Abstract
BACKGROUND AND PURPOSE
NF-κB has been implicated as a therapeutic target for the treatment of rheumatoid arthritis. We previously synthesized a thiourea analogue, SPA0355, which suppressed NF-κB activity. Here we have assessed the anti-inflammatory and anti-arthritic effects of SPA0355.
EXPERIMENTAL APPROACH
We evaluated the effects of SPA0355 on human rheumatoid fibroblast-like synoviocytes in vitro and on collagen-induced arthritis (CIA) in mice in vivo.
KEY RESULTS
In vitro experiments demonstrated that SPA0355 suppressed chemokine production, matrix metalloproteinase secretion and cell proliferation induced by TNF-α in rheumatoid fibroblast-like synoviocytes. In addition, SPA0355 inhibited osteoclast differentiation induced by macrophage colony-stimulating factor and the receptor activator of NF-κB ligand, in bone marrow macrophages. Mice with CIA that were pretreated with SPA0355 had a lower cumulative disease incidence and severity of arthritis, based on hind paw thickness, radiological and histopathological findings, and inflammatory cytokine levels, than mice treated with vehicle. Mice treated with SPA0355, after the onset of CIA, also showed significantly decreased disease incidence and joint oedema. The in vitro and in vivo protective effects of SPA0355 were mediated by inhibition of the NF-κB signalling pathway.
CONCLUSION AND IMPLICATIONS
Taken together, these results suggested that using SPA0355 to block the NF-κB pathway in rheumatoid joints reduced both the inflammatory responses and tissue destruction. Therefore, SPA0355 may have therapeutic value in preventing or delaying joint destruction in patients with rheumatoid arthritis.
Keywords: NF-κB, thiourea, synoviocytes, CIA, RA, TNF-α, inflammation
Introduction
Rheumatoid arthritis (RA) is a chronic destructive disease of the joints that is characterized by proliferative synovitis, infiltration of inflammatory cells into synovial tissue and joint destruction (Smolen et al., 2007). One of the major transcriptional circuits implicated in joint inflammation is the NF-κB pathway (McInnes and Schett, 2007). Within the joints, fibroblast-like synoviocytes (FLS) and inflammatory cells produce proinflammatory cytokines such as IL-1β and TNF-α. These cytokines activate NF-κB and can also be induced by it, and this positive regulatory loop leads to the expression of mediators of inflammation. Examples of proinflammatory mediators include cytokines, chemokines such as CCL5 (RANTES) and CXCL5 (ENA-78), and adhesion molecules (Koch, 2005; McInnes and Schett, 2007). NF-κB activation in FLS also contributes to the pathogenesis of RA by activating the transcription of matrix metalloproteinases (MMPs) (Vincenti et al., 1998). The MMP family includes over 20 members that differentially mediate the degradation of each component of the extracellular matrix, including MMP-1 and MMP-3, thought to be the major enzymes involved in tissue destruction (Pretzel et al., 2009; Rooney et al., 2010).
NF-κB activity is increased in synovial tissue from RA patients (Handel et al., 1995) and in mice with collagen-induced arthritis (CIA) (Hah et al., 2010) compared with synovium from normal controls. Mice that lack the p50 subunit of NF-κB do not show joint destruction (Campbell et al., 2000). Activation of NF-κB is inhibited by several antirheumatic drugs currently in clinical use, including glucocorticoids (Auphan et al., 1995), aspirin (Yin et al., 1998), gold salts (Jeon et al., 2000), leflunomide (Manna et al., 2000) and methotrexate (Majumdar and Aggarwal, 2001). In addition, intraarticular injection of NF-κB decoy oligonucleotides or adenoviral gene transfer of dominant negative IκB kinaseβ (IKKβ) suppresses CIA in mice (Tomita et al., 1999). We recently demonstrated that adenoviral gene transfer of A20, a universal NF-κB inhibitor, attenuated CIA in mice (Hah et al., 2010). Furthermore, in vitro overexpression of IκBα super-repressor in FLS from patients with RA (RA-FLS) inhibits the expression of proinflammatory mediators (Lee et al., 2009). Taken together, these studies confirm the importance of NF-κB activation in the development of RA.
We have synthesized a series of thiourea analogues and evaluated them with respect to their NF-κB-suppressing activities (Cheon et al., 2010). These molecules were composed of a thiourea moiety as a core structure, with heterocycles such as indole, phenoxazine or carbazole. Among them, SPA0355 (Fig 1A) was found to be the most potent NF-κB suppressor in the macrophage cell line RAW264.7, stimulated with LPS. These observations suggested that SPA0355 could be used to treat NF-κB-related inflammatory diseases including RA. Here we investigated the effects of SPA0355 on cultured human RA-FLS and mice with CIA. We found that SPA0355 was effective at suppressing the expression of proinflammatory mediators in vitro. Furthermore, prophylactic and therapeutic administration of SPA0355 significantly reduced the severity of CIA in mice.
Figure 1.
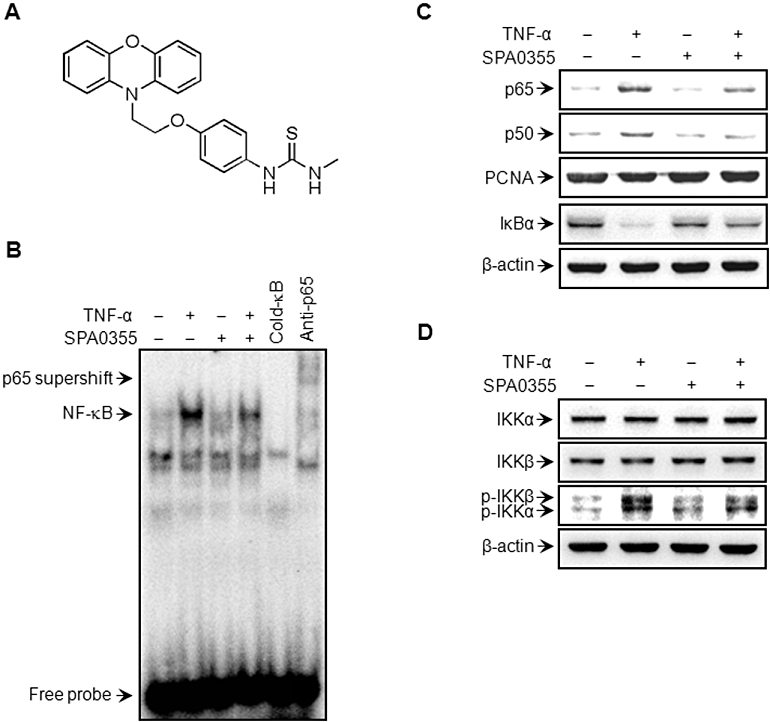
Suppression of TNF-α-induced NF-κB activation in human RA-FLS by SPA0355. (A) Chemical structure of SPA0355. FLS (2 × 106 cells) from RA patients were pretreated with 10 µM SPA0355 for 1 h and then treated with TNF-α (10 ng·mL−1). Following 3 h of incubation, binding of NF-κB to DNA was analysed by EMSA (B), and the translocation of p65 and p50 to the nucleus and IκBα degradation in the cytoplasm were determined by Western blotting (C). β-Actin and PCNA were used as loading controls for cytoplasmic and nuclear proteins respectively. Protein levels and phosphorylation of IKKα and IKKβ were determined by Western blotting (D). Results are representative of three independent experiments.
Methods
Isolation and culture of RA-FLS from patients
Human tissue was obtained with informed consent from all patients, and the study protocol was approved by the Chonbuk National University Hospital Ethics Committee. RA-FLS were isolated from primary synovial tissue obtained from 12 patients with RA who met the revised criteria of the American College of Rheumatology and had undergone total joint replacement surgery or synovectomy, as previously described (Lee et al., 2008). Cells were used at passages four to eight, at which time they consisted of a homogeneous population. Cells were grown at 37°C under a humidified atmosphere containing 5% CO2, in high glucose-containing Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 units·mL−1 penicillin, 100 µg·mL−1 streptomycin and 2.5 µg·mL−1 amphotericin B. All treatments were performed in serum-free medium.
