Abstract
BACKGROUND AND PURPOSE
We investigated the effect of the phosphodiesterase-5 inhibitor, tadalafil, on the acute hypernociception in rat models of arthritis.
EXPERIMENTAL APPROACH
Rats were treated with either an intra-articular injection of zymosan (1 mg) or surgical transection of the anterior cruciate ligament (as an osteoarthritis model). Controls received saline intra-articular or sham operation respectively. Joint pain was evaluated using the articular incapacitation test measured over 6 h following zymosan or between 4 and 7 days after anterior cruciate ligament transection. Cell counts, tumour necrosis factor-α (TNF-α), interleukin-1 (IL-1), and the chemokine, cytokine-induced neutrophil chemoattractant-1 (CINC-1) were measured in joint exudates 6 h after zymosan. Groups received tadalafil (0.02–0.5 mg·kg−1per os) or saline 2 h after intra-articular zymosan. Other groups received the µ-opioid receptor antagonist naloxone or the cGMP inhibitor 1H-[1,2,4] oxadiazolo [4,3-a] quinoxalin-1-one (ODQ) before tadalafil.
KEY RESULTS
Tadalafil dose-dependently inhibited hypernociception in zymosan and osteoarthritis models. In zymosan-induced arthritis, tadalafil significantly decreased cell influx and TNF-α release but did not alter IL-1 or CINC-1 levels. Pretreatment with ODQ but not with naloxone prevented the anti-inflammatory effects of tadalafil.
CONCLUSIONS AND IMPLICATIONS
Therapeutic oral administration of tadalafil provided analgesia mediated by guanylyl cyclase and was independent of the release of endogenous opioids. This effect of tadalafil was associated with a decrease in neutrophil influx and TNF-α release in inflamed joints.
Keywords: tadalafil, PDE inhibitors, pain, cytokines, chemokines, neutrophils, arthirits, inflammation
Introduction
Modulation of pro-inflammatory and anti-inflammatory mediators by specific phosphodiesterase (PDE) inhibitors is a growing strategy for the treatment of inflammatory diseases (Boswell-Smith et al., 2006). Cilomilast, a cAMP-specific PDE inhibitor, has been evaluated in clinical trials for inflammatory airway diseases (Rennard et al., 2006). Our group has shown that the PDE-4 inhibitor rolipram promoted antinociception and anti-inflammatory effects in a rat model of adjuvant-induced arthritis, effects associated with reduction of local and systemic TNF-α production (Francischi et al., 2000), raising the possibility that other PDE inhibitors exert similar effects.
The NO – cGMP pathway participates in nociception in inflammatory arthritis. Intracellular cGMP concentrations are controlled by the action of guanylyl cyclases and its degradation rate by specific PDEs; PDE-5, -6 and -9 degrade cGMP, but PDE-5 seems to be the most relevant in cGMP inactivation inside cells (Beavo, 1995). As a consequence, the antinociceptive effects of PDE inhibitors are associated with an increase of intracellular cGMP levels in nociceptive neurons. There is considerable evidence that sildenafil, a PDE-5 inhibitor used in the treatment of erectile dysfunction, produces antinociception in pain models (Asomoza-Espinosa et al., 2001; Jain et al., 2001). On the other hand, it has also been reported that zaprinast, another PDE-5 inhibitor, had no effect on prostaglandin E2 (PGE2) and carrageenan – induced hyperalgesia (Amarante and Duarte, 2002) suggesting that the antinociceptive effect of PDE-5 inhibitors may vary among different models.
The biological effects of PDE inhibitors have been associated with inhibition of cytokine production. Zaprinast reduced both TNF-α and IL-6 production by murine alveolar epithelial cells exposed to lipopolysaccharide (Haddad et al., 2002), and it also reduced PGE2 release by porcine epithelial cells exposed to arachidonic acid while not affecting IL-8 release induced by TNF-α in bronchial cells (Fuhrmann et al., 1999).
Presently, there are three PDE-5 inhibitors in clinical practice to treat erectile dysfunction: sildenafil, tadalafil and vardenafil (Shabsigh, 2004). Although they present similar mechanisms of action variations in the structure, pharmacokinetics and PDE specificity may account for differences among them (Blount et al., 2004). In keeping with this possibility, tadalafil, but not sildenafil or vardenafil, significantly decreased hypoxia-induced up-regulation of TNF-α and IL-1β in rat isolated pulmonary arteries (Tsai et al., 2006).
Transection of the anterior cruciate ligament has been frequently used in different species, as an experimental model of osteoarthritis. We have recently standardized a method to reproducibly and quantitatively study joint pain that occurs in this model (Castro et al., 2006). In the present study, we investigated the antinociceptive activity of tadalafil in two models of arthritis, induced by zymosan or by transection of the anterior cruciate ligament. Our data demonstrated that oral administration of tadalafil provided therapeutic antinociception and was associated with decreased neutrophil influx into inflamed joints and with a reduction of intra-articular TNF-α in the zymosan model of arthritis.
Methods
Animals
All animal care and experimental protocols followed the guidelines of the Brazilian College of Animal Experimentation and was approved by our local ethics committee. Male Wistar rats (180–200 g) from the animal colony of the Ceará State University were used throughout the experiments.
