Abstract
BACKGROUND AND PURPOSE
The role of hydrogen sulphide (H2S) as a putative endogenous signalling molecule in the gastrointestinal tract has not yet been established. We investigated the effect of D,L-propargylglycine (PAG), an inhibitor of cystathionine γ-lyase (CSE), amino-oxyacetic acid (AOAA) and hydroxylamine (HA), inhibitors of cystathionine β-synthase (CBS) on rat colonic motility.
EXPERIMENTAL APPROACH
Immunohistochemistry, H2S production, microelectrode and organ bath recordings were performed on rat colonic samples without mucosa and submucosa to investigate the role of endogenous H2S in motility.
KEY RESULTS
CSE and CBS were immunolocalized in the colon. H2S was endogenously produced (15.6 ± 0.7 nmol·min−1·g−1 tissue) and its production was strongly inhibited by PAG (2 mM) and AOAA (2 mM). PAG (2 mM) caused smooth muscle depolarization and increased spontaneous motility. The effect was still recorded after incubation with tetrodotoxin (TTX, 1 µM) or Nω-nitro-L-arginine (L-NNA, 1 mM). AOAA (2 mM) caused a transient (10 min) increase in motility. In contrast, HA (10 µM) caused a ‘nitric oxide-like effect’, smooth muscle hyperpolarization and relaxation, which were antagonized by 1H-[1,2,4]oxadiazolo[4,3-α]quinoxalin-1-one (ODQ, 10 µM). Neither spontaneous nor induced inhibitory junction potentials were modified by AOAA or PAG.
CONCLUSIONS AND IMPLICATIONS
We demonstrated that H2S is endogenously produced in the rat colon. PAG and AOAA effectively blocked H2S production. Our data suggest that enzymatic production of H2S regulates colonic motility and therefore H2S might be a third gaseous inhibitory signalling molecule in the gastrointestinal tract. However, possible non-specific effects of the inhibitors should be considered.
Keywords: hydrogen sulphide, nitric oxide, smooth muscle, gastrointestinal, inhibitory neuromuscular transmission
Introduction
Hydrogen sulphide (H2S) is an endogenous gaseous signalling molecule similar to NO and carbon monoxide (CO). The biology of H2S is an emerging topic of research and H2S potentially plays important roles in several areas including the CNS, cardiovascular, renal, respiratory and digestive systems (Fiorucci et al., 2006). In mammals, two pyridoxal phosphate-dependent enzymes are responsible for H2S synthesis: cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE). These two enzymes are expressed in a great variety of organs and tissues including the liver, kidney, vascular and central nervous system. Both enzymes use L-cysteine as a substrate to produce H2S (review by Szabo, 2007). A third route of H2S synthesis involves 3-mercaptopyruvate sulphurtransferase in combination with cysteine aminotransferase (Shibuya et al., 2009a; Shibuya et al., 2009b). Three inhibitors of these enzymes are commonly used to inhibit H2S synthesis: D,L-propargylglycine (PAG), an inhibitor of CSE and both amino-oxyacetic acid (AOAA) and hydroxylamine (HA), inhibitors of CBS. These inhibitors are important pharmacological tools for investigating the effects of endogenous production of hydrogen sulphide (reviewed by Szabo, 2007).
Little is known as yet about the physiological function of H2S in the gastrointestinal (GI) tract (Linden et al., 2010; Wallace, 2010) but it might play a role as a ‘gasotransmitter’ and the criteria to consider it as such have recently been reviewed: ‘The gasotransmitter must: 1. be small molecules of gas, 2. be freely permeable to membranes, 3. be endogenously and enzymatically generated in a regulated manner, 4. have well-defined specific functions at physiologically relevant concentrations, and 5. act at specific cellular targets’ (Linden et al., 2010). H2S fulfils part of these criteria.
H2S is endogenously produced in the colon of the mouse (Linden et al., 2008). Smooth muscle homogenates produce H2S through CBS and CSE enzymes (Hosoki et al., 1997). Both enzymes have been detected immunohistochemically in rat colonic smooth muscle cells (Hennig and Diener, 2009). CSE immunoreactivity (IR) has been found in neurons of the mouse and guinea-pig myenteric plexus and in neurons of the guinea-pig and human submucous plexus as well as in certain subclasses of interstitial cells of Cajal (ICC) in the guinea-pig colon (Schicho et al., 2006; Linden et al., 2008). CBS-IR has also been detected in guinea-pig myenteric and submucous plexus and in human submucous plexus (Schicho et al., 2006). These data suggest that several cell structures are able to synthesize H2S, which is therefore a potential signalling molecule regulating GI motility.
Sodium hydrosulphide (NaHS) is the source of H2S usually employed to study H2S functions. The effects induced by NaHS and its mechanisms of action, however, differ between species and might also depend on the concentration used in the experiments. NaHS exerts a prosecretory effect through a neurally mediated mechanism [tetrodotoxin (TTX)-sensitive] both in the guinea-pig and human colon (Schicho et al., 2006; Krueger et al., 2010). In the rat colon, a TTX-sensitive and also an insensitive response, probably due to direct stimulation of apical as well as basolateral epithelial K+ channels, have been reported (Hennig and Diener, 2009; Pouokam and Diener, 2011). NaHS causes concentration-dependent relaxation of the guinea-pig and rabbit ileum (Hosoki et al., 1997; Teague et al., 2002). We have recently demonstrated that NaHS inhibits the peristaltic activity in the mouse colon and small intestine and also inhibits the spontaneous contractions in rat and human colon measured in organ bath (Gallego et al., 2008a). NaHS also caused inhibition of pre-contracted gastric and colonic strips in mice (Dhaese and Lefebvre, 2009; Dhaese et al., 2010). H2S donors are drugs that could be used to treat several GI disorders such as ulcers and colonic inflammation (Fiorucci et al., 2007; Wallace et al., 2007; Wallace et al., 2009), although their putative beneficial and toxic effects are much debated in the literature (Coffey et al., 2009; Matsunami et al., 2009; Schemann and Grundy, 2009).
Little is known about the enzymatic endogenous production of H2S and colonic motility. In the present paper, we characterized the effects of H2S synthesis inhibitors, PAG, AOAA and HA, as well as the effect of L-cysteine, which is a precursor of H2S synthesis, on rat colonic motility. Briefly, we found that PAG and AOAA effectively blocked H2S production and increased motility, suggesting that H2S is an endogenous inhibitory signalling molecule in the GI tract. However, non-specific effects of these compounds should be considered.
Methods
Animals and tissue samples
Male Sprague-Dawley rats (8–10 weeks old, 300–350 g) were purchased from Charles River (Lyon, France). Animals were housed under controlled conditions: temperature 22°C ± 2°C, humidity 55% ± 10%, 12:12 h light–dark cycle and access to water and food ad libitum. Animals were stunned and killed by decapitation and exsanguination 2–3 s afterwards. The colon was quickly removed and placed in carbogenated (95% O2 and 5% CO2), ice-cold physiological saline solution. Then, it was opened along the mesenteric border and pinned to a Sylgard® base (mucosa side up). The mid colon was identified according to anatomical criteria previously described (Alberti et al., 2005). The mucosal and submucosal layers were removed and muscle strips were cut 1 cm × 2 cm for the endogenous production of H2S and 1 cm × 0.3 cm for the intracellular microelectrode and organ bath experiments. All procedures were approved by the Ethics Committee of the Universitat Autònoma de Barcelona.
Immunohistochemistry
Mid colon samples were fixed with paraformaldehyde (4%) in PBS (0.2 M) and embedded in paraffin. Paraffin sections were cut, mounted on glass slides, deparaffinized and rehydrated. To reduce autofluorescence, a 30 min pretreatment with NH4Cl (50 mM; pH 8.0) was performed. After the sections had been washed in PBS-T (PBS containing; 0.01% Triton X-100; 0.02% Tween 20), they were incubated at 100°C in citrate buffer (10 mM; pH 6.0) for 20 min to retrieve antigens, cooled for 30 min and rinsed again with PBS-T. Non-specific binding was blocked by incubating the samples in PBS-T containing normal goat serum (10%). Mouse monoclonal anti-CSE or mouse polyclonal anti-CBS (both 1:50; ABNOVA, Taipei, Taiwan) diluted in PBS were incubated with the sections overnight at 4°C. These antibodies were co-incubated with a rabbit polyclonal Anti-HuD (1:200; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) to label enteric neurons. After 24 h, the samples were rinsed in PBS-T and then incubated with the secondary antibodies (both 1:200; Alexa Fluor® 488 goat anti-mouse and Alexa Fluor® 568 goat anti-rabbit from Invitrogen Ltd, Paisley, UK) diluted in PBS for 1 h at room temperature. Negative controls were performed by leaving out the primary antibodies during the staining procedure. Additional controls with pre-absorbed antisera with CSE or CBS recombinant proteins (both 1 µM; ABNOVA, Taipei, Taiwan) were performed as well. The samples were examined on an Axioskop 40 FL fluorescence microscope equipped with a AxioCam MRm camera and the AxioVision Release 4.8.1 software for image acquisition (all of them from Carl Zeiss, Inc., Göttingen, Germany).
