Abstract
BACKGROUND AND PURPOSE
In the current study, we investigated the role of both kinin B1 and B2 receptors in peripheral neuropathy induced by the chronic treatment of mice with paclitaxel a widely used chemotherapeutic agent.
EXPERIMENTAL APPROACH
Chemotherapy-evoked hyperalgesia was induced by i.p. injections of paclitaxel (2 mg·kg−1) over 5 consecutive days. Mechanical and thermal hyperalgesia were evaluated between 7 and 21 days after the first paclitaxel treatment.
KEY RESULTS
Treatment with paclitaxel increased both mechanical and thermal hyperalgesia in mice (C57BL/6 and CD1 strains). Kinin receptor deficient mice (B1,or B2 receptor knock-out and B1B2 receptor, double knock-out) presented a significant reduction in paclitaxel-induced hypernociceptive responses in comparison to wild-type animals. Treatment of CD1 mice with kinin receptor antagonists (DALBK for B1 or Hoe 140 for B2 receptors) significantly inhibited both mechanical and thermal hyperalgesia when tested at 7 and 14 days after the first paclitaxel injection. DALBK and Hoe 140 were also effective against paclitaxel-induced peripheral neuropathy when given intrathecally or i.c.v.. A marked increase in B1 receptor mRNA was observed in the mouse thalamus, parietal and pre-frontal cortex from 7 days after the first paclitaxel treatment.
CONCLUSIONS AND IMPLICATIONS
Kinins acting on both B1 and B2 receptors, expressed in spinal and supra-spinal sites, played a crucial role in controlling the hypernociceptive state caused by chronic treatment with paclitaxel.
Keywords: neuropathic pain, chemotherapy, paclitaxel, kinin, B1 receptor, B2 receptor
Introduction
Kinins are potent endogenous algogenic peptides, and their role in pain transmission has been extensively reviewed (Couture et al., 2001; Calixto et al., 2004; Huang and Player, 2010). Once formed from their precursors, the kininogens, by the action of kallikrein enzymes, kinins are released and exert their actions via the activation of two subtypes of GPCRs, named B1 and B2 receptors (nomenclature follows Alexander et al., 2009). The B2 receptor displays higher affinity for bradykinin (BK) and kallidin peptides, while the B1 receptor presents high affinity for the kinin metabolites, des-Arg9-BK (DABK) and Lys-des-Arg9-BK. B2 receptors are usually expressed in a constitutive manner throughout peripheral and central tissues, mediating most of the physiological effects of the kinins and the acute phase of inflammatory and nociceptive responses. In contrast, B1 receptors are generally absent under physiological conditions, being quickly up-regulated after tissue injury or during certain inflammatory process. Therefore, they might represent important players in the chronic phase of pain and inflammation (Calixto et al., 2004; Marceau and Regoli, 2004; Huang and Player, 2010). Nevertheless, the constitutive expression of B1 receptors in sensory neurons has been reported (Ma and Heavens, 2000; Wothersponn and Winter, 2000; Ma, 2001).
Many groups have reported that both kinin receptors are involved in the onset and/or maintenance of neuropathic pain (Petersen et al., 1998; Levy and Zochodne, 2000; Yamaguchi-Sase et al., 2003; Rashid et al., 2004; Ferreira et al., 2005; Lai et al., 2006; Quintão et al., 2008), a chronic condition characterized by spontaneous pain, allodynia and hyperalgesia, which remains without satisfactory treatment and compromises the quality of life (Woolf and Mannion, 1999; Jensen and Baron, 2003). Nerve injuries (caused by surgery or trauma), some pathological states (e.g. diabetes mellitus, herpes zoster or HIV infection) and chemotherapy are the main causes of peripheral neuropathy in humans (Woolf and Mannion, 1999). Chemotherapy-induced peripheral neuropathy is a common side effect of several anticancer drugs, including vincristine, oxaliplatin and paclitaxel (Wolf et al., 2008). Paclitaxel, derived from Taxus brevifolia and commercially known as Taxol, is one of the most effective and commonly used anti-neoplastic drugs. Its major dose-limiting side effect is the appearance of peripheral sensory neuropathy characterized by painful paresthesias of the hands and feet (Polomano and Bennett, 2001; Dougherty et al., 2004). In accordance with these clinical findings, chronic treatment with paclitaxel in rodents induced mechanical and thermal hyperalgesia, and has been used as a reproducible model to evaluate chemotherapy-induced peripheral neuropathy (Cliffer et al., 1998; Dina et al., 2001; Polomano et al., 2001).
Recent evidence has suggested that kinins, and their receptors, might play a critical role in peripheral neuropathy induced by chemotherapy (Bujalska et al., 2008; Bujalska and Makulska-Nowak, 2009a,b;). However, the mechanisms underlying these actions still remain unclear. Hence, in the present study, in order to provide new evidence on the relevance of both kinin B1 and B2 receptors in chemotherapy-induced neuropathy, we sought to analyse, by the use of kinin receptor knock-out mice in combination with selective kinin receptor antagonists and molecular analysis, the contribution of these receptors to the thermal and mechanical hyperalgesia induced by paclitaxel.
Methods
Animals
All animal care and experimental procedures complied with the National Institutes of Health Animal Care Guidelines (NIH publications  80-23), and were approved by the Ethics Committee of the Universidade Federal de Santa Catarina (protocol number PP00032). The animals were housed in a room with controlled temperature (22 ± 2°C) and humidity (around 60–80%) under a 12:12 h light–dark cycle (lights on 0600 h). Food and water were provided ad libitum. Adult male CD1 mice (8–10 weeks) were used in this study. In some experiments, male C57BL/6 wild-type mice, C57BL/6 kinin B1- or B2 receptor-deficient mice (B1R−/− and B2R−/−, respectively) and mice lacking the genes encoding both kinin receptors (double knock-out mice, B1B2R−/−) were also used. Wild-type and knock-out mice were originally obtained from the Centro de Desenvolvimento de Modelos Experimentais para Medicina e Biologia, from the Universidade Federal de São Paulo (São Paulo, Brazil). Deletion of the entire coding sequence for kinin B1 and B2 receptors was achieved according to the methodology previously described by Pesquero et al. (2000) and Rupniak et al. (1997) respectively. Mice lacking both kinin receptors (B1B2R−/−) were generated according to the methodology described by Cayla et al. (2007). The animals were randomly distributed between the experimental groups (six animals per group), and all behavioural experiments were conducted without knowledge of the treatments in order to reduce experimental bias. The number of animals and the intensity of noxious stimuli used were the minimum necessary to demonstrate consistent effects. There were no withdrawals or exclusions in this study.
80-23), and were approved by the Ethics Committee of the Universidade Federal de Santa Catarina (protocol number PP00032). The animals were housed in a room with controlled temperature (22 ± 2°C) and humidity (around 60–80%) under a 12:12 h light–dark cycle (lights on 0600 h). Food and water were provided ad libitum. Adult male CD1 mice (8–10 weeks) were used in this study. In some experiments, male C57BL/6 wild-type mice, C57BL/6 kinin B1- or B2 receptor-deficient mice (B1R−/− and B2R−/−, respectively) and mice lacking the genes encoding both kinin receptors (double knock-out mice, B1B2R−/−) were also used. Wild-type and knock-out mice were originally obtained from the Centro de Desenvolvimento de Modelos Experimentais para Medicina e Biologia, from the Universidade Federal de São Paulo (São Paulo, Brazil). Deletion of the entire coding sequence for kinin B1 and B2 receptors was achieved according to the methodology previously described by Pesquero et al. (2000) and Rupniak et al. (1997) respectively. Mice lacking both kinin receptors (B1B2R−/−) were generated according to the methodology described by Cayla et al. (2007). The animals were randomly distributed between the experimental groups (six animals per group), and all behavioural experiments were conducted without knowledge of the treatments in order to reduce experimental bias. The number of animals and the intensity of noxious stimuli used were the minimum necessary to demonstrate consistent effects. There were no withdrawals or exclusions in this study.
Peripheral neuropathy induced by paclitaxel
The neuropathy induced by paclitaxel was induced according to the methodology described previously by Polomano et al. (2001) and adapted for use in mice. Briefly, mice were injected i.p. with paclitaxel (2 mg·kg−1 per injection) for 5 consecutive days (days 1–5), using an injection volume of 10 mL·kg−1. The cumulative paclitaxel dose was 10 mg·kg−1. Control animals received only the vehicle (0.9% NaCl). In order to assess general toxicity, mouse body weight and rectal temperature were measured at regular intervals of time, for 21 days after the first paclitaxel administration. Tests for altered pain sensitivity began on day 7 and continued until day 14 or 21.
Mechanical hyperalgesia in hind paws
To assess the mechanical hypernociceptive response, mice were placed individually in clear Plexiglas boxes (9 × 7 × 11 cm) on elevated wire-mesh platforms to allow access to the ventral surface of the right hind paw (Ugo Basile, Comerio, VA, Italy). The animals were acclimatized for 1 h before behavioural testing. The withdrawal response frequency (in %) was measured following 10 applications (with a duration of ∼3 s each, and an interval of ∼20 s among each) of von Frey hairs (VFHs, Stoelting, Chicago, IL, USA). Stimuli were delivered from below to the plantar surface of the right hind paw. The 0.6 g VFH filament produces a mean withdrawal frequency of about 20%, which is considered to be an adequate value for the measurement of mechanical hyperalgesia (Quintão et al., 2008). Hence, the 0.6 g VFH was used throughout this study. All the groups were evaluated before vehicle or paclitaxel injections, in order to determine basal mechanical thresholds. The incidence of mechanical hyperalgesia was ∼90% in paclitaxel-treated animals.
