Abstract
BACKGROUND AND PURPOSE
Atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP) decrease vascular volume and pressure by activating guanylyl cyclase-A (GC-A). C-type natriuretic peptide (CNP) activation of guanylyl cyclase-B (GC-B) stimulates long bone growth. This study investigated the effects of the indolocarbazole, Gö6976, on the guanylyl cyclase activity of GC-A and GC-B as a first step towards developing small molecule regulators of these enzymes.
EXPERIMENTAL APPROACH
Whole cell cGMP concentrations or 32P-cGMP accumulation in membrane preparations measured the effects of indolocarbazoles on the enzymatic activity GC-A and GC-B from transfected 293T or endogenously expressing 3T3-L1 cells.
KEY RESULTS
Gö6976 blocked cellular CNP-dependent cGMP elevations in 293T-GC-B cells. The t½ for Gö6976 inhibition was 7 s and IC50 was 380 nM. Gö6976 increased the EC50 for CNP 4.5-fold, but increasing the CNP concentration did not overcome the inhibition. Half of the inhibition was lost 1 h after removal of Gö6976 from the medium. Cellular exposure to Gö6976 reduced basal and natriuretic peptide-dependent, but not detergent-dependent, GC-A and GC-B activity. Inhibition was also observed when Gö6976 was added directly to the cyclase assay. A constitutively phosphorylated form of GC-B was similarly inhibited.
CONCLUSIONS AND IMPLICATIONS
These data demonstrate that Gö6976 potently, rapidly and reversibly inhibited GC-A and GC-B via a process that did not require intact cells, known phosphorylation sites or inactivation of all catalytic sites. This is the first report of an intracellular inhibitor of a transmembrane guanylyl cyclase and the first report of a non-kinase target for Gö6976.
Keywords: indolocarbazole, guanylate cyclase, Gö6850, protein kinase C, cGMP
Introduction
Natriuretic peptides are pleiotropic factors that regulate the cardiovascular and skeletal systems (Potter et al., 2009). Humans express three structurally related but genetically distinct natriuretic peptides known as atrial natriuretic peptide (ANP), B-type natriuretic peptide (BNP) and C-type natriuretic peptide (CNP). Four natriuretic peptide receptors (NPR1-3 and GUCY2C; nomenclature follows Alexander et al., 2009) have been identified and two of these, guanylyl cyclase-A (GC-A; NPR1) and guanylyl cyclase-B (GC-B; NPR2) are composed of large extracellular ligand binding domains, single membrane-spanning regions and intracellular kinase homology, dimerization, and carboxyl-terminal guanylyl cyclase catalytic domains (Potter et al., 2009; Potter, 2009; 2011;). GC-A is activated by ANP and BNP, whereas GC-B is activated by CNP. Gene inactivation experiments in mice indicate that GC-A mediates the blood pressure and volume reducing effects of ANP and BNP, whereas GC-B mediates the effects of CNP on skeletal growth (Lopez et al., 1995; Tamura et al., 2004).
Homozygous inactivating mutations in GC-B cause a severe form of human dwarfism called acromesomelic dysplasia, type Maroteaux (AMDM). Human chromosomal translocations that increase CNP concentrations are associated with Marfanoid-like skeletal overgrowth (Bocciardi et al., 2007; Moncla et al., 2007). Recently, CNP infusions were shown to increase long bone growth in a murine model of fibroblast growth factor receptor-3-dependent dwarfism (Yasoda et al., 2009). Together, these data indicate that GC-B inactivation causes dwarfism, that GC-B overactivation causes Marfanoid-like skeletal overgrowth, and that CNP rescues long bone growth in a murine model of the most common form of human dwarfism. Thus, identifying small molecule inhibitors and activators of GC-B may lead to a new class of drugs for skeletal diseases.
In broken cell preparations, ATP increases the guanylyl cyclase activity of GC-A and GC-B when activated by natriuretic peptides but inhibits when activity is measured in the presence of manganese and non-ionic detergent (Kurose et al., 1987; Gazzano et al., 1991). Whether ATP is required for the initial activation of GC-A and GC-B by natriuretic peptide is controversial. We found that initial activation does not require ATP (Antos et al., 2005) but that activities measured at longer time periods are increased in the presence of ATP due to reduction of the Michaelis constant for GTP (Antos and Potter, 2007).
Both GC-A and GC-B are phosphorylated on multiple residues in resting cells (Yoder et al., 2010). Prolonged exposure to natriuretic peptide causes the dephosphorylation and desensitization of the receptors (Potter and Garbers, 1992; Potter, 1998). Conversion of known phosphorylation sites to alanine or glutamate yields hormonally unresponsive receptors or responsive receptors respectively (Potter and Hunter, 1999). Thus, phosphorylation is required for hormonal activation and dephosphorylation is a mechanism of desensitization.
