Abstract
A rapid recruitment of neutrophils to sites of injury or infection is a hallmark of the inflammatory response and is required for effective host defense against pathogenic stimuli. However, neutrophil-mediated inflammation can also lead to chronic tissue destruction; therefore, a better understanding of the mechanisms underlying neutrophil influx and activation is of critical importance. We have previously shown that the acute phase protein α1-antitrypsin (AAT) inhibits neutrophil chemotaxis. In this study, we examine mechanisms related to the effect of AAT on neutrophil responses. We report a previously unknown function of AAT to inactivate calpain I (μ-calpain) and to induce a rapid cell polarization and random migration. These effects of AAT coincided with a transient rise in intracellular calcium, increase in intracellular lipids, activation of the Rho GTPases, Rac1 and Cdc42, and extra-cellular signal-regulated kinase (ERK1/2). Furthermore, AAT caused a significant inhibition of nonstimulated as well as formyl-met-leu-phe (fMLP)-stimulated neutrophil adhesion to fibronectin, strongly inhibited lipopolysaccharide-induced IL-8 release and slightly delayed neutrophil apoptosis. The results presented here broaden our understanding of the regulation of calpain-related neutrophil functional activities, and provide the impetus for new studies to define the role of AAT and other acute phase proteins in health and disease.
INTRODUCTION
Neutrophils are early responders to inflammatory stimuli, and their response involves adhesion to the microvasculature, subsequent migration into the affected tissue and the retention within tissue. Understanding the mechanisms underlying the recruitment of neutrophils to inflammatory sites and modulation of their function is of critical importance for the prevention of neutrophil-induced tissue injury and the resultant organ dysfunction.
Acute-phase proteins (APPs) play an important defensive role that is linked to the magnitude and rapidity of changes in their concentrations, together with their short half-life (1). Current knowledge clearly indicates that diverse APPs regulate neutrophil activities. For example, α1-antitrypsin (AAT)—which is an archetypal member of the SERPIN superfamily, a main inhibitor of neutrophil elastase (2) and an α1 acid glycoprotein, a member of the lipocalin family, carrying hydrophobic molecules (3)—inhibits formyl-met-leu-phe (fMLP) or interleukin (IL)-8–induced neutrophil activation, chemotaxis and adhesion; induces macrophage-derived IL-1 receptor antagonist release; and protects mice from endotoxin-induced septic shock (4–10). Similarly, C-reactive protein (opsonin) and haptoglobin (hemoglobin binder) inhibit neutrophil chemotaxis, superoxide production and degranulation (11–13). Together, these findings strengthen the role of APPs in neutrophil-mediated inflammation and encourage new studies regarding the mechanisms by which APPs regulate neutrophil function.
AAT is one of the major APPs in humans and it is used for the augmentation therapy of patients with AAT deficiency–related emphysema. Both clinical and experimental studies in animals have shown that AAT augmentation therapy decreases neutrophil infiltration during acute and chronic inflammation (14,15). AAT has also been shown to reduce neutrophil infiltration into kidneys during ischemia/reperfusion (10). Until recently, the most beneficial effects of AAT were attributed to its inhibition of neutrophil elastase activity (16). However, novel studies show that AAT directly inhibits the activity of caspase-3, an intracellular cysteine protease that plays an essential role in cell apoptosis (17,18), and the catalytic domain of matriptase (19), a cell surface serine protease involved in the activation of epithelial sodium channels. A recent study by Bergin et al. (20) provided new evidence that AAT is associated with neutrophil membrane lipid rafts, interacting with the glycosylphosphatidylinositol-linked (GPI-linked) membrane protein FcγRIIIb and modulates neutrophil chemotaxis in response to soluble immune complexes by inhibiting ADAM-17 activity, also called tumor necrosis factor-α–converting enzyme (TACE). Thus, AAT has broader antiprotease and neutrophil-regulating activities than previously anticipated.
In our present investigations, we discovered that in the absence of any exogenous stimuli, AAT inactivates calpain I (μ-calpain) and concomitantly induces random neutrophil migration and polarization and inhibits neutrophil adhesion to fibrinogen. We believe that this finding broadens our knowledge of AAT as an inhibitor of various proteases and suggests the use of AAT therapy in relevant clinical indications.
MATERIALS AND METHODS
AAT Preparations
We used purified human plasma pooled AAT (Prolastin®) HS (Talecris Biotherapeutics, Research Triangle Park, NC, USA). Before the experiments, to remove polymers of AAT and possible contaminations of lower molecular size, Prolastin® was repurified by ultrafiltration using a centricon-30 and centricon-100 cutoff, diluted in phosphate-buffered saline (PBS) and stored at −80°C.
In the same experiments, highly purified human plasma AAT protein purchased from Calbiochem (San Diego, CA, USA) was used, or recombinant AAT was expressed in the novel human neuronal cell line AGE1.HN® (a gift from Dr. Véronique Blanchard: Glycodesign and Glycoanalytics, Central Institute of Laboratory Medicine and Pathobiochemistry, Charité Medical University, Berlin, Germany). AAT preparations were also tested for quality to control endotoxin contamination using the limulus amebocyte lysate endochrome kit (Charles River Endosafe, Charleston, SC, USA). Endotoxin levels were <0.1 enzyme units/mg protein in all preparations used.
Neutrophil Isolation
Human neutrophils were isolated from the peripheral blood of healthy volunteers using Polymorphprep (Axis-Shield PoC AS, Oslo, Norway) according to the manufacturer’s recommendations as previously described (9). The neutrophil purity was typically ≥90%, judged by examination of cytospins, and cell viability exceeded 97% according to staining with 0.4% trypan blue solution staining (Sigma-Aldrich, St. Louis, MO, USA).
Determination of Calpain Activity
Calpain I activity was determined by using two different Calpain Activity Assay Kits (QIA120, Calbiochem, and ab65308, Abcam, Cambridge, MA, USA) according to the manufacturers’ recommendations. Briefly, highly purified human calpain I was incubated with various concentrations of AAT (from 0.05 to 2 mg/mL) and the substrate Suc Leu-Leu-Val-Tyr-AMC (Suc-LLVY-AMC) for 15 min at room temperature, and fluorescence was measured using a fluorescence plate reader (Spectrafluor Plus Fluorimeter, Tecan, Germany) at an excitation wavelength of 380 nm and an emission wavelength of 460 nm. Human blood neutrophils (3 × 105 cells/mL) were incubated for 20 min at 37°C 5% CO2 either alone or in the presence of various amounts of AAT (from 0.25 to 1 mg/mL). Subsequently, the cells were lysed and incubated with substrate Ac-Leu-Leu-Tyr-AFC (Ac-LLY-AFC) at 37°C for 1 h in the dark. Active calpain I was used as a positive control. Fluorescence was measured at an excitation wavelength of 400 nm and emission of 505 nm using a fluorescence plate reader.
Neutrophil Adhesion Assay
The adherence of calcein-labeled neutrophils to human fibroblast cellular fibronectin (Sigma-Aldrich) was measured by a method modified from that of Howard et al. (21). Purified neutrophils (5 × 106 cells/mL) were labeled in suspension in PBS supplemented with 5 μg/mL calcein-AM (Molecular Probes, Van Allen Way, CA, USA) at 37°C. Labeled neutrophils were then washed with PBS and resuspended at 2 × 106 cells/mL in RPMI supplemented with bovine serum albumin. Aliquots of cells (100,000 cells/well) were then added to the fibronectin-coated plates containing either AAT (0.5 mg/mL), fMLP (100 nmol/L), an AAT/fMLP combination or medium alone. The plates were then incubated at 37°C for 25 min under static conditions. Nonadherent cells were aspirated, and the adherent cells were analyzed with a fluorescence spectrophotometer (Spectrafluor Plus Fluorimeter, Tecan, Germany) using an excitation λ of 485 nm and emission λ of 520 nm. Triplicate determinations were used for each condition in three independent experiments.
Time-Lapse Recording and Analysis of Cell Migration
Cells (3.5 × 105/mL) suspended in RPMI were plated in a glass-bottom dish (MatTek, Ashland, MA, USA), precoated with fetal calf serum to prevent spontaneous neutrophil adherence to the glass surface. Cell migration was monitored at 30°C using a digital image correlation (DIC) setup on an OlympusX81 inverted microscope with a 63× oil immersion objective. The videos were recorded with a temporal resolution of 5 s using a Hamamatsu ORCA-R2 charge-coupled device (CCD) camera and Cell-R software (Hamamatsu Photonics Deutschland GmbH, Herrsching am Ammersee, Germany). Cell migration rates were determined using Time-Lapse Analyzer software (22). Tracks of the cells that remained rounded after stimulation were manually excluded from the analysis. Values were expressed as mean ± SD.
Determination of Actin Reorganization and Analysis of Lipid Bodies
Actin reorganization was analyzed using confocal laser-scanning microscopy. Neutrophils (1 × 107/mL, 200 μL) suspended in RPMI were treated with AAT (0.5 mg/mL) for different lengths of time at 37°C. After incubation, cells were fixed with 4.4% paraformaldehyde and permeabilized with 0.2% Triton X-100 in PBS. Actin filaments were labeled with phalloidin conjugated with AlexaFluor 488 (Invitrogen Life Technologies, Carlsbad, CA, USA) followed by DNA staining with 2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride (DAPI) (Sigma-Aldrich). Specimens were analyzed using an Olympus FluoView confocal laser scanning microscope in a sequential scanning mode for three channels. The third channel was used to record the intrinsic fluorescence of lipid droplets excited with the 559-nm laser line with the emission barrier filter from 575 to 675 nm.