Hind paw thermal hyperalgesia (paw flick)
A radiant heat analgesiometer (Tail-Flick Analgesia Meter, Albarsch, Porto Alegre, Brazil) was used to measure latencies for paw withdrawal according to the method described by Menéndez et al. (2002). All the animals were evaluated to determine the basal thermal threshold (I.R. intensity of 15), and then they were submitted to paclitaxel injections, as described earlier. Thermal hyperalgesia was evaluated at several time intervals after the initiation of vehicle or paclitaxel treatment. Twenty seconds was adopted as the maximal time of reaction to avoid possible tissue damage. The development of thermal hyperalgesia by paclitaxel treatments was not reproduced in all experiments conducted in CD1 animals, and its incidence was variable among experiments (from 10 to 80%). The effect of drug treatments on this parameter was assessed only when the incidence of thermal hyperalgesia reached ∼80%.
Overt nociception
The procedure used was similar to that described previously (Ferreira et al., 2005). Twenty microlitres of BK (10 nmol per paw) or DABK solution (20 nmol per paw) was injected intraplantarly (i.pl.) under the surface of the right hindpaw 7 days after the first treatment with paclitaxel or vehicle in CD1 mice. Separate groups of animals received an i.pl. injection of saline (0.9% NaCl). The animals were placed individually in chambers (transparent glass cylinders of 20 cm diameter) and were allowed to adapt to the chambers for 20 min before algogen or saline injection. After challenge, the mice were observed individually for 10 min. The amount of time spent licking the injected paw was measured with a chronometer and was considered as indicative of overt nociception.
Mechanical and thermal hyperalgesia after paclitaxel treatment in kinin receptor knock-out mice
The relevance of kinin B1 or B2 receptors for the mechanical and thermal hyperalgesia induced by paclitaxel was analysed using kinin B1 and B2 receptor knock-out mice (B1R−/− and B2R−/−), and the corresponding wild-type mice (C57BL/6 strains). The full functional contribution of the kallikrein–kinin system was checked using C57BL/6 double knock-out mice lacking the two kinin receptors (B1B2R−/−). Briefly, the animals were submitted to five paclitaxel injections as described earlier, and the mechanical and thermal hyperalgesia were evaluated at several times after paclitaxel injections. Each set of experiments used four groups: wild-type vehicle- and paclitaxel-injected mice, B1R−/−, B2R−/− or B1B2R−/− paclitaxel-treated mice.
Intrathecal (i.t.) and i.c.v. drug injections
The i.t. drug injections were performed in accordance with the method described by Hylden and Wilcox (1980), with minor modifications (Ferreira et al., 2002a). The animals were lightly anaesthetized with isoflurane, and a needle connected to a microsyringe by polyethylene tubing was introduced through the skin. Subsequently, 5 µL of saline solution (0.9% NaCl) alone (control) or containing the drugs was injected between the L5 and L6 vertebral spaces. For i.c.v. injections, the animals were lightly anaesthetized with isoflurane, and 5 µL of sterile saline containing the drugs was injected directly into the lateral ventricle (coordinates from bregma: 1 mm lateral; 1 mm rostral; 3 mm vertical), as described previously by Laursen and Belknap (1986). The control animals received the same volume of saline.
Effect of selective kinin receptor antagonists on the hypernociceptive responses induced by paclitaxel
To assess the contribution of kinin B1 and B2 receptors to the development of mechanical and thermal hyperalgesia induced by paclitaxel, different groups of CD1 mice were treated with the selective peptide kinin B1 or B2 receptor antagonists, des-Arg9-Leu8-BK (DALBK; 100 nmol·kg−1) and Hoe 140 (50 nmol·kg−1), respectively, administered by the i.p. route twice a day (each 12 h) for 6 days (days 1–6), starting at the time of the first (day 1) paclitaxel treatment. Mechanical and thermal hyperalgesia were evaluated between days 7 and 9 after the first paclitaxel injection.
To analyse the involvement of kinin B1 or B2 receptors on the established mechanical and thermal hyperalgesia induced by paclitaxel, CD1 mice were treated with the selective peptide B1 or B2 receptor antagonists, DALBK or Hoe 140, respectively, 7 or 14 days after the first paclitaxel treatment by different pathways of administration. First, DALBK (100–300 nmol·kg−1) or Hoe 140 (30–100 nmol·kg−1) was given by the i.p. route in order to evaluate its systemic effect. In other experimental groups, to evaluate the peripheral effect of the antagonists, DALBK (3 nmol per paw) or Hoe 140 (3 nmol per paw) was injected by the i.pl. pathway. Finally, the central effect of single injections of DALBK (10 pmol) or Hoe 140 (100 pmol) was tested by the i.t. or i.c.v. route. Mechanical and/or thermal hyperalgesia were evaluated, as described previously, between 1 and 6 h after drug treatment.
To check the effect of repeated administration of kinin receptor antagonists on the established mechanical hyperalgesia induced by paclitaxel, CD1 mice were treated with DALBK (100 nmol·kg−1, i.p.) or Hoe 140 (50 nmol·kg−1, i.p.) twice a day (every 12 h) for 2 days (days 7 and 8). Mechanical hyperalgesia was evaluated between days 7 and 11 after the first paclitaxel injection.
The protocols of all tested drugs (doses and time of injections) were chosen in accordance with previous publications of our group (Ferreira et al., 2002a,b; 2004; 2008; Costa et al., 2006; 2010; Quintão et al., 2008).
Quantitative real-time PCR
The expression of B1 receptor mRNA was measured using quantitative real-time PCR according to the method described previously (Ferreira et al., 2005). Seven and fourteen days after the first injection of vehicle or paclitaxel, mice (four to six in each group) were killed, and the plantar skin of the right hind paw, lumbar dorsal root ganglia (DRG) (between the L4 and L6 segments), lumbar spinal cord segments (L4–L6), thalamus, hypothalamus, parietal cortex and pre-frontal cortex were isolated, dissected, frozen in liquid nitrogen and stored at −80°C until use. Thawed tissues were homogenized in 0.3–1 mL of TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and total RNA was isolated according to the instructions of the manufacturer. RNA concentration in the samples was determined by a NanoDrop 1100 (Nanodrop Technologies, Wilmington, DE, USA). Reverse transcription assay was carried out using M-MLV Reverse Transcriptase (Invitrogen) according to the manufacturer's instructions. cDNA was amplified in duplicate using a TaqMan Universal PCR Master Mix Kit (Applied Biosystems, Foster City, CA, USA) with specific TaqMan Gene Expression target genes (Applied Biosystems): the 3′ quencher FAM-labelled probe for mouse B1R (Mm00432059_s1) and the 3′ quencher VIC-labelled probe for mouse GAPDH (Mm03302249_g1), the latter being used as an endogenous control for normalization. PCR was performed in a 96-well Optical Reaction Plate (Applied Biosystems). The thermocycler parameters were as follows: 50°C for 2 min, 95°C for 10 min, 60 cycles of 95°C for 15 s and 60°C for 1 min. Both FAM and VIC correspondent fluorescence were acquired at the end of each extension phase. The PCR cycle (when a given fluorescence threshold is crossed by the amplification curve) was considered our first parameter to analyse mRNA expression and named Ct. ΔCt values were calculated by subtracting GAPDH Ct from kinin B1R Ct to obtain the 2−ΔΔCt parameter, which represents relative B1R/GAPDH expression.
Data analysis
Results are presented as the mean ± SEM of six to eight animals for each experimental group. The percentages of inhibition are reported as the difference (in percentage) between the areas under the time–response curve of the test group in relation to the corresponding control group. Statistical comparisons of the data were performed by two-way anova followed by Bonferroni's post-test, one-way anova followed by the Newman–Keuls post-test or Student's t-test, using GraphPad Prism software version 5.01 (GraphPad Software Inc., La Jolla, CA, USA). P values <0.05 were considered significant.
Materials
The following drugs were used: Cremophor EL, DABK, BK and DALBK were purchased from Sigma Chemical Company (St. Louis, MO, USA). Hoe 140 was kindly donated by Sanofi-Aventis (Bridgewater, NJ, USA). Paclitaxel (6 mg·mL−1 in Cremophor EL) was obtained from Dosa S.A. Laboratory (Buenos Aires, Argentina). All drugs were diluted in saline (0.9% NaCl). The paclitaxel stock solution (6 mg·mL−1 in Cremophor EL) was diluted in saline to a concentration of 0.2 mg·mL−1 (solution for injection).
