although immune responses are generated to DC-based vaccine approaches in patients, only in a few instances has tumor regression been observed in phase I clinical trials (28,29). Clearly, this result could have many explanations including the DC subtypes used, the maturation stimuli, the suppressive tumor microenvironment, treatment starting too late in the disease, and other explanations. An alternative possibility is that the ex vivo–generated DCs are not optimal for in vivo function because of the applied culture conditions. Optimization of the in vitro culture conditions should allow for the generation of DCs that will give the desired and maximal Th1-type immune response in vivo. In studies using ex vivo differentiated DCs, the DCs are generally differentiated in incubators that maintain atmospheric oxygen levels (21% O2 and 5% CO2). However, in vivo, most cells including DCs do not encounter such high oxygen levels. DCs exist both in blood and tissue, and most of them reside in the tissue where the oxygen levels are 3–5% (30–32). The effect of physiological and atmospheric oxygen levels has recently been compared with regard to its effect on T cells (32–34). At physiological oxygen levels, primary T cells proliferated less in response to CD3/CD28 stimulation, which correlated with higher intracellular nitric oxide levels (33). Furthermore, cytotoxic T cells developed under 2.5% O2 were more lytic but secreted lower amounts of IL-2 and interferon (IFN)-γ (32). Although the effect of hypoxia on DCs has been investigated (35,36), since this is of interest to understand the effect of hypooxygeneation on, for example, DCs in tumor tissue, no data exist on the effect of physiological oxygen levels on the differentiation of human DCs from progenitors and their maturation. Thus, we compared functionally and phenotypically monocyte-derived DCs that have been differentiated under physiological oxygen with those differentiated under atmospheric oxygen conditions with a goal to evaluate if we can generate more potent DCs for tumor immunotherapy.
MATERIALS AND METHODS
Cells
HeLa cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). HeLa cells that express human CD154 (HeLa-CD154) were generated by electroporation of an expression vector containing the full-length human CD154 into HeLa cells, followed by selection and cloning as described (37).
Generation of Human Monocyte–Derived DCs
Peripheral blood mononuclear cells (PBMCs) were isolated from the blood of normal volunteers (San Diego Blood Bank) over a Ficoll-Hypaque (Amersham Biosciences, Uppsala, Sweden) density gradient. To generate DCs, PBMCs were allowed to adhere to culture plates for 1 h. The nonadherent cells were washed off, and the adherent cells were cultured in RPMI 1640 medium supplemented with 2 mmol/L l-glutamine (GIBCO-BRL Life Technologies, Grand Island, NY, USA), 50 μmol/L 2-mercaptoethanol (Sigma, St. Louis, MO, USA), 10 mmol/L HEPES (GIBCO-BRL), penicillin (100 U/mL)-streptomycin (100 μg/mL) (GIBCO-BRL) and 5% human serum (HS; Human AB serum, Gemini Bio Products, West Sacramento, CA, USA), supplemented with 1,000 units GM-CSF/mL (Bayer HealthCare Pharmaceuticals, Wayne, NJ, USA) and 200 units IL-4/mL (R&D Systems, Minneapolis, MN, USA) at days 0, 2 and 4. Immature DCs were harvested on days 5–7. If not otherwise, the DCs were differentiated starting from day 0 under two different oxygen levels. Physiological oxygen tensions, or 5% O2, were generated in a Sanyo MCO-18M O2/CO2 incubator (Sanyo Scientific, Bensenville, IL, USA). Gas phase O2 levels were controlled by continuous injection of medical grade N2 to reach the target oxygen level. DCs cultured in atmospheric oxygen levels (20% O2) were incubated in a standard incubator with-out the addition of N2. All cells were exposed to 5% CO2.
Endocytosis Assay
Immature DCs were collected on day 7 and incubated with 1 mg/mL Dextran-FITC (Molecular Probes, Eugene, OR, USA) for 30 min at 4°C or 37°C. The caps of the tubes were left opened to ensure that the cells were exposed to the respective oxygen levels. DCs were washed twice with 5% HS/RPMI, fixed in 3.7% formaldehyde in phosphate-buffered saline (PBS) (pH 7.2–7.4) and analyzed by flow cytometry using a FACSCalibur (Beckon Dickinson, San Jose, CA, USA). Data were analyzed using the FlowJo 7.2.2 software (Tree Star, Ashland, OR, USA).
Stimulation of DCs
At day 5 of culture, immature DCs were either left untreated (immature [IM]), stimulated with indicated doses of lipopolysaccharide (LPS) (E. coli serotype 026:B6; Sigma) or a cocktail of cytokines (CyC) consisting of TNF-α at 10 ng/mL, IL-1β at 10 ng/mL (all from R&D Systems) and PGE2 at 1 μg/mL (Sigma). At 48 h after stimulation, cell-free culture supernatants were collected, and cytokines were measured by enzyme-linked immunosorbent assay (ELISA) (eBioscience, San Diego, CA, USA).
