Abstract
Lipid rafts are cholesterol- and sphingomyelin-enriched microdomains that provide a highly saturated and viscous physicochemical microenvironment to promote protein–lipid and protein–protein interactions. We purified lipid rafts from human frontal cortex from normal, early motor stages of Parkinson’s disease (PD) and incidental Parkinson’s disease (iPD) subjects and analyzed their lipid composition. We observed that lipid rafts from PD and iPD cortices exhibit dramatic reductions in their contents of n-3 and n-6 long-chain polyunsaturated fatty acids, especially docosahexaenoic acid (22:6-n3) and arachidonic acid (20:4n-6). Also, saturated fatty acids (16:0 and 18:0) were significantly higher than in control brains. Paralleling these findings, unsaturation and peroxidability indices were considerably reduced in PD and iPD lipid rafts. Lipid classes were also affected in PD and iPD lipid rafts. Thus, phosphatidylserine and phosphatidylinositol were increased in PD and iPD, whereas cerebrosides and sulfatides and plasmalogen levels were considerably diminished. Our data pinpoint a dramatic increase in lipid raft order due to the aberrant biochemical structure in PD and iPD and indicate that these abnormalities of lipid rafts in the frontal cortex occur at early stages of PD pathology. The findings correlate with abnormal lipid raft signaling and cognitive decline observed during the development of these neurodegenerative disorders.
INTRODUCTION
Parkinson’s disease (PD) is a multisystemic neurodegenerative disease that affects selected nuclei of the medulla oblongata and pons, olfactory bulb and tract, intestinal ganglionic plexus, substantia nigra pars compacta, amygdala, nucleus basalis of Meynert and cerebral cortex. The main neuropathological hallmark is the presence of intraneuronal inclusions named Lewy bodies (LB) and aberrant neurites (LN) filled with abnormal protein aggregates, of which the most important component is α-synuclein, which is abnormally phosphorylated, nitrated and oxidized and shows abnormal solubility, aggregation and facility to fibril formation (1–3). Classic PD is manifested as a complex motor disorder that results from the reduced dopaminergic input of the substantia nigra to the striatum and the resultant altered basal ganglia modulation of motor control (4). Cases with Lewy body pathology in the brain stem without motor symptoms are considered as having incidental PD (iPD) because they are unexpectedly discovered after appropriate postmortem neuropathological study (5–8). Whether these cases constitute premotor PD has been a matter of controversy for some years, because it cannot be confirmed that these cases would have progressed to Parkinsonism if they had survived longer. However, the study of consecutive cases in a large series and the recognition of several intermediate degrees of involvement of the brain stem, limbic structures and, eventually, the cerebral cortex make it clear that a prediction of iPD as an anterior stage of PD seems more than reasonable (2,9–11).
Cortical involvement in PD has long been neglected. Yet cognitive impairment occurs in most cases with advanced PD. More importantly, altered cortical function can be detected in some individuals before the appearance of motor symptoms, and cortical dysfunction is common in classic Parkinsonism when appropriately examined (12). Recent neuroimaging approaches have supported the involvement of the cerebral cortex with disease progression (13–15).
However, an important aspect is that altered cortical function is not related to the presence of LB and LN in the cerebral cortex (16). Therefore, factors other than the accumulation of abnormal α-synuclein in the form of LB and LN are causative of the abnormal cortical function (17). Molecular neuropathology has elucidated a series of modifications that may result in impaired function of selected metabolic pathways, these including altered mitochondrial function, glycolysis and protein degradation pathways in the frontal cortex in PD and iPD (17,18). There is some agreement that molecular damage in PD is due to oxidative stress, which enhances oxidative DNA damage and increases the expression levels of lipoxidation, nitration and glycoxidation markers and lipid peroxides and increases the expression of RAGE (advanced glycation end products receptor) in the frontal cortex of PD and iPD (19–21).
Recent evidence has demonstrated that molecular alterations in PD are not limited to specific proteins but also to abnormal levels of fatty acids in the frontal cortex in iPD and PD compared with age-matched controls. Thus, the highly peroxidizable docosahexaenoic acid (DHA) appears to be significantly increased in the frontal cortex in iPD and PD (20). Whether altered lipid composition affects highly specialized structures such as lipid rafts in PD and iPD is not known. Lipid rafts are membrane microdomains characterized by their high content in sphingolipids, cholesterol and saturated fatty acids, as well as by reduced content in polyunsaturated fatty acids (PUFAs). These micro -domains provide a highly saturated and liquid-ordered physicochemical micro -environment that promotes protein–lipid and protein–protein interactions (22–24). Lipid rafts comprise a highly dynamic clustering of proteins and lipids that play a central role in signal transduction and intercellular communication. Their alterations have been associated with altered neuronal function, synaptic transmission and neurotransmitter signaling (17,25–27). We have recently demonstrated that lipid composition, in particular, long-chain polyunsaturated fatty acids (LCPUFAs), are profoundly altered in the frontal cortex of late stages of Alzheimer’s disease (28), which presumably would underlie, at least in part, neuronal dysfunction and cognitive deterioration. However, potential alterations in the lipid structure and spatial organization of lipid rafts in PD have not been explored to date. In the present study, we aimed at isolating highly purified lipid rafts from human frontal cortex from control, PD and iPD subjects to analyze in detail their lipid profiles and distribution.
MATERIALS AND METHODS
Human Brain Tissue
Human samples analyzed in the present study are summarized in Table 1 and include eight cases with PD (group PD: five males and three females, average age 73.9 years), eight cases with incidental PD (group iPD: six males and two females, average age 72.7 years) and 11 age-matched controls (group No Significant Lesions [NSL]: five males and six females, average age 75.5 years). Control cases had no neurological symptoms and signs, and the neuropathological examination did not show abnormalities. Cases with PD showed a short history of motor symptoms. Modified Hoehn and Yahr staging was between 1.5 (unilateral plus axial involvement) and 2.5 (mild bilateral disease with recovery on pull test). The selection of PD cases with relatively mild or moderate motor symptoms was made to minimize changes related to a long course of the disease and the concomitant combination of variegated drugs used for the treatment. Cases with iPD did not complain of neurological deficits, although PD-related lesions were found in the neuropathological postmortem study. The causes of death in both groups were similar and included infectious diseases (mainly pneumonia), heart infarction and carcinoma.
Table 2.
Lipid class comparison between lipid rafts and nonraft membranes in control subjects (NSL).
| Nonrafts | Lipid rafts | |
|---|---|---|
| Sphingomyelin | 1.97 ± 0.86 | 11.43 ± 1.44a |
| PC | 9.86 ± 0.87 | 5.35 ± 0.49a |
| PS | 10.85 ± 1.08 | 6.82 ± 0.50a |
| PI | 3.67 ± 0.59 | 2.16 ± 0.17a |
| Phosphatidylglycerol | 2.71 ± 0.58 | 0.70 ± 0.10a |
| PE | 28.72 ± 1.82 | 20.97 ± 0.57a |
| Sulfatides | 0.00 ± 0.00 | 10.56 ± 0.68a |
| Cerebrosides | 0.00 ± 0.00 | 5.10 ± 0.79a |
| Cholesterol | 27.68 ± 1.98 | 33.04 ± 1.18a |
| Free fatty acids | 10.68 ± 0.92 | 2.17 ± 0.28a |
| Sterol esters | 0.00 ± 0.00 | 1.68 ± 0.60a |
| Total neutral lipids | 42.23 ± 2.66 | 36.90 ± 1.41 |
| Total polar lipids | 57.77 ± 2.66 | 63.09 ± 1.41 |
| Phospholipid/cholesterol | 2.15 ± 0.18 | 1.11 ± 0.06a |
Results are expressed as mole % and represent mean ± SEM.
P < 0.05 compared with nonrafts.
At autopsy, one cerebral hemisphere, alternate sections of the brain stem and one cerebellar hemisphere were fixed in buffered formalin and processed for neuropathological study. The other hemisphere, cut on coronal sections 1-cm thick, alternate sections of the brain stem and sections of the cerebellum were immediately stored at −80°C until use for biochemical studies. The time of postmortem delay varied between 2 h, 15 min, and 10 h, 50 min. The neuropathological diagnosis and staging was carried out according to well-established criteria for Alzheimer’s disease, PD and argyrophilic grain disease (9,29).
Lipid Raft Isolation
Lipid raft fractions from cortical gray matter were isolated following the protocol described by Mukherjee et al. (30) with slight modifications (28). Briefly, 0.1 g frontal cortex gray matter was homogenized at 4°C in 8 vol of isolation buffer (50 mmol/L Tris-HCl, pH 8.0, 10 mmol/L MgCl2, 20 mmol/L NaF, 1 mmol/L Na3VO4, 5 mmol/L β-mercaptoethanol and 1 mmol/L PMSF) and a cocktail of protease inhibitors (Roche Diagnostics, Barcelona, Spain) containing 1% Triton X-100 and 5% glycerol in a glass homogenizer for 5 min and then centrifuged at 500g for another 5 min. About 800 μL of the supernatant was mixed with an equal volume of 80% sucrose in isolation buffer and overlayed with 7.5 mL of a 36% sucrose solution and 2.7 mL of a 15% sucrose solution in isolation buffer, in 10-mL ultracentrifuge tubes (Ultraclear, Beckman). Sucrose gradients were centrifuged at 150,000g for 18 h at 4°C using a Beckman SW41Ti rotor. Then 2-mL fractions were collected from the top to the bottom, and the final pellet, corresponding to nonraft fractions, was collected and resuspended in 2 mL isolation buffer. All fractions were frozen at −80°C until analyses. Purity of lipid rafts (fraction 1) was assessed by analyses of protein markers and lipid composition (see below).