Endogenous production of hydrogen sulphide
Production of H2S was measured as previously described (Linden et al., 2008). Briefly, the tissue was placed in a sealed polypropylene vial containing physiological saline solution with L-cysteine (10 mM) and pyridoxal 5′-phosphate (2 mM) which was connected to a second vial containing 0.5 mL of 1% (w v-1) zinc acetate. The zinc acetate solution did not come in contact with the tissue. The first vial was bubbled with a gas mixture of 95% O2 and 5% CO2 at a rate of 1–4 mL·min−1 in order to minimize the degradation of H2S (Linden et al., 2008). The increase in pressure in the first vial forced the gases to move into the second vial where they bubbled through the zinc acetate solution and H2S was trapped as zinc sulphide. The incubation mixture was prepared on ice and the reaction was started by transferring the vials to a water bath at 37 ± 1°C. The reaction was stopped at 30 min by injecting 0.5 mL of 50% (w v-1) trichloroacetic acid through a stainless steel needle. Gas flow was allowed to continue for an additional 30 min to ensure complete trapping of H2S. In some experiments, PAG (2 mM) or AOAA (2 mM) were added to the incubation mixture in order to block CSE and CBS activity, respectively.
H2S was measured by using a colorimetric method (Siegel, 1965; Stipanuk and Beck, 1982; Abe and Kimura, 1996). The content of the second vial was transferred to test tubes and 3.5 mL of distilled water, 0.4 mL of N,N-dimethyl-p-phenylenediamine sulphate (20 mM) in HCl (7.2 M) and 0.4 mL of FeCl3 (30 mM) in HCl (1.2 M) were added to each tube. After 20 min of incubation at room temperature, the absorbance of the resulting solution at 670 nm was determined with a Ultrospec 2000 spectrophotometer [Pharmacia Biotech (Biochrom) Ltd, Cambrige, UK]. A linear regression was set up with defined concentrations of NaHS and the concentration of H2S was estimated and expressed in nmol·min−1·g−1 tissue.
Intracellular microelectrode recording
The tissue was pinned with the circular muscle layer facing upward in a Sylgard-coated chamber and continuously perfused with carbogenated physiological saline solution at 37°C ± 1°C and was allowed to equilibrate for 1 h. Circular smooth muscle cells were impaled with glass microelectrodes filled with 3 M KCl (30–60 MΩ of resistance). Membrane potential was measured by using standard electrometer Duo773 (WPI Inc., Sarasota, FL, USA). Tracings were displayed on an oscilloscope 4026 (Racal-Dana Ltd, Windsor, UK) and simultaneously digitalized (100 Hz) with PowerLab 4/30 system and Chart 5 software for Windows (both from ADInstruments, Castle Hill, NSW, Australia). In order to stabilize impalements, experiments were performed in the presence of nifedipine (1 µM). The spontaneous inhibitory neural tone was characterized as we have described previously (Gil et al., 2010). Briefly, the resting membrane potential (RMP) (expressed in mV) was estimated as the most probable bin of the frequency distribution of the membrane potential (0.1 mV bins; 30–60 s recordings). Spontaneous inhibitory junction potentials (sIJP) were illustrated with the frequency distribution (0.5 mV bins) of the values of the membrane potential (30 to 60 s) expressed as bin probability (from 0 to 1), and for statistical purposes we calculated the mean SD of the distribution of the membrane potential: SD of the recording inside the cell minus SD of the recording outside the cell (expressed in mV). IJP were induced by electrical field stimulation (EFS) using the following parameters: total train duration 100 ms, frequency 20 Hz, pulse duration 0.3 ms and increasing amplitude voltage (5, 10, 12, 15, 17, 20, 25, 30 and 50 V). Both IJP amplitude (from RMP to the most hyperpolarized value) and duration (measured at the baseline) were estimated for each voltage of stimulation.
Muscle bath studies
Muscle strips were mounted in a 10 mL organ bath containing carbogenated physiological saline solution maintained at 37 ± 1°C. Motility was measured using an isometric force transducer (Harvard VF-1, Harvard Apparatus Inc., Holliston, MA, USA) connected to a computer through an amplifier. Data were digitalized (25 Hz) using Data 2001 software (Panlab, Barcelona, Spain) coupled to an A/D converter installed in the computer. A tension of 1 g was applied and tissues were allowed to equilibrate for 1 h. After this period, strips displayed spontaneous phasic activity. The release of inhibitory neurotransmitters was studied by using EFS applied for 4 min; pulse duration 0.3 ms; frequency 5 Hz; amplitude 30 V. The area under the curve (AUC) of contractions from the baseline was measured to estimate the mechanical activity and the result was expressed in g·min−1.
Solutions and drugs
The composition of the physiological saline solution was (in mM) glucose 10.10; NaCl 115.48; NaHCO3 21.90; KCl 4.61; NaH2PO4 1.14; CaCl2 2.50 and MgSO4 1.16 (pH 7.3–7.4). All the chemicals employed in the H2S production were purchased from Sigma Chemicals (St. Louis, MO, USA). The following drugs were used: TTX (Latoxan, Valence, France); AOAA, PAG, HA, L-cysteine, nifedipine, Nω-nitro-L-arginine (L-NNA), 1H-[1,2,4]oxadiazolo[4,3-α]quinoxalin-1-one (ODQ) (Sigma Chemicals, St. Louis, MO, USA); D-(-)-2-amino-5-phosphonopentanoic acid (D-AP5) (Tocris, Bristol, UK). Stock solutions were made by dissolving drugs in distilled water except for: (i) nifedipine which was dissolved in 96% ethanol; (ii) L-NNA which was dissolved in physiological saline solution by sonication; and (iii) AOAA which was dissolved in physiological saline solution and the pH was adjusted to 7.4 by using NaOH. Drug and receptor nomenclature conform to the guidelines of the British Journal of Pharmacology (Alexander et al., 2009).
Data analysis and statistics
Differences in the RMP, sIJP, spontaneous motility (AUC) and EFS-induced relaxation were compared before and after drug addition by Student's paired t-test. One-way anova followed by Bonferroni post hoc test was used to evaluate the effect of PAG and AOAA on the endogenous H2S production. Differences between the amplitude and duration of the electrically-elicited IJPs before and after drug infusion were compared by two-way anova (drug and voltage). IC50 values were calculated using a conventional sigmoid concentration–response curve with variable slope.
Data are expressed as mean ± SEM. A P < 0.05 was considered statistically significant; n values indicate the number of samples. Statistical analysis and curve fit were performed with GraphPad Prism version 4.00 (GraphPad Software, San Diego, CA, USA).
Results
CSE and CBS expression in the rat mid colon and endogenous production of H2S
CSE-IR was mainly observed in the circular and longitudinal smooth muscle layers. Double-labelling with the neuronal marker anti-HuD showed that CSE was expressed in neurons of the enteric nervous system as well. Furthermore, diffuse CSE-IR was also present in the mucosa and submucosa layers (Figure 1A). A completely different pattern was found for CBS. Positive IR for this enzyme was mainly localized in the colonic epithelium, although a diffuse pattern was also observed in the muscular layers. Colocalization between CBS-IR and HuD-IR was not observed showing that CBS was not expressed in neurons (Figure 1B). No CSE-IR or CBS-IR was detected when primary antibodies were left out or pre-absorption with recombinant proteins was performed (data not shown).
Figure 1.
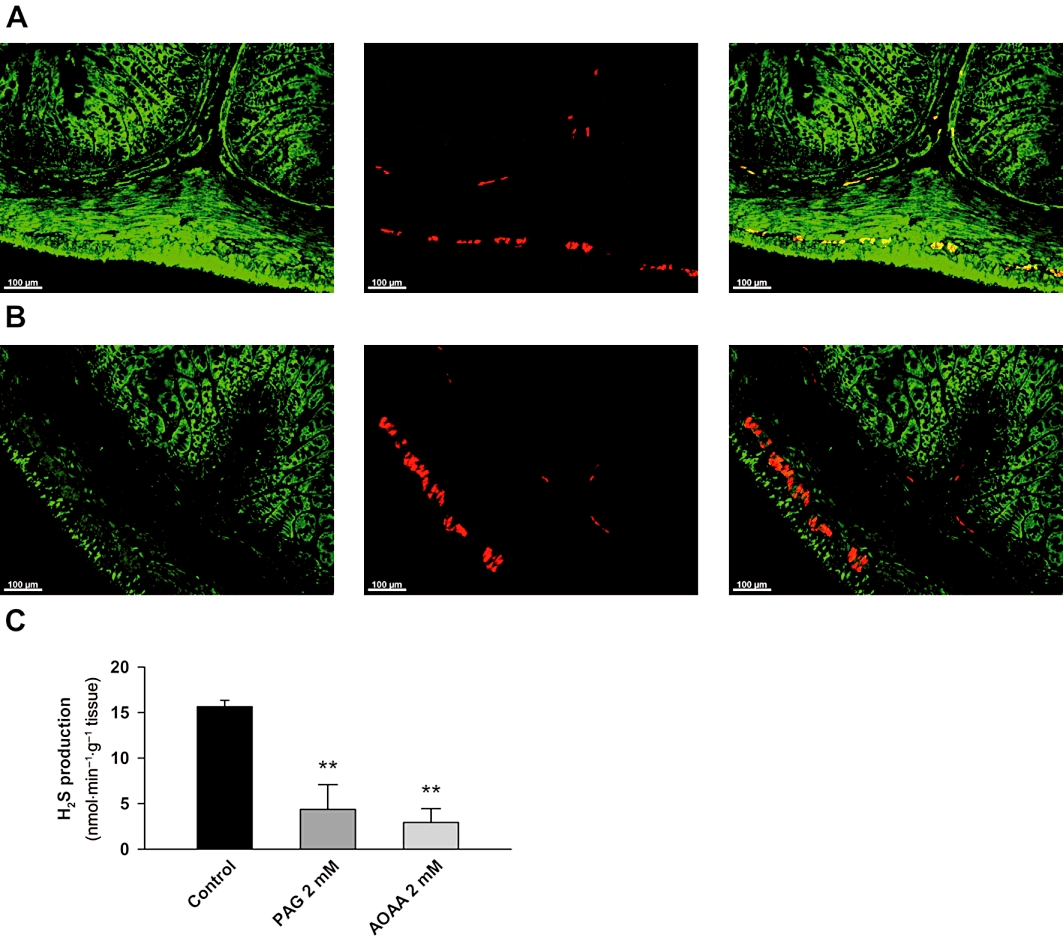
Distribution of cystathionine γ-lyase (CSE) (A) and cystathionine β-synthase (CBS) (B) in the rat mid colon. Left: CSE/CBS-immunoreactivity (IR); middle: HuD-IR; right: merged. Scale bar = 100 µm. Histogram showing the production of H2S in rat colonic samples devoid of mucosa and submucosa in control conditions and in the presence of PAG (2 mM) and AOAA (2 mM) (C). All values are mean ± SEM. Significant differences were assessed using one-way anova, followed by Bonferroni post hoc test. **P < 0.01; significant difference from control.