Evaluation of joint pain in zymosan arthritis
Rats were given one intra-articular injection of 1 mg zymosan (50 µL total volume), dissolved in sterile saline, into their right knee joints. A control group received only intra-articular saline. We used the rat knee-joint incapacitation test, as a measure of inflammatory joint hypernociception (Rocha et al., 1999). Briefly, after injection of the zymosan, animals were put to walk on a steel rotary drum (30 cm wide × 50 cm diameter), covered with a fine-mesh non-oxidizable wire screen, which rotates at 3 r.p.m. Specially designed metal gaiters were wrapped around both hind-paws. After placement of the gaiters, the animals were allowed to walk freely for habituation. The right paw was then connected via a simple circuit to a microcomputer data input/output port. The paw elevation time is defined as the time that, during a 60 s period, the inflamed hind-paw was not in contact with the cylinder. This is directly proportional to the articular incapacitation. The paw elevation time was measured at baseline and then hourly, until the end of the experiment, at 6 h after injection of the zymosan, irrespective of the drug treatment. Results (s·min−1) are reported as the maximal paw elevation time that occurred between 3 and 4 h after injection of the zymosan.
Assessment of articular hypernociception in the anterior cruciate ligament transection model
Animals were anaesthetized with chloral hydrate (400 mg·kg−1) and the anterior cruciate ligament of the right hind-paw transected. A sham group was given the same surgical procedure, without transection of the ligament. Joint pain (hypernociception) was assessed as described above. However, the paw elevation time was measured over a 10 min period during the first 7 days following anterior cruciate ligament transection. In this model, data are presented as Δ paw elevation time, which was calculated as the elevation times greater than 115 s per 10 min, established as a cut-off baseline mean value for naïve rats. Values between day 1 and day 3 after anterior cruciate ligament transection were attributed to surgical trauma. Data obtained between day 4 and 7 were used in analysis of nociception related to the arthritis model (Castro et al., 2006). Results (s per 10 min) are reported as the maximal Δ paw elevation time that occurred between days 4 and 7 after anterior cruciate ligament transection.
Assessment of cell influx and cytokine release in the joint lavage
At 6 h after injection of zymosan, the animals were anaesthetized (chloral hydrate 400 mg·kg−1, i.p.), killed by cervical dislocation, and exsanguinated. The synovial cavity of the knee joints was then washed with 0.4 mL saline containing 10 mM EDTA. The synovial exudates were collected by aspiration and total cell counts were performed using a Neubauer chamber. After centrifuging (500x g, for 10 min), the supernatants were used for measuring the concentrations of TNF-α, IL-1β and the chemokine cytokine-induced neutrophil chemoattractant-1 (CINC-1) which is the rat orthologue of IL-8 (CXCL1), using elisa. Briefly, 96-well microtiter plates (NUNC-Immuno™ Plate) were coated overnight at 4°C with sheep polyclonal anti-rat TNF-α, sheep polyclonal anti-rat IL-1β or sheep polyclonal anti-rat CINC-1, diluted in 50 µL PBS buffer. These antibodies were provided by Dr Stephen Poole [National Institute for Biological Standards and Control (NIBSC) – UK]. Blocking of non-specific binding sites was accomplished by incubating plates with PBS containing 2% BSA for 90 min at 37°C. A secondary rabbit biotinylated immunoaffinity-purified antibody was added, followed by incubation for 1 h (22°C). Finally, 100 µL of avidin-horseradish peroxidase (1:5000 dilution; DAKO A/S, Denmark) was added to each well; after 30 min the plates were washed and the colour reagent o-phenylenediamine (40 µg·well−1) was added. After 15 min, the reaction was stopped with 1 M H2SO4 and the optical density was measured at 490 nm. Cytokine concentration was expressed as pg·mL−1.
Pharmacological interventions
Groups received tadalafil (Cialis®– purchased from Eli Lilly do Brasil Ltda. São Paulo, SP, Brasil) (0.02–0.5 mg·kg−1) per os, dissolved in sterile saline, 2 h after injection of 1 mg zymosan into the right hind-paw (therapeutic intervention). Controls received the vehicle (saline). Other groups received naloxone, morphine sulphate (Laboratórios Cristália do Brasil Ltda. São Paulo, SP, Brasil) or the cGMP inhibitor 1H-[1,2,4] oxadiazolo [4,3-a] quinoxalin-1-one (ODQ) (Cayman Chem. Co. Ann Arbor, MI, USA) before tadalafil administration. To assess the antinociceptive effect of an NO donor, a group received 3-morpholinosydnonimine (SIN-1) 10 µg intra-articularly 2 h after zymosan. Another group received a 50 µL injection of a sheep anti-rat TNF antiserum (NIBSC, UK) into the knee joint 2 h after the injection of 1 mg zymosan.
Data analysis
Results are expressed as means ± SEM. Differences between means were evaluated using one-way anova, followed by Tukey's test. Significance was set at P < 0.05.