Western Blot Analyses
For protein characterization of lipid rafts, all fractions were resolved on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and incubated with different antibodies directed to raft (caveolin-1 and flotillin-1), nonraft (Na+/K+ ATPase α1 subunit and clathrin heavy chain), cytosolic (Cu/Zn superoxide dismutase, SOD-1) and mitochondrial (Mn superoxide dismutase, SOD-2) markers.
Equal volumes of all fractions isolated were resuspended in SDS loading buffer (625 mmol/L Tris-HCl, pH 6.8; 1% SDS; 10% glycerol; 5% β-mercaptoethanol; 0.01% bromophenol blue) and boiled at 95°C for 5 min to avoid protein aggregates. Protein contents were quantified by the bicinchoninic acid assay (BCA, Fisher Scientific, Madrid, Spain). Samples were run on SDS-PAGE, transferred to polyvinylidene fluoride (PVDF) transfer membranes (GE Healthcare) and processed for immunoblotting with the different lipid raft and nonraft markers used in this study. Membranes were first incubated with mouse monoclonal anti–caveolin-1 antibody (BD Transduction, 1:500) and then incubated with the corresponding horseradish peroxidase (HRP)-linked antimouse antibody (1:5,000). Afterward, the same membrane was successively reblotted with the antibodies specifically directed to flotillin-1 (BD Biosciences, 1:500), Na+/K+ ATPase α1 subunit (Santa Cruz Biotechnology, 1:1,000), clathrin heavy chain (BD Transduction 1:1,000), superoxide dismutase-1 (SOD-1, Novocastra, 1:1,000) and super-oxide dismutase-2 (SOD-2, StressGen, 1:5,000), followed by incubation with the corresponding HRP-linked secondary antibodies. When required, before reblotting, antibody stripping was performed by incubating the PVDF membrane in stripping buffer (2% SDS, 100 mmol/L β-mercaptoethanol, 50 mmol/L Tris, pH 6.8) at 50°C for 30 min with gentle agitation. Then membranes were rinsed several times before incubation with antibodies. Finally, the specific antibody signals were visualized by using the enhanced chemiluminescence (ECL) kit (GE Healthcare, Spain), followed by apposition of the membranes to autoradiographic films (GE Healthcare, Spain).
Lipid Analyses
Total lipids were extracted with chloroform/methanol (2:1 v/v). The organic solvent was evaporated under a stream of nitrogen, and the lipid content was determined gravimetrically and stored in chloroform/methanol (2:1 v/v) containing 0.01% of butylated hydroxytoluene (BHT) as an antioxidant (31). Lipid classes were separated from a fraction of total lipid by one-dimensional double development high-performance thin-layer chromatography using methyl acetate/isopropanol/chloroform/methanol/0.25% (w/v) KCl (5:5:5:2:1.8 by volume) as the developing solvent system for the polar lipid classes and hexane/diethyl ether/acetic acid (22.5:2.5:0.25 by volume) as the developing solvent system for the neutral lipid classes (31). Lipid classes were quantified by charring with 3% (w/v) aqueous cupric acetate containing 8% (v/v) phosphoric acid followed by calibrated scanning densitometry using a Shimadzu CS-9001PC dual-wavelength spot scanner. Equal amounts of total lipids were used in all analyses.
Lipids from cortical gray matter and from lipid rafts and nonrafts fractions were subjected to acid-catalyzed transmethylation for 16 h at 50°C, using 1 mL toluene and 2 mL 1% sulfuric acid (v/v) in methanol. The resultant fatty acid methyl esters were purified by thin-layer chromatography and visualized under spraying with 1% iodine in chloroform (31). Fatty acid methyl esters were separated and quantified by using a Shimadzu GC-14A gas chromatograph equipped with a flame ionization detector (250°C) and a fused silica capillary column Supelcowax™ 10 (30 m × 0.32 mm internal diameter). Individual fatty acid methyl esters were identified by referring to authentic standards.
Statistical Analyses
Comparison between groups was assessed either by one-way analysis of variance followed by the Tukey post hoc test or Kruskal-Wallis test followed by the Games-Howell post hoc test, depending on the homocedasticity and normality of experimental data. Results illustrated in Figure 4 and Table 2 were assessed using the Student t test. Data were arcsin transformed (percent lipid content) to attain the assumption of normality. Pearson correlation coefficients were used to express bivariate relationships between fatty acids and lipid classes. Multivariate statistics were performed using discriminant function analysis. Predictive variables were chosen according to the number of cases in each group to fulfill the assumptions of discriminant analysis (32). Individual canonical scores for each case and centroids were calculated using the SPSS statistical package, and the results were plotted to predict to which group a particular individual case belonged.
Figure 4.
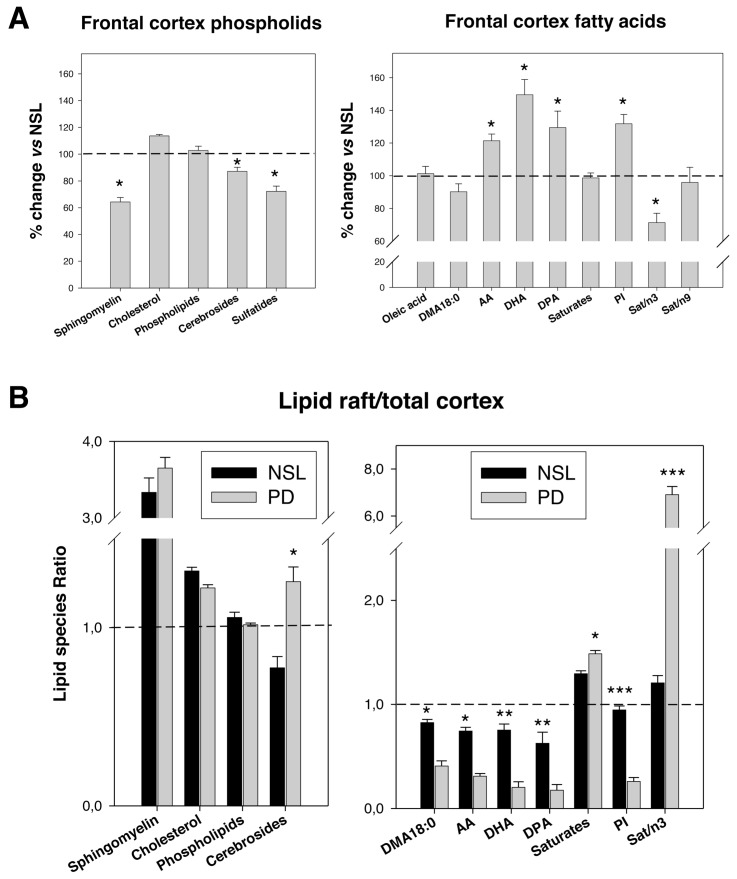
(A) Comparative analyses of main lipid classes and fatty acid contents and relevant indices between control and PD frontal cortex gray matter. Results are expressed as percent of change versus NSL group. Eight cases were analyzed in each group. *P < 0.1 and P < 0.05, respectively. (B) Analyses of the lipid raft-to-cortex ratios for representative lipid classes and fatty acid contents as well as for representative indices in the NSL and PD groups. Cortical and lipid raft were processed individually for each case. *P < 0.05, ** P < 0.01 and ***P < 0.005 compared with NSL group.
RESULTS
Characterization of Lipid Rafts from Normal Human Frontal Cortex
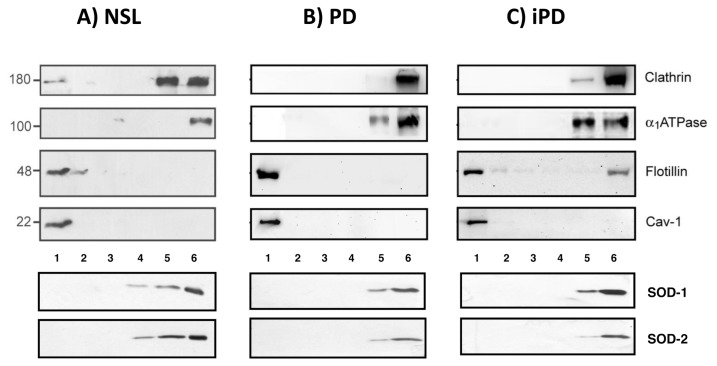
We first tested for the purity of lipid rafts isolated from the frontal cortex of human brains from control, PD or iPD, using different lipid raft and nonraft markers in immunoblotting assays. Thus, specific antibodies directed to lipid raft markers, caveolin-1 and flotillin-1 were used to identify raft-enriched fractions, observing that these two markers were mostly present in fraction 1 (Figure 1A). The same membranes were then used to reblot with antibodies directed to Na+/K+ ATPase α1 subunit and clathrin heavy chain, two proteins highly represented in nonraft fractions of the plasma membrane, observing that these antibodies recognized some bands exclusively in fractions 5 and 6. Similarly, cytosolic and mitochondrial markers SOD-1 and SOD-2, respectively, were present in fractions 5 and 6 but absent in fractions 1–4. Therefore, fraction 1 was selected for lipid analyses.
