Abstract
BACKGROUND AND PURPOSE
Vitamin D receptor (VDR) modulators (VDRMs) such as calcitriol, paricalcitol and doxercalciferol are commonly used to manage hyperparathyroidism secondary to chronic kidney disease (CKD). CKD patients experience extremely high risks of cardiovascular morbidity and mortality. Clinical observations show that VDRM therapy may be associated with cardio-renal protective and survival benefits for CKD patients. However, hypercalcaemia remains a serious side effect for current VDRMs, which leads to the need for frequent dose titration and serum Ca (calcium) monitoring. Significant clinical benefits can be derived from a VDRM with cardiovascular protective effects without the hypercalcaemic liability.
EXPERIMENTAL APPROACH
Male Sprague–Dawley rats were 5/6 nephrectomized and 6 weeks later, after they had established uraemia, elevated parathyroid hormone levels, endothelial dysfunction and left ventricular hypertrophy, the rats were treated with VS-105, a novel VDRM. The effects of VS-105 were also tested in cultured HL-60 cells.
KEY RESULTS
VS-105 induced HL-60 cell differentiation with an EC50 value at 11.8 nM. Treatment (i.p., 3× a week over a period of 2 weeks) of the 5/6 nephrectomized rats by VS-105 (0.004–0.64 µg·kg−1) effectively suppressed serum parathyroid hormone without raising serum Ca or phosphate levels. Furthermore, 2 weeks of treatment with VS-105 improved endothelium-dependent aortic relaxation and attenuated left ventricular abnormalities in a dose range that did not affect serum Ca levels. Similar results were obtained when VS-105 was administered i.p. or by oral gavage.
CONCLUSIONS AND IMPLICATIONS
VS-105 exhibits an overall therapeutic product profile that supports expanded use in CKD to realize the cardiovascular protective effects of VDR activation.
Keywords: PTH, vitamin D receptor, vitamin D analogue, endothelial dysfunction, left ventricular hypertrophy
Introduction
The synthesis of vitamin D3 occurs naturally in the skin with adequate sunlight exposure. However, vitamin D3 is not active and needs to be converted to 1,25-dihydroxyvitamin D3 (1,25(OH)2D3, calcitriol). Calcitriol is a secosteroid hormone that binds to the vitamin D receptor (VDR), a nuclear receptor, to activate multiple signalling pathways in various cells and tissues (Wu-Wong, 2009).
Chronic kidney disease (CKD) is a growing epidemic. Globally more than 500 million individuals, or about one adult in 10 in the general population, have some form of CKD (World Kidney Day Website). CKD progresses through five stages and stage 5 CKD requires renal replacement therapy (dialysis or transplantation). CKD patients experience an extremely high rate of cardiovascular complications and mortality (Foley et al., 1998; Baigent et al., 2000; Reddan et al., 2003; Go et al., 2004; Foley, 2010). Deficient calcitriol production is an early sign of CKD (Levin et al., 2007) and may be linked to complications in CKD patients such as secondary hyperparathyroidism (SHPT), bone and cardiovascular disorders (Gal-Moscovici and Sprague, 2010). Clinical observations suggest that vitamin D receptor modulators (VDRMs) such as calcitriol, paricalcitol and doxercalciferol may be associated with cardiovascular and survival benefits for CKD patients (Teng et al., 2003; 2005; Kalantar-Zadeh et al., 2006; Kim et al., 2006; Tentori et al., 2006; Lee et al., 2007; Wolf et al., 2007; 2008; Kovesdy et al., 2008; Levin et al., 2008; Naves-Diaz et al., 2008; Shinaberger et al., 2008; Shoben et al., 2008; Barreto et al., 2009; Covic et al., 2010; Verhave and Siegert, 2010; Biggar et al., 2011), but randomized trials are needed to confirm the benefits. Currently in the CKD field VDRM is only used for managing SHPT (Gal-Moscovici and Sprague, 2010; Mirkovic et al., 2011), and current VDRM therapy requires frequent dose titration and serum Ca monitoring. A narrow therapeutic window (efficacy vs. the hypercalcaemic side effect) and lack of cardio-renal benefits in the non-hypercalcaemic dose range are some of the limiting factors for expanding the usage of on-market VDRMs. Hence, a novel VDRM with cardiovascular benefits and minimal hypercalcaemic toxicity would provide substantial benefits to CKD patients.
This report demonstrates that a novel VDRM, VS-105, either administered by i.p. or oral gavage, not only suppresses parathyroid hormone (PTH) effectively without affecting serum Ca, but also improves endothelial function and regresses left ventricular hypertrophy (LVH) in the 5/6 nephrectomized (NX) uraemic rats.
Methods
Materials
VS-105 ((1R,3R)-5-((E)-2-((3αS,7αS)-1-((R)-1-((S)-3-hydroxy-2,3-dimethylbutoxy)ethyl)-7α-methyldihydro-1H-inden-4(2H,5H,6H,7H,7αH)-ylidene)ethylidene)-2-methylenecyclohexane-1,3-diol) and paricalcitol (19-nor-1α,25(OH) 2D2) were made by Vidasym (Chicago, IL, USA). Two different lots of VS-105 with >95% purity were tested yielding identical results. The synthesis scheme of VS-105 was published previously (Kawai, 2010). Other reagents were of analytical grade.
Differentiation of HL-60 cells
HL-60 promyelocytic leukaemia cells (ATCC, Manassas, VA, USA) were cultured in HEPES-buffered RPMI 1640 medium (Invitrogen, Grand Island, NY, USA) supplemented with 10% heat-inactivated fetal bovine serum at 37°C in a humidified 5% CO2 : 95% air atmosphere. Cells were treated with test agent for 4 days. Cell differentiation was determined by the nitroblue tetrazolium (NBT) reduction assay (Segal, 1974). Briefly, cells in 96-well plates (0.5 × 106 cells per well, 75 µL per well) were incubated for 2 h at 37°C with an equal volume of freshly made NBT solution containing 2 mg·mL−1 of NBT and 200 ng·mL−1 PMA (phorbol 12-myrystate 13-acetate). Then, 150 µL of lysing buffer (45% dimeththylformamide and 0.135 g·mL−1 SDS in water) was added to each well and the plates were left for 4 h at room temperature before the optical density (OD) at 560 nm was determined.