Animals
All animal care and experimental procedures in this study complied with the protocol approved by the Institutional Animal Care and Use Committee at Chonbuk National University. Pathogen-free male DBA/1 mice were purchased from Orient Bio (Seoul, Korea). Mice were housed in a laminar flow cabinet with a 12 h light/dark cycle and maintained on standard laboratory chow ad libitum.
Preparation of SPA0355
Preparation of SPA0355 was performed as previously described (Cheon et al., 2010). Briefly, N-hydroxyethylation of phenoxazine and mesylation of the resulting alcohol gave the corresponding mesylate. The alkylation of p-nitrophenol with the mesylate in the presence of base, reduction of the obtained nitro compound to amine and subsequent condensation of the amine with methyl isothiocyanate provided SPA0355. The product was purified by column chromatography on SiO2 to afford pure SPA0355 as a pale yellow solid. The structure and purity of SPA0355 were identified by 1H NMR, 13C NMR, FT-IR and HRMS. The spectral data for SPA0355 were as follows: IR (neat, cm−1) 3387, 3211, 1540, 1508, 1490, 1464, 1376, 1273, 1042; 1H NMR (400 MHz, CDCl3) δ 7.78 (br s, 1H), 7.07 (d, 2H, J = 8.8 Hz), 6.85 (d, 2H, J = 8.8 Hz), 6.76 (td, 2H, J = 7.6, 1.6 Hz), 6.64 (td, 2H, J = 7.6, 1.6 Hz), 6.59–6.00 (m, 4H), 5.83 (br s, 1H), 4.17 (t, 2H, J = 6.4 Hz), 3.94 (t, 2H, J = 6.4 Hz), 3.06 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 182.3, 158.0, 145.0, 133.2, 129.2, 128.1, 123.8, 121.6, 116.1, 115.8, 111.9, 64.3, 44.0, 32.3; MS (FAB) m/z 392.14 [M + H]+; HRMS (FAB) Calculated for C22H22N3O2S [M + H]+ 392.1433, Found 392.1400. Stock solutions of SPA0355 were prepared in dimethyl sulphoxide at 50 mM.
Bromo-2-deoxyuridine (BrdU)-labelling cell proliferation assay
A cell proliferation elisa (BrdU kit; Amersham Biosciences, Piscataway, NJ, USA) was used to measure the incorporation of BrdU during DNA synthesis, following the manufacturer's protocols. Briefly, after treatment with TNF-α for 72 h, BrdU (10 µM) was added to the culture medium for 2 h, the BrdU-labelled cells were fixed, and the DNA was denatured in fixative solution for 30 min at room temperature. The cells were then incubated with peroxidase-conjugated anti-BrdU antibody for 2 h at room temperature, followed by washing three times with washing solution. The immune complex was detected using a 3,3′,5,5′-tetramethylbenzidine substrate reaction and absorbance was measured at 405 nm.
Osteoclast differentiation
Bone marrow cells from 5-week-old ICR mice were isolated by flushing the long bones with α-minimum essential medium and suspended in α-minimum essential medium supplemented with 10% fetal bovine serum. Cells were plated and cultured overnight in the presence of macrophage colony-stimulating factor (M-CSF, 10 ng·mL−1). Non-adherent cells were collected and cultured for 3 days in the presence of M-CSF (30 ng·mL−1). Floating cells were removed and adherent cells were used as bone marrow-derived macrophages (BMMs). BMMs were seeded at 3.5 × 104 cells per well in 48-well plates and cultured with M-CSF (30 ng·mL−1) and the receptor activator of NF-κB ligand (RANKL, 50 ng·mL−1) for 4 days in the presence or absence of SPA0355. Osteoclasts were visualized by staining for tartrate-resistant acid phosphatase (TRAP) activity. TRAP-positive cells were counted as osteoclasts.
Collagen-induced arthritis
Male DBA/1 mice (7–9 weeks old) were immunized with 150 µg of bovine type II collagen emulsified with an equal volume of complete Freund's adjuvant (Chondrex, Redmond, WA, USA). The day of the first immunization was defined as day 0. The mice were then boosted by an equal amount of bovine type II collagen emulsified in Freund's incomplete adjuvant on day 21. A single i.p. injection of SPA0355 (10 mg·kg−1 body weight in 100 µL of corn oil) or vehicle was administered on day 20. To examine the therapeutic effect, arthritic mice (mean arthritis score of 3 on day 27) were injected with the same doses of SPA0355 on days 27 and 30. Clinical arthritis scores were evaluated using a scale of 0–3 for each limb: grade 0, no swelling; grade 1, slight swelling and erythema; grade 2, pronounced swelling; grade 3, severe swelling and/or joint rigidity. Hind paw thickness was measured with an electric caliper placed across the ankle joint at the widest point. The increase in diameter of the arthritic ankle at specific time points over that on day 0 was defined as the paw thickness index, and this value is presented as a percentage. On day 45, the mice were killed and synovial tissues were harvested from each animal for end-point histology.
Chemokine, MMP and cytokine analyses
Following TNF-α treatment, levels of the chemokines CCL5 (RANTES) and CXCL5 (ENA-78) and of MMP-3 in the cell culture supernatants were determined using Quantikine elisa kits (R&D Systems, Minneapolis, MN, USA). MMP-1 was quantified by a fluorescent assay using the Fluorokine E human active MMP-1 fluorescent assay kit (R&D Systems) according to the manufacturer's protocol. For determination of cytokine levels in mice with CIA, serum and ankle joint samples were obtained on day 45. Protein extracts of ankle joints were prepared by homogenization of joints (50 mg tissue·mL−1) in lysis buffer (T-PER Tissue protein extraction reagent; Thermo) containing a proteinase inhibitor cocktail. TNF-α, IL-1β, IL-17 (all from Invitrogen, Carlsbad, CA, USA), IL-10 (BD Biosciences, Franklin Lakes, NJ, USA) and RANKL (PeproTech, Rocky Hill, NJ, USA) were measured by specific elisa kits. Serum alanine aminotransferase, aspartate aminotransferase, creatine phosphokinase, lactate dehydrogenase (LDH) and creatinine levels were measured using a commercial kit from Asan Pharm (Seoul, Korea).
Preparation of cytoplasmic and nuclear protein extracts
The RA-FLS (2 × 106 cells) or synovial tissue pieces (1 mm3) were washed twice by centrifugation in PBS, and then pelleted at 500× g for 5 min. The pellets were then lysed in CytoBuster protein extraction buffer (Novagen, Madison, WI, USA). Cytoplasmic and nuclear extracts were prepared from cells or tissues using the NE-PER nuclear and cytoplasmic extraction reagents (Pierce, Rockford, IL, USA).
EMSA
Nuclear extracts prepared from the RA-FLS or from joint tissues were incubated with a proteinase inhibitor cocktail (Thermo, San Diego, CA, USA) to inhibit endogenous protease activity. Oligonucleotides corresponding NF-κB and AP-1 sites (5′-CCGGTTAACAGAGGGGGCTTTCCGAG-3′ and 5′-CGCTTGATGAGTCAGCCGGAA-3′ respectively) were synthesized and used as a probe for a gel retardation assay. The two complementary strands were then annealed and labelled with α-32P-dCTP. Labelled oligonucleotides (10 000 counts·min−1), 10 µg of nuclear extracts and binding buffer (10 mM Tris-HCl, pH 7.6, 500 mM KCl, 10 mM EDTA, 50% glycerol, 100 ng poly[dI·dC], 1 mM dithiothreitol) were then incubated for 30 min at room temperature in a final volume of 20 µL. Next, the reaction mixtures were analysed by electrophoresis on 4% polyacrylamide gels in a 0.5 × Tris-borate buffer. The gels were dried and examined by autoradiography. The specificity of the DNA–protein interactions for NF-κB and AP-1 was confirmed by competition assays using a 50-fold excess of unlabelled oligonucleotide.