Results
Antinociceptive effect of tadalafil in zymosan arthritis in rats
Figure 1 shows that treatment with tadalafil dose-dependently reduced the paw elevation time in zymosan-induced arthritis in rats. The paw elevation time in the group that received tadalafil (0.5 mg·kg−1) was significantly reduced (up to 49% reduction) at both 3 and 4 h following injection of the zymosan, compared with vehicle-treated rats. Therefore, tadalafil (0.5 mg·kg−1) was the dose chosen to be studied in further experiments.
Figure 1.
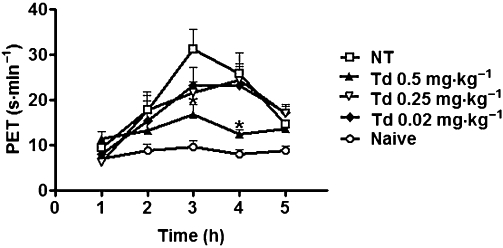
Antinociceptive effects of tadalafil in zymosan-induced arthritis. Joint pain was measured hourly as the increase in the paw elevation time (PET; shown as s·min−1) over 5 h after injection of the zymosan (1 mg intra-articularly). Naïve animals received only saline. Groups received tadalafil (Td 0.02–0.5 mg·kg−1) or saline (non-treated NT) per os 2 h after zymosan. Results are expressed as the mean ± SEM of the maximal PET; n = 6 animals for each group. *P < 0.05, significantly different from NT.
Effect of pretreatment with naloxone upon the anti-nociceptive activity of tadalafil in zymosan arthritis
Naloxone (1 mg·kg−1, i.p.) given 30 min before tadalafil (0.5 mg·kg−1per os) did not reverse the anti-nociceptive effect of tadalafil in the zymosan arthritis model, suggesting that endogenous opioids did not contribute to the analgesic effect of tadalafil (Table 1). As a control of the efficacy of naloxone, a group of animals with zymosan-induced arthritis were given morphine before the zymosan and these animals showed less joint hypernociception. This analgesic effect was reversed by the co-administration of naloxone, at the same dose as was used with tadalafil (Table 1).
Table 1.
Effect of naloxone or the guanylyl cyclase inhibitor ODQ on the anti-inflammatory activity of tadalafil or SIN-1 in zymosan-induced arthritis
| Group | PET (s·min−1) | Cells mm−3 |
|---|---|---|
| Naïve | 12.1 ± 1.2 | 133 ± 42.2 |
| Saline | 30.6 ± 3.7# | 14 022 ± 2 429# |
| ODQ | 30.1 ± 3.5 | 10 855 ± 1 226 |
| Tadalafil | 14.6 ± 1.1* | 8 400 ± 2 728* |
| ODQ + tadalafil | 37.2 ± 3.8 | 16 640 ± 1 141 |
| SIN | 15.6 ± 3.8* | 12 150 ± 3 545 |
| ODQ + SIN | 28.4 ± 3.3 | 10 350 ± 368.6 |
| Morphine | 14.3 ± 0.9* | 11 300 ± 1 659 |
| Naloxone | 29.1 ± 1.2 | 17 200 ± 2 175 |
| Naloxone + morphine | 33.7 ± 1.5 | 16 340 ± 1 615 |
| Naloxone + tadalafil | 17.4 ± 2.7* | 18 200 ± 3 018 |
#,* P < 0.05 significantly different from naïve or saline respectively.
Rats received 1 mg zymosan by intra-articular injection. Joint pain was measured hourly as the increase in the maximal paw elevation time (PET) between 3–4 h after injection of the zymosan. Cell counts were measured in the joint exudates at 6 h. Naïve animals received only saline. Groups received either tadalafil (0.5 mg·kg−1per os), SIN-1 (10 µg intra-articularly) or saline (intra-articularly) 2 h after zymosan; ODQ (4 µg intra-articularly) was given either 2 h after zymosan or before tadalafil (0.5 mg·kg−1) or SIN-1. Another group received 1 mg naloxone i.p. before tadalafil (0.5 mg·kg−1). Results are expressed as the means ± SEM of the maximal PET or total leukocytes; n = 6 animals for each group.
Effect of a cGMP inhibitor on the antinociceptive effect of tadalafil
Intra-articular administration of the cGMP inhibitor ODQ did not alter joint pain in rats with zymosan arthritis. However, ODQ given intra-articularly before 0.5 mg·kg−1 tadalafil per os significantly blocked the antinociceptive effect of the latter compound, indicating that the analgesic effect of tadalafil was mediated through synthesis of cGMP (Table 1). Table 1 also shows that the NO donor SIN-1 (10 µg) given intra-articularly 2 h after the zymosan significantly inhibited joint pain. This therapeutic antinociceptive effect of SIN-1 effect was reversed by the co-administration of ODQ.
Effect of tadalafil in the acute cell influx in zymosan arthritis
As reported previously (Rocha et al., 1999), the cell influx into the joint cavity assessed at 6 h of zymosan arthritis is mostly (>85%) composed of neutrophils (data not shown). As seen in joint hypernociception, tadalafil (0.5 mg·kg−1per os) significantly reduced this neutrophil infiltration (Figure 2). We should stress that the same effective dose of tadalafil that provided analgesia (see Figure 1) also reduced neutrophil influx into the joints in zymosan arthritis. As shown in Table 1, pretreatment with the µ-opioid receptor antagonist naloxone did not prevent the inhibition of neutrophil migration provided by tadalafil (0.5 mg·kg−1) whereas co-administration of the inhibitor of guanylyl cyclase, ODQ, significantly reversed the inhibitory effect of tadalafil on neutrophil migration in zymosan arthritis (Table 1). Table 1 also shows that the NO donor SIN-1 (10 µg) given intra-articularly 2 h after the zymosan did not alter cell influx.