Sub-totally NX rats
The nephrectomy was performed on male, Sprague–Dawley rats weighing ∼200 g with a standard two-step surgical ablation procedure (Slatopolsky et al., 1995). Rats were maintained on normal diet containing 1% Ca and 0.7% phosphorus. Rats of established uraemia were studied on Week 6 after surgery. For oral gavage studies, rats on Week 6 after surgery were treated with vehicle (20% hydroxypropyl-β-cyclodextrin, 1.65 mL·kg−1) or VS-105 (in vehicle), p.o. by gavage, daily for 12 days. For i.p. studies, Week 6 after surgery rats were treated with vehicle (5% ethanol + 95% propylene glycol, 0.4 mL·kg−1) or VS-105 (prepared into vehicle from a stock solution of 1 mg·mL−1 in ethanol), i.p., 3× a week for 12 days. On Day 0 (before the first dosing) and Day 13 (24 h after the last dosing), blood was collected and physiological parameters measured. In some experiments, the heart, left ventricle and aorta were collected for additional studies. The animal studies were conducted under the auspice of Office of Animal Care and Institutional Biosafety, University of Illinois at Chicago (approval reference number: A08-051). The animal care and experimental procedures complied with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication no. 85–23, revised 1996).
Measurements of physiological parameters
Serum Ca, Pi (phosphate), creatinine, and blood urea nitrogen (BUN) concentrations were measured using a chemistry analyser. Serum PTH was measured using a rat intact PTH elisa kit obtained from ALPCO (Windham, NH, USA).
Histological assessment
The left ventricular tissue was fixed in a 4% formaldehyde-PBS (pH 7.4) solution overnight. The samples were embedded in wax and cut into 4 µm sections. The sections were stained with haematoxylin-eosin and examined under a microscope. For fibrosis, sections were stained with Masson trichrome and examined using a Leica DM IL LED-Inverted fluorescence microscope. Image analysis was done by Image-Pro Plus 6.0. To determine the diameter of cardiomyocytes, a previously published method was followed (Xiang et al., 2005). The sections of left ventricles were stained with fluorescein isothiocyanate (FITC)-labelled wheat germ agglutinin (1:5 dilution) for 2 h at room temperature and then examined under a fluorescence microscope to visualize the myocyte membrane. The relative size of the cardiomyocytes was quantified by measuring the diameter of the myocytes, which was the distance between the two plasma membranes of a cell in longitudinal section.
Vascular function studies
Thoracic aortas from rats were excised in a cold modified Krebs solution (see below) and a 3 mm aortic ring was suspended in 10 mL tissue baths under 0.5 g of resting tension in a modified Krebs solution containing (g·L−1): NaCl 6.9169, KCl 0.3499, NaHCO3 2.0998, MgSO4 0.2901, KH2PO4 0.1604, CaCl2 0.2663, glucose 1.9994, EDTA 0.026, equilibrated with 5% CO2 : 95% O2 (pH 7.4 at 37°C). Aortas were sensitized by the addition of phenylephrine (PE, 3 µM) with 10 min washouts between intervals. Aortas were precontracted with PE (3 µM), and the endothelium-dependent vasodilator acetylcholine (ACh) was added in half-log increments (10−9 mol·L−1 : 10−4.5 mol·L−1) at 3–5 min intervals, allowing time for the effect of ACh to plateau. After a 60 min washout, aortas were precontracted with PE (3 µM) and subsequently treated with endothelium-independent vasodilator sodium nitroprusside (SNP; 10−9 mol·L−1 : 10−6 mol·L−1) at 3–5 min intervals, allowing time for the effect to plateau. Data were recorded with the BL-420F Data Acquisition & Analysis System.
Data analysis
Differences between Sham and uraemic rats with different treatments were assessed using a one-way anova followed by a Dunnett's post hoc test. A t-test was used to assess differences between Day 0 (before treatment) and Day 13 (after treatment) parameters or as indicated. For vascular function, ACh- and SNP-induced relaxations were calculated as the % relaxation of the PE-induced precontraction. Differences in vascular function were determined using a two-way anova, followed by a Bonferroni post hoc test.
Results
Figure 1 shows the structure of VS-105 and the typical absorbance profile from a spectrophotometer scan. The maximal absorbance wavelength (ODmax) for VS-105 is at 253 nm and the extinction coefficient is 48 360.
Figure 1.
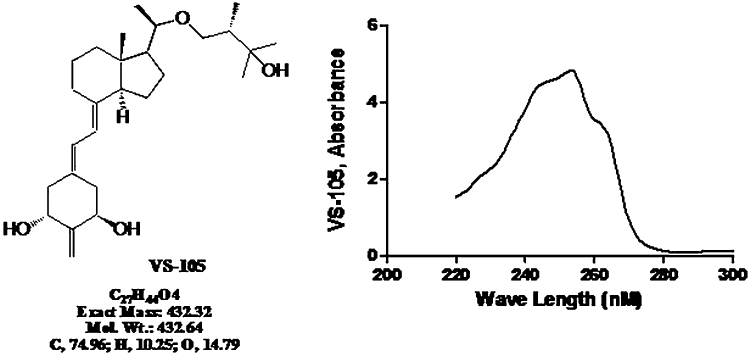
Structure and absorbance wavelength profile of VS-105. To determine the maximal absorbance wavelength (ODmax) and the extinction coefficients for the compound, VS-105 was dissolved in a 50:50 solution (by volume) of de-ionized water and ethanol at 100 µM and scanned by a spectrophotometer.
It is well-documented that VDRMs induce the differentiation of HL-60 promyelocytic leukaemia cells into monocytes and macrophages (Mangelsdorf et al., 1984; Koeffler et al., 1985). The potency of VS-105, paricalcitol and calcitriol in inducing HL-60 differentiation was compared. Figure 2 shows that all three compounds induced HL-60 differentiation effectively with EC50 values of 11.8 ± 0.25, 12.5 ± 0.20 and 17.9 ± 0.13 nM for VS-105, paricalcitol and calcitriol, respectively.
Figure 2.
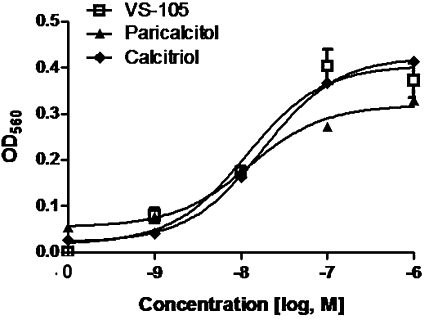
Effect of VS-105 on HL-60 differentiation. HL-60 cells were treated with different concentrations of VS-105, calcitriol or paricalcitol for 4 days. Cell differentiation was determined as described in Methods. Mean ± SD are shown. Results shown (with triplicate samples in this experiment) are representative of three independent experiments.