Intracellular Free Cholesterol Detection
Human neutrophils were seeded into glass coverslips alone or in the presence of AAT (0.5 mg/mL) for various time points (5, 15 and 30 min and 1 h). Cells were fixed with 4% formaldehyde. The intracellular free cholesterol was stained with filipin III for 45 min according to the recommendation of the manufacturer (Cholesterol Cell-Based Detection Assay Kit, Cayman Chemical Company, Ann Arbor, MI, USA). The images were recorded using an excitation of 340–380 nm and an emission of 385–470 nm with an Olympus inverted fluorescence microscope with a 63× oil immersion objective in an Olympus ×81 Cell-R epifluorescence microscope equipped with a Hamamatsu ORCA-R2 CCD camera. The filipin fluorescence was visualized using the DAPI settings from the DAPI/FITC/Cy3 filter set (Chroma Technology, Bellow Falls, VT, USA) and recorded with equal exposure time for all samples.
Red Oil O Staining
Neutrophils were plated on coverslips alone and in the presence of AAT (0.5 mg/mL) for 5, 15 and 30 min and 2 h. At the end of the incubation period, cells were washed with PBS and fixed with 4% PBS-buffered formaldehyde. In the next step, cells were rinsed with water, dipped for a few seconds in 60% isopropanol and stained with Red Oil O. Cell nuclei were stained with hematoxylin. Samples were analyzed under the microscope (Zeiss Axiophot, Jena, Germany). Images were taken using a digital camera Olympus DP71 at a magnification of 63×.
Calcium Analysis
Neutrophils were incubated for 30 min at 37°C with the acetometoxy ester of fluo-3 (Fluo-3 AM, 2 μmol/L). After incubation, cells were centrifuged and resuspended at approximately 3 × 106 cells/mL in 10 mmol/L HEPES [4-(2- hydroxyethyl)-1-piperazineethanesulfonic acid], pH 7.4, without or with 1 mmol/L CaCl2. The cell suspension was kept at room temperature until use. Fluo-3 fluorescence was kinetically analyzed during addition of various concentrations of AAT by means of flow cytometry (FACSCalibur; Becton Dickinson, USA). We used excitation at 488 nm and measured emission at 530 nm. Neutro phils were gated on the basis of their scatter profile, and Fluo-3 fluorescence was measured logarithmically. Data analysis was performed using Summit V5.2 software (Becton Dickinson, Franklin Lakes, NJ, USA).
Electrophoresis and Western Blot Analysis
Neutrophils were incubated alone or with AAT for different lengths of time up to 2 h and lysed, and the protein concentration in the lysates was determined by Bradford assay. Equal amounts of analyzed protein were separated to 7.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel. For the assessment of calpain activity, neutrophils were treated with AAT (0.5 mg/mL) for 18 h and analyzed using 6.5% SDS-PAGE as described by New-comb et al. (23). Proteins were transferred to a polyvinylidene fluoride (PVDF) membrane and detected using the following primary antibodies: anti–α-spectrin mono clonal antibody (Millipore, Billerica, MA, USA); anti–extracellular signal- regulated kinase (ERK)-1/2 anti–mitogen activated protein kinase (MAP kinase [MAPK], ERK1/2, catalog no. M5670); mouse monoclonal anti–p-ERK1/2 (MAPK-YT, Cat. No. M8159 [Sigma-Aldrich]); and rabbit polyclonal anti–Rac 1 (C-14) (sc-217), mouse monoclonal anti-Cdc42 (B-9) (Sc-8401), mouse monoclonal anti–CD16 IgG1 (3H1029, sc-70553) and polyclonal goat anti-actin (I-19 sc:1616) (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The immuno-complexes were visualized with appropriate secondary horseradish peroxidase–conjugated antibodies (DAKO A/S, Denmark) and developed with the enhanced chemiluminescence (ECL) Western blot analysis system (Thermo Fisher Scientific, Rockford, IL, USA).
RNA Isolation, cDNA Synthesis and Real-Time Polymerase Chain Reaction
Neutrophils were isolated from six donors and treated with either AAT (0.5 mg/mL) or PBS (as a control) for 30 min, and RNA was prepared using the RNeasy Micro kit (Qiagen Sample and Assay Technologies, Qiagen Inc., Valencia, CA, USA). For cDNA synthesis, 1 μg total RNA was transcribed using the High Capacity Kit RNA to cDNA from Applied Biosystems (Darmstadt, Germany) after the protocol supplied by the manufacturer. mRNA levels of ERK were determined using TaqMan Gene Expression Assays™ and TaqMan Mastermix™ from Applied Biosystems following the protocol supplied by the manufacturer. As endogenous references, the mRNA levels of GAPDH, GUS and Pol2a were measured in the same run, also using TaqMan Gene Expression Assays™ and TaqMan Mastermix™. All real-time polymerase chain reaction experiments were performed on an ABI7500 from Applied Biosystems. For calculation of relative mRNA expression levels, the target gene was normalized in a first step to the mean of the endogenous controls (calculation of ΔCT values). The ΔCT value of the control sample of individual number 1 was set equal to a relative expression level of 1. All (n = 6) individual measurements were normalized in a second step to this value. All measurements were performed in duplicate with two independent cDNA preparations.
IL-8 Assay
Neutrophils were resuspended in RPMI 1640 medium at a concentration of 3 × 106 cells/mL and stimulated with lipopolysaccharide (LPS) (10 ng/mL), AAT (0.5 mg/mL) alone and LPS/AAT together for 4 h at 37°C, 5% CO2. Supernatants of cell cultures were analyzed to determine the levels of IL-8 using DuoSet enzyme-linked immunosorbent assay sets (R&D Systems Inc., Minneapolis, MN, USA); detection limits 31.25 pg/mL).
Apoptosis Assay
Neutrophil apoptosis was monitored using an Annexin V–FITC (fluorescein isothiocyanate) apoptosis detection kit (BD Biosciences Pharmingen, San Diego, CA, USA) according to the protocol provided by the manufacturer. Cells were analyzed by flow cytometry (FACS -Calibur). Unstained cells, and cells stained with FITC Annexin V or propidium iodine (PI), were used as controls.
Statistical Analysis
The differences in the means of experimental results were analyzed for their statistical significance using one-way analysis of variance combined with a multiple-comparison procedure (Scheffe multiple range test), with an overall significance level of α = 0.05. An independent two-sample t test was also used. A statistical package (SPSS for Windows, release 17.0) was used for the statistical calculations.
RESULTS
AAT Inhibits Activity of Calpain I
Previous studies have shown that AAT inhibits fMLP-mediated neutrophil adhesion and directed migration (9). On the other hand, it is well documented that a constitutively active calpain I is expressed in human neutrophils, and, as a result of the inhibition of calpain I, neutrophils exhibit decreased adhesion and enhanced chemokinesis (24,25). This prompted us to investigate whether the effects of AAT on neutrophils are linked to inactivation of calpain I. In the first set of experiments, we pre-incubated neutrophils with AAT (0.25–1 mg/mL) for 30 min and measured calpain I activity in cell lysates using the Ac-LLY-AFC substrate. As illustrated in Figure 1, the treatment of neutrophils with various concentrations of AAT resulted in a significant suppression of calpain I activity. Under these experimental conditions, the activity of cellular calpain I was inhibited by 40 ± 5.6% and 96 ± 3% [mean (SD), n = 6] with the specific calpain I inhibitor z-Leu-Leu-Tyr-fluoromethyl ketone (z-LLY-FMK) (10 nmol/L) and AAT (19.2 μmol/L), respectively.
Figure 1.
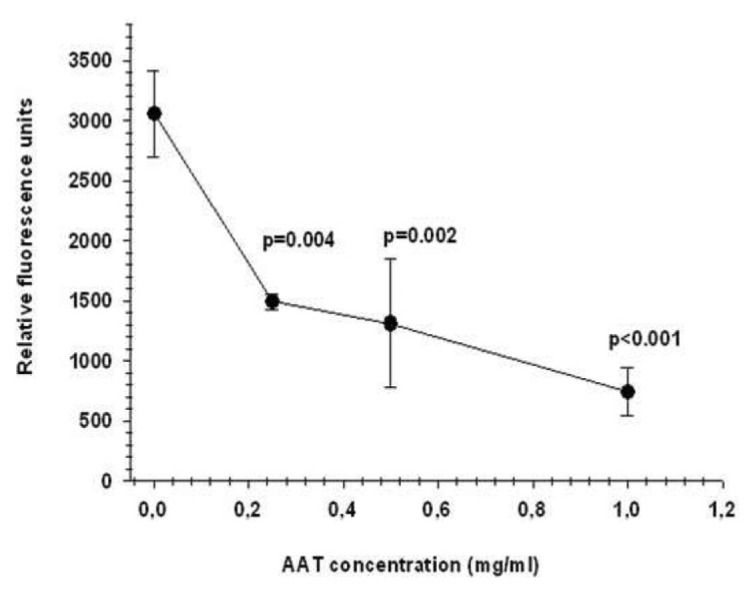
AAT inhibits neutrophil calpain I activity. Dose-dependent inhibitory effects of AAT on the enzymatic activity (expressed as relative fluorescence units) of calpain I on the specific substrate Ac-LLY-AFC in human neutrophils are shown. The fluorescence value of all samples is corrected by subtracting the value of the blank and then calculating the mean fluorescence value (mean ± SEM) for each sample from duplicate readings from six independent experiments.