Figure 2.
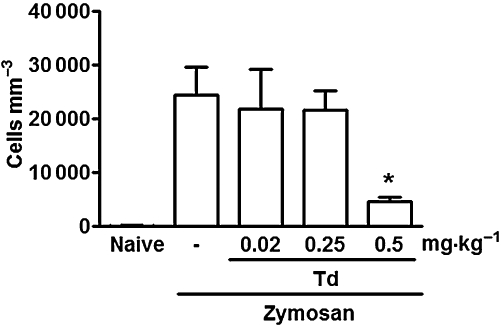
Effects of tadalafil on the acute cell influx in zymosan arthritis. Rats received 1 mg zymosan intra-articularly. and the cell influx was measured in the joint exudates after 6 h. Different doses of either tadalafil (Td) or saline (-) were given per os 2 h after zymosan. Naïve animals received only saline. Results are expressed as the mean ± SEM of total leukocytes (n = 6 animals per group). *P < 0.05, significantly different from saline (-).
Effect of tadalafil on cytokine release in zymosan-induced arthritis
The effects of tadalafil (0.5 mg·kg−1per os) on the release of IL-1β, TNF-α and CINC-1 into the joints is shown in Figure 3A–C. There was a significant decrease of TNF-α release, whereas levels of IL-1β and CINC-1 were not altered.
Figure 3.
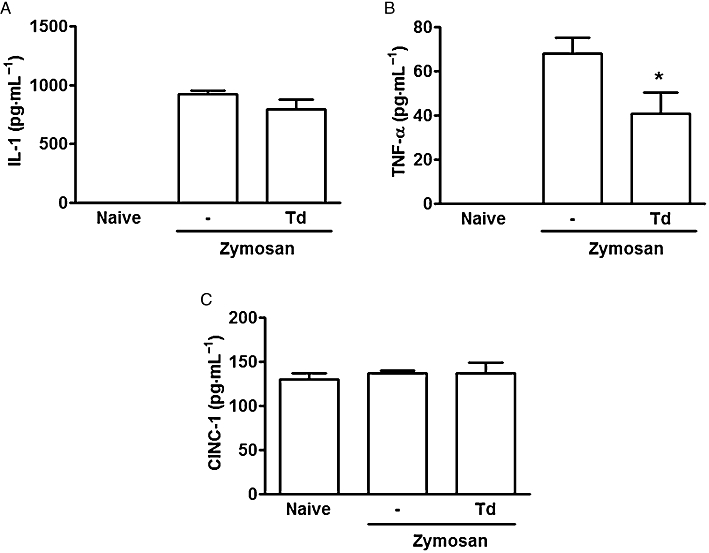
Effect of tadalafil on cytokine release in zymosan arthritis. Zymosan (1 mg) or saline (-) were injected intra-articularly. IL-1 (A), TNF-α (B) and CINC-1 (C) levels were determined in joint exudates at 6 h, using elisa. Naïve animals received saline intra-articularly. Groups received tadalafil (Td) (0.5 mg·kg−1) or saline (-) per os 2 h after zymosan. Results are expressed as means ± SEM of n = 6 animals for each group.*P < 0.05 significantly different from saline (-).
Effect of the local administration of an anti-TNF antiserum
As expected, the intra-articular injection of an anti-TNF antiserum significantly reduced both hypernociception and cell influx into the joints of rats with zymosan arthritis (Figure 4A and B).
Figure 4.
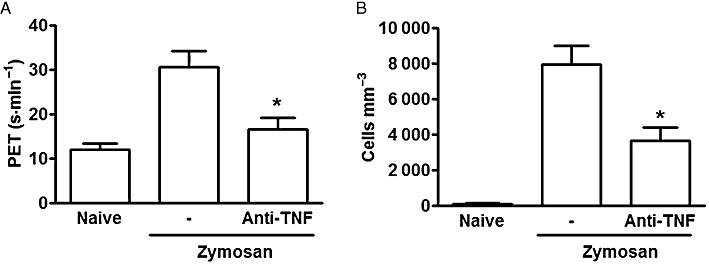
Effect of an anti-TNF antiserum on joint pain and cell infiltration in zymosan arthritis. Rats received 1 mg zymosan or saline (-)intra-articularly. Groups received an anti-TNF antiserum (50 µL) or saline (-)intra-articularly, 2 h after the zymosan. Naïve animals received only saline; (A) joint pain is expressed as the maximal increase in paw elevation time (PET) occurring 3–4 h after injection of the zymosan; (B) cell influx was measured in joint exudates collected at 6 h. Results are expressed as the mean ± SEM of maximal PET and total leukocytes; n = 6 animals per group. *P < 0.05 significantly different from saline (-).