Figure 1.
Western blot analyses of protein markers in fractions 1–6 from NSL, PD and PDi brain cortices. Equal amounts of total protein were used for NSL, PD and iPD samples. Standard molecular weight values are indicated (left).
Lipid class analyses of lipid rafts from control subjects are detailed in Table 2. The results revealed a high content of sphingomyelin (11.4% ± 1.4%), cholesterol (33.0% ± 1.2%) and saturated fatty acids (53.86% ± 1.53%, shown in Table 3), which is consistent with the notion that lipid rafts are sphingomyelin- and cholesterol-enriched membrane micro -domains containing high levels of saturated acyl chains. Within phospholipid groups, phosphatidylethanolamine (PE) was the most abundant (21% ± 0.6%) glycerophospholipid, followed by phosphatidylserine (PS) (6.8% ± 0.5%), phosphatidylcholine (PC) (5.3% ± 0.5%) and phosphatidylinositol (PI) (2.2% ± 0.2%). Glycosphingolipid sulfates (sulfatides) and monoglycosylceramides (cerebro-sides) were also present in significant amounts in lipid rafts (10.6% ± 0.7% and 5.1% ± 0.8%, respectively). Comparison of lipid classes between lipid raft and nonraft fractions from control subjects (Table 2) confirmed the heterogeneous distribution of lipids between these two membrane domains. Thus, sphingomyelin content in nonraft fractions was around 17% that observed in lipid rafts (Table 2). Likewise, cholesterol in lipid rafts was significantly higher than in nonrafts (Table 2). Among phospholipids, nonraft fractions displayed higher contents of PC, PE, PS and PI than lipid raft fractions. Consequently, the phospholipid-to-cholesterol ratio was considerably higher in nonrafts (2.15) than in lipid rafts (1.11). Taken together, the outcomes from protein and lipid analyses confirm the high purity of lipid rafts isolated from brain cortex.
Table 3.
Fatty acid composition of lipid rafts from control subjects, PD and iPD.
| NSL | PD | iPD | |
|---|---|---|---|
| Fatty acids | |||
| 14:0 | 0.52 ± 0.04 | 0.62 ± 0.07 | 0.58 ± 0.04 |
| 14:1 | 0.13 ± 0.10ab | 0.18 ± 0.08a | 0.00 ± 0.00b |
| 15:0 | 0.91 ± 0.14ab | 1.42 ± 0.06a | 0.42 ± 0.15b |
| 15:1 | 0.10 ± 0.03a | 0.17 ± 0.04a | 0.00 ± 0.00b |
| 16:0 DMA | 1.82 ± 0.25 | 1.73 ± 0.26 | 1.44 ± 0.15 |
| 16:0 | 24.10 ± 1.31b | 28.66 ± 2.07ab | 31.98 ± 1.06a |
| 16:1 | 1.08 ± 0.08b | 3.94 ± 0.63a | 1.17 ± 0.13b |
| 16:2 | 0.20 ± 0.03b | 0.53 ± 0.21ab | 0.39 ± 0.02a |
| 17:0 | 0.31 ± 0.01a | 0.36 ± 0.02a | 0.05 ± 0.03b |
| 17:1 | 0.05 ± 0.02b | 0.35 ± 0.12a | 0.00 ± 0.00b |
| 18:0 DMA | 3.47 ± 0.11a | 1.54 ± 0.20b | 3.75 ± 0.27a |
| 18:1n-9 DMA | 0.76 ± 0.13a | 0.84 ± 0.09a | 0.29 ± 0.05b |
| 18:1n-7 DMA | 1.49 ± 0.38a | 0.79 ± 0.16ab | 0.56 ± 0.12b |
| 18:0 | 22.05 ± 0.43b | 26.35 ± 0.73a | 28.18 ± 0.65a |
| 18:1 n-9 | 17.64 ± 1.10a | 14.88 ± 1.33a | 10.09 ± 0.53b |
| 18:2 n-6 | 0.85 ± 0.09a | 0.38 ± 0.06b | 0.50 ± 0.10b |
| 20:1 | 1.08 ± 0.19 | 1.25 ± 0.19 | 0.68 ± 0.09 |
| 20:2 n-6 | 0.13 ± 0.04 | 0.07 ± 0.04 | 0.04 ± 0.03 |
| 20:3 n-6 | 0.53 ± 0.02a | 0.31 ± 0.05b | 0.44 ± 0.06ab |
| 20:4 n-6 | 3.70 ± 0.18a | 1.47 ± 0.12b | 1.26 ± 0.26b |
| 22:2 n-6 | 0.68 ± 0.09b | 2.77 ± 0.36a | 1.63 ± 0.28b |
| 23:0 | 2.40 ± 0.12a | 1.21 ± 0.12b | 1.32 ± 0.12b |
| 22:5 n-6 | 0.46 ± 0.06a | 0.09 ± 0.04b | 0.08 ± 0.04b |
| 22:5 n-3 | 0.14 ± 0.03a | 0.06 ± 0.03ab | 0.01 ± 0.01b |
| 22:6 n-3 | 6.87 ± 0.34a | 1.71 ± 0.22b | 1.41 ± 0.41b |
| Totals | |||
| Saturates | 53.86 ± 1.53b | 61.04 ± 2.41a | 66.96 ± 1.56a |
| n-3 | 7.11 ± 0.37a | 2.10 ± 0.24b | 1.95 ± 0.47b |
| n-6 | 6.35 ± 0.24a | 5.11 ± 0.48b | 4.18 ± 0.20b |
| n-9 | 19.63 ± 1.26a | 17.33 ± 1.27a | 10.88 ± 0.58b |
| n-3 LCPUFA | 7.11 ± 0.37a | 2.10 ± 0.24b | 1.91 ± 0.46b |
| Monoenes | 28.92 ± 1.65a | 29.21 ± 2.53a | 21.18 ± 0.97b |
| Ratios | |||
| n-3/n-6 | 1.12 ± 0.05a | 0.42 ± 0.03b | 0.45 ± 0.08b |
| 18:1/n-3 LCPUFA | 2.57 ± 0.22b | 8.26 ± 1.81a | 7.35 ± 1.77a |
| Saturates/n-3 | 7.75 ± 0.40b | 31.62 ± 3.43a | 47.01 ± 9.72a |
| Saturates/n-9 | 2.91 ± 0.26b | 3.71 ± 0.37b | 6.28 ± 0.36a |
| Indices | |||
| Unsaturation index | 93.73 ± 5.07a | 57.35 ± 2.47b | 44.86 ± 3.52c |
| Peroxidability index | 73.88 ± 3.35a | 26.70 ± 2.41b | 23.08 ± 4.48b |
Results are expressed as mole % and represent means ± SEM. Values in the same row bearing different letters are significantly different (P < 0.05). Unsaturation and peroxidability indices were calculated following the equations in Cosgrove et al. (63).
Individual fatty acid contents of lipid rafts from control subjects (group NSL) shown in Table 3 revealed the presence of significant contents of monoene fatty acids (principally oleic acid, 18:1n-9) and n-3 LCPUFA, specifically DHA (22:6n-3), whereas eicosapentaenoic acid (20:5n-3) level was negligible. PUFAs of the n-6 series accounted for 6.35% of total lipid raft fatty acids, of which arachidonic acid (AA) (22:4n-6) was the main constituent. Lipid rafts also exhibited significant amounts of dimethyl acetal (DMA) fatty acids, in particular, 18:0 DMA and 16:0 DMA. These ether fatty acids are formed exclusively from neutral plasmalogens upon acidic transesterification methods used to prepare fatty acid methyl esters (as used here). In the plasmalogen molecule, the aliphatic moieties at the sn-1 position consist of C18:0 (and to a lesser extent, C16:0 and C18:1-n9) carbon chains forming a vinyl-ether bond, whereas the sn-2 position is occupied by PUFAs, specifically AA or DHA (33,34). Therefore, the amount of DMA fatty acids stoichiometrically reflects that of plasmalogens, being commonly used as a quantitative measure of total plasmalogens, mainly represented by the PE class (plasmenylethanolamines) with rather less in PC, and commonly little or none in other phospholipids such as PI (33,35).
Lipid Composition of PD and iPD Lipid Rafts
Results shown in Tables 3 and 4 reveal that lipid rafts isolated from PD and iPD frontal cortices exhibit profound alterations in their lipid composition compared with control healthy subjects. First, lipid rafts from PD and iPD brains contain significantly higher contents of saturates (mainly 16:0 and 18:0) and lower unsaturated fatty acids than age-matched controls (as reflected in the unsaturation and peroxidability indices) (Table 3). In fact, the unsaturation index was reduced by 38% in PD and 52% in iPD compared with controls, indicating that lipid rafts in PD and iPD are significantly more viscous and liquid-ordered structures than in controls.