Activation of protein kinase C (PKC) by phorbol 12-myristate, 13-acetate (PMA) inhibits hormone-dependent activation of GC-A and GC-B in a manner that correlates with site-specific receptor dephosphorylation (Potter and Garbers, 1994; Potter and Hunter, 2000; Potthast et al., 2004). Guanylyl cyclase inhibition and dephosphorylation by PMA is blocked by the general PKC inhibitor GF-109203X, which competes for ATP binding to the active site of PKC (Potter and Garbers, 1994; Abbey-Hosch et al., 2005). GF-109203X has no inhibitory effect on natriuretic peptide receptors in the absence of PKC activators. To identify the PKC isoform required for PMA-dependent inhibition of GC-B, cells were incubated with Gö6976, a compound that inhibits the conventional subgroup of the PKC family. However, an unexpected inhibitory effect of Gö6976 on GC-B activation was observed in the absence of PMA, which led to the studies described in this report. We found that Gö6976 potently, rapidly and reversibly inhibits GC-B by a mechanism that did not require changes in known phosphorylation sites, cellular architecture or complete disruption of the active site.
Methods
Cells
Human 293T cells stably expressing rat GC-A or GC-B were cultured as described (Potter and Hunter, 2000; Fan et al., 2005). Mouse 3T3-L1 cells were cultured as described (Student et al., 1980).
Whole cell stimulations
Cells were seeded on poly-D-lysine-coated 48-well plates and incubated >5 h in serum-free media upon reaching 70% confluency. The medium was aspirated and replaced with 0.25 mL Dulbecco's modified Eagle's medium (DMEM) containing 1 mM 1-methyl-3-isobutylxanthine (IBMX) and 25 mM HEPES pH 7.4 for 10 min. Following pretreatment, the medium was aspirated and cells were incubated with the new medium with or without 1 µM CNP for 1 min. The medium was aspirated and the reaction was stopped with 0.5 mL ice-cold 80% ethanol. The cGMP content of the extract was determined by radioimmunoassay as described (Abbey and Potter, 2002).
Guanylyl cyclase assays
Crude membranes were prepared in phosphatase inhibitor buffer as previously described (Bryan and Potter, 2002). Assays were performed at 37°C for 5 min in a buffer containing 25 mM HEPES pH 7.4, 50 mM NaCl, 0.1% BSA, 0.5 mM IBMX, 1 mM GTP, 10–30 µCi of α-32P-GTP, 1 mM EDTA, 0.5 mM microcystin, 1 mM ATP and 5 mM MgCl2 in the presence (stimulated) or absence (basal) of the indicated natriuretic peptide. In some assays, Mn2+ was substituted for Mg2+ and Triton X-100 was added to a final concentration of 1% to artificially activate the enzyme. Synthesized 32P-cGMP was purified and quantified as described (Bryan and Potter, 2002).
Concentration response assays
Cells in 48-well plates were exposed to different concentrations of Gö6976 or an equivalent volume of DMSO for 1 h at 37°C. The medium was aspirated and new medium with or without 1 µM CNP for was added. After 1 min, the medium was aspirated and the reaction was stopped with 0.5 mL ice-cold 80% ethanol and the cGMP content of the extract was determined.
Time course assays
Cells in 48-well plates were exposed to either 10 µM Gö6976 or an equivalent volume of DMSO for the periods of time indicated. The medium was then aspirated and replaced with new medium with or without 1 µM CNP. After 1 min, the medium was aspirated and the reaction was stopped with 0.5 mL ice-cold 80% ethanol and cGMP concentrations were determined.
Wash out experiments
293T GC-B cells were incubated with medium containing 1 µM Gö6976, 10 µM Gö6976 or DMSO for 5 min. After 5 min the cells were washed twice with medium and then new medium containing 1 µM CNP was added for 1 min at the indicated post-wash time points. The medium was aspirated and the reaction was stopped with 0.5 mL ice-cold 80% ethanol and cellular cGMP concentrations of cell extracts determined.
Statistical analysis
All curves were fitted using GraphPad Prism 5.0 software. All P-values were obtained using a paired Student's t-test in Microsoft Excel. Error bars represent the standard error of the mean and when not visible, are contained within the symbol.
Materials
Cyclic GMP radioimmunoassay kits and 32P-α GTP were from Perkin Elmer (Waltham, MA). Gö6976 was acquired from Sigma-Aldrich (Saint Louis, MO). GF-109203X, also known as Gö6850, was purchased from EMD chemicals (Gibbstown, NJ).
Results
Gö6976 inhibits CNP-dependent elevation of intracellular cGMP
Initial studies investigated the ability of PMA, Gö6976 and GF-109203X to modulate CNP-dependent cGMP elevations in 293T-GC-B cells. The structural similarity between Gö6976 and GF-109203X is shown in Figure 1A. CNP (1 µM) elevated intracellular cGMP concentrations 48-fold, but prior PMA exposure reduced these increases by more than 60% (Figure 1B). Exposure to GF-109203X for 60 min prior to CNP stimulation yielded intracellular cGMP concentrations that were elevated 55-fold higher than those from control cells. PMA did not significantly reduce CNP-dependent cGMP concentrations in cells exposed to GF-109203X, consistent with PMA inhibiting GC-B through a PKC-dependent mechanism. Surprisingly, Gö6976 alone completely blocked CNP-dependent cGMP concentrations, and exposure to both Gö6976 and PMA reduced CNP-dependent intracellular cGMP concentrations to levels that were below basal values.
Figure 1.