In the next set of experiments, highly purified active human calpain I (25 μmol/L) was pre-incubated with vehicle (PBS) or AAT for 15 min, and cleavage of the calpain substrate, Suc-LLVY-AMC, was measured fluorometrically. AAT inhibited the activity of cal-pain I in a concentration-dependent manner by up to 90% (Figure 2A). We also incubated active calpain I at a molar ratio of 1:1.3 with calpastatin or AAT and measured AMC release upon Suc-LLVY-AMC cleavage with calpain I. Both calpastatin and AAT significantly inhibited calpain I activity (Figure 2B). We also confirmed this finding by using recombinant AAT (9.6 μmol/L), which inhibited calpain I by 89 ± 7.2% [mean (SD), n = 4].
Figure 2.
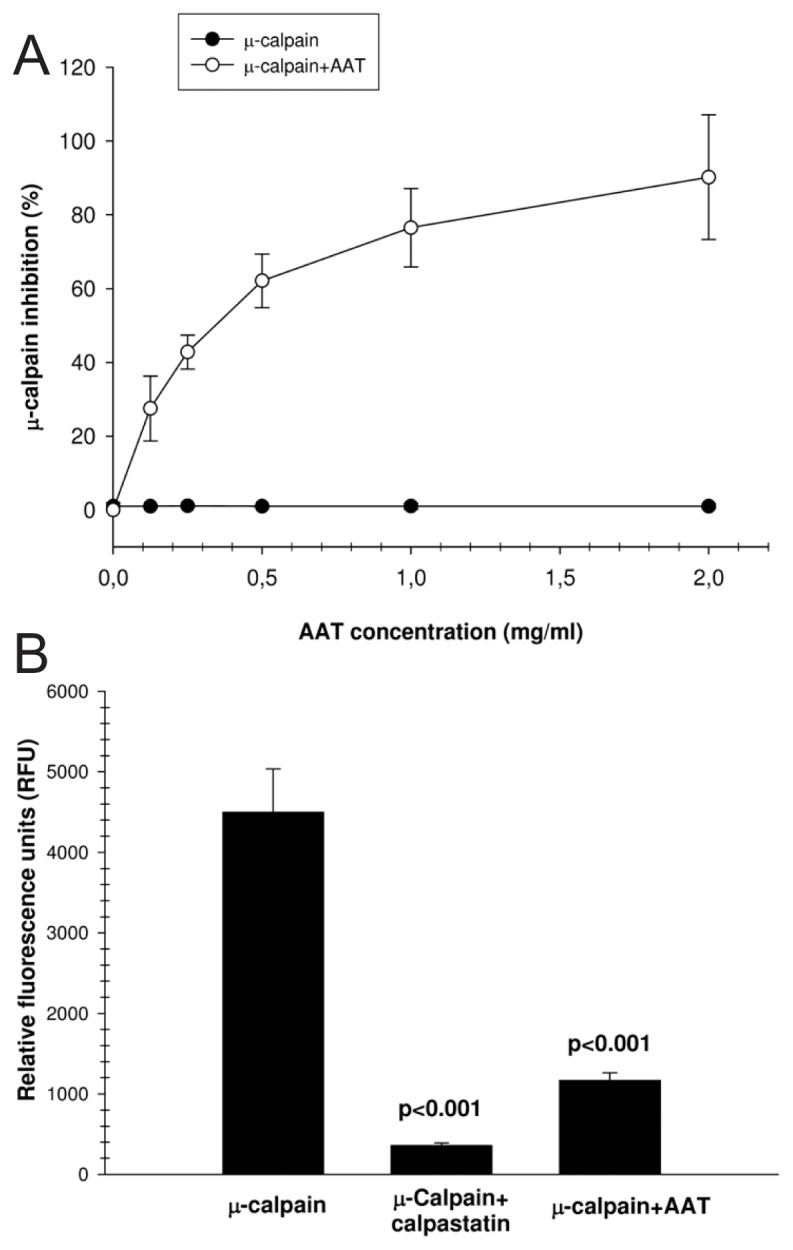
AAT inhibits calpain I activity in vitro. Dose-dependent inhibitory effects of AAT on the enzymatic activity of purified human active calpain I on the specific substrate Suc-LLVY-AMC are shown. (A) The activity of purified calpain I was inhibited by AAT up to 90% in a concentration-dependent manner. Each point represents the mean ± SD of four independent experiments. (B) Active calpain I was incubated at a molar ratio of 1:1.3 with calpastatin or AAT for 5 min, and AMC release was measured upon Suc-LLVY-AMC cleavage with calpain I. The fluorescence value of all samples is corrected by subtracting the value of the blank and then calculating the mean fluorescence value (mean ± SD) for each sample from five independent experiments.
AAT Inhibits α-Spectrin Proteolysis
Calpain I activity leads to α-spectrin proteolysis and production of a breakdown product of 145 kDa, which has been proposed to contribute to cell death (26). As illustrated in Figure 3, neutrophil exposure to AAT for 18 h results in a total inhibition of α-spectrin proteolysis compared with the vehicle control. At the same time, on the basis of the Trypan blue dye assay, as a measure of cytotoxicity, numbers of dead neutrophils were reduced by about 30–40% in the presence of AAT, compared with the untreated cells (data not shown).
Figure 3.
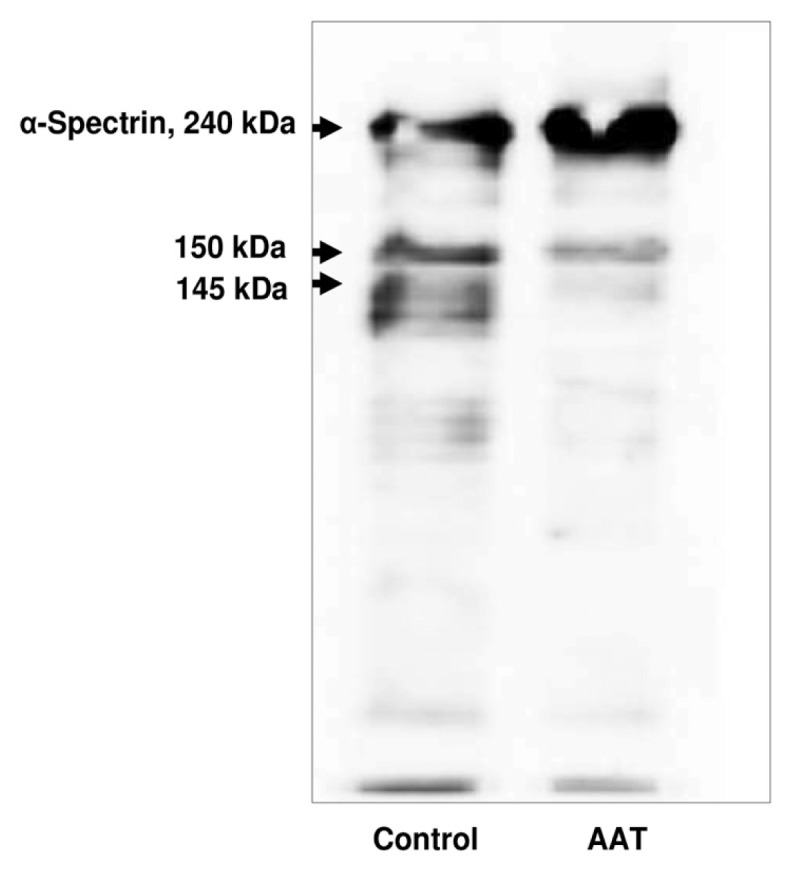
AAT effects on α-spectrin cleavage in neutrophils. Neutrophils were cultured alone or exposed to AAT for 18 h. Equal amounts of cell lysates were separated on 6.5% SDS-PAGE after Western blot analysis. Blots were immunostained with an anti–human α-spectrin monoclonal antibody (1:2,000 dilution) followed by incubation with horseradish peroxidase–conjugated rabbit anti–mouse secondary antibody diluted 1:10,000. The enhanced chemiluminescence kit was used to visualize immunolabeling. A 145-kDa calpain-mediated and 150-kDa (calpain- and caspase 3–mediated) fragments are clearly detectable in controls cells. The 145-kDa calpain-mediated fragment is not detectable in neutrophils treated with AAT. Weak staining for the 150-kDa (calpain- and caspase 3–mediated) fragment is present. A representative Western blot is shown (n = 3).
AAT Inhibits Neutrophil Adhesion
To further confirm the AAT effect of calpain I inhibition, cell adhesion assays were performed on nontreated and fMLP-stimulated neutrophils. Cells were fluorescently labeled with calcein-AM and allowed to adhere to fibrinogen-coated 96-well plates for 30 min in the presence or absence of AAT, and adhesion was quantified by fluorescence detection. As expected, fMLP significantly increased adhesion of neutrophils to fibrinogen relative to vehicle controls, whereas AAT either alone or in the presence of fMLP inhibited neutrophil adhesion by about 50% (Figure 4).
Figure 4.