The effects of VS-105 on physiological parameters in the 5/6 NX rats were determined. Figure 3A and B shows that the serum creatinine and BUN levels were significantly elevated in the 5/6 NX rats (vs. Sham-vehicle), indicating a uniform uraemic state. VS-105 at the highest dose (0.64 µg·kg−1) exhibited a modest effect on reducing creatinine or BUN. Figure 4A and B shows that both serum Ca and Pi were not significantly changed in the 5/6 NX-vehicle group (vs. Sham). VS-105 at 0.004–0.64 µg·kg−1 had no significant effect on serum Ca and Pi. Figure 4C shows that serum PTH was elevated in the 5/6 NX rat, and was effectively suppressed by VS-105. Other VDRMs such as paricalcitol were used as control in the studies. The results for paricalcitol on serum creatinine, BUN, Ca, Pi and PTH were similar to those in our previous report (Wu-Wong et al., 2010a).
Figure 3.
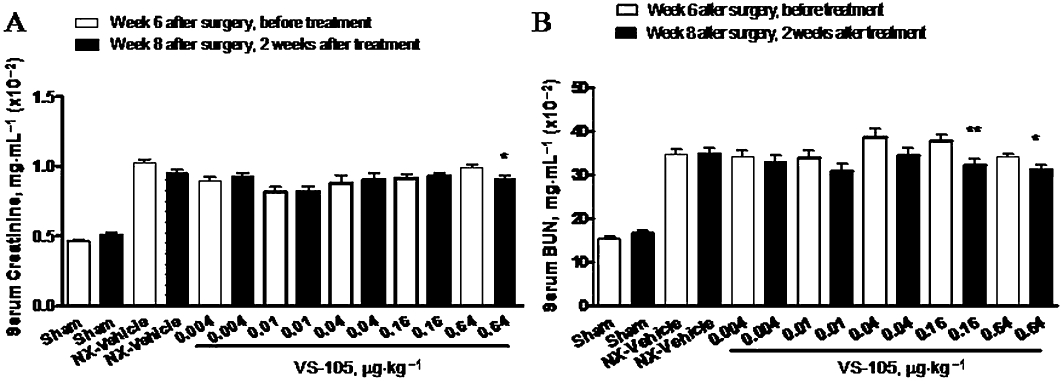
Effects of VS-105 on serum creatinine and BUN after 2 weeks of i.p. dosing in the 5/6 NX rats. Sham and 5/6 NX rats were treated with vehicle or VS-105 (i.p., 3× a week) for 12 days as described in Methods (n = 7–10 per group). On Day 0 (before dosing) and Day 13 (24 h after the last dosing), blood samples were collected for the measurement of serum creatinine (A) and BUN (B). Means ± SEM were calculated for each group. Unpaired t-test with 95% confidence intervals of difference was performed to assess differences between baseline Day 0 and Day 13. *P < 0.05, **P < 0.01 versus before treatment.
Figure 4.
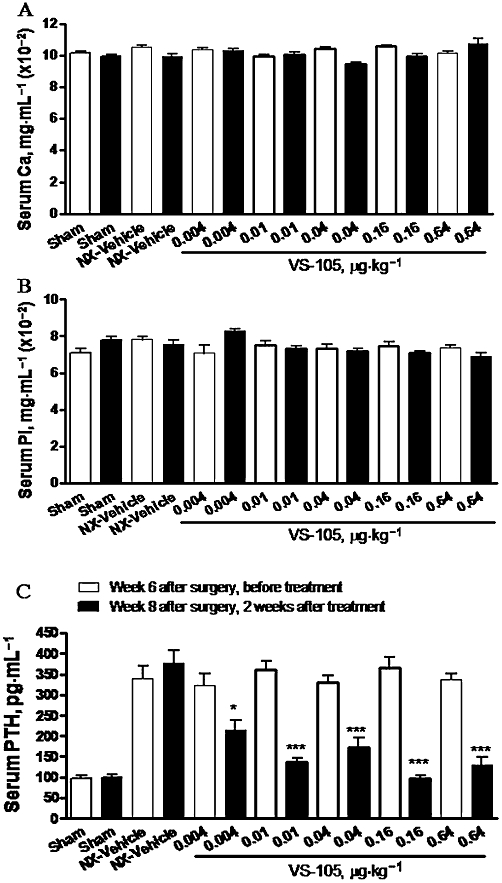
Effects of VS-105 on serum Ca, Pi and PTH levels after 2 weeks of i.p. dosing in the 5/6 NX rats. Rats were treated as in Figure 3. Blood samples were collected for the measurement of serum Ca (A), Pi (B) and PTH levels (C). Means ± SEM were calculated for each group. Unpaired t-test with 95% confidence intervals of difference was performed to assess differences between baseline Day 0 (before treatment) and Day 13 (after treatment). *P < 0.05, ***P < 0.001 versus before treatment.
The oral efficacy of VS-105 was demonstrated in the 5/6 NX rats. Figure 5A shows that VS-105 at 0.01 or 0.5 µg·kg−1 by oral gavage daily dosing for 12 days had no effect on serum Ca. Figure 5B shows that VS-105 at the two doses tested effectively suppressed serum PTH in the 5/6 NX rats. Table 1 summarizes the results showing that VS-105 did not have significant effects on serum BUN, creatinine and Pi. The results, consistent with those shown in Figure 4, demonstrate that VS-105 is equally efficacious when administered either by the i.p. or oral route.
Figure 5.
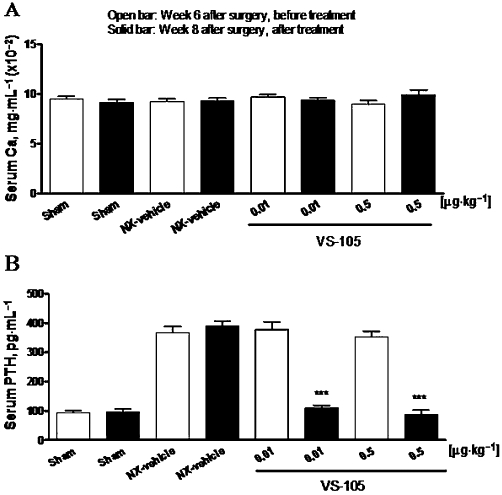
Effects of VS-105 on serum Ca and PTH after 2 weeks of oral dosing in the uraemic rats. Sham and 5/6 NX rats were treated with vehicle or VS-105 (oral gavage, once daily) for 12 days as described in Methods (n = 8–11 per group). On Day 0 (before dosing) and Day 13 (24 h after the last dosing), blood samples were collected for the measurement of serum Ca (A) and PTH (B). Means ± SEM were calculated for each group. Unpaired t-test with 95% confidence intervals of difference was performed to assess differences between baseline Day 0 and Day 13. ***P < 0.0001 versus own control group at Week 6.
Table 1.