Table 4.
Lipid class composition of lipid rafts from control subjects, PD and iPD.
| NSL | PD | iPD | |
|---|---|---|---|
| Sphingomyelin | 11.43 ± 1.44 | 8.05 ± 0.64 | 10.30 ± 1.52 |
| PC | 5.35 ± 0.49 | 6.42 ± 0.49 | 7.11 ± 0.76 |
| PS | 6.82 ± 0.50b | 7.54 ± 0.55ab | 9.31 ± 0.46a |
| PI | 2.16 ± 0.17b | 2.20 ± 0.20b | 3.00 ± 0.24a |
| Phosphatidylglycerol | 0.70 ± 0.10 | 0.86 ± 0.19 | 1.09 ± 0.21 |
| PE | 20.97 ± 0.57b | 23.54 ± 0.36a | 22.14 ± 0.53ab |
| Sulfatides | 10.56 ± 0.68a | 10.35 ± 0.98ab | 7.37 ± 0.84b |
| Cerebrosides | 5.10 ± 0.79a | 5.98 ± 0.69a | 2.52 ± 0.60b |
| Diacylglycerols | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.0 ± 0.0 |
| Cholesterol | 33.04 ± 1.18 | 30.43 ± 1.17 | 35.16 ± 1.96 |
| Free fatty acids | 2.17 ± 0.28a | 2.70 ± 0.32a | 0.88 ± 0.31b |
| Triglycerides | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Sterol esters | 1.68 ± 0.60 | 1.94 ± 0.31 | 1.11 ± 0.57 |
| Neutral lipids | 36.90 ± 1.41 | 35.08 ± 1.23 | 37.15 ± 1.35 |
| Polar lipids | 63.09 ± 1.41 | 64.92 ± 1.23 | 62.85 ± 1.35 |
| Phospholipid/cholesterol | 1.11 ± 0.06b | 1.35 ± 0.09a | 1.26 ± 0.12ab |
Results are expressed as mole % and represent means ± SEM. Values in the same row bearing different letters are significantly different (P < 0.05).
Second, detailed analyses of unsaturated fatty acids in PD and iPD lipid rafts revealed abnormally low amounts of LCPUFAs (Table 3). Thus, in PD subjects, DHA (22:6n-3) and AA (22:4n-6) were reduced by 75% and 60%, respectively. Similar reductions were observed in the levels of these two fatty acids in iPD lipid rafts (79% and 66% for DHA and AA, respectively). Interestingly, the reduction of DHA in PD lipid rafts took place without a compensatory increase in docosapentaenoic acid (DPA) (22:5n-6). Instead, DPA content was significantly diminished by 80% in PD and 92% in iPD brains. Also, linoleic acid (18:2n-6), the metabolic precursor of AA, was significantly reduced by 55% and 41% in PD and iPD lipid rafts, respectively, which might resemble a limited ability to synthesize AA in degenerating nerve tissue. Regarding monoenes, no statistical differences were observed in the content of oleic acid (18:1n-9) or in the total n-9 series in PD, although a reduction of 15% in 18:1n-9 was evident. On the contrary, 18:1n-9 and n-9 fatty acids were considerably diminished in iPD (around 43% for both lipid parameters). As a consequence, the ratios saturates/n-3, saturates/n-9 and 18:1/n-3 were all dramatically increased in PD and iPD lipid rafts, whereas the ratio n-3/n-6 was significantly reduced (Table 3). Also, PD and iPD lipid rafts exhibited a considerably reduced unsaturation index (that could be entirely attributable to the reductions in LCPUFAs) as well as a dramatic reduction in the peroxidability of membrane lipids (63% and 68% in PD and iPD, respectively).
Third, lipid classes were also affected in PD and iPD lipid rafts (Table 4). Thus, PE was found to be significantly increased (12%) in PD lipid rafts (but not in iPD), whereas PS and PI levels were increased by 36% in iPD (but not in PD) compared with control cases (Table 4). Conversely, the amounts of cerebrosides and sulfatides were dramatically reduced by 30% and 51%, respectively, in iPD (but not in PD). In addition, clear trends were also observed for the reduction in sphingomyelin and the increase in PC in PD and iPD frontal cortex lipid rafts, although in these cases, changes were not statistically significant. In view of these findings, the alteration of lipid classes from lipid rafts was considerably more pronounced in iPD than in PD. As a consequence of the alterations in the contents of PE, PC and PS, the proportion of phospholipids to cholesterol in the lipid rafts from frontal cortex of PD and iPD brains appeared increased when compared with control subjects (Table 4), indicating a net reduction in the proportion of cholesterol in membrane rafts.
Regarding lipid rafts protein markers, there were no significant changes of flotillin or caveolin contents in lipid rafts from PD or iPD compared with age-matched controls, a finding that indicates that resident protein contents of rafts remained unaffected in PD or iPD (Figure 1).
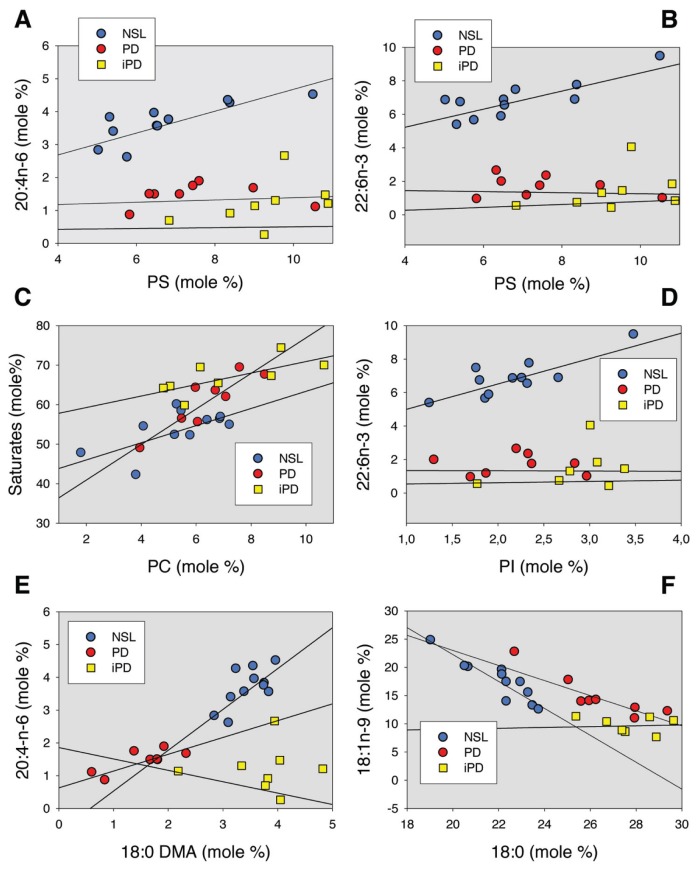
We have also explored the relevant bivariate relationships between lipid classes and fatty acids in control, PD and iPD lipid rafts. The results indicate that PD-induced neurodegeneration is accompanied by altered relationships between n-3 or n-6 and main LCPUFAs containing phospholipids (Figure 2). Thus, the observed linear association between PS and AA (Figure 2A) or DHA (Figure 2B) in control subjects (r = 0.792, P < 0.01, and r = 0.603, P < 0.05, respectively) completely disappeared in PD and iPD. The same applies to the relationship between PI and DHA (r = 0.815, P < 0.01 in control rafts), which vanished in PD and iPD (Figure 2D). These changes are specific for LCPUFAs, since other relationships, such as saturates (namely 16:0 + 18:0) versus PC (Figure 2C) or PS (not shown), remained unaffected in the neuropathological lipid rafts. Despite most alterations in bivariate lipid relationships being similar in PD and iPD, some differences could be detected between these two pathologies. Thus, there appeared to be a gradual change in the relationships between plasmalogen-associated 18:0 DMA and AA consisting on a progressive diminution of regression slopes with NSL (s = 1,245, r = 0.667, P < 0.05) > PD (s = 0.512, r = 0.780, P < 0.05) > iPD (s = −0.347, r = −0.014, P > 0.05) (Figure 2E). A similar interesting finding was observed for the saturated 18:0 versus monounsaturated 18:1n-9 relationship (Figure 2F), where NSL (s = −2.383, r = −0.907, P < 0.01) < PD (s = −1.335, r = −0.900, P < 0.01) < iPD (s = −0.072, r = 0.16, P > 0.05).
Figure 2.
Linear relationships for a subset of lipid parameters in lipid rafts from NSL, PD and iPD frontal cortices. Correlation coefficients and statistical significance are indicated in the text. Units are expressed as mole percentage for all variables.