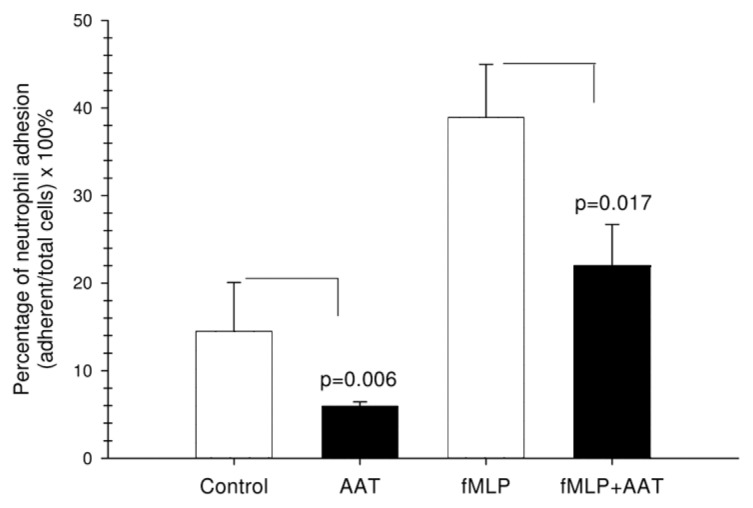
Effects of AAT on neutrophil adhesion to fibronectin. Calcein-AM–labeled neutrophils were added to fibronectin-coated 96-well plates and stimulated with AAT, fMLP (100 nmol/L), fMLP/AAT or medium alone, as described in Materials and Methods. Adherent cells were analyzed with a fluorescence spectrophotometer using an excitation λ of 485 nm and emission λ of 520 nm. The results are expressed as a percentage of neutrophil adhesion ([adherent cells/total cells] × 100%). Each bar represents the mean ± SD of three independent experiments, each performed in four repeats.
AAT Induces Time-Dependent Polarized Spreading of Adherent Neutrophils
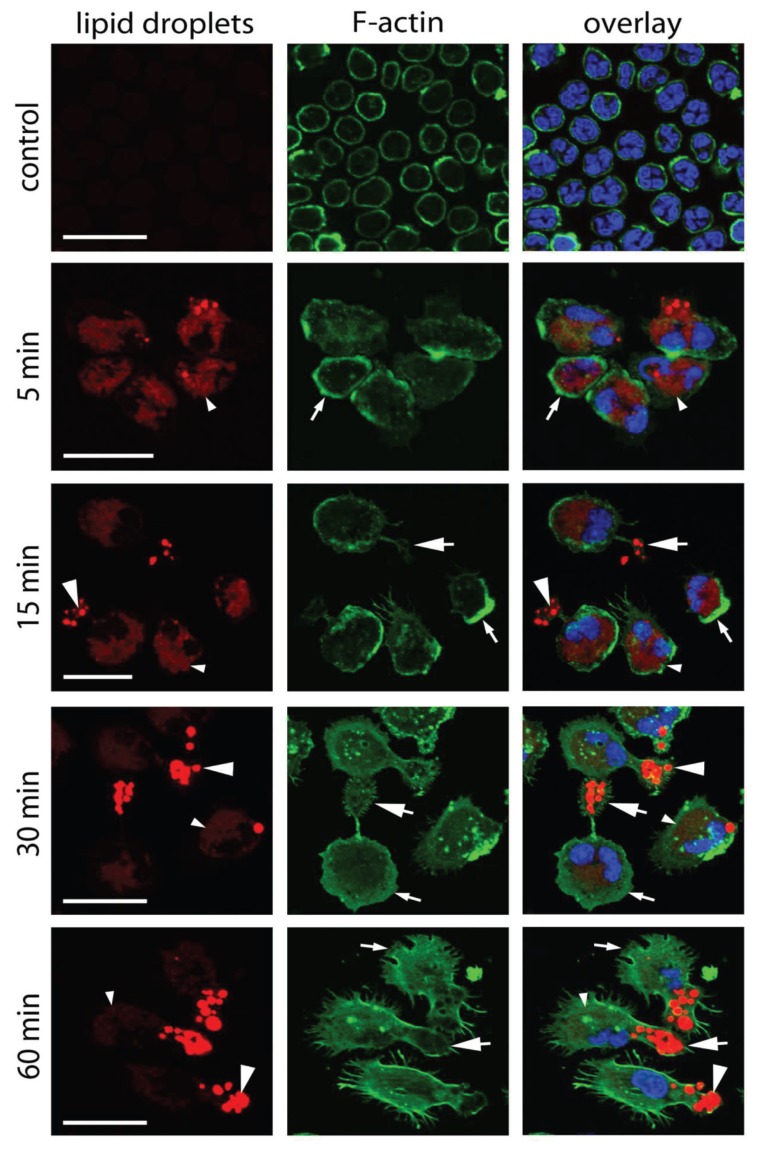
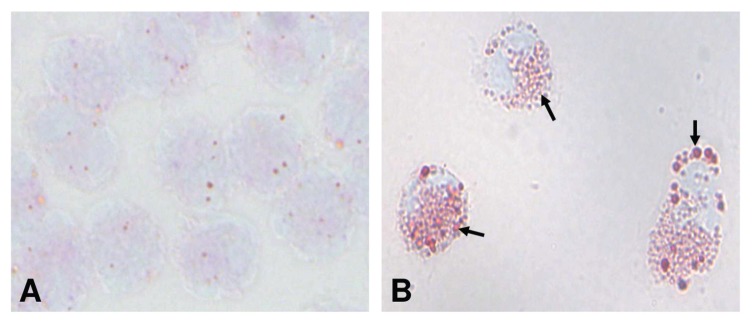
Previous studies have shown that neutrophils treated with calpain inhibitors alone exhibit polarized cell spreading, with actin polarized to the leading edge (27,28). Although AAT strongly impaired neutrophil adhesion, a marked reorganization of the actin cytoskeleton was observed in the relatively small number of adherent neutrophils. As illustrated in Figure 5, nontreated neutrophils have a rounded spherical shape with subcortical actin assembly and show no intrinsic fluorescence. After exposure to AAT for 5 min, the overall adherent cell shape remains round, and some cells begin to show the first signs of intrinsic lipid fluorescence and a redistribution of F-actin to one side of the cell. Neutrophils incubated with AAT for 15 min become strongly polarized bearing uropods, which are often connected to the cell body via a narrow cytoplasmic bridge (see Figure 5). Cell bodies nevertheless remain rounded rather than elongated. Leading edges with broad lamellipodia enriched for F-actin rarely show filopodia. After 30 min of AAT stimulation, neutrophils acquire an elongated shape with prominent lamellipodia often supplemented with filopodia, as spike-like protrusions beyond the leading edge. Notably, at this time point, the leading edges do not show as much F-actin as at the earlier stages. Finally, after 60 min of treatment, the leading edges of elongated neutrophils acquired more filopodia, but became less enriched with F-actin compared to the earlier stages. At this time, the fluorescence intensity of the lipids in the central part of the cell is reduced relative to the intensive fluorescence intensity at the tail. Increased lipid content in the adherent AAT-treated neutrophils was also confirmed by filipin-fluorescent staining (Figure 6). As expected, the increase in filipin-cholesterol staining was time dependent and correlated with neutrophil polarization. In support of this finding, we found high amounts of cytosolic lipid droplets stained with Red Oil O in adherent neutrophils treated with AAT (Figure 7).
Figure 5.
AAT-induced neutrophil polarization and reorganization of F-actin cytoskeleton. Controls and AAT-stimulated cells were labeled with Alexa Fluor 488-phalloidin for F-actin (green) and DAPI for DNA (blue). Intrinsic lipid fluorescence (red) was recorded as described in Materials and Methods. Spherical nontreated neutrophils with a subcortical F-actin arrangement showed no lipid fluorescence. After 5 min of exposure to AAT, cells spread out, acquiring wide F-actin–positive lamellas (small arrows) and accumulated lipid droplets in the cell body (small arrowheads). Gradually stimulated neutrophils obtained polarized elongated shape with uropods (large arrows) and F-actin redistribution from leading edges to F-actin foci in the central part of the cell. At the same time, larger lipid bodies (large arrowheads) accumulated at the rear of the cell. Representative images of three independent experiments are shown. Scale bar = 20 μm.
Figure 6.
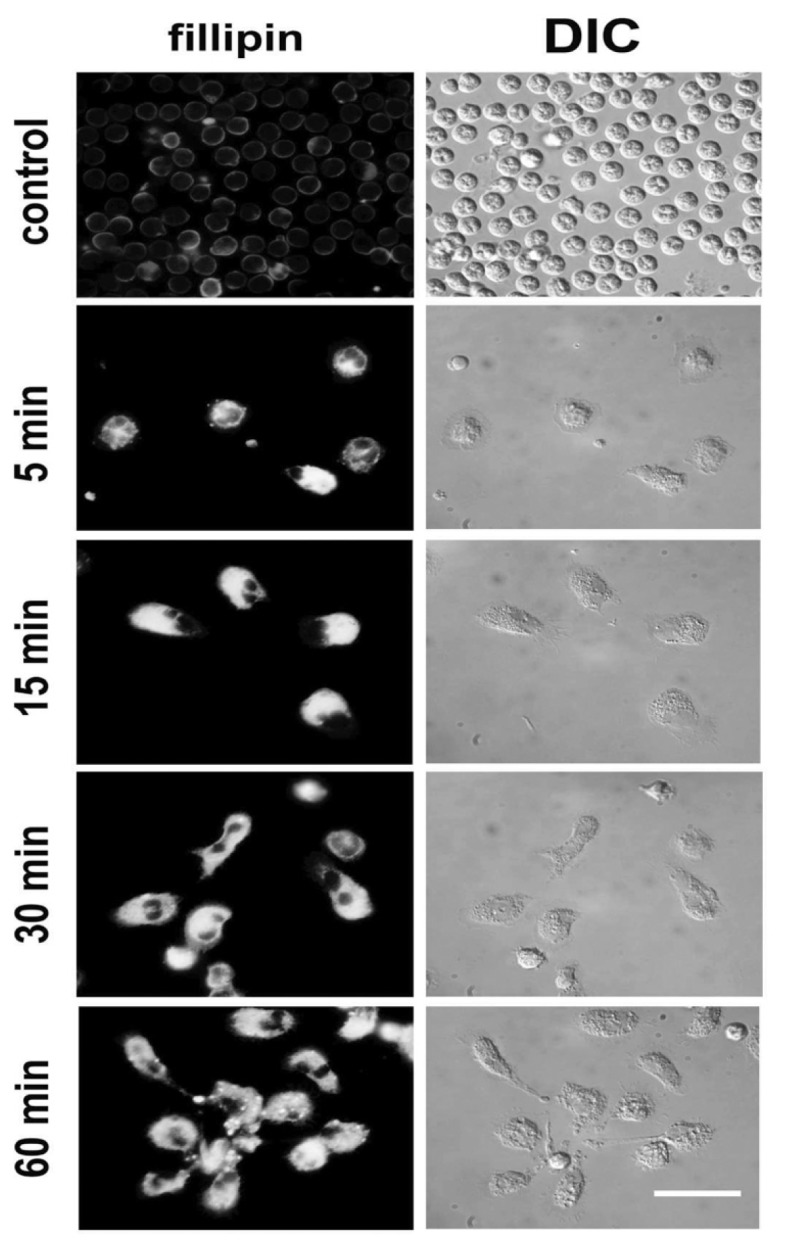
AAT increases intracellular cholesterol levels. Cells were incubated with AAT for the indicated time periods. The intra-cellular cholesterol was stained with filipin, as described in the Materials and Methods. Filipin fluorescence appears shortly after stimulation with AAT and enhances in a time-dependent manner. Images were acquired with constant exposure time and under the same magnification for the whole series. Representative images of three independent experiments are shown. Scale bar = 20 μm.