Effects of VS-105 oral dosing on physiological parameters in Sham versus 5/6 nephrectomized rats
| Sham | 5/6 NX-vehicle | 5/6 NX-VS-105 (0.01 µg·kg−1) | 5/6 NX-VS-105 (0.5 µg·kg−1) | |||||
|---|---|---|---|---|---|---|---|---|
| Parameters | Day 0 | Day 13 | Day 0 | Day 13 | Day 0 | Day 13 | Day 0 | Day 13 |
| Creatinine (mg·dL−1) | 0.45 ± 0.02 | 0.51 ± 0.03 | 1.01 ± 0.06*** | 1.12 ± 0.09*** | 0.87 ± 0.05*** | 0.93 ± 0.08*** | 0.83 ± 0.03*** | 0.81 ± 0.04*** |
| BUN (mg·dL−1) | 13.3 ± 1.2 | 14.1 ± 0.8 | 44.3 ± 3.6*** | 45.3 ± 5.0*** | 41.2 ± 4.7*** | 39.0 ± 3.9*** | 37.5 ± 2.8*** | 34.5 ± 3.0*** |
| Serum Pi (mg·dL−1) | 7.71 ± 0.16 | 8.09 ± 0.43 | 7.72 ± 0.12 | 8.03 ± 0.31 | 7.40 ± 0.15 | 8.01 ± 0.23 | 8.34 ± 0.20 | 8.48 ± 0.25 |
Rats were treated as in Figure 5. Data presented are means ± SEM (n = 8–11 per group).
One-way anova Dunnett's test with 95% confidence intervals of difference was performed for statistical comparisons:
P < 0.001 versus Sham Day 0.
The endothelium-dependent and -independent relaxation of aortic rings was examined. Figure 6A shows that the maximal aorta relaxation response to ACh in Sham rats was −74.5 ± 3.6%. Relaxation was significantly reduced in the aorta from the 5/6 NX vehicle-treated rats (−31.4 ± 4.7%), which indicates compromised endothelial function. As shown in Figure 6B, the aorta relaxation produced by SNP (an endothelium-independent vasodilator) was not significantly different between the Sham and 5/6 NX rats, indicating that the smooth muscle relaxation response is intact and functional. Figure 6A demonstrates that a 2 week treatment with VS-105 (i.p., 3× a week) produced a dose-dependent improvement in ACh-induced endothelium-dependent relaxation. The maximal relaxation response to ACh with 0.01 and 0.16 µg·kg−1 of VS-105 was −54.3 ± 8.2% and −64.0 ± 8.3%, respectively. Treatment with 0.004 µg·kg−1 VS-105 exhibited a modest effect on the maximal relaxation response to ACh of −48.6 ± 4.9%. Similar results were obtained when VS-105 was administered by oral gavage daily for 12 days in the 5/6 NX rats (data not shown). Figure 6B shows that VS-105 had no significant effect on SNP-induced endothelium-independent relaxation.
Figure 6.
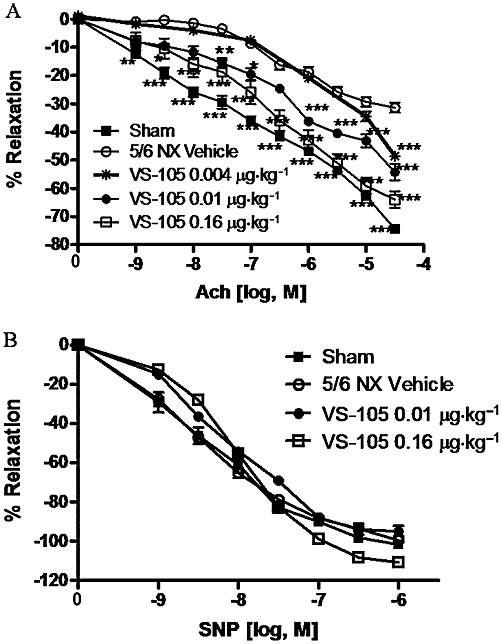
Endothelial dysfunction in 5/6 NX rats and the effect of VS-105. Sham and 5/6 NX rats were treated with vehicle or VS-105 at indicated doses as described in Figure 3. Vascular function was determined as described in Methods (n = 7–10 per group). (A) Acetylcholine-evoked relaxation. (B) SNP-evoked relaxation. Group mean ± SEM are presented. Statistical analysis was determined using a two-way anova, followed by a Bonferroni post hoc test. *P < 0.05, **P < 0.01, ***P < 0.001 versus vehicle.
The 5/6 NX rats are known to develop LVH (Wolf et al., 2000). Figure 7A shows that, at 8 weeks after the renal ablation surgery, the left ventricle weight (LVW) versus body weight (BW) ratio as a percentage of control was significantly higher in the 5/6 NX rats (vs. Sham). Another control study found that LVH was present in the 5/6 NX rats 6 weeks after the second surgery (data not shown). Figure 7A also demonstrates that a 2 week treatment with VS-105 (i.p., 3× a week) at the tested doses produced a significant effect on reducing the LVW/BW ratio. Similar results were obtained when VS-105 was administered by oral gavage daily for 12 days in the 5/6 NX rats (data not shown). The sections of left ventricles were prepared and stained with FITC-labelled wheat germ agglutinin to determine the diameter of cardiomyocytes. Figure 7B shows that VS-105 at 0.01–0.64 µg·kg−1 significantly reduced the cardiomyocyte diameter.
Figure 7.
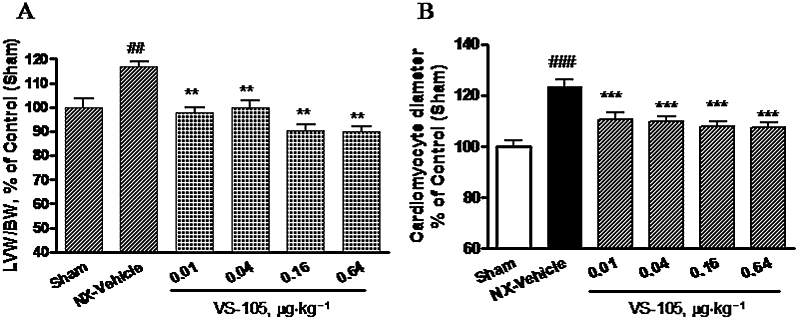
Left ventricular hypertrophy in 5/6 NX rats and the effect of VS-105. Sham and 5/6 NX rats were treated with vehicle or VS-105 at the doses indicated, as described in Figure 3. (A) Heart was collected and weighed. Left ventricle (LV) was then dissected and weighed (n = 7–10 per group). Heart LV weight (LVW) was first normalized by body weight (BW) and then expressed as % of control (Sham). (B) The diameter of cardiomyocytes was determined as described in Methods. Data were obtained from 30 cells randomly selected from 10 microscopic fields across different rats in each treatment group and expressed as % of control (Sham). Group means ± SEM are presented. One-way anova Dunnett's test with 95% confidence intervals of difference was performed for statistical comparisons. ##P < 0.01, ###P < 0.001 versus Sham; **P < 0.01, ***P < 0.001 versus NX-vehicle.