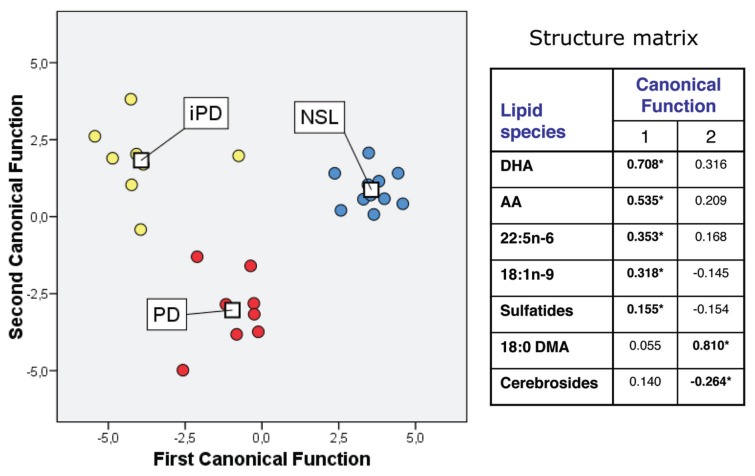
We also performed discriminant analysis on lipid raft composition to assess whether lipid composition per se could provide a predictive determination of the pathological state. Our results showed that the contribution to overall variance of the first and second canonical functions was 72.0% (χ2 = 87.15, P < 0.001) and 28.0% (χ2 = 35.22, P < 0.001), respectively. The combination of first and second discriminant functions clearly separates the NSL group from the PD and iPD groups (Figure 3). Structure matrix revealed that the variables that showed the highest absolute correlation (around ±0.3) with respect to every discriminate function were DHA, AA, 22:5n-6 and 18:1-n9 (for the first discriminant function), whereas the second function (which allows a neat discrimination between PD and iPD) was defined by the 18:0 DMA and cerebroside contents. One important outcome from this analysis was that PD and iPD groups could be resolved in the two- dimensional plot on the basis of subtle differences on gross fatty acids and lipid class composition.
Figure 3.
Discriminant function analyses of groups NSL, PD and iPD. In the scatterplot, centroids are represented (□). Table in the right panel shows the variable matrix structure for each canonical function. For details and interpretation, see Results and Discussion.
Lipid Composition of Frontal Brain Cortex
To obtain a macroscopic view of the changes in lipid composition in control and PD, we also performed lipid analyses on frontal cortex phospholipids from the same control and PD samples where lipid rafts were obtained. The main results are summarized in Figure 4 as percentage of change in PD compared with NSL. As it can be seen, no changes were observed in the proportion of phospholipids in the frontal gray matter between NSL and PD. However, a significant reduction in sphingomyelin (−35.7%), sulfatides (−12.8%) and cerebrosides (−27.8%) was evident in the PD cortex (Figure 4A). Regarding fatty acids, the results showed increased levels of AA (+21.4%) and DHA (+49.5 %) in PD cortex compared with age-matched controls (Figure 4A). Likewise, DPA (22:5n-6) content and peroxidability index were significantly augmented in the PD cortex, whereas total saturates (18:0 and 16:0), 18:1n-9 and 18:0 DMA (an index of plasmalogen content, see below) remained unaltered compared with control subjects. These results demonstrate that, while in whole cortical gray matter from PD brains levels of LCPUFAs are increased, in particular for DHA, their presence in lipid rafts are dramatically reduced, indicating the complexity of the deregulation cell membrane lipid metabolism during the development of PD.
To get a deeper insight into the molecular alterations in PD nerve cell membranes, we analyzed the relationship between individual lipid species in lipid raft and cortex in NSL and PD brains (Figure 4B) case by case. The results showed that, as expected, the sphingolipids and cholesterol ratios were considerably increased in lipid rafts compared with cortical tissues in both groups. Other lipid classes appeared equally distributed between lipid rafts and total cortex from either group (that is, total phospholipids), but fatty acids were dramatically affected in PD (Figure 4B). Thus, the ratios for AA, DHA, DPA and 18:0 DMA (as well as total n-3, total n-6 PUFA and peroxidability index) were considerably reduced in PD compared with NSL subjects. Moreover, because saturated fatty acids were significantly higher in NSL lipid rafts, the ratio saturates/n-3 LCPUFAs suffered a spectacular increase (more than six-fold) in PD.
DISCUSSION
The existence of lipid alterations in PD, especially in the cortex, has been poorly studied. Only a few studies have addressed the biochemical status of brain lipids in PD and related diseases, and none have previously assessed the extent of such lipid alterations in the molecular structure of lipid rafts from the neocortex. In the present work, we isolated and characterized highly purified lipid rafts from the human frontal cortex. Our data show that lipid rafts from control, PD and iPD subjects are enriched in sphingomyelin, cholesterol and saturated fatty acids compared with nonraft fractions. Likewise, protein markers flotillin-1 and caveolin-1 were only detected in fraction 1, which correspond to the lipid rafts fraction. These characteristics fit perfectly with the widely assumed composition of lipid rafts in cell membranes including nerve cells (22–24).
Interestingly, no differences were observed in any of the lipid raft protein markers or in the contents of sphingomyelin or cholesterol between control and iPD and PD, indicating that the basic supporting structure of lipid rafts remained unaltered in the frontal cortex of subjects suffering from either neurodegenerative disease. However, we cannot discard the possibility that impaired protein–protein and/or lipid–protein interactions may take place in these micro -structures of PD neurons, since it has been claimed for other neuropathologies such as Alzheimer’s disease (36). Conversely, our analyses point to massive modification of fatty acid contents in polar lipids from lipid rafts in PD and iPD. Thus, n-6 PUFA levels (principally AA and its metabolic precursor linoleic acid) were dramatically reduced both in PD and iPD (>60% for AA) compared with age-matched control subjects and, indeed, n-3 LCPUFA levels (mainly DHA) were reduced even to a larger extent (>75%), but without concomitant increase in DPA, which represented a minor component of total fatty acids in all groups. Significant reductions of monoene 18:1n-9 were also detected in lipid rafts from PD and iPD lipid rafts, and these alterations occurred in combination with significant increases in stearic acid (18:0). The stoichiometric consequence of these changes is that the significant negative relationship between 18:0 and 18:1n-9 observed in lipid rafts from healthy brains is progressively reduced in PD and completely absent in iPD samples (Figure 2F). Such a relationship is physicochemically relevant, since the ratio between both fatty acids represents an evolutionary conserved mechanism to preserve the homeoviscous state of cell membranes in response to different forms of physical and/or chemical stress (37,38). It can be surmised that the significant increase in saturated 18:0 and the reduction in 18:1n-9, along with a disappearance of the relationship between 18:0 and 18:1n-9 in iPD lipid rafts, may be indicative of a downregulation of Δ9-desaturase, the enzyme responsible for the synthesis of n-9 monounsaturated fatty acids from their saturated precursors (39). This occurrence would result in a concomitant loss of the ability to adjust the lipid raft physical order. Both aspects are currently under investigation in our laboratories.
The abnormalities in the fatty acid profiles of PD and iPD lipid rafts provoke a considerable reduction in the unsaturation (>40%) and peroxidability indices (>60%), also accompanied by dramatic increases in saturates/n-3 (>300%) and saturates/n-9 ratios (>30%), which indicates that raft microdomains from PD and iPD brain cortices are notably more viscous and liquid ordered than in the age-matched control group. Indeed, it has been experimentally demonstrated that n-3 LCPUFAs, mainly DHA, are incorporated into both cholesterol and sphingolipid-rich detergent-resistant liquid-ordered (lo) and liquid-disordered (ld) plasma membrane microdomains in many cell types (40,41). However, the poor affinity of DHA, and perhaps other LCPUFAs for cholesterol, provides a lipid-driven mechanism for lateral-phase separation of cholesterol- and sphingolipid-rich lipid microdomains from the surrounding ld phase in model membranes altering the size, physical order, elastic compressibility, lateral segregation and clustering of cell surface lipid microdomains (42–44). Furthermore, it has been proposed that PUFA impoverishment in microdomains may have profound consequences on the dynamic partitioning of acylated proteins, membrane fusion, rapid flip-flop, receptor binding and resident protein function, thereby altering signal transduction and neurotransmission (27,42,44).
Oxidative stress is considered to be one of the key factors in the pathogenesis of PD (45,46). Studies in the substantia nigra and midbrain have shown increased levels of lipid hydroperoxides (47) and 4-hydroxy-2-nonenal (an end product of lipid peroxidation) (48), as well as changes in PUFAs susceptible for lipid peroxidation, leading to increased generation of malondialdehyde and hydroperoxides (20,49). Interestingly, oxidative damage in the substantia nigra is present in iPD (20). Only recently, evidence for lipid peroxidation and oxidative damage in cortical structures was demonstrated in PD and iPD (20,50). Further, studies performed in the neocortex of subjects with iPD revealed the existence of oxidative damage, particularly lipoxidation of different proteins (including α-synuclein and Mn-superoxide dismutase), advanced glycation and oxidative DNA damage (as inferred by increased 8-hydroxyguanine levels) in early stages of PD neuropathology (19,20), indicating the priming role of oxidative stress in the generation of the disease. In agreement, we found that oxidative stress–related fatty acid modifications (such as the reduction of LCPUFAs and monoene contents, and the depression of unsaturation and peroxidability indices), as well as the loss of significant correlations between (i) PS and AA or DHA, (ii) PI and DHA and (iii) 18:0 and 18:1n-9, in lipid rafts from frontal cortex in PD and iPD, point to the generation of oxidative stress as a central causative factor for lipid raft lipid damage. The fact that most changes can be observed in iPD indicates that lipid raft lipid alterations represent early events in the development of the pathology.