Antinociceptive effect of tadalafil in experimental osteoarthritis in rats
Tadalafil, at the same dose (0.5 mg·kg−1) that produced antinociception in the zymosan arthritis model, was also analgesic in the osteoarthritis model. As can be seen in Figure 5, in rats with anterior cruciate ligament transection, treatment with tadalafil significantly reduced the paw elevation time, compared with rats that received saline. As seen in the zymosan model (Figure 1), ODQ given intra-articularly before 0.5 mg·kg−1 tadalafil per os significantly blocked the antinociceptive effect of tadalafil.
Figure 5.
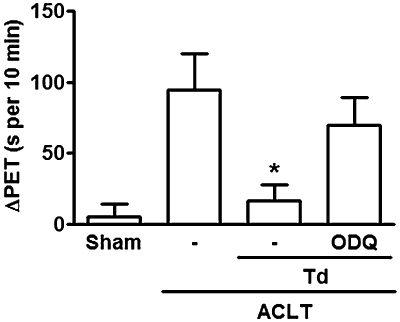
Antinociceptive effect of tadalafil in experimental osteoarthritis, following anterior cruciate ligament transection (ACLT). Joint pain was assessed daily as the increase in the paw elevation time (PET), using the articular incapacitation test. A sham group was subjected to the surgical procedure without ACLT and received saline by gavage. Groups received tadalafil (Td) (0.5 mg·kg−1) or saline (-) per os daily, starting on day 4, until day 7 after ACLT; ODQ (4 µg) or saline intra-articularly were given 30 min before Td (0.5 mg·kg−1). Data for ΔPET (s per 10 min) represent the means ± SEM of the difference between the maximal increase in PET and the baseline value, achieved between days 4 and 7 after ACLT; n = 6 animals per group; *P < 0.05 significantly different from saline. Data analysed using one-way anova, followed by Tukey's test.
Discussion
In the present study, we have demonstrated that the PDE-5 inhibitor tadalafil exhibited systemic anti-inflammatory activity in vivo by providing antinociception and inhibition of neutrophil migration in a model of zymosan-induced arthritis. Tadalafil was effective when administered per os in ongoing zymosan arthritis and those effects were accompanied by a reduction of the intra-articular levels of TNF-α. Additionally, the antinociceptive activity of tadalafil was observed in both the severely inflamed joints of the zymosan model, with a prominent participation of neutrophils, and the milder inflammatory osteoarthritis model, where there was no detectable cell influx (Castro et al., 2006). Moreover, the fact that we obtained similar analgesia in two mechanistically different arthritis models indicates a common pathway involving activation of PDE-5 in joint nociception. To our knowledge, there are no data reporting intrinsic analgesia linked to tadalafil. However, a very recently published randomized clinical trial has shown that tadalafil alleviated symptoms in patients with the complex regional pain syndrome. This effect was attributed to microvascular changes promoted by tadalafil, rather than to direct actions of this compound on pain mechanisms (Groeneweg et al., 2008).
The role of the L-arginine : NO system in pain development in arthritis is still a matter of debate, as it can be either pro- or anti-nociceptive. This is similar to that reported for NO actions in other tissues. For instance, NO per se is essential to normal osteoblast metabolism. On the other hand, peroxynitrite formed during inflammatory reactions by the combination of NO and superoxide can be deleterious to these cells (da Rocha and de Brum-Fernandes, 2002). In addition, NO can have different effects depending on the tissue. While intracutaneous NO in humans can induce pain, administration of transdermal glyceryl trinitrate, an NO donor, provided analgesia in patients with shoulder periarthritis (Groeneweg et al., 2008). In the zymosan-arthritis model in rats, we have shown that NOS inhibitors do not prevent analgesia in ongoing arthritis, whereas exogenous NO donors displayed intrinsic antinociceptive activity (da S Rocha et al., 2002). We reproduced these data in the present study, showing that SIN-1 inhibited ongoing hypernociception in zymosan arthritis, while not significantly altering cell infiltration into the joints, suggesting that these phenomena are modulated by different mechanisms. Aa observed in other tissues, joint derived pain is not necessarily linked to the presence of inflammatory cells. In fact, although both zymosan arthritis and the anterior cruciate ligament transection models display hypernociception, there is no detectable cell influx in the osteoarthritis model whereas a prominent neutrophil infiltration is characteristic of zymosan-induced arthritis. As we observed with tadalafil, the antinociceptive effect of SIN-1 was reversed by the co-administration of SIN-1 and ODQ, suggesting that the intrinsic analgesic activity of SIN-1 depended on cGMP synthesis by guanylyl cyclase.
Intra-articular levels of NO increase as osteoarthritis develops following transection of the anterior cruciate ligament and this increase is associated with enhanced levels of immuno-reactive inducible NOS isoform in synovial cells. However, NOS inhibitors were able to block the hypernociceptive response in this model only when given as a pre-treatment (Castro et al., 2006). One of the proposed mechanisms for an antinociceptive effect of NO in osteoarthritis would be increased local blood flow promoted by the low NO levels produced by endothelial NOS. On the other hand, hyperalgesia could result from high NO levels leading to the formation of potent oxidant species, for example, peroxynitrite. In keeping with this proposal, scavenging of peroxynitrite provided analgesia in the zymosan-induced arthritis model in rats (Bezerra et al., 2004). Considering that osteoarthritis may share common inflammatory pathways with other arthritis models, it seems likely that reactive nitrogen/oxygen species are also involved in the hypernociceptive response associated with osteoarthritis. However, this remains to be demonstrated.