Colonic tissue in which mucosa and submucosa had been removed was able to produce H2S (15.6 ± 0.7 nmol·min−1·g−1 tissue; n = 4; Figure 1C). H2S production was reduced when the experiments were performed in the presence of PAG (2 mM) (4.4 ± 2.7 nmol·min−1·g−1 tissue; n = 4; P < 0.001; Figure 1C) and AOAA (2 mM) (2.9 ± 1.5 nmol·min−1·g−1 tissue; n = 3; P < 0.01; Figure 1C), showing that it was due to CSE and CBS activity. We did not test HA on H2S production due to the ‘NO-like effects’ described below.
Effect of PAG on RMP and spontaneous mechanical activity
Effect of PAG was evaluated on the RMP and mechanical activity. PAG induced a concentration-dependent increase in motility (IC50 = 1.55 mM; 95% confidence interval 1.26–1.90 mM; log IC50 = −2.81 ± 0.09; n = 4; Figure 2A). A time-dependent control was performed and the spontaneous motility remained stable during the experiment (not shown). Furthermore, administration of PAG (2 mM) depolarized smooth muscle cells and increased mechanical activity (Table 1 and Figure 2B,C). In order to test whether the depolarization and increase in motility were due to a neural effect, we performed experiments with the tissue pre-incubated with TTX (1 µM) and L-NNA (1 mM). As previously reported (Gil et al., 2010), both TTX and L-NNA caused depolarization (Control: −48.2 ± 1.2 mV vs. TTX: −43.6 ± 1.2 mV; n = 19; P < 0.001 and Control: −47.1 ± 1.8 mV vs. L-NNA: −41.2 ± 1.8 mV; n = 8; P < 0.001) and an increase in spontaneous motility (Control: 9.5 ± 1.1 g·min−1 vs. TTX: 24.1 ± 2.5 g·min−1 AUC; n = 39; P < 0.001 and Control: 15.2 ± 4.3 g·min−1 vs. L-NNA: 39.7 ± 7.1 g·min−1 AUC; n = 8; P < 0.01). In the presence of TTX (1 µM), PAG (2 mM) induced smooth muscle depolarization and an increase in spontaneous motility (Table 1 and Figure 2D,E). In the presence of L-NNA (1 mM), PAG (2 mM) also caused smooth muscle depolarization and increased spontaneous mechanical activity (Table 1 and Figure 2F,G). The depolarization induced by PAG (2 mM) was repetitive. Four consecutive transient (5 min) incubations with PAG (2 mM) produced the same depolarization (1st addition: 8.0 ± 0.4 mV, 2nd addition: 9.6 ± 1.4 mV, 3rd addition: 9.2 ± 0.4 mV, 4th addition: 9.0 ± 0.5 mV; n = 3; not significant; Figure 2H). Note that after each incubation, the membrane potential of smooth muscle cells returned to a value similar to control levels (Control: −43.5 ± 5.3 mV; 1st addition −43.9 ± 5.6 mV, 2nd addition −44.1 ± 5.0 mV, 3rd addition −44.5 ± 5.1 mV and 4th addition −45.0 ± 4.9 mV n = 3; not significant; Figure 2H).
Figure 2.
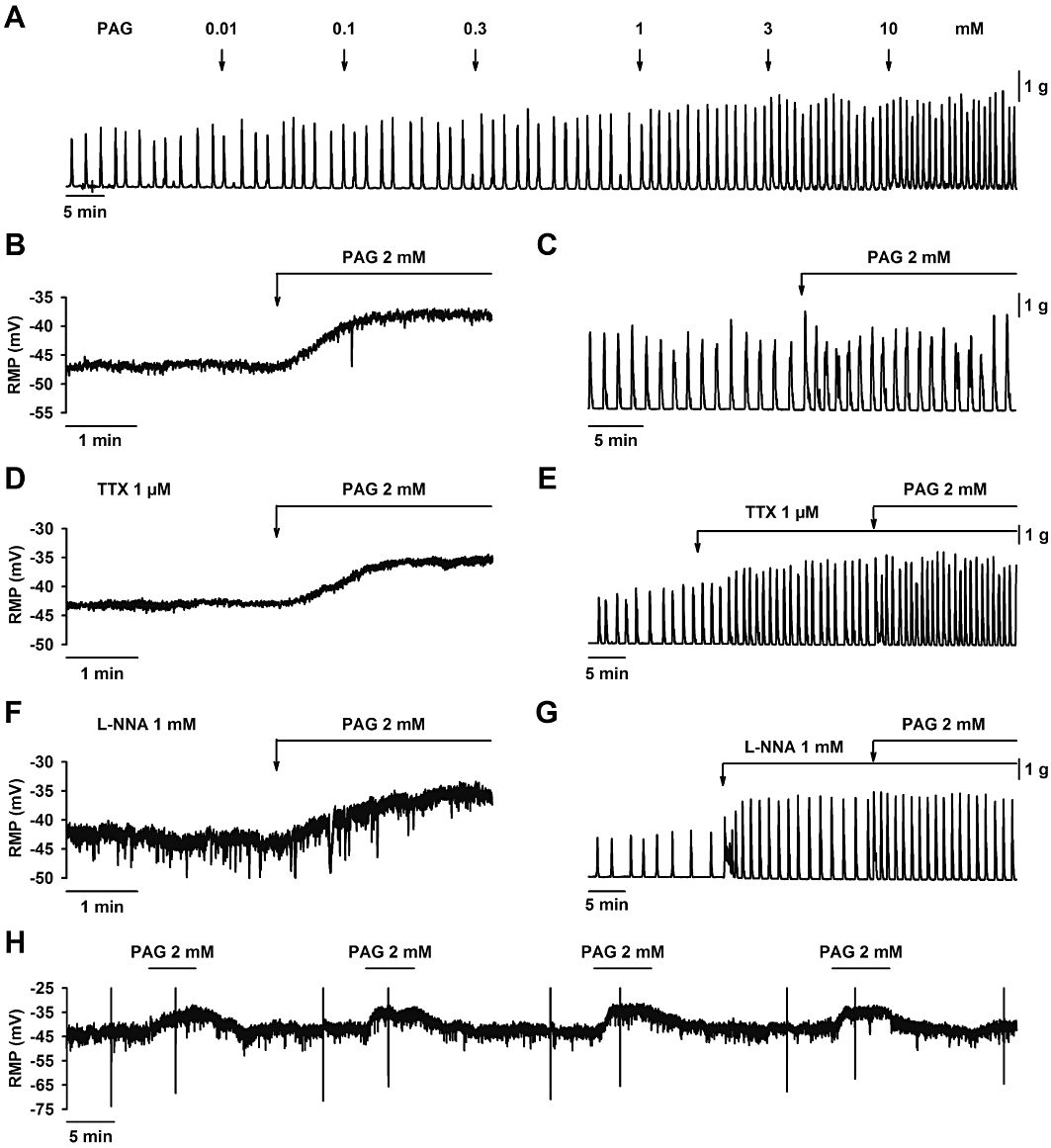
Effect of D,L-propargylglycine (PAG) on smooth muscle resting membrane potential and spontaneous mechanical activity. (A) Mechanical recording showing that PAG increased spontaneous motility in a concentration-dependent manner. PAG (2 mM) induced smooth muscle depolarization (B) and an increase in spontaneous motility (C) which was still recorded in the presence of TTX (1 µM) (D, E) and L-NNA (1 mM) (F, G). Four consecutive incubations with PAG (2 mM) produced four consecutive depolarizations (H). For statistics, see Table 1.
Table 1.
