Abstract
Ciliary Neurotrophic Factor (CNTF) was first characterized as a trophic factor for motor neurons in the ciliary ganglion and spinal cord, leading to its evaluation in humans suffering from motor neuron disease. In these trials, CNTF caused unexpected and substantial weight loss, raising concerns that it might produce cachectic-like effects. Countering this possibility was the suggestion that CNTF was working via a leptin-like mechanism to cause weight loss, based on the findings that CNTF acts via receptors that are not only related to leptin receptors, but also similarly distributed within hypothalamic nuclei involved in feeding. However, although CNTF mimics the ability of leptin to cause fat loss in mice that are obese because of genetic deficiency of leptin (ob/ob mice), CNTF is also effective in diet-induced obesity models that are more representative of human obesity, and which are resistant to leptin. This discordance again raised the possibility that CNTF might be acting via nonleptin pathways, perhaps more analogous to those activated by cachectic cytokines. Arguing strongly against this possibility, we now show that CNTF can activate hypothalamic leptin-like pathways in diet-induced obesity models unresponsive to leptin, that CNTF improves prediabetic parameters in these models, and that CNTF acts very differently than the prototypical cachectic cytokine, IL-1. Further analyses of hypothalamic signaling reveals that CNTF can suppress food intake without triggering hunger signals or associated stress responses that are otherwise associated with food deprivation; thus, unlike forced dieting, cessation of CNTF treatment does not result in binge overeating and immediate rebound weight gain.
Ciliary Neurotrophic Factor (CNTF) was first described as a trophic factor for motor neurons in the ciliary ganglion (1) and subsequently found to act on multiple other motor neuron populations (2), leading to its evaluation in humans suffering from motor neuron disease. In these trials, CNTF caused unexpected and substantial weight loss (3), raising concerns that it was acting in a manner akin to cachectic cytokines such as IL-1. However, other findings raised the possibility that CNTF induced weight loss via a leptin-like mechanism. The first suggestion that CNTF might act via a leptin-like mechanism came following the molecular cloning of the leptin receptor (ObR) (4, 5), which revealed that it was closely related to components of the CNTF receptor complex (6–8). Further studies found that CNTF and leptin activate overlapping signaling molecules, notably the STAT3 transcription factor (9–13), and that, like ObR, CNTF receptors are located in hypothalamic nuclei involved in feeding (14). Consistent with this, CNTF mimics the ability of leptin to cause preferential loss of fat in mice that are obese because of genetic deficiency of leptin (ob/ob mice) (14). However, CNTF is also effective in diet-induced obesity (DIO) models that are more representative of human obesity, and which are resistant to leptin (14). This discordance once again raised the possibility that CNTF might be acting via nonleptin pathways, perhaps more analogous to those activated by cachectic cytokines.
Arguing strongly against the possibility that CNTF acts like a cachectic cytokine, we now show that CNTF can activate hypothalamic leptin-like pathways even in DIO models unresponsive to leptin, that CNTF improves prediabetic parameters (such as hyperinsulinemia and hyperlipidemia) in these mouse models, and that CNTF acts very differently than the prototypical cachectic cytokine IL-1. Most notably, IL-1 does not activate hypothalamic leptin-like pathways while efficacious doses of CNTF do not induce the muscle wasting, proinflammatory responses, conditioned taste aversion, or corticosterone release seen with doses of IL-1 that cause comparable weight loss. Further analyses of hypothalamic signaling revealed that CNTF can suppress food intake without triggering hunger signals or associated stress responses otherwise associated with food deprivation. Thus, unlike forced dieting or other treatments for obesity, CNTF treatment may reset the hypothalamic weight setpoint, such that cessation of CNTF administration does not result in binge overeating and immediate rebound weight gain.
Methods
Animals.
Male C57BL/6 mice (Taconic Farms), C57BL/6J-Lepob (ob/ob), and AKR/J were obtained at 7–8 wk of age and housed in 12 h of light per day at 69–74°F and 40–60% humidity. All experiments began at 10 wk of age. Mice were provided with Rodent Laboratory Chow 5001 (Purina, St. Louis, MO) ad libitum, except for pair-fed mice, which were restricted to the same amount of food as eaten by the treatment group or AKR/J mice placed on a high fat diet (45% of the calories as fat, Research Diets, New Brunswick, NJ) ad libitum for 7 wk to produce a DIO. After 7 wk, DIO mice weighed ≈30% more than littermates eating standard chow. Water was provided ad libitum to all mice. All animal procedures were conducted in strict compliance with protocols approved by the Regeneron Institutional Animal Care and Use Committee.
Experimental Procedures.
Before the start of treatment, mice were transferred from group housing to single housing to facilitate food intake measurements. Body weight and food consumption were monitored daily. In some studies, carcass analysis was performed (Covance Laboratory, Princeton, NJ) to determine body composition. In other studies, mice were killed by cervical dislocation, and wet weights of the epididymal fat pads (bilateral) and the tibialis anterior, extensor digitorum longus, and/or gastrocnemius muscles were obtained as measurements of visceral adiposity and lean muscle mass, respectively. Tissues were collected 18–20 h after the last injection. Terminal blood samples were collected and serum corticosterone levels were measured by using a commercially available RIA kit (Biotrak, Amersham). Activity was measured as “mobile time” in a 21 × 33 cm monitoring chamber (IITC, Woodland Hills, CA; model AM1051). Mobile time was defined as the percentage of a 10-min test period during which more than two horizontally displaced photocell beams were interrupted per 5 s.
Immunohistochemistry.
Mice were deeply anesthetized (120 mg/kg ketamine, 24 mg/kg xylazine, i.m.), exsanguinated, and perfused transcardially with heparinized saline followed by 4% buffered paraformaldehyde. The brains were removed, postfixed, and stored in sucrose solution at 4°C. Then 40-μm thick sections were cut through the hypothalamus and stored in cryoprotectant at −20°C before staining.
