Abstract
In cochlear implants (CIs), simultaneous or sequential stimulation of adjacent electrodes can produce intermediate pitch percepts between those of the component electrodes. However, it is unclear whether simultaneous and sequential virtual channels (VCs) can be discriminated. In this study, CI users were asked to discriminate simultaneous and sequential VCs; discrimination was measured for monopolar (MP) and bipolar + 1 stimulation (BP + 1), i.e., relatively broad and focused stimulation modes. For sequential VCs, the interpulse interval (IPI) varied between 0.0 and 1.8 ms. All stimuli were presented at comfortably loud, loudness-balanced levels at a 250 pulse per second per electrode (ppse) stimulation rate. On average, CI subjects were able to reliably discriminate between sequential and simultaneous VCs. While there was no significant effect of IPI or stimulation mode on VC discrimination, some subjects exhibited better VC discrimination with BP + 1 stimulation. Subjects’ discrimination between sequential and simultaneous VCs was correlated with electrode discrimination, suggesting that spatial selectivity may influence perception of sequential VCs. To maintain equal loudness, sequential VC amplitudes were nearly double those of simultaneous VCs, presumably resulting in a broader spread of excitation. These results suggest that perceptual differences between simultaneous and sequential VCs might be explained by differences in the spread of excitation.
INTRODUCTION
Although cochlear implant (CI) users are able to understand speech in quiet, speech in background noise and music perception are difficult for even the best CI performers. CI performance in difficult listening conditions is primarily limited by the poor spectral resolution provided by the implant device and signal processing (Shannon et al., 2004). Contemporary CI devices typically utilize 12 to 22 “physical” channels (i.e., the implanted intracochlear electrodes). To increase the number of stimulation sites beyond the number of implanted electrodes, virtual channels (VCs) have been implemented by simultaneously delivering in-phase stimulation to two adjacent electrodes, creating a current field with a peak located between the two electrodes (Busby et al., 2008; Miyoshi et al., 1996). The ratio of current delivered to the two electrodes is designated α, which ranges in value from 0 (100% of the current is delivered to the apical electrode) to 1 (100% of the current is delivered to the basal electrode). When α = 0.5, 50% of the current is delivered to each of the electrodes, resulting in a peak in the current field located midway between the two electrodes.
Simultaneous VC stimulation has been shown to produce a similar amount of current spread as with stimulation of a single electrode (Busby et al., 2008; Bonham and Litvak, 2008). For a fixed amount of current, stimulation of a single electrode and a simultaneous VC have approximately the same loudness (Donaldson et al., 2005; Luo et al., 2010; Frijns et al., 2009). CI users are often able to perceive simultaneous VC pitches intermediate to those of the component electrodes (Donaldson et al., 2005; Firszt et al., 2007). From their data, Firszt et al. (2007) extrapolated that CI users could discriminate ∼5 VC pitches between electrodes spaced approximately 1 mm apart. Advanced Bionics has implemented simultaneous VCs in their Fidelity 120 signal processing strategy.
Simultaneous VCs require multiple independent current sources, which are provided by the Advanced Bionics (CI, CII, and HiRes 90k) and MED-El (Pulsar, Sonata, and Concerto) implant devices. Currently, the majority of CI patients have been implanted with Cochlear Corp. devices (e.g., Nucleus-22, Nucleus-24, or Freedom implants), which utilize a single current source. However, previous studies (McDermott and McKay, 1994; Kwon and van den Honert, 2006a; Galvin et al., 2009) found that rapid sequential stimulation of adjacent electrodes produced pitch percepts intermediate to those of the adjacent electrodes, i.e., “sequential VCs.” While simultaneous VCs are created by shaping the current field, sequential VCs are created by shaping the neural excitation pattern. Even though simultaneous and sequential VCs are produced by very different patterns of electrical stimulation, they may not be discriminable if the neural excitation patterns are sufficiently similar.
Frijns et al. (2009) recently modeled excitation patterns for simultaneous and sequential VCs. According to the model, when current was adjusted to provide equal loudness, simultaneous and sequential VCs produced very similar excitation patterns. Assuming that pitch is derived from the centroid of the excitation pattern, they predicted that simultaneous and sequential VCs should provide a similar continuum of VC pitch percepts. The modeling data were confirmed in terms of loudness comparisons between simultaneous and sequential VCs; pitch perception was not directly measured. Saoji et al. (2009) measured spread of neural excitation using electrically evoked compound action potentials (ECAPs) for simultaneous and sequential stimulation of non-adjacent electrodes, i.e., separated by 1 electrode. The area and center of gravity for the simultaneous VCs were similar to those evoked by single-channel stimulation of the intermediate electrode. For sequential VCs, the area and center of gravity were different from those of the intermediate electrode, and were influenced by the stimulation order of the component channels (i.e., apical-first or basal-first). Thus, the modeling data of Frijns et al. (2009) seem to conflict with the ECAP data from Saoji et al. (2009). Further, it is unclear whether simultaneous and sequential VCs can be discriminated, as no previous studies have directly compared simultaneous and sequential VCs, other than in terms of loudness (Frijns et al., 2009). If simultaneous and sequential VCs are not discriminable, then either could be used to increase the number of pitch percepts.
In this study, discrimination was measured between equally-loud simultaneous and sequential VCs. VC discrimination was measured for monopolar (MP) and bipolar + 1 (BP + 1) stimulation modes. MP stimulation is thought to produce broader current spread than bi- or tri-polar stimulation (e.g., Bierer and Middlebrooks, 2002), which could potentially influence the discrimination of simultaneous and sequential VCs. Similarly, some CI users could be sensitive to the pulse timing within sequential VCs (e.g., McKay et al., 1996; Galvin et al., 2009; Machery and Carlyon, 2010), which may affect discrimination of sequential and simultaneous VCs. However, if the IPI is sufficiently short (i.e., within the neural refractory period), pulse timing seems less likely to contribute to the sequential VC percept, potentially making discrimination more difficult between sequential and simultaneous VCs. In this study, VC discrimination was measured between simultaneous and sequential VCs with inter-pulse intervals (IPIs) ranging from 0 to 1.8 ms.