Analysis of DC Phenotype
The 1 × 104 DCs were incubated for at least 20 min at 4°C in 100 μL PBS/5% fetal calf serum/0.1% sodium azide (staining buffer) with phycoerythrin (PE)-conjugated IgG specific for cluster of differentiation 54 (CD54), human leukocyte antigen (HLA)-DR (all from Becton Dickinson Immunostaining Systems, San Jose, CA, USA), CD83 (Immunotech-Beckman-Coulter, Marseille, France) and CD184 (C-X-C chemokine receptor type 4 [CXCR4]) (BD Biosciences, Franklin Lakes, NJ, USA) or fluorescein isothiocyanate (FITC)-conjugated IgG monoclonal antibody (mAb) specific for CD40 and CD58 (all from Becton Dickinson Immunostaining Systems). Cells were washed 4× with staining buffer, fixed in 3.7% formaldehyde in PBS and examined by flow cytometry using a FACScan(Calibur) (BD Biosciences). In all experiments, isotype controls were included using PE-or FITC-conjugated irrelevant mAb of the same Ig class.
T-Cell Isolation
T cells were isolated by negative selection using the RosetteSep antibody cocktail from StemCell Technologies (Vancouver, CA, USA) according to the manufacturer’s instructions. The purity of the isolated T cells was routinely ~99%.
Mixed Leukocyte Reaction
To assess levels of cellular activation and proliferation, cells were plated at 2 × 105 cells per well in a flat-bottomed 96-well tray at DC:T-cell ratios of 1:10 for 5 d in medium described above. T-cell proliferation was measured using the CellTrace CFSE Cell Proliferation Kit from Invitrogen (Eugene, Oregon, USA). Cells were stained in 0.1% bovine serum albumin/PBS for at least 10 min in a 37°C water bath, washed 3× with culture media and then plated. Cells were harvested on days 2 and 5, fixed in 10% formaldehyde in PBS and analyzed by flow cytometry.
Activation of DCs Using CD154-Expressing Hela Cells
Immature DCs were exposed to media or LPS at day 5 for 24 h. On day 6, immature and LPS matured DCs were collected and cocultured with HeLa or HeLa-CD154 cells. HeLa cells were plated in a flat-bottomed 96-well plate at 1.5 × 104 cells per well 24 h before addition of 2.5 × 105 DCs. After 12 h, cell culture supernatants were collected and IL-12(p70) was measured by ELISA. HeLa-only controls did not contain measurable levels of IL-12.
ELISPOT
Ninety-six–well polyvinylidene fluoride (PVDF) plates (Millipore, Billerica, MA, USA) were coated overnight at 4°C with 5 μg/mL of the primary anti–human IFN-γ mAb (Mabtech, Cincinnati, OH, USA). The antibody-coated plates were washed 5× with PBS and blocked with RPMI 1640 containing 5% human serum for 1 h at 37°C. Immature and mature DCs were pulsed with 100 ng/mL MART-1 peptide (ELAGIGILTV) for 1 h at 37°C. Un-pulsed and peptide-pulsed DCs were cocultured with MART-1 specific T cells (generated in our laboratory from normal donor PBMCs) at a ratio of 103 DCs:105 T cells and incubated overnight (approximately 16–18 h) at 37°C. Enzyme-linked immunosorbent spot (ELISPOT) plates were washed 5× with PBS containing 0.05% Tween-20 followed by a 2-h incubation at room temperature with 1 μg/mL biotinylated anti–IFN-γ mAB (Mabtech). Plates were washed 5× in PBS with 0.1% Tween-20. Streptavidin–horseradish peroxidase (1:500) was added to wells and incubated for 1 h at room temperature. The plates were washed 5× in PBS with 0.1% Tween-20 and then 2× in PBS only, followed by a 2- to 3-min incubation in tetramethylkbenzidine (TMB [Mabtech]) to develop the reaction. Plates were washed with tap water to stop the reaction. IFN-γ-secreting T cells were counted using an automated image analysis ELISpot reader (ImmunoSpot Series 1 Analyzer; Cellular Technology, Cleveland, OH, USA).
Statistical Analysis
Data are represented as mean ± SD. Data were analyzed for statistical significance using a Student t test. P values <0.05 were considered statistically significant.
RESULTS
Immature DCs Differentiated Under Physiological Oxygen Levels Show Decreased Endocytic Activity
Because cell death during the culture period can negatively influence the outcome of an immunization, we first evaluated the yield/recovery of viable DCs after differentiation from blood monocyte precursors at physiological oxygen (physO2) levels or atmospheric oxygen (atmosO2) levels. The yield of viable immature and mature DCs generated under physO2 or atmosO2 levels were comparable (Table 1).