Effects of hydrogen sulphide synthesis inhibitors (PAG, AOAA and HA) and the precursor of its synthesis (L-cysteine), in the absence and presence of TTX, L-NNA or ODQ, on smooth muscle membrane potential and motility
| Controla | Membrane potential (mV) | Motility (g·min−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Drug | In the presence of: | n | Controla | Drug | P-value | n | Control* | Drug | P-value |
| PAG | – | 9 | −47.4 ± 2.8 | −39.2 ± 2.8 | <0.001 | 9 | 10.3 ± 0.9 | 17.7 ± 1.6 | <0.001 |
| PAG | TTX | 5 | −43.8 ± 0.6 | −37.6 ± 1.5 | <0.01 | 8 | 18.6 ± 4.0 | 25.2 ± 4.9 | <0.05 |
| PAG | L-NNA | 4 | −42.7 ± 1.1 | −37.0 ± 1.7 | <0.01 | 6 | 32.3 ± 6.8 | 41.4 ± 6.6 | <0.001 |
| AOAA | – | 5 | −44.5 ± 1.5 | −42.4 ± 1.7 | n.s. | 11 | 12.8 ± 2.3 | 18.2 ± 3.2 | <0.05 |
| AOAA | TTX | 5 | −45.7 ± 3.0 | −43.9 ± 3.1 | n.s. | 14 | 27.3 ± 4.3 | 15.6 ± 2.4 | <0.01 |
| AOAA | L-NNA | 4 | −39.8 ± 3.6 | −39.0 ± 3.9 | n.s. | 4 | 48.0 ± 9.8 | 21.0 ± 4.2 | <0.05 |
| HA | – | 4 | −45.8 ± 2.2 | −51.5 ± 1.6 | <0.05 | 5 | 22.0 ± 4.0 | 0.2 ± 0.2 | <0.01 |
| HA | TTX | 4 | −44.0 ± 2.6 | −53.1 ± 3.4 | <0.05 | 6 | 34.0 ± 10.2 | 0.0 ± 0.0 | <0.05 |
| HA | ODQ | 4 | −41.9 ± 1.3 | −42.8 ± 1.4 | n.s. | 5 | 32.0 ± 6.8 | 28.4 ± 6.0 | <0.05 |
| L-cys | – | 4 | −52.5 ± 1.7 | −42.8 ± 1.3 | <0.01 | 9 | 11.4 ± 2.1 | 21.3 ± 3.7 | <0.001 |
| L-cys | TTX | 5 | −40.9 ± 2.6 | −36.0 ± 2.3 | <0.05 | 7 | 23.9 ± 5.0 | 38.1 ± 6.8 | <0.001 |
Control are values of the membrane potential or motility before drug addition but in the presence of TTX, L-NNA, L-cysteine, etc.
Values are means ± SE. n, no. of samples; PAG, D,L-propargylglycine (2 mM); TTX, tetrodotoxin (1 µM); L-NNA, Nω-nitro-L-arginine (1 mM); L-cys, L-cysteine (1 mM); AOAA, amino-oxyacetic acid (2 mM); HA, hydroxylamine (10 µM); ODQ, 1H-[1,2,4]oxadiazolo[4,3-α]quinoxalin-1-one (10 µM). The statistical significance of differences was assessed by using Student's paired t-test. n.s., not significant.
Effect of AOAA on RMP and motility
AOAA is a CBS inhibitor. The effect of AOAA was tested on membrane potential and spontaneous motility. AOAA (2 mM) did not depolarize the smooth muscle cells and produced a transient increase in motility, which lasted for 10 min (Table 1 and Figure 3A,B). After 10 min, the spontaneous motility returned to basal values. In order to investigate the putative neural effects of AOAA, experiments were performed in the presence of the neural blocker TTX or the NOS inhibitor L-NNA. Surprisingly, AOAA (2 mM) induced a significant reduction of spontaneous motility in the presence of TTX (1 µM) or L-NNA (1 mM) (Table 1 and Figure 3D,F). No change in the RMP was observed in the presence of TTX (1 µM) or L-NNA (1 mM) (Table 1 and Figure 3C,E).
Figure 3.
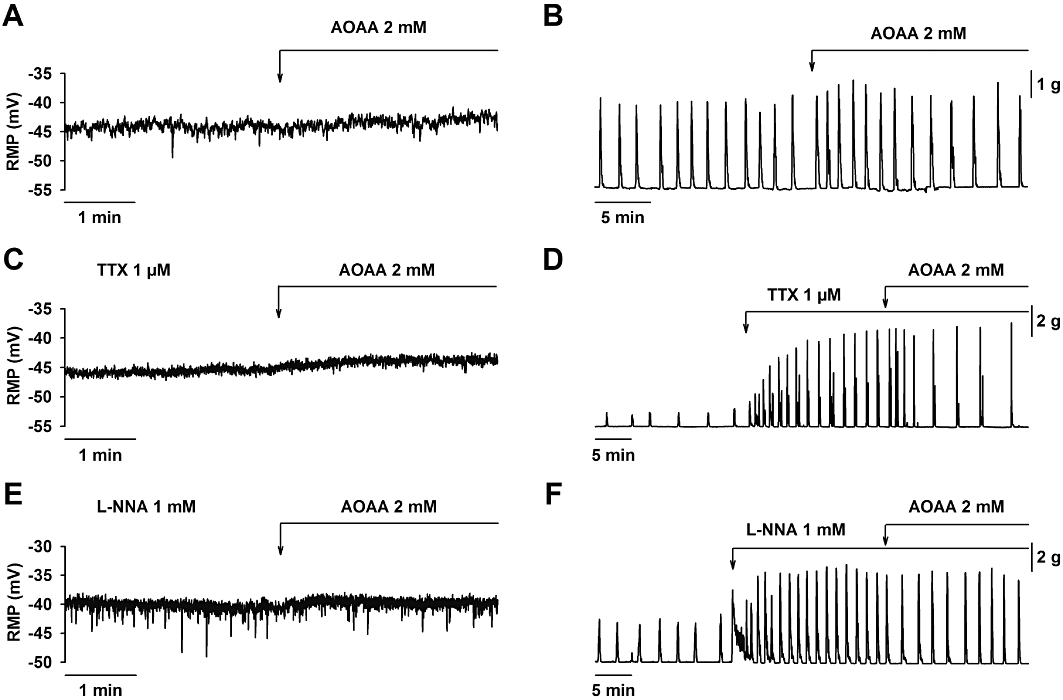
Effect of aminooxyacetic acid (AOAA) on smooth muscle resting membrane potential and spontaneous mechanical activity. AOAA (2 mM) did not modify the resting membrane potential in control (A), in the presence of TTX (1 µM) (C) or L-NNA (1 mM) (E). AOAA (2 mM) caused a transient increase in spontaneous motility (B). In the presence of TTX (1 µM) (D) or L-NNA (1 mM) (F), AOAA (2 mM) caused an inhibition of spontaneous motility. For statistics, see Table 1.
Effect of HA on RMP and motility
HA inhibited the mechanical spontaneous activity in a concentration-dependent manner (IC50 = 0.31 µM; 95% confidence interval 0.21–0.45 µM; log IC50 = −6.51 ± 0.08; n = 4. Figure 4A). HA (10 µM) hyperpolarized the smooth muscle cells and almost abolished spontaneous mechanical activity (Table 1 and Figure 4B,C). In the presence of TTX (1 µM), HA (10 µM) also hyperpolarized smooth muscle cells and abolished the spontaneous mechanical activity (Table 1 and Figure 4D,E). HA might act as an NO donor (Southam and Garthwaite, 1991; Correia et al., 2000) and so we tested HA in the presence of ODQ, a cGMP inhibitor. ODQ (10 µM) prevented both the hyperpolarization [HA (10 µM): 5.7 ± 1.1 mV vs. ODQ (10 µM) + HA (10 µM): 0.9 ± 0.5 mV hyperpolarization; n = 4; P < 0.05; Table 1 and Figure 4F] and the inhibition of spontaneous motility [HA (10 µM): 98.9 ± 1.1 % vs. ODQ (10 µM) + HA (10 µM): 10.9 ± 1.5 % of inhibition; n = 5; P < 0.001; Table 1 and Figure 4G].
Figure 4.
