Abstract
BACKGROUND AND PURPOSE
The fatty acid amide hydrolase inhibitor URB 597 increases brain anandamide levels, suggesting that URB 597 could enhance the behavioural effects of anandamide. The goal of the current study was to examine and characterize the in vivo pharmacology of URB 597 alone and in combination with anandamide and Δ9-tetrahydrocannabinol (Δ9-THC) in two drug discrimination assays in rhesus monkeys.
EXPERIMENTAL APPROACH
The effects of URB 597 alone and in combination with anandamide were investigated in one group of monkeys (n = 4) that discriminated Δ9-THC (0.1 mg·kg−1 i.v.) from vehicle, and in another group (n = 5) receiving chronic Δ9-THC (1 mg·kg−112 h−1 s.c.) that discriminated the cannabinoid antagonist rimonabant (1 mg·kg−1 i.v.).
KEY RESULTS
Intravenous anandamide fully substituted for, and had infra-additive effects with, Δ9-THC. URB 597 (up to 3.2 mg·kg−1 i.v.) did not substitute for or modify the effects of Δ9-THC but markedly increased the potency (32-fold) and duration of action of anandamide. The rimonabant discriminative stimulus in Δ9-THC-treated monkeys (i.e. Δ9-THC withdrawal) was attenuated by both Δ9-THC (at doses larger than 1 mg·kg−1 per 12 h) and anandamide but not by URB 597 (3.2 mg·kg−1). URB 597 did not increase the potency of anandamide to attenuate the rimonabant-discriminative stimulus.
CONCLUSIONS AND IMPLICATIONS
URB 597 enhanced the behavioural effects of anandamide but not other CB1 agonists. However, URB 597 did not significantly enhance the attenuation of Δ9-THC withdrawal induced by anandamide. Collectively, these data suggest that endogenous anandamide in primate brain does not readily mimic the behavioural effects of exogenously administered anandamide.
Keywords: anandamide, cannabinoid, Δ9-tetrahydrocannabinol, drug discrimination, rhesus monkey, rimonabant, URB 597
Introduction
Cannabinoid CB1 receptors are widely expressed (Herkenham et al., 1991) and the most abundant G-protein-coupled receptor (GPCR) in brain (Gifford et al., 1999). Δ9-tetrahydrocannabinol (Δ9-THC), a chemical in cannabis or marijuana, stimulates CB1 receptors to produce a variety of behavioural effects, including anti-emetic (Darmani, 2001), anti-nociceptive (Compton et al., 1996), memory-impairing (Lichtman and Martin, 1996), discriminative stimulus (Järbe et al., 2001) and positive reinforcing effects (Justinova et al., 2008; Panlilio et al., 2010). Δ9-THC and other cannabinoid receptor agonists differ from each other in CB1 agonist efficacy in vitro, with Δ9-THC having relatively low efficacy (e.g. less than anandamide) as indexed by stimulation of G-proteins (Childers, 2006). Cannabinoid agonists also differ in relative binding affinity at CB1 versus CB2 receptors (Miller and Stella, 2008) and activity at orphan GPCRs (Pertwee et al., 2010; Sharir and Abood, 2010). Despite this heterogeneity, CB1 agonists generally share behavioural effects including non-preferred effects (i.e. sedation), resulting in efforts to develop novel pharmacological approaches for activating cannabinoid signalling.
The naturally occurring brain lipid N-arachidonoylethanolamine (anandamide) and its synthetic and metabolic pathways are targets for novel therapeutics. However, despite being a cannabinoid receptor agonist (Devane et al., 1992), anandamide does not always share in vivo effects with Δ9-THC. In drug discrimination procedures, for example, anandamide did not fully substitute for the discriminative stimulus effects of Δ9-THC (Wiley et al., 1995; 1997; Järbe et al., 2001; Solinas et al., 2007). When anandamide and Δ9-THC did share effects (e.g. hypothermic, anti-nociceptive and motor-impairing effects), CB1 receptors appeared to mediate the effects Δ9-THC, as evidenced by antagonism with rimonabant, but not the effects of anandamide (Adams et al., 1998). One interpretation is that anandamide itself acts at non-CB1 receptors to produce in vivo effects, although an alternative explanation is that anandamide is rapidly metabolized to non-CB1 receptor ligands that, in turn, mimic the effects of anandamide (Wiley et al., 2006). Evidence against the former interpretation is provided by studies with i.v. Δ9-THC as a discriminative stimulus in rhesus monkeys; those studies demonstrated that i.v. anandamide shared effects with Δ9-THC and strongly suggested that both ligands acted at the same (e.g. CB1) receptors (McMahon, 2009). This Δ9-THC discrimination assay appears to be especially sensitive to the CB1 receptor-mediated effects of anandamide.
Fatty acid amide hydrolase inhibitors, such as URB 597, block the enzyme primarily responsible for the metabolism of anandamide and increase brain anandamide levels in rodents (Kathuria et al., 2003; Fegley et al., 2005), marmosets (Johnston et al., 2011) and squirrel monkeys (Justinova et al., 2008). In pre-clinical studies, URB 597 does not have effects predictive of the abuse potential and the ‘high’ associated with marijuana. Anandamide, but not URB 597, maintained self-administration behaviour in squirrel monkeys (Justinova et al., 2008). When studied separately, URB 597 and anandamide did not substitute for a Δ9-THC discriminative stimulus in rats, although substitution was obtained when URB 597 and anandamide were combined (Solinas et al., 2007). URB 597 produces some effects indicative of therapeutic potential, including anti-nociception (Jhaveri et al., 2007; Naidu et al., 2009) and attenuation of cannabinoid withdrawal (Clapper et al., 2009; Schlosburg et al., 2009). The goal of the current study was to examine and characterize the in vivo pharmacology of URB 597 by combining it with anandamide in rhesus monkeys. URB 597 and anandamide were studied in a drug discrimination assay sensitive to anandamide, that is the discriminative stimulus effects of Δ9-THC (0.1 mg·kg−1 i.v.), and in a drug discrimination assay sensitive to cannabinoid withdrawal, that is the discriminative stimulus effects of the cannabinoid antagonist rimonabant (1 mg·kg−1i.v.) in rhesus monkeys dependent on Δ9-THC (1 mg·kg−1 per 12 h s.c.). To examine whether URB 597 selectively interacts with anandamide, URB 597 was also combined with Δ9-THC. To better understand the combined effects of URB 597 and Δ9-THC, which conceivably involve an increase in endogenous anandamide, the combined effects of Δ9-THC and anandamide were quantified by isobolographic analysis (Tallarida, 2006).
Methods
Subjects
One female and three male (Δ9-THC discrimination assay) as well as two male and three female (rimonabant discrimination assay) rhesus monkeys (Macaca mulatta) were housed individually and kept on a 14 h light/10 h dark schedule. They were maintained at 95% free-feeding weight (range 5–10 kg) with a diet consisting of primate chow (High Protein Monkey Diet, Harlan Teklad, Madison, WI), fresh fruit and peanuts; water was provided in the home cage. The monkeys had received cannabinoids and non-cannabinoids in previous studies (Stewart and McMahon, 2010; McMahon, 2011). Monkeys were maintained in accordance with the Institutional Animal Care and Use Committee, The University of Texas Health Science Center at San Antonio and with the ‘Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research’ (National Research Council, 2003).