Western blot analysis
The RA-FLS (2 × 106 cells) or joint tissues were homogenized with protease and phosphatase inhibitors in protein extraction solution (Pro-Prep, Intron Biotechnology, Sungnam, Korea). The homogenates, which contained 30 µg of protein, were separated by 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis, and transferred to polyvinylidene difluoride membranes. The blot was probed with 1 µg·mL−1 of primary antibody against p50, p65, IκBα, PCNA, and β-actin (all from Santa Cruz Biotechnology, Santa Cruz, CA, USA), IKKα, IKKβ, phosphorylated-IKKα (p-IKKα), p-IKKβ, p38, p-p38, JNK, p-JNK, ERK, and p-ERK (all from Cell Signaling, Beverly, MA, USA), or MMP-1 and MMP-3 (both from R&D Systems). Horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG (Santa Cruz Biotechnology) was used as a secondary antibody.
Histopathological assessment
Joint tissues were fixed with 10% formalin, decalcified for 3 weeks in 10% EDTA, dehydrated and embedded in paraffin. Sections (5 µm) were stained with haematoxylin and eosin or Safranin O for light microscopy. To investigate osteoclast bone resorption activity in mice with CIA, sections were stained with a TRAP staining kit (Sigma, St. Louis, MO, USA). Histopathological assessment of arthritis was carried out, using a scoring system as follows: 0, normal; 1, minimal synovitis without cartilage and bone erosion; 2, synovitis with some marginal erosion but joint architecture maintained; 3, severe synovitis and erosion with loss of normal joint architecture. For inflammation, scores were as follows: 0, no infiltration; 1, mild infiltration; 2, moderate infiltration; 3, severe infiltration of inflammatory cells.
Radiographic evaluations
Plain radiographs of paws were obtained using a mammographic imager based on a direct detection flat panel array design (Mammomat NovationDR, Siemens Medical Solutions, Erlangen, Germany), using exposure settings of 30 kVp and 90 mA. All images were scored according to a previously described scoring system (Joosten et al., 1999). The degree of joint destruction and bone erosion was scored on a scale of 0–5, where 0, no damage; 1, minor bone destruction observed in one enlightened spot; 2, moderate changes, with 2–4 spots in one area; 3, marked changes with 2–4 spots in more areas; 4, severe erosions afflicting the joint; and 5, complete destruction of the joints.
Statistical analysis
Data are expressed as the mean ± SEM. Statistical comparisons were performed using one-way anova, followed by the Fisher's post hoc analysis. The significance of differences between groups was determined using Student's unpaired t-test. A P-value of less than 0.05 was considered significant.
Results
Effects of SPA0355 on TNF-α-induced NF-κB activity in RA-FLS
The chemical structure of SPA0355 (1-methyl-3-[4-(2-phenoxazin-10-ylethoxy)phenyl]thiourea) is presented in Figure 1A. To determine the effects of SPA0355 on RA, we first investigated whether SPA0355 could affect the NF-κB signalling pathway in cultured human RA-FLS. Nuclear extracts prepared from these cells, 3 h after TNF-α treatment, were used to analyse NF-κB-DNA binding activity and cytoplasmic IκBα degradation. The DNA-bound p65 subunit of NF-κB was resolved by supershift (Figure 1B, lane 6).
Binding activity of the p65 subunit to an NF-κB consensus sequence was increased in TNF-α-stimulated RA-FLS, compared with unstimulated cells (Figure 1B), and nuclear levels of p65 and p50 subunits were increased (Figure 1C). In contrast, nuclear extracts prepared from SPA0355-treated RA-FLS revealed markedly suppressed nuclear translocation and DNA binding of NF-κB.
We previously reported that IκBα, but not IκBβ, is the major participant in cytokine-induced NF-κB activation in RA-FLS (Lee et al., 2009). We therefore investigated IκBα protein levels in the cytoplasmic fraction following TNF-α treatment. TNF-α-treated RA-FLS showed decreased levels of IκBα protein in the cytoplasm due to IκBα degradation, compared with a similar fraction from unstimulated cells, but IκBα degradation was markedly suppressed by SPA0355 (Figure 1C).
Next we tested the effect of SPA0355 on IKK activation, which is required for IκB phosphorylation. SPA0355 had no effect on IKKα and IKKβ protein levels, but suppressed TNF-α-induced IKK activity, as shown by decreased levels of phosphorylated forms of IKKα and IKKβ (Figure 1D). Together, these results suggested that SPA0355 inhibited the degradation of IκBα by reducing IKK activities, thereby preventing subsequent NF-κB activation.
Effect of SPA0355 on TNF-α-induced activation of MMPs and chemokines production in RA-FLS
The effects of SPA0355 on TNF-α-induced MMP secretion were investigated by elisa. Incubation of RA-FLS with TNF-α resulted in a 3.4-fold increase in the production of MMP-1 and a 3.5-fold increase in the production of MMP-3 in culture supernatants (Figure 2A). However, pretreatment with SPA0355 significantly diminished TNF-α-mediated MMP-1 and MMP-3 production in a concentration-dependent manner. The effect of SPA0355 on TNF-α-induced MMP activation was further confirmed by Western blotting. As shown in Figure 2B, TNF-α treatment led to an increase in MMP-1 and MMP-3 protein levels in total cell lysates, whereas pretreatment with SPA0355 blocked the activity of TNF-α and resulted in a decrease in MMP-1 and MMP-3 levels.
Figure 2.
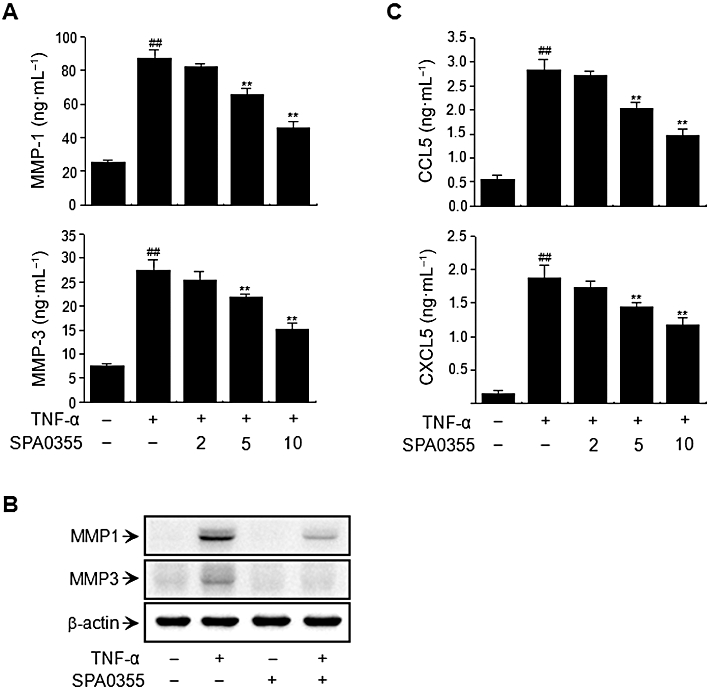
Inhibition of TNF-α-induced MMP and chemokine production by RA-FLS by SPA0355. FLS from RA patients were pretreated with 10 μM SPA0355 for 1 h and exposed to TNF-α (10 ng·mL−1) for 24 h. The presence of MMP-1 and MMP-3 (A) and CXCL5 and CCL5 (C) in the cell-free culture supernatants were then evaluated by elisa. The expression levels of MMP-1 and MMP-3 in the total cell lysates were evaluated by Western blotting (B). Values are the mean ± SEM of three independent experiments. ##P < 0.01 versus untreated control; **P < 0.01, significantly different from TNF-α.
To determine the effects of SPA0355 on chemokine production, RA-FLS were incubated for 24 h with TNF-α, at which time the levels of chemokines present in the culture supernatants were determined using an elisa kit. TNF-α treatment led to a 5.1-fold increase in the production of CCL5 and a 13.3-fold increase in the production of CXCL5 (Figure 2C). In contrast, pretreatment with SPA0355 significantly diminished TNF-α-mediated chemokine production in a concentration-dependent manner.