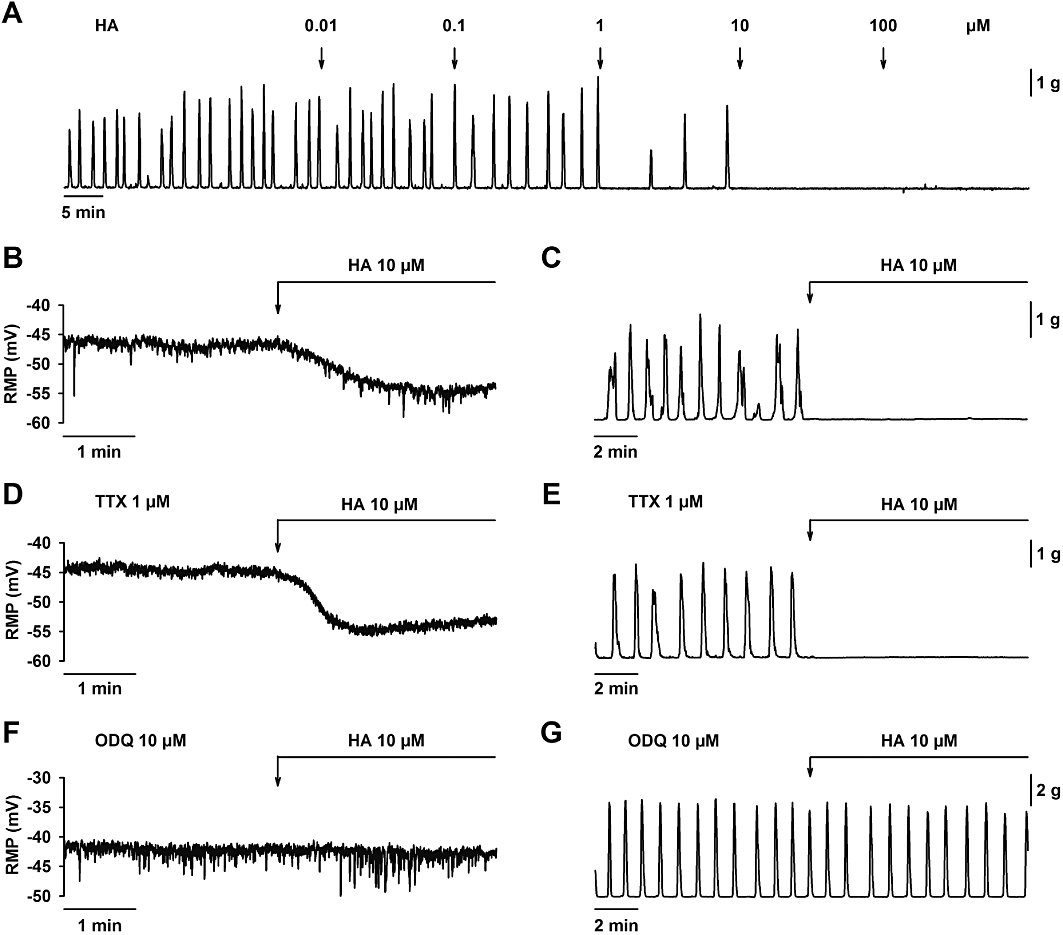
Effect of hydroxylamine (HA) on smooth muscle resting membrane potential and spontaneous mechanical activity. (A) Mechanical recording showing that HA decreased spontaneous motility in a concentration-dependent manner. HA (10 µM) induced smooth muscle hyperpolarization (B) and abolished spontaneous motility (C). Both effects were still recorded in the presence of TTX (1 µM) (D, E) but ODQ (10 µM) inhibited HA (10 µM) induced hyperpolarization (F) and inhibition of spontaneous motility (G). For statistics, see Table 1.
Effect of AOAA and PAG on neural-mediated responses
sIJPs were recorded in samples of rat mid colon (0.72 ± 0.06 mV SD; n = 14). AOAA (2 mM) did not modify these sIJPs [Control: 0.65 ± 0.08 mV vs. AOAA (2 mM): 0.66 ± 0.07 mV SD; n = 5; not significant; Figure 5A]. Similar results were obtained with PAG (2 mM) (Control: 0.76 ± 0.08 mV vs. PAG (2 mM): 0.75 ± 0.09 mV SD; n = 9; not significant; Figure 5B). It is important to note that the shift to the right in the frequency distribution is indicative of smooth muscle depolarization. The effect of AOAA and PAG was tested on the IJP and relaxation induced by EFS. AOAA (2 mM) did not modify the IJP or the inhibition of motility induced by EFS (Figure 6A,D). PAG (2 mM) slightly increased IJP amplitude at the highest voltages but did not modify IJP duration or EFS-induced relaxation (Figure 6B,E). Combined addition of AOAA (2 mM) and PAG (2 mM) produced very similar effects to those observed when PAG (2 mM) was administered alone (Figure 6C,F).
Figure 5.
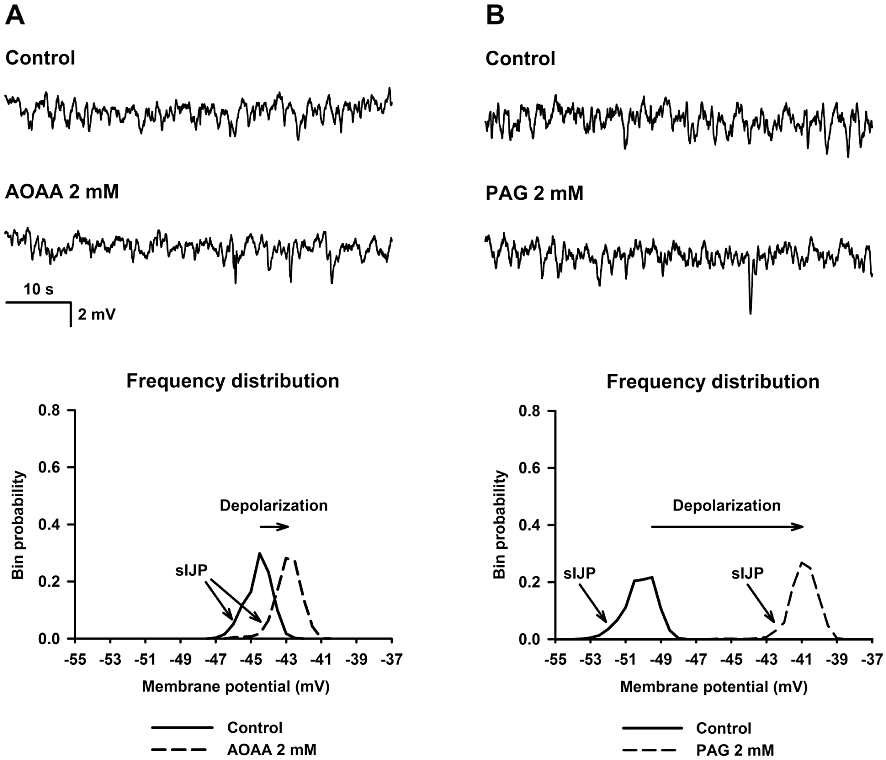
Effect of amino-oxyacetic acid (AOAA, 2 mM) (A) and D,L-propargylglycine (PAG, 2 mM) (B) on resting membrane potential and spontaneous inhibitory junction potentials (sIJP). Top: representative microelectrode recordings. Bottom: representative frequency distribution (0.5 mV bins) of the membrane potential in control and after drug addition.
Figure 6.
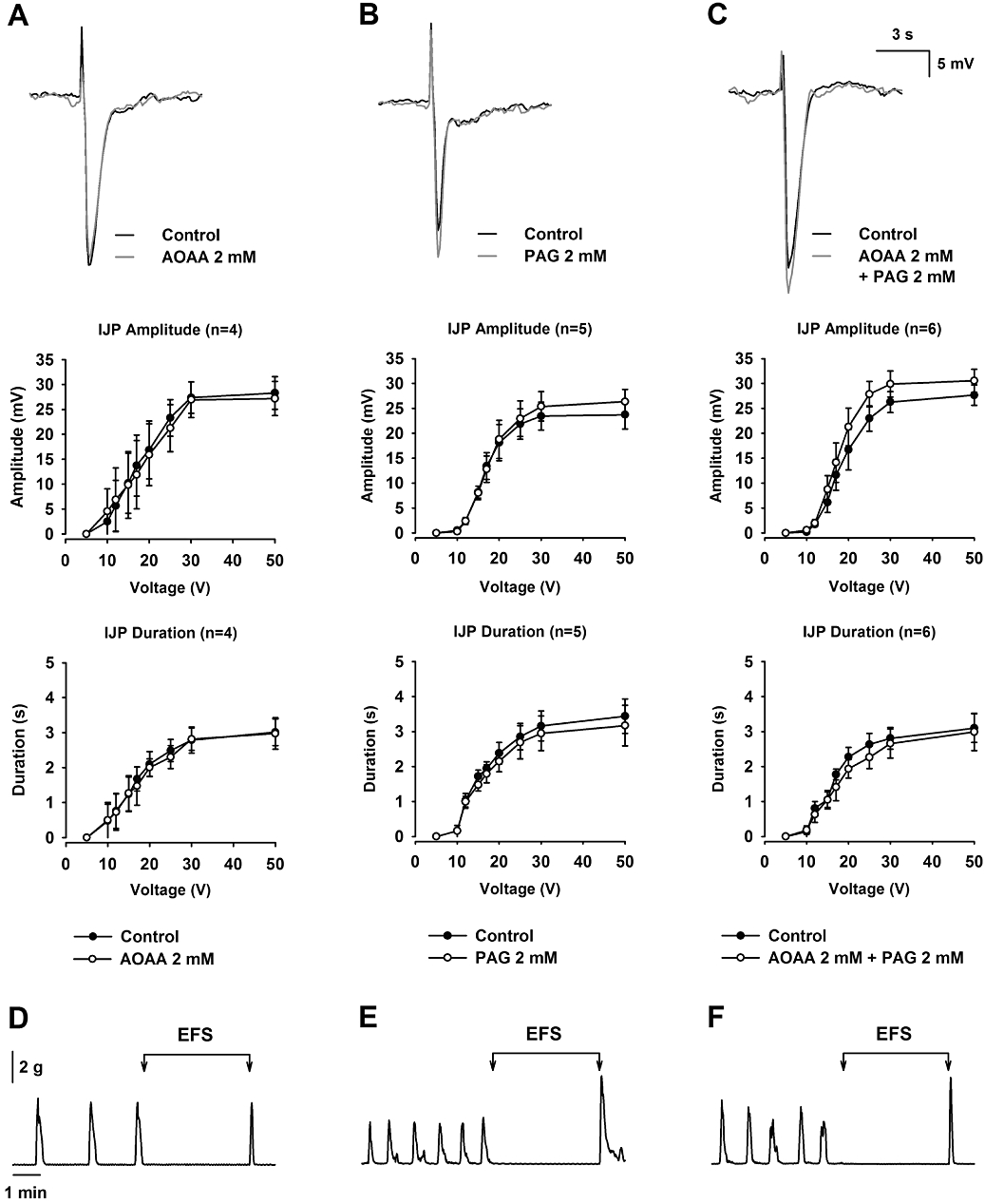
Effect of amino-oxyacetic acid (AOAA) and D,L-propargylglycine (PAG) on EFS-induced IJP and mechanical relaxation. (A, D): AOAA (2 mM); (B, E): PAG (2 mM); (C, F): AOAA (2 mM) + PAG (2 mM). Note that none of the treatments modified the amplitude or duration of the IJP (A, B and C) or the mechanical relaxation induced by EFS (D, E and F). IJPs shown in the figure were elicited with a pulse of 30 V.