Phospho-STAT3 (pSTAT3)/Cyclooxygenase-2 (COX-2) Immunocytochemistry.
Sections were rinsed in KPBS (50 mM; 80 ml 0.5 M K2HPO4/20 ml 0.5 M KH2PO4/8.8 g NaCl/900 ml of distilled water, pH 7.2–7.4) to remove cryoprotectant and for pSTAT3-immunostaining were then pretreated for 20 min in 1% NaOH + 1% H2O2 in dH2O. The tissue was rinsed again in KPBS before immersion in 0.3% glycine for 10 min. Following a further rinse, sections were placed in 0.03% SDS for 10 min. For COX-2 immunostaining, sections were pretreated in 1% H2O2 for 20 min. All sections were rinsed once more and placed in 4% normal serum + 0.4% Triton X-100 + 1% BSA (fraction V) for 20 min before incubation with a polyclonal primary antibody for phosphorylated STAT3 (New England Biolabs, 1:3,000) or COX-2 (Chemicon, 1:5,000) overnight at 4°C in 1% normal serum + 0.4% Triton X-100 + 1% BSA. The protocol on day 2 used a biotinylated goat anti-rabbit secondary antibody at a concentration of 1:5,000 followed by application of an Avidin–Biotin peroxidase complex (Vector Elite ABC Kit). The diaminobenzidine reaction product was intensified with nickel sulfate.
Neuropeptide Y (NPY)/pCREB Immunocytochemistry.
Brain slices were rinsed to remove cryoprotectant and then incubated in 4.0% normal serum + 0.4% Triton X-100 + 1.0% BSA for 20 min. Tissue was then incubated overnight at 4°C in primary antisera (Upstate Biotechnology rabbit pCREB polyclonal, 1:1,000; Peninsula rabbit NPY polyclonal, 1:5,000) + 0.4% Triton X-100 + 1.0% normal serum + 1.0% BSA. The protocol on day 2 used a goat anti-rabbit secondary antibody (1:600 for pCREB; 1:1,500 for NPY), which was visualized with an Avidin–Biotin peroxidase procedure as described above.
After staining, all sections were mounted on slides, dehydrated, and coverslipped. Image analysis (NIH Image) was conducted to quantify relative density of NPY-immunoreactivity and number of pCREB-positive cells within the paraventricular nucleus of the hypothalamus (PVN). NPY density in the parvocellular nucleus of the PVN was measured from one section for each brain. Sections containing the parvocellular nucleus were selected and matched by reference to Nissl-stained adjacent sections.
Northern Blot Analysis.
Total RNA was prepared from hypothalamic tissue by using Trizol Reagent (Life Technologies, Grand Island, NY). RNA (10 μg) was separated on 1.2% agarose formamide-formaldehyde gels, transferred to nylon membrane, and immobilized by UV crosslinking. After prehybridization, 32P-labeled Agouti-related peptide (Agrp)-, NPY-, or glyceraldehyde-3-phosphate dehydrogenase-specific probes were added, and the filters were hybridized at 42°C overnight. The stringency washing was performed by standard protocols, and an autoradiograph was obtained after 48-h exposure to x-ray film with intensifying screens.
Conditioned Taste Aversion.
Mice were switched to a reverse light–dark cycle and acclimatized to the presence of two water bottles. They were then subjected to water deprivation training with water available for 1 h per day for 3 days. The following day, mice were given 0.1% saccharin solution in place of water and 1 h later were injected with CNTF, IL-1, lithium chloride, or vehicle. After dosing, water was returned and the mice had free access for 2 days. On the day of testing, water was removed 24 h before presentation of one bottle of water and one bottle of saccharin and the volumes of each solution consumed in 1 h were measured.
Proteins.
Human leptin was purchased from R & D Systems and CNTFAx15 and human IL-1β were manufactured by Regeneron Pharmaceuticals. All proteins were dissolved in 5 mM phosphate buffer (pH 8.3). CNTFAx15, under the name AXOKINE, is being developed by Regeneron for the treatment of obesity. CNTFAx15 is a truncated form of CNTF with the last 15 c-terminal amino acids removed. To enhance stability of the molecule, glutamine is replaced by arginine at position 63 and the free cysteine at position 17 is replaced by alanine.
Statistical Analysis.
Data collected at the end of the experiments were analyzed by one-way ANOVA and Dunnett post hoc comparison. P values <0.05 were considered to be significant.
Results
CNTF Mimics Leptin Action in ob/ob Mice but Is Also Effective in DIO Models.