Suppression of synovial inflammation and joint destruction in mice with CIA by pretreatment with SPA0355
We next assessed the anti-inflammatory role of SPA0355 in mice with CIA. Vehicle-treated mice had an increased incidence of disease and developed severe swelling, erythema and joint rigidity of the hind paws (Figure 3A and B). In contrast, in mice treated with SPA0355, the incidence and severity of CIA were attenuated in a dose-dependent manner. Furthermore, we observed significant differences in the clinical scores in SPA0355-treated mice (Figure 3C). Hind paw swelling was measured and these results correlated with those of the clinical scores (Figure 3D). Consistent with in vitro results, SPA0355 treatment also resulted in a decrease in MMP-1 and MMP-3 levels in joint tissues (Figure 3E).
Figure 3.
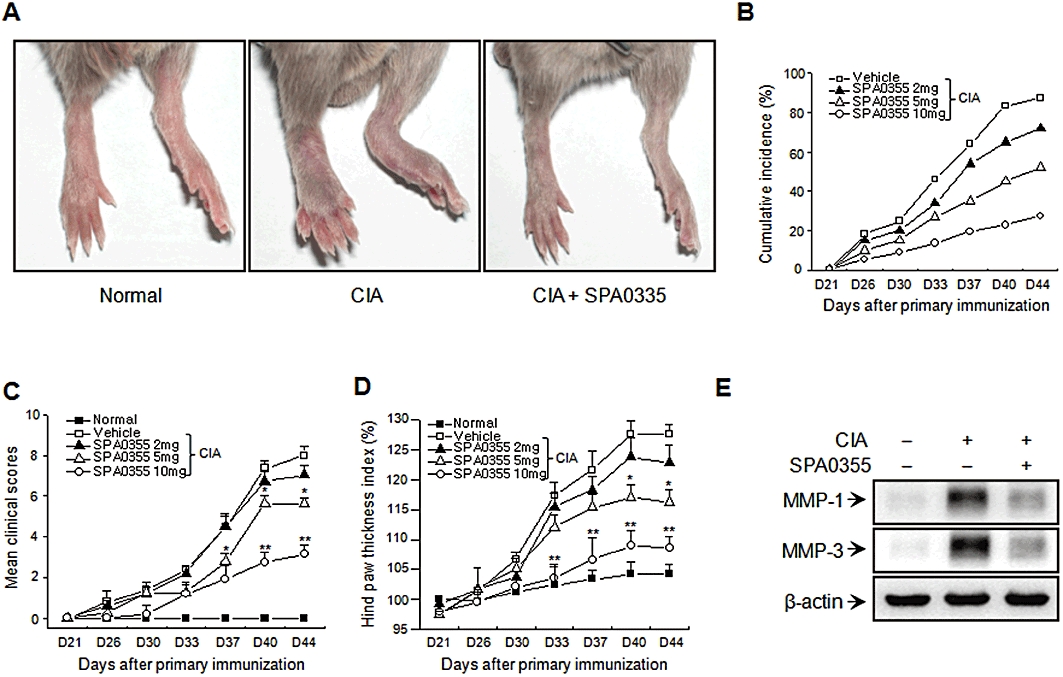
Prevention of CIA in mice pretreated with SPA0355. Mice were injected with vehicle or indicated concentrations of SPA0355 on day 20 (1 day before booster immunization on day 21). (A) Representative photographs of the hind paws of CIA mice on day 45. The cumulative incidence of arthritis (B), the mean clinical scores (C) and the hind paw thicknesses (D) were determined on the indicated days after the primary immunization. (E) The presence of MMP-1 and MMP-3 in the joint tissues was evaluated by Western blotting. Values in (B)–(D) are the mean ± SEM of three independent experiments (n = 8–9 mice per group). *P < 0.05, **P < 0.01, significantly different from untreated CIA.
Radiographs of hind paws of vehicle-treated mice showed changes typical of CIA, with articular destruction, joint displacement and irregular bony proliferation covering the entire ankle region (Figure 4A). However, radiographic findings in the hind paws and the mean radiologic scores showed markedly less bone destruction in SPA0355-treated mice (Figure 4A and B).
Figure 4.
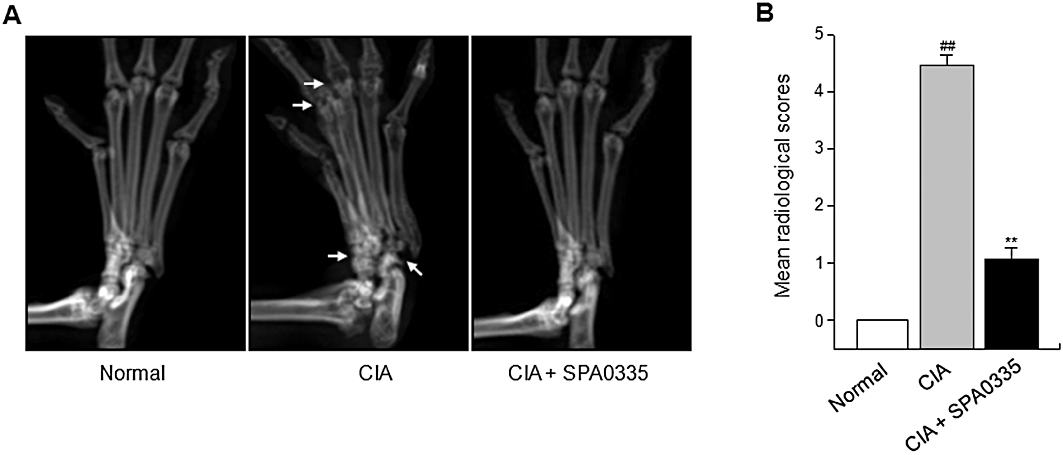
Reduced severity of radiological changes in mice with CIA and pretreated with SPA0355. Mice were injected with vehicle or SPA0355 (10 mg·kg−1) on day 20. (A) Representative radiographs of the hind paws of CIA mice on day 45. Note the enhanced bone erosion and joint damage (arrows). (B) Radiological scores were determined as described. Values are the mean ± SEM of three independent experiments (n = 8–9 mice per group). ##P < 0.01, significantly different from normal; **P < 0.01, significantly different from untreated CIA.
Histopathological assessment revealed synovial proliferation, cartilage damage, inflammatory cell infiltration and bone erosion in the ankle joints of vehicle-treated mice. In contrast, ankle joints from the SPA0355-treated mice showed a remarkable improvement in inflammation and joint destruction (Figure 5A and B).
Figure 5.
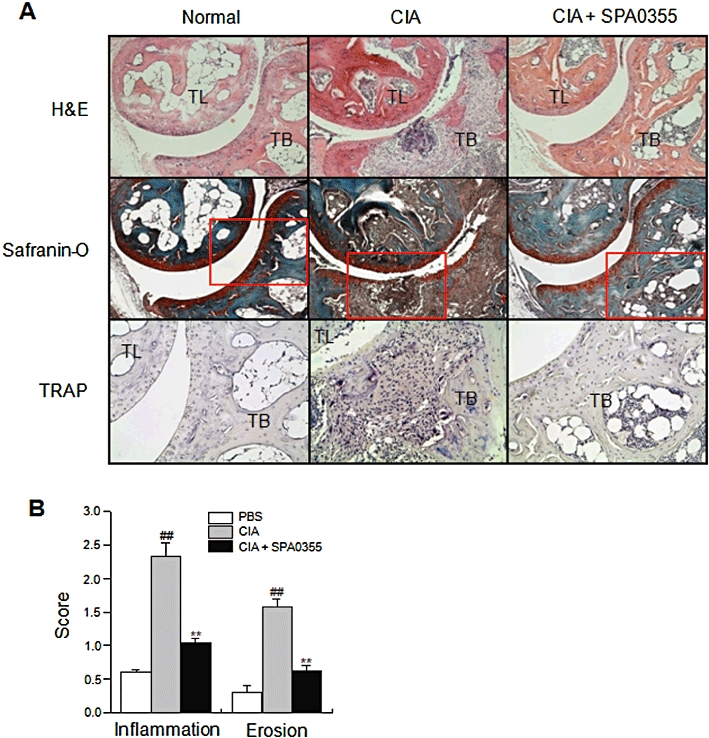
Reduced severity of histopathological changes in mice with CIA and pretreated with SPA0355. Mice were injected with vehicle or SPA0355 (10 mg·kg−1) on day 20. (A) Hind paws from mice with CIA were obtained on day 45, sectioned and stained with haematoxylin and eosin (H&E), Safranin O or TRAP. The boxed area in each Safranin O-stained section is shown at higher magnification in the TRAP-stained sections. TB, tibia; TL, talus. Photomicrographs are representative of three independent experiments. (Original magnification ×100 for H&E- and Safranin O-stained sections; ×200 for TRAP-stained sections.) (B) Inflammatory and lesion scores were determined as described in Methods. Values are the mean ± SEM of three independent experiments (n = 8–9 mice per group). ##P < 0.01, significantly different from normal; **P < 0.01, significantly different from untreated CIA.