Results
Mechanical and thermal hyperalgesia following paclitaxel treatment in kinin receptor-deficient mice
As illustrated in Figures 1A,B and 2, the 5 day treatment with daily i.p. injections of paclitaxel (2 mg·kg−1) induced a significant decrease in both mechanical and thermal (heat) withdrawal threshold in C57BL/6 and CD1 mice strains compared with vehicle-treated groups. Mechanical and thermal hyperalgesia was significant 7 days after the initial injection of paclitaxel, and persisted for up to 21 and 14 days, respectively (Figure 1). When B1R−/−, B2R−/− or B1B2R−/− mice were treated with paclitaxel, both mechanical and thermal hypernociceptive responses were notably reduced during almost the entire period of evaluation in comparison to wild-type mice (Figure 1A,B). As expected, the inhibition of paclitaxel-induced hyperalgesia by concomitant deficiency of both kinin B1 and B2 receptors (B1B2R−/−, double knock-out mice) was greater than that caused by the single ablation of B1 or B2 receptors (Figure 1C,D). Of note, the inhibition of paclitaxel-induced mechanical hyperalgesia by double deletion of both kinin receptors persisted up to 21 days, while single ablations (B1 or B2 receptors) were effective only for the period of 14 days (Figure 1A). On the other hand, there was no significant difference in the percentage of weight gain and rectal temperature between vehicle- and paclitaxel-treated animals in both CD1 and C57BL/6 mice during 21 days of testing (data not shown).
Figure 1.
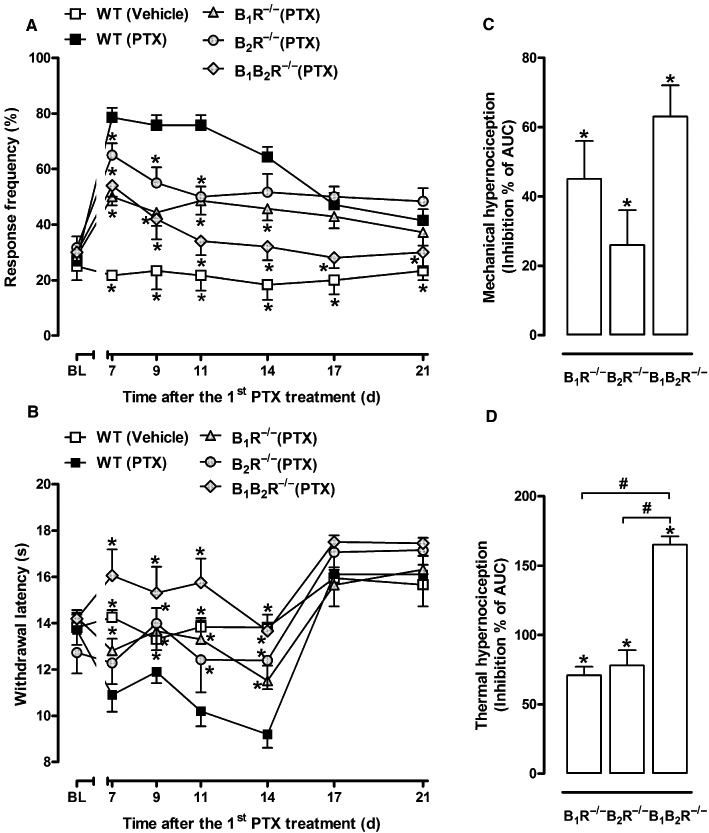
Paclitaxel-induced hyperalgesia in kinin receptor-deficient mice. Mechanical (A) and thermal (B) withdrawal threshold of vehicle-treated wild-type (WT vehicle) mice, paclitaxel-treated WT (WT PTX) mice, paclitaxel-treated B1R−/−, (B1R−/− PTX) B2R−/− (B2R−/− PTX) and B1B2R−/− (B1B2R−/− PTX) mice were evaluated at different time intervals after the first paclitaxel treatment. (C, D) Inhibition of the AUC 0–6 h (from day 0 to 21) of paclitaxel-induced mechanical (C) and thermal (D) hyperalgesia in kinin receptor-deficient mice. The inhibition is shown as the AUC of the test group (receptor knock-out PTX animals), as a percentage of the control AUC (WT PTX animals). Each group represents the mean of five to six animals, and the error bars indicate the SEM. *P < 0.05 significantly different from paclitaxel-treated WT mice (two-way anova followed by the Bonferroni post-test). BL, baseline withdrawal threshold. #P < 0.05, significantly different from B1R−/− or B2R−/− group (one-way anova followed by the Newman–Keuls post-test).
Figure 2.
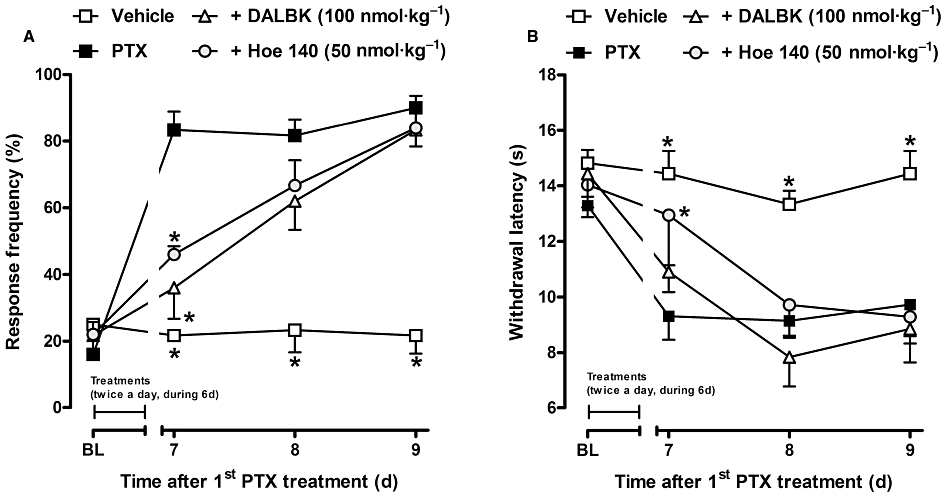
Effect of treatment with selective kinin B1 or B2 receptor antagonists, DALBK (100 nmol·kg−1, i.p.) and Hoe 140 (50 nmol·kg−1, i.p.), respectively, on the genesis of paclitaxel-induced mechanical (A) and thermal (B) hyperalgesia in CD1 mice. The drugs were given twice a day (every 12 h) for 6 days, starting at the time of the first paclitaxel treatment. Each group represents the mean of five to six animals, and the error bars indicate the SEM. *P < 0.05, significantly different from paclitaxel-treated mice (two-way anova followed by the Bonferroni post-test). BL, baseline withdrawal threshold.
Effect of selective kinin B1R or B2R antagonists on the genesis of hypernociceptive responses induced by paclitaxel treatment
The involvement of kinin receptors in the onset of mechanical and thermal hyperalgesia induced by paclitaxel treatment was assessed by treating CD1 mice with selective kinin B1 or B2 receptor antagonists, DALBK (100 nmol·kg−1) or Hoe 140 (50 nmol·kg−1) respectively. The antagonists were given by the i.p. route twice a day (every 12 h) for 6 days (between days 1 and 6), starting at the time of the first paclitaxel treatment. As can be seen in Figure 2A, DALBK (B1 receptor antagonist) or Hoe 140 (B2 receptor antagonist) treatments were able to prevent the mechanical hyperalgesia only at the initial time point (day 7) after paclitaxel injection (77 ± 15% and 61 ± 4% of inhibition respectively). In addition, Hoe 140 treatment prevented the paclitaxel-induced thermal hypernociceptive response at the initial stage (69 ± 13% of inhibition), while the B1 receptor antagonist (DALBK) was ineffective on this parameter (Figure 2B).
Effect of systemic treatment with selective kinin B1 or B2 receptor antagonists on the established mechanical and thermal hyperalgesia induced by paclitaxel treatment
In an attempt to further evaluate the participation of kinin receptors in the maintenance of mechanical and thermal hyperalgesia induced by paclitaxel, CD1 mice were intraperitoneally treated with the selective kinin B1 (DALBK) or B2 receptor (Hoe 140) antagonists, 7 and 14 days after the first paclitaxel injection. The results depicted in Figure 3 demonstrate that systemic treatment with DALBK (150 and 300 nmol·kg−1, i.p.) or Hoe 140 (50 and 100 nmol·kg−1, i.p.) was effective in inhibiting the mechanical hyperalgesia induced by paclitaxel for up to 2–3 h after drug administration when assessed at days 7 (Figure 3A,B) and 14 (Figure 3C). The inhibition values obtained for mechanical hyperalgesia are shown in Table 1 and are expressed as the area under the time–response curve (AUC 0–6 h), as a percentage of the control AUC. The administration of DALBK (150 nmol·kg−1, i.p.) or Hoe 140 (100 nmol·kg−1, i.p.) reduced the paclitaxel-induced thermal hyperalgesia for 3–4 h (Figure 3D,E; Table 1). Repeated treatments with DALBK (100 nmol·kg−1, i.p.) or Hoe 140 (50 nmol·kg−1, i.p.), twice a day (every 12 h) for 2 days (between days 7 and 8), were effective in inhibiting the established mechanical hyperalgesia induced by paclitaxel at all periods of treatment and for nearly 48 h after the last treatment (37 ± 9% and 30 ± 3% inhibition of AUC, respectively) (Figure 3F).
Figure 3.