As mentioned in the Introduction, antinociception following the administration of PDE-5 inhibitors depends on pharmacokinetic differences, route of administration, animal species and pain models studied. Among the mechanisms responsible for the anti-inflammatory effect of tadalafil, we should include modulation of cGMP levels, release of endogenous opioids and the release of inflammatory mediators.
Our data demonstrate that pretreatment with the specific soluble guanylyl cyclase inhibitor, ODQ, completely blocked the antinociceptive activity as well as the inhibition of neutrophil migration provided by tadalafil. Similar data were reported with sildenafil, where the enhancement of the antinociceptive activity of morphine provoked by sildenafil was reversed by the co-administration of methylene blue, used as a cGMP inhibitor (Jain et al., 2003). In the same study, naloxone, an µ-opioid receptor antagonist, also reversed the antinociceptive effect of sildenafil. However, in our models, co-administration of tadalafil and naloxone did not reverse the antinociceptive effect, leading us to conclude that the analgesic effect of tadalafil in arthritis does not depend on the release of endogenous opioids.
TNF-α is considered a pivotal cytokine in the hypernociception of inflamed joints . As expected, blockade of TNF-α by the local administration of an anti-TNF-α antiserum significantly reduced both joint hypernociception and cell influx. Interestingly, the administration of tadalafil, in a dose that significantly prevented joint hypernociception as well as inhibiting neutrophil migration, reduced TNF-α levels release into the joint exudates, compared with vehicle-treated rats. On the other hand, intra-articular levels of both IL-1β and CINC-1 were not altered. This is the first demonstration that a PDE-5 inhibitor decreases the local in vivo release of cytokines, specifically TNF-α. The fact that the same dose of tadalafil that was both anti-inflammatory and effective in reducing intra-articular TNF-α levels, implies that these phenomena are associated.
Inhibition of neutrophil migration by tadalafil could be a consequence of the reduction of rolling and adhesion of leukocytes to the endothelium. In addition, we have recently demonstrated that neutrophil-derived peroxynitrite, a reactive nitrogen species, is involved in joint hypernociception in zymosan arthritis in rats (Bezerra et al., 2007). Also, the antinociceptive effect of TNF-α blockade, either with antibodies or with thalidomide, pentoxyfylline and chlorpromazine, in experimental arthritis was shown to be linked to a decreased neutrophil influx into the joints (Bombini et al., 2004).
Sensitization of nociceptors occurs as a secondary process following the initiation of a cytokine cascade (Cunha et al., 1991). For instance, during inflammation, TNF-α release induces the liberation of IL-1β and IL-6 as well as the production of cyclooxygenase products. TNF-α may also trigger the release of IL-8, that induces the production of sympathomimetic mediators (Cunha et al., 1992). Rats do not express IL-8 but the chemokine CINC-1 is considered to be the orthologue of IL-8 in rats, and CINC-1 and IL-8 are ligands for the same receptor. Recently, our group demonstrated that CINC-1 was directly associated with the development of inflammatory hyperalgesia in rats (Cunha et al., 2008). As CINC-1 participates in inflammatory pain in rats, we assessed the effects of tadalafil on CINC-1 levels in arthritis. In addition, it was recently reported that tadalafil decreased IL-8 production by endothelial cells stimulated in vitro with TNF and myeloperoxidase-modified low-density lipoprotein (Roumeguère et al., 2010). This effect could explain a beneficial anti-inflammatory activity of tadalafil in the endothelium. However, in the present in vivo study, intra-articular levels of CINC-1 were not altered by treatment with tadalafil, arguing against a role for CINC-1 to explain the anti-inflammatory effects of tadalafil in zymosan arthritis.
As discussed above, there is little evidence for the anti-inflammatory effect of PDE-5 inhibitors. Some in vitro experiments have suggested that tadalafil can reduce the up-regulation of TNF-α and IL-1β (Tsai et al., 2006), which are pivotal cytokines in inflammatory arthritis, and the same effect was not demonstrated for sildenafil or vardenafil. Our data are the first demonstration that PDE-5 inhibitors modulate cytokine release in vivo in experimental arthritis. In addition to the possible participation of cytokines other than IL-1, TNF and CINC-1 in this mechanism, the intracellular pathways involved should be further explored.
The synovium is a richly vascularised tissue. Therefore, endothelial cells are natural candidates as the main source for PDE-5 in the joints. However, PDE-5 has never been shown to be expressed in the synovial endothelium. On the other hand, chondrocytes specifically express the mRNA for PDE-5 and the activity of PDE in these cells is increased following stimulation with IL-1 (Geng et al., 1998). More recently, using SW982 cells, a human synovial cell line, cGMP hydrolysis was specifically due to the PDE-5 isoform (Kim et al., 2008). Hence, considering the acute nature of the joint hypernociception in zymosan arthritis we would suggest that synoviocytes, in addition to endothelial cells in the synovium, are probably the loci of the PDE-5 activity found in the joints.