Inhibition of osteoclast differentiation by SPA0355
Recent studies suggest that bone-resorbing osteoclasts in the synovium play an important role in bone destruction in RA (Boyle et al., 2003). We therefore stained the mouse joint tissues with TRAP. As shown in Figure 5A, there were more TRAP-positive cells in the joint tissues of vehicle-treated mice compared with the SPA0355-treated mice.
Osteoclasts were generated from mouse BMMs in the presence of M-CSF and RANKL to verify the effects of SPA0355 in osteoclastogenesis. The BMMs of the control group differentiated into mature TRAP-positive multinucleated osteoclasts while SPA0355 reduced the formation and numbers of TRAP-positive multinucleated cells in a concentration-dependent manner (Figure 6).
Figure 6.
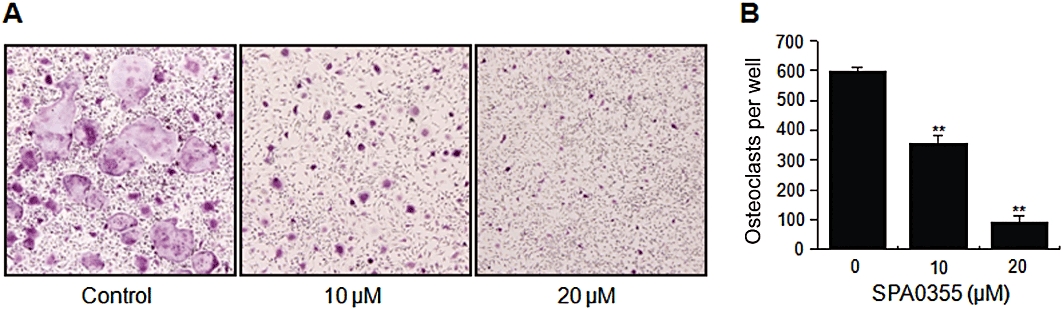
Effect of SPA0355 on RANKL-induced osteoclast differentiation. (A) BMMs were cultured for 4 days with M-CSF (30 ng·mL−1) and RANKL (50 ng·mL−1) in the presence of the indicated concentrations of SPA0355. Cells were fixed with 3.7% formalin, permeabilized with 0.1% Triton X-100 and stained with TRAP solution. (B) TRAP-positive cells were counted as osteoclasts. Each value represents the mean ± SEM of the three independent experiments. **P < 0.01, significantly different from untreated control.
Inhibition of NF-κB signal pathway in mice with CIA by pretreatment with SPA0355
To clarify the mechanism underlying the in vivo protective effect of SPA0355, we investigated the NF-κB signalling pathway in nuclear extracts prepared 3 days after booster injection. An increase in binding activity of nuclear extracts to a NF-κB consensus sequence (Figure 7A), nuclear translocation of NF-κB subunits and cytoplasmic degradation of IκBα (Figure 7B) were observed compared with nuclear extracts from control mice. However, pretreatment with SPA0355 completely inhibited NF-κB activation, suggesting that SPA0355 induced a protective effect in mice with CIA through suppression of NF-κB activation.
Figure 7.
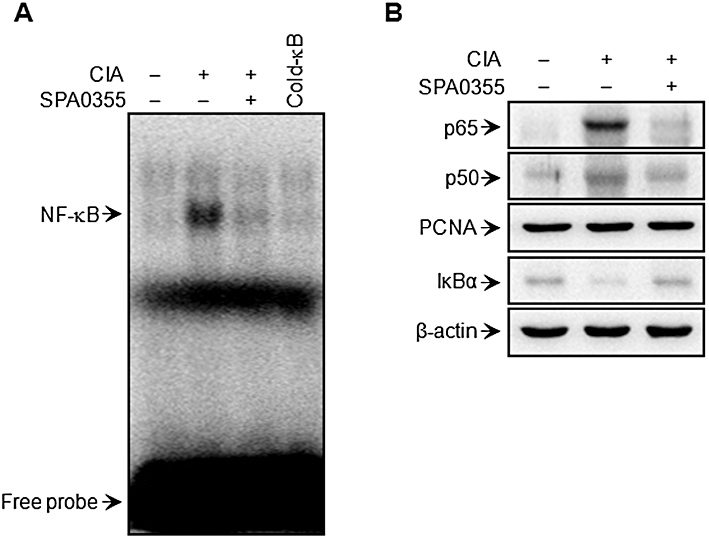
Suppression of NF-κB activation in mice with CIA and pretreated with SPA0355. Mice were injected with vehicle or SPA0355 (10 mg·kg−1) on day 20. Joint homogenates were prepared at day 24. NF-κB DNA binding activity (A) and nuclear translocation of p65 and p50 and cytoplasmic IκBα degradation (B) were determined by EMSA and Western blotting, respectively. PCNA and β-actin were used as loading controls for nuclear and cytoplasmic proteins, respectively.
Suppression of proinflammatory cytokine production involved in the pathophysiology of CIA by pretreatment with SPA0355
It is well known that proinflammatory cytokines play key roles in the pathogenesis of RA. Cytokine levels were therefore measured by elisa using joint tissues and serum obtained on day 45. Compared with vehicle-treated mice with CIA, significant decreases of TNF-α, IL-1β, RANKL and IL-17 were observed in the joint tissues of SPA0355-treated mice (Figure 8A). Because RA is a systemic autoimmune disease, cytokine levels were also measured in the serum (Figure 8B). Consistent with the tissue elisa results, SPA0355 led to a decrease in serum levels of cytokines, suggesting SPA0355 might provide beneficial effects by down-regulating the synthesis of a number of cytokines.
Figure 8.
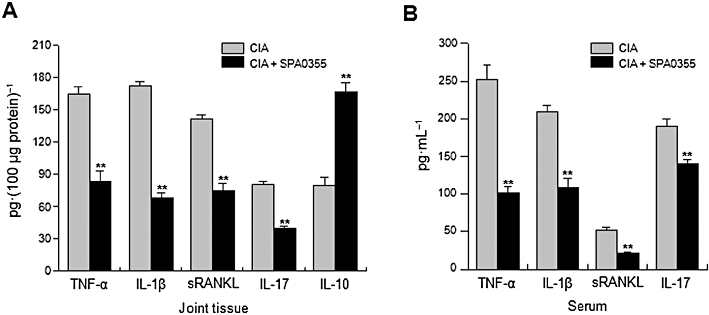
Inhibition of the production of proinflammatory cytokines in joint tissues and sera from mice with CIA and pretreated with SPA0355. Mice were injected with vehicle or SPA0355 (10 mg·kg−1) on day 20. Hind paws and sera were collected on day 45 from mice with CIA as well as from normal mice. Cytokines in joint homogenates (A) and sera (B) were measured by specific elisa. Values are the mean ± SEM of three independent experiments (n = 8–9 mice per group). *P < 0.05, **P < 0.01 significantly different from untreated CIA.