Nonetheless, changes affecting lipid structure of lipid rafts in PD and iPD appear to indicate a more complex deregulation of lipid metabolism than merely oxidative stress–induced lipid oxidation. Indeed, we have found that, compared with control cases, anionic phospholipid PS and PI levels were increased by 36% in iPD (but not in PD), whereas neutral phospholipids remained unaffected. Further, the amounts of cerebrosides and sulfatides were dramatically reduced by >30% in iPD (but not in PD). The reduction of sulfatides and cerebrosides would affect nerve cell metabolism, since their deficiency is associated with increased ceramide production, which is formed upon hydrolysis by sulfatidase and galactsylcerebrosidase activities. Accumulation of ceramide has deleterious biological results, including upregulation of cytokines, generation of reactive oxygen species, interruption of the mitochondrial respiratory chain and apoptosis (reviewed in [51]). Further, elevation of ceramide and depletion of sulfatides has contributed to the neurodegeneration that occurs in Alzheimer’s disease (52).
Interestingly, the contents of lipid hallmarks in lipid raft structure, namely sphingolipids and cholesterol, remained unaltered in PD and iPD. These changes cannot be explained under an oxidative stress framework exclusively, but indicate that phospholipid metabolism was selectively affected since the early stages of the disease. It can be inferred that the increase in LCPUFA-containing neutral and anionic phospholipids (PE and PS, respectively) might reflect a transient compensatory mechanism involving phospholipid synthesis linked to early reduction in AA and DHA levels. In agreement with this notion, activity of phospholipid biosynthetic enzymes phosphoethanolamine cytidylyltrans-ferase, phosphocholine cytidylyltrans-ferase, and PS synthase have been found to be elevated in the substantia nigra of subjects suffering from idiopathic PD (53), a finding that has been interpreted as a compensatory response to repair membrane phospholipids in nigrastriatal neurons.
On the other hand, previous studies on lipid composition of PD and iPD brains have shown that highly peroxidable LCP-UFAs, especially DHA, are not only not reduced but are significantly increased in PD frontal cortex (20), which has lead to the hypothesis that DHA would be a major source of lipoperoxides promoting additional oxidative damage to cortical neurons. The increase in DHA contents in PD brains appeared to be restricted to the frontal cortex, since no changes are present in the amygdala, whereas a considerable reduction of DHA contents was observed in the substantia nigra from the same subjects (20). In principle, these observations in the frontal cortex are difficult to reconcile, with the results in this study showing a reduction of DHA in frontal cortex lipid rafts. Therefore, we found it worthwhile to perform lipid analyses on frontal cortex phospholipids from the same samples where lipid rafts were obtained. We could confirm increased levels of DHA and AA in the PD cortex compared with age-matched controls (Figure 4A). Likewise, DPA (22:5n-6) content and peroxidability index were significantly augmented in the PD cortex, whereas total saturates, monoenes and 18:0 DMA remained unaltered compared with control subjects. Also, PD gray matter exhibited lower levels of sphingolipids, cerebrosides and sulfatides than age-matched control subjects, but it is noteworthy that levels of individual phospholipids and cholesterol were similar to those of control brains.
Our results are well in agreement with previous data published for frontal cortex in PD pathology (20,54) and indicate increased levels of peroxidable fatty acids. These results demonstrate that, while in whole cortical tissues from PD brains, there are increased levels of LCP-UFAs (particularly DHA), their presence in lipid rafts was dramatically reduced. The opposite trends of lipid alteration between lipid rafts and brain cortex was further explored by analyzing case by case the content ratio (raft content/cortex content) for each molecular species. The outcomes of these analyses showed that the ratios for AA, DHA, DPA and 18:0 DMA (as well as total n-3 and n-6 PUFAs) were considerably reduced in PD compared with NSL subjects. These data demonstrate a differential deregulation of fatty acid metabolism and phospholipid remodeling between distinct membrane microdomains associated with the progression of the disease.
The fact that major lipid changes in PD and iPD frontal cortex lipid rafts affect fatty acid contents in phospholipids strongly indicates the abnormal function or expression of deacylation-reacylation enzymes during the development of Parkinson’s diseases. These include acyl-CoA synthetase (which converts free fatty acids into acyl-CoA esters), acyl-CoA:lysophospholipase acyltransferase (which catalyzes the transfer of acyl groups from acyl-CoA to a lysophospholipid acceptor) and phospholipase A2 (which is responsible for the hydrolysis of sn-2 fatty acids, especially AA and DHA, of a preexisting glycerophospholipid with the formation of a lysophospholipid) (55,56). A meta-analysis of our data suggests the abnormal function/expression of at least acyl-CoA:lysophospholipase acyltransferase (probably acyl-CoA:1-acyl-2-lysophospholipid acyltransferase, which prefers polyunsaturated fatty acyl-CoAs as acyl donors) and phospholipase A2, which would explain the reduction of DHA, DPA and AA contents in lipid raft phospholipids. Interestingly, cytosolic phospholipase A2 (cPLA2) activity was shown to be up-regulated in several types of neurode-generation, including spinal cord injury, ischemic injury and Alzheimer’s disease (57). In addition, mice deficient in cPLA2 activity are resistant to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) neurotoxicity, an experimental model of Parkinsonism, and this resistance strongly suggests that cPLA2 is closely associated with the pathophysiology of PD (58). Also, our data indicate that fatty acid metabolism in lipid rafts and nerve cell membranes from frontal cortex depends on the type of membrane microdomain considered (rafts versus nonrafts) and was profoundly altered during the development of PD pathology. In agreement, fatty acid synthase was recently shown to be rate-limiting in production of lipid molecules partitioning into Triton X-100 or Lubrol- resistant membrane microdomains. It was also pointed out that overexpression of fatty acid synthase is linked to deregulation of raft membrane composition and function (59).
One important conclusion emerging from our lipid raft analyses is the presence of significant amounts of dimethyl acetals fatty acids (~7.5 % of total fatty acids in polar lipids from NSL lipid rafts), which correlates with that of plasmalogens. One interesting property of plasmalogens is that they have the ability to form nonlamellar structures and can allow the transition of membranes from a lamellar gel to liquid-crystalline form. Obviously, these physical properties contribute to, or modulate, the formation and/or maintenance of lipid rafts (34). On the basis of these assumptions, the considerable reduction of plasmalogen levels in PD strongly indicates altered physicochemical properties and protein–lipid dynamics within frontal cortex lipid rafts in PD neuropathology. Interestingly, in light of the multivariate analyses performed here, it can be concluded that subtle changes observed in the plasmalogens (that is, 18:0 DMA) and cerebroside contents are sufficient to discriminate between PD and iPD lipid rafts. Moreover, it was suggested that plasmalogens can act as storage depots of DHA and AA given their high levels in plasmalogens when compared with their diacyl counterparts (34,35). In agreement, we could demonstrate that the impoverishment of plasmalogens correlates with that of AA (and DHA) in PD and iPD lipid rafts. Given that AA is the precursor for eicosanoid formation, its reduction would affect signal transduction in the frontal cortex.
Another crucial aspect whereby lipid raft alteration might be involved in the pathogenesis of PD is through its interaction with α-synuclein. It was shown that interaction with lipid rafts is essential for the normal, presynaptic localization of α-synuclein (60). The binding of α-synuclein to lipid rafts requires acidic phospholipids, with a preference for PS (60,61). Moreover, α-synuclein interaction with lipid rafts requires a combination of PS with oleic (18:1n-9) and polyunsaturated (either 20:4n-6 or 22:6n-3) fatty acyl chains (61). Thus, according to our present results, the depletion of DHA and AA in PD and iPD lipid rafts would force lipid rafts to exclude α-synuclein from this microdomain, eventually altering the dynamics of the aggregation/fibrillation state of α-synuclein (62), likely facilitating the formation of neurotoxic aggregates. Whether changes in LCPUFAs in lipid rafts are the cause or consequence of PD neurodegeneration cannot be ascertained from the present study but, in view of present results in iPD, it might constitute an early causative event.
In summary, our results demonstrate the existence of massive lipid alterations in lipid rafts from human frontal cortex in PD and iPD. The marked reductions of n-3 and n-6 LCPUFAs, plasmalogens and unsaturation index, together with the increase of saturated fatty acids, indicate that lipid rafts in PD pathologies exist in a notable viscous liquid-ordered state. Such physicochemical alterations would have a profound impact on lipid raft thermodynamic properties, spatial organization and signal transduction. The mechanisms responsible for the deregulation of plasma membrane lipid metabolism in PD appear to be complex and differentially affect the lipid structure of lipid rafts and other plasma membrane domains.
Table 1.