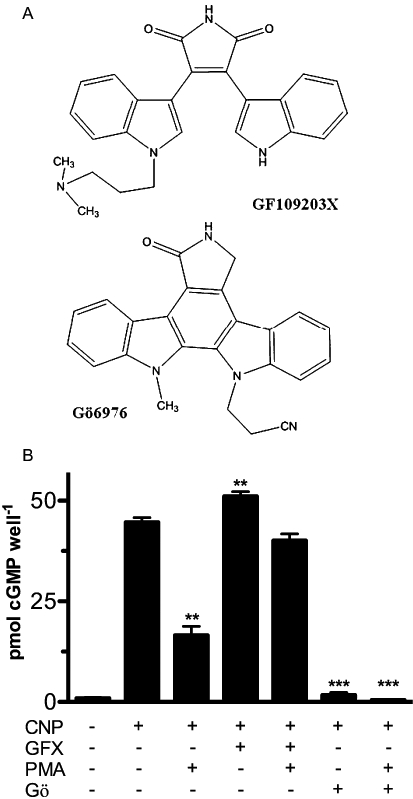
The protein kinase C inhibitor Gö6976 (Gö), but not GF-109203X (GFX), inhibits C-type natriuretic peptide (CNP)-dependent activation of GC-B in whole cells. A. The structures of GF-109203X and Gö6976 are shown. B. 293T-GC-B cells were incubated with or without the indicated compounds for 30 min at 37°C and then cells were stimulated with 1 µM CNP for 1 min and intracellular cGMP concentrations were determined. **P < 0.02; ***P < 0.001, compared with cGMP concentrations measured in cells treated with CNP alone; n = 6 from two experiments. PMA, phorbol 12-myristate 13-acetate.
Gö6976 inhibits the guanylyl cyclase activity of GC-B
To directly measure the effect of Gö6976 on the enzymic activity of GC-B, intact 293T-GC-B cells were incubated with 10 µM Gö6976 for 1 h and guanylyl cyclase activities in crude membranes were determined under basal, CNP-stimulated or detergent-stimulated conditions (Figure 2A). Exposure to Gö6976 (10 µM) reduced basal and CNP-dependent guanylyl cyclase activities by 51 and 49% respectively, but failed to significantly reduce activity measured in the presence of Triton X-100 and Mn2+GTP.
Figure 2.
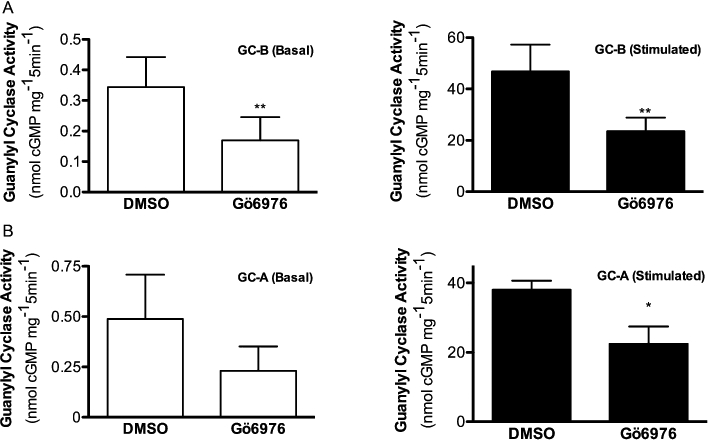
Gö6976 inhibits basal and hormone- but not detergent-dependent guanylyl cyclase-A and B activity. A. 293T-GC-B cells were incubated with DMSO (vehicle) or 10 µM Gö6976 for 1 h at 37°C and then crude membranes were prepared and guanylyl cyclase activities measured under basal (Left) or C-type natriuretic peptide (CNP)-stimulated (Right) conditions and presented as shown. Activities measured in the presence of Mn2+GTP and 1% Triton X-100 were 112.7 ± 18.7 and 101.5 ± 13.2 for DMSO and Gö6976 respectively. **P < 0.002, n = 12 from six experiments. B. 293T-GC-A cells were incubated with 10 µM Gö6976 and assayed for guanylyl cyclase activity under basal (Left) or CNP-stimulated (Right) conditions and presented as shown. Activities measured in the presence of Mn2+GTP and 1% Triton X-100 were 49.8 ± 8.5 and 45.2 ± 10.1 for DMSO and Gö6976 respectively, *P < 0.02, n≥ 4 from three experiments.
Gö6976 inhibits GC-A
Intact 293T cells stably expressing GC-A (293T-GC-A) were also incubated with 10 µM Gö6976 for 1 h and guanylyl cyclase activities in crude membranes were determined as described above except that ANP was substituted for CNP (Figure 2B). Incubation with Gö6976 (10 µM) reduced basal and ANP-dependent guanylyl cyclase activities by 53 and 40% respectively, but failed to significantly reduce activity measured in the presence of Triton X-100 and Mn2+GTP.
Gö6976 is a potent inhibitor of GC-B
293T-GC-B cells were incubated with increasing concentrations of Gö6976 and then CNP-dependent increases in cGMP were determined (Figure 3A). The concentration required to inhibit half of the maximal CNP-dependent cGMP response (IC50) was approximately 380 nM.
Figure 3.