Figure 8 shows the cardiomyocytes morphology. Cardiomyocytes were markedly hypertrophic in the 5/6 NX-vehicle treated animals. VS-105 at 0.01 µg·kg−1 improved the condition, and VS-105 at 0.16 µg·kg−1 nearly restored the morphology of the cardiomyocytes back to that in Sham. These results are consistent with those shown in Figure 7 that demonstrate treatment with VS-105 regresses LVH in the 5/6 NX uraemic rats.
Figure 8.
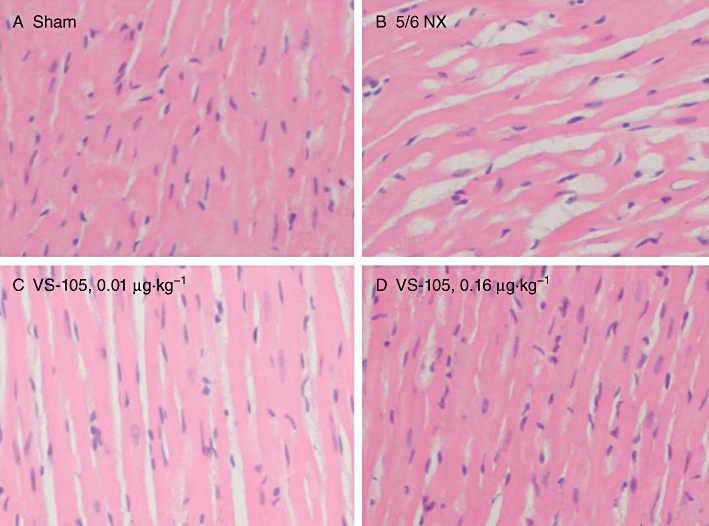
Cardiomyocyte morphology in 5/6 NX rats and the effect of VS-105. Sham rats were treated with vehicle and 5/6 NX rats were treated with vehicle or VS-105 at the indicated doses as described in Figure 3. The left ventricular tissue sections were prepared and stained with haematoxylin-eosin as described in Methods. Randomly selected areas under 200 × magnification were examined. Pictures shown are representative of 10 fields per section per rat, four rats per treatment group. (A) Sham; (B) 5/6 NX-vehicle; (C) 5/6 NX treated with VS-105 at 0.01 µg·kg−1; (D) 5/6 NX treated with VS-105 at 0.16 µg·kg−1.
Figure 9A–D shows that, compared with Sham, a significant increase in collagen deposition was observed in the left ventricle of the 5/6 NX rat treated with vehicle. Treatment with VS-105 at 0.01 and 0.5 µg·kg−1 substantially reduced the fibrosis staining.
Figure 9.
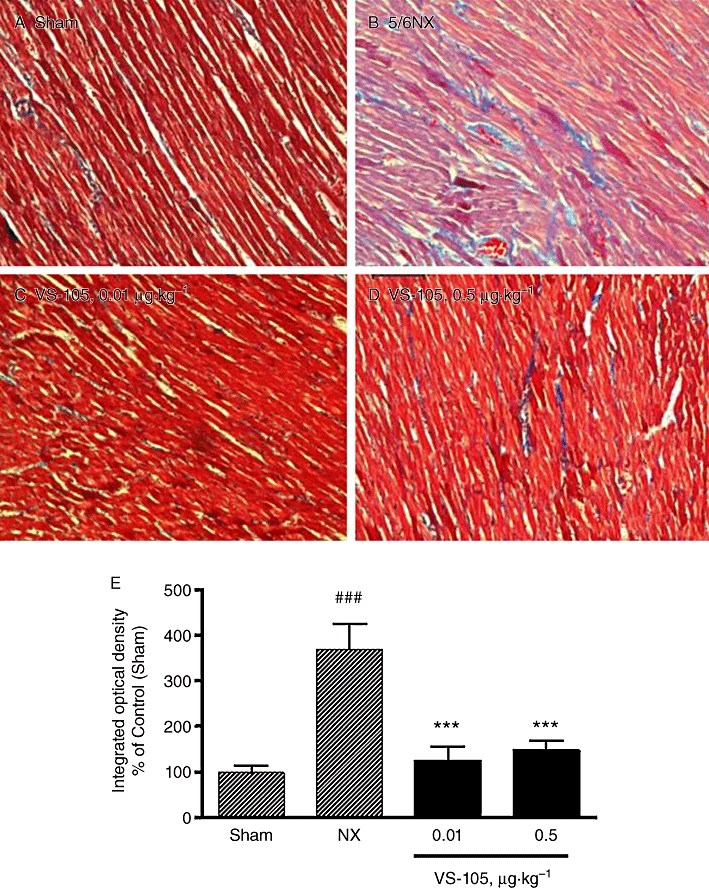
Left ventricle fibrosis in 5/6 NX rats and the effect of VS-105. The Sham rats were treated with vehicle and 5/6 NX rats were treated with vehicle or VS-105 at the indicated doses as described in Figure 5. The left ventricular tissue sections were prepared and stained with Masson trichrome as described in Methods. Randomly selected areas under 200 × magnification were examined. Pictures shown are representative of 10 fields per section per rat, four rats per treatment group. (A) Sham; (B) 5/6 NX-vehicle; (C) 5/6 NX treated with VS-105 at 0.01 µg·kg−1; (D) 5/6 NX treated with VS-105 at 0.5 µg·kg−1. (E) Quantitative determination of tissue collagen abundance; group means ± SEM are presented. One-way anova Dunnett's test with 95% confidence intervals of difference was performed for statistical comparisons. ###P < 0.001 versus Sham; ***P < 0.001 versus NX-vehicle.
Discussion and conclusions
Data from the in vitro studies demonstrated that VS-105 is as potent as paricalcitol and calcitriol in inducing the differentiation of HL-60 cells into monocytes and macrophages. More importantly, data from in vivo studies show that VS-105 is efficacious in improving PTH and cardiovascular parameters in the 5/6 NX uraemic rats without affecting serum Ca.