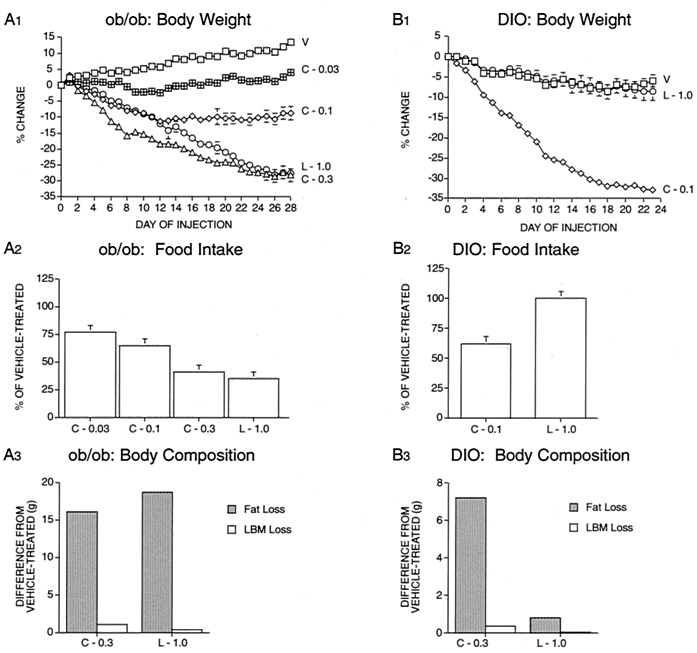
Confirming previous findings (14), daily injections of CNTFAx15 caused weight loss in ob/ob mice (Fig. 1A1) as well as in a DIO model in which AKR/J mice were maintained on a high fat diet (Fig. 1B1). In both models, weight loss was associated with decreased food intake (Fig. 1 A2 and B2), and characterized by a preferential loss of fat as opposed to lean body mass (Fig. 1 A3 and B3). In ob/ob mice, daily injections of leptin at a dose of 1.0 mg/kg resulted in weight loss equivalent to that seen in animals treated with CNTFAx15 at a dose of 0.3 mg/kg/d (Fig. 1A1). However, this dose of leptin was completely ineffective in DIO mice (Fig. 1B1), consistent with the previous finding that DIO animals are “leptin-resistant” (15). In contrast, CNTFAx15 was, if anything, more potent in DIO than in ob/ob mice (compare Fig. 1 A1 vs. B1). Furthermore, as had previously been shown in ob/ob mice (14), we found that CNTF administration could ameliorate the hyperinsulinemia and hyperlipidemia seen in DIO mice (Table 1). These effects could not be attributed solely to weight loss due to food restriction because insulin and triglyceride levels were not lowered in animals provided with an amount of food equal to that consumed by animals treated with CNTFAx15. Of interest, these “pair-fed” DIO mice lost similar amounts of weight when compared to animals treated with the corresponding dose of CNTFAx15 (0.1 mg/kg/d), supporting the idea that weight loss caused by CNTFAx15 is largely attributable to decreased food intake (Table 1). Thus, CNTF treatment not only reduces body weight in leptin resistant DIO models, but can produce improvements in hyperinsulinemia and hyperlipidemia in these settings, beyond that achieved by equivalent reduction in food intake.
Figure 1.
Treatment with leptin or CNTFAx15 in ob/ob and DIO mice [starting body weight ≈50 g]. Groups of ob/ob mice (n = 8) or DIO mice (n = 7) received a daily s.c. injection of vehicle (V), leptin (L; 1 mg/kg/day), or CNTFAx15 (C; 0.03, 0.1, 0.3 mg/kg/day). Body weight was measured daily and is shown as the percentage difference from body weight on the first day of injection (A1 and B1). A 24-h food intake was recorded and is shown as percentage of vehicle-treated control (A2 and B2). Body compositions were determined by carcass analysis at the end of the study, and data are shown as difference in fat and lean body mass (LBM) relative to vehicle-treated controls (A3 and B3). All data are mean ± SEM.
Table 1.
Effects of treatment with CNTFAx15 (C-0.1 or 1.0 mg/kg/day s.c. for 7 days) or pair feeding (PFC-0.1) on change in body weight, serum levels, and activity in DIO mice
| Treatment | BW, % change | Insulin, pg/ml | Triglycerides, mg/dl | Activity, % | Corticosterone, ng/ml |
|---|---|---|---|---|---|
| lean | −3.6 ± 1* | 680 ± 175*† | 137 ± 16 | 94.6 ± 0.6 | 33 ± 10* |
| DIO | −3.9 ± 1.2* | 2701 ± 564 | 146 ± 12 | 90.4 ± 1.5 | 28 ± 5* |
| DIO + C-0.1 | −14.3 ± 0.6† | 1103 ± 234*† | 67 ± 3*† | 89.9 ± 1.6 | 38 ± 5* |
| DIO + PFC-0.1 | −11.2 ± 0.3† | 2846 ± 887 | 126 ± 4 | 91.3 ± 1 | 92 ± 26† |
| DIO + C-1.0 | −32.9 ± 1.3*† | 683 ± 227*† | 42 ± 9*† | 83.2 ± 4*† | 118 ± 22† |
Mean ± SEM (n = 7) for change in body weight, serum level of insulin, triglycerides, corticosterone, and locomotor activity are shown. ANOVA: percentage of BW, P < 0.0001; insulin, P < 0.01; triglycerides, P < 0.0001; corticosterone, P < 0.001; and activity, P < 0.05.
Difference from PFC-0.1 by Dunnett post hoc test.
Difference from ad libitum fed DIO control by Dunnett post hoc test.
CNTF Induces Hypothalamic STAT3 Activation in Both Leptin-Responsive and Leptin-Resistant Obese Mice.
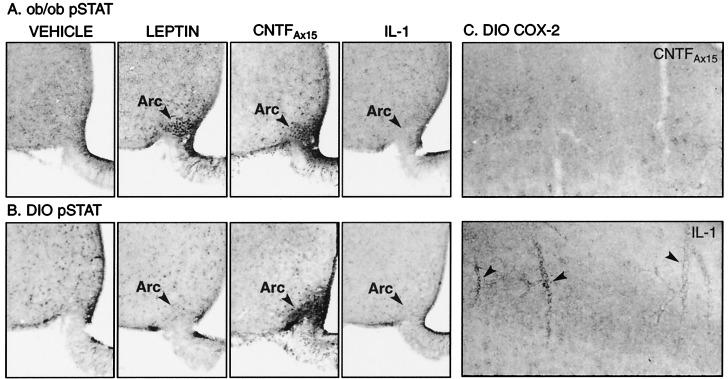
To elucidate molecular mechanisms that might explain the efficacy of CNTFAx15 in leptin-resistant DIO mice, we evaluated intracellular signaling cascades activated by leptin and CNTFAx15 within the hypothalami of both ob/ob and DIO mice. In ob/ob mice, both CNTFAx15 (0.1 mg/kg) and leptin (1.0 mg/kg) induced robust phosphorylation of STAT3 in neurons of the hypothalamic arcuate nucleus (Fig. 2A). However, in DIO mice, CNTFAx15 produced robust STAT3 activation within the arcuate nucleus, whereas leptin failed to induce any detectable STAT3 phoshorylation at this site (Fig. 2B). These immunostaining results were confirmed by Western blot analysis for STAT3 phosphorylation in the basal hypothalamus of ob/ob and DIO mice (data not shown). Thus, the ability of CNTFAx15 to cause weight loss in both ob/ob and DIO mice correlates precisely with its ability to activate STAT3 in the hypothalamic arcuate nuclei of these animals.