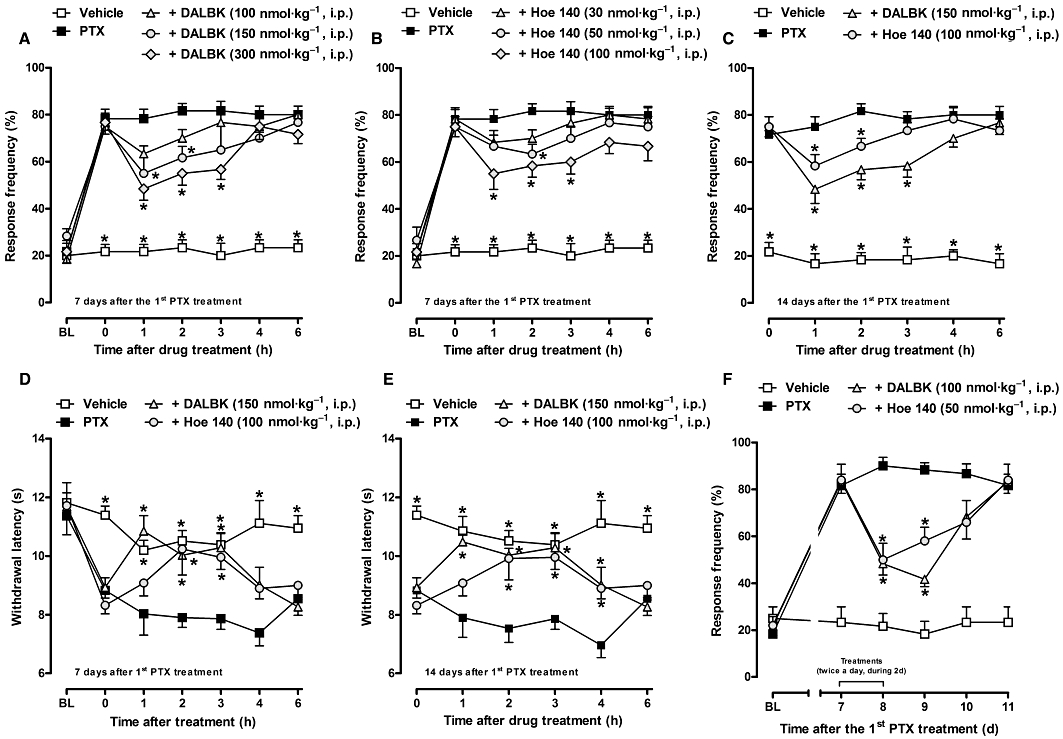
Effect of treatment with selective kinin B1R or B2R antagonists, DALBK (100–300 nmol·kg−1, i.p.) and Hoe 140 (30–100 nmol·kg−1, i.p.), respectively, on established mechanical (A, B, C) and thermal (D, E) hyperalgesia induced by paclitaxel in CD1 mice. A single injection of the drugs was given 7 (A, B, D) and 14 (C, E) days after the first paclitaxel (PTX) injection. (F) Effect of the repeated treatment with DALBK (100 nmol·kg−1, i.p.) or Hoe 140 (50 nmol·kg−1, i.p.), every 12 h for 2 days, on the sustained mechanical hyperalgesia in CD1 mice. Each group represents the mean of five to six animals, and the error bars indicate the SEM. *P < 0.05, significantly different from paclitaxel-treated mice (two-way anova followed by the Bonferroni post-test). BL, baseline withdrawal threshold.
Table 1.
Effect of selective kinin receptor antagonists on the established mechanical and thermal hypersensitivities induced by paclitaxel in mice
| Inhibition (%) | |||||
|---|---|---|---|---|---|
| Mechanical hyperalgesia | Thermal hyperalgesia | ||||
| Drug/Route | Dose | Day 7 | Day 14 | Day 7 | Day 14 |
| DALBK (B1R)/i.p. | 150 nmol·kg−1 | 23 ± 9* | 25 ± 7* | 39 ± 5* | 37 ± 5* |
| Hoe 140 (B2R)/i.p. | 100 nmol·kg−1 | 28 ± 6* | 10 ± 5ns | 54 ± 10* | 50 ± 9* |
| DALBK (B1R)/i.t. | 10 pmol | 40 ± 8* | 27 ± 8* | ne | ne |
| Hoe 140 (B2R)/i.t. | 100 pmol | 23 ± 4* | 27 ± 12* | ne | ne |
| DALBK (B1R)/i.c.v. | 10 pmol | 17 ± 5ns | 34 ± 5* | ne | ne |
| Hoe 140 (B2R)/i.c.v. | 100 pmol | 7 ± 2ns | 10 ± 7ns | ne | ne |
Inhibition of hyperalgesia is expressed as the mean (±SEM) AUC (1–6 h) of the drug treated group as a percentage of the AUC from the vehicle-treated group (control values). The selectivity of the antagonists is shown as: B1R, selective for B1 receptors; B2R, selective for B2 receptors.
P < 0.05, significantly different from control values.
ns, no significant inhibition; ne, not evaluated.
Effect of peripheral and central blockade of kinin B1 or B2 receptors on the mechanical hyperalgesia induced by paclitaxel treatment
As can be seen in Figure 4, the local administration of DALBK (3 nmol per paw, i.pl.) or Hoe 140 (3 nmol per paw, i.pl.) was not able to alter the mechanical hypernociceptive response induced by paclitaxel (Figure 4A,B), suggesting that kinin receptors do not contribute to paclitaxel-induced mechanical hyperalgesia at the peripheral level. In fact, the nociceptive response (overt nociception) evoked by i.pl. injection of the selective kinin B1 or B2 receptor agonists, DABK (20 nmol per paw) or BK (10 nmol per paw), respectively, was very similar between vehicle- and paclitaxel-treated mice (Figure 4C), suggesting no functional up-regulation of kinin receptors.
Figure 4.
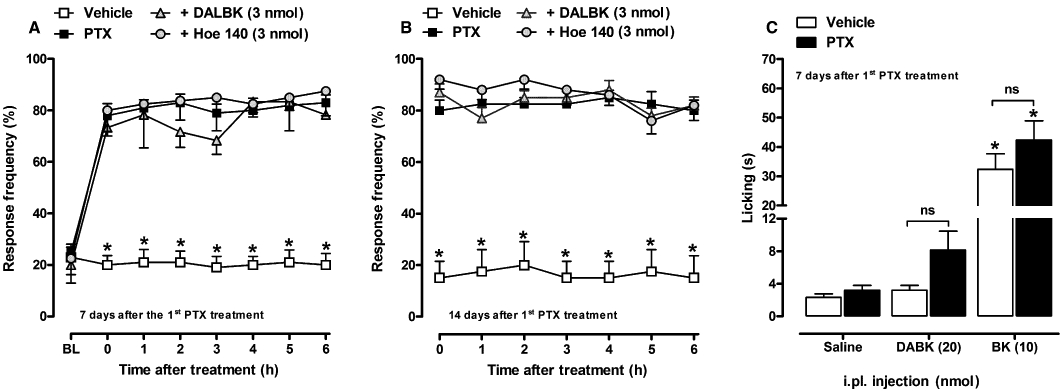
Effect of i.pl. treatment with selective kinin B1 or B2 receptor antagonists, DALBK (3 nmol per paw, i.pl.) and Hoe 140 (3 nmol per paw, i.pl.), respectively, on paclitaxel-induced mechanical hyperalgesia in CD1 mice. A single injection of the drugs was given 7 (A) and 14 (B) days after the first paclitaxel (PTX) injection. (C) Effect of the i.pl. injection of kinin B1 or B2 receptor agonists, DABK (20 nmol per paw, i.pl.) and BK (10 nmol per paw, i.pl.), respectively, on licking behaviour in vehicle- and paclitaxel-treated CD1 mice. Each group represents the mean of five to six animals, and the error bars indicate the SEM. (A, B) *P < 0.05, significantly different from paclitaxel-treated mice (two-way anova followed by Bonferroni post-test). BL, baseline withdrawal threshold. (C) *P < 0.05, significantly different from saline- (i.pl.) injected mice (one-way anova followed by the Newman–Keuls post-test).
In order to verify the possible involvement of central pathways in the modulatory actions of kinin receptors on the established hyperalgesia induced by paclitaxel, animals were treated by the i.t. or i.c.v. route with the selective kinin receptor antagonists. The administration of DALBK (10 pmol) or Hoe 140 (100 pmol) by the i.t. route, at 7 or 14 days after the first paclitaxel injection, significantly inhibited mechanical hyperalgesia for up to 4–5 h after the treatment (Figure 5A,B; Table 1). Interestingly, i.c.v. treatment with DALBK (10 pmol) or Hoe 140 (100 pmol) failed to significantly alter paclitaxel-induced mechanical hyperalgesia, when administered on the seventh day (Figure 5C). However, when the same group of mice received a second i.c.v. injection of DALBK (10 pmol), at 14 days after the first paclitaxel treatment, the inhibitory effect of the drug was observed for up to 5 h (Figure 5D; Table 1), suggesting an over-expression of kinin B1 receptor protein at mouse supra-spinal sites. Hoe 140 (100 pmol) i.c.v. treatment remained ineffective at day 14 (Figure 5D).
Figure 5.