Therapeutic effect of SPA0355 in mice with CIA
To test the possible therapeutic effect of SPA0355 on the CIA animal model, we included only those mice that had apparently begun to develop arthritis and injected SPA0355 twice. Significant differences in the clinical score and hind paw thickness were observed between SPA0355-treated mice and vehicle-treated mice (Figure 9A and B).
Figure 9.
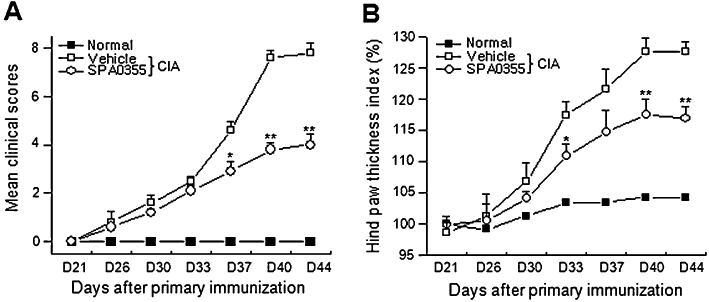
Therapeutic effects of SPA0355 on established arthritis. Following the development of clinical arthritis (average clinical score of 3) on day 27, CIA mice were randomized and injected with SPA0355 (10 mg·kg−1) on days 27 and 30. Clinical scores (A) and hind paw thickness (B) were measured. The results of three independent experiments with 4 mice per group are expressed as mean ± SEM. *P < 0.05, **P < 0.01, significantly different from untreated CIA.
Inhibition of cell proliferation in RA-FLS
Synovial hyperplasia is believed to play a central role in the development of pannus, a thickening of synovial tissue responsible for the cartilage and bone erosion seen in RA, and such synovial hyperplasia is probably caused by an increased rate of proliferation of RA-FLS. TNF-α stimulates the proliferation of RA-FLS in vitro and is known to act via signal transduction pathways including NF-κB (Youn et al., 2002). To investigate whether SPA0355 could affect the proliferation of TNF-α-stimulated RA-FLS, cellular proliferation was measured using the BrdU incorporation assay (Figure 10). Treatment with TNF-α for 72 h significantly increased the cell proliferative potential (P < 0.01) and SPA0355 reduced this increased proliferation of TNF-α-treated RA-FLS. Incubation with SPA0355 in the absence of TNF-α did not affect proliferation.
Figure 10.
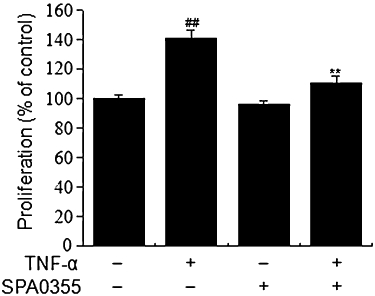
Effect of SPA0355 on TNF-α-induced cell proliferation. RA-FLS (1 × 105 cells) were pretreated with 10 µM SPA0355 for 1 h and exposed to TNF-α (10 ng·mL−1) for 72 h. Cellular proliferation was measured using the BrdU incorporation assay. Each value represents the mean ± SEM of the three independent experiments. ##P < 0.01, significantly different from untreated control; **P < 0.01, significantly different from TNF-α.
Discussion
In this study, we demonstrated that SPA0355 effectively suppressed inflammatory responses and joint destruction in both in vitro and in vivo RA models. We found that the i.p. administration of SPA0355 suppressed joint destruction in collagen-induced arthritic mice. In addition, SPA0355 suppressed TNF-α-induced cytokine and chemokine production, MMP secretion and cell proliferation in cultured human RA-FLS. Several mechanisms may account for these effects. First, SPA0355 inhibited local and systemic levels of proinflammatory cytokines and chemokines. Second, SPA0355 suppressed production of MMP. Finally, SPA0355 impaired osteoclastogenesis and reduced the number of osteoclasts in SPA0355-treated mice. These beneficial effects of SPA0355 correlated with decreased activity of NF-κB.
NF-κB is a pleiotropic transcription factor that plays an important role in regulating the expression of many genes involved in immune responses, including the cytokines TNF-α and IL-1β; the chemokines CXCL5 and CCL5; and MMP-1 and MMP-3, all of which are closely involved in the pathogenesis of RA. Products of these genes coordinately enhance inflammatory reactions resulting in further activation of NF-κB. Indeed, persistent NF-κB activation has been observed in both human and animal models of RA (Handel et al., 1995; Hah et al., 2010). It is clear, therefore, that NF-κB is an important target molecule for RA therapy.
Some urea and thiourea analogues are known to inhibit NO production and/or iNOS expression in LPS-activated macrophages (Prabhakar et al., 1997; Goodyer et al., 2003). We synthesized a series of novel urea and thiourea analogues as small molecule inhibitors of NF-κB, and found that thioureas were more potent inhibitors than ureas (Kim et al., 2007; Jin et al., 2009; Cheon et al., 2010). Our thiourea analogues were composed of a heterocycle as a lipophilic tail, attached to the nitrogen (N3) of a thiourea head by two methylene units of linker. We further investigated the structure–function relationship of thiourea analogues for the inhibition of NF-κB activity. Functional activity was affected by the type of heterocycle, the linker size and the substituent of the other nitrogen (N1) (Cheon et al., 2010). Among the prepared compounds, SPA0355 was the most potent inhibitor of the NF-κB pathway.
As a first step towards application of SPA0355, we tested the efficacy of SPA0355 as an NF-κB inhibitor. In EMSA and Western blotting assays, 10 µM SPA0355 almost totally suppressed NF-κB activation in TNF-α-stimulated human RA-FLS through the inhibition of IKK activity and IκBα degradation. Furthermore, we demonstrated that NF-κB activation was mediated through a traditional pathway from IKKα/IKKβ linking to NF-κB in RA-FLS. The effective dose of SPA0355 (10 mg·kg−1) used in the mouse model of CIA was comparable to those of other NF-κB inhibitors (Wakamatsu et al., 2005; Rice et al., 2008).
The transcriptional activity of NF-κB can be further modulated in the nucleus by three MAPK pathways, including p42/p44 MAPK, p38 MAPK and JNK. In addition, TNF-α has been reported to activate all of the pathways (Vanden Berghe et al., 1998). Concomitant with activation of the NF-κB pathway, TNF-α activates another transcription factor, activator protein-1 (AP-1) (Baud and Karin, 2001). The AP-1 transcription factor consists of homodimers or heterodimers of the Jun and Fos family, and its activity is also regulated by MAPK-mediated phosphorylation (Yoshizawa et al., 2008). In this way, an additional level of regulation is created, providing a basis for the crosstalk with other signalling pathways. In this respect, we looked for evidence that SPA0355 could affect MAPKs and AP-1 pathways in addition to NF-κB. Results revealed that TNF-α-stimulated MAPKs activation (Figure S1) and AP-1 transcriptional activity (Figure S2) were not inhibited by pretreatment with SPA0355. Thus, it is not likely that the MAPKs and AP-1 pathways mediated the suppressive effects of SPA0355. However, we cannot exclude the possibility that SPA0355 may interfere with other signal transduction pathways that are independent of NF-κB.
The balance between proinflammatory cytokines and anti-inflammatory cytokines contributes to the onset and progression of joint destruction in RA. Proinflammatory cytokines such as TNF-α, IL-1β and IL-17 activate the NF-κB pathway, leading to the expression of proinflammatory mediators. For example, TNF-α and IL-1β induce and/or enhance the production of MMPs that in turn mediate tissue destruction (Joosten et al., 1999; Kokkonen et al., 2010). IL-17 interacts with TNF-α and, to a lesser extent, with IL-1β and an injection of IL-17 alone into a normal knee was sufficient to induce tissue destruction and bone erosion (Miossec, 2007). In contrast, anti-inflammatory cytokines such as IL-4 and IL-10 suppress joint destruction in RA (Juarranz et al., 2005). In this study, we confirmed that SPA0355 reduced the concentration of TNF-α, IL-1β, sRANKL and IL-17 at local and systemic levels. However, anti-inflammatory IL-10 was increased by SPA0355 treatment, suggesting that SPA0355 shifts the cytokine balance towards protecting joint destruction. Measurement of CCL5 and CXCL5 levels in cultured RA-FLS further supported the results of the in vivo study.