Figure 7.
AAT induces the cytosolic lipid droplet formation. High amounts of cytosolic lipid droplets stained with Red Oil O (indicated by arrows) were found in neutrophils treated with AAT for 30 min (A) compared with nontreated control cells (B). The images were taken by digital camera (Sony, DKC-5000) at a magnification of 100×. Pictures are representative of five independent experiments. The arrow is pointing to lipid droplets stained with Red Oil O.
AAT Induces Random Neutrophil Migration
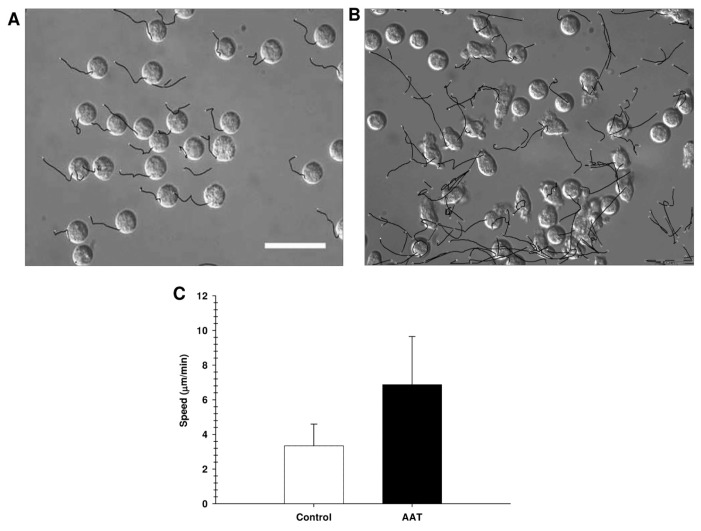
Previous studies have shown that calpain I inhibition affects migration of un-stimulated neutrophils (29,30). Therefore, we monitored migratory responses of neutrophils challenged with AAT under a video microscope equipped for DIC. In the presence of AAT, neutrophils acquired a polarized shape and significantly increased motility within 5 min (Figure 8), which was sustained for over 45 min. Nontreated cells retained rounded morphology and were moving slowly. Recombinant AAT at the concentrations of 0.1 and 0.2 mg/mL also induced an increase in neutrophil motility and transient polarization (data not shown).
Figure 8.
Neutrophil random migration induced by AAT. The videos were recorded with the temporal resolution of 5 s using a Hamamatsu ORCA-R2 CCD camera. Cell migration rates were determined using the Time Lapse Analyzer (TLA) software. Tracks of the cells that remained round after stimulation were manually excluded from the analysis. Values are expressed as mean ± SD, with track numbers n = 35 for control cells and n = 119 for AAT stimulated cells. In the presence of AAT, cell migration was significantly enhanced compared with nontreated controls.
AAT Induces Ca2+ Release from Intracellular Storage
Existing data provide clear evidence that neutrophil polarization is linked to intracellular Ca2+ mobilization (31–33). Therefore, we performed flow cytometric measurement of intracellular Ca2+ with Fluo-3–loaded neutrophils. Addition of AAT to Fluo-3–loaded neutrophils in Ca2+-free buffer resulted in a concentration-dependent and transient increase in cytoplasmic Ca2+ levels (Figure 9). There was no significant difference in the kinetics of intracellular Ca2+ changes induced by AAT when Ca2+-containing buffer was used (Figure 10), suggesting that AAT induces cellular Ca2+ rise from the mobilization of intracellular stores.
Figure 9.
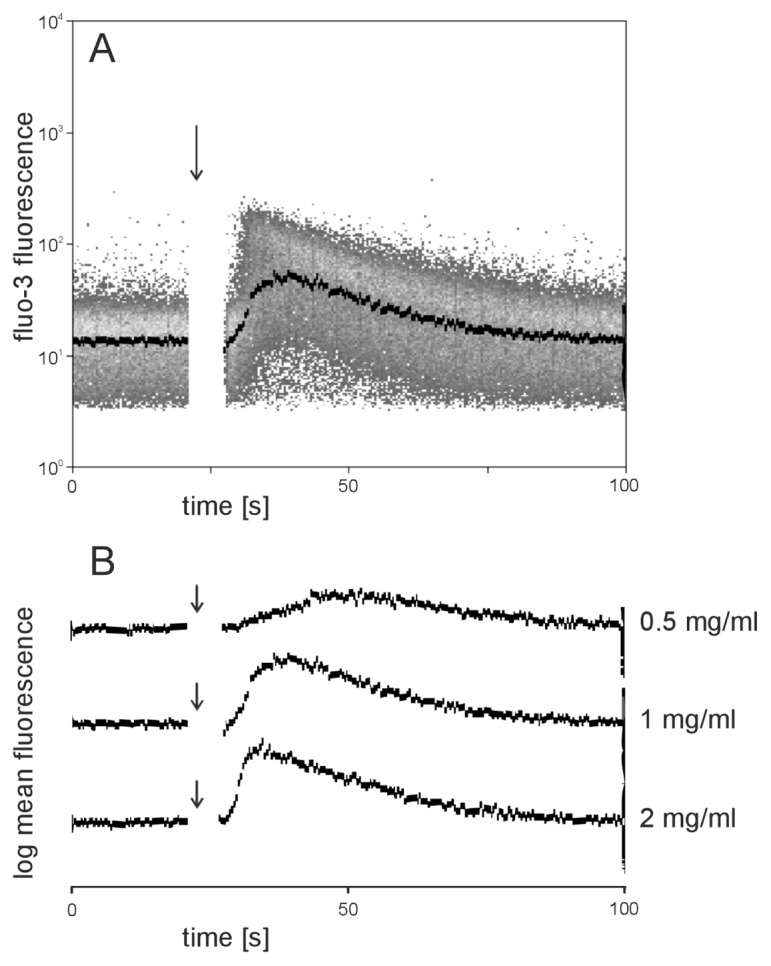
Mobilization of intracellular calcium induced by AAT. Neutrophils loaded with the fluorescent Ca2+ indicator Fluo-3 were measured flow cytometrically in a buffer without Ca2+ containing 1 mmol/L EGTA. Fluo-3 fluorescence of neutrophils was measured for 20 s on unstimulated cells before the injection of AAT at various concentrations (arrows). After addition of AAT, measurement was continued until a total time of 100 s. (A) Fluo-3 fluorescence of single cells (gated on neutrophils on the basis of scatter properties) was plotted against time. The black curve indicates the median fluorescence of neutrophils. AAT (1 mg/mL) addition is marked by the arrow. (B) Comparison of Ca2+ kinetics after stimulation with various concentrations of AAT.
Figure 10.

Comparison of Ca2+ mobilization by AAT in Ca2+-free versus Ca2+-containing buffer. Neutrophils loaded with the fluorescent Ca2+ indicator Fluo-3 were measured flow cytometrically in a buffer containing 1 mmol/L Ca2+ (A, C) or Ca2+-free buffer containing 1 mmol/L EGTA (B, D). Measurement was as described in Materials and Methods and in Figure 4. The black curves indicate the median fluorescence of neutrophils over time. The arrows indicate addition of 1 mg/mL (A, B) or 2 mg/mL (C, D) AAT.
AAT Induces Rapid Activation of Rho GTPases and ERK Phosphorylation
Consistent with the previous reports showing that the Rho GTPases are rapidly activated in polarized neutrophils (24,25,34), we found a marked activation of Rac1 and Cdc42 in AAT-treated neutrophils within 5–15 min (Figure 11A). No activation of GTPases was seen after 30 min (data not shown). Furthermore, as shown in Figure 11B, exposure of neutrophils to AAT resulted in a significant phosphorylation of ERK, which peaked between 15 and 30 min. Notably, AAT had no effect on Rho GTPases and total ERK mRNA expression (data not shown).
Figure 11.