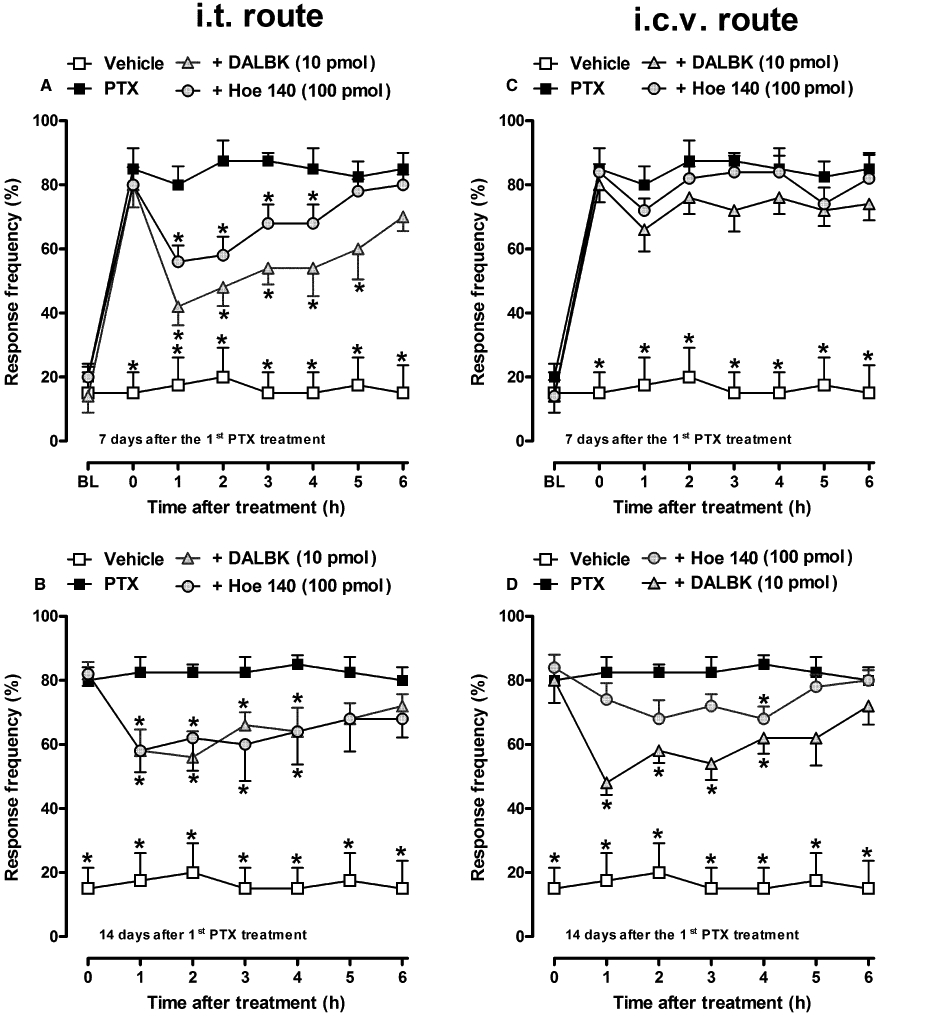
Effect of i.t. (A, B) or i.c.v. (C, D) treatment with selective kinin B1 or B2 receptor antagonists, DALBK (10 pmol) and Hoe 140 (100 pmol), respectively, on paclitaxel-induced mechanical hyperalgesia in CD1 mice. A single injection of the drugs was given 7 (A, C) and 14 (B, D) days after the first paclitaxel (PTX) injection. Each group represents the mean of five to six animals, and the error bars indicate the SEM. *P < 0.05, significantly different from paclitaxel-treated mice (two-way anova followed by Bonferroni post-test). BL, baseline withdrawal threshold.
Effect of treatment with paclitaxel on the levels of kinin B1 receptor mRNA in peripheral and central tissues
To evaluate the effects of paclitaxel injections on the expression of kinin B1 receptors in peripheral (plantar skin and DRG) and central (spinal cord, thalamus, hypothalamus, parietal cortex and pre-frontal cortex) structures, the mRNA levels of these receptors were evaluated by means of real-time RT-PCR in vehicle- and paclitaxel-treated mice at 7 and 14 days after the first paclitaxel treatment (Figure 6). Basal expression of B1 receptors was detected in plantar hind paw skin, DRG (L4–L6), spinal cord (L4–L6) and supra-spinal structures of vehicle-treated mice (Figure 6A–F). The 5 day treatment with single paclitaxel injections induced an over-expression of kinin B1 receptor transcripts in the mouse thalamus and pre-frontal cortex from 7 days after the first paclitaxel treatment (Figure 6E,F) when compared to the control animals. Curiously, paclitaxel administration reduced the basal level of kinin B1 receptor expression in the mouse hypothalamus from 7 days. However, no significant difference was observed for kinin B1 receptor mRNA levels between vehicle- and paclitaxel-treated groups in plantar skin, DRG, spinal cord (Figure 6A–C) or parietal cortex (data not shown).
Figure 6.
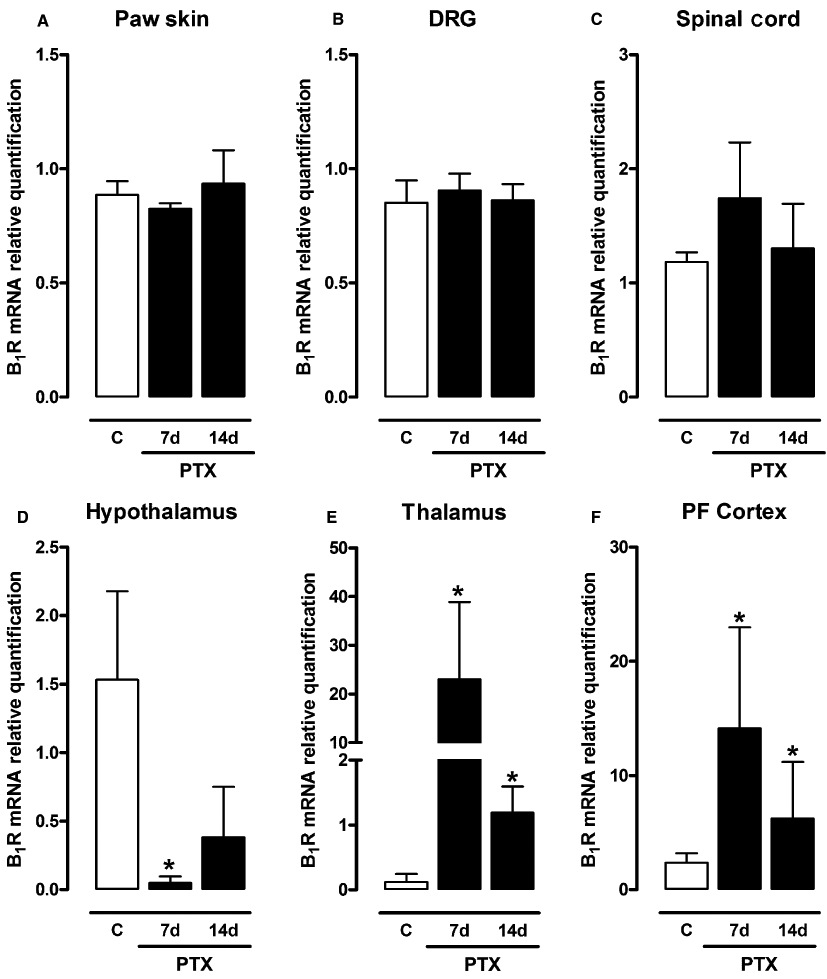
Levels of expression of kinin B1R mRNA in mouse paw skin (A), DRG (L4–L6) (B), spinal cord (L4–L6) (C), hypothalamus (D), thalamus (E) and pre-frontal (PF) cortex (D), 7 and 14 days after the first paclitaxel (PTX) treatment in CD1 mice, assessed by real-time RT-PCR assay. All data have been normalized for levels of GAPDH expression within the same sample. Each bar represents the mean SEM of three to four mice. *P < 0.05, significantly different from vehicle-treated mice (Student's t-test).
Discussion
Peripheral neurotoxicity induced by chemotherapy is one of the leading causes of neuropathic pain in humans (Wolf et al., 2008). Despite much effort, there are so far no available pharmacotherapies providing satisfactory pain relief for patients with persistent pain (O'Connor and Dworkin, 2009). Hence, understanding the mechanisms underlying this syndrome is critical to permit the discovery of new molecular targets with the intent to develop effective analgesic drugs. Relevantly, it has been proposed that chronic pain is perhaps the most promising area where kinin receptor antagonists could prove to be useful, although the B2 receptor subtype has become less attractive than the B1 receptor because of the potential detrimental consequences of its antagonism, especially in the cardiovascular system (Alfie et al., 1997; 1999;). On the other hand, B1 receptors can be up-regulated in inflamed or damaged tissues, and might constitute a more attractive target to the development of analgesic drugs (Campos et al., 2006; Huang and Player, 2010). In this study, we provide convincing evidence implicating both kinin receptors in mechanical and thermal hyperalgesia induced by the chemotherapeutic agent paclitaxel. We have made the following major findings: (i) after chronic treatment with paclitaxel, kinin B1 or B2 receptor-deficient mice exhibited a lower frequency of response to both mechanical and thermal stimuli when compared to wild-type littermates; (ii) the treatment of mice with the selective kinin B1 or B2 receptor antagonists, given by different routes, reduced paclitaxel-induced hypernociceptive responses; and (iii) repeated injections of paclitaxel induced an over-expression of kinin B1 receptor mRNA in mouse supra-spinal structures.