Figure 2.
pSTAT3 and COX-2 immunostaining in the brain of ob/ob and DIO mice. The ob/ob mice (A) exhibited nuclear pSTAT3 immunostaining in neurons of the arcuate nucleus (arrowheads) 30 min after i.v. treatment with CNTFAx15 (0.1 mg/kg) or leptin (1.0 mg/kg). However, DIO mice showed STAT3-phosphorylation in response to CNTFAx15 but not leptin (B). No pSTAT immunostaining was seen in the arcuate nucleus of ob/ob (A) or DIO (B) mice 30 min after i.v. treatment with a dose of IL-1 (0.01 mg/kg) known to reduce body weight. Note that CNTFAx15 also induced pSTAT3 expression in the median eminence and in tanycytes and ependyma of the ventral part of the third ventricle. STAT3-phosphorylation was also noted within and adjacent to other circumventricular organs (area postrema, subfornical organ, and organum vasculosum of the lamina terminalis), which contain a fenestrated vasculature (data not shown). However, pSTAT3-immunoreactivity was not evident in other areas of the brain where CNTF receptor α is equally abundant, suggesting that peripherally administered CNTFAx15 does not freely cross the blood–brain barrier. The cortex of DIO mice (C) exhibited COX-2-immunostaining in blood vessels (arrowheads) 6 h after i.v. administration of IL-1 (0.01 mg/kg) but not CNTFAx15 (0.1 mg/kg). Arc, arcuate nucleus.
CNTF Acts Much Differently Than the Prototypical Cachectic Cytokine, IL-1.
Weight loss produced by cachectic cytokines is characterized not only by anorexia, but also by induction of proinflammatory responses, muscle wasting, activation of the pituitary-adrenal axis, and general malaise(16). Given the similarities in the physiological effects and central signaling pathways used by CNTF and leptin, we next sought to determine whether CNTF acts in a manner which is similar to, or fundamentally different from that of classical cachectic cytokines, such as IL-1.
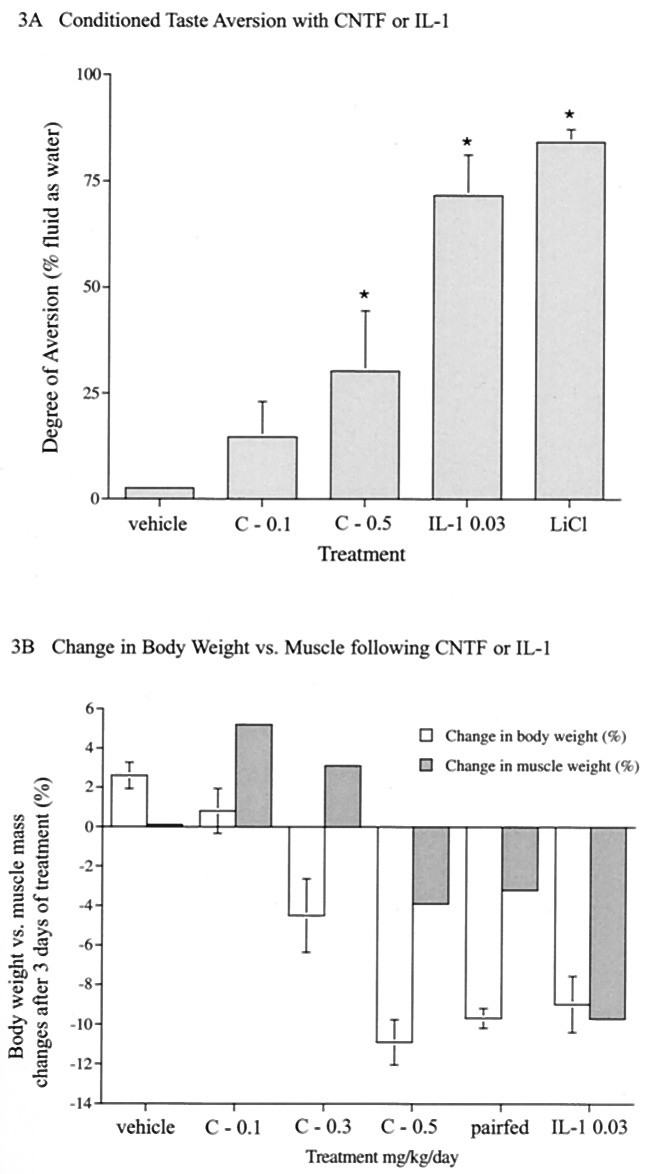
Our findings suggest that CNTF and IL-1 produce decreases in food intake by way of distinct mechanisms. In contrast to CNTFAx15, IL-1 did not activate hypothalamic STAT3 in either ob/ob or DIO mice, even at doses that had profound effects on food intake (Fig. 2 A and B). This finding is consistent with a lack of STAT activation motifs within IL-1 receptor (9) and supports the notion that cachectic cytokines produce anorexia by way of neural mechanisms, which are distinct from those of either leptin or CNTF. Along these lines, decreased food intake in rodents is often seen in response to agents that produce unpleasant visceral sensations, akin to nausea. To determine whether treatment with CNTFAx15 or IL-1 might be perceived by animals as aversive, we determined whether these factors were capable of producing a conditioned taste aversion (CTA) response. CNTFAx15 did not support a CTA when administered at a dose known to produce significant weight loss in obese animals (0.1 mg/kg), although administration of a higher dose did produce a small but significant response (Fig. 3A, and see below). In contrast, IL-1 produced a prominent CTA equivalent to that seen with the classically aversive agent, lithium chloride (Fig. 3A).