Table 1.
The number of DCs recovered per well at atmospheric and physiological oxygen levels.
| atmosO2 | physO2 | |
|---|---|---|
| Media | 3.3 × 105 ± 2.2 × 105 | 4.1 × 105 ± 2.7 × 105 |
| LPS | 5.5 × 105 ± 3.6 × 105 | 4.5 × 105 ± 1.9 × 105 |
| CyC | 4.4 × 105 ± 2.4 × 105 | 5.4 × 105 ± 2.7 × 105 |
Data are the mean ± SD from five separate donors.
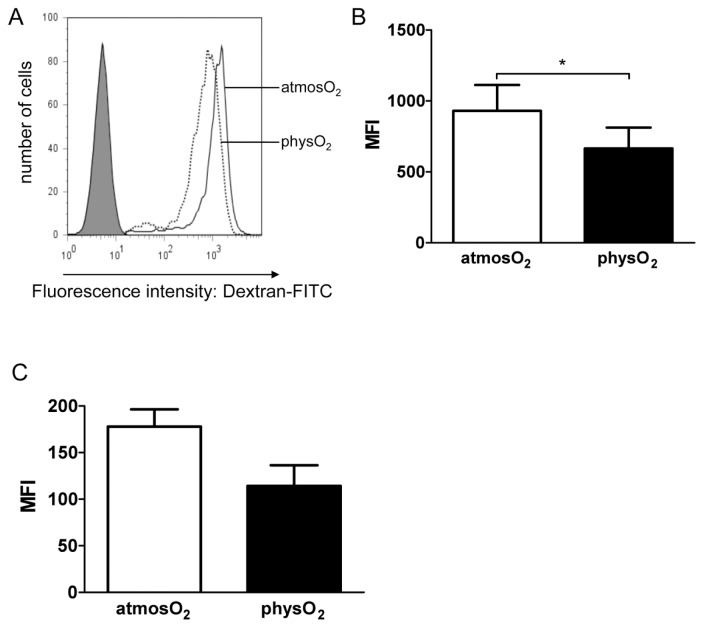
Next, the endocytic capacity of immature DCs differentiated at physO2 or at-mosO2 was compared by measuring the uptake of Dextran-FITC at both oxygen levels. Although 100% of the immature DCs differentiated under the two oxygen conditions took up Dextran-FITC (Figure 1A), DCs differentiated under physO2 conditions endocytosed less Dextran-FITC per DC than atmosO2 DCs when endocytosis took place at atmospheric oxygen levels (Figure 1B, P < 0.05). However, when the uptake of Dextran-FITC was evaluated in physO2 levels, no significant difference was noted between DCs differentiated at physO2 and at atmosO2 (Figure 1C).
Figure 1.
Endocytic capacity of immature DCs generated at atmosO2 and physO2 conditions. Immature DCs differentiated at atmospheric or physiological oxygen levels were harvested and incubated with 1 mg/mL Dextran-FITC for 30 min at 37°C at atmospheric oxygen levels (A, B) or at physiological oxygen levels (C). Cells were subsequently washed, fixed and analyzed by flow cytometry. (A) Immature DCs exposed to media are shown in the shaded histogram as the control. PhysO2 immature DCs (dotted line) and atmosO2 immature DCs (solid line) were exposed to Dextran-FITC at atmosO2. One representative experiment out of two is shown. (B) The mean fluorescence intensities (MFI) ± SD of four experiments, where DCs were exposed to Dextran-FITC at atmosO2 using DCs from different donors. *Statistically significant difference, P < 0.05, paired Student t test. (C) MFI ± SD of four experiments conducted where DCs were exposed to Dextran-FITC at physO2 levels using DCs from different donors. No significance was observed.