Catheter insertion
Following anaesthesia with ketamine (10 mg·kg−1 i.m.) and isoflurane (1.5–3.0% inhaled via facemask), a chronic indwelling catheter (heparin-coated polyurethane, od = 1.68 mm, id = 1.02 mm; Instech Solomon, Plymouth Meeting, PA) was inserted and advanced 5 cm into a subclavian or femoral vein. Suture silk (coated vicryl; Ethicon Inc., Somerville, NJ) was used to anchor the catheter to the vessel and to ligate the section of the vessel proximal to the catheter insertion. The other end of the catheter was passed s.c. to the mid-scapular region of the back and was attached to a vascular access port (Mida-cbas-c50, Instech Solomon).
Apparatus
Monkeys were seated in chairs (Model R001, Primate Products, Miami, FL) and were placed in ventilated, sound-attenuating chambers equipped with two levers and a light positioned above each lever. Feet were placed in shoes containing brass electrodes to which a brief electric stimulus (3 mA, 250 ms) could be delivered from an a/c generator. The chambers were connected to the computer with an interface (Med Associates, St. Albans, VT); experimental events were controlled and recorded with Med-PC software (Med Associates).
Drug discrimination procedure
Monkeys (n = 4) discriminated Δ9-THC (0.1 mg·kg−1 i.v.) from vehicle (1 part absolute ethanol, 1 part Emulphor-620 and 18 parts saline) while responding under a fixed ratio 5 (FR5) schedule of stimulus-shock termination. A separate group of monkeys (n = 5) received 1 mg·kg−1 every 12 h of Δ9-THC (at 0600 and 1800 h) and discriminated rimonabant (1 mg·kg−1 i.v.) from the same vehicle at 1200 h under an FR5 schedule of stimulus-shock termination. Experimental sessions were divided into multiple cycles; each cycle began with a timeout, which was 5 min for the Δ9-THC discrimination and 15 min for the rimonabant discrimination; responses during the timeout had no programmed consequence. For both discrimination procedures, the timeout was followed by a 5 min schedule of stimulus-shock termination; therefore, cycle duration was 10 min for the Δ9-THC discrimination and 20 min for the rimonabant discrimination. The schedule was signalled by illumination of red lights (one positioned above each lever); five consecutive responses on the correct lever extinguished the red lights, prevented delivery of an electric stimulus and initiated a 30 s timeout. Otherwise, an electric stimulus was delivered every 40 s (Δ9-THC discrimination) or 10 s (rimonabant discrimination). Responding on the incorrect lever reset the response requirement on the correct lever. Determination of correct levers varied among monkeys (i.e. left lever associated with drug; right lever associated with vehicle) and remained the same for that monkey for the duration of the study.
Training sessions were conducted by administering the training drug (Δ9-THC or rimonabant) or vehicle within the first minute of a cycle followed by vehicle or sham (dull pressure applied to the skin overlying the vascular access port) within the first minute of subsequent cycles. Δ9-THC training consisted of three cycles and was preceded by zero to three vehicle-training cycles; rimonabant training consisted of two cycles and was preceded by zero to four vehicle-training cycles. Training sessions with vehicle alone consisted of two to six cycles. Completion of the FR on the correct lever was required to prevent the delivery of the electric stimulus during each training cycle. The monkeys had previously satisfied the criteria for testing, that is at least 80% of the total responses occurred on the correct lever and fewer than five responses occurred on the incorrect lever before completing the FR on the correct lever for all cycles during five consecutive or six of seven training sessions. Tests were conducted after performance for consecutive training sessions, including both vehicle and drug training sessions, had satisfied the test criteria. The type of training session preceding test sessions varied non-systematically.
During the test sessions, five consecutive responses on either lever postponed the schedule of stimulus presentations. In monkeys discriminating Δ9-THC, dose–effect functions for Δ9-THC and anandamide were determined by administering vehicle in the first cycle followed by doses increasing by 0.25 or 0.5 log unit in subsequent cycles. The dose–effect function included ineffective doses (i.e. doses producing less than 20% of responses on the Δ9-THC lever) up to doses that produced greater than 80% of responses on the Δ9-THC lever. The combined effects of Δ9-THC and anandamide were examined using a fixed-ratio design, whereby doses of the two drugs were maintained in a constant proportion of their respective ED50 values (Tallarida, 2000). In the first cycle, vehicle for each compound was administered in two separate injections, followed by cumulative doses of Δ9-THC and anandamide in two separate injections in subsequent cycles. The largest dose combination was the ED50 value of Δ9-THC and anandamide; smaller doses included one-half the ED50 value of each drug, etc. URB 597 was studied by administering a dose (0.32–3.2 mg·kg−1) in the first cycle followed by cumulative doses of Δ9-THC or anandamide in subsequent cycles. The time course of anandamide alone (5.6 and 10 mg·kg−1) and anandamide (5.6 mg·kg−1) in combination with URB 597 (3.2 mg·kg−1) was examined by administering vehicle or URB 597 in the first cycle followed by anandamide in the second cycle of a six-cycle test.
In Δ9-THC-treated monkeys discriminating rimonabant, dose–effect functions for rimonabant were determined by administering vehicle in the first cycle followed by doses increasing by 0.25 or 0.5 log unit in subsequent cycles. The effects of Δ9-THC (0.32–3.2 mg·kg−1) and URB 597 (3.2 mg·kg−1) were studied by administering a dose prior to cumulative doses of rimonabant. Because anandamide has a short duration of action (i.e. less than 15 min; see Results), the combined effects of anandamide and rimonabant were examined by administering a dose of each during separate tests consisting of a single cycle. Vehicle or a dose of rimonabant was administered within the first minute of the cycle, followed by vehicle or a dose of anandamide (10 and 32 mg·kg−1) 10 min later (i.e. 5 min before the response period). Similarly, the combined effects of URB 597 and anandamide were studied by administering URB 597 (3.2 mg·kg−1) or vehicle in combination with a dose of rimonabant at the beginning of a single cycle; anandamide (10 mg·kg−1) was administered 10 min later. The concentrations of rimonabant studied varied from ineffective doses up to doses that produced greater than 80% of responses on the rimonabant lever.
Drugs
Drug/molecular target nomenclature conforms to the British Journal of Pharmacology Guide to Receptors and Channels (Alexander et al., 2009). Δ9-THC (100 mg·mL−1 in absolute ethanol), rimonabant and URB 597 were obtained from The Research Technology Branch of the National Institute on Drug Abuse (Rockville, MD). Anandamide was synthesized from arachidonoyl chloride (Nu-Check Prep, Inc., Elysian, MN), dichloromethane and ethanolamine (Sigma-Aldrich, St. Louis, MO) according to a protocol developed by Giuffrida and Piomelli (1998a). The product was analysed for purity with gas chromatography and mass spectrometry as described previously (Giuffrida and Piomelli, 1998b). Drugs were dissolved in a mixture of 1 part absolute ethanol, 1 part Emulphor-620 (Rhodia Inc., Cranbury, NJ) and 18 parts physiological saline. Drugs were administered i.v. in a volume of 0.1–1 mL·kg−1. All doses are expressed as the weight of the forms listed above in mg·kg−1 body weight.
Data analyses
Four monkeys discriminating Δ9-THC and five monkeys discriminating rimonabant were included using a within-subjects design. Discrimination data were expressed as a percentage of responses on the drug lever out of total responses on both the drug and vehicle levers. Rate of responding on both levers (i.e. drug and vehicle) was calculated as responses s−1 excluding responses during timeouts. Rate of responding during a test was expressed as the percentage of the control response rate for individual animals. The control was defined as the average response rate for all cycles during the five previous vehicle training sessions excluding sessions during which the test criteria were not satisfied. Discrimination and rate data were averaged among subjects (±SEM) and plotted as a function of dose. Effects of drugs on response rate were examined with analyses of variance (anovas) for repeated measures; Dunnett's test was used to examine significant differences relative to the vehicle control (P < 0.05).