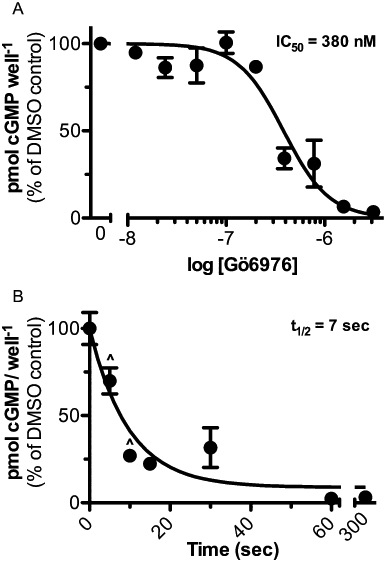
Gö6976 is a potent and rapid inhibitor of GC-B. A. 293T-GC-B cells were incubated with increasing concentrations of Gö6976 for 1 h at 37°C and then the cells were stimulated with 1 µM C-type natriuretic peptide (CNP) for 1 min and cGMP accumulation was determined and plotted as a function of log (Gö6976 concentration). IC50 = 380 nM, r2 = 0.81, n = 6 from two experiments. B. 293T-GCB cells were treated with 10 µM Gö6976 for the indicated periods of time and then stimulated with 1 µM CNP for 1 min. cGMP accumulation was measured and plotted as a function of time in the presence of Gö6976. t½ = 7 s, r2 = 0.82, n≥ 3 from two experiments. ^ indicates an n of 3, for all other points n = 6.
Gö6976 is a rapid inhibitor of GC-B
The time required for Gö6976 to reduce CNP-dependent concentrations to half of control values (t½) was also determined. 293T-GC-B cells were incubated with 10 µM Gö6976 for the periods shown and then the cells were incubated with 1 µM CNP for 1 min and intracellular cGMP concentrations were determined. The t½ was estimated to be 7 s (Figure 3B).
Gö6976 modestly increases the EC50 but inhibition is maintained at saturating CNP concentrations
The ability of Gö6976 to competitively antagonize CNP activation of GC-B was investigated. Whole 293T-GC-B cells were incubated with or without 10 µM Gö6976 for 30 min and then exposed to various concentrations of CNP for 1 min and intracellular cGMP concentrations were determined (Figure 4A). Absolute cGMP concentrations are shown in Figure 4A and the data are replotted with two Y-axes, as shown in Figure 4B. The calculated EC50s for control (DMSO) and Gö6976-treated cells were 40 nM and 180 nM respectively. A slight but significant 4.5-fold rightward shift in the dose–response curve in Gö6976-treated cells was observed (Figure 4B). However, the inhibitory effect was not overcome at saturating CNP concentrations.
Figure 4.
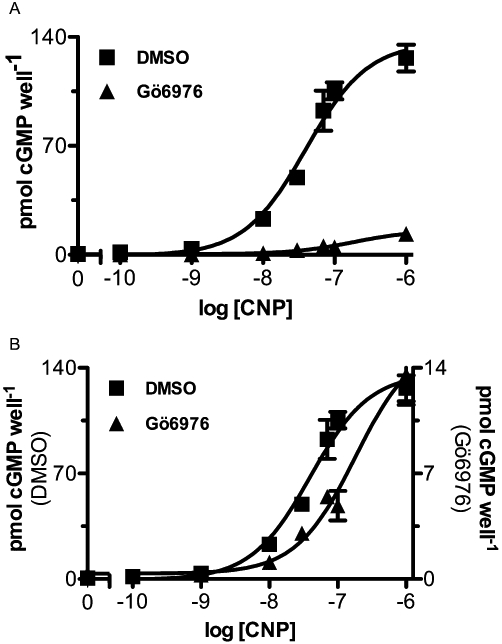
Gö6976 reduces maximum C-type natriuretic peptide (CNP)-dependent cGMP elevation and increases the EC50 for CNP. 293T-GCB cells were treated with DMSO or 10 µM Gö6976 for 30 min and then stimulated with the indicated concentrations of CNP for 1 min and intracellular cGMP concentrations were determined. A. Absolute values are plotted as pmol cGMP per well. B. Data are replotted with two Y-axes to show the rightward shift in the CNP dose–response curve in presence of Gö6976. DMSO EC50 = 40 nM, r2 = 0.91; Gö6976 EC50 = 181 nM, r2 = 0.83, where n = 6 from two experiments.
Gö6976 inhibition of GC-B is concentration-dependent and rapidly reversible
293T-GC-B cells were incubated with 10 µM Gö6976 for 5 min, washed twice with DMEM, and incubated for the indicated periods of time before exposing the cells to CNP for 1 min to elevate intracellular cGMP concentrations (Figure 5A). Approximately half of the activity was recovered 1 h after removal of Gö6976. When the concentration of Gö6976 was reduced 10-fold to 1 µM, recovery was more rapid (Figure 5B). The apparent half-time for recovery was 9 min and complete recovery was observed by 30 min in cells exposed to 1 µM Gö6976.
Figure 5.