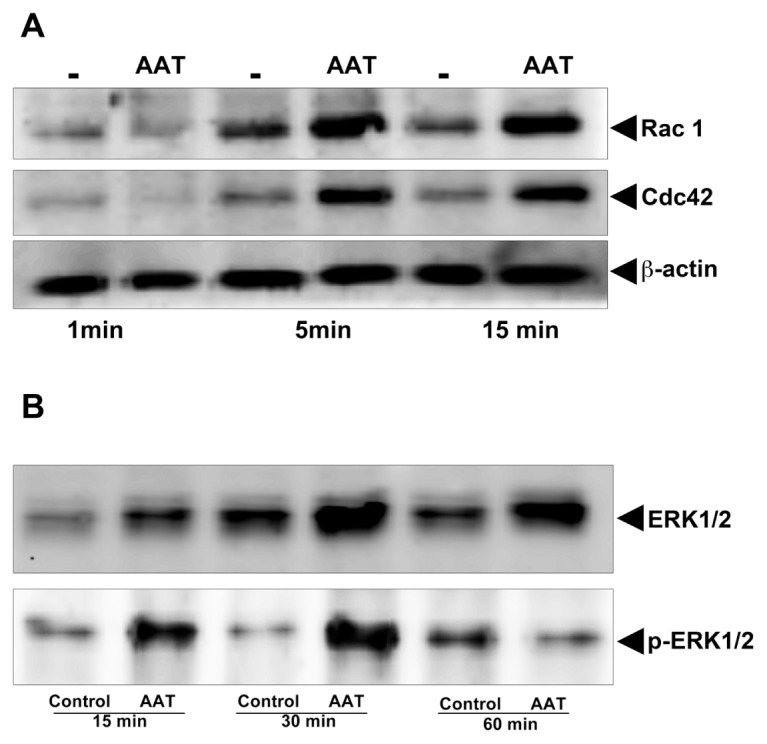
AAT induces activation of Rac1/Cdc42 (A) and ERK1/2 (B). Neutrophils were treated with AAT (0.5 mg/mL) for the indicated periods at 37°C, 5% CO2. Cell lysates from 3 × 106 neutrophils were separated on 7.5% SDS-PAGE followed by immunoblotting, as described in Materials and Methods. Immunoblotting was performed using antibodies against Rac1 and Cdc42 and against the phosphorylated and unphosphorylated forms of ERK. Presumably because of the stress of the cell culture, the phosphorylation of ERK was also detected in unstimulated control cells to a lesser degree than in AAT-treated neutrophils incubated for 60 min. The results shown are representative of three or five independent experiments.
AAT Effects on IL-8 Release from Neutrophils, CD16 Expression and Apoptosis
To investigate the potential harmful effects of AAT on neutrophils, we analyzed the release of IL-8. Under the experimental conditions used, neutrophils exposed to AAT for 4 h did not increase IL-8 levels, but in contrast, AAT inhibited LPS-induced IL-8 release (Figure 12).
Figure 12.
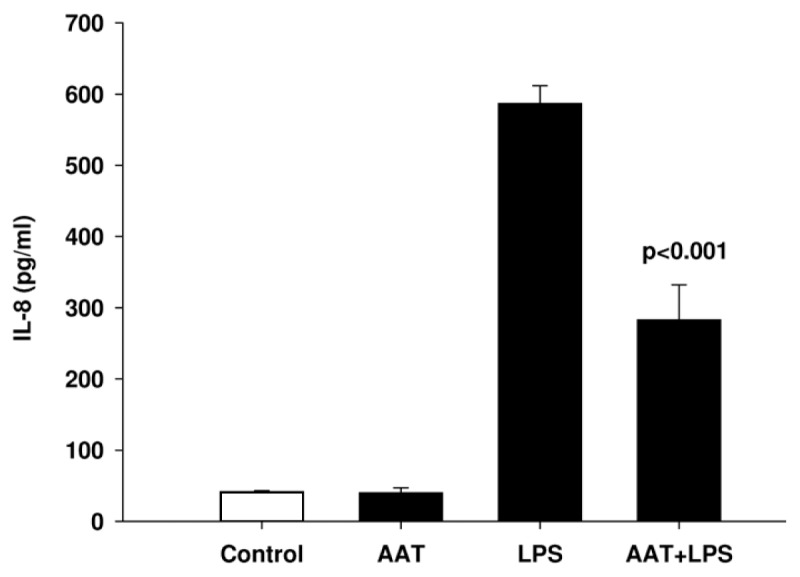
AAT inhibits LPS-induced IL-8 release from neutrophils. Neutrophils were re-suspended in RPMI 1640 medium at a concentration of 3 × 106 cells/mL and stimulated with LPS (10 ng/mL), AAT (0.5 mg/mL) alone and LPS/AAT together for 4 h at 37°C, 5% CO2. Supernatants of cell cultures were analyzed to determine the levels of IL-8. Each bar represents the mean ± SD from four independent experiments.
It is well documented that the loss of CD16 expression is a major factor contributing to loss of neutrophil functions and apoptosis (35). According to our findings, AAT did not reduce CD16 levels nor induce neutrophil apoptosis (Figures 13A, B).
Figure 13.
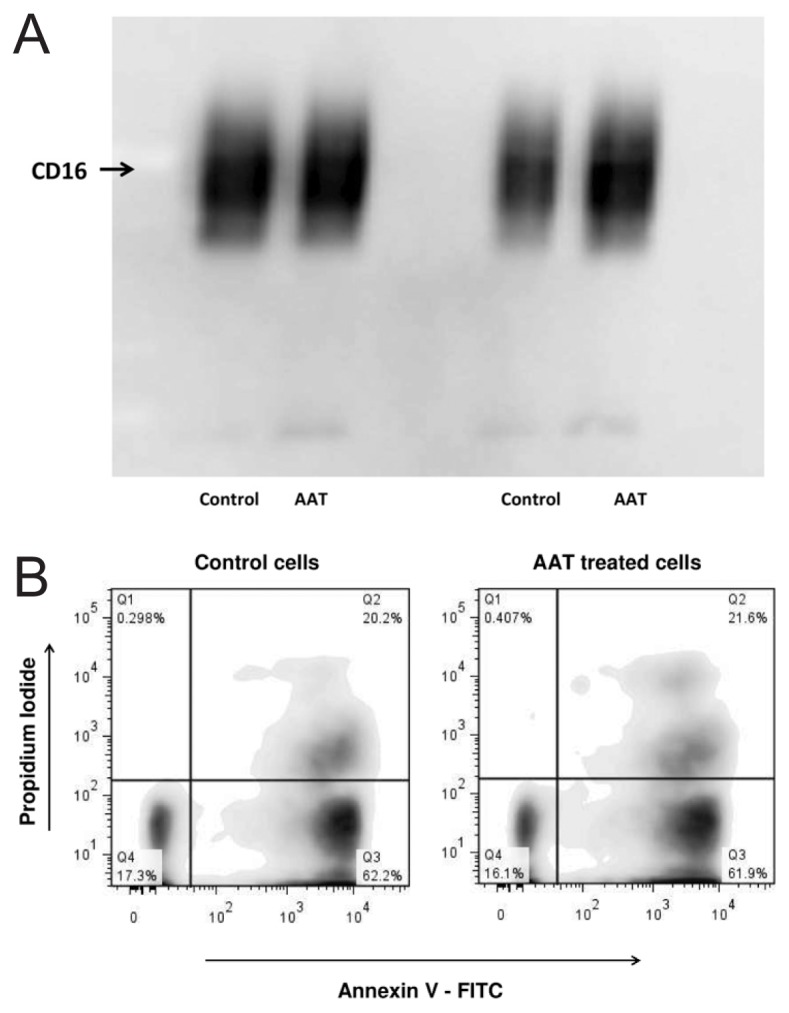
AAT does not decrease CD16 expression (A) and does not induce neutrophil apoptosis (B). Neutrophils were treated with AAT (0.5 mg/mL) for 4 h at 37°C, 5% CO2. (A) Cell lysates from 3 × 106 neutrophils were separated on 7.5% SDS-PAGE, followed by immunoblotting, as described in Materials and Methods. Immunoblotting was performed using a monoclonal antibody against human CD16. The results shown are representative of two independent experiments. (B) Neutrophil apoptosis was monitored using an Annexin V–FITC apoptosis detection kit (BD Biosciences PharmingenTM). Cells were analyzed by flow cytometry. Unstained cells, and cells stained with FITC Annexin V or PI, were used controls.