Individual dose–response data were analysed with linear regression (GraphPad Prism version 5.0 for Windows, San Diego, CA) including doses spanning the linear portion of the dose–response curve. If the slopes of dose–response curves were not significantly different, as determined by an F-ratio test, then a common, best-fitting slope was used for further analyses (Kenakin, 1997). Doses corresponding to the 50% level of effect (ED50 value), potency ratios and their 95% confidence limits were calculated by parallel line analyses of data from individual subjects (Tallarida, 2000). Potencies were considered significantly different when the 95% confidence limits of the potency ratio did not include 1.
The experimentally derived dose–response data for the combined effects of Δ9-THC and anandamide were used to calculate a theoretical line of additivity (i.e. composite additive curve) according to the method described by Tallarida (2000). F-ratio tests in GraphPad were used to compare the slopes and intercepts of the experimentally derived and composite-additive dose–response data, which were derived from the group-averaged data. For example, a significant F-ratio test for slopes and intercepts shows that the dose–response curves cannot be described by a single line; that is, the lines are significantly different from each another. To compare agonist potency in Δ9-THC-treated monkeys, dose–response data for rimonabant, alone and in combination with a dose of agonist, were analysed with linear regression for individual monkeys. Dose-ratios (i.e. ED50 value of rimonabant in the presence of agonist divided by the ED50 value of rimonabant alone) are expressed as a function of agonist dose and analysed with linear regression to estimate the dose producing a twofold shift in the rimonabant dose–response function.
Results
The separate and combined effects of Δ9-THC and anandamide in monkeys discriminating Δ9-THC from vehicle
Δ9-THC dose-dependently increased mean (±SEM) responding on the Δ9-THC lever, from 4 ± 4% at a dose of 0.01 mg·kg−1 to 96 ± 4% at the training dose (0.1 mg·kg−1) (Figure 1, top). Following vehicle, mean (±SEM) responding on the Δ9-THC lever was 0% (Figure 1, top, above V). Anandamide dose-dependently increased responding on the Δ9-THC lever (Figure 1, top), from 0% at a dose of 1 mg·kg−1 to a maximum (mean ± SEM) of 90 ± 10% at 17.8 mg·kg−1 anandamide. The slopes of the dose–response curves for Δ9-THC and anandamide were not significantly different, and their ED50 values (95% confidence limits) were 0.039 (0.022–0.072) mg·kg−1 and 5.8 (2.7–13) mg·kg−1, respectively (Table 1).
Figure 1.

Effects of Δ9-THC and anandamide, alone (top) and in combination (bottom), in monkeys discriminating Δ9-THC (0.1 mg·kg−1 i.v.) from vehicle. Abscissae: vehicle (V) or dose in mg·kg−1 body weight. Ordinates: mean (±SEM) percentage of responding on the Δ9-THC lever. The composite additive line was determined from linear regression of all data from the individual dose–response curves (top panel) expressed as a function of the theoretically derived, combined total dose of Δ9-THC and anandamide (bottom panel, composite additive). The solid line in the bottom panel was determined from linear regression of the experimentally derived data produced by the combination of Δ9-THC and anandamide.
Table 1.
ED50 values and 95% confidence limits for Δ9-THC and anandamide, alone and in combination with URB 597, in monkeys discriminating Δ9-THC (0.1 mg·kg−1 i.v.) from vehicle
| Drug | ED50 value (95% CL) in mg·kg−1 | Potency ratio (95% CL)† |
|---|---|---|
| Δ9-THC | 0.039 (0.022–0.072) | |
| +URB 597 (1 mg·kg−1) | 0.024 (0.015–0.038) | 1.6 (0.8–3.5) |
| +URB 597 (3.2 mg·kg−1) | 0.072 (0.029–0.18) | 0.5 (0.2–1.4) |
| Anandamide | 5.8 (2.7–13) | |
| +URB 597 (0.32 mg·kg−1) | 1.9 (0.38–9.0) | 3.1 (0.3–28) |
| +URB 597 (1 mg·kg−1) | 0.45 (0.11–1.8)* | 13 (2.1–99) |
| +URB 597 (3.2 mg·kg−1) | 0.18 (0.056–0.57)* | 32 (7.8–140) |
Significant increase in potency.
Potency ratios and 95% confidence limits (CL) are the ED50 values of the agonist alone divided by the ED50 value of the agonist in combination with URB 597.
Δ9-THC and anandamide were combined at doses that were a fixed ratio of their respective ED50 values, yielding a proportion in the total mixture of 0.007 for Δ9-THC and 0.993 for anandamide. The individual Δ9-THC and anandamide dose–response data (Figure 1, top) were expressed as a function of the combined, total dose of Δ9-THC and anandamide (Figure 1, bottom), which was calculated on the basis of additivity using isobolographic analysis. Linear regression of these data yielded the composite additive curve (Figure 1, bottom). When Δ9-THC and anandamide were administered together, doses one-half of their respective ED50 values (i.e. 0.02 mg·kg−1 of Δ9-THC and 2.9 mg·kg−1 of anandamide) produced 5% responding on the Δ9-THC lever (Figure 1, bottom panel, value above 3.2 mg·kg−1). Increasing the dose to the ED50 values produced 91% responding on the Δ9-THC lever. Linear regression of the experimentally derived points yielded a line with a slope that was significantly greater than the slope of the composite additive curve (F1,6 = 12.62; P < 0.05). Moreover, the y-intercepts of the lines were significantly different (F1,4 = 11.08; P < 0.05). This significant difference between the composite additive and experimentally derived curves rejects the null hypothesis that Δ9-THC and anandamide have additive effects; rather, the effects appear to be infra-additive, especially at lower levels of effect.
The effects of URB 597, alone and in combination with Δ9-THC and anandamide, in monkeys discriminating Δ9-THC from vehicle
URB 597, up to a dose of 3.2 mg·kg−1, produced a maximum of 1% responding on the Δ9-THC lever (Figure 2, top, V). In the presence of URB 597 (1 and 3.2 mg·kg−1), Δ9-THC dose-dependently increased drug lever responding (Figure 2, top left). The slopes of the Δ9-THC dose–response curves, alone and in combination with URB 597 (1 and 3.2 mg·kg−1), were not significantly different (P > 0.05). URB 597 did not significantly modify the discriminative stimulus effects of Δ9-THC, as evidenced by ED50 values determined in the presence of URB 597 that were not significantly different from control (Table 1). In contrast, URB 597 markedly and dose-dependently increased the potency of anandamide to produce Δ9-THC-like discriminative stimulus effects (Figure 2, top right). The slopes of the anandamide dose–response curves, alone and in combination with URB 597, were not significantly different. Doses of 0.32, 1 and 3.2 mg·kg−1 URB 597 decreased the ED50 value of anandamide 3.1-, 13-, and 32-fold respectively (Table 1). Absolute rate of responding for individual monkeys was 0.86, 1.02, 1.57 and 1.71 responses s−1. Up to the largest doses studied, URB 597, Δ9-THC and anandamide did not significantly modify response rate, either when administered alone or in combination (P > 0.05) (Figure 2, bottom).
Figure 2.

Effects of URB 597, alone and in combination with Δ9-THC (left) or anandamide (right), in monkeys discriminating Δ9-THC (0.1 mg·kg−1 i.v.) from vehicle. Abscissae: vehicle (V) or dose in mg·kg−1 body weight. Ordinates: mean (±SEM) percentage of responding on the Δ9-THC lever (top) and mean (±SEM) response rate expressed as a percentage of control (V training days) rate [Rate (% control)] (bottom).