In summary, we have shown that tadalafil has in vivo therapeutic anti-inflammatory effects by promoting antinociception and reducing the leukocyte influx into the synovial cavity in zymosan arthritis. Moreover, tadalafil was effective when given per os, thus indicating a therapeutic potential in humans. The analgesic effect is probably secondary to the activation of cGMP in nociceptive neurons and did not appear to depend on the release of endogenous opioids. In addition to the specific direct effect via cGMP modulation, we propose that the antinociceptive effect of tadalafil is also linked to a decreased release of TNF-α associated with the reduction of neutrophil migration into the joints. Usually, the patients prescribed PDE-5 inhibitors to treat erectile dysfunction are those also affected by osteoarthritis. The possibility of relief of joint pain provided by these compounds, as a side effect, could possibly increase patient compliance with the therapy.
Acknowledgments
This work was supported by grants from CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), CAPES (Fundação Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) and FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo). The authors thank Giuliana Bertozi for assistance with the elisa procedures.
Glossary
Abbreviations
- CINC-1
(cytokine-induced neutrophil chemoattractant-1)/CXCL1
- ODQ
1H-[1,2,4] oxadiazolo [4,3-a] quinoxalin-1-one
- PGE2
prostaglandin E2
Conflicts of interest
None.
References
- Amarante LH, Duarte ID. The kappa-opioid agonist (+/−)-bremazocine elicits peripheral antinociception by activation of the L-arginine/nitric oxide/cyclic GMP pathway. Eur J Pharmacol. 2002;454:19–23. doi: 10.1016/s0014-2999(02)02275-6. [DOI] [PubMed] [Google Scholar]
- Asomoza-Espinosa R, Alonso-López R, Mixcoatl-Zecuatl T, Aguirre-Bañuelos P, Torres-López JE, Granados-Soto V. Sildenafil increases diclofenac antinociception in the formalin test. Eur J Pharmacol. 2001;418:195–200. doi: 10.1016/s0014-2999(01)00956-6. [DOI] [PubMed] [Google Scholar]
- Beavo JA. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol Rev. 1995;75:725–748. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- Bezerra MM, Brain SD, Greenacre S, Jerônimo SM, de Melo LB, Keeble J, et al. Reactive nitrogen species scavenging, rather than nitric oxide inhibition, protects from articular cartilage damage in rat zymosan-induced arthritis. Br J Pharmacol. 2004;141:172–182. doi: 10.1038/sj.bjp.0705600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezerra MM, Brain SD, Girão VC, Greenacre S, Keeble J, Rocha FA. Neutrophils-derived peroxynitrite contributes to acute hyperalgesia and cell influx in zymosan arthritis. Naunyn Schmiedebergs Arch Pharmacol. 2007;374:265–273. doi: 10.1007/s00210-006-0123-9. [DOI] [PubMed] [Google Scholar]
- Blount MA, Beasley A, Zoraghi R, Sekhar KR, Bessay EP, Francis SH, et al. Binding of tritiated sildenafil, tadalafil, or vardenafil to the phosphodiesterase-5 catalytic site displays potency, specificity, heterogenicity and cGMP stimulation. Mol Pharmacol. 2004;66:144–152. doi: 10.1124/mol.66.1.144. [DOI] [PubMed] [Google Scholar]
- Bombini G, Canetti C, Rocha FA, Cunha FQ. Tumour necrosis factor-alpha mediates neutrophil migration to the knee synovial cavity during immune inflammation. Eur J Pharmacol. 2004;496:197–204. doi: 10.1016/j.ejphar.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Boswell-Smith V, Spina D, Page CP. Phosphodiesterase inhibitors. Br J Pharmacol. 2006;147(Suppl 1):S252–S257. doi: 10.1038/sj.bjp.0706495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro RR, Cunha FQ, Silva FS, Jr, Rocha FA. A quantitative approach to measure joint pain in experimental osteoarthritis – evidence of a role for nitric oxide. Osteoarthritis Cartilage. 2006;14:769–776. doi: 10.1016/j.joca.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Cunha FQ, Lorenzetti BB, Poole S, Ferreira SH. Interleukin-8 as a mediator of sympathetic pain. Br J Pharmacol. 1991;104:765–767. doi: 10.1111/j.1476-5381.1991.tb12502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH. The pivotal role of tumor necrosis factor alpha in the development of inflammatory hyperalgesia. Br J Pharmacol. 1992;107:660–664. doi: 10.1111/j.1476-5381.1992.tb14503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha TM, Verri WA, Jr, Schivo IR, Napimoga MH, Parada CA, Poole S, et al. Crucial role of neutrophils in the development of mechanical inflammatory hypernociception. J Leukoc Biol. 2008;83:824–832. doi: 10.1189/jlb.0907654. [DOI] [PubMed] [Google Scholar]