DISCUSSION
In contrast to many other cell types examined to date, the inhibition of neutrophil μ-calpain (calpain I) activity promotes rapid cell polarization, spreading and random migration. For example, Lokuta et al. (24) clearly showed that neutrophil treatment with calpain I inhibitors, but not inhibitors of other pro-teases, promotes random cell migration and polarization of cells in the absence of any exogenous activators. Calpain I inhibition–mediated neutrophil polarization also depends on the activation of Rho GTPases and Rac-dependent downstream signaling pathways, e.g., the ERK1/2 pathway (25). Thus, according to our current knowledge, constitutively active calpain I expressed in human neutrophils most likely contributes to their spherical phenotype. On the other hand, the random migration of neutrophils induced by calpain inhibition is associated with their decreased directional migration toward chemotactic stimuli (24, 36–38). This latter finding and the fact that chemotaxis of activated neutrophils is inhibited by AAT (9) prompted us to investigate the putative ability of AAT to inactivate calpain I activity. Indeed, we found that AAT inhibits calpain I in isolated neutrophils and when added to the active form of calpain I, in vitro. Remarkably, calpain I inhibition occurred irrespectively of the transient increase in the intracellular Ca2+ level in response to AAT treatment. Importantly, other calpain inhibitors have also been found to induce levels of intracellular Ca2+ (38,39). This transient increase in calcium may be a common property of calpain inhibitors because of the homeostatic feedback control of basal calpain activity on intracellular free Ca2+. However, this increase is small enough to escape detection and is not large enough to alter the calpain inhibition. Furthermore, the elevation of cytoplasmic Ca2+ is shown to regulate the activities of Rho GTPases (40), whereas Rho GTPases also regulate Ca2+ dynamics (41,42). According to our data, a transient increase in Ca2+ after neutrophil exposure to AAT paralleled with a rapid activation of Rac1, Cdc42 and ERK1/2. Presently, we are not able to delineate the direct relationship between AAT-induced transient intracellular Ca2+ elevation and activation of Rac1/Cdc42. However, Rac1 activity has been shown to promote random cell motility (42,43), suggesting that AAT-induced random neutrophil migration is most likely a result of the synergistic and transient changes in intracellular Ca2+ dynamics and GTPase activation. It is important to note that, under our experimental conditions, AAT induced no changes in ERK and GTPases mRNA expression. These findings further show that the activity of calpain in vivo is under tight regulation, which may result from the direct and coordinated interaction of inhibitory and stimulatory molecules with calpain, modulated by changes in the intracellular Ca2+ concentration.
Several studies have shown that calpain may be activated in the cytosol and subsequently translocated to the membrane after interaction with phospholipids (44–47). Because AAT is shown to be localized in lipid rafts (20,48) and AAT increases cellular lipid content, it is plausible that the transient elevation of cytoplasmic Ca2+ and lipid redistribution, as induced by exogenous AAT, is required for the calpain I translocation to the membranes, where it is ultimately inhibited by AAT. Interestingly, we observed that AAT does not inhibit calpain I when added to neutrophil lysates and that both AAT and calpain are localized in the neutrophil membrane fractions (unpublished data). The direct relationship between the transient increase in Ca2+ levels, intracellular lipid redistribution, calpain translocation to the membrane and inactivation by exogenous AAT needs to be investigated in more detail.
The facts that the inhibition of calpain I, but not calpain II, in resting neutrophils induces random motility in the absence of exogenous activators and that calpain II is not required for chemoattractant-induced random migration (36) further support our finding that AAT induces neutrophil random motility by inhibiting calpain I. As mentioned above, the increase in neutrophil motility caused by calpain I inhibition is accompanied by an impaired chemotactic response. This result again is keeping in line with previous results showing that AAT inhibits IL-8 and fMLP-induced neutrophil chemotaxis (9,20) and our current observation that AAT significantly inhibits nonstimulated or fMLP-induced neutrophil adhesion to fibronectin. It is noteworthy that, in our experimental model, AAT itself did not induce any cytotoxic effects (as determined by Trypan blue staining and Annexin V assay) and did not reduce neutrophil functions (as analyzed by CD16 expression). The addition of AAT to resting neutrophil cultures did not elicit an inflammatory response, as can be deduced from the fact that AAT significantly inhibited IL-8 release either when added directly to the neutrophils or during activation with LPS.
Calpain is implicated in numerous pathological conditions including Alzheimer’s disease; demyelination events of multiple sclerosis; neuronal damage after spinal cord injury and hypoxic/ischemic injury to brain, kidney and heart organs; and tumor development and invasion (49–53). Thus, the elucidation of the mechanisms by which calpain activity is regulated is of interest for the development of therapeutics for a wide range of pathological states. Under physiological conditions, calpain and its classic inhibitor, calpastatin, exist as relatively stable proteins (54). However, in response to stimuli, calpain can cause proteolysis of its inhibitor calpastatin, thereby enhancing its own activity by a positive feedback loop (55). It is possible that AAT, as an APP that can easily enter various cells, plays an important role in maintaining calpain/calpastatin balance and regulation of cell function and viability during inflammation or infection.
Collectively, our results extend current knowledge into biological activities of AAT and provide new evidence as to how AAT may offer substantial protection against neutrophilic inflammation owing to its ability to inhibit calpain I. In view of these findings, it is clear that AAT is an antiinflammatory (56) and immunoregulatory protein (57), but its optimal concentration and the best functional molecular form are still unknown. It is noteworthy that various preparations of human purified plasma AAT are used for augmentation therapy; therefore, further studies would be required to confirm whether the above-described anti–calpain I activity of AAT are consistent across preparations and whether this activity is reproducible in other cell models. Our findings point to the critical importance of the further characterization of the AAT molecule and improvement of its application for augmentation therapy. We believe that the results presented here should provide the impetus for new studies to define the role of AAT and other APPs in health and disease.
ACKNOWLEDGMENTS
This study was supported by the Swedish Research Council (SJ) and Hannover Medical School. RM was supported by the Cambridge National Institute for Health Research (NIHR) Biomedical Research Centre.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 2.Köhnlein T, Welte T. Alpha-1 antitrypsin deficiency: pathogenesis, clinical presentation, diagnosis, and treatment. Am J Med. 2008;121:123–9. doi: 10.1016/j.amjmed.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 3.Fournier T, Medjoubi NN, Porquet D. Alpha-1-acid glycoprotein. Biochim Biophys Acta. 2000;1482:157–71. doi: 10.1016/s0167-4838(00)00153-9. [DOI] [PubMed] [Google Scholar]
- 4.Lainé E, et al. Modulation of human polymorphonuclear neutrophil functions by alpha 1-acid glycoprotein. Inflammation. 1990;14:1–9. doi: 10.1007/BF00914025. [DOI] [PubMed] [Google Scholar]
- 5.Vasson MP, Roch-Arveiller M, Couderc R, Baguet JC, Raichvarg D. Effects of alpha-1 acid glycoprotein on human polymorphonuclear neutrophils: influence of glycan microheterogeneity. Clin Chim Acta. 1994;224:65–71. doi: 10.1016/0009-8981(94)90121-x. [DOI] [PubMed] [Google Scholar]
- 6.Rinaldi M, Ceciliani F, Lecchi C, Moroni P, Bannerman DD. Differential effects of alpha1-acid glycoprotein on bovine neutrophil respiratory burst activity and IL-8 production. Vet Immunol Immunopathol. 2008;26:199–210. doi: 10.1016/j.vetimm.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Bucurenci N, Blake DR, Chidwick K, Winyard PG. Inhibition of neutrophil superoxide production by human plasma alpha 1-antitrypsin. FEBS Lett. 1992;300:21–4. doi: 10.1016/0014-5793(92)80156-b. [DOI] [PubMed] [Google Scholar]
- 8.Tilg H, Vannier E, Vachino G, Dinarello CA, Mier JW. Antiinflammatory properties of hepatic acute phase proteins: preferential induction of interleukin 1 (IL-1) receptor antagonist over IL-1 beta synthesis by human peripheral blood mononuclear cells. J Exp Med. 1993;178:1629–36. doi: 10.1084/jem.178.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janciauskiene S, Zelvyte I, Jansson L, Stevens T. Divergent effects of alpha1-antitrypsin on neutrophil activation, in vitro. Biochem Biophys Res Commun. 2004;315:288–96. doi: 10.1016/j.bbrc.2004.01.055. [DOI] [PubMed] [Google Scholar]
- 10.Daemen MA, et al. Functional protection by acute phase proteins alpha(1)-acid glycoprotein and alpha(1)-antitrypsin against ischemia/reperfusion injury by preventing apoptosis and inflammation. Circulation. 2000;102:1420–6. doi: 10.1161/01.cir.102.12.1420. [DOI] [PubMed] [Google Scholar]
- 11.Zhong W, et al. Effect of human C-reactive protein on chemokine and chemotactic factor-induced neutrophil chemotaxis and signaling. J Immunol. 1998;161:2533–40. [PubMed] [Google Scholar]