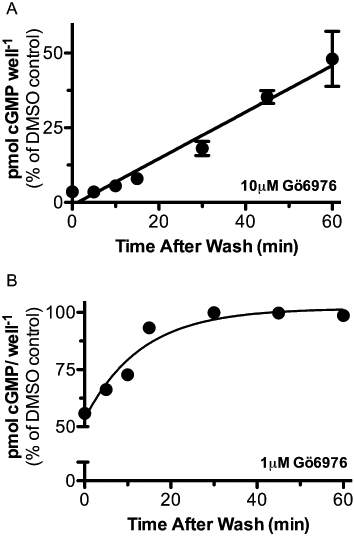
Inhibition of GC-B by Gö6976 is concentration-dependent and reversible. A. 293T-GC-B cells were treated with 10 µM Gö6976 for 5 min and then washed twice with Dulbecco's modified Eagle's medium (DMEM). At the indicated times, cells were stimulated with 1 µM C-type natriuretic peptide (CNP) for 1 min and cGMP accumulation was determined and plotted as a function of time after wash. Time required to recover half of the initial maximal activity was approximately 1 h, r2 = 0.93, where n = 6 from two experiments. B. 293T-GC-B cells were treated with 1 µM Gö6976 for 5 min and then washed twice with DMEM. At the indicated times, cells were stimulated with 1 µM CNP for 1 min and cGMP accumulation was determined and plotted as a function of time after wash. Time required to recover half of the initial maximal activity was approximately 9 min, r2 = 0.94, where n≥ 3 from two experiments.
Gö6976 inhibition does not require changes in known GC-B phosphorylation sites
Since Gö6976 inhibits several protein kinases and phosphorylation of GC-B is required for CNP-dependent activation (Potter, 1998; Potter and Hunter, 1998), we examined if Gö6976 reduces CNP-dependent activation by blocking GC-B phosphorylation (Figure 6). A cell line stably expressing a version of GC-B containing glutamate substitutions for all six known GC-B phosphorylation sites (293-GC-B-6E) was used in these experiments (Yoder et al., 2010). Gö6976 inhibited GC-B-6E similarly to the wild-type receptor, consistent with a mechanism that is independent of changes in the phosphorylation of known sites.
Figure 6.
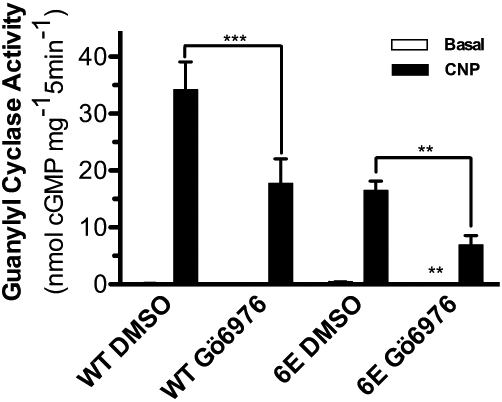
Gö6976 inhibition does not involve known GC-B phosphorylation sites. 293T-GC-B or 293T-GC-B 6E cells were incubated with DMSO or 10 µM Gö6976 for 1 h at 37°C. Crude membranes were prepared and guanylyl cyclase assays were determined under basal or stimulated conditions. **P < 0.01; ***P < 0.0005; n = 6 from three experiments.
Gö6976 inhibits GC-B in broken cell preparations
In all previous experiments, measuring cGMP concentrations in whole cells or cyclase activity in crude membranes assessed the effect of incubating whole cells with Gö6976. Here, the ability of Gö6976 to inhibit GC-B activity when directly added to membranes was examined. As a positive control for Gö6976 inhibition, whole 293T-GC-B cells were incubated in the presence or absence of Gö6976 for 1 h and then crude membranes were prepared and assayed for basal, CNP or detergent-dependent guanylyl cyclase activity (Figure 7A, Whole Cell). To test for direct inhibitory effects, crude membranes from 293T-GC-B cells that were not previously exposed to Gö6976 were incubated with Gö6976 or vehicle during the cyclase assay (Figure 7B, Membrane). Addition of Gö6976 to whole cells or crude membranes inhibited CNP-dependent GC-B activity to a similar extent, consistent with Gö6976 acting in a manner that does not require intact intracellular architecture or cellular incubation. We also investigated whether Gö6976 inhibited GC-B in a separate cell line. Gö6976 (10 µM) decreased CNP-dependent guanylyl cyclase activities at 5 and 10 min by 68 and 71% respectively, in membranes from mouse 3T3-L1 cells. We have previously shown that 3T3 cells express GC-B, but not GC-A (Abbey and Potter, 2003).
Figure 7.

Gö6976 inhibits C-type natriuretic peptide (CNP)-dependent activity in broken cell preparations. A. Intact 293T-GC-B cells were incubated with 10 µM Gö6976 or DMSO for 1 h at 37°C and then membranes were prepared and assayed for guanylyl activity (Whole Cell). Alternatively, 10 µM Gö6976 or DMSO was directly added to membranes prepared from naive 293T-GC-B cells and guanylyl cyclase activity was measured under stimulated conditions (Membrane). *P < 0.05; **P < 0.004, n = 11 from six experiments (Whole Cell) or n = 4 from two experiments (Membrane). B.10 µM Gö6976 or DMSO was directly added to membranes prepared from 3T3-L1 cells and then guanylyl cyclase activity was measured in the presence of 1 µM CNP for the periods of time indicated. n = 10 from two experiments.
Discussion
Little is known about the inhibition of GC-A and GC-B. HS-142-1, a microbial polysaccharide, was shown to block CNP-dependent bone growth (Yasoda et al., 1998) and to inhibit CNP-dependent cGMP elevations in podocytes (Lewko et al., 2004), but no direct studies on GC-B guanylyl cyclase activities have been reported. Studies by Lewko and colleagues indicated an IC50 of HS-142-1 between 0.25 and 2.5 µM, which is similar to the IC50 of 380 nM determined for Gö6976. Both known GC-A antagonists, HS-142-1 and A71915, block ANP binding to the extracellular domain of GC-A (Delporte et al., 1992; Sano et al., 1992). In contrast, Gö6976 does not inhibit GC-B by only reducing CNP binding since the shift in the EC50 was relatively small and the inhibition was maintained at high CNP concentrations (Figure 4A). Thus, Gö6976 uses a novel inhibitory mechanism compared to other antagonists of guanylyl cyclase-linked natriuretic peptide receptors.