When administered at the beginning of multiple cycles, 5.6 and 10 mg·kg−1 anandamide produced 24% and 82% responding on the Δ9-THC lever, respectively, at 5–10 min; both doses resulted in 0% responding on the Δ9-THC lever at 15–20 min (Figure 3, top). Anandamide (5.6 and 10 mg·kg−1) did not significantly alter rate of responding (Figure 3, bottom). When combined with 3.2 mg·kg−1 URB 597, both the potency and duration of action of anandamide (5.6 mg·kg−1) were increased; that is, responding on the Δ9-THC lever was 100% at 5–10 min and 50% or greater for up to 45–50 min (Figure 3, top). The combination of anandamide (5.6 mg·kg−1) and URB 597 (3.2 mg·kg−1) significantly decreased response rate to 66% of control at 5–10 min (Figure 3, bottom).
Figure 3.
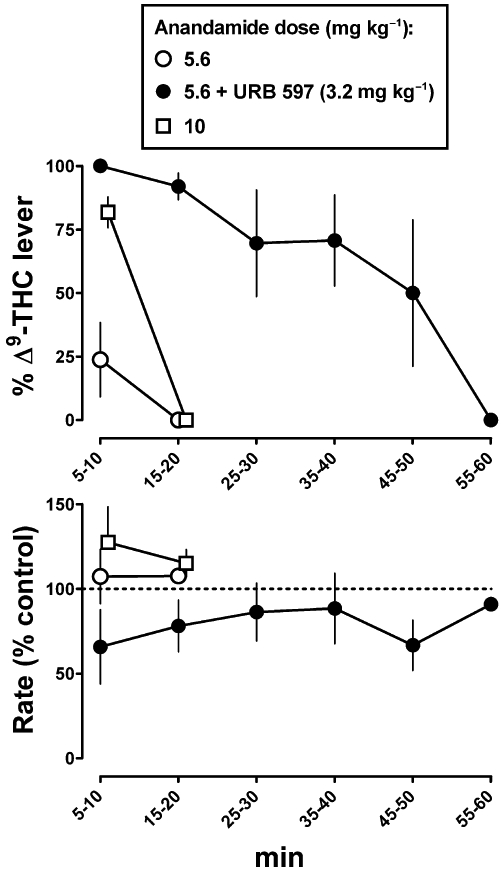
Time course for anandamide, alone and in combination with URB 597, in monkeys discriminating Δ9-THC (0.1 mg·kg−1 i.v.) from vehicle. Abscissae: time in minutes. Ordinates: mean (±SEM) percentage of responding on the Δ9-THC lever (top) and mean (±SEM) response rate expressed as a percentage of control rate [Rate (% control)] (bottom).
The effects of Δ9-THC, anandamide and URB 597 in Δ9-THC-treated monkeys discriminating rimonabant
Rimonabant dose-dependently increased drug lever responding, with 0.32 and 1 mg·kg−1 rimonabant producing 47% and 91% drug lever responding, respectively (Figures 4 and 5, top left). The ED50 value was 0.35 mg·kg−1 (Table 2). Vehicle produced 0% responding on the rimonabant lever. When the rimonabant dose–response function was determined by administering a single dose per test session, potency was slightly greater than that determined from cumulative dosing (Figures 4 and 5, top right). A single, bolus dose of 0.32 mg·kg−1 rimonabant produced 77% responding on the drug lever versus 47% with cumulative dosing. The ED50 value of rimonabant from single, bolus dosing was 0.18 mg·kg−1 (Table 2).
Figure 4.
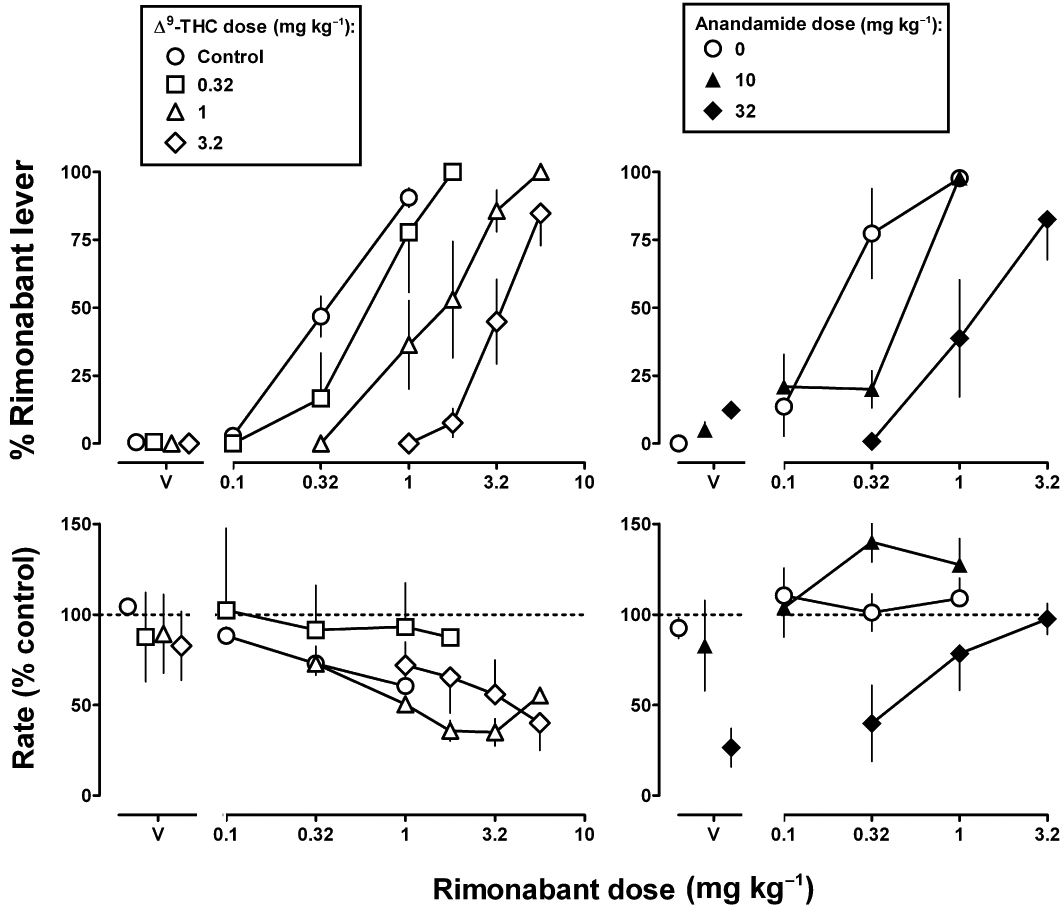
Effects of Δ9-THC (left) and anandamide (right), alone and in combination with rimonabant, in Δ9-THC-treated (1 mg·kg−1 12 h−1 s.c.) monkeys discriminating rimonabant (1 mg·kg−1 i.v.). Abscissae: vehicle (V) or dose in mg·kg−1 body weight of rimonabant. Ordinates: mean (±SEM) percentage of responding on the rimonabant lever (top) and mean (±SEM) response rate expressed as a percentage of control (V training days) rate [Rate (% control)] (bottom).
Figure 5.