Effect of L-cysteine on spontaneous motility and RMP
L-cysteine is the substrate of both CBS and CSE enzymes so we wanted to test its effect on membrane potential and spontaneous motility. L-cysteine increased spontaneous motility in a concentration-dependent manner (IC50 = 0.72 mM; 95% confidence interval 0.55–0.93 mM; log IC50 = −3.14 ± 0.11; n = 4. Figure 7A). Addition of L-cysteine (1 mM) depolarized smooth muscle cells and increased spontaneous motility (Table 1 and Figure 7B,C). It is important to note that the effect of L-cysteine was immediately observed after its addition and it was not affected by neural blocking by TTX (1 µM) (Table 1 and Figure 7D,E). L-Cysteine is an agonist of NMDA receptors (Schicho et al., 2006). Therefore, to evaluate this putative pathway, organ bath experiments were performed using D-AP5, a NMDA receptor antagonist. However, pre-incubation with D-AP5 (100 µM) did not modify the effect of L-cysteine on spontaneous motility (Control: 26.2 ± 6.1 g·min−1 vs. L-cysteine (1 mM): 38.9 ± 7.1 g·min−1 AUC; n = 4; P < 0.01), showing that NMDA receptors are not involved in the response to L-cysteine.
Figure 7.
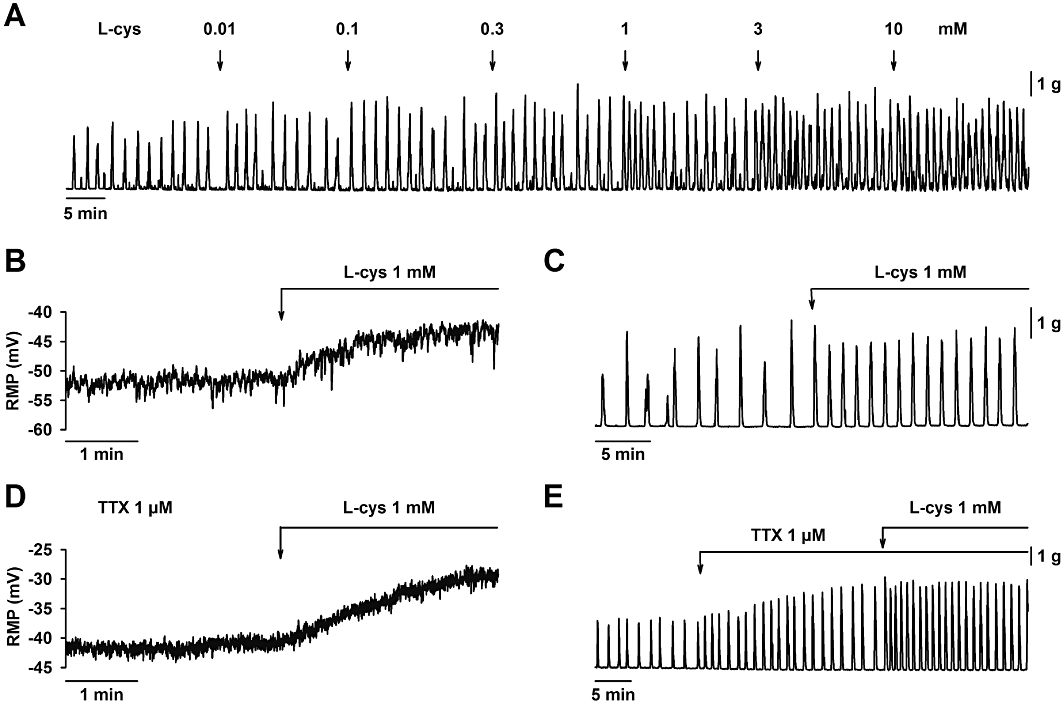
Effect of L-cysteine on smooth muscle resting membrane potential and spontaneous mechanical activity. (A) Mechanical recording showing that L-cysteine increased spontaneous motility in a concentration-dependent manner. L-cysteine (1 mM) induced smooth muscle depolarization (B) and an increase in spontaneous motility (C) which were both still recorded in the presence of TTX (1 µM) (D, E). For statistics, see Table 1.
Discussion
H2S is a gaseous mediator that is endogenously produced in several systems of the body including the GI tract (Linden et al., 2008; Martin et al., 2010). Although its physiological function is still unknown (Linden et al., 2010), it has been shown that H2S donors cause colonic secretion (Schicho et al., 2006; Hennig and Diener, 2009; Krueger et al., 2010; Pouokam and Diener, 2011) and regulate motility, causing inhibition of peristalsis and smooth muscle relaxation in several areas of the GI tract (Hosoki et al., 1997; Teague et al., 2002; Gallego et al., 2008a; Dhaese and Lefebvre, 2009; Dhaese et al., 2010). H2S donors have been proposed as potential therapeutic agents to treat colonic inflammation and improve ulcer healing (Fiorucci et al., 2007; Wallace et al., 2007), so H2S might have important therapeutic potential (Szabo, 2007; Bannenberg and Vieira, 2009). However, high concentrations of H2S are known to be toxic, causing inhibition of mitochondrial cytochrome C oxidase (Li and Moore, 2007). To be able to distinguish between physiological, pharmacological and toxicological effects, one possible experimental approach is to block the endogenous synthesis of H2S. PAG has been commonly used as a putative inhibitor of CSE and both AOAA and HA have been employed as putative inhibitors of CBS. These inhibitors are usually employed to inhibit H2S production in cell homogenates at the tissue level and also in some in vivo experiments using animal models of disease (see for review Szabo, 2007).
In the present study, we indentified strong CSE-IR in smooth muscle cells of the lamina propia in the rat colon whereas CBS-IR was less intense but still positive in muscle layers. A similar staining was described in the rat colon (Hennig and Diener, 2009). Note that major differences between species might exist. For example, a ‘diffuse staining’ in the lamina propia was detected in the mouse colon (Linden et al., 2008) and positive staining was detected in neurons of the mouse, guinea-pig and human colon, but the precise neurons expressing each particular enzyme might vary between species (Schicho et al., 2006; Linden et al., 2008). Moreover, in the present study we also found positive CSE-IR in neurons of both the myenteric and submucous plexus. Whether these major differences in the distribution of the enzymes are due to different technical approaches or are real species differences needs further study. In the current study, we used the methodology developed by Linden et al., (2008) to measure endogenous production of H2S in several mouse tissues. Note that the measurements of H2S production were performed in colonic samples where the mucosa and submucosa had been previously removed and consequently H2S production was not due to the activity of the epithelium. Effective inhibition of H2S production was obtained with PAG (2 mM) or AOAA (2 mM) suggesting that both CSE and CBS participate in the endogenous production of H2S synthesis in the rat colon. Effective blockade of H2S production in intact tissue was obtained using a combination of PAG (2 mM) and HA (2 mM) in the mouse colon (Linden et al., 2008). However, due to the NO-like effects of HA (see below), we did not test this inhibitor on H2S production. Note that AOAA was the only inhibitor that was able to block H2S production in rat colon homogenates (Martin et al., 2010). However, under our experimental conditions – the strip devoid of mucosa and submucosa – (this is a major difference between the present and other studies), the tissue was able to endogenously produce H2S and both AOAA and PAG were effective inhibitors of this H2S production.
In the present study, we analysed the effect of PAG, AOAA and HA on GI motility. Our first report using NaHS as a source of H2S showed that NaHS inhibited human and rat colonic motility (Gallego et al., 2008a). However, little is known about the blockade of the endogenous production of H2S on GI motility. Our results demonstrated that PAG was able to cause a smooth muscle depolarization and increase spontaneous motility. Accordingly PAG was mimicking the effect of NO inhibition with L-NNA (Gil et al., 2010). AOAA caused a minor and transient effect on membrane potential and spontaneous motility. These results suggest that H2S might be a third inhibitory signalling molecule in the GI tract, regulating GI motility. However, it is important to distinguish between a neural and a non-neural origin of endogenous H2S. In the first case, H2S should be considered a ‘gasotransmitter’, whereas in the second H2S might be an endogenous signalling molecule produced in non-neural cell types (i.e. smooth muscles and/or ICC) able to regulate motility.