DCs Differentiated and Matured Under Atmospheric and Physiological Oxygen Levels Show Similar Expression of Maturation Markers
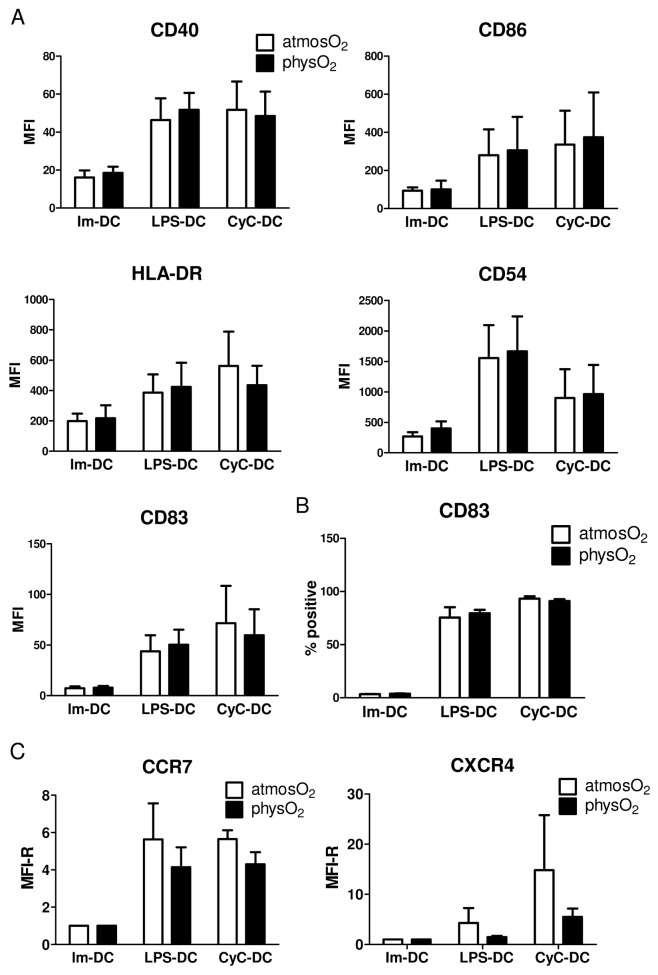
To examine whether oxygen levels influence DC maturation, the expression of cell surface molecules characteristic of DC maturation and functionality was analyzed in DCs that were differentiated under and subsequently matured by exposure to LPS or a cocktail of inflammatory cytokines (CyC: containing PGE2, TNF-α and IL-1β) under physO2 or atmosO2 conditions. As expected, increased expression of CD54, CD40, CD83, CD86 and major histocompatibility complex class II was observed in response to both stimuli (Figure 2). However, no difference in the expression of these molecules was observed between immature or mature physO2 DCs or atmosO2 DCs with regard to mean fluorescence intensity (see Figure 2A) or percentage of cells positive for the respective molecules (see Figure 2B). One of the noted deficiencies in DC-based therapies is the inability of the majority of injected DCs to migrate to the draining lymphoid tissue. Expression of C-C chemokine receptor type 7 (CCR7), a receptor needed for lymph node homing of DCs, was upregulated by LPS and CyC exposure as expected. However, no difference in expression was noted between physO2 DCs or atmosO2 DCs (see Figure 2C). In addition, similar expression of the receptor CXCR4 was seen in immature DCs differentiated at both oxygen levels. Although mature atmosO2 DCs showed higher expression of CXCR4, the difference was not significant (see Figure 2C).
Figure 2.
DCs differentiated and activated under atmosO2 and physO2 conditions show similar levels of surface molecule expression. Immature DCs were differentiated at atmosO2 and physO2 for 5 d. Subsequently, DCs were exposed to 10 ng/mL LPS (LPS-DC), a cytokine cocktail (CyC) containing IL-1β/TNF-α/PGE2 (CyC-DC) or media only (Im-DCs) for 48 h. DCs were collected, stained and analyzed by flow cytometry for the expression of the depicted surface molecules (A). Data shown are the MFI ± SD from three independent experiments using DCs from different donors. (B) The percentage of CD83-positive DCs. Data shown are mean ± SD from three independent experiments using DCs from different donors. (C) Data shown are the MFI ± SD from five independent experiments using DCs from different donors.
Mature DCs Differentiated Under Physiological Oxygen Secrete Larger Amounts of IL-12
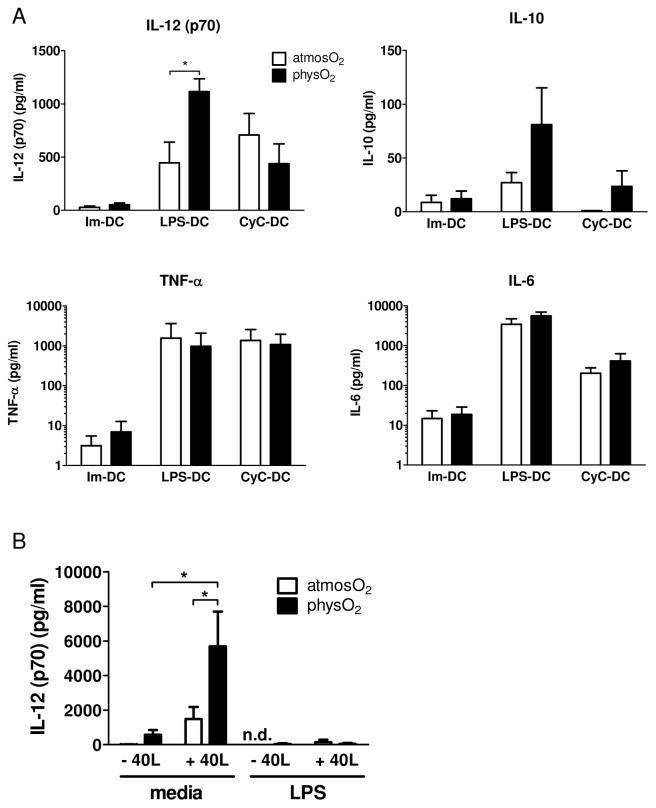
In addition to expression changes in cell surface molecules, mature DCs are also characterized by the secretion of inflammatory cytokines promoting the immune response. DCs differentiated under physO2 or atmosO2 when exposed to either LPS or CyC showed increased production of IL-6, IL-12(p70) and TNF-α under both conditions (Figure 3). No significant difference was observed in IL-6, TNF-α and IL-10 secretion between immature or mature DCs differentiated at the different oxygen levels. However, physO2 DCs showed a significant increase in IL-12(p70) expression after exposure to LPS but not CyC (see Figure 3A). Although DCs differentiated at physO2 or atmosO2 conditions show similar expression of