Summary of cases.
| Case | Age | Sex | Postmortem delay | Neuropathology |
|---|---|---|---|---|
| NSL | ||||
| 1 | 78 | M | 2 h 15 min | No lesions |
| 2 | 79 | M | 7 h | No lesions |
| 3 | 85 | M | 5 h 45 min | ADII/A, status cribosus |
| 4 | 70 | M | 13 h | ADI/A |
| 5 | 71 | M | 12 h | Lacunes |
| 6 | 73 | F | 5 h 30 min | ADI/0 |
| 7 | 82 | F | 11 h | ADI/A |
| 8 | 75 | F | 3 h | ADI/A, status cribosus |
| 9 | 69 | F | 2 h 30 min | ADI/0 |
| 10 | 66 | F | 8 h | No lesions |
| 11 | 65 | F | 4 h | No lesions |
| PD | ||||
| 12 | 70 | M | 9 h | PD4, ADII/B, lacunes |
| 13 | 68 | M | 4 h 45 min | PD4, ADIII/A, lacune |
| 14 | 76 | M | 4 h 30 min | PD4 |
| 15 | 85 | M | 3 h 15 min | PD4, ADIII/A, status cribosus |
| 16 | 69 | M | 5 h 55 min | PD4, ADI/A |
| 17 | 74 | F | 10 h 15 min | PD3, ADII/0, AGD1, status cribosus |
| 18 | 70 | F | 10 h 50 min | PD3, ADII/A |
| 19 | 70 | F | 5 h 15 min | PD4, ADII/A |
| iPD | ||||
| 20 | 72 | M | 8 h 55 min | PD1, ADII/A |
| 21 | 74 | M | 10 h 50 min | PD1, ADII/0, AGD3 |
| 22 | 83 | M | 3 h 30 min | PD2, ADIII/A, AGD3 |
| 23 | 83 | M | 4 h 30 min | PD2, ADII/A, AGD1 |
| 24 | 67 | M | 2 h 30 min | PD3, ADI/0 |
| 25 | 78 | M | 10 h 45 min | PD3, ADI/0 |
| 26 | 77 | F | 3 h 15 min | PD1, ADII/A, lacunes |
| 27 | 70 | F | 10 h 50 min | PD3, ADI/A, lacunes |
NL, no lesions; 1–4 refer to Braak stages of PD-related pathology. ADI, ADII and ADIII refer to Braak stages of neurofibrillary tangle pathology; 0, A and B refer to Braak stages of β amyloid plaques; AGD, argyrophilic grain disease; 1, 2 and 3 refers to AGD stages. Cases 1–11 had not suffered from neurological deficits, and the neuropathological examination did not reveal brain lesions. Cases 12–19 had suffered from PD, and the neuropathological examination revealed the presence of LB in selected brain stem nuclei and the limbic system but not in the cerebral neocortex. Cases 20–27 did not refer neurological deficits but showed LB in the medulla oblongata, pons and substantia nigra (depending on the stage), but neuron loss in the substantia nigra did not exceed the 50% of the total number of neurons in the pars compacta. Cases 1–11 were considered as controls cases 12–19 as PD with Parkinsonism and cases 20–27 as incidental PD.
ACKNOWLEDGMENTS
This study was supported by grants SAF2007-66148-C02-02 and SAF2010-22114-C02-01/02 from the Spanish Ministry of Science and Innovation and FIS-PI080582 from the Spanish Ministry of Health.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Forno LS. Neuropathology of Parkinson’s disease. J Neuropathol Exp Neurol. 1996;55:259–72. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Jellinger K, Mizuno Y. Parkinson’s disease. In: Dickson D, editor. Neurodegeneration: The Molecular Pathology of Dementia and Movement Disorders. Basel, Switzerland: ISN Neuropath Press; 2003. pp. 159–87. [Google Scholar]
- 3.Iwatsubo T. Aggregation of α-synuclein in the pathogenesis of Parkinson’s disease. J. Neurol. 2003;250(Suppl 3):11–4. doi: 10.1007/s00415-003-1303-x. [DOI] [PubMed] [Google Scholar]
- 4.Braak H, del Tredici K. Cortico-basal ganglia-cortical circuitry in Parkinson’s disease reconsidered. Exp Neurol. 2008;212:226–9. doi: 10.1016/j.expneurol.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Jellinger KA. Lewy body-related synucleinopathy in the aged human brain. J Neural Transm. 2004;111:1219–35. doi: 10.1007/s00702-004-0138-7. [DOI] [PubMed] [Google Scholar]
- 6.Saito Y, et al. Lewy body-related α-synucleinopathy in aging. J Neuropathol Exp Neurol. 2004;63:742–9. doi: 10.1093/jnen/63.7.742. [DOI] [PubMed] [Google Scholar]
- 7.Jellinger KA. A critical reappraisal of current staging of Lewy-related pathology in human brain. Acta Neuropathol. 2008;116:1–16. doi: 10.1007/s00401-008-0406-y. [DOI] [PubMed] [Google Scholar]
- 8.Jellinger KA. A critical evaluation of current staging of alpha-synuclein pathology in Lewy body disorders. Biochim Biophys Acta. 2009;1792:730–40. doi: 10.1016/j.bbadis.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Braak H, et al. Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson’s disease (preclinical and clinical stages) J. Neurol. 2002;249(Suppl 3):1–5. doi: 10.1007/s00415-002-1301-4. [DOI] [PubMed] [Google Scholar]
- 10.Braak H, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neuro-biol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 11.Dickson DW, et al. Evidence that incidental Lewy body disease is pre-symptomatic Parkinson’s disease. Acta Neuropathol. 2008;115:437–44. doi: 10.1007/s00401-008-0345-7. [DOI] [PubMed] [Google Scholar]
- 12.Metzler-Baddeley C. A review of cognitive impairments in dementia with Lewy bodies relative to Alzheimer’s disease and Parkinson’s disease with dementia. Cortex. 2007;43:583–600. doi: 10.1016/s0010-9452(08)70489-1. [DOI] [PubMed] [Google Scholar]
- 13.Rango M, Bonifati C, Bresolin N. Parkinson’s disease and brain mitochondrial dysfunction: a functional phosphorus magnetic resonance spectroscopy study. J Cereb Blood Flow Metab. 2006;26:283–90. doi: 10.1038/sj.jcbfm.9600192. [DOI] [PubMed] [Google Scholar]
- 14.Wallin A, et al. Posterior cortical brain dysfunction in cognitively impaired patients with Parkinson’s disease: a rCBF scintigraphic study. Acta Neurol Scand. 2007;116:347–54. doi: 10.1111/j.1600-0404.2007.00887.x. [DOI] [PubMed] [Google Scholar]
- 15.Tessa C, et al. A whole-brain analysis in de novo Parkinson disease. Am J Neuroradiol. 2008;29:674–80. doi: 10.3174/ajnr.A0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parkkinen L, Kauppinen T, Pirttila T, Autere JM, Alafuzoff I. Alpha-synuclein pathology does not predict extrapyramidal symptoms or dementia. Ann Neurol. 2005;57:82–91. doi: 10.1002/ana.20321. [DOI] [PubMed] [Google Scholar]
- 17.Ferrer I. Early involvement of the cerebral cortex in Parkinson’s disease: convergence of multiple metabolic defects. Prog Neurobiol. 2009;88:89–103. doi: 10.1016/j.pneurobio.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Navarro A, et al. Human brain cortex: mitochondrial oxidative damage and adaptive response in Parkinson’s disease and in dementia with Lewy bodies. Free Radic Biol Med. 2009;46:1574–80. doi: 10.1016/j.freeradbiomed.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez-Ramos JR, Overvik E, Ames BN. A marker of oxyradical-mediated DNA damage (8-hydroxy-2′-deoxyguanosine) is increased in nigrostriatum of Parkinson’s disease brain. Neurodegeneration. 1994;3:197–204. [Google Scholar]
- 20.Dalfó E, et al. Evidence of oxidative stress in the neocortex in incidental Lewy body disease. J Neuropathol Exp Neurol. 2005;64:816–30. doi: 10.1097/01.jnen.0000179050.54522.5a. [DOI] [PubMed] [Google Scholar]
- 21.Gómez A, Ferrer I. Increased oxidation of certain glycolysis and energy metabolism enzymes in the frontal cortex in Lewy body diseases. J Neurosci Res. 2009;87:1002–13. doi: 10.1002/jnr.21904. [DOI] [PubMed] [Google Scholar]
- 22.Brown DA, London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem. 2000;275:17221–4. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- 23.Pike LJ. Lipid rafts: bringing order to chaos. J Lipid Res. 2003;44:655–67. doi: 10.1194/jlr.R200021-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Pike LJ. The challenge of lipid rafts. J. Lipid Res. 2009;50(Suppl):S323–8. doi: 10.1194/jlr.R800040-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramírez CM, et al. VDAC and ERα interaction in caveolae from human cortex is altered in Alzheimer’s disease. Mol Cell Neurosci. 2009;42:172–83. doi: 10.1016/j.mcn.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Marín R, et al. Voltage-dependent anion channel (VDAC) participates in amyloid beta-induced toxicity and interacts with plasma membrane estrogen receptor α in septal and hippocampal neurons. Mol Membr Biol. 2007;24:148–60. doi: 10.1080/09687860601055559. [DOI] [PubMed] [Google Scholar]