Inhibitory motor neurons are predominant in human colonic circular muscle (Gallego et al., 2006; Gallego et al., 2008b). In the rat colon, inhibitory neurons spontaneously release NO and a purine acting on P2Y1 receptors. NO regulates the membrane potential whereas the purine acting on P2Y1 receptors is responsible for the spontaneous IJP (Gil et al., 2010). EFS-evoked IJP show a fast component followed by a slow one, being blocked by P2Y1 receptor antagonists and NOS inhibitors respectively (Grasa et al., 2009). For this reason we wanted to investigate the effect of AOAA and PAG on neuromuscular interaction. AOAA, PAG or a combination of both did not modify the spontaneous IJP or the EFS-induced IJP either (neither the fast nor the slow component were affected). Complete mechanical relaxation was still present after incubation with both antagonists. These results suggest that H2S does not participate in neurally mediated relaxation or alternatively we have not been able to use the appropriate parameters of stimulation to elicit H2S release. Further studies using EFS and H2S detection are needed to evaluate the possible neural release of H2S.
Note that depolarization and increase in motility induced by PAG occur in the presence of TTX and L-NNA. This result suggests that the effect is not related to NO production and to TTX-sensitive neurally mediated responses. According to the distribution of the enzyme, ongoing production of H2S in smooth muscle cells (and/or ICC) may keep the membrane potential hyperpolarized and therefore the inhibition of H2S production would depolarize smooth muscle cells, increasing the motility. Our experiments showed that this effect was more consistent with PAG than AOAA, suggesting that the endogenous source of H2S was due to CSE with a minor contribution of CBS.
At least part of the observed effects could be unrelated to inhibition of H2S production and other ‘side’ effects of the drugs might explain part of our results. This is not new and researchers working in the field are aware that, for example, AOAA has been used as a pharmacological tool to investigate multiple pharmacological actions including several pyridoxal phosphate-dependent enzymes such as aspartate transaminase, 4-aminobutyrate transaminase or dopa-decarboxylase (John and Charteris, 1978). Regarding colonic motility, possible side effects of the drugs might be due to the following findings:
(1) In the rat colon, L-cysteine, the precursor of H2S synthesis, depolarizes smooth muscles cells and increases motility, not mimicking the effect of exogenous NaHS (Gallego et al., 2008a). Moreover, the effect of L-cysteine was probably not due to activation of the NMDA receptor because pre-incubation with D-AP5, an NMDA receptor antagonist, did not modify the motor response (Schicho et al., 2006). It has been demonstrated that L-cysteine at the concentrations used in the present study blocks stretch-dependent potassium channels in the murine colon (Park et al., 2005). These stretch-dependent potassium channels participate in background potassium conductance and are expressed in a variety of excitable cells including smooth muscle (see for review: Sanders and Koh, 2006). It might be possible that the smooth muscle depolarization and the increase in spontaneous motility in the rat colon might be due to a direct effect of L-cysteine on these channels. Note, however, that L-cysteine mimics the effects of exogenous H2S donors in other studies, acting like a prosecretory molecule in the guinea-pig and human colon (Schicho et al., 2006), enhancing ulcer healing in the rat stomach (Wallace et al., 2007) and exerting antinociceptive effects in the rat colon (Distrutti et al., 2006). In addition, in these studies its effects are reversed by AOAA and/or PAG.
(2) HA, another CBS inhibitor, is causing opposite effects to those found with AOAA. HA is usually used at high concentrations, up to 1 mM, to inhibit the CBS enzyme. Our preliminary results showed that at 1 mM, HA completely inhibited spontaneous motility. Therefore, we decided to perform a concentration–response curve to investigate its effects and the IC50 was close to 1 µM. Furthermore, the hyperpolarization and inhibition of spontaneous motility was antagonized by ODQ, showing that HA was probably causing its effects through cGMP activation. HA is an NO-generating compound that relaxes smooth muscle (Iversen et al., 1994) and it has been previously demonstrated in the rat duodenum that HA mimics the NO effect (Correia et al., 2000). Our results confirm these results which means HA is unlikely to be a selective H2S inhibitor.
(3) In the presence of TTX, PAG and AOAA caused an opposite effect. AOAA alone caused a slight but consistent increase in motility but, in the presence of TTX or L-NNA, AOAA inhibited spontaneous motility. We still do not know why this dual effect occurs. This result suggests that in the absence of NO, which is ‘spontaneously’ released from inhibitory neurons (Gil et al., 2010), AOAA causes smooth muscle inhibition. AOAA is a pharmacological tool widely used to inhibit NADH shuttles (see e.g.: Eto et al., 1999; Casimir et al., 2009). These shuttles participate in the transfer of reducing equivalents between the cytosolic compartment and the mitochondrial matrix. In vascular smooth muscle, AOAA caused inhibition of O2 consumption, inhibition of the tricarboxylic acid cycle, increase in lactate production and reduction of contractility (Barron et al., 1998; Barron et al., 1999). The reduction in spontaneous motility without a change in membrane potential might be due to an effect on a shuttle. Unfortunately, octanoate, which partially reverses the effect of AOAA on malate-aspartate shuttle (Barron et al., 1998), did not reverse the inhibitory effect of AOAA (not shown).
(4) Finally, it is important to note that PAG exerts a covalent and irreversible inhibition of CSE (Sun et al., 2009). However, repetitive depolarizations observed with PAG are not consistent with an irreversible effect on CSE.
In the present study, we demonstrated that rat colonic strips devoid of mucosa and submucosa are able to enzymatically produce H2S, in which both CBS and CSE enzymes are involved. Effects of PAG, AOAA, HA and L-cysteine on rat colonic RMP and motility were reported as well. Both PAG and AOAA increased motility, suggesting that H2S is an endogenous inhibitory signalling molecule in the GI tract. However, it is important for future investigations that possible side effects of the drugs are presented that might (or might not) be related to H2S production, so as to encourage further research into the design of novel inhibitors with higher selectivity, potency and cell-membrane permeability.
Acknowledgments
The authors would like to thank Antonio Acosta and Claudia Arenas for their technical assistance. This work has been funded by the following grant: BFU2009-11118. Victor Gil is supported by the Ministerio de Ciencia e Innovación (Spain) (AP2007-01583). Diana Gallego is supported by the Instituto de Salud Carlos III, Centro de Investigación Biomédica en red de enfermedades hepáticas y digestivas (Ciberehd).
Glossary
Abbreviations
- AOAA
amino-oxyacetic acid
- AUC
area under the curve
- CBS
cystathionine β-synthase
- CSE
cystathionine γ-lyase
- D-AP5
D-(-)-2-amino-5-phosphonopentanoic acid
- EFS
electrical field stimulation
- GI
gastrointestinal
- HA
hydroxylamine
- ICC
interstitial cells of Cajal
- IJP
inhibitory junction potential
- IR
immunoreactivity
- L-NNA
Nω-nitro-L-arginine
- ODQ
1H-[1,2,4]oxadiazolo[4,3-α]quinoxalin-1-one
- PAG
D,L-propargylglycine
- RMP
resting membrane potential
- TTX
tetrodotoxin
Conflict of interest
None.