Previous studies have demonstrated the contribution of the kallikrein–kinin system to the development and/or maintenance of neuropathic pain resulting from nerve injury or diabetes in rodents (Petersen et al., 1998; Eckert et al., 1999; Levy and Zochodne, 2000; Gabra and Sirois, 2002; 2003; Yamaguchi-Sase et al., 2003; Rashid et al., 2004; Ferreira et al., 2005; Lai et al., 2006; Werner et al., 2007; Petcu et al., 2008; Quintão et al., 2008). Recently, an important role played by kinin receptor activation has also been suggested in peripheral neuropathy induced by chemotherapy (Bujalska et al., 2008; Bujalska and Makulska-Nowak, 2009a,b;). Besides, the use of kinin receptor knock-out mice has led to a better understanding of the role played by kinins in chronic pain of inflammatory and neuropathic origin, supporting the notion that kinin receptors could be new targets for the development of analgesic drugs used for chronic pain relief (Ferreira et al., 2002b; 2005; Lai et al., 2006; Quintão et al., 2008).
In agreement with these earlier findings, our first set of results clearly implicated both kinin B1 and B2 receptors in the hypernociceptive responses caused by paclitaxel in mice. Accordingly, mechanical and thermal hyperalgesia induced by paclitaxel were clearly reduced in B1R−/−, B2R−/− and B1B2R−/− mice (Figure 1). B2 receptor activation is responsible for mediating kinin effects mainly in the acute phases of inflammation or pain, while the B1 receptor normally mediates its actions in later stages (see Marceau and Regoli, 2004). In contrast, the results presented here indicate an equivalent contribution of both kinin B1 and B2 receptors during the entire evaluation period of paclitaxel-induced hyperalgesia (Figure 1A,B). In line with these findings, previous studies have demonstrated a similar importance of both kinin receptors for long-lasting neuropathic hypernociceptive responses in rodents (Lai et al., 2006; Werner et al., 2007; Petcu et al., 2008). Of relevance, the deletion of both kinin receptors was more efficacious in inhibiting paclitaxel-induced hyperalgesia than the single ablation of B1 receptors or B2 receptor (Figure 1C,D).
Our next step was to verify the contribution of B1 or B2 receptors to the genesis of paclitaxel-induced nociceptive responses in CD1 mice. The results presented here permitted us to suggest that kinin receptors are not implicated in the development of paclitaxel-induced hyperalgesia in mice by the fact that the preventive actions of selective antagonists (DALBK or Hoe 140) on mechanical and thermal hyperalgesia were manifested for only 24 h after the last drug treatment and disappeared after 48 h (Figure 2). In spite of their ineffectiveness on the establishment of mechanical and thermal hyperalgesia, both kinin receptor antagonists, given as a single or repeated injections (twice a day for 2 days), had a prominent anti-nociceptive effect on established hyperalgesia caused by paclitaxel, as assessed 7 and 14 days after the first chemotherapeutic injection (Figure 3). These findings partially conflict with our previous data showing that the kinin B1 receptor was involved not only in the maintenance, but also in the generation of chronic pain induced by peripheral nerve injury in mice (Ferreira et al., 2005; Quintão et al., 2008). However, the present results are in full accordance with several studies demonstrating the anti-nociceptive effect of kinin receptor antagonists on the maintenance of long-lasting pain in experimental models (Ferreira et al., 2002b; Werner et al., 2007; Petcu et al., 2008).
To our surprise, despite good reproduction of mechanical hyperalgesia (∼90%) in all experiments performed in C57BL/6 and CD1 mice, the development of thermal hypernociceptive response was not well reproduced in all experiments conducted in the CD1 strain (data not shown). Indeed, the development of thermal hyperalgesia in paclitaxel-induced peripheral neuropathy is still a variable finding among different studies, which have reported thermal hyperalgesia, thermal hypoalgesia or no change in thermal nociception (Cavaletti et al., 1995; Campana et al., 1998; Cliffer et al., 1998). Thus, to avoid excessive repetition of experiments and the consequent overuse of CD1 mice, we decided to assess the peripheral and central effects of kinin receptor antagonists only on mechanical hyperalgesia.
To examine the involvement of peripheral kinin receptors in paclitaxel-evoked hyperalgesia, we next assessed the local (i.pl.) effect of selective kinin B1 or B2 receptor antagonists on the mechanical hypernociceptive response. The peripheral blockade of B1 or B2 receptors by the i.pl. treatment with DALBK or Hoe 140, respectively, did not prevent paclitaxel-induced mechanical hyperalgesia (Figure 4A,B), discounting the contribution of both kinin receptors located in peripheral tissues. Corroborating these results, the 5 day treatment with paclitaxel was not able to significantly alter overt nociception induced by the i.pl. injection of kinin B1 or B2 receptor agonists DABK and BK, respectively (Figure 4C), suggesting no functional up-regulation of kinin B1 or B2 receptors in mouse paw skin. In fact, our molecular analysis showed that B1 receptor mRNA was not up-regulated in the mouse paw skin or DRG after paclitaxel treatment (Figure 6A,B), despite its well-documented inducible nature in inflammatory and nociceptive conditions (Calixto et al., 2004). These findings contrast with previous data from our and other groups showing both functional and molecular (mRNA and protein) up-regulation of B1 receptors in peripheral tissues after nerve injury in rodents (Petersen et al., 1998; Rashid et al., 2004; Ferreira et al., 2005; Werner et al., 2007).
The next aim of the present study was to investigate the possible involvement of CNS pathways in the modulatory actions of kinin receptors in paclitaxel-induced hyperalgesia in mice by the use of selective antagonists directly injected into central structures. Noticeably, i.t. administration of the kinin receptor antagonists, DALBK (for B1 receptors) or Hoe 140 (for B2 receptors), reduced the mechanical hypernociceptive response evoked by paclitaxel (Figure 5A,B). These findings are supported by evidence showing that both kinin B1 and B2 receptors are functionally expressed at the level of the spinal cord (Chapman and Dickenson, 1992; Corrêa and Calixto, 1993; Pesquero et al., 2000; Ferreira et al., 2002a; 2004; Fox et al., 2003). Of relevance, i.t. injection of DABK or Tyr8-BK (selective kinin B1 or B2 receptor agonists, respectively) caused thermal and mechanical hyperalgesia in mice (Ferreira et al., 2002a; Fox et al., 2003). Moreover, i.t. treatment with kinin B1 or B2 receptor antagonists was effective against the overt nociception caused by formalin in rodents and scratching behaviour evoked by proteinase-activated receptor (PAR)-2 agonists in mice (Chapman and Dickenson, 1992; Costa et al., 2010). Furthermore, Lai et al. (2006) demonstrated that mechanical and thermal hyperalgesia evoked by spinal nerve ligation in rats was mediated by dynorphin A release at the spinal cord, which acts on both kinin B1 and B2 receptors. Corroborating these data, it has been shown that B1R−/− mice show hypoalgesia in chemical models of nociception, probably related to a reduction in activity-dependent facilitation (the ‘wind-up’ phenomenon) of spinal nociceptive reflexes (Pesquero et al., 2000). Indeed, i.t. treatment with selective kinin B1 receptor antagonists (R-715 or SSR240612) reduced mechanical hyperalgesia caused by brachial plexus avulsion (ABP) in mice or by streptozotocin in rats (Quintão et al., 2008; Talbot et al., 2010). Another interesting result showed here was the inhibitory effect of DALBK, given i.c.v., on paclitaxel-induced mechanical hyperalgesia at later stages (14 days) (Figure 5D). In agreement with these data, Quintão et al. (2008) have previously shown the anti-nociceptive effect of i.c.v. injected kinin B1 receptor antagonists on ABP in mice.
Collectively, our results strongly suggest a central involvement (at both spinal and supra-spinal levels) of kinin receptors in the hyperalgesia caused by paclitaxel. However, it is well known that the blood–brain barrier (BBB) is only permeable to small peptide molecules (Begley and Brightman, 2003). Therefore, an intriguing question raised in this study is how systemic (i.p.) treatment with the kinin receptor antagonists, themselves peptides, could inhibit the paclitaxel-evoked hypernociceptive responses. First, we might infer that paclitaxel treatments had broken down the integrity of the BBB, allowing the passage of kinin receptor antagonists to central structures. Indeed, it has previously been reported that neuropathic pain states alter permeability of cerebral and spinal cord BBB in experimental models (Gordh et al., 2006; Beggs et al., 2010). Another plausible explanation for systemic (but not i.pl.) effect of kinin receptor antagonists is that these drugs would be acting on sites other than plantar nociceptors, such as DRG. In fact, interactions between neurons and satellite glial cells and/or leucocytes in the DRG can contribute to neuropathic pain in rodents (Hu and McLachlan, 2002; Capuano et al., 2009). However, further experiments are needed to better clarify these hypotheses.