The kinetics of the Gö6976-dependent inhibition of GC-B (t½ = 7 s) were striking. We have never seen any compound inhibit GC-B as rapidly as Gö6976. The fast kinetics of inhibition, relatively rapid reversibility and ability to inhibit GC-B in broken cell preparations are consistent with a model where Gö6976 binds directly to the receptor, but this remains to be determined.
Gö6976 is structurally similar to ATP and competitively inhibits ATP binding to various PKCs (Martiny-Baron et al., 1993). Therefore, it is possible that Gö6976 inhibits GC-B by blocking the ability of ATP to activate the receptor. Two reports have identified putative ATP binding sites in the kinase homology domain (KHD) of GC-A (Joubert et al., 2005; Burczynska et al., 2007), but whether these sites are conserved in GC-B and are required for ATP-dependent activation of GC-A is not known. An alternative hypothesis is that ATP binds one of the two putative nucleotide-binding sites in the catalytic domain of GC-B. Structural and functional data on a guanylyl cyclase isolated from cyanobacteria indicates two nucleotide-binding sites that have similar affinities for GTP and ATP (Rauch et al., 2008). When manganese is used as the divalent metal, GC-A, GC-B and the cyanobacteria guanylyl cyclase exhibit positive cooperative kinetics with respect to increasing GTP concentrations, consistent with two catalytic sites. However, under physiological conditions where magnesium is the divalent cofactor, linear kinetics are observed for GC-A and GC-B, consistent with a single catalytic site. We found that ATP decreases the Michaelis constant for Mg2+GTP, which is in agreement with a model where ATP increases the affinity of the catalytic site for GTP (Antos and Potter, 2007). Thus, it is possible that Gö6976 inhibits GC-A and GC-B by blocking binding of ATP to a regulatory site in the catalytic domain. The fact that Gö6976 does not inhibit detergent-dependent activity suggests that it does not directly inhibit the catalytic site, but we cannot rule out the possibility that the catalytic sites differ in the detergent-activated and natriuretic peptide-activated forms of the enzymes.
Recently Duda et al. (2010) reported that staurosporine activates GC-A similarly to AMP-PNP in a broken cell assay, which suggests that staurosporine mimics the ability of ATP to activate the receptor. However, we failed to observe activation of GC-B or GC-A by any of the indolocarbazoles that we tested in this report, including staurosporine (unpublished work; Potter et al.). Thus, in our hands, indolocarbazoles do not mimic the ability of ATP to activate receptor guanylyl cyclases.
In addition to the classic forms of PKC (Martiny-Baron et al., 1993), Gö6976 has been shown to inhibit the tyrosine kinases associated with the neurotrophin receptors, trk A and trk B, and the JAK2 and FLT3 tyrosine kinases (Behrens et al., 1999; Grandage et al., 2006). However, GC-A and GC-B are the first reported non-kinase enzymic targets of Gö6976. The IC50 of Gö6976 for whole cell inhibition of GC-B was similar to that reported for inhibition of JAK3 but higher than that reported for inhibition of the trk A and trk B receptors (Behrens et al., 1999; Grandage et al., 2006). Our data indicate that inhibition of the natriuretic peptide receptor guanylyl cyclases should be considered when interpreting the effects of Gö6976 on cell function.
In conclusion, we have shown for the first time that Gö6976 is a rapid, potent and reversible inhibitor of GC-A and GC-B that does not require changes in phosphorylation sites or modification of the active site. Future studies will investigate the mechanism of action of Gö6976 on GC-A and GC-B.
Acknowledgments
This work was supported by National Institutes of Health Heart, Lung, and Blood Institute (Grant R21HL093402) to L. R. Potter and National Institute of Arthritis and Musculoskeletal and Skin Diseases (Grant T32AR050938) to J. W. Robinson. We thank Dr Ann Hertzel and Dr David Bernlohr for 3T3-L1 cells.
Glossary
Abbreviations
- ANP
atrial natriuretic peptide
- BNP
B-type (brain) natriuretic peptide
- CNP
C-type natriuretic peptide
- DMEM
Dulbecco's modified Eagle's medium
- GC-A
guanylyl cyclase-A
- GC-B
guanylyl cyclase-B
- PKC
protein kinase C
- PMA
phorbol 12-myristate 13-acetate
Conflicts of interest
The authors declare no conflicts of interest.