Figure 3.
Evaluation of CTA, expressed as percentage of fluid intake as water after administration of CNTFAx15 (0.1, 0.5 mg/kg) or IL-1 (0.03 mg/kg) in C57BL/6 mice (n = 6, A). B illustrates changes in tibialis anterior (TA) muscle mass following a 10% reduction in body weight induced by CNTFAx15, IL-1, or food restriction (n = 5, B) in C57BL/6 mice (starting BW ≈24 g). The percentage difference in TA muscle weight from control (closed bar) is plotted alongside percentage change in body weight from day 0 to day 3 (open bar). Equivalent changes were observed in the relative weights of other muscles. Similarly distinct effects of IL-1 and CNTFAx15 on CTA response and muscle mass also were observed in AKR mice (data not shown).
IL-1 also differs from CNTF in its ability to cause inflammatory responses and muscle wasting. As previously shown (17), even low doses of IL-1 induced an inflammatory response in the brain, as determined by induction of COX-2 in brain vasculature (Fig. 2C). In contrast, COX-2 was not induced by CNTFAx15 at doses that produced comparable weight loss and robustly activated hypothalamic STAT3. Moreover, weight loss induced by IL-1 administration produced a significantly greater loss of muscle mass compared to that seen after equivalent weight loss produced by food restriction or CNTF administration (Fig. 3B).
CNTF Differs from IL-1 as Well as Forced Dieting in That Efficacious Doses Do Not Increase Corticosterone Levels.
Another way in which weight loss produced by CNTF administration can be distinguished from that produced by cachectic cytokines, such as IL-1, is that the latter trigger activation of the pituitary adrenal axis and dramatic induction of circulating corticosteroids (16, 18). In contrast, administration of efficacious doses of CNTFAx15 (0.1 mg/kg/d) produced a marked reduction in food intake and bodyweight and improved insulin and lipid homeostasis in DIO mice, without elevating corticosterone levels (Table 1). Of interest, the ability to induce weight loss without a corticosterone response also distinguished CNTF treatment from enforced dieting. Animals subjected to extended food restriction exhibit increases in food-seeking behavior, and appear to be under significant stress as evidenced by associated increases in circulating adrenal corticosteroids (refs. 19 and 20; Table 1). This finding suggests that, in contrast to an equivalent degree of food-restriction, CNTFAx15 treatment was not experienced as a general stressor, supporting the notion that CNTF-mediated weight loss is not attributable to malaise or general toxicity (14). The ability of CNTFAx15 to cause substantial weight loss without increasing corticosterone could have important clinical benefits because increased corticosteroids can result in muscle wasting, bone resorption, and other unwanted effects.
Although Efficacious Doses of CNTF Are Well-Tolerated and Do Not Cause Cachexia, Excessively High Doses Can Elicit Stress Responses.
The above findings indicate that doses of CNTFAx15 that cause profound weight loss in obese mice can be well-tolerated and not accompanied by cachectic-like effects on muscle wasting, CTA, and corticosterone induction. These findings stand in contrast to previous data suggesting a cachectic action of CNTF (16, 21, 22). However, it should be noted that much higher doses of CNTF were used in those studies. Thus, we explored the effects of delivering superefficacious doses of CNTFAx15. Repeated administration of a 10-fold higher dose of CNTFAx15 (1.0 mg/kg/d) did indeed produce a significant elevation in corticosterone levels and a decrease in spontaneous motor activity, as well as very rapid and severe weight loss, (33% of body weight within 7 days), and significant loss of lean body mass (Table 1); it should be noted that, unlike IL-1, muscle loss seen with even high doses of CNTF does not exceed that seen following equivalent levels of food deprivation (e.g., Fig. 3B). A small CTA response also was noted following high doses of CNTFAx15 (0.5 mg/kg), although the response was not nearly as robust as that seen with IL-1 or lithium chloride (Fig. 3A). It should also be noted that, at high doses, CNTF can signal through receptors for related cytokines such as IL-6 and leukemia inhibitory factor (23), potentially explaining some of the side effects at superefficacious doses.
Taken together, our findings indicate that doses of CNTF that mediate substantial weight loss can be very well-tolerated but that there are limits to the dose of CNTF and to the rate of weight loss, which can be achieved without adverse effect; importantly, substantial absolute weight loss (≈30–35%) can be achieved without adverse effect in DIO mice when the animals are given a lower dose of CNTFAx15 over an extended period (Fig. 1 A2 and B2 and Table 1).
CNTF May Alter the Body Weight Set-Point, Preventing Rebound Weight Gain After Treatment Cessation.