One major hurdle in developing new drugs for treating human diseases is that animal models often fall short of predicting human responses. The CKD field has the advantage of the 5/6 NX uraemic rat model that, albeit a difficult model to handle (it takes >2 months to complete one study), it is highly predictive of the human conditions for VDRMs. For example, in CKD clinical studies, paricalcitol's therapeutic index (efficacy vs. hypercalcaemic toxicity) is about threefold to fourfold better than calcitriol, which is reproduced in this animal model of kidney disease (Slatopolsky et al., 1998; Martin and Gonzalez, 2001). Consistent with reports by others (Slatopolsky et al., 1998; 2002;), we have previously demonstrated that paricalcitol and doxercalciferol effectively suppressed serum PTH at 0.021–0.33 µg·kg−1 in the uraemic rats, but both drugs also induced an increase in serum Ca in the same dose range (Wu-Wong et al., 2010a,b;). As a comparison, VS-105 significantly suppressed serum PTH at 0.004–0.64 µg·kg−1 without affecting serum Ca. Calculating from PTH suppressing efficacy versus hypercalcaemic toxicity, VS-105 provides a therapeutic index at a minimum of 50-fold versus twofold to fourfold for paricalcitol and doxercalciferol.
Endothelial dysfunction, very common in CKD (Damman et al., 2010; Malyszko, 2011), is usually present before clinical LVH, and is a risk factor for coronary heart disease, stroke and peripheral vascular disease (Yeboah et al., 2011). Previously, we (Wu-Wong et al., 2010b) and others (Hasdan et al., 2002) have reported that ACh-evoked relaxation is significantly reduced in arteries prepared from the 5/6 NX uraemic rats, indicating endothelial dysfunction. We have also demonstrated that endothelial function in the 5/6 NX rats was significantly improved by paricalcitol in the 0.042–0.083 µg·kg−1 dose range, but no clear separation between the efficacious and hypercalcaemic dose range was observed for paricalcitol. As a comparison, our results from this study demonstrate that VS-105 improves endothelial function in a non-hypercalcaemic dose range at 0.01–0.16 µg·kg−1. Previously we have also reported that paricalcitol is less hypercalcaemic than calcitriol because it was slightly less potent than calcitriol in inducing an upregulation of the Ca transporter genes, TRPV6 (the gene for CaT1 and ECaC) and Calb3 (the gene for calbindin D9k), in the intestine (Nakane et al., 2007). Consistent with our previous results, preliminary mechanistic studies found that VS-105 was significantly less potent than paricalcitol at inducing the expression of TRPV6 and Calb3 in the intestine of 5/6 NX rats (data not shown).
Like endothelial dysfunction, LVH is a common condition in CKD, which often leads to heart failure (Bluemke et al., 2008) and increased risks of hospitalization and mortality (Gwadry-Sridhar et al., 2004; Sciacqua et al., 2006; Pons et al., 2010). Mizobuchi et al. (2010) previously demonstrated that paricalcitol at 40 ng per injection (equivalent to ∼0.1 µg·kg−1 in 400 g rats) had an improving effect on LVH and myocardial and perivascular fibrosis in 5/6 NX uraemic rats. Our results from this study demonstrate that VS-105 regresses LVH and myocardial fibrosis in a dose range that does not affect serum Ca. Currently both i.v. and oral formulations of VDRMs such as paricalcitol are used to treat CKD patients. A desirable VDRM should have this characteristic. Results from this study demonstrate that VS-105 exhibits similar effects after both i.p. and oral dosing in the 5/6 NX rats.
One of the limitations of the current study is the lack of data on the FGF23 status before and after VS-105 treatment. FGF23 is a phosphorus regulating factor (Wolf, 2010). Excessive FGF23 levels, which increase progressively beginning in early stages of kidney disease in order to maintain normophosphataemia despite decreased nephron mass, may be partially responsible for early calcitriol deficiency and SHPT in CKD (Gutierrez et al., 2005). Furthermore, several clinical studies have demonstrated that VDRMs are potentially useful for reducing proteinuria/albuminuria (Agarwal et al., 2005; Alborzi et al., 2008; Szeto et al., 2008; Fishbane et al., 2009; de Zeeuw et al., 2010); it will be important to study the effect of VS-105 on reducing proteinuria/albuminuria and kidney fibrosis. Also, data on cardiac function to confirm the efficacy of VS-105 on improving cardiovascular parameters are needed. Follow-up studies investigating the effect of VS-105 on the FGF-23 status, cardiac function, proteinuria/albuminuria and kidney fibrosis are planned.
In summary, VS-105 effectively suppresses PTH, improves endothelial function and regresses LVH in the 5/6 NX uraemic rats in a dose range that does not affect serum Ca. VS-105 has the potential to treat not only SHPT but also cardiovascular complications in CKD in order to improve patient outcomes.
Acknowledgments
We thank Dr Terry Opgenorth for his comments and suggestions. We are grateful for the help and support from the staff at the University of Illinois at Chicago Animal Care Facility.
Glossary
Abbreviations
- CKD
chronic kidney disease
- LV
left ventricle
- NX
nephrectomized
- Pi
phosphate
- PTH
parathyroid hormone
- SHPT
secondary hyperparathyroidism
- VDR
vitamin D receptor
- VDRMs
VDR modulators
Conflicts of interest
The study was funded in full by Vidasym. The authors hold option units in Vidasym, LLC., a privately held company focusing on developing VDRM drugs.