Over 20 members of the MMP family have been identified in humans to date. Of these, MMP-1 and MMP-3 are particularly important in RA because they are produced by RA-FLS and macrophages in the synovium and are known to play a key role in tissue destruction. The levels of MMP-1 and MMP-3 are significantly higher in synovial fluid of RA patients (Rooney et al., 2010). We found that SPA0355 significantly interfered with the TNF-α-augmented gene expression and secretion of MMP-1 and MMP-3 in cultured RA-FLS. In vivo data also demonstrated that SPA0355 down-regulated the protein levels of MMP-1 and MMP-3 in CIA mice. This finding corroborated those of previous studies showing that NF-κB activation is a necessary step for MMP expression (Vincenti et al., 1998; Pretzel et al., 2009). Therefore, it is likely that SPA0355 may block joint destruction mediated by MMPs in the inflamed joints of RA.
The most important goal in RA therapy is the prevention of bone destruction in order to maintain normal joint function. Studies suggest that bone-resorbing osteoclasts in the synovium play an important role in bone and cartilage destruction in RA (Boyle et al., 2003; Cronstein, 2007). Osteoclast differentiation and activation is regulated by RANKL and RANK signalling (Choi et al., 2009). Activation of RANK by its ligand RANKL leads to activation of various signalling pathways in osteoclasts. Among them, the NF-κB signalling pathway appears to be crucial for osteoclast differentiation (Boyle et al., 2003). Peripheral blood T cells or NK cells express RANKL, which triggers osteoclastogenesis and bone destruction in arthritis (Miranda-Carus et al., 2006; Soderstrom et al., 2010). Consistent with these reports, we found here that SPA0355 suppressed the formation and maturation of osteoclasts induced by M-CSF and RANKL in mouse BMMs. In addition, SPA0355 treatment led to a decrease in the level of RANKL and the number of TRAP-positive osteoclasts in joint tissue of mice with CIA. Given the essential role of NF-κB in RANK signalling for the expression of genes required for osteoclastogenesis (Boyle et al., 2003), we suggest that SPA0355 minimizes joint destruction through suppressing the NF-κB pathway and decreasing the number and activity of osteoclasts.
A number of targets are being pursued for the development of new therapeutic agents for RA, including pro-inflammatory cytokines and chemokines, MMPs and osteoclastogenesis. The fact that many of these molecules are regulated by the NF-κB pathway makes this signalling pathway a more effective therapeutic target. Here we have shown that SPA0355 was a potent NF-κB inhibitor and significantly reduced the severity of inflammation and joint destruction in CIA mice. Although we argue NF-κB suppression is the principal therapeutic effect of SPA0355, we do not exclude the possibility of unidentified effects of SPA0355 in other cell types. Along with efficacy, safety issues need to be addressed. Throughout the study, SPA0355 was well tolerated at the given dose with no evidence of drug toxicity (Table 1). Additionally, there were no apparent differences in body weight change among the groups (Figure S3). However, further safety studies of SPA0355 are warranted. Taken together, this study suggests that SPA0355 could be used as a therapeutic agent for the treatment of joint destruction occurring in arthritis.
Table 1.
Effects of SPA0355 on the liver, heart and kidney functions of mice
| Normal | CIA | CIA + SPA0355 (10 mg·kg−1) | |
|---|---|---|---|
| Alanine aminotransferase (IU·L−1) | 55 ± 7 | 74 ± 4 | 74 ± 6 |
| Aspartate aminotransferase (IU·L−1) | 5.2 ± 1.1 | 5.2 ± 0.8 | 5.4 ± 1.2 |
| LDH (IU·L−1) | 148 ± 4 | 159 ± 3 | 151 ± 2 |
| Creatine phosphokinase (IU·L−1) | 151 ± 23 | 160 ± 32 | 153 ± 25 |
| Creatinine (mg·L−1) | 4 ± 1 | 5 ± 1 | 4 ± 1 |
All values are presented as means ± SEM (n = 5 per group).
Acknowledgments
This work was supported by the National Research Foundation of Korea grant funded by the Korean government (2010–0029463 and R11-2005-017–07005-0).
Glossary
Abbreviations
- BMM
bone marrow-derived macrophage
- BrdU
bromo-2-deoxyuridine
- CIA
collagen-induced arthritis
- FLS
fibroblast-like synoviocytes
- IKK
IκB kinase
- M-CSF
macrophage colony-stimulating factor
- RA
rheumatoid arthritis
- RANKL
receptor activator of NF-κB ligand
- TRAP
tartrate-resistant acid phosphatase
Conflicts of interest
The authors declare no conflicts of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Effect of SPA0355 on TNF-α-induced MAPKs activation in RA-FLS. FLS (2 × 106) from RA patients were pretreated with 10 µM SPA0355 for 30 min and then treated with TNF-α (10 ng·mL−1). The phosphorylation levels of MAPKs in the total cell lysates were evaluated by Western blotting. Results are representative of three independent experiments.
Figure S2 Effect of SPA0355 on TNF-α-induced AP-1 activation in RA-FLS. FLS (2 × 106) from RA patients were pretreated with 10 µM SPA0355 for 1 h and then treated with TNF-α (10 ng·mL−1). Following 3 h of incubation, AP-1 DNA binding activity was analysed by EMSA. Results are representative of three independent experiments.
Figure S3 Effect of SPA0355 administration on the change in body weight of mice with CIA. Values are the mean and SEM (n = 8–9 mice per group).