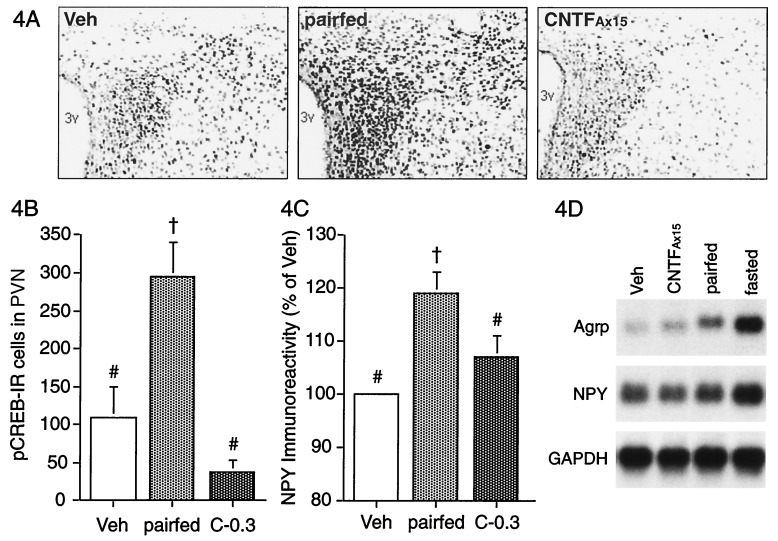
The above observations suggested that, at optimal doses, CNTFAx15 suppresses food intake without triggering hunger signals or associated stress responses otherwise associated with food deprivation. To explore this possibility further, we examined known hypothalamic hunger-signaling systems. NPY is a potent orexigenic peptide and NPY mRNA levels are elevated in the arcuate nucleus during food deprivation, with a concomitant increase in NPY within the terminals of arcuate neurons in the PVN (24, 25). NPY mRNA and peptide levels return to baseline only after the animals are again allowed to feed freely (26, 27). Thus, NPY is thought to contribute to the “memory” of the calories that need to be replenished to restore prefast body weight. Phosphorylation of the transcription factor, CREB, is also elevated in the PVN after food deprivation, suggesting that pCREB is also involved in signal-transduction processes mediating postfast feeding (28). Therefore, we examined NPY and pCREB immunostaining in the PVN of C57BL/6 mice treated for 3 days with CNTFAx15 (0.3 mg/kg/d) compared to vehicle-treated animals that were pair-fed to CNTFAx15 or allowed free access to food. Mice treated with CNTFAx15 or pair-fed lost approximately 5% of their body weight compared to ad libitum fed controls. As anticipated, the density of NPY-immunoreactive fibers and the number of pCREB-immunoreactive nuclei in the PVN were significantly elevated in pair-fed mice compared to the ad libitum fed control, whereas neither marker was elevated in the PVN of CNTFAx15-treated mice (Fig. 4). We also show that treatment with CNTFAx15 does not result in increased hypothalamic NPY and Agrp mRNA levels of the magnitude seen after food restriction or fasting (Fig. 4D). These data confirm and extend previous findings that CNTF treatment blunts increases in NPY mRNA seen with fasting (29) and that NPY administration can counteract the anorectic and weight reducing effects of CNTF (30) Importantly, these data demonstrate that enforced food-restriction activates hypothalamic hunger signals, whereas CNTFAx15-treatment can cause equivalent decreases in food intake and associated weight loss without activating these hypothalamic hunger signals.
Figure 4.
Effect of CNTFAx15 and pair-feeding on hypothalamic markers of hunger in C57BL6 mice. Digitized images of pCREB immunostaining in the PVN after 3 days of s.c. treatment with vehicle, CNTFAx15 (C, 0.3 mg/kg/day) or pair-feeding (A). 3v, third ventricle. Histograms show mean number of pCREB-immunoreactive cells and NPY immunoreactivity (percentage of vehicle group) in the PVN after treatment (B and C; n = 4). ANOVA: pCREB, P < 0.01; NPY, P = 0.01. †, Difference from vehicle (veh); #, difference from pair-fed by Dunnett post hoc test. Northern blot analysis for Agrp, NPY, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA from hypothalamus of AKR mice after 4 days of treatment with CNTFAx15 (0.3 mg/kg/day), pair-feeding or a 48 h fast also is shown (D). Each lane represents pooled tissue from three mice.
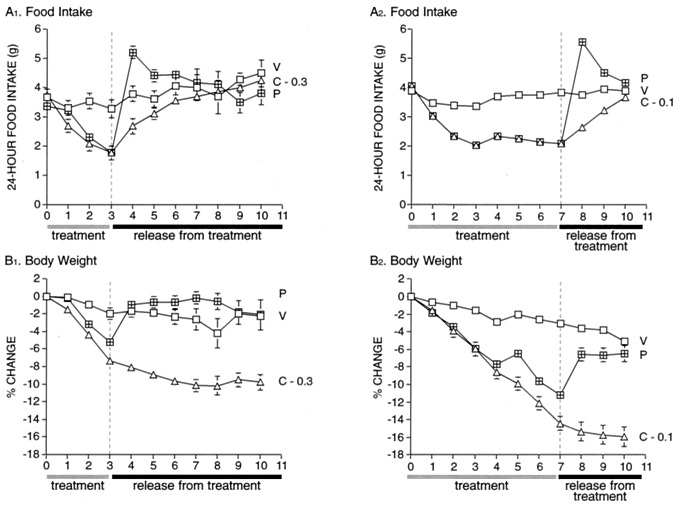
Because these hypothalamic changes are thought to contribute to the memory of missed calories and promote a rapid return to prediet body weight, these findings predict that, in contrast to release from enforced food restriction, cessation of CNTFAx15 treatment might not be accompanied by binge overeating and a rapid, rebound weight gain. To test this hypothesis, DIO mice were treated with CNTFAx15, or were pair-fed to the CNTFAx15-treated animals, for 3 or 7 days. After this treatment phase, all animals were allowed free access to food. Cessation of forced dieting in pair-fed mice resulted in immediate binge over-eating (Fig. 5 A1 and A2) and rapid return of body weight to prerestriction levels (Fig. 5 B1 and B2). At that point, food intake normalized, suggesting that the pair-fed animals had retained a memory of the calories lost during the food restriction and had made up for these missed calories when allowed free access to food. In contrast, CNTFAx15-treated DIO animals did not over-eat after cessation of treatment (Fig. 5 A1 and A2), and thus did not exhibit immediate rebound weight gain (Fig. 5 B1 and B2). The lack of binge overeating and rapid rebound weight gain after termination of CNTFAx15 treatment confirms that CNTF acts very differently from forced dieting, perhaps by altering the hypothalamic body weight set-point.
Figure 5.