References
- Agarwal R, Acharya M, Tian J, Hippensteel RL, Melnick JZ, Qiu P, et al. Antiproteinuric effect of oral paricalcitol in chronic kidney disease. Kidney Int. 2005;68:2823–2828. doi: 10.1111/j.1523-1755.2005.00755.x. [DOI] [PubMed] [Google Scholar]
- Alborzi P, Patel NA, Peterson C, Bills JE, Bekele DM, Bunaye Z, et al. Paricalcitol reduces albuminuria and inflammation in chronic kidney disease: a randomized double-blind pilot trial. Hypertension. 2008;52:249–255. doi: 10.1161/HYPERTENSIONAHA.108.113159. [DOI] [PubMed] [Google Scholar]
- Baigent C, Burbury K, Wheeler D. Premature cardiovascular disease in chronic renal failure. Lancet. 2000;356:147–152. doi: 10.1016/S0140-6736(00)02456-9. [DOI] [PubMed] [Google Scholar]
- Barreto DV, Barreto FC, Liabeuf S, Temmar M, Boitte F, Choukroun G, et al. Vitamin D affects survival independently of vascular calcification in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:1128–1135. doi: 10.2215/CJN.00260109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggar PH, Liangos O, Fey H, Brandenburg VM, Ketteler M. Vitamin D, chronic kidney disease and survival: a pluripotent hormone or just another bone drug? Pediatr Nephrol. 2011;26:7–18. doi: 10.1007/s00467-010-1526-x. [DOI] [PubMed] [Google Scholar]
- Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–2155. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covic A, Voroneanu L, Goldsmith D. The effects of vitamin D therapy on left ventricular structure and function – are these the underlying explanations for improved CKD patient survival? Nephron Clin Pract. 2010;116:c187–c195. doi: 10.1159/000317198. [DOI] [PubMed] [Google Scholar]
- Damman K, Kalra PR, Hillege H. Pathophysiological mechanisms contributing to renal dysfunction in chronic heart failure. J Ren Care. 2010;36(Suppl 1):18–26. doi: 10.1111/j.1755-6686.2010.00172.x. [DOI] [PubMed] [Google Scholar]
- Fishbane S, Chittineni H, Packman M, Dutka P, Ali N, Durie N. Oral paricalcitol in the treatment of patients with CKD and proteinuria: a randomized trial. Am J Kidney Dis. 2009;54:647–652. doi: 10.1053/j.ajkd.2009.04.036. [DOI] [PubMed] [Google Scholar]
- Foley RN. Clinical epidemiology of cardiovascular disease in chronic kidney disease. J Ren Care. 2010;36(Suppl 1):4–8. doi: 10.1111/j.1755-6686.2010.00171.x. [DOI] [PubMed] [Google Scholar]
- Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32(Suppl 3):S112–S119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- Gal-Moscovici A, Sprague SM. Use of vitamin D in chronic kidney disease patients. Kidney Int. 2010;78:146–151. doi: 10.1038/ki.2010.113. [DOI] [PubMed] [Google Scholar]
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16:2205–2215. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- Gwadry-Sridhar FH, Flintoft V, Lee DS, Lee H, Guyatt GH. A systematic review and meta-analysis of studies comparing readmission rates and mortality rates in patients with heart failure. Arch Intern Med. 2004;164:2315–2320. doi: 10.1001/archinte.164.21.2315. [DOI] [PubMed] [Google Scholar]
- Hasdan G, Benchetrit S, Rashid G, Green J, Bernheim J, Rathaus M. Endothelial dysfunction and hypertension in 5/6 nephrectomized rats are mediated by vascular superoxide. Kidney Int. 2002;61:586–590. doi: 10.1046/j.1523-1755.2002.00166.x. [DOI] [PubMed] [Google Scholar]
- Kalantar-Zadeh K, Kuwae N, Regidor DL, Kovesdy CP, Kilpatrick RD, Shinaberger CS, et al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006;70:771–780. doi: 10.1038/sj.ki.5001514. [DOI] [PubMed] [Google Scholar]
- Kawai M. Vitamin D receptor agonists and uses thereof. U.S. Provisional Patent Application No. 61/170,160, Publication No. WO 2010/120698 A1 (published October 21, 2010) 2010.
- Kim HW, Park CW, Shin YS, Kim YS, Shin SJ, Kim YS, et al. Calcitriol regresses cardiac hypertrophy and QT dispersion in secondary hyperparathyroidism on hemodialysis. Nephron Clin Pract. 2006;102:c21–c29. doi: 10.1159/000088295. [DOI] [PubMed] [Google Scholar]
- Koeffler HP, Hirji K, Itri L. 1,25-Dihydroxyvitamin D3: in vivo and in vitro effects on human preleukemic and leukemic cells. Cancer Treat Rep. 1985;69:1399–1407. [PubMed] [Google Scholar]
- Kovesdy CP, Ahmadzadeh S, Anderson JE, Kalantar-Zadeh K. Association of activated vitamin D treatment and mortality in chronic kidney disease. Arch Intern Med. 2008;168:397–403. doi: 10.1001/archinternmed.2007.110. [DOI] [PubMed] [Google Scholar]
- Lee GH, Benner D, Regidor DL, Kalantar-Zadeh K. Impact of kidney bone disease and its management on survival of patients on dialysis. J Ren Nutr. 2007;17:38–44. doi: 10.1053/j.jrn.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71:31–38. doi: 10.1038/sj.ki.5002009. [DOI] [PubMed] [Google Scholar]
- Levin A, Djurdjev O, Beaulieu M, Er L. Variability and risk factors for kidney disease progression and death following attainment of stage 4 CKD in a referred cohort. Am J Kidney Dis. 2008;52:661–671. doi: 10.1053/j.ajkd.2008.06.023. [DOI] [PubMed] [Google Scholar]
- Malyszko J. Mechanism of endothelial dysfunction in chronic kidney disease. Clin Chim Acta. 2011;411:1412–1420. doi: 10.1016/j.cca.2010.06.019. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Koeffler HP, Donaldson CA, Pike JW, Haussler MR. 1,25-Dihydroxyvitamin D3-induced differentiation in a human promyelocytic leukemia cell line (HL-60): receptor-mediated maturation to macrophage-like cells. J Cell Biol. 1984;98:391–398. doi: 10.1083/jcb.98.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KJ, Gonzalez EA. Vitamin D analogues for the management of secondary hyperparathyroidism. Am J Kidney Dis. 2001;38(Suppl 5):S34–S40. doi: 10.1053/ajkd.2001.28109. [DOI] [PubMed] [Google Scholar]
- Mirkovic K, van den Born J, Navis G, de Borst MH. Vitamin D in chronic kidney disease: new potential for intervention. Curr Drug Targets. 2011;12:42–53. doi: 10.2174/138945011793591572. [DOI] [PubMed] [Google Scholar]