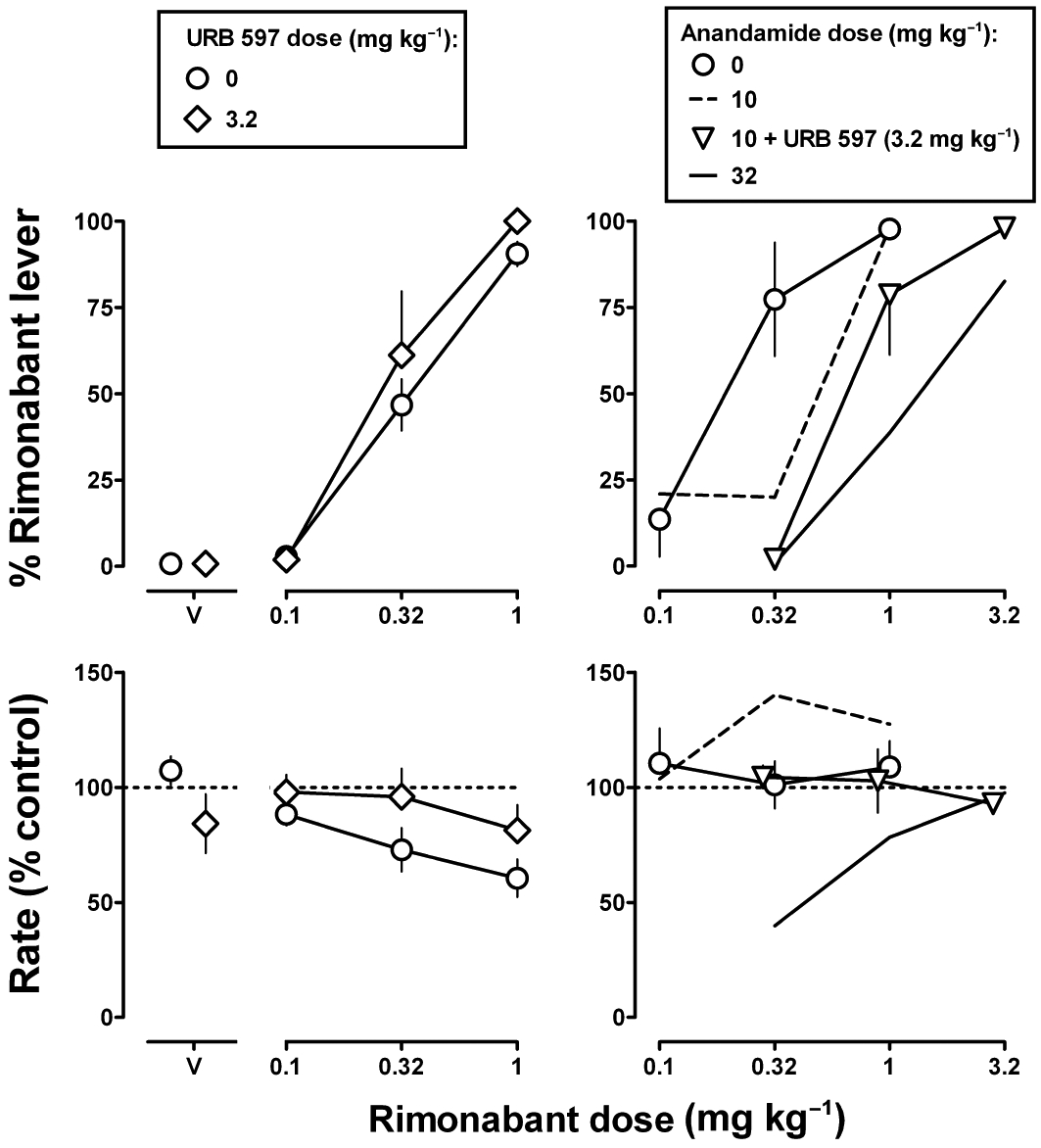
Effects of URB 597, alone (left) and in combination with anandamide (right), in Δ9-THC-treated (1 mg·kg−1 12 h−1 s.c.) monkeys discriminating rimonabant (1 mg·kg−1 i.v.). Abscissae: vehicle (V) or dose in mg·kg−1 body weight of rimonabant. Ordinates: mean (±SEM) percentage of responding on the rimonabant lever (top) and mean (±SEM) response rate expressed as a percentage of control (V training days) rate [Rate (% control)] (bottom). The dashed and solid lines not connected to symbols are the effects of 10 and 32 mg·kg−1 of anandamide, respectively, in combination with rimonabant, which are re-plotted from Figure 4.
Table 2.
ED50 values and 95% confidence limits for rimonabant, alone and in combination with Δ9-THC, anandamide and URB 597, in Δ9-THC-treated (1 mg·kg−112 h−1) monkeys discriminating rimonabant (1 mg·kg−1 i.v.)
| Drug | ED50 value (95% CL) in mg·kg−1 | Potency ratio (95% CL)† |
|---|---|---|
| Rimonabant (cumulative dosing) | 0.35 (0.30–0.40) | |
| +Δ9-THC (0.32 mg·kg−1) | 0.52 (0.25–1.1) | 1.5 (0.9–2.7) |
| +Δ9-THC (1 mg·kg−1) | 1.3 (0.89–1.9)* | 3.7 (2.5–5.7) |
| +Δ9-THC (3.2 mg·kg−1) | 3.4 (2.3–5.0)* | 9.7 (6.7–14) |
| +URB 597 (3.2 mg·kg−1) | 0.27 (0.18–0.44) | 0.8 (0.5–1.2) |
| Rimonabant (single bolus dosing) | 0.18 (0.07–0.46) | |
| +Anandamide (10 mg·kg−1) | 0.35 (0.22–0.55) | 1.9 (0.9–4.2) |
| +Anandamide (32 mg·kg−1) | 1.24 (0.66–2.3)* | 6.9 (2.6–17) |
| +Anandamide (10 mg·kg−1) +URB 597 (3.2 mg·kg−1) | 0.71 (0.38–1.3)* | 3.9 (1.6–9.7) |
Significant decrease in potency.
Potency ratios and 95% confidence limits (CL) are the ED50 value of rimonabant in combination with test drug divided by the control ED50 value of rimonabant.
Δ9-THC (0.32–3.2 mg·kg−1 i.v. in addition to 1 mg·kg−1 every 12 h s.c.) produced no greater than 1% responding on the rimonabant lever and induced dose-dependent, rightward shifts in the rimonabant dose–response curve for discriminative stimulus effects (Figure 4, top left). Doses of 1 and 3.2 mg·kg−1Δ9-THC significantly increased the ED50 value of rimonabant 3.7- and 9.7-fold, respectively (Table 2). Anandamide (10 and 32 mg·kg−1) produced a maximum of 12% responding on the rimonabant lever and dose-dependently attenuated the rimonabant discriminative stimulus (Figure 4, top right). The ED50 value of rimonabant in combination with 32 mg·kg−1 of anandamide was significantly greater (i.e. 6.9-fold) than the control ED50 value of rimonabant (Table 2).
URB 597 (3.2 mg·kg−1) produced 1% responding on the rimonabant lever and did not modify the rimonabant dose–response curve (Figure 5, top left), as evidenced by ED50 values that were not significantly different from each other (Table 2). When URB 597 (3.2 mg·kg−1) was combined with anandamide (10 mg·kg−1), the ED50 value of rimonabant was increased significantly (i.e. 3.9-fold) (Figure 5, top right). The ED50 value of rimonabant determined in the presence of URB 597 (3.2 mg·kg−1) in combination with anandamide (10 mg·kg−1) was not significantly different from the ED50 values of rimonabant determined in the presence of either dose (10 or 32 mg·kg−1) of anandamide alone (P > 0.05).