Lack of rebound food intake in DIO mice after cessation of daily s.c. injection with CNTFAx15. DIO mice (n = 7; starting BW ≈50 g) were treated for 3 (A1 and B1) or 7 days (A2 and B2) with vehicle (V) or CNTFAx15 (C, 0.1, 0.3 mg/kg/day), or were pair-fed to the food intake of CNTFAx15-treated mice (P) after which all treatment was stopped and all animals were returned to ad lib feeding. The 24-h food intake (A1 and A2) and change in body weight (percentage from day 0; B1 and B2) was measured throughout both studies. The overeating and rapid return to prerestriction body weight seen after returning pair-fed mice to ad lib feeding was not seen after cessation of treatment with CNTFAx15.
Discussion
The discovery that CNTF could cause weight loss in animals and humans occurred rather serendipitously during its evaluation for another indication (3). At that time, the lack of a clear understanding of how CNTF caused weight loss led to initial concerns that it might be acting in a deleterious manner. However, recent studies (14, 29) together with those described herein have shown that CNTF is likely working, at least in part, via a leptin-like pathway, on appropriate hypothalamic cellular targets, to cause selective loss of fat as opposed to lean body mass, and that it acts very differently from prototypical cachectic cytokines such as IL-1. Thus, efficacious doses of CNTFAx15 activate hypothalamic STAT3 and cause preferential fat loss, whereas IL-1 does not activate hypothalamic STAT3 and causes muscle wasting, inflammatory changes, CTA, and increased corticosterone release. Of interest, excessively high doses of CNTFAx15, far above those necessary to cause weight loss in obese mice, can induce stress responses as well. These stress responses are more reminiscent of those seen following extreme food deprivation, as opposed to those induced by cachectic cytokines, as even superefficacious doses of CNTFAx15 do not produce muscle loss in excess of that seen with equivalent food restriction. Thus, our findings indicate that although CNTFAx15 is generally well-tolerated, there is a limit to the dose of CNTF and the rate of weight loss per se that can be achieved without adverse effects.
Importantly, CNTF differs from leptin in that it does not appear to normally play a physiologic role in weight control; mice and humans lacking CNTF are not obese (31–33). Thus, activation of CNTF receptors in the hypothalamus seems to fortuitously mimic many of the effects induced by activation of related and similarly localized leptin receptors. That CNTF is a fortuitous pharmacological mimic of leptin also may explain, in part, its undiminished ability to produce weight loss in forms of obesity that are profoundly leptin resistant, including MC4R knock-out mice (34), Agouti mice (data not shown), and DIO mice. That is, the effects of pharmacologically administered CNTF may not be subject to normal physiological counterregulatory mechanisms.
Another remarkable property that distinguishes CNTF-mediated weight loss from forced dieting is that cessation of treatment does not result in binge overeating and immediate rebound weight gain. This is apparently because of the ability of CNTF to reduce food intake without triggering hypothalamic hunger signals and associated stress responses. Thus, CNTF may alter, at least for a time, body weight settings encoded in the hypothalamus. Hopefully, ongoing clinical studies of CNTFAx15 will confirm that it can indeed produce substantial weight loss in obese human patients at doses that are well tolerated.
Acknowledgments
We thank Evan Burrows for expert production of figures for the manuscript.
Abbreviations
- CNTF
ciliary neurotrophic factor
- CNTFAx15, a truncated form of CNTF
DIO, diet-induced obesity
- STAT3
signal transducer and activator of transcription-3
- pSTAT3
phospho-STAT3
- pCREB
phospho-cyclic AMP response element-binding protein
- CTA
conditioned taste aversion
- COX-2
cyclooxygenase-2
- NPY
neuropeptide Y
- PVN
paraventricular nucleus
Footnotes
See commentary on page 4279.
References
- 1.Lin L, Armes L, Sommer A, Smith D, Collins F. J Biol Chem. 1990;265:8942–8947. [PubMed] [Google Scholar]
- 2.Sendtner M, Arakawa Y, Stockli K, Kreutzberg G, Thoenen H. J Cell Sci Suppl. 1991;15:103–109. doi: 10.1242/jcs.1991.supplement_15.14. [DOI] [PubMed] [Google Scholar]
- 3.Amyotrophic Lateral Sclerosis CNTF Treatment Study Group. Neurology. 1996;46:1244–1249. doi: 10.1212/wnl.46.5.1244. [DOI] [PubMed] [Google Scholar]
- 4.Chen H, Charlat O, Tartaglia L A, Woolf E A, Weng X, Ellis S J, Lakey N D, Culpepper J, Moore K J, Breitbart R E, et al. Cell. 1996;84:491–495. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- 5.Tartaglia L A, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards G J, Campfield L A, Clark F T, Deeds J, et al. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 6.Davis S, Aldrich T H, Valenzuela D M, Wong V V, Furth M E, Squinto S P, Yancopoulos G D. Science. 1991;253:59–63. doi: 10.1126/science.1648265. [DOI] [PubMed] [Google Scholar]