- Mizobuchi M, Nakamura H, Tokumoto M, Finch J, Morrissey J, Liapis H, et al. Myocardial effects of VDR activators in renal failure. J Steroid Biochem Mol Biol. 2010;121:188–192. doi: 10.1016/j.jsbmb.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane M, Ma J, Rose AE, Osinski MA, Wu-Wong JR. Differential effects of Vitamin D analogs on calcium transport. J Steroid Biochem Mol Biol. 2007;103:84–89. doi: 10.1016/j.jsbmb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Naves-Diaz M, Alvarez-Hernandez D, Passlick-Deetjen J, Guinsburg A, Marelli C, Rodriguez-Puyol D, et al. Oral active vitamin D is associated with improved survival in hemodialysis patients. Kidney Int. 2008;74:1070–1078. doi: 10.1038/ki.2008.343. [DOI] [PubMed] [Google Scholar]
- Pons F, Lupon J, Urrutia A, Gonzalez B, Crespo E, Diez C, et al. Mortality and cause of death in patients with heart failure: findings at a specialist multidisciplinary heart failure unit. Rev Esp Cardiol. 2010;63:303–314. doi: 10.1016/s1885-5857(10)70063-3. [DOI] [PubMed] [Google Scholar]
- Reddan DN, Szczech LA, Tuttle RH, Shaw LK, Jones RH, Schwab SJ, et al. Chronic kidney disease, mortality, and treatment strategies among patients with clinically significant coronary artery disease. J Am Soc Nephrol. 2003;14:2373–2380. doi: 10.1097/01.asn.0000083900.92829.f5. [DOI] [PubMed] [Google Scholar]
- Sciacqua A, Borrello F, Vatrano M, Grembiale RD, Perticone F. Effect of interaction between left ventricular dysfunction and endothelial function in hypertension. Curr Hypertens Rep. 2006;8:212–218. doi: 10.1007/s11906-006-0053-4. [DOI] [PubMed] [Google Scholar]
- Segal AW. Nitroblue-tetrazolium tests. Lancet. 1974;2:1248–1252. doi: 10.1016/s0140-6736(74)90758-2. [DOI] [PubMed] [Google Scholar]
- Shinaberger CS, Kopple JD, Kovesdy CP, McAllister CJ, van Wyck D, Greenland S, et al. Ratio of paricalcitol dosage to serum parathyroid hormone level and survival in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2008;3:1769–1776. doi: 10.2215/CJN.01760408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoben AB, Rudser KD, de Boer IH, Young B, Kestenbaum B. Association of oral calcitriol with improved survival in nondialyzed CKD. J Am Soc Nephrol. 2008;19:1613–1619. doi: 10.1681/ASN.2007111164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slatopolsky E, Finch J, Ritter C, Denda M, Morrissey J, Brown A, et al. A new analog of calcitriol, 19-nor-1,25-(OH)2D2, suppresses parathyroid hormone secretion in uremic rats in the absence of hypercalcemia. Am J Kidney Dis. 1995;26:852–860. doi: 10.1016/0272-6386(95)90455-7. [DOI] [PubMed] [Google Scholar]
- Slatopolsky E, Finch J, Ritter C, Takahashi F. Effects of 19-nor-1,25(OH)2D2, a new analogue of calcitriol, on secondary hyperparathyroidism in uremic rats. Am J Kidney Dis. 1998;32(Suppl 2):S40–S47. doi: 10.1053/ajkd.1998.v32.pm9808142. [DOI] [PubMed] [Google Scholar]
- Slatopolsky E, Cozzolino M, Finch JL. Differential effects of 19-nor-1,25-(OH)(2)D(2) and 1alpha-hydroxyvitamin D(2) on calcium and phosphorus in normal and uremic rats. Kidney Int. 2002;62:1277–1284. doi: 10.1111/j.1523-1755.2002.kid573.x. [DOI] [PubMed] [Google Scholar]
- Szeto CC, Chow KM, Kwan BC, Chung KY, Leung CB, Li PK. Oral calcitriol for the treatment of persistent proteinuria in immunoglobulin A nephropathy: an uncontrolled trial. Am J Kidney Dis. 2008;51:724–731. doi: 10.1053/j.ajkd.2007.12.038. [DOI] [PubMed] [Google Scholar]
- Teng M, Wolf M, Lowrie E, Ofsthun N, Lazarus JM, Thadhani R. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med. 2003;349:446–456. doi: 10.1056/NEJMoa022536. [DOI] [PubMed] [Google Scholar]
- Teng M, Wolf M, Ofsthun MN, Lazarus JM, Hernan MA, Camargo CA, et al. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. J Am Soc Nephrol. 2005;16:1115–1125. doi: 10.1681/ASN.2004070573. [DOI] [PubMed] [Google Scholar]
- Tentori F, Hunt WC, Stidley CA, Rohrscheib MR, Bedrick EJ, Meyer KB, et al. Mortality risk among hemodialysis patients receiving different vitamin D analogs. Kidney Int. 2006;70:1858–1865. doi: 10.1038/sj.ki.5001868. [DOI] [PubMed] [Google Scholar]
- Verhave G, Siegert CE. Role of vitamin D in cardiovascular disease. Neth J Med. 2010;68:113–118. [PubMed] [Google Scholar]
- Wolf M. Forging forward with 10 burning questions on FGF23 in kidney disease. J Am Soc Nephrol. 2010;21:1427–1435. doi: 10.1681/ASN.2009121293. [DOI] [PubMed] [Google Scholar]
- Wolf SC, Gaschler F, Brehm S, Klaussner M, Amann K, Risler T, et al. Endothelin-receptor antagonists in uremic cardiomyopathy. J Cardiovasc Pharmacol. 2000;36(Suppl 1):S348–S350. doi: 10.1097/00005344-200036051-00101. [DOI] [PubMed] [Google Scholar]
- Wolf M, Shah A, Gutierrez O, Ankers E, Monroy M, Tamez H, et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007;72:1004–1013. doi: 10.1038/sj.ki.5002451. [DOI] [PubMed] [Google Scholar]
- Wolf M, Betancourt J, Chang Y, Shah A, Teng M, Tamez H, et al. Impact of activated vitamin D and race on survival among hemodialysis patients. J Am Soc Nephrol. 2008;19:1379–1388. doi: 10.1681/ASN.2007091002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu-Wong JR. Potential for vitamin D receptor agonists in the treatment of cardiovascular disease. Br J Pharmacol. 2009;158:395–412. doi: 10.1111/j.1476-5381.2009.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu-Wong JR, Nakane M, Gagne GD, Brooks KA, Noonan WT. Comparison of the pharmacological effects of paricalcitol and doxercalciferol on the factors involved in mineral homeostasis. Int J Endocrinol. 2010a;2010:621687–621694. doi: 10.1155/2010/621687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu-Wong JR, Noonan W, Nakane M, Brooks KA, Segreti JA, Polakowski JS, et al. Vitamin d receptor activation mitigates the impact of uremia on endothelial function in the 5/6 nephrectomized rats. Int J Endocrinol. 2010b;2010:625852–625861. doi: 10.1155/2010/625852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang W, Kong J, Chen S, Cao LP, Qiao G, Zheng W, et al. Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrinol Metab. 2005;288:E125–E132. doi: 10.1152/ajpendo.00224.2004. [DOI] [PubMed] [Google Scholar]
- Yeboah J, Crouse JR, Bluemke DA, Lima JA, Polak JF, Burke GL, et al. Endothelial dysfunction is associated with left ventricular mass (assessed using MRI) in an adult population (MESA) J Human Hypertens. 2011;25:25–31. doi: 10.1038/jhh.2010.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zeeuw D, Agarwal R, Amdahl M, Audhya P, Coyne D, Garimella T, et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet. 2010;376:1543–1551. doi: 10.1016/S0140-6736(10)61032-X. [DOI] [PubMed] [Google Scholar]