the costimulatory molecule CD40, we wanted to determine the influence of oxygen levels on CD40 stimulation with CD40L, which is mimicking the receptor and ligand interaction occurring between DCs and T cells. DCs were differentiated and LPS matured at the different oxygen levels and was subsequently cocultured with HeLa or HeLaCD154 cells. Significantly higher levels of IL-12(p70) secretion were observed in physO2 DCs after stimulation with CD40L (see Figure 3B). DCs that were exposed prior to LPS for 24 h, washed and subsequently stimulated with CD40L showed reduced secretion of IL-12(p70) compared with those only exposed to CD40L (see Figure 3B). This result is consistent with findings by Sinistro et al., showing that LPS desensitizes macrophages to CD40L stimulation (38). PhysO2 DCs stimulated with LPS for 24 h, washed and cultured for an additional 12 h after the wash in new media showed low levels of IL-12(p70) (42.9 pg/mL) (see Figure 3B, sixth bar from left) in contrast to DCs stimulated in the presence of LPS for 48 h (see Figure 3A). Because IL-12(p70) production peaks around 12–18 h, the reason is most likely that after the removal of all accumulated IL-12 24 h after the first stimulation, no significant amount of additional IL-12 was released in the following 12 h of culture, which is 36 h after the initial stimulation.
Figure 3.
Effect of oxygen levels on the cytokine profile of immature and mature DCs. (A) DCs were differentiated and activated as above with LPS or CyC. Cell culture supernatants were collected at 48 h, and the presence of the indicated cytokines and chemokines was measured by ELISA. Results shown are mean ± SD of six independent experiments using DCs from different donors. *Statistically significant difference; P < 0.05, paired Student t test. (B) Immature DCs differentiated at physO2 showed higher secretion of IL-12 when stimulated with CD40L. DCs were differentiated and LPS matured at both oxygen levels and then were cocultured with HeLa (data not shown) or HeLa CD154 cells for 12 h. Sups were collected and IL-12p(70) was measured by ELISA. Results are of six independent experiments using DCs generated from different donors. n.d., IL-12 was not detected in those samples.
DCs Differentiated Under Physiological or Atmospheric Oxygen Levels Induce Similar Levels of T-Cell Activation
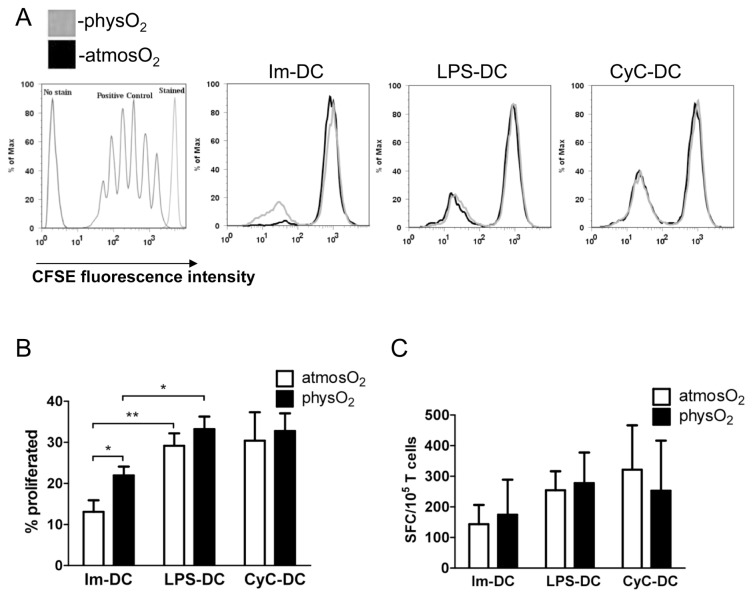
Mature cytokine-producing DCs elicit T-cell activation and proliferation, leading to the development of adaptive immunity (38,39). DCs differentiated and matured under physO2 or atmosO2 conditions were cocultured with allogeneic T cells for 3 d (data not shown) and 5 d (Figures 4A, B). As expected, increased T-cell proliferation was observed with mature DCs compared with immature DCs at day 5 of the coculture at both oxygen levels. Interestingly, immature physO2 DCs induced significantly higher proliferation in allogeneic T cells than immature atmosO2 DCs when the proliferation was performed at atmospheric oxygen levels. Immature physO2 DCs induced proliferation in 22 ± 4% of T cells and immature atmosO2 DCs in 13 ± 5% of T cells (see Figures 4A, B). However, no difference was noted in mature DCs.