Absolute rate of responding for individual monkeys was 1.86, 2.07, 2.36, 2.43 and 2.80 responses s−1. When administered in cumulative doses, rimonabant dose-dependently decreased the rate of responding (Figures 4 and 5, bottom left); 0.32 and 1 mg·kg−1 of rimonabant decreased response rate significantly to 73% and 61% of the vehicle control, respectively (F3,9 = 10.09, P < 0.01). In contrast, response rate was not significantly decreased by rimonabant when single, bolus doses (0.1–1 mg·kg−1) were administered per test session (Figures 4 and 5, bottom right) (P > 0.05). Δ9-THC (0.32–3.2 mg·kg−1) did not significantly modify response rate. Rimonabant decreased response rate in the presence of the larger doses (1 and 3.2 mg·kg−1) of Δ9-THC, whereas rate-decreasing effects were not observed when rimonabant was combined with 0.32 mg·kg−1Δ9-THC (Figure 4, bottom left). Anandamide dose-dependently decreased response rate to 26% of control at 32 mg·kg−1 (P < 0.05) (Figure 4, bottom right, V). The rate-decreasing effects of anandamide (32 mg·kg−1) were dose-dependently antagonized by rimonabant, with significant attenuation obtained with 3.2 mg·kg−1 rimonabant (P < 0.05). Rate of responding was not significantly modified by anandamide (10 mg·kg−1) in combination with rimonabant (Figure 4, bottom right), URB 597 (3.2 mg·kg−1) alone or in combination with rimonabant or when all three drugs were combined (P > 0.05) (Figure 5, bottom).
Figure 6 shows the magnitude of the shift in the rimonabant dose–response curve expressed as a function of dose of Δ9-THC and anandamide, calculated for individual monkeys and expressed as an average (±SEM). The slopes of the lines were not significantly different from each other (P > 0.05), and the dose of each agonist producing a twofold shift in the rimonabant dose–response curve was estimated to be 0.31 mg·kg−1 for Δ9-THC and 8.7 mg·kg−1 for anandamide. When compared with the ED50 in substituting for the Δ9-THC discriminative stimulus, 0.039 mg·kg−1 for Δ9-THC and 1.7 mg·kg−1 for anandamide using parameters identical to those used to examine the effects of anandamide in combination with rimonabant (i.e. single-cycle tests; McMahon, 2009); the potency was decreased 7.9- and 5.1-fold, respectively. When combined with URB 597 at the dose (3.2 mg·kg−1) producing 32-fold enhancement of anandamide in monkeys discriminating Δ9-THC from vehicle, the potency of anandamide in Δ9-THC-treated monkeys was increased 1.6-fold although this effect was not significant (Figure 6).
Figure 6.
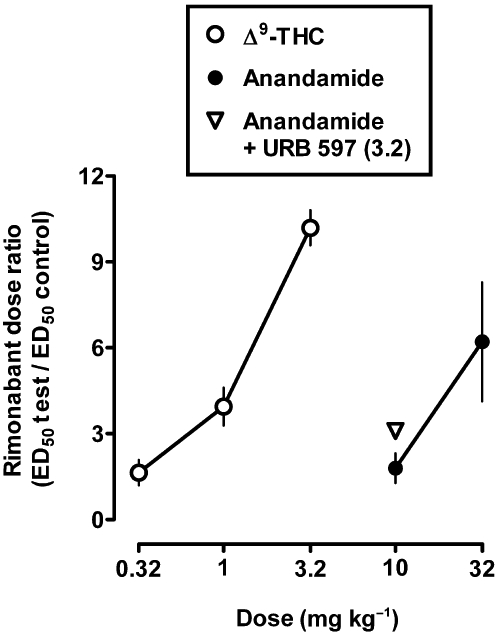
Magnitude of rightward shift in the rimonabant dose-response function expressed as a function of dose of Δ9-THC, anandamide alone and anandamide plus URB 597. Abscissa: dose in mg·kg−1 body weight. Ordinate: mean (±SEM) rightward shift in the rimonabant dose-response function, calculated as rimonabant ED50 following pretreatment with a cannabinoid agonist divided by the control rimonabant ED50.
Discussion
By increasing brain anandamide levels, URB 597 might be expected to enhance the behavioural effects of anandamide. In rhesus monkeys discriminating Δ9-THC from vehicle, URB 597 markedly enhanced (i.e. produced synergistic effects with) and extended the duration of action of anandamide; however, by itself, URB 597 did not share effects with exogenous anandamide. Although URB 597 failed to modify the effects of Δ9-THC, there was a tendency for URB 597 to attenuate the Δ9-THC-discriminative stimulus, consistent with a tendency for the combined effects of Δ9-THC and anandamide to be infra-additive, especially at relatively low levels of effect. Anandamide attenuated the discriminative stimulus effects of rimonabant in Δ9-THC-treated monkeys (i.e. Δ9-THC withdrawal). In contrast, URB 597 did not attenuate Δ9-THC withdrawal and did not significantly enhance the withdrawal-attenuating effects of anandamide. Collectively, these results suggest that inhibition of endogenous anandamide metabolism in primate brain does not mimic the behavioural effects obtained with exogenous anandamide administration.
URB 597 produced dose-dependent, parallel, leftward shifts in the anandamide dose–response curve and increased the duration of action of anandamide, consistent with inhibition of fatty acid amide hydrolase-mediated metabolism of anandamide in rhesus monkeys. The maximum shift (32-fold) in the anandamide dose–response curve was similar to the leftward shifts of the dose–response curve for the inhibitory effect of anandamide on electrically-evoked contractions of smooth muscle (Makwana et al., 2010); those shifts were 20- and 49-fold for rat and guinea-pig, respectively, at a concentration of URB 597 that was presumed to fully saturate fatty acid amide hydrolase. Whether complete inactivation of fatty acid amide hydrolase in rhesus monkeys was achieved (i.e. greater than a 32-fold leftward shift could be obtained) is not known due to limitations in solubility and the large injection volumes required for doses of URB 597 larger than 3.2 mg·kg−1. Despite the marked enhancement of the effects of anandamide, URB 597 alone did not substitute for or modify the effects of Δ9-THC. The failure of URB 597 to modify the effects of Δ9-THC is consistent with the metabolism of Δ9-THC by enzymes other than fatty acid amide hydrolase (Grotenhermen, 2003). That anandamide and Δ9-THC tended to have infra-additive effects might suggest that a sufficient increase in endogenous anandamide (i.e. URB 597) in primate brain would actually attenuate, rather than enhance, the effects of Δ9-THC.
Abrupt discontinuation of frequent marijuana use can result in a withdrawal syndrome, the magnitude of which positively correlates with resumption of use (Chung et al., 2008; Cornelius et al., 2008). Fatty acid amide hydrolase inhibitors have been proposed as treatments for marijuana withdrawal. However, URB 597 did not modify the discriminative stimulus effects of rimonabant in Δ9-THC-treated monkeys (i.e. Δ9-THC withdrawal). Failure of URB 597 to attenuate Δ9-THC withdrawal was not due to an inability of anandamide to attenuate Δ9-THC withdrawal; rather, anandamide produced dose-dependent, rightward shifts in the rimonabant dose–effect curve. A previous study demonstrated that URB 597 attenuated the directly observable signs of Δ9-THC withdrawal in mice (Schlosburg et al., 2009). For other classes of drug (e.g. opioids), discriminative stimulus effects and directly observable signs of withdrawal differ qualitatively, inasmuch as a drug that attenuates one type of sign does not always attenuate the other (McMahon et al., 2004; Sell et al., 2005). Similar qualitative differences and/or other factors (e.g. species) could be responsible for attenuation of Δ9-THC withdrawal in some studies and not others.