References
- Abbey SE, Potter LR. Vasopressin-dependent inhibition of the C-type natriuretic peptide receptor, NPR-B/GC-B, requires elevated intracellular calcium concentrations. J Biol Chem. 2002;277:42423–42430. doi: 10.1074/jbc.M206686200. [DOI] [PubMed] [Google Scholar]
- Abbey SE, Potter LR. Lysophosphatidic acid inhibits C-type natriuretic peptide activation of guanylyl cyclase-B. Endocrinology. 2003;144:240–246. doi: 10.1210/en.2002-220702. [DOI] [PubMed] [Google Scholar]
- Abbey-Hosch SE, Smirnov D, Potter LR. Differential regulation of NPR-B/GC-B by protein kinase c and calcium. Biochem Pharmacol. 2005;70:686–694. doi: 10.1016/j.bcp.2005.04.034. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edn. Br J Pharmacol. 2009;158(Suppl. 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antos LK, Potter LR. Adenine nucleotides decrease the apparent Km of endogenous natriuretic peptide receptors for GTP. Am J Physiol Endocrinol Metab. 2007;293:E1756–E1763. doi: 10.1152/ajpendo.00321.2007. [DOI] [PubMed] [Google Scholar]
- Antos LK, Abbey-Hosch SE, Flora DR, Potter LR. ATP-independent activation of natriuretic peptide receptors. J Biol Chem. 2005;280:26928–26932. doi: 10.1074/jbc.M505648200. [DOI] [PubMed] [Google Scholar]
- Behrens MM, Strasser U, Choi DW. Go 6976 is a potent inhibitor of neurotrophin-receptor intrinsic tyrosine kinase. J Neurochem. 1999;72:919–924. doi: 10.1046/j.1471-4159.1999.0720919.x. [DOI] [PubMed] [Google Scholar]
- Bocciardi R, Giorda R, Buttgereit J, Gimelli S, Divizia MT, Beri S, et al. Overexpression of the C-type natriuretic peptide (CNP) is associated with overgrowth and bone anomalies in an individual with balanced t(2;7) translocation. Hum Mutat. 2007;28:724–731. doi: 10.1002/humu.20511. [DOI] [PubMed] [Google Scholar]
- Bryan PM, Potter LR. The atrial natriuretic peptide receptor (NPR-A/GC-A) is dephosphorylated by distinct microcystin-sensitive and magnesium-dependent protein phosphatases. J Biol Chem. 2002;277:16041–16047. doi: 10.1074/jbc.M110626200. [DOI] [PubMed] [Google Scholar]
- Burczynska B, Duda T, Sharma RK. ATP signaling site in the ARM domain of atrial natriuretic factor receptor guanylate cyclase. Mol Cell Biochem. 2007;301:93–107. doi: 10.1007/s11010-006-9400-7. [DOI] [PubMed] [Google Scholar]
- Delporte C, Winand J, Poloczek P, Von Geldern T, Christophe J. Discovery of a potent atrial natriuretic peptide antagonist for ANPA receptors in the human neuroblastoma NB-OK-1 cell line. Eur J Pharmacol. 1992;224:183–188. doi: 10.1016/0014-2999(92)90803-c. [DOI] [PubMed] [Google Scholar]
- Duda T, Yadav P, Sharma RK. ATP allosteric activation of atrial natriuretic factor receptor guanylate cyclase. FEBS J. 2010;277:2550–2553. doi: 10.1111/j.1742-4658.2010.07670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D, Bryan PM, Antos LK, Potthast RJ, Potter LR. Down-regulation does not mediate natriuretic peptide-dependent desensitization of natriuretic peptide receptor (NPR)-A or NPR-B: guanylyl cyclase-linked natriuretic peptide receptors do not internalize. Mol Pharmacol. 2005;67:174–183. doi: 10.1124/mol.104.002436. [DOI] [PubMed] [Google Scholar]
- Gazzano H, Wu HI, Waldman SA. Adenine nucleotide regulation of particulate guanylate cyclase from rat lung. Biochim Biophys Acta. 1991;1077:99–106. doi: 10.1016/0167-4838(91)90531-4. [DOI] [PubMed] [Google Scholar]
- Grandage VL, Everington T, Linch DC, Khwaja A. Go6976 is a potent inhibitor of the JAK 2 and FLT3 tyrosine kinases with significant activity in primary acute myeloid leukaemia cells. Br J Haematol. 2006;135:303–316. doi: 10.1111/j.1365-2141.2006.06291.x. [DOI] [PubMed] [Google Scholar]
- Joubert S, Jossart C, McNicoll N, De Lean A. Atrial natriuretic peptide-dependent photolabeling of a regulatory ATP-binding site on the natriuretic peptide receptor-A. Febs J. 2005;272:5572–5583. doi: 10.1111/j.1742-4658.2005.04952.x. [DOI] [PubMed] [Google Scholar]
- Kurose H, Inagami T, Ui M. Participation of adenosine 5′-triphosphate in the activation of membrane-bound guanylate cyclase by the atrial natriuretic factor. Febs Lett. 1987;219:375–379. doi: 10.1016/0014-5793(87)80256-9. [DOI] [PubMed] [Google Scholar]
- Lewko B, Endlich N, Kriz W, Stepinski J, Endlich K. C-type natriuretic peptide as a podocyte hormone and modulation of its cGMP production by glucose and mechanical stress. Kidney Int. 2004;66:1001–1008. doi: 10.1111/j.1523-1755.2004.00848.x. [DOI] [PubMed] [Google Scholar]
- Lopez MJ, Wong SK, Kishimoto I, Dubois S, Mach V, Friesen J, et al. Salt-resistant hypertension in mice lacking the guanylyl cyclase-A receptor for atrial natriuretic peptide. Nature. 1995;378:65–68. doi: 10.1038/378065a0. [DOI] [PubMed] [Google Scholar]
- Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, et al. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J Biol Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- Moncla A, Missirian C, Cacciagli P, Balzamo E, Legeai-Mallet L, Jouve JL, et al. A cluster of translocation breakpoints in 2q37 is associated with overexpression of NPPC in patients with a similar overgrowth phenotype. Hum Mutat. 2007;12:1183–1188. doi: 10.1002/humu.20611. [DOI] [PubMed] [Google Scholar]
- Potter LR. Phosphorylation-dependent regulation of the guanylyl cyclase-linked natriuretic peptide receptor B: dephosphorylation is a mechanism of desensitization. Biochemistry. 1998;37:2422–2429. doi: 10.1021/bi972303k. [DOI] [PubMed] [Google Scholar]
- Potter LR. Guanylyl Cyclases. In: Bradshaw RA, Dennis EA, editors. Handbook of Cell Signaling. 2nd edn. Oxford: Academic Press; 2009. pp. 1399–1407. [Google Scholar]
- Potter LR. Regulation and therapeutic targeting of peptide-activated receptor guanylyl cyclases. Pharmacol Ther. 2011;130:71–82. doi: 10.1016/j.pharmthera.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter LR, Garbers DL. Dephosphorylation of the guanylyl cyclase-A receptor causes desensitization. J Biol Chem. 1992;267:14531–14534. [PubMed] [Google Scholar]
- Potter LR, Garbers DL. Protein kinase C-dependent desensitization of the atrial natriuretic peptide receptor is mediated by dephosphorylation. J Biol Chem. 1994;269:14636–14642. [PubMed] [Google Scholar]
- Potter LR, Hunter T. Identification and characterization of the major phosphorylation sites of the B-type natriuretic peptide receptor. J Biol Chem. 1998;273:15533–15539. doi: 10.1074/jbc.273.25.15533. [DOI] [PubMed] [Google Scholar]
- Potter LR, Hunter T. A constitutively “Phosphorylated” guanylyl cyclase-linked atrial natriuretic peptide receptor mutant is resistant to desensitization. Mol Biol Cell. 1999;10:1811–1820. doi: 10.1091/mbc.10.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter LR, Hunter T. Activation of PKC stimulates the dephosphorylation of natriuretic peptide receptor-B at a single serine residue: a possible mechanism of heterologous desensitization. J Biol Chem. 2000;275:31099–31106. doi: 10.1074/jbc.M005506200. [DOI] [PubMed] [Google Scholar]
- Potter LR, Yoder AR, Flora DR, Antos LK, Dickey DM. Natriuretic peptides: their structures, receptors, physiologic functions and therapeutic applications. Handb Exp Pharmacol. 2009;191:341–366. doi: 10.1007/978-3-540-68964-5_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potthast R, Abbey-Hosch SE, Antos LK, Marchant JS, Kuhn M, Potter LR. Calcium-dependent dephosphorylation mediates the hyperosmotic and lysophosphatidic acid-dependent inhibition of natriuretic peptide receptor-B/Guanylyl Cyclase-B. J Biol Chem. 2004;279:48513–48519. doi: 10.1074/jbc.M408247200. [DOI] [PubMed] [Google Scholar]
- Rauch A, Leipelt M, Russwurm M, Steegborn C. Crystal structure of the guanylyl cyclase Cya2. Proc Natl Acad Sci USA. 2008;105:15720–15725. doi: 10.1073/pnas.0808473105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano T, Imura R, Morishita Y, Matsuda Y, Yamada K. HS-142-1, a novel polysaccharide of microbial origin, specifically recognizes guanylyl cyclase-linked ANP receptor in rat glomeruli. Life Sci. 1992;51:1445–1451. doi: 10.1016/0024-3205(92)90539-2. [DOI] [PubMed] [Google Scholar]
- Student AK, Hsu RY, Lane MD. Induction of fatty acid synthetase synthesis in differentiating 3T3-L1 preadipocytes. J Biol Chem. 1980;255:4745–4750. [PubMed] [Google Scholar]
- Tamura N, Doolittle LK, Hammer RE, Shelton JM, Richardson JA, Garbers DL. Critical roles of the guanylyl cyclase B receptor in endochondral ossification and development of female reproductive organs. Proc Natl Acad Sci USA. 2004;101:17300–17305. doi: 10.1073/pnas.0407894101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasoda A, Ogawa Y, Suda M, Tamura N, Mori K, Sakuma Y, et al. Natriuretic peptide regulation of endochondral ossification. Evidence for possible roles of the C-type natriuretic peptide/guanylyl cyclase-B pathway. J Biol Chem. 1998;273:11695–11700. doi: 10.1074/jbc.273.19.11695. [DOI] [PubMed] [Google Scholar]
- Yasoda A, Kitamura H, Fujii T, Kondo E, Murao N, Miura M, et al. Systemic administration of C-type natriuretic peptide as a novel therapeutic strategy for skeletal dysplasias. Endocrinology. 2009;150:3138–3144. doi: 10.1210/en.2008-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder AR, Stone MD, Griffin TJ, Potter LR. Mass spectrometric identification of phosphorylation sites in guanylyl cyclase A and B. Biochemistry. 2010;49:10137–10145. doi: 10.1021/bi101700e. [DOI] [PMC free article] [PubMed] [Google Scholar]