Figure 4.
DCs differentiated at atmosO2 and physO2 induced similar levels of T-cell activation. Immature and mature atmosO2 DCs and physO2 DCs were analyzed for the ability to stimulate allogeneic T-cell proliferation in a mixed lymphocyte reaction (MLR). Allogeneic T cells were stained with carboxyfluorescein succinimidyl ester (CFSE) and cocultured with IM-DCs, LPS-DCs or CyC-DCs for 5 d at atmosO2. Cells were collected and T-cell proliferation was analyzed by flow cytometry gating on lymphocytes. (A) A representative result of three is shown. (B) The mean ± SD percentage of proliferating allogeneic T cells from three independent experiments using DCs and T cells from different donors. *Statistically significant difference, P < 0.05, Student t test. (C) Immature and mature DCs were generated and activated as above from HLA-A*0201–positive donors, pulsed with 100 ng/mL HLA-A*0201–specific MART1 peptide and cocultured with a MART-1–specific T-cell clone at a DC:T-cell ratio of 1:100 for 18 h at atmosO2. Controls included non–peptide-pulsed DCs. The number of spot-forming cells (SFCs) for IFN-γ secretion was assessed by ELISPOT. Data are shown as mean ± SD of the number of SFCs per 105 T cells from three independent experiments.
To examine whether antigen-specific CD8+ T-cell activation was affected by DCs differentiated under different oxygen conditions, we used HLA-A*0201–positive donors. Immature DCs, LPS-DCs and CyC-DCs generated at the different oxygen conditions were pulsed with MART-1 peptide and cocultured with a MART-1–specific CD8+ T-cell clone for 18 h, and secretion of IFN-γ was measured by ELISPOT (see Figure 4C). The result shows that oxygen levels had no influence on the assay. No difference was observed between immature physO2 or atmosO2 DCs or mature physO2 or at-mosO2 DCs with regard to their ability to induce MART1-specific T-cell responses.
DISCUSSION
Because DCs are the most potent initiators of antigen-specific T-cell responses, they have been applied toward immunotherapy of cancer and chronic infectious diseases. For DC-based adoptive immunotherapy, DCs are differentiated ex vivo from CD14+ or CD34+ progenitors, pulsed with tumor antigen, exposed to a maturation stimulus and reinjected back into patients (7,40). For these studies, DCs were differentiated in CO2 incubators containing atmospheric oxygen levels of 20–21%. However, these levels are two to four times higher than physiological oxygen levels, which are approximately 12% in arterial blood and 3–5% in tissue (30–32), where most DCs reside.
In light of a recent finding showing altered T-cell responses at atmosO2 compared with physO2 (33), we sought to determine if differentiation of DCs from CD14+ progenitors under physiological oxygen levels would alter their physiology and antigen-presenting capacity, with the hope to identify improved conditions for DC-based studies. Surprisingly, no difference in expression of the surface molecules CD54, CD40, CD83, CD86, HLA-DR, CXCR4 and CCR7 was observed in immature DCs or mature DCs. In addition, the secretion of TNF-α, IL-6 and IL-10 from either immature or mature DCs did not differ between the different oxygen culture conditions.
We found that LPS-matured physO2 DCs secreted higher levels of IL-12(p70) than LPS-matured atmosO2 DCs. IL-12 is an important cytokine mediating Th1 polarization of CD4+ T cells, which provides help for the activation of cytotoxic CD8+ T cells. Although physO2 DCs did not elicit increased CD8+ T-cell responses in vitro, as measured by IFN-γ secretion, this could be due to the use of a CD8+ T-cell clone, which is easier to activate than, for instance, naive T cells. Another possibility is that the levels of IL-12 secreted in the two different oxygen conditions were sufficiently high to support T-cell activation; thus, no difference could be observed. The increased capacity of immature physO2 DCs to activate allogeneic T cells could be due to their increased secretion of IL-12 after ligation with CD40L on the T cells, as seen when DCs were stimulated by CD40L.
We found that the cytokine cocktail–matured DCs, when generated under physiological or atmospheric oxygen conditions, did not differ with regard to their ability to induce proliferation of allogeneic T cells or activation of antigen-specific CD8+ T cells. It was recently shown that cytokine cocktail–matured DCs can also induce immunosuppressive regulatory T cells (Tregs) (41), and it remains to be tested what effect oxygen levels will have on the induction of Tregs by DCs.