URB 597 did not significantly enhance the ability of anandamide to attenuate Δ9-THC withdrawal, in contrast to the marked enhancement of anandamide effects in monkeys discriminating Δ9-THC from vehicle. This could be related to a difference between the two discrimination assays in their sensitivity to CB1 receptor agonism. The potency of Δ9-THC and anandamide in the rimonabant discrimination assay was estimated from the dose producing a twofold rightward shift in the rimonabant dose–response curve. Although relative potency was similar in the two procedures (compare Figure 1, top and Figure 6), Δ9-THC and anandamide were eight- and fivefold less potent, respectively, in the rimonabant discrimination assay as compared with their ED50 value in substituting for the Δ9-THC discriminative stimulus. This difference in potency could reflect greater tolerance and dependence in the rimonabant discrimination assay as compared with the Δ9-THC discrimination assay. Based on previous studies in rodents, tolerance to Δ9-THC is accompanied by increased brain anandamide (Di Marzo et al., 2000), which, in turn, is consistent with a decrease in metabolism, perhaps reflecting a decrease in fatty acid amide hydrolase (Cravatt et al., 2001; Castelli et al., 2007). Loss of fatty acid amide hydrolase is a parsimonious explanation for loss of sensitivity to the anandamide-enhancing effects of URB 597. However, the corresponding increase in anandamide would potentially offset a loss of sensitivity to URB 597. Further studies are required to explore the mechanisms responsible for this loss of sensitivity to the combined effects of anandamide and URB 597 in Δ9-THC-treated animals.
Anandamide, in contrast to some other neurotransmitters, enters the brain following systemic administration (Willoughby et al., 1997) and can produce CB1 receptor-mediated behavioural effects. Although the behavioural effects of ligands that cross the blood–brain barrier can be studied with systemic routes of administration (e.g. i.v.), it cannot be assumed that this approach is predictive of the normal physiology of an endogenous ligand. Exogenous administration could result in greater concentrations and more widespread distribution in brain as compared with the endogenous ligand, resulting in both quantitative and qualitative differences in effect. The failure of URB 597 to share behavioural effects with exogenously administered anandamide in rhesus monkeys, even up to doses that produced a 32-fold enhancement of anandamide levels, strongly suggests that there is a limited amount and/or distribution of endogenous anandamide in brain. There is consensus among different studies in this regard, with URB 597 failing to mimic the ability of anandamide to increase nucleus accumbens dopamine in rats (Solinas et al., 2006) or to maintain self-administration behaviour in squirrel monkeys (Justinova et al., 2008).
In summary, URB 597 markedly enhanced the behavioural effects of anandamide, but by itself did not share effects with anandamide in rhesus monkeys discriminating Δ9-THC from vehicle. In Δ9-THC-dependent monkeys, URB 597 neither attenuated Δ9-THC withdrawal nor enhanced the effects of anandamide to attenuate Δ9-THC withdrawal, suggesting that URB 597 has limited utility as a treatment for marijuana dependence and withdrawal and, further, that Δ9-THC treatment decreases fatty acid amide hydrolase and the effectiveness of fatty acid amide hydrolase inhibitors. The numerous behavioural effects of Δ9-THC are probably due to widespread activation of brain cannabinoid receptors. That fatty acid amide hydrolase inhibition does not mimic all of the behavioural effects of direct-acting cannabinoid receptor agonists could be an advantage inasmuch as non-preferred effects (abuse liability and the ‘high’ associated with marijuana use) are absent. However, the extent to which fatty acid amide hydrolase inhibition produces therapeutic effects remains to be established.
Acknowledgments
The authors thank Dr M. Leland for assistance with surgical procedures; Drs A. Giuffrida and A. Seillier for assistance with synthesis and GC/MS analysis of anandamide; Dr W. Koek for assistance with isobolographic analysis; and D. Aguirre, W. Holbein, C. Rock and D. Schulze for assistance with behavioural experiments. This research was supported by grants from the U.S. Public Health Service, National Institutes of Health, National Institute on Drug Abuse [R01-DA19222 and R01-DA26781]
Glossary
Abbreviations
- Δ9-THC
Δ9-tetrahydrocannabinol
- CB
cannabinoid
- FR
fixed ratio
- URB
597, cyclohexylcarbamic acid 3′-carbamoylbiphenyl-3-yl ester
Conflict of interest
None.
References
- Adams IB, Compton DR, Martin BR. Assessment of anandamide interaction with the cannabinoid brain receptor: SR 141716A antagonism studies in mice and autoradiographic analysis of receptor binding in rat brain. J Pharmacol Exp Ther. 1998;284:1209–1217. [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edition. Br J Pharmacol. 2009;158:S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli MP, Paola Piras A, D'Agostino A, Pibiri F, Perra S, Gessa GL, et al. Dysregulation of the endogenous cannabinoid system in adult rats prenatally treated with the cannabinoid agonist WIN 55,212-2. Eur J Pharmacol. 2007;573:11–19. doi: 10.1016/j.ejphar.2007.06.047. [DOI] [PubMed] [Google Scholar]
- Childers SR. Activation of G-proteins in brain by endogenous and exogenous cannabinoids. AAPS J. 2006;8:E112–E117. doi: 10.1208/aapsj080113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung T, Martin CS, Cornelius JR, Clark DB. Cannabis withdrawal predicts severity of cannabis involvement at 1-year follow-up among treated adolescents. Addiction. 2008;103:787–799. doi: 10.1111/j.1360-0443.2008.02158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapper JR, Mangieri RA, Piomelli D. The endocannabinoid system as a target for the treatment of cannabis dependence. Neuropharmacology. 2009;56:235–243. doi: 10.1016/j.neuropharm.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton DR, Aceto MD, Lowe J, Martin BR. In vivo characterization of a specific cannabinoid receptor antagonist (SR141716A): inhibition of Δ9-tetrahydrocannabinol-induced responses and apparent agonist activity. J Pharmacol Exp Ther. 1996;277:586–594. [PubMed] [Google Scholar]
- Cornelius JR, Chung T, Martin C, Wood DS, Clark DB. Cannabis withdrawal is common among treatment-seeking adolescents with cannabis dependence and major depression, and is associated with rapid relapse to dependence. Addict Behav. 2008;33:1500–1505. doi: 10.1016/j.addbeh.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, et al. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci USA. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmani NA. Δ9-tetrahydrocannabinol differentially suppresses cisplatin-induced emesis and indices of motor function via cannabinoid CB1 receptors in the least shrew. Pharmacol Biochem Behav. 2001;69:239–249. doi: 10.1016/s0091-3057(01)00531-7. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Berrendero F, Bisogno T, González S, Cavaliere P, Romero J, et al. Enhancement of anandamide formation in the limbic forebrain and reduction of endocannabinoid contents in the striatum of Δ9-tetrahydrocannabinol-tolerant rats. J Neurochem. 2000;74:1627–1635. doi: 10.1046/j.1471-4159.2000.0741627.x. [DOI] [PubMed] [Google Scholar]