- 12.Heuertz RM, Tricomi SM, Ezekiel UR, Webster RO. C-reactive protein inhibits chemotactic peptide-induced p38 mitogen-activated protein kinase activity and human neutrophil movement. J Biol Chem. 1999;274:17968–74. doi: 10.1074/jbc.274.25.17968. [DOI] [PubMed] [Google Scholar]
- 13.Quaye IK. Haptoglobin, inflammation and disease. Trans R Soc Trop Med Hyg. 2008;102:735–42. doi: 10.1016/j.trstmh.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Griese M, et al. Alpha1-antitrypsin inhalation reduces airway inflammation in cystic fibrosis patients. Eur Respir J. 2007;29:240–50. doi: 10.1183/09031936.00047306. [DOI] [PubMed] [Google Scholar]
- 15.Subramaniyam D, et al. Effects of alpha 1-antitrypsin on endotoxin-induced lung inflammation in vivo. Inflamm Res. 2010;59:571–8. doi: 10.1007/s00011-010-0164-x. [DOI] [PubMed] [Google Scholar]
- 16.Hill AT, Campbell EJ, Bayley DL, Hill SL, Stockley RA. Evidence for excessive bronchial inflammation during an acute exacerbation of chronic obstructive pulmonary disease in patients with alpha(1)-antitrypsin deficiency (PiZ) Am J Respir Crit Care Med. 1999;160:1968–75. doi: 10.1164/ajrccm.160.6.9904097. [DOI] [PubMed] [Google Scholar]
- 17.Petrache I, et al. Alpha-1 antitrypsin inhibits caspase-3 activity, preventing lung endothelial cell apoptosis. Am J Pathol. 2006;169:1155–66. doi: 10.2353/ajpath.2006.060058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang B, et al. Alpha1-antitrypsin protects beta-cells from apoptosis. Diabetes. 2007;56:1316–23. doi: 10.2337/db06-1273. [DOI] [PubMed] [Google Scholar]
- 19.Janciauskiene S, et al. Alpha1-antitrypsin inhibits the activity of the matriptase catalytic domain in vitro. Am J Respir Cell Mol Biol. 2008;39:631–7. doi: 10.1165/rcmb.2008-0015RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bergin DA, et al. α-1 Antitrypsin regulates human neutrophil chemotaxis induced by soluble immune complexes and IL-8. J Clin Invest. 2010;120:4236–50. doi: 10.1172/JCI41196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howard OM, Dong HF, Shirakawa AK, Oppen-heim JJ. LEC induces chemotaxis and adhesion by interacting with CCR1 and CCR8. Blood. 2000;96:840–5. [PubMed] [Google Scholar]
- 22.Huth J, et al. Significantly improved precision of cell migration analysis in time-lapse video microscopy through use of a fully automated tracking system. BMC Cell Biol. 2010;11:24. doi: 10.1186/1471-2121-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newcomb JK, Pike BR, Zhao X, Hayes RL. Concurrent assessment of calpain and caspase-3 activity by means of Western blots of protease-specific spectrin breakdown products. Methods Mol Biol. 2000;144:219–23. doi: 10.1385/1-59259-050-0:219. [DOI] [PubMed] [Google Scholar]
- 24.Lokuta MA, Nuzzi PA, Huttenlocher A. Calpain regulates neutrophil chemotaxis. Proc Natl Acad Sci U S A. 2003;100:4006–11. doi: 10.1073/pnas.0636533100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katsube M, et al. Calpain-mediated regulation of the distinct signaling pathways and cell migration in human neutrophils. J Leukoc Biol. 2008;84:255–63. doi: 10.1189/jlb.0907664. [DOI] [PubMed] [Google Scholar]
- 26.Czogalla A, Sikorski AF. Spectrin and cal-pain: a ‘target’ and a ‘sniper’ in the pathology of neuronal cells. Cell Mol Life Sci. 2005;62:1913–24. doi: 10.1007/s00018-005-5097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Servant G, et al. Polarization of chemo-attractant receptor signaling during neutrophil chemotaxis. Science. 2000;287:1037–40. doi: 10.1126/science.287.5455.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang KK. Developing selective inhibitors of calpain. Trends Pharmacol Sci. 1990;11:139–42. doi: 10.1016/0165-6147(90)90060-L. [DOI] [PubMed] [Google Scholar]
- 29.Xu J, et al. Divergent signals and cyto-skeletal assemblies regulate self-organizing polarity in neutrophils. Cell. 2003;114:201–14. doi: 10.1016/s0092-8674(03)00555-5. [DOI] [PubMed] [Google Scholar]
- 30.Niggli V. Signaling to migration in neutrophils: importance of localized pathways. Intern J Biochem Cell Biol. 2003;35:1619–38. doi: 10.1016/s1357-2725(03)00144-4. [DOI] [PubMed] [Google Scholar]
- 31.Kruskal BA, Shak S, Maxfield FR. Spreading of human neutrophils is immediately preceded by a large increase in cytoplasmic free calcium. Proc Natl Acad Sci U S A. 1986;83:2919–23. doi: 10.1073/pnas.83.9.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marks PW, Maxfield FR. Local and global changes in cytosolic free calcium in neutrophils during chemotaxis and phagocytosis. Cell Calcium. 1990;11:181–90. doi: 10.1016/0143-4160(90)90069-7. [DOI] [PubMed] [Google Scholar]
- 33.Kindzelskii AL, Petty HR. Intracellular calcium waves accompany neutrophil polarization, formylmethionylleucylphenylalanine stimulation, and phagocytosis: a high speed microscopy study. J Immunol. 2003;170:64–72. doi: 10.4049/jimmunol.170.1.64. [DOI] [PubMed] [Google Scholar]
- 34.Weiner OD, et al. A PtdInsP(3)- and Rho GTPase-mediated positive feedback loop regulates neutrophil polarity. Nat Cell Biol. 2002;4:509–13. doi: 10.1038/ncb811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dransfield I, et al. Neutrophil apoptosis is associated with a reduction in CD16 (FcyRIII) expression. J. Immunol. 1994;153:1254–63. [PubMed] [Google Scholar]
- 36.Nuzzi PA, Senetar MA, Huttenlocher A. Asymmetric localization of calpain 2 during neutrophil chemotaxis. Mol Biol Cell. 2007;18:795–805. doi: 10.1091/mbc.E06-09-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ray SK, et al. Calpain inhibitor prevented apoptosis and maintained transcription of proteolipid protein and myelin basic protein genes in rat spinal cord injury. J Chem Neuroanat. 2003;26:119–24. doi: 10.1016/s0891-0618(03)00044-9. [DOI] [PubMed] [Google Scholar]
- 38.Butler J, et al. Involvement of calpain in the process of Jurkat T cell chemotaxis. J Neurosci Res. 2009;87:626–35. doi: 10.1002/jnr.21882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiemer AJ, Lokuta M, Surfus JC. Calpain inhibition impairs TNF-α-mediated neutrophil adhesion, arrest and oxidative burst. Mol Immunol. 2010;47:894–902. doi: 10.1016/j.molimm.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Price LS, et al. Calcium signaling regulates translocation and activation of Rac. J Biol Chem. 2003;278:39413–21. doi: 10.1074/jbc.M302083200. [DOI] [PubMed] [Google Scholar]
- 41.Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Dev Biol. 2004;265:23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 42.Pankov R, et al. A Rac switch regulates random versus directionally persistent cell migration. J Cell Biol. 2005;170:793–802. doi: 10.1083/jcb.200503152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chung CY, Funamoto S, Firtel RA. Signaling pathways controlling cell polarity and chemotaxis. Trends Biochem Sci. 2001;26:557–66. doi: 10.1016/s0968-0004(01)01934-x. [DOI] [PubMed] [Google Scholar]
- 44.Arthur JS, Crawford C. Investigation of the interaction of m calpain with phospholipids: calpain-phospholipid interactions. Biochim Biophys Acta. 1996;1293:201–6. doi: 10.1016/0167-4838(95)00243-x. [DOI] [PubMed] [Google Scholar]
- 45.Pontremoli S, et al. Role of phospholipids in the activation of the Ca2+-dependent neutral proteinase of human erythrocytes. Biochem Biophys Res Commun. 1985;129:389–95. doi: 10.1016/0006-291x(85)90163-9. [DOI] [PubMed] [Google Scholar]
- 46.Ducharme NA, Bickel PE. Lipid droplets in lipogenesis and lipolysis. Endocrinology. 2008;149:942–9. doi: 10.1210/en.2007-1713. [DOI] [PubMed] [Google Scholar]
- 47.Pierini LM, et al. Membrane lipid organization is critical for human neutrophil polarization. J Biol Chem. 2003;278:10831–41. doi: 10.1074/jbc.M212386200. [DOI] [PubMed] [Google Scholar]
- 48.Subramaniyam D, et al. Cholesterol rich lipid raft microdomains are gateway for acute phase protein, SERPINA1. Int J Biochem Cell Biol. 2010;42:1562–70. doi: 10.1016/j.biocel.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 49.Nixon RA. The calpains in aging and aging-related diseases. Ageing Res Rev. 2003;2:407–18. doi: 10.1016/s1568-1637(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 50.Vosler PS, Brennan CS, Chen J. Calpain-mediated signaling mechanisms in neuronal injury and neurodegeneration. Mol Neurobiol. 2008;38:78–100. doi: 10.1007/s12035-008-8036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andersen SS, Bi GQ. Axon formation: a molecular model for the generation of neuronal polarity. Bioessays. 2000;22:172–9. doi: 10.1002/(SICI)1521-1878(200002)22:2<172::AID-BIES8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 52.Braun C, et al. Expression of calpain I messenger RNA in human renal cell carcinoma: correlation with lymph node metastasis and histological type. Int J Cancer. 1999;84:6–9. doi: 10.1002/(sici)1097-0215(19990219)84:1<6::aid-ijc2>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 53.Kimura Y, Saya H, Nakao M. Calpain-dependent proteolysis of NF2 protein: involvement in schwannomas and meningiomas. Neuropathology. 2000;20:153–60. doi: 10.1046/j.1440-1789.2000.00326.x. [DOI] [PubMed] [Google Scholar]
- 54.Zhang W, Lane RD, Mellgren RL. The major calpain isozymes are long-lived proteins: design of an antisense strategy for calpain depletion in cultured cells. J Biol Chem. 1996;271:18825–30. doi: 10.1074/jbc.271.31.18825. [DOI] [PubMed] [Google Scholar]
- 55.Carragher NO, et al. v-Src-induced modulation of the calpain-calpastatin proteolytic system regulates transformation. Mol Cell Biol. 2002;22:257–69. doi: 10.1128/MCB.22.1.257-269.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Janciauskiene S, et al. The discovery of α1-antitrypsin and its role in health and disease. Respir Med. 2011 Feb 28; doi: 10.1016/j.rmed.2011.02.002. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 57.Lewis EC, et al. Alpha1-antitrypsin monotherapy prolongs islet allograft survival in mice. Proc Natl Acad Sci U S A. 2005;102:12153–8. doi: 10.1073/pnas.0505579102. [DOI] [PMC free article] [PubMed] [Google Scholar]