- Francischi JN, Yokoro CM, Poole S, Tafuri WL, Cunha FQ, Teixeira MM. Anti-inflammatory and analgesic effects of the phosphodiesterase 4 inhibitor rolipram in a rat model of arthritis. Eur J Pharmacol. 2000;399:243–249. doi: 10.1016/s0014-2999(00)00330-7. [DOI] [PubMed] [Google Scholar]
- Fuhrmann M, Jahn HU, Seybold J, Neurohr C, Barnes PJ, Hippenstiel S, et al. Identification and function of cyclic nucleotide phosphodiesterase isoenzymes in airway epithelial cells. Am J Respir Cell Mol Biol. 1999;20:292–302. doi: 10.1165/ajrcmb.20.2.3140. [DOI] [PubMed] [Google Scholar]
- Geng Y, Zhou L, Thompson WJ, Lotz M. Cyclic GMP and cGMP-binding phosphodiesterase are required for interleukin-1-induced nitric oxide synthesis in human articular chondrocytes. J Biol Chem. 1998;273:27484–27491. doi: 10.1074/jbc.273.42.27484. [DOI] [PubMed] [Google Scholar]
- Groeneweg G, Huygen FJ, Niehof SP, Wesseldijk F, Bussmann JB, Schasfoort FC, et al. Effect of tadalafil on blood flow, pain, and function in chronic cold complex regional pain syndrome: a randomized controlled trial. BMC Musculoskelet Disord. 2008;9:143. doi: 10.1186/1471-2474-9-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad JJ, Land SC, Tarnow-Mordi WO, Zembala M, Kowalczyk D, Lauterbach R. Imunopharmacological potential of selective phosphodiesterase inhibition. I. Differential regulation of lipopolysaccharide-mediated proinflammatory cytokine (interleukin-6 and tumor necrosis factor-alpha) biosynthesis in alveolar epithelial cells. J Pharmacol Exp Ther. 2002;300:559–566. doi: 10.1124/jpet.300.2.559. [DOI] [PubMed] [Google Scholar]
- Jain NK, Patil CS, Singh A, Kulkarni SK. Sildenafil-induced peripheral analgesia and activation of the nitric oxide–cyclic GMP pathway. Brain Res. 2001;909:170–178. doi: 10.1016/s0006-8993(01)02673-7. [DOI] [PubMed] [Google Scholar]
- Jain NK, Patil CS, Singh A, Kulkarni SK. Sildenafil, a phosphodiesterase-5 inhibitor, enhances the antinociceptive effect of morphine. Pharmacology. 2003;67:150–156. doi: 10.1159/000067802. [DOI] [PubMed] [Google Scholar]
- Kim KO, Park SY, Han CW, Chung HK, Yoo DH, Han JS. Effect of sildenafil citrate on interleukin-1beta-induced nitric oxide synthesis and iNOS expression in SW982 cells. Exp Mol Med. 2008;40:286–293. doi: 10.3858/emm.2008.40.3.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennard SI, Schachter N, Strek M, Rickard K, Amit O. Cilomilast for COPD: results of a 6-month, placebo-controlled study of a potent, selective inhibitor of phosphodiesterase 4. Chest. 2006;129:56–66. doi: 10.1378/chest.129.1.56. [DOI] [PubMed] [Google Scholar]
- Rocha FA, Aragão AG, Jr, Oliveira RC, Pompeu MM, Vale MR, Ribeiro RA. Periarthritis promotes gait disturbance in zymosan-induced arthritis in rats. Inflamm Res. 1999;48:485–490. doi: 10.1007/s000110050491. [DOI] [PubMed] [Google Scholar]
- da Rocha FA, de Brum-Fernandes AJ. Evidence that peroxynitrite affects human osteoblast proliferation and differentiation. J Bone Miner Res. 2002;17:434–442. doi: 10.1359/jbmr.2002.17.3.434. [DOI] [PubMed] [Google Scholar]
- Roumeguère T, Zouaoui Boudjeltia K, Babar S, Nuyens V, Rousseau A, Van Antwerpen P, et al. Effects of phosphodiesterase inhibitors on the inflammatory response of endothelial cells stimulated by myeloperoxidase-modified low-density lipoprotein or tumor necrosis factor alpha. Eur Urol. 2010;57:522–528. doi: 10.1016/j.eururo.2009.01.030. [DOI] [PubMed] [Google Scholar]
- da S Rocha JC, Peixoto ME, Jancar S, de Q Cunha F, de A Ribeiro R, da Rocha FA. Dual effect of nitric oxide in articular inflammatory pain in zymosan-induced arthritis in rats. Br J Pharmacol. 2002;136:588–596. doi: 10.1038/sj.bjp.0704755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabsigh R. Therapy of ED: PDE-5 inhibitors. Endocrine. 2004;23:135–141. doi: 10.1385/ENDO:23:2-3:135. [DOI] [PubMed] [Google Scholar]
- Tsai BM, Turrentine MW, Sheridan BC, Wang M, Fiore AC, Brown JW, et al. Differential effects of phosphodiesterease-5 inhibitors on hypoxic pulmonary vasoconstriction and pulmonary artery cytokine expression. Ann Thorac Surg. 2006;81:272–278. doi: 10.1016/j.athoracsur.2005.06.040. [DOI] [PubMed] [Google Scholar]