MATERIALS AND METHODS
Subjects
Seven users of the Advanced Bionics Clarion II or HiRes 90K implant device participated in the experiment. All subjects were postlingually deafened. Subjects used the HiRes or the Fidelity 120 speech processing strategies in their clinical processors. All subjects provided informed consent in accordance with local IRB regulations, and all subjects were compensated for their participation. Table TABLE I. shows subject demographics, the experimental electrodes and the stimulation modes. Due to logistical problems, subject C16 was able to complete only the MP condition.
Table 1.
CI subject demographics. The component electrodes used for the MP and BP+1 VCs are listed at right.
| VC component electrodes | ||||||||
|---|---|---|---|---|---|---|---|---|
| Subject | Age | Gender | Etiology | Prosthesis | Strategy | CI experience (years) | MP | BP+1 |
| C1 | 76 | M | Sudden sensorineural hearing loss | CII | HiRes-P w∕Fidelity 120 | 7 | (14) + (15) | (14,16)+ (15,17) |
| C3 | 54 | F | Genetic | HiRes 90K | HiRes-S w∕Fidelity 120 | 3 | (2) + (3) | (2,4) + (3,5) |
| C4 | 62 | F | Cochlear otosclerosis | CII | HiRes-S | 3.8 | (2) + (3) | (2,4) + (3,5) |
| C7 | 59 | F | Fever + streptomycin | CII | HiRes-P w∕Fidelity 120 | 3 | (7) + (8) | (7,9) + (8,10) |
| C14 | 44 | M | Maternal rubella | HiRes 90k | HiRes-P w∕Fidelity 120 | 4 | (7) + (8) | (7,9) + (8,10) |
| C16 | 56 | F | Unknown | HiRes 90K | HiRes-P w∕Fidelity 120 | 0.7 | (2) + (3) | |
| C17 | 47 | M | Traumatic Head Injury | HiRes 90K | HiRes-S w∕Fidelity 120 | 1 | (2) + (3) | (2,4) + (3,5) |
Stimuli
Stimuli were 300 ms bi-phasic (100 μs∕phase, 0 μs interphase gap), cathodic-first pulse trains; the stimulation rate for each electrode was 250 pulses per second (pps). The stimulation mode was MP or BP + 1. In MP stimulation, there is an “active” intracochlear electrode and an extra-cochlear ground. In BP +1 stimulation, there is an active intracochlear electrode and an intracochlear return, separated by one electrode. For the remainder of this paper, we will refer only to the active electrode for either stimulation mode, i.e., the electrode that provides the cathodic stimulation in the first phase of the bi-phasic pulse. The experimental stimulation modes were selected to compare the effects of presumably different spreads of excitation on VC discrimination. These modes are commonly used in past and present CI clinical speech processing strategies. BP + 1 was also used in a related study by Galvin et al. (2009) that measured sequential VC pitch perception for different IPIs.
Experimental electrodes were selected for each subject according to the electrode pairs that provided the best electrode discrimination in Landsberger and Srinivasan (2009) or Luo et al. (2010), and are listed in Table TABLE I.. For all simultaneous VCs, α = 0.5. For MP stimuli, equal current amplitudes were delivered to adjacent electrodes. For BP + 1 stimuli, current associated with equal loudness was delivered to adjacent electrodes (see loudness-balancing procedures below). For sequential VCs, the IPI (from offset of the first pulse to onset of the second pulse) was 0.0, 0.1, 0.2, 0.3, 0.8, 1.3, or 1.8 ms. All stimuli were presented via standard clinical fitting hardware, controlled by Advanced Bionics’ Bionic Ear Data Collection System (BEDCS, Advanced Bionics Corporation, Sylmar, CA).
Dynamic range estimation and loudness balancing
MP stimuli
For MP VCs (simultaneous or sequential), equal current was delivered to each component electrode. The dynamic range (DR) was estimated by increasing the total current of a 300-ms pulse train in 5 μA steps (2.5 μA∕electrode) from a level below threshold until obtaining the maximal comfort level (MCL). After each stimulus presentation, subjects indicated loudness by pointing to a level along the 11-point scale used in Advanced Bionics’ clinical fitting procedure: 0 — No Sound, 1 — Barely Audible, 2 — Very Soft, 3 — Soft, 4 — Medium Soft, 5 — Medium, 6 — Most Comfortable, 7 —Loud But Comfortable, 8 — Maximal Comfort, 9 — Uncomfortable, 10 — Very Uncomfortable. The minimum current required to reach levels 1 (Barely Audible), 3 (Soft), 6 (Most Comfortable), 8 (Maximal Comfort) were recorded. The procedure ended when “Maximal Comfort” was indicated.
At each IPI, sequential MP VCs were loudness-balanced to the simultaneous MP VC at the “Most Comfortable” listening level. During loudness balancing, the simultaneous and sequential VCs were repeatedly played; the interstimulus interval (ISI) was 300 ms. The subject adjusted the amplitude of the sequential VC by turning a knob (Griffin Powermate) until achieving equal loudness. The current delivered to each channel was globally adjusted in 1 μA steps. The procedure was repeated at least three times for each sequential VC, and the mean loudness-balanced amplitudes were used (along with a ±0.5 dB level rove) as the presentation levels for the subsequent VC discrimination experiment.