References
- Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti E, Mikkelsen HB, Larsen JO, Jimenez M. Motility patterns and distribution of interstitial cells of Cajal and nitrergic neurons in the proximal, mid- and distal-colon of the rat. Neurogastroenterol Motil. 2005;17:133–147. doi: 10.1111/j.1365-2982.2004.00603.x. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edn. Br J Pharmacol. 2009;158(Suppl 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannenberg GL, Vieira HL. Therapeutic applications of the gaseous mediators carbon monoxide and hydrogen sulfide. Expert Opin Ther Pat. 2009;19:663–682. doi: 10.1517/13543770902858824. [DOI] [PubMed] [Google Scholar]
- Barron JT, Gu L, Parrillo JE. Malate-aspartate shuttle, cytoplasmic NADH redox potential, and energetics in vascular smooth muscle. J Mol Cell Cardiol. 1998;30:1571–1579. doi: 10.1006/jmcc.1998.0722. [DOI] [PubMed] [Google Scholar]
- Barron JT, Gu L, Parrillo JE. Relation of NADH/NAD to contraction in vascular smooth muscle. Mol Cell Biochem. 1999;194:283–290. doi: 10.1023/a:1006928516648. [DOI] [PubMed] [Google Scholar]
- Casimir M, Rubi B, Frigerio F, Chaffard G, Maechler P. Silencing of the mitochondrial NADH shuttle component aspartate-glutamate carrier AGC1/Aralar1 in INS-1E cells and rat islets. Biochem J. 2009;424:459–466. doi: 10.1042/BJ20090729. [DOI] [PubMed] [Google Scholar]
- Coffey JC, Docherty NG, O'Connell PR. Hydrogen sulphide: an increasing need for scientific equipoise. Gastroenterology. 2009;137:2181–2182. doi: 10.1053/j.gastro.2009.06.073. [DOI] [PubMed] [Google Scholar]
- Correia NA, Oliveira RB, Ballejo G. Pharmacological profile of nitrergic nerve-, nitric oxide-, nitrosoglutathione- and hydroxylamine-induced relaxations of the rat duodenum. Life Sci. 2000;68:709–717. doi: 10.1016/s0024-3205(00)00957-7. [DOI] [PubMed] [Google Scholar]
- Dhaese I, Lefebvre RA. Myosin light chain phosphatase activation is involved in the hydrogen sulfide-induced relaxation in mouse gastric fundus. Eur J Pharmacol. 2009;606:180–186. doi: 10.1016/j.ejphar.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Dhaese I, Van Colen I, Lefebvre RA. Mechanisms of action of hydrogen sulfide in relaxation of mouse distal colonic smooth muscle. Eur J Pharmacol. 2010;628:179–186. doi: 10.1016/j.ejphar.2009.11.024. [DOI] [PubMed] [Google Scholar]
- Distrutti E, Sediari L, Mencarelli A, Renga B, Orlandi S, Antonelli E, et al. Evidence that hydrogen sulfide exerts antinociceptive effects in the gastrointestinal tract by activating KATP channels. J Pharmacol Exp Ther. 2006;316:325–335. doi: 10.1124/jpet.105.091595. [DOI] [PubMed] [Google Scholar]
- Eto K, Tsubamoto Y, Terauchi Y, Sugiyama T, Kishimoto T, Takahashi N, et al. Role of NADH shuttle system in glucose-induced activation of mitochondrial metabolism and insulin secretion. Science. 1999;283:981–985. doi: 10.1126/science.283.5404.981. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Distrutti E, Cirino G, Wallace JL. The emerging roles of hydrogen sulfide in the gastrointestinal tract and liver. Gastroenterology. 2006;131:259–271. doi: 10.1053/j.gastro.2006.02.033. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Orlandi S, Mencarelli A, Caliendo G, Santagada V, Distrutti E, et al. Enhanced activity of a hydrogen sulphide-releasing derivative of mesalamine (ATB-429) in a mouse model of colitis. Br J Pharmacol. 2007;150:996–1002. doi: 10.1038/sj.bjp.0707193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego D, Hernandez P, Clave P, Jimenez M. P2Y1 receptors mediate inhibitory purinergic neuromuscular transmission in the human colon. Am J Physiol Gastrointest Liver Physiol. 2006;291:G584–G594. doi: 10.1152/ajpgi.00474.2005. [DOI] [PubMed] [Google Scholar]
- Gallego D, Clave P, Donovan J, Rahmati R, Grundy D, Jimenez M, et al. The gaseous mediator, hydrogen sulphide, inhibits in vitro motor patterns in the human, rat and mouse colon and jejunum. Neurogastroenterol Motil. 2008a;20:1306–1316. doi: 10.1111/j.1365-2982.2008.01201.x. [DOI] [PubMed] [Google Scholar]
- Gallego D, Gil V, Aleu J, Auli M, Clave P, Jimenez M. Purinergic and nitrergic junction potential in the human colon. Am J Physiol Gastrointest Liver Physiol. 2008b;295:G522–G533. doi: 10.1152/ajpgi.00510.2007. [DOI] [PubMed] [Google Scholar]
- Gil V, Gallego D, Grasa L, Martin MT, Jimenez M. Purinergic and nitrergic neuromuscular transmission mediates spontaneous neuronal activity in the rat colon. Am J Physiol Gastrointest Liver Physiol. 2010;299:G158–G169. doi: 10.1152/ajpgi.00448.2009. [DOI] [PubMed] [Google Scholar]
- Grasa L, Gil V, Gallego D, Martin MT, Jimenez M. P2Y(1) receptors mediate inhibitory neuromuscular transmission in the rat colon. Br J Pharmacol. 2009;158:1641–1652. doi: 10.1111/j.1476-5381.2009.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig B, Diener M. Actions of hydrogen sulphide on ion transport across rat distal colon. Br J Pharmacol. 2009;158:1263–1275. doi: 10.1111/j.1476-5381.2009.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun. 1997;237:527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- Iversen HH, Gustafsson LE, Leone AM, Wiklund NP. Smooth muscle relaxing effects of NO, nitrosothiols and a nerve-induced relaxing factor released in guinea-pig colon. Br J Pharmacol. 1994;113:1088–1092. doi: 10.1111/j.1476-5381.1994.tb17107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John RA, Charteris A. The reaction of amino-oxyacetate with pyridoxal phosphate-dependent enzymes. Biochem J. 1978;171:771–779. doi: 10.1042/bj1710771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger D, Foerster M, Mueller K, Zeller F, Slotta-Huspenina J, Donovan J, et al. Signaling mechanisms involved in the intestinal pro-secretory actions of hydrogen sulfide. Neurogastroenterol Motil. 2010;22:1224–e320. doi: 10.1111/j.1365-2982.2010.01571.x. [DOI] [PubMed] [Google Scholar]
- Li L, Moore PK. An overview of the biological significance of endogenous gases: new roles for old molecules. Biochem Soc Trans. 2007;35:1138–1141. doi: 10.1042/BST0351138. [DOI] [PubMed] [Google Scholar]
- Linden DR, Sha L, Mazzone A, Stoltz GJ, Bernard CE, Furne JK, et al. Production of the gaseous signal molecule hydrogen sulfide in mouse tissues. J Neurochem. 2008;106:1577–1585. doi: 10.1111/j.1471-4159.2008.05502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DR, Levitt MD, Farrugia G, Szurszewski JH. Endogenous production of H2S in the gastrointestinal tract: still in search of a physiologic function. Antioxid Redox Signal. 2010;12:1135–1146. doi: 10.1089/ars.2009.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GR, McKnight GW, Dicay MS, Coffin CS, Ferraz JG, Wallace JL. Hydrogen sulphide synthesis in the rat and mouse gastrointestinal tract. Dig Liver Dis. 2010;42:103–109. doi: 10.1016/j.dld.2009.05.016. [DOI] [PubMed] [Google Scholar]
- Matsunami M, Tarui T, Mitani K, Nagasawa K, Fukushima O, Okubo K, et al. Luminal hydrogen sulfide plays a pronociceptive role in mouse colon. Gut. 2009;58:751–761. doi: 10.1136/gut.2007.144543. [DOI] [PubMed] [Google Scholar]
- Park KJ, Baker SA, Cho SY, Sanders KM, Koh SD. Sulfur-containing amino acids block stretch-dependent K+ channels and nitrergic responses in the murine colon. Br J Pharmacol. 2005;144:1126–1137. doi: 10.1038/sj.bjp.0706154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouokam E, Diener M. Mechanisms of actions of hydrogen sulphide on rat distal colonic epithelium. Br J Pharmacol. 2011;162:392–404. doi: 10.1111/j.1476-5381.2010.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM, Koh SD. Two-pore-domain potassium channels in smooth muscles: new components of myogenic regulation. J Physiol. 2006;570:37–43. doi: 10.1113/jphysiol.2005.098897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schemann M, Grundy D. Role of hydrogen sulfide in visceral nociception. Gut. 2009;58:744–747. doi: 10.1136/gut.2008.167858. [DOI] [PubMed] [Google Scholar]
- Schicho R, Krueger D, Zeller F, Von Weyhern CW, Frieling T, Kimura H, et al. Hydrogen sulfide is a novel prosecretory neuromodulator in the Guinea-pig and human colon. Gastroenterology. 2006;131:1542–1552. doi: 10.1053/j.gastro.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Shibuya N, Mikami Y, Kimura Y, Nagahara N, Kimura H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J Biochem. 2009a;146:623–626. doi: 10.1093/jb/mvp111. [DOI] [PubMed] [Google Scholar]
- Shibuya N, Tanaka M, Yoshida M, Ogasawara Y, Togawa T, Ishii K, et al. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal. 2009b;11:703–714. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]
- Siegel LM. A direct microdetermination for sulfide. Anal Biochem. 1965;11:126–132. doi: 10.1016/0003-2697(65)90051-5. [DOI] [PubMed] [Google Scholar]
- Southam E, Garthwaite J. Comparative effects of some nitric oxide donors on cyclic GMP levels in rat cerebellar slices. Neurosci Lett. 1991;130:107–111. doi: 10.1016/0304-3940(91)90239-p. [DOI] [PubMed] [Google Scholar]
- Stipanuk MH, Beck PW. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem J. 1982;206:267–277. doi: 10.1042/bj2060267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Collins R, Huang S, Holmberg-Schiavone L, Anand GS, Tan CH, et al. Structural basis for the inhibition mechanism of human cystathionine gamma-lyase, an enzyme responsible for the production of H(2)S. J Biol Chem. 2009;284:3076–3085. doi: 10.1074/jbc.M805459200. [DOI] [PubMed] [Google Scholar]
- Szabo C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- Teague B, Asiedu S, Moore PK. The smooth muscle relaxant effect of hydrogen sulphide in vitro: evidence for a physiological role to control intestinal contractility. Br J Pharmacol. 2002;137:139–145. doi: 10.1038/sj.bjp.0704858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace JL. Physiological and pathophysiological roles of hydrogen sulfide in the gastrointestinal tract. Antioxid Redox Signal. 2010;12:1125–1133. doi: 10.1089/ars.2009.2900. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Dicay M, McKnight W, Martin GR. Hydrogen sulfide enhances ulcer healing in rats. FASEB J. 2007;21:4070–4076. doi: 10.1096/fj.07-8669com. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Vong L, McKnight W, Dicay M, Martin GR. Endogenous and exogenous hydrogen sulfide promotes resolution of colitis in rats. Gastroenterology. 2009;137:569–578. doi: 10.1053/j.gastro.2009.04.012. [DOI] [PubMed] [Google Scholar]