In spite of the inducible nature of the kinin B1 receptor, its constitutive presence in rodent and monkey sensory neurons has been frequently described (Levy and Zochodne, 2000; Ma and Heavens, 2000; Wothersponn and Winter, 2000; Yamaguchi-Sase et al., 2003; Ferreira et al., 2005; Quintão et al., 2008). Additionally, after peripheral nerve injury (by partial sciatic nerve ligation or ABP) kinin B1 receptor mRNA or protein is up-regulated in mouse plantar surface tissue, DRG, spinal cord, hypothalamus, hippocampus, thalamus and cortex at distinct time points after nerve damage (Rashid et al., 2004; Ferreira et al., 2005; Quintão et al., 2008). Thus, as a final goal of this study, we sought to investigate the effects of paclitaxel on the expression of kinin B1 receptor mRNA in peripheral and central tissues at 7 and 14 days after paclitaxel injection. Interestingly, no significant change in the expression of B1 receptor mRNA in the mouse parietal cortex (data not shown), plantar skin, DRG or spinal cord (Figure 6A–C) was observed between vehicle- and paclitaxel-treated animals, implicating spinal constitutive B1 receptor expression in the anti-nociceptive actions of i.t. injected DALBK (Figure 5A,B). On the other hand, we have shown a marked increase in B1 receptor mRNA in the mouse thalamus and pre-frontal cortex from 7 days after the first paclitaxel treatment (Figure 6E,F), which probably precedes the augmented expression of B1 receptor protein, which could explain the anti-nociceptive effect of i.c.v. injected DALBK only at 14 days (Figure 5D). Curiously, paclitaxel treatment caused a significant decrease in B1 receptor transcript in the mouse hypothalamus from 7 days. So far, we are unable to explain this result; however, it is unlikely that this fact is related to the pro-nociceptive role of kinin B1 receptors in paclitaxel-induced peripheral neuropathy. Unlike our findings on kinin B1 receptor, we did not obtain pharmacological evidence for increased function and/or expression of B2 receptors in either peripheral or central tissues after paclitaxel administration (Figures 4 and 5). Thus, the expression level of B2 receptor transcript was not evaluated in the present study.
A major novelty of this study are the findings that kinins acting on both receptors play a critical role in controlling nociceptive signalling in the model of paclitaxel-induced neuropathic hyperalgesia in mice. First, the deletion of kinin B1 or B2 receptors prevented mechanical and thermal hyperalgesia induced by paclitaxel. Second, the treatment of mice with the selective kinin B1 or B2 receptor antagonists potently inhibited paclitaxel-induced nociceptive responses, when given by the systemic, i.t. or i.c.v. route. Finally, paclitaxel treatments caused an over-expression of B1 receptors in the mouse supra-spinal structures. This evidence supports the notion that selective kinin receptor antagonists (mainly against the B1 receptor subtype) might represent new and attractive therapeutic options for treating chronic pain generated by chemotherapy.
Acknowledgments
This work was supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), the Programa de Apoio aos Núcleos de Excelência (PRONEX) and the Fundação de Apoio à Pesquisa do Estado de Santa Catarina (FAPESC). R.C., R.C.D. and A.F.B. are PhD pharmacology students funded by CNPq. M.N.M. is a PhD pharmacology student supported by CAPES. E.M.M. is a recipient of a post-doctoral grant from CNPq. The authors gratefully thank Ana Paula Luiz and Maíra Bicca for i.t and i.c.v. injections respectively.
Glossary
Abbreviations
- ABP
brachial plexus avulsion
- B1R−/−
B1 receptor deficient
- B2R−/−
B2 receptor deficient
- B1B2R−/−
B1 and B2 receptor deficient
- BBB
blood-brain barrier
- DABK
des-Arg9-BK
- DALBK
des-Arg9-Leu8-BK
- DRG
dorsal root ganglia
Conflict of interest
The authors state no conflicts of interest.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edn. Br J Pharmacol. 2009;158(Suppl. 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfie ME, Sigmon DH, Pomposiello SI, Carretero OA. Effect of high salt intake in mutant mice lacking bradykinin-B2 receptors. Hypertension. 1997;29:483–487. doi: 10.1161/01.hyp.29.1.483. [DOI] [PubMed] [Google Scholar]
- Alfie ME, Alim S, Mehta D, Shesely EG, Carretero OA. An enhanced effect of arginine vasopressin in bradykinin B2 receptor null mutant mice. Hypertension. 1999;33:1436–1440. doi: 10.1161/01.hyp.33.6.1436. [DOI] [PubMed] [Google Scholar]
- Beggs S, Liu XJ, Kwan C, Salter MW. Peripheral nerve injury and TRPV1-expressing primary afferent C-fibers cause opening of the blood–brain barrier. Mol Pain. 2010;6:74. doi: 10.1186/1744-8069-6-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley DJ, Brightman MW. Structural and functional aspects of the blood–brain barrier. Prog Drug Res. 2003;61:39–78. doi: 10.1007/978-3-0348-8049-7_2. [DOI] [PubMed] [Google Scholar]
- Bujalska M, Makulska-Nowak H. Bradykinin receptor antagonists and cyclooxygenase inhibitors in vincristine- and streptozotocin-induced hyperalgesia. Pharmacol Rep. 2009a;61:631–640. doi: 10.1016/s1734-1140(09)70115-x. [DOI] [PubMed] [Google Scholar]
- Bujalska M, Makulska-Nowak H. Bradykinin receptors antagonists and nitric oxide synthase inhibitors in vincristine and streptozotocin induced hyperalgesia in chemotherapy and diabetic neuropathy rat model. Neuro Endocrinol Lett. 2009b;30:144–152. [PubMed] [Google Scholar]
- Bujalska M, Tatarkiewicz J, Gumułka SW. Effect of bradykinin receptor antagonists on vincristine- and streptozotocin-induced hyperalgesia in a rat model of chemotherapy-induced and diabetic neuropathy. Pharmacology. 2008;81:158–163. doi: 10.1159/000110788. [DOI] [PubMed] [Google Scholar]
- Calixto JB, Medeiros R, Fernandes ES, Ferreira J, Cabrini DA, Campos MM. Kinin B1 receptors: key G-protein-coupled receptors and their role in inflammatory and painful processes. Br J Pharmacol. 2004;143:803–818. doi: 10.1038/sj.bjp.0706012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campana WM, Eskeland N, Calcutt NA, Misasi R, Myers RR, O'Brien JS. Prosaptide prevents paclitaxel neurotoxicity. Neurotoxicology. 1998;19:237–244. [PubMed] [Google Scholar]
- Campos MM, Leal PC, Yunes RA, Calixto JB. Non-peptide antagonists for kinin B1 receptors: new insights into their therapeutic potential for the management of inflammation and pain. Trends Pharmacol Sci. 2006;27:646–651. doi: 10.1016/j.tips.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Capuano A, De Corato A, Lisi L, Tringali G, Navarra P, Dello Russo C. Proinflammatory-activated trigeminal satellite cells promote neuronal sensitization: relevance for migraine pathology. Mol Pain. 2009;5:43. doi: 10.1186/1744-8069-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaletti G, Tredici G, Braga M, Tazzari S. Experimental peripheral neuropathy induced in adult rats by repeated intraperitoneal administration of taxol. Exp Neurol. 1995;133:64–72. doi: 10.1006/exnr.1995.1008. [DOI] [PubMed] [Google Scholar]
- Cayla C, Todiras M, Iliescu R, Saul VV, Gross V, Pilz B, et al. Mice deficient for both kinin receptors are normotensive and protected from endotoxin-induced hypotension. FASEB J. 2007;21:1689–1698. doi: 10.1096/fj.06-7175com. [DOI] [PubMed] [Google Scholar]
- Chapman V, Dickenson AH. The spinal and peripheral roles of bradykinin and prostaglandins in nociceptive processing in the rat. Eur J Pharmacol. 1992;219:427–433. doi: 10.1016/0014-2999(92)90484-l. [DOI] [PubMed] [Google Scholar]
- Cliffer KD, Siuciak JA, Carson SR, Radley HE, Park JS, Lewis DR, et al. Physiological characterization of taxol-induced large-fiber sensory neuropathy in the rat. Ann Neurol. 1998;43:46–55. doi: 10.1002/ana.410430111. [DOI] [PubMed] [Google Scholar]
- Corrêa CR, Calixto JB. Evidence for participation of B1 and B2 kinin receptors in formalin-induced nociceptive response in the mouse. Br J Pharmacol. 1993;110:193–198. doi: 10.1111/j.1476-5381.1993.tb13791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa R, Fernandes ES, Menezes-de-Lima O, Jr, Campos MM, Calixto JB. Effect of novel selective non-peptide kinin B(1) receptor antagonists on mouse pleurisy induced by carrageenan. Peptides. 2006;27:2967–2975. doi: 10.1016/j.peptides.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Costa R, Manjavachi MN, Motta EM, Marotta DM, Juliano L, Torres HA, et al. The role of kinin B1 and B2 receptors in the scratching behaviour induced by proteinase-activated receptor-2 agonists in mice. Br J Pharmacol. 2010;159:888–897. doi: 10.1111/j.1476-5381.2009.00571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couture R, Harrisson M, Vianna RM, Cloutier F. Kinin receptors in pain and inflammation. Eur J Pharmacol. 2001;429:161–176. doi: 10.1016/s0014-2999(01)01318-8. [DOI] [PubMed] [Google Scholar]