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of NF-κB activity through induction of IκB synthesis. Science. 1995;270:286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11:372–377. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- Campbell IK, Gerondakis S, O'Donnell K, Wicks IP. Distinct roles for the NF-κB1 (p50) and c-Rel transcription factors in inflammatory arthritis. J Clin Invest. 2000;105:1799–1806. doi: 10.1172/JCI8298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheon YJ, Gim HJ, Jang HR, Ryu JH, Jeon R. Synthesis of heterocyte-linked thioureas and their inhibitory activities of NO production in LPS activated macrophages. Bull Korean Chem Soc. 2010;31:27–30. [Google Scholar]
- Choi Y, Arron JR, Townsend MJ. Promising bone-related therapeutic targets for rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:543–548. doi: 10.1038/nrrheum.2009.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein BN. Interleukin-6 – a key mediator of systemic and local symptoms in rheumatoid arthritis. Bull NYU Hosp Jt Dis. 2007;65(Suppl 1):S11–S15. [PubMed] [Google Scholar]
- Goodyer CL, Chinje EC, Jaffar M, Stratford IJ, Threadgill MD. Synthesis of N-benzyl- and N-phenyl-2-amino-4,5-dihydrothiazoles and thioureas and evaluation as modulators of the isoforms of nitric oxide synthase. Bioorg Med Chem. 2003;11:4189–4206. doi: 10.1016/s0968-0896(03)00451-6. [DOI] [PubMed] [Google Scholar]
- Hah YS, Lee YR, Jun JS, Lim HS, Kim HO, Jeong YG, et al. A20 suppresses inflammatory responses and bone destruction in fibroblast-like synoviocytes and collagen-induced arthritic mice. Arthritis Rheum. 2010;62:2313–2321. doi: 10.1002/art.27545. [DOI] [PubMed] [Google Scholar]
- Handel ML, McMorrow LB, Gravallese EM. Nuclear factor-κB in rheumatoid synovium. Localization of p50 and p65. Arthritis Rheum. 1995;38:1762–1770. doi: 10.1002/art.1780381209. [DOI] [PubMed] [Google Scholar]
- Jeon KI, Jeong JY, Jue DM. Thiol-reactive metal compounds inhibit NF-κB activation by blocking IκB kinase. J Immunol. 2000;164:5981–5989. doi: 10.4049/jimmunol.164.11.5981. [DOI] [PubMed] [Google Scholar]
- Jin GH, Lee DY, Cheon YJ, Gim HJ, Kim DH, Kim HD, et al. Synthesis of phenylisothiourea derivatives as inhibitors of NO production in LPS activated macrophages. Bioorg Med Chem Lett. 2009;19:3088–3092. doi: 10.1016/j.bmcl.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Joosten LA, Helsen MM, Saxne T, van De Loo FA, Heinegard D, van Den Berg WB. IL-1αβ blockade prevents cartilage and bone destruction in murine type II collagen-induced arthritis, whereas TNF-α blockade only ameliorates joint inflammation. J Immunol. 1999;163:5049–5055. [PubMed] [Google Scholar]
- Juarranz Y, Abad C, Martinez C, Arranz A, Gutierrez-Canas I, Rosignoli F, et al. Protective effect of vasoactive intestinal peptide on bone destruction in the collagen-induced arthritis model of rheumatoid arthritis. Arthritis Res Ther. 2005;7:R1034–R1045. doi: 10.1186/ar1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Ryu JH, Cheon YJ, Lim HJ, Jeon R. Design and synthesis of urea and thiourea derivatives and their inhibitory activities on lipopolysaccharide-induced NO production. Bioorg Med Chem Lett. 2007;17:3317–3321. doi: 10.1016/j.bmcl.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Koch AE. Chemokines and their receptors in rheumatoid arthritis: future targets? Arthritis Rheum. 2005;52:710–721. doi: 10.1002/art.20932. [DOI] [PubMed] [Google Scholar]
- Kokkonen H, Soderstrom I, Rocklov J, Hallmans G, Lejon K, Rantapaa Dahlqvist S. Up-regulation of cytokines and chemokines predates the onset of rheumatoid arthritis. Arthritis Rheum. 2010;62:383–391. doi: 10.1002/art.27186. [DOI] [PubMed] [Google Scholar]
- Lee YR, Lee JH, Noh EM, Kim EK, Song MY, Jung WS, et al. Guggulsterone blocks IL-1β-mediated inflammatory responses by suppressing NF-κB activation in fibroblast-like synoviocytes. Life Sci. 2008;82:1203–1209. doi: 10.1016/j.lfs.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Lee YR, Kweon SH, Kwon KB, Park JW, Yoon TR, Park BH. Inhibition of IL-1β-mediated inflammatory responses by the IκBα super-repressor in human fibroblast-like synoviocytes. Biochem Biophys Res Commun. 2009;378:90–94. doi: 10.1016/j.bbrc.2008.11.002. [DOI] [PubMed] [Google Scholar]
- McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- Majumdar S, Aggarwal BB. Methotrexate suppresses NF-κB activation through inhibition of IκBα phosphorylation and degradation. J Immunol. 2001;167:2911–2920. doi: 10.4049/jimmunol.167.5.2911. [DOI] [PubMed] [Google Scholar]
- Manna SK, Mukhopadhyay A, Aggarwal BB. Leflunomide suppresses TNF-induced cellular responses: effects on NF-κB, activator protein-1, c-Jun N-terminal protein kinase, and apoptosis. J Immunol. 2000;165:5962–5969. doi: 10.4049/jimmunol.165.10.5962. [DOI] [PubMed] [Google Scholar]
- Miossec P. Interleukin-17 in fashion, at last: ten years after its description, its cellular source has been identified. Arthritis Rheum. 2007;56:2111–2115. doi: 10.1002/art.22733. [DOI] [PubMed] [Google Scholar]
- Miranda-Carus ME, Benito-Miguel M, Balsa A, Cobo-Ibanez T, Perez de Ayala C, Pascual-Salcedo D, et al. Peripheral blood T lymphocytes from patients with early rheumatoid arthritis express RANKL and interleukin-15 on the cell surface and promote osteoclastogenesis in autologous monocytes. Arthritis Rheum. 2006;54:1151–1164. doi: 10.1002/art.21731. [DOI] [PubMed] [Google Scholar]
- Prabhakar SS, Zeballos GA, Montoya-Zavala M, Leonard C. Urea inhibits inducible nitric oxide synthase in macrophage cell line. Am J Physiol. 1997;273(6)(Pt 1):C1882–C1888. doi: 10.1152/ajpcell.1997.273.6.C1882. [DOI] [PubMed] [Google Scholar]
- Pretzel D, Pohlers D, Weinert S, Kinne RW. In vitro model for the analysis of synovial fibroblast-mediated degradation of intact cartilage. Arthritis Res Ther. 2009;11:R25. doi: 10.1186/ar2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JW, Veal JM, Fadden RP, Barabasz AF, Partridge JM, Barta TE, et al. Small molecule inhibitors of Hsp90 potently affect inflammatory disease pathways and exhibit activity in models of rheumatoid arthritis. Arthritis Rheum. 2008;58:3765–3775. doi: 10.1002/art.24047. [DOI] [PubMed] [Google Scholar]
- Rooney T, Roux-Lombard P, Veale DJ, FitzGerald O, Dayer JM, Bresnihan B. Synovial tissue and serum biomarkers of disease activity, therapeutic response and radiographic progression: analysis of a proof-of-concept randomised clinical trial of cytokine blockade. Ann Rheum Dis. 2010;69:706–714. doi: 10.1136/ard.2009.108324. [DOI] [PubMed] [Google Scholar]
- Smolen JS, Aletaha D, Koeller M, Weisman MH, Emery P. New therapies for treatment of rheumatoid arthritis. Lancet. 2007;370:1861–1874. doi: 10.1016/S0140-6736(07)60784-3. [DOI] [PubMed] [Google Scholar]
- Soderstrom K, Stein E, Colmenero P, Purath U, Muller-Ladner U, Teixeira de Matos C, et al. Natural killer cells trigger osteoclastogenesis and bone destruction in arthritis. Proc Natl Acad Sci USA. 2010;107:13028–13033. doi: 10.1073/pnas.1000546107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T, Takeuchi E, Tomita N, Morishita R, Kaneko M, Yamamoto K, et al. Suppressed severity of collagen-induced arthritis by in vivo transfection of nuclear factor κB decoy oligodeoxynucleotides as a gene therapy. Arthritis Rheum. 1999;42:2532–2542. doi: 10.1002/1529-0131(199912)42:12<2532::AID-ANR5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Vanden Berghe W, Plaisance S, Boone E, De Bosscher K, Schmitz ML, Fiers W, et al. p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways are required for nuclear factor-κB p65 transactivation mediated by tumor necrosis factor. J Biol Chem. 1998;273:3285–3290. doi: 10.1074/jbc.273.6.3285. [DOI] [PubMed] [Google Scholar]
- Vincenti MP, Coon CI, Brinckerhoff CE. Nuclear factor κB/p50 activates an element in the distal matrix metalloproteinase 1 promoter in interleukin-1β-stimulated synovial fibroblasts. Arthritis Rheum. 1998;41:1987–1994. doi: 10.1002/1529-0131(199811)41:11<1987::AID-ART14>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Wakamatsu K, Nanki T, Miyasaka N, Umezawa K, Kubota T. Effect of a small molecule inhibitor of nuclear factor-κB nuclear translocation in a murine model of arthritis and cultured human synovial cells. Arthritis Res Ther. 2005;7:R1348–R1359. doi: 10.1186/ar1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin MJ, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of IκB kinase-β. Nature. 1998;396:77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- Yoshizawa T, Hammaker D, Sweeney SE, Boyle DL, Firestein GS. Synoviocyte innate immune responses: I. Differential regulation of interferon responses and the JNK pathway by MAPK kinases. J Immunol. 2008;181:3252–3258. doi: 10.4049/jimmunol.181.5.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn J, Kim HY, Park JH, Hwang SH, Lee SY, Cho CS, et al. Regulation of TNF-α-mediated hyperplasia through TNF receptors, TRAFs, and NF-κB in synoviocytes obtained from patients with rheumatoid arthritis. Immunol Lett. 2002;83:85–93. doi: 10.1016/s0165-2478(02)00079-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