- 27.Allen JA, Halverson-Tamboli RA, Rasenick MM. Lipid raft microdomains and neurotransmitter signalling. Nat Rev Neurosci. 2007;8:128–40. doi: 10.1038/nrn2059. [DOI] [PubMed] [Google Scholar]
- 28.Martín V, et al. Lipid alterations in lipid rafts from Alzheimer’s disease human brain cortex. J Alzheimers Dis. 2010;19:489–502. doi: 10.3233/JAD-2010-1242. [DOI] [PubMed] [Google Scholar]
- 29.Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318:121–34. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- 30.Mukherjee A, Arnaud L, Cooper JA. Lipid-dependent recruitment of neuronal Src to lipid rafts in the brain. J Biol Chem. 2003;278:40806–14. doi: 10.1074/jbc.M306440200. [DOI] [PubMed] [Google Scholar]
- 31.Christie WW. Lipids Analysis. Oxford, UK: Pergamon Press; 1982. [Google Scholar]
- 32.Huberty CJ. Applied Discriminant Analysis. New York: Wiley-Interscience; 1994. [Google Scholar]
- 33.Christie WW, Han X. Lipid Analysis. 3rd ed. Bridgewater, UK: Oily Press; 2003. [Google Scholar]
- 34.Brites P, Waterham HR, Wanders RJ. Functions and biosynthesis of plasmalogens in health and disease. Biochim Biophys Acta. 2004;1636:219–31. doi: 10.1016/j.bbalip.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 35.Ford DA, Gross RW. Plasmenylethanolamine is the major storage depot for arachidonic acid in rabbit vascular smooth muscle and is rapidly hydrolyzed after angiotensin II stimulation. Proc Natl Acad Sci U S A. 1989;86:3479–83. doi: 10.1073/pnas.86.10.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferrer I. Altered mitochondria, energy metabolism, voltage-dependent anion channel, and lipid rafts converge to exhaust neurons in Alzheimer’s disease. J Bioenerg Biomembr. 2009;41:425–31. doi: 10.1007/s10863-009-9243-5. [DOI] [PubMed] [Google Scholar]
- 37.McDonald AG. Application of the theory of homeoviscous adaptation to excitable membranes: pre-synaptic processes. Biochem J. 1988;256:313–27. doi: 10.1042/bj2560313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pamplona R, Barja G, Portero-Otín M. Membrane fatty acid unsaturation, protection against oxidative stress, and maximum life span: a homeoviscous-longevity adaptation. Ann N Y Acad Sci. 2002;959:475–90. doi: 10.1111/j.1749-6632.2002.tb02118.x. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura MT, Nara TY. Structure, function, and dietary regulation of delta6, delta5, and delta9 desaturases. Annu Rev Nutr. 2004;24:345–76. doi: 10.1146/annurev.nutr.24.121803.063211. [DOI] [PubMed] [Google Scholar]
- 40.Fan YY, McMurray DN, Ly LH, Chapkin RS. Dietary (n-3) polyunsaturated fatty acids remodel mouse T-cell lipid rafts. J Nutr. 2003;133:1913–2190. doi: 10.1093/jn/133.6.1913. [DOI] [PubMed] [Google Scholar]
- 41.Ma DW, et al. n-3 PUFA and membrane microdomains: a new frontier in bioactive lipid research. J Nutr Biochem. 2004;15:700–6. doi: 10.1016/j.jnutbio.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 42.Stillwell W, Wassall SR. Docosahexaenoic acid: membrane properties of a unique fatty acid. Chem Phys Lipids. 2003;126:1–27. doi: 10.1016/s0009-3084(03)00101-4. [DOI] [PubMed] [Google Scholar]
- 43.Shaikh SR, Cherezov V, Caffrey M, Stillwell W, Wassall SR. Interaction of cholesterol with a docosahexaenoic acid-containing phosphatidylethanolamine: trigger for microdomain/raft formation. Biochemistry. 2003;42:12028–37. doi: 10.1021/bi034931+. [DOI] [PubMed] [Google Scholar]
- 44.Shaikh SR, Rockett BD, Salameh M, Carraway K. Docosahexaenoic acid modifies the clustering and size of lipid rafts and the lateral organization and surface expression of MHC class I of EL4 cells. J Nutr. 2009;139:1632–9. doi: 10.3945/jn.109.108720. [DOI] [PubMed] [Google Scholar]
- 45.Jenner P. Oxidative stress in Parkinson’s disease. Ann. Neurol. 2003;53(Suppl 3):S26–38. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- 46.Kidd PM. Parkinson’s disease as multifactorial oxidative neurodegeneration: implications for integrative management. Altern Med Rev. 2000;5:502–29. [PubMed] [Google Scholar]
- 47.Dexter DT, et al. Lipid peroxidation as a cause of nigral death in Parkinson’s disease. Lancet. 1986;2:639–40. doi: 10.1016/s0140-6736(86)92471-2. [DOI] [PubMed] [Google Scholar]
- 48.Yoritaka A, et al. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc Natl Acad Sci U S A. 1996;93:2696–701. doi: 10.1073/pnas.93.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dexter DT, et al. Basal lipid peroxidation in substantia nigra is increased in Parkinson’s disease. J Neurochem. 1989;52:381–9. doi: 10.1111/j.1471-4159.1989.tb09133.x. [DOI] [PubMed] [Google Scholar]
- 50.Dexter DT, et al. Indices of oxidative stress and mitochondrial function in individuals with incidental Lewy body disease. Ann Neurol. 1994;35:38–44. doi: 10.1002/ana.410350107. [DOI] [PubMed] [Google Scholar]
- 51.Hannun YA, Luberto C. Ceramide in the eukaryotic stress response. Trends Cell Biol. 2000;10:73–80. doi: 10.1016/s0962-8924(99)01694-3. [DOI] [PubMed] [Google Scholar]
- 52.Cheng H, Xu J, McKeel DW, Jr, Han X. Specificity and potential mechanism of sulfatide deficiency in Alzheimer’s disease: an electro-spray ionization mass spectrometric study. Cell Mol Biol (Noisy-le-grand) 2003;49:809–18. [PubMed] [Google Scholar]
- 53.Ross BM, Mamalias N, Moszczynska A, Rajput AH, Kish SJ. Elevated activity of phospholipid biosynthetic enzymes in substantia nigra of patients with Parkinson’s disease. Neuroscience. 2001;102:899–904. doi: 10.1016/s0306-4522(00)00501-7. [DOI] [PubMed] [Google Scholar]
- 54.Sharon R, Bar-Joseph I, Mirick GE, Serhan CN, Selkoe DJ. Altered fatty acid composition of dopaminergic neurons expressing alpha-synuclein and human brains with alpha-synucleinopathies. J Biol Chem. 2003;278:49874–81. doi: 10.1074/jbc.M309127200. [DOI] [PubMed] [Google Scholar]
- 55.Farooqui AA, Horrocks LA, Farooqui T. Deacylation and reacylation of neural membrane glycerophospholipids. J Mol Neurosci. 2000;14:123–35. doi: 10.1385/jmn:14:3:123. [DOI] [PubMed] [Google Scholar]
- 56.Farooqui AA, Horrocks LA, Farooqui T. Glycerophospholipids in the brain: their metabolism, incorporation into membranes, functions, and involvement in neurological disorders. Chem Phys Lipids. 2000;106:1–29. doi: 10.1016/s0009-3084(00)00128-6. [DOI] [PubMed] [Google Scholar]
- 57.Farooqui AA, Ong WY, Horrocks LA. Inhibitors of brain phospholipase A2 activity: their neuropharmacological effects and therapeutic importance for the treatment of neurologic disorders. Pharmacol Rev. 2006;58:591–620. doi: 10.1124/pr.58.3.7. [DOI] [PubMed] [Google Scholar]
- 58.Klivenyi P, et al. Mice deficient in group IV cytosolic phospholipase A2 are resistant to MPTP neurotoxicity. J Neurochem. 1998;71:2634–37. doi: 10.1046/j.1471-4159.1998.71062634.x. [DOI] [PubMed] [Google Scholar]
- 59.Swinnen JW, et al. Fatty acid synthase drives the synthesis of phospholipids partitioning into detergent-resistant membrane domains. Biochem Biophys Res Commun. 2003;302:898–903. doi: 10.1016/s0006-291x(03)00265-1. [DOI] [PubMed] [Google Scholar]
- 60.Fortin DL, et al. Lipid rafts mediate the synaptic localization of alpha-synuclein. J Neurosci. 2004;24:6715–23. doi: 10.1523/JNEUROSCI.1594-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kubo S, et al. A combinatorial code for the interaction of alpha-synuclein with membranes. J Biol Chem. 2005;280:31664–72. doi: 10.1074/jbc.M504894200. [DOI] [PubMed] [Google Scholar]
- 62.Davidson WS, Jonas A, Clayton DF, George JM. Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem. 1998;273:9443–9. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- 63.Cosgrove JP, Church DF, Pryor WA. The kinetics of the autoxidation of polyunsaturated fatty acids. Lipids. 1987;22:299–304. doi: 10.1007/BF02533996. [DOI] [PubMed] [Google Scholar]