- Dina OA, Chen X, Reichling D, Levine JD. Role of protein kinase Cepsilon and protein kinase A in a model of paclitaxel-induced painful peripheral neuropathy in the rat. Neuroscience. 2001;108:507–515. doi: 10.1016/s0306-4522(01)00425-0. [DOI] [PubMed] [Google Scholar]
- Dougherty PM, Cata JP, Cordella JV, Burton A, Weng HR. Taxol-induced sensory disturbance is characterized by preferential impairment of myelinated fiber function in cancer patients. Pain. 2004;109:132–142. doi: 10.1016/j.pain.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Eckert A, Segond von Banchet G, Sopper S, Petersen M. Spatio-temporal pattern of induction of bradykinin receptors and inflammation in rat dorsal root ganglia after unilateral nerve ligation. Pain. 1999;83:487–497. doi: 10.1016/S0304-3959(99)00152-9. [DOI] [PubMed] [Google Scholar]
- Ferreira J, Campos MM, Araújo R, Bader M, Pesquero JB, Calixto JB. The use of kinin B1 and B2 receptor knockout mice and selective antagonists to characterize the nociceptive responses caused by kinins at the spinal level. Neuropharmacology. 2002a;43:1188–1197. doi: 10.1016/s0028-3908(02)00311-8. [DOI] [PubMed] [Google Scholar]
- Ferreira J, Campos MM, Pesquero JB, Araújo RC, Bader M, Calixto JB. Evidence for the participation of kinins in Freund's adjuvant-induced inflammatory and nociceptive responses in kinin B1 and B2 receptor knockout mice. Neuropharmacology. 2002b;41:1006–1012. doi: 10.1016/s0028-3908(01)00142-3. [DOI] [PubMed] [Google Scholar]
- Ferreira J, Da Silva GL, Calixto JB. Contribution of vanilloid receptors to the overt nociception induced by B2 kinin receptor activation in mice. Br J Pharmacol. 2004;141:787–794. doi: 10.1038/sj.bjp.0705546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira J, Beirith A, Mori MAS, Araújo RC, Bader M, Pesquero JB, et al. Reduced nerve injury-induced neuropathic pain in kinin B1 receptor knock-out mice. J Neurosci. 2005;25:2405–2415. doi: 10.1523/JNEUROSCI.2466-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira J, Trichês KM, Medeiros R, Cabrini DA, Mori MA, Pesquero JB, et al. The role of kinin B1 receptors in the nociception produced by peripheral protein kinase C activation in mice. Neuropharmacology. 2008;54:597–604. doi: 10.1016/j.neuropharm.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Fox A, Wotherspoin G, McNair K, Hudson L, Patel S, Gentry C, et al. Regulation and function of spinal and peripheral neuronal B1 bradykinin receptors in inflammatory mechanical hyperalgesia. Pain. 2003;104:683–691. doi: 10.1016/S0304-3959(03)00141-6. [DOI] [PubMed] [Google Scholar]
- Gabra BH, Sirois P. Role of bradykinin B(1) receptors in diabetes-induced hyperalgesia in streptozotocin-treated mice. Eur J Pharmacol. 2002;457:115–124. doi: 10.1016/s0014-2999(02)02658-4. [DOI] [PubMed] [Google Scholar]
- Gabra BH, Sirois P. Kinin B1 receptor antagonists inhibit diabetes-induced hyperalgesia in mice. Neuropeptides. 2003;37:36–44. doi: 10.1016/s0143-4179(02)00148-8. [DOI] [PubMed] [Google Scholar]
- Gordh T, Chu H, Sharma HS. Spinal nerve lesion alters blood–spinal cord barrier function and activates astrocytes in the rat. Pain. 2006;124:211–221. doi: 10.1016/j.pain.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Hu P, McLachlan EM. Macrophage and lymphocyte invasion of dorsal root ganglia after peripheral nerve lesions in the rat. Neuroscience. 2002;112:23–38. doi: 10.1016/s0306-4522(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Huang H, Player MR. Bradykinin B1 receptor antagonists as potential therapeutic agents for pain. J Med Chem. 2010;53:5383–5389. doi: 10.1021/jm1000776. [DOI] [PubMed] [Google Scholar]
- Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- Jensen TS, Baron R. Translation of symptoms and signs into mechanisms in neuropathic pain. Pain. 2003;102:1–8. doi: 10.1016/s0304-3959(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Lai J, Luo MC, Chen Q, Ma S, Gardell LR, Ossipov MH, et al. Dynorphin A activates bradykinin receptors to maintain neuropathic pain. Nat Neurosci. 2006;9:1534–1540. doi: 10.1038/nn1804. [DOI] [PubMed] [Google Scholar]
- Laursen SE, Belknap JK. Intracerebroventricular injections in mice: some methodological refinements. J Pharmacol Methods. 1986;16:355–357. doi: 10.1016/0160-5402(86)90038-0. [DOI] [PubMed] [Google Scholar]
- Levy D, Zochodne DW. Increased mRNA expression of the B1 and B2 bradykinin receptors and antinociceptive effects of their antagonists in na animal model of neuropathic pain. Pain. 2000;86:265–271. doi: 10.1016/S0304-3959(00)00256-6. [DOI] [PubMed] [Google Scholar]
- Ma QP. The expression of bradykinin B1 receptors on primary sensory neurones that give rise to small calibre sciatic nerve fibres in rats. Neuroscience. 2001;107:665–673. doi: 10.1016/s0306-4522(01)00387-6. [DOI] [PubMed] [Google Scholar]
- Ma QP, Heavens R. Basal expression of bradykinin B(1) receptor in the spinal cord in humans and rats. Neuroreport. 2000;12:2311–2314. doi: 10.1097/00001756-200108080-00006. [DOI] [PubMed] [Google Scholar]
- Marceau F, Regoli D. Bradykinin receptor ligands: therapeutic perspectives. Nat Rev Drug Discov. 2004;3:845–852. doi: 10.1038/nrd1522. [DOI] [PubMed] [Google Scholar]
- Menéndez L, Lastra A, Hidalgo A, Baamonde A. Unilateral hot plate test: a simple and sensitive method for detecting central and peripheral hyperalgesia in mice. J Neurosci Methods. 2002;113:91–97. doi: 10.1016/s0165-0270(01)00483-6. [DOI] [PubMed] [Google Scholar]
- O'Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med. 2009;122:S22–S32. doi: 10.1016/j.amjmed.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Pesquero JB, Araujo RC, Heppenstall PA, Stucky CL, Silva JA, Jr, Walther T, et al. Hypoalgesia and altered inflammatory responses in mice lacking kinin B1 receptors. Proc Natl Acad Sci U S A. 2000;97:8140–8145. doi: 10.1073/pnas.120035997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petcu M, Dias JP, Ongali B, Thibault G, Neugebauer W, Couture R. Role of kinin B1 and B2 receptors in a rat model of neuropathic pain. Int Immunopharmacol. 2008;8:188–196. doi: 10.1016/j.intimp.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Petersen M, Eckert AS, Segond von Banchet G, Heppelmann B, Klusch A, Kniffki KD. Plasticity in the expression of bradykinin binding sites in sensory neurons after mechanical nerve injury. Neuroscience. 1998;83:949–959. doi: 10.1016/s0306-4522(97)00465-x. [DOI] [PubMed] [Google Scholar]
- Polomano RC, Bennett GJ. Chemotherapy-evoked painful peripheral neuropathy. Pain Med. 2001;2:8–14. doi: 10.1046/j.1526-4637.2001.002001008.x. [DOI] [PubMed] [Google Scholar]
- Polomano RC, Mannes AJ, Clark US, Bennett GJ. A painful peripheral neuropathy in the rat produced by the chemotherapeutic drug, paclitaxel. Pain. 2001;94:293–230. doi: 10.1016/S0304-3959(01)00363-3. [DOI] [PubMed] [Google Scholar]
- Quintão NL, Passos GF, Medeiros R, Paszcuk AF, Motta FL, Pesquero JB, et al. Neuropathic pain-like behavior after brachial plexus avulsion in mice: the relevance of kinin B1 and B2 receptors. J Neurosci. 2008;28:2856–2863. doi: 10.1523/JNEUROSCI.4389-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid MH, Inoue M, Matsumoto M, Ueda H. Switching of bradykinin-mediated nociception following partial sciatic nerve injury in mice. J Pharmacol Exp Ther. 2004;308:1158–1164. doi: 10.1124/jpet.103.060335. [DOI] [PubMed] [Google Scholar]
- Rupniak NM, Boyce S, Webb JK, Williams AR, Carlson EJ, Hill RG, et al. Effects of the bradykinin B1 receptor antagonist des-Arg9[Leu8]bradykinin and genetic disruption of the B2 receptor on nociception in rats and mice. Pain. 1997;71:89–97. doi: 10.1016/s0304-3959(97)03343-5. [DOI] [PubMed] [Google Scholar]
- Talbot S, Chahmi E, Dias JP, Couture R. Key role for spinal dorsal horn microglial kinin B1 receptor in early diabetic pain neuropathy. J Neuroinflammation. 2010;7:36. doi: 10.1186/1742-2094-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner MF, Kassuya CA, Ferreira J, Zampronio AR, Calixto JB, Rae GA. Peripheral kinin B(1) and B(2) receptor-operated mechanisms are implicated in neuropathic nociception induced by spinal nerve ligation in rats. Neuropharmacology. 2007;53:48–57. doi: 10.1016/j.neuropharm.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C. Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. Eur J Cancer. 2008;44:1507–1515. doi: 10.1016/j.ejca.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptons, mechanisms, and management. Lancet. 1999;353:1959–1965. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- Wothersponn G, Winter J. Bradykinin B1 receptor is constitutively expressed in the rat sensory nervous system. Neurosci Lett. 2000;294:175–178. doi: 10.1016/s0304-3940(00)01561-5. [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Sase S, Hayashi I, Okamoto H, Nara Y, Matsuzaki S, Hoka S, et al. Amelioration of hyperalgesia by kinin receptor antagonists or kininogen deficiency in chronic constriction nerve injury in rats. Inflamm Res. 2003;52:164–169. doi: 10.1007/s000110300067. [DOI] [PubMed] [Google Scholar]