Overall, we show that DCs differentiated and stimulated with LPS or CD40L under physO2 conditions secreted higher amounts of IL-12(p70), suggesting that it may be more beneficial to culture and activate DCs at physO2 levels because higher IL-12 secretion may cause a more pronounced T-cell response in vivo. Overall, only a few differences were observed owing to oxygen levels and other parameters, such as the DC subtype, the maturation stimulus, or the presence of the immunosuppressive microenvironment of the tumor are more likely influencing the poor clinical outcome of the currently used adoptive DC immunotherapy protocols.
ACKNOWLEDGMENTS
This work was supported by the U.S. Army Medical Research and Materiel Command under agreement number W81XWH-07-1-0412 to D Messmer, the Swedish Research Council AI52731 and the Swedish International Development Cooperation Agency (SIDA and VINNMER [Vinnova]) to M Larsson. The authors thank Jessie F Fecteau and Dan Seible for the critical reading of the manuscript.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–26. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 2.Steinman RM. Dendritic cells: understanding immunogenicity. Eur. J. Immunol. 2007;37(Suppl 1):S53–60. doi: 10.1002/eji.200737400. [DOI] [PubMed] [Google Scholar]
- 3.Steinman RM, Mellman I. Immunotherapy: bewitched, bothered, and bewildered no more. Science. 2004;305:197–200. doi: 10.1126/science.1099688. [DOI] [PubMed] [Google Scholar]
- 4.Dhodapkar KM, Banerjee D, Steinman RM. Harnessing the immune system against human glioma. Ann N Y Acad Sci. 2005;1062:13–21. doi: 10.1196/annals.1358.003. [DOI] [PubMed] [Google Scholar]
- 5.Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15:138–47. doi: 10.1016/s0952-7915(03)00015-3. [DOI] [PubMed] [Google Scholar]
- 6.Gilboa E. The promise of cancer vaccines. Nat Rev Cancer. 2004;4:401–11. doi: 10.1038/nrc1359. [DOI] [PubMed] [Google Scholar]
- 7.Gilboa E. DC-based cancer vaccines. J Clin Invest. 2007;117:1195–203. doi: 10.1172/JCI31205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5:296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 9.Nestle FO, Farkas A, Conrad C. Dendritic-cell-based therapeutic vaccination against cancer. Curr Opin Immunol. 2005;17:163–9. doi: 10.1016/j.coi.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Steinman RM, Pope M. Exploiting dendritic cells to improve vaccine efficacy. J Clin Invest. 2002;109:1519–26. doi: 10.1172/JCI15962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palucka AK, et al. Boosting vaccinations with peptide-pulsed CD34+ progenitor-derived dendritic cells can expand long-lived melanoma peptide-specific CD8+ T cells in patients with metastatic melanoma. J Immunother. 2005;28:158–68. doi: 10.1097/01.cji.0000154249.74383.17. [DOI] [PubMed] [Google Scholar]
- 12.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–8. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palucka AK, et al. Single injection of CD34+ progenitor-derived dendritic cell vaccine can lead to induction of T-cell immunity in patients with stage IV melanoma. J Immunother. 2003;26:432–9. doi: 10.1097/00002371-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Dhodapkar MV, et al. Rapid generation of broad T-cell immunity in humans after a single injection of mature dendritic cells. J Clin Invest. 1999;104:173–80. doi: 10.1172/JCI6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu JS, et al. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64:4973–9. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]
- 16.Lee WC, et al. Vaccination of advanced hepatocellular carcinoma patients with tumor lysate-pulsed dendritic cells: a clinical trial. J Immunother. 2005;28:496–504. doi: 10.1097/01.cji.0000171291.72039.e2. [DOI] [PubMed] [Google Scholar]
- 17.Chang AE, et al. A phase I trial of tumor lysate-pulsed dendritic cells in the treatment of advanced cancer. Clin Cancer Res. 2002;8:1021–32. [PubMed] [Google Scholar]
- 18.Su Z, et al. Telomerase mRNA-transfected dendritic cells stimulate antigen-specific CD8+ and CD4+ T cell responses in patients with metastatic prostate cancer. J Immunol. 2005;174:3798–807. doi: 10.4049/jimmunol.174.6.3798. [DOI] [PubMed] [Google Scholar]
- 19.Van Tendeloo VF, et al. Highly efficient gene delivery by mRNA electroporation in human hematopoietic cells: superiority to lipofection and passive pulsing of mRNA and to electroporation of plasmid cDNA for tumor antigen loading of dendritic cells. Blood. 2001;98:49–56. doi: 10.1182/blood.v98.1.49. [DOI] [PubMed] [Google Scholar]