- 7.Ip N Y, Nye S H, Boulton T G, Davis S, Taga T, Li Y, Birren S J, Yasukawa K, Kishimoto T, Anderson D J, et al. Cell. 1992;69:1121–1132. doi: 10.1016/0092-8674(92)90634-o. [DOI] [PubMed] [Google Scholar]
- 8.Davis S, Aldrich T H, Stahl N, Pan L, Taga T, Kishimoto T, Ip N Y, Yancopoulos G D. Science. 1993;260:1805–1808. doi: 10.1126/science.8390097. [DOI] [PubMed] [Google Scholar]
- 9.Stahl N, Farruggella T J, Boulton T G, Zhong Z, Darnell J E, Jr, Yancopoulos G D. Science. 1995;267:1349–1353. doi: 10.1126/science.7871433. [DOI] [PubMed] [Google Scholar]
- 10.Baumann H, Morella K K, White D W, Dembski M, Bailon P S, Kim H, Lai C F, Tartaglia L A. Proc Natl Acad Sci USA. 1996;93:8374–8378. doi: 10.1073/pnas.93.16.8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaisse C, Halaas J L, Horvath C M, Darnell J E, Jr, Stoffel M, Friedman J M. Nat Genet. 1996;14:95–97. doi: 10.1038/ng0996-95. [DOI] [PubMed] [Google Scholar]
- 12.Ghilardi N, Ziegler S, Wiestner A, Stoffel R, Heim M H, Skoda R C. Proc Natl Acad Sci USA. 1996;93:6231–6235. doi: 10.1073/pnas.93.13.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carpenter L R, Farruggella T J, Symes A, Karow M L, Yancopoulos G D, Stahl N. Proc Natl Acad Sci USA. 1998;95:6061–6066. doi: 10.1073/pnas.95.11.6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gloaguen I, Costa P, Demartis A, Lazzaro D, Di Marco A, Graziani R, Paonessa G, Chen F, Rosenblum C I, Van der Ploeg L H, et al. Proc Natl Acad Sci USA. 1997;94:6456–6461. doi: 10.1073/pnas.94.12.6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campfield L A, Smith F J, Guisez Y, Devos R, Burn P. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 16.Matthys P, Billiau A. Nutrition. 1997;13:763–770. doi: 10.1016/s0899-9007(97)00185-8. [DOI] [PubMed] [Google Scholar]
- 17.Cao C, Matsumura K, Yamagata K, Watanabe Y. Brain Res. 1996;733:263–272. doi: 10.1016/0006-8993(96)00575-6. [DOI] [PubMed] [Google Scholar]
- 18.Fantuzzi G, Benigni F, Sironi M, Conni M, Carelli M, Cantoni L, Shapiro L, Dinarello C, Sipe J, Ghezzi P. Cytokine. 1995;7:150–156. doi: 10.1006/cyto.1995.1020. [DOI] [PubMed] [Google Scholar]
- 19.Campfield L A, Smith F J. Brain Res Bull. 1986;17:427–433. doi: 10.1016/0361-9230(86)90250-9. [DOI] [PubMed] [Google Scholar]
- 20.Heiderstadt K M, McLaughlin R M, Wright D C, Walker S E, Gomez-Sanchez C E. Lab Anim. 2000;34:20–28. doi: 10.1258/002367700780578028. [DOI] [PubMed] [Google Scholar]
- 21.Henderson J T, Seniuk N A, Richardson P M, Gauldie J, Roder J C. J Clin Invest. 1994;93:2632–2638. doi: 10.1172/JCI117276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin D, Merkel E, Tucker K K, McManaman J L, Albert D, Relton J, Russell D A. Am J Physiol. 1996;271:R1422–R1428. doi: 10.1152/ajpregu.1996.271.5.R1422. [DOI] [PubMed] [Google Scholar]
- 23.Davis S, Aldrich T, Ip N, Stahl N, Scherer S, Farruggella T, DiStefano P, Curtis R, Panayotatos N, Gascan H, et al. Science. 1993;259:1736–1739. doi: 10.1126/science.7681218. [DOI] [PubMed] [Google Scholar]
- 24.Brady L S, Smith M A, Gold P W, Herkenham M. Neuroendocrinology. 1990;52:441–447. doi: 10.1159/000125626. [DOI] [PubMed] [Google Scholar]
- 25.Sahu A, Kalra P, Kalra S. Peptides. 1988;9:83–86. doi: 10.1016/0196-9781(88)90013-7. [DOI] [PubMed] [Google Scholar]
- 26.Marks J L, Davies L. Am J Physiol. 1994;266:R1687–R1691. doi: 10.1152/ajpregu.1994.266.5.R1687. [DOI] [PubMed] [Google Scholar]
- 27.Beck B, Jhanwar-Uniyal M, Burlet A, Chapleur-Chateau M, Leibowitz S F, Burlet C. Brain Res. 1990;528:245–249. doi: 10.1016/0006-8993(90)91664-3. [DOI] [PubMed] [Google Scholar]
- 28.Sheriff S, Chance W T, Fischer J E, Balasubramaniam A. Mol Pharmacol. 1997;51:597–604. doi: 10.1124/mol.51.4.597. [DOI] [PubMed] [Google Scholar]
- 29.Xu B, Dube M G, Kalra P S, Farmerie W G, Kaibara A, Moldawer L L, Martin D, Kalra S P. Endocrinology. 1998;139:466–473. doi: 10.1210/endo.139.2.5723. [DOI] [PubMed] [Google Scholar]
- 30.Pu S, Dhillon H, Moldawer L L, Kalra P S, Kalra S P. Neuroendocrinology. 2000;12:827–832. doi: 10.1046/j.1365-2826.2000.00526.x. [DOI] [PubMed] [Google Scholar]
- 31.DeChiara T, Vejsada R, Poueymirou W, Acheson A, Suri C, Conover J, Friedman B, McClain J, Pan L, Stah L N, et al. Cell. 1995;83:313–322. doi: 10.1016/0092-8674(95)90172-8. [DOI] [PubMed] [Google Scholar]
- 32.Masu Y, Wolf E, Holtmann B, Sendtner M, Brem G, Thoenen H. Nature (London) 1993;365:27–32. doi: 10.1038/365027a0. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi R, Yokoji H, Misawa H, Hayashi M, Hu J, Deguchi T. Nat Genet. 1994;7:79–84. doi: 10.1038/ng0594-79. [DOI] [PubMed] [Google Scholar]
- 34.Marsh D, Hollopeter G, Huszar D, Laufer R, Yagaloff K, Fisher S, Burn P, Palmiter R. Nat Genet. 1999;21:119–122. doi: 10.1038/5070. [DOI] [PubMed] [Google Scholar]