- Fegley D, Gaetani S, Duranti A, Tontini A, Mor M, Tarzia G, et al. Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3′-carbamoyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. J Pharmacol Exp Ther. 2005;313:352–358. doi: 10.1124/jpet.104.078980. [DOI] [PubMed] [Google Scholar]
- Gifford AN, Bruneus M, Gatley SJ, Lan R, Makriyannis A, Volkow ND. Large receptor reserve for cannabinoid actions in the central nervous system. J Pharmacol Exp Ther. 1999;288:478–483. [PubMed] [Google Scholar]
- Giuffrida A, Piomelli D. Purification and high-resolution analysis of anandamide and other fatty acylethanolamides. In: Laychock SG, Rubin RP, editors. Lipid Second Messengers. Boca Raton, FL: CRC; 1998a. pp. 113–133. [Google Scholar]
- Giuffrida A, Piomelli D. Isotope dilution GC/MS determination of anandamide and other fatty acylethanolamides in rat blood plasma. FEBS Lett. 1998b;422:373–376. doi: 10.1016/s0014-5793(98)00046-5. [DOI] [PubMed] [Google Scholar]
- Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet. 2003;42:327–360. doi: 10.2165/00003088-200342040-00003. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järbe TU, Lamb RJ, Lin S, Makriyannis A. (R)-methanandamide and Δ9-THC as discriminative stimuli in rats: tests with the cannabinoid antagonist SR-141716 and the endogenous ligand anandamide. Psychopharmacology. 2001;156:369–380. doi: 10.1007/s002130100730. [DOI] [PubMed] [Google Scholar]
- Jhaveri MD, Richardson D, Chapman V. Endocannabinoid metabolism and uptake: novel targets for neuropathic and inflammatory pain. Br J Pharmacol. 2007;152:624–632. doi: 10.1038/sj.bjp.0707433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston TH, Huot P, Fox SH, Wakefield JD, Sykes KA, Bartolini WP, et al. Fatty acid amide hydrolase (FAAH) inhibition reduces L-DOPA-induced hyperactivity in the MPTP-lesioned non-human primate model of Parkinson's disease. J Pharmacol Exp Ther. 2011;336:423–430. doi: 10.1124/jpet.110.169532. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Mangieri RA, Bortolato M, Chefer SI, Mukhin AG, Clapper JR, et al. Fatty acid amide hydrolase inhibition heightens anandamide signaling without producing reinforcing effects in primates. Biol Psychiatry. 2008;64:930–937. doi: 10.1016/j.biopsych.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valiño F, Duranti A, Tontini A, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Pharmacologic Analysis of Drug-receptor Interaction. Philadelphia, PA: Lippincott-Raven; 1997. [Google Scholar]
- Lichtman AH, Martin BR. Δ9-tetrahydrocannabinol impairs spatial memory through a cannabinoid receptor mechanism. Psychopharmacology. 1996;126:125–131. doi: 10.1007/BF02246347. [DOI] [PubMed] [Google Scholar]
- Makwana R, Molleman A, Parsons ME. Evidence for both inverse agonism at the cannabinoid CB1 receptor and the lack of an endogenous cannabinoid tone in the rat and guinea-pig isolated ileum myenteric plexus-longitudinal muscle preparation. Br J Pharmacol. 2010;160:615–626. doi: 10.1111/j.1476-5381.2010.00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon LR. Apparent affinity estimates of rimonabant in combination with anandamide and chemical analogs of anandamide in rhesus monkeys discriminating Δ9-tetrahydrocannabinol. Psychopharmacology. 2009;203:219–228. doi: 10.1007/s00213-008-1230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon LR. Chronic Δ9-tetrahydrocannabinol treatment in rhesus monkeys: differential tolerance and cross-tolerance among cannabinoids. Br J Pharmacol. 2011;162:1060–1073. doi: 10.1111/j.1476-5381.2010.01116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon LR, Sell SL, France CP. Cocaine and other indirect-acting monoamine agonists differentially attenuate a naltrexone discriminative stimulus in morphine-treated rhesus monkeys. J Pharmacol Exp Ther. 2004;308:111–119. doi: 10.1124/jpet.103.058917. [DOI] [PubMed] [Google Scholar]
- Miller AM, Stella N. CB2 receptor-mediated migration of immune cells: it can go either way. Br J Pharmacol. 2008;153:299–308. doi: 10.1038/sj.bjp.0707523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu PS, Booker L, Cravatt BF, Lichtman AH. Synergy between enzyme inhibitors of fatty acid amide hydrolase and cyclooxygenase in visceral nociception. J Pharmacol Exp Ther. 2009;329:48–56. doi: 10.1124/jpet.108.143487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. Washington, DC: National Academies Press; 2003. [PubMed] [Google Scholar]
- Panlilio LV, Justinova Z, Goldberg SR. Animal models of cannabinoid reward. Br J Pharmacol. 2010;160:499–510. doi: 10.1111/j.1476-5381.2010.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev. 2010;62:588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE, Carlson BL, Ramesh D, Abdullah RA, Long JZ, Cravatt BF, et al. Inhibitors of endocannabinoid-metabolizing enzymes reduce precipitated withdrawal responses in THC-dependent mice. AAPS J. 2009;11:342–352. doi: 10.1208/s12248-009-9110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell SL, McMahon LR, Koek W, France CP. Monoaminergic drugs and directly observable signs of LAAM withdrawal in rhesus monkeys. Behav Pharmacol. 2005;16:53–58. doi: 10.1097/00008877-200502000-00006. [DOI] [PubMed] [Google Scholar]
- Sharir H, Abood ME. Pharmacological characterization of GPR55, a putative cannabinoid receptor. Pharmacol Ther. 2010;126:301–313. doi: 10.1016/j.pharmthera.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Justinova Z, Goldberg SR, Tanda G. Anandamide administration alone and after inhibition of fatty acid amide hydrolase (FAAH) increases dopamine levels in the nucleus accumbens shell in rats. J Neurochem. 2006;98:408–419. doi: 10.1111/j.1471-4159.2006.03880.x. [DOI] [PubMed] [Google Scholar]
- Solinas M, Tanda G, Justinova Z, Wertheim CE, Yasar S, Piomelli D, et al. The endogenous cannabinoid anandamide produces Δ9-tetrahydrocannabinol-like discriminative and neurochemical effects that are enhanced by inhibition of fatty acid amide hydrolase but not by inhibition of anandamide transport. J Pharmacol Exp Ther. 2007;321:370–380. doi: 10.1124/jpet.106.114124. [DOI] [PubMed] [Google Scholar]
- Stewart JL, McMahon LR. Rimonabant-induced Δ9-tetrahydrocannabinol withdrawal in rhesus monkeys: discriminative stimulus effects and other withdrawal signs. J Pharmacol Exp Ther. 2010;334:347–356. doi: 10.1124/jpet.110.168435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallarida RJ. Drug Synergism and Dose-effect Data Analysis. Boca Raton, FL: Chapman and Hall/CRC; 2000. pp. 44–50. [Google Scholar]
- Tallarida RJ. An overview of drug combination analysis with isobolograms. J Pharmacol Exp Ther. 2006;319:1–7. doi: 10.1124/jpet.106.104117. [DOI] [PubMed] [Google Scholar]
- Wiley J, Balster R, Martin B. Discriminative stimulus effects of anandamide in rats. Eur J Pharmacol. 1995;276:49–54. doi: 10.1016/0014-2999(95)00010-i. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Golden KM, Ryan WJ, Balster RL, Razdan RK, Martin BR. Evaluation of cannabimimetic discriminative stimulus effects of anandamide and methylated fluoroanandamide in rhesus monkeys. Pharmacol Biochem Behav. 1997;58:1139–1143. doi: 10.1016/s0091-3057(97)00327-4. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Razdan RK, Martin BR. Evaluation of the role of the arachidonic acid cascade in anandamide's in vivo effects in mice. Life Sci. 2006;80:24–35. doi: 10.1016/j.lfs.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Willoughby KA, Moore SF, Martin BR, Ellis EF. The biodisposition and metabolism of anandamide in mice. J Pharmacol Exp Ther. 1997;282:243–247. [PubMed] [Google Scholar]