BP +1 stimuli
For BP +1 VCs, the DR was first estimated for each component electrode using the same method for estimating the MP DR. The initial current level was 5 μA, and the current was increased in 5 μA steps until the subject indicated the target loudness levels. Next, the basal component electrode was loudness-balanced to the apical component electrode at the “Most Comfortable” listening level. The apical and basal component electrodes were repeatedly played (ISI = 300 ms) and the subject adjusted the level of the basal electrode (by turning the knob) until it was equally loud as the apical electrode. Once equal loudness was obtained, the level difference between component channels was fixed (in dB) for all BP +1 VCs. The DRs for simultaneous and sequential BP +1 VCs were estimated using the same method as for MP VCs. Similarly, all sequential BP +1 VCs were loudness balanced to the simultaneous BP +1 VCs using the same method as for MP VCs.
Equally loud (rather than equal-amplitude) stimuli were used for BP VC stimuli for two reasons. First, BP stimulation typically results in greater variability in thresholds and MCL levels than observed with MP stimulation, even for adjacent electrodes. Due to the relatively broad current spread, MP stimulation typically results in more consistent threshold and MCL levels across electrodes; as such, equal amplitude would be expected to produce equal loudness. Thus, to compensate for the variability across electrodes with BP stimulation, component channels were presented at an equal loudness to ensure that both contributed equally to the VC percept. Second, equally loud component channels for BP VCs were used to be consistent with previous, related work (Galvin et al., 2009), while equal-amplitude component channels were used for MP VCs to be consistent with previous studies (e.g., Donaldson et al., 2005; Landsberger and Srinivasan, 2009).
Virtual channel discrimination
VC discrimination was tested for each stimulation mode within different test blocks; the test order for the mode conditions was randomized within and across subjects. Within each mode condition, each sequential VC was compared to the simultaneous VC 30 times. All IPIs for the sequential VCs were included in the stimulus set. VC discrimination was measured using a three-interval forced-choice (3IFC) task. In half of the trials, two of the stimuli were simultaneous VCs and the third was the sequential VC; in the other half, two of the stimuli were sequential VCs (with the same IPI) and the third was the simultaneous VC. Stimuli were randomly assigned to each interval from trial to trial. The stimulus duration was 300 ms and the ISI was 300 ms. All stimuli were presented at loudness-balanced levels with a ±0.5 dB level rove (applied equally to both electrodes) to reduce the possibility of using loudness cues to discriminate between sequential and simultaneous VCs. Note that the ±0.5 dB level rove was more than double the mean range of current adjustments required to loudness balance all stimuli (mean = ±0.22 dB). Subjects were instructed to indicate which of the three stimuli was different in any way other than loudness by pressing a button on a response box. Subjects did not report what cues they used for the discrimination task. No feedback was provided.
RESULTS
Dynamic range and loudness balancing
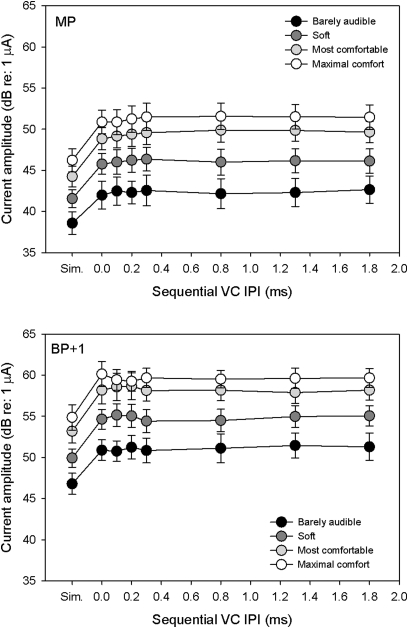
Figure 1 shows mean “Barely Audible,” “Soft,” “Most Comfortable,” and “Maximal Comfort” levels for MP and BP + 1 VCs, as a function of IPI; recall that we were unable to collect BP +1 VC data for subject C16. Within each stimulation mode, and within each loudness level, there was a sharp rise in current between the simultaneous and sequential VC (0 ms IPI), followed by nearly constant current across the sequential VC IPIs. As shown in Fig. 1, the DRs for VCs were largely unaffected by VC type (simultaneous or sequential), stimulation mode or IPI. These results are consistent with other studies that showed little to no change in DR across stimulation modes (e.g., Galvin and Fu, 2005; Litvak et al., 2007; Landsberger and Srinivasan, 2009).
Figure 1.
Mean current levels (across subjects) needed to obtain “Barely audible,” “Soft,” “Most comfortable,” and “Maximal comfort” loudness for MP (top panel) and BP + 1 VCs (bottom panel), as a function of IPI. The error bars show ±1 standard error.
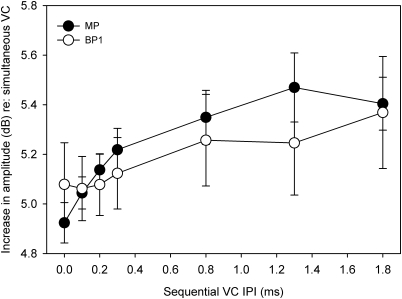
Figure 2 shows the mean amount of additional current needed to balance the loudness of a sequential VC to that of a simultaneous VC, for MP and BP + 1 stimulation modes. For MP stimulation, sequential VCs required an additional 5.22 dB of current to maintain equal loudness to the reference simultaneous VC. Similarly, for BP + 1 stimulation, sequential VCs required an additional 5.17 dB of current to maintain equal loudness to the reference simultaneous VC. For both modes, as the IPI was increased, the amount of compensatory current also increased. A two-way repeated-measures analysis of variance (RM ANOVA) showed a significant effect for IPI (F6,30 = 7.68. p < 0.0005), but not for stimulation mode (F1,5 = 0.006, p > 0.05); there were no significant interactions (F6,30 = 2.13, p > 0.05). Loudness-balanced amplitudes are shown in Table TABLE II.. Post hoc t-tests using Hochberg’s method to control type I error (Hochberg, 1988) revealed significant differences between IPIs of 0.0 and 0.8 ms, 0.0 and 1.8 ms, and 0.2 and 1.8 ms.
Figure 2.
Additional current (in dB re: 1 μa) required for a sequential VC to maintain equal loudness to a simultaneous VC (α = 0.5). Mean data (across subjects) are shown for MP and BP +1 VCs. The error bars show ±1 standard error.
Table 2.
Loudness-balanced amplitudes (in dB re: 1μa) used for VC discrimination. The simultaneous VC column shows the “Most Comfortable” reference amplitudes for MP (top) and BP + 1 stimulation (bottom). The loudness-balanced amplitudes for sequential VCs for the experimental IPIs are shown at right. For each cell for the MP stimuli, the total amplitude (on both the apical and basal electrodes) is presented. For each cell for the BP + 1 stimulation, two values are presented: (1) the apical channel amplitude, and (2) basal channel amplitude.
| Sequential VC - IPI (ms) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Subject | Simultaneous VC | 0.00 | 0.10 | 0.20 | 0.30 | 0.80 | 1.30 | 1.80 | |
| MP | C01 | 46.24 | 51.17 | 51.32 | 51.32 | 51.53 | 51.83 | 51.57 | 51.66 |
| C03 | 39.65 | 44.71 | 44.51 | 44.91 | 45.01 | 45.20 | 45.39 | 45.39 | |
| C04 | 46.44 | 51.11 | 51.25 | 51.37 | 51.24 | 51.27 | 51.30 | 51.43 | |
| C07 | 47.16 | 51.91 | 52.34 | 52.17 | 52.34 | 52.26 | 53.03 | 52.51 | |
| C14 | 49.40 | 54.06 | 54.68 | 54.40 | 54.47 | 54.76 | 54.62 | 54.56 | |
| C16 | 42.92 | 48.15 | 47.89 | 48.32 | 48.38 | 48.51 | 48.50 | 48.45 | |
| C17 | 42.01 | 47.00 | 47.08 | 47.16 | 47.38 | 47.31 | 47.82 | 47.75 | |
| BP+1 | C01 | 48.94∕45.93 | 54.74∕51.73 | 54.51∕51.50 | 54.61∕51.61 | 54.75∕51.75 | 54.96∕51.95 | 55.17∕52.17 | 55.34∕52.34 |
| C03 | 41.43∕41.21 | 46.40∕46.23 | 46.52∕46.36 | 46.40∕46.23 | 46.23∕46.06 | 46.92∕46.76 | 46.52∕46.36 | 46.88∕46.72 | |
| C04 | 44.60∕49.33 | 50.01∕54.74 | 49.95∕54.69 | 49.77∕54.50 | 49.78∕54.52 | 49.95∕54.68 | 50.00∕54.73 | 49.86∕54.63 | |
| C07 | 50.10∕48.29 | 54.65∕52.83 | 54.79∕52.98 | 54.90∕53.08 | 54.84∕53.02 | 54.85∕53.03 | 54.97∕53.16 | 55.09∕53.27 | |
| C14 | 52.04∕50.04 | 56.84∕54.86 | 56.85∕54.87 | 56.79∕54.81 | 57.03∕55.05 | 56.74∕54.75 | 56.62∕54.64 | 56.63∕54.65 | |
| C17 | 48.62∕46.44 | 53.55∕51.35 | 53.46∕51.26 | 53.73∕51.50 | 53.81∕51.61 | 53.83∕51.62 | 53.90∕51.70 | 54.10∕51.89 | |
Virtual channel discrimination
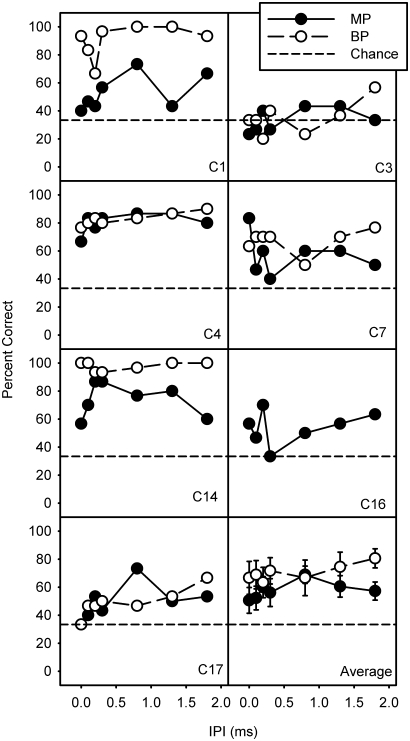
Figure 3 shows the percent correct discrimination between simultaneous and sequential VCs for MP and BP + 1 stimulation, as a function of IPI. VC discrimination was highly variable across subjects, although most subjects (except C3) were able to reliably discriminate between simultaneous and sequential VCs. One sample t-tests showed that discrimination was significantly better than chance for MP (t6 = 4.087, p < 0.006) and BP + 1 (t5 = 3.682, p < 0.014) stimulation modes, suggesting that the subject population was able to discriminate between simultaneous and sequential VCs. Within individual subjects for a given IPI, performance was considered significantly better than chance when discrimination was 53% correct higher, corresponding to the upper limit of the 95% confidence interval (CI) for chance. However, when collapsing across IPI (using the average percent correct for a given subject and stimulation mode), the upper limit of the 95% CI was reduced to 40% correct, suggesting that all subjects (except C3) could discriminate between sequential and simultaneous VCs, for both MP and BP stimulation modes. Among subjects who completed all tests, VC discrimination was similar with MP and BP + 1 stimulation for subjects C3, C4, C7, and C17, but better with BP + 1 stimulation for subjects C1 and C14. A two-way RM ANOVA showed no significant effect for IPI (F1,5 = 3.93, p > 0.05) or stimulation mode (F6,30 = 2.081, p > 0.05); however, there was a significant interaction between mode and IPI (F6,30 = 2.915, p < 0.023). To further investigate the interaction between stimulation mode and IPI, we performed a one-way RM ANOVA within each stimulation mode. There was no significant effect for IPI for MP stimulation (F6,36 = 1.89, p > 0.05); note that data from all 7 subjects were included in the MP data analysis. However, there was a significant effect for IPI for BP + 1 stimulation (F6,30 = 3.515, p < 0.01). Using the Holm-Sidak method for pairwise multiple comparisons to examine the effect of IPI on BP + 1 VCs, only a significant difference between an IPI of 1.8 ms and 0.2 ms was detected, with better discrimination with the 1.8 ms than with the 0.2 ms IPI. Paired t-tests comparing MP and BP + 1 VC discrimination was measured for each IPI. After Bonferroni correction, there was a significant difference between modes only when the IPI = 1.8 ms (t5 = 5.32, p = 0.003).
Figure 3.
Individual and mean discrimination scores between simultaneous and sequential VCs for MP and BP + 1 stimulation, as a function of IPI. The error bars representing ±1 standard error. The dashed line indicates chance level (33% correct). Note that the average data (in the bottom right panel) excludes subject C16, as no BP data was collected for C16.
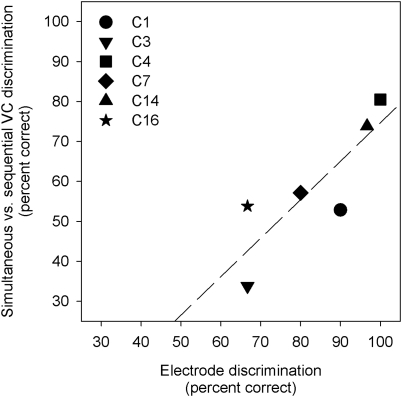
As noted above, there was a significant effect of IPI only for BP +1 stimulation. Nonetheless, subjects were often able to discriminate between sequential and simultaneous VCs with either stimulation mode. The present MP VC discrimination data were compared to MP electrode discrimination data collected from the same subjects in Landsberger and Srinivasan (2009; subjects C1, C3, C4, and C7) and Luo et al. (2010; subjects C14 and C16) ; subject C17 did not participate in the previous studies. Electrode discrimination was measured using the same electrodes as used for VC discrimination in the current study. Figure 4 shows the present sequential vs. simultaneous VC discrimination scores as a function of the previous electrode discrimination scores. VC discrimination was significantly correlated with electrode discrimination (r2 = 0.72, p = 0.034), suggesting some relationship between this simple measure of spatial selectivity and discrimination between simultaneous and sequential VCs.
Figure 4.
Discrimination scores between simultaneous and sequential MP VCs, as a function of MP electrode discrimination scores for the same electrode pairs. The electrode discrimination data are from Landsberger and Srinivasan (2009) and Luo et al. (2010). The dashed line indicates the best linear fit.
DISCUSSION
While previous studies have shown that both simultaneous (Busby and Plant, 2005; Donaldson et al., 2005; Busby et al., 2008) and sequential VCs (Kwon and van den Honert, 2006a; Galvin et al., 2009) can produce intermediate pitch percepts between those of the component electrodes, this is the first study in which simultaneous and sequential VCs have been directly compared (except in terms of loudness by Frijns et al., 2009). The present data suggest that simultaneous and sequential VCs can be discriminated. CI subjects were able to discriminate between simultaneous and sequential VCs significantly better than chance level (33.3% correct).
There was only a weak effect (at best) of IPI for BP + 1 stimulation, and then only between the 0.2 and 1.8 ms IPIs. However, CI subjects were able to reliably discriminate between a simultaneous VC and a sequential VC with no temporal offset (IPI = 0 ms). In general, increasing the IPI well beyond the absolute neural refractory period (∼0.5 ms, according to Miller et al., 2001) did not significantly affect VC discrimination. It is possible that CI subjects may have been able to discriminate simultaneous and sequential VCs without being sensitive to the IPI in the sequential VCs. Previous studies (Galvin et al., 2009; Macherey and Carlyon, 2010) have shown that IPI can significantly affect pitch perception for sequential VCs. In the Galvin et al. (2009) and Macherey and Carlyon (2010) studies, pitch perception was compared between IPIs; in this study, discrimination was measured between simultaneous and sequential VCs for a wide range of IPIs. Overall, temporal cues had a weak, inconsistent, or nonexistent effect on the present data, suggesting that discrimination was driven by other factors. As discussed in greater detail below, discrimination between simultaneous and sequential VCs may be explained by: (1) differences in the spread of excitation due to differences in amplitude between equally loud sequential and simultaneous VCs, (2) differences in place-pitch between sequential and simultaneous, as observed by Saoji et al. (2009), or (3) higher level temporal asynchrony detection in sequential VCs, as documented by Carlyon et al. (2000).
Spread of excitation may influence discrimination of simultaneous and sequential VCs
In order to maintain equal loudness between simultaneous and sequential VCs when α = 0.5, the sequential VC current levels had to be increased (on average) by 5.17 dB for MP stimulation and 5.22 dB for BP + 1 stimulation, as shown in Fig. 1 and Table TABLE II.. The present loudness balancing data are consistent with Frijns et al. (2009); when α = 0.5, sequential VCs required almost a doubling of current to maintain equal loudness to a simultaneous VC. Increased amplitude results in increased current spread (e.g., Chatterjee and Shannon, 1998). Thus, given the greater current needed to maintain a fixed loudness, one would expect a greater current spread for sequential VCs, relative to simultaneous VCs.
The loudness model of Frijns et al. (2009) predicts the peak in the excitation pattern to be similarly located for equally loud simultaneous and sequential VCs, suggesting that differences in excitation peak most likely did not contribute to discrimination of simultaneous and sequential VCs. Instead, the present subjects may have attended to the “skirts” (edges) of the excitation patterns (which would be presumably broader with sequential VCs) when discriminating equally loud simultaneous and sequential VCs. Given the much larger difference in amplitude between simultaneous and sequential VCs (∼5.2 dB) than between sequential VC IPIs (∼0.4 dB), differences in excitation patterns among IPIs were most likely obscured by the generally broader excitation associated with sequential stimulation. Thus, the effect of IPI on VC discrimination was minor at best and subjects most likely attended to the strong contrasts in the spread of excitation between sequential and simultaneous VCs.
The hypothesis that patients are attending to the skirts rather than the peak of the excitation patterns is consistent with Laneau and Wouters (2004), who found that with multichannel stimulation, pitch ranking can be reliably performed by attending to differences in the edges of stimulation. To the extent that the IPI might influence the spread of excitation in a sequential VC, it might be worthwhile to compare pitch-ranking across small α steps for simultaneous and sequential VCs. Pitch ranking could also be directly compared between simultaneous and sequential VCs for fixed α values. If pitch differences were observed, this might explain why simultaneous and sequential VCs could be discriminated, even at short IPIs. Note that Frijns et al. (2009) did not explicitly model selectivity between simultaneous and sequential VCs. ECAP data from Saoji et al. (2009), using dual-channel stimulation of nonadjacent electrodes, showed a broader spread of activation for sequential than for simultaneous stimulation, suggesting that spatial selectivity may be broader with sequential stimulation. For stimulation of adjacent electrodes (as in this study), it is unclear whether this cue may have contributed to the present results.
While there was no significant effect of stimulation mode on VC discrimination, some subjects performed much better with BP +1 than MP stimulation. Focused stimulation modes are thought to produce more spatially compact excitation patterns (e.g., Bierer and Middlebrooks, 2002; Srinivasan et al., 2010). However, for a fixed loudness (and therefore different current amplitudes), the spread of excitation is not consistently narrower with bipolar than with monopolar stimulation (Kwon and van den Honert, 2006b). Four of the present subjects (C3, C4, C7, C17) performed similarly with MP and BP stimulation. If subjects were indeed attending to the skirts of the excitation pattern, it is possible that the spread of excitation was similar for these equally-loud simultaneous and sequential VCs. Conversely, for two subjects (C1 and C14) who performed better with BP + 1 than with MP stimulation, the spread of excitation may have been different between stimulation modes. These subjects may have better attended to the skirts of focused BP + 1 excitation than to the skirts of broad MP excitation. It is worth noting that although BP is generally considered a narrower spread of excitation than MP stimulation, the BP VC configuration consisted of two physically overlapping BP stimuli, it is unclear whether or not there would be more or less overlap between the BP or MP VCs used in this experiment.
Place pitch cues may influence discrimination of simultaneous and sequential VCs
Contrary to the Frijns et al. (2009) model predictions, ECAP data from Saoji et al. (2009) showed that the peak of the forward-masking functions with sequential VCs was shifted relative to that with simultaneous VCs, and that the direction of the shift depended on which electrode was stimulated first. Note that the IPI for sequential stimulation was 0 ms, which might be expected to maximize a pitch shift in the direction of the electrode that was stimulated first due to refractory effects. Assuming there are some overlapping neural populations, when IPI = 0 ms, neurons stimulated by the first pulse from the first electrode cannot respond to the second pulse from the second electrode. As the IPI is increased, one would expect greater neural recovery and a corresponding shift toward the second electrode. However, the present data showed little to no effect of IPI, suggesting few if any of the putative pitch cues inferred from the Saoji et al. (2009) study. Note that Saoji et al. (2009) used non-adjacent component electrodes for VCs and that forward-masking functions were measured for single-pulse stimuli, both of which may limit easy comparison to the present results.
Alternatively, the firing probability across neurons may have also contributed to the sequential VC percept. For example, if there was a low average firing probability, a smaller number of neurons might respond to the first pulse, leaving a larger number to respond to the second pulse, after which the amount of recovery for either neural population would be mediated by the IPI. Neurons in the overlapping region might not be fully charged by the first pulse (from the first channel) and instead respond to the second pulse (from the second channel). It is unclear how differences in firing probability not predicted by neural recovery might interact with sequential stimulation.
Central asynchrony detection may influence discrimination of simultaneous and sequential VCs
Carlyon et al. (2000) measured CI users’ discrimination between a “nearly synchronous” dual-electrode stimulus (IPI = 20 μs, offset to onset, as termed in the present study) and dual-electrode stimuli with larger IPIs (up to 1.6 ms). The electrode separation for the component channels in Carlyon et al. (2000) was much larger (6.2 to 11.1 mm) than was used in the present experiment (approximately 1 mm), and therefore the dual-electrode stimuli would not typically be considered sequential VCs. However, despite the much larger electrode separation and the not-quite simultaneous timing in the reference stimulus, results were similar between Carlyon et al. (2000) and the present study. Carlyon et al. (2000) found that most patients could discriminate near-simultaneous stimulation from stimulation with a greater IPI. However, no further effect of IPI was observed for longer IPIs. The authors suggested that it is possible that CI users’ ability to detect differences between synchronous and asynchronous stimulation may originate in more central neurons. However, despite the similarity in protocol (and results) between Carlyon et al. (2000) and the present study, a higher-level asynchrony detector may not fully explain the present results. Such an asynchrony detector would require sufficiently independent outputs from each spectral channel. While this might be true for the electrode separations of Carlyon et al. (2000) of 6.2 mm or more, it seems unlikely that broad MP stimulation of adjacent electrodes would provide sufficiently independent information, as an overlapping population would respond to stimulation from both electrodes.
Differences between BP and MP discrimination
Individual CI subjects’ spatial selectivity may underlie this sensitivity to stimulation mode. Given poor spatial selectivity, some CI subjects may have been only sensitive to large differences between the excitation patterns, e.g., the greater current spread associated with the higher-amplitude sequential stimulation. Differences in excitation due to stimulation mode or IPI may not have targeted sufficiently different neural populations. As shown in Fig. 4, subjects’ discrimination between simultaneous and sequential MP VCs was significantly correlated to their MP electrode discrimination measured in previous studies (Landsberger and Srinivasan, 2009; Luo et al., 2010), suggesting that spatial selectivity may have influenced discrimination of sequential and simultaneous VCs.
It is also possible that the different methods for setting component channel amplitudes (equal-current for MP VCs, equal loudness for BP VCs) may have contributed to the present results. Large current differences between equally loud component channels for BP VCs may indicate that the equal current amplitudes used for MP VCs might not have been equally loud. As such, the pitch percept might have shifted for MP VCs (relative to BP VCs), because stimulation for α = 0.5 was not perceptually midway between the component channels. However, this seems unlikely to have strongly contributed to the present results. The two subjects who performed most differently across MP and BP stimulation (C1 and C14) had relatively small current differences across equally loud BP VC component channels (3.01 and 2.00 dB, respectively), well within 1 standard deviation of the mean current differences across equally loud BP VC component channels (2.33 ± 1.49 dB).
Relevance to clinical rates of stimulation
The stimulation rate used in this study (250 ppse) is the same as that used in the SPEAK processing strategy (Seligman and McDermott, 1995) for the Nucleus-22 device, but lower than that used in most contemporary clinical processors (typically, 900 ppse or higher). The 250 ppse rate was used to study the effects of a wide range of IPIs on simultaneous versus sequential VC discrimination. High rates necessarily require shorter IPIs, especially when focused stimulation such as BP + 1 necessitates long pulse phase durations to achieve adequate loudness. While simultaneous and sequential VCs were not compared at the higher rates typically used in clinical processors, the results with short IPIs would most likely predict performance with high-rate sequential VCs. For example, given the default stimulation parameters (900 ppse, 25 μs phase duration, 8 μs interphase gap and 8 maxima), the IPI for Cochlear Corp.’s ACE strategy is 0.0655 ms, which is between the 0 and 0.1 ms IPIs tested in the present study. Even with this short IPI (0.1 ms), sequential and simultaneous VCs could be discriminated, suggesting that at the higher rates used in clinical processors, sequential and simultaneous VCs would most likely sound different.
While simultaneous and sequential VCs may be discriminated, either could be (potentially) used to increase the number of pitch percepts beyond the number of implanted electrodes. If discrimination is driven by a shift in the peak of the excitation pattern (as suggested by the ECAP data from Saoji et al., 2009), then sequential VCs may provide an equivalent range of pitches (albeit spectrally shifted) as simultaneous VCs.
SUMMARY AND CONCLUSIONS
Discrimination between simultaneous and sequential VCs was measured in CI users for different IPIs (for sequential VCs) and different stimulations modes (MP vs BP + 1). Results showed that:
-
1.
Simultaneous and sequential VCs could be discriminated, regardless of IPI or stimulation mode. Performance was not significantly affected by IPI, suggesting that temporal cues did not contribute to discrimination of sequential and simultaneous VCs.
-
2.
Discrimination was not significantly affected by stimulation mode. However, two of the six CI subjects exhibited better discrimination performance with BP + 1 than with MP stimulation.
-
3.
For a fixed loudness and fixed α value (0.5), sequential VCs required ∼5.2 dB more current than simultaneous VCs. The higher current and associated broader spread of excitation most likely contributed to the perceptual discrimination of sequential and simultaneous VCs.
-
4.
Discrimination between simultaneous and sequential VCs was correlated with subjects’ electrode discrimination measured in previous studies, suggesting that subjects’ spatial selectivity may have influenced discrimination of sequential and simultaneous VCs.
ACKNOWLEDGMENTS
We thank all the CI subjects for their gracious participation. We also thank Emily Buss and two anonymous reviewers for their insightful comments and suggestions. Work was supported by NIH grants R03-DC010064 and R01-DC004993.
References
- Bierer, J. A., and Middlebrooks, J. C. (2002). “Auditory cortical images of cochlear-implant stimuli: dependence on electrode configuration,” J. Neurophysiol. 87, 478–492. [DOI] [PubMed] [Google Scholar]
- Bonham, B. H., and Litvak, L. M. (2008). “Current focusing and steering: modeling, physiology, and psychophysics,” Hear. Res. 242, 141–153. 10.1016/j.heares.2008.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busby, P. A., Battmer, R. D., and Pesch, J. (2008). “Electrophysiological spread of excitation and pitch perception for dual and single electrodes using the Nucleus Freedom cochlear implant,” Ear Hear. 29, 853–864. 10.1097/AUD.0b013e318181a878 [DOI] [PubMed] [Google Scholar]
- Busby, P. A., and Plant, K. L. (2005). “Dual electrode stimulation using the nucleus CI24RE cochlear implant: electrode impedance and pitch ranking studies,” Ear Hear. 26, 504–511. 10.1097/01.aud.0000179693.32989.84 [DOI] [PubMed] [Google Scholar]
- Carlyon, R. P., Geurts, L., and Wouters, J. (2000). “Detection of small across-channel timing differences by cochlear implantees,” Hear. Res. 141, 140–54. 10.1016/S0378-5955(99)00215-4 [DOI] [PubMed] [Google Scholar]
- Chatterjee, M., and Shannon, R. V. (1998). “Forward masked excitation patterns in multielectrode electrical stimulation,” J. Acoust. Soc. Am. 103, 2565–2572. 10.1121/1.422777 [DOI] [PubMed] [Google Scholar]
- Donaldson, G. S., Kreft, H. A., and Litvak, L. (2005). “Place-pitch discrimination of single- versus dual-electrode stimuli by cochlear implant users (L),” J. Acoust. Soc. Am. 118, 623–626. 10.1121/1.1937362 [DOI] [PubMed] [Google Scholar]
- Firszt, J. B., Koch, D. B., Downing, M., and Litvak, L. (2007). “Current steering creates additional pitch percepts in adult cochlear implant recipients,” Otol. Neurotol. 28, 629–636. 10.1097/01.mao.0000281803.36574.bc [DOI] [PubMed] [Google Scholar]
- Frijns, J. H., Kalkman, R. K., Vanpoucke, F. J., Bongers, J. S., and Briaire, J. J. (2009). “Simultaneous and non-simultaneous dual electrode stimulation in cochlear implants: evidence for two neural response modalities,” Acta Otolaryngol. 129, 433–439. 10.1080/00016480802610218 [DOI] [PubMed] [Google Scholar]
- Galvin, J. J., 3rd, and Fu, Q. J. (2005). “Effects of stimulation rate, mode and level on modulation detection by cochlear implant users,” J. Assoc. Res. Otolaryngol. 6, 269–279. 10.1007/s10162-005-0007-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin, J. J., Fu, Q.-J., and Oba, S. I. (2009). “Virtual Channels with Sequential Stimulation in Cochlear Implant Users” in ARO Midwinter Meeting (Baltimore, MD: ). [Google Scholar]
- Hochberg, Y. (1988). “A sharper Bonferroni procedure for multiple tests of significance,” Biometrika 75, 800–803. 10.1093/biomet/75.4.800 [DOI] [Google Scholar]
- Kwon, B. J., and van den Honert, C. (2006a). “Dual-electrode pitch discrimination with sequential interleaved stimulation by cochlear implant users,” J. Acoust. Soc. Am. 120, EL1–6. 10.1121/1.2208152 [DOI] [PubMed] [Google Scholar]
- Kwon, B. J., and van den Honert, C. (2006b). “Effect of electrode configuration on psychophysical forward masking in cochlear implant listeners,” J. Acoust. Soc. Am. 119, 2994–3002. 10.1121/1.2184128 [DOI] [PubMed] [Google Scholar]
- Landsberger, D. M., and Srinivasan, A. G. (2009). “Virtual channel discrimination is improved by current focusing in cochlear implant recipients,” Hear Res. 254, 34–41. 10.1016/j.heares.2009.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laneau, J., and Wouters, J. (2004). “Multichannel place pitch sensitivity in cochlear implant recipients,” J. Assoc. Res. Otolaryngol. 5, 285–294. 10.1007/s10162-004-4049-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak, L. M., Spahr, A. J., and Emadi, G. (2007). “Loudness growth observed under partially tripolar stimulation: model and data from cochlear implant listeners,” J. Acoust. Soc. Am. 122, 967–981. 10.1121/1.2749414 [DOI] [PubMed] [Google Scholar]
- Luo, X., Landsberger, D. M., Padilla, M., and Srinivasan, A. G. (2010). “Encoding pitch contours using current steering,” J. Acoust. Soc. Am. 128, 1215–1223. 10.1121/1.3474237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macherey, O., and Carlyon, R. P. (2010). “Temporal pitch percepts elicited by dual-channel stimulation of a cochlear implant,” J. Acoust. Soc. Am. 127, 339–349. 10.1121/1.3269042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott, H. J., and McKay, C. M. (1994). “Pitch ranking with nonsimultaneous dual-electrode electrical stimulation of the cochlea,” J. Acoust. Soc. Am. 96, 155–162. 10.1121/1.410475 [DOI] [PubMed] [Google Scholar]
- McKay, C. M., McDermott, H. J., and Clark, G. M. (1996). “The perceptual dimensions of single-electrode and nonsimultaneous dual-electrode stimuli in cochlear implantees,” J. Acoust. Soc. Am. 99, 1079–1090. 10.1121/1.414594 [DOI] [PubMed] [Google Scholar]
- Miller, C. A., Abbas, P. J., and Robinson, B. K. (2001). “Response properties of the refractory auditory nerve fiber,” J. Assoc. Res. Otolaryngol. 2, 216–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi, S., Iida, Y., Shimizu, S., Matsushima, J., and Ifukube, T. (1996). “Proposal of a new auditory nerve stimulation method for cochlear prosthesis,” Artif. Organs 20, 941–946. 10.1111/j.1525-1594.1996.tb04574.x [DOI] [PubMed] [Google Scholar]
- Saoji, A. A., Litvak, L. M., and Hughes, M. L. (2009). “Excitation patterns of simultaneous and sequential dual-electrode stimulation in cochlear implant recipients,” Ear Hear. 30, 559–567. 10.1097/AUD.0b013e3181ab2b6f [DOI] [PubMed] [Google Scholar]
- Seligman, P., and McDermott, H. (1995). “Architecture of the Spectra 22 speech processor,” Ann. Otol. Rhinol. Laryngol. Suppl. 166, 139–141. [PubMed] [Google Scholar]
- Shannon, R. V., Fu, Q.