- 20.Nair SK, et al. Induction of primary carci-noembryonic antigen (CEA)-specific cytotoxic T lymphocytes in vitro using human dendritic cells transfected with RNA. Nat Biotechnol. 1998;16:364–9. doi: 10.1038/nbt0498-364. [DOI] [PubMed] [Google Scholar]
- 21.Gilboa E, Vieweg J. Cancer immunotherapy with mRNA-transfected dendritic cells. Immunol Rev. 2004;199:251–63. doi: 10.1111/j.0105-2896.2004.00139.x. [DOI] [PubMed] [Google Scholar]
- 22.Bubenik J. Genetically engineered dendritic cell-based cancer vaccines (Review) Int J Oncol. 2001;18:475–8. [PubMed] [Google Scholar]
- 23.Steinman RM. The control of immunity and tolerance by dendritic cell. Pathol Biol (Paris) 2003;51:59–60. doi: 10.1016/s0369-8114(03)00096-8. [DOI] [PubMed] [Google Scholar]
- 24.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 25.De Vries IJ, et al. Effective migration of antigen-pulsed dendritic cells to lymph nodes in melanoma patients is determined by their maturation state. Cancer Res. 2003;63:12–7. [PubMed] [Google Scholar]
- 26.Schuler-Thurner B, et al. Rapid induction of tumor-specific type 1 T helper cells in metastatic melanoma patients by vaccination with mature, cryopreserved, peptide-loaded monocyte-derived dendritic cells. J Exp Med. 2002;195:1279–88. doi: 10.1084/jem.20012100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jonuleit H, et al. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27:3135–42. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 28.Palucka AK, et al. Dendritic cells loaded with killed allogeneic melanoma cells can induce objective clinical responses and MART-1 specific CD8+ T-cell immunity. J Immunother. 2006;29:545–57. doi: 10.1097/01.cji.0000211309.90621.8b. [DOI] [PubMed] [Google Scholar]
- 29.O’Rourke MG, et al. Durable complete clinical responses in a phase I/II trial using an autologous melanoma cell/dendritic cell vaccine. Cancer Immunol Immunother. 2003;52:387–95. doi: 10.1007/s00262-003-0375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell JA. The influence of O(2)- tension in the inspired air upon the O(2)-tension in the tissues. J Physiol. 1925;60:20–9. doi: 10.1113/jphysiol.1925.sp002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laser H. Tissue metabolism under the influence of low oxygen tension. Biochem J. 1937;31:1671–6. doi: 10.1042/bj0311671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caldwell CC, et al. Differential effects of physiologically relevant hypoxic conditions on T lymphocyte development and effector functions. J Immunol. 2001;167:6140–9. doi: 10.4049/jimmunol.167.11.6140. [DOI] [PubMed] [Google Scholar]
- 33.Atkuri KR, Herzenberg LA, Niemi AK, Cowan T. Importance of culturing primary lymphocytes at physiological oxygen levels. Proc Natl Acad Sci U S A. 2007;104:4547–52. doi: 10.1073/pnas.0611732104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atkuri KR, Herzenberg LA. Culturing at atmospheric oxygen levels impacts lymphocyte function. Proc Natl Acad Sci U S A. 2005;102:3756–9. doi: 10.1073/pnas.0409910102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang M, et al. Hypoxia skews dendritic cells to a T helper type 2-stimulating phenotype and promotes tumour cell migration by dendritic cell-derived osteopontin. Immunology. 2009;128:e237–49. doi: 10.1111/j.1365-2567.2008.02954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Q, et al. Reoxygenation of hypoxia-differentiated dentritic cells induces Th1 and Th17 cell differentiation. Mol Immunol. 2010;47:922–31. doi: 10.1016/j.molimm.2009.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chu P, Wierda WG, Kipps TJ. CD40 activation does not protect chronic lymphocytic leukemia B cells from apoptosis induced by cytotoxic T lymphocytes. Blood. 2000;95:3853–8. [PubMed] [Google Scholar]
- 38.Sinistro A, et al. Lipopolysaccharide desensitizes monocytes–macrophages to CD40 ligand stimulation. Immunology. 2007;122:362–70. doi: 10.1111/j.1365-2567.2007.02648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 40.Osada T, Clay TM, Woo CY, Morse MA, Lyerly HK. Dendritic cell-based immunotherapy. Int Rev Immunol. 2006;25:377–413. doi: 10.1080/08830180600992456. [DOI] [PubMed] [Google Scholar]
- 41.Banerjee DK, Dhodapkar MV, Matayeva E, Stein-man RM, Dhodapkar KM. Expansion of FOXP3high regulatory T cells by human dendritic cells (DCs) in vitro and after injection of cytokine-matured DCs in myeloma patients. Blood. 2006;108:2655–61. doi: 10.1182/blood-2006-03-011353. [DOI] [PMC free article] [PubMed] [Google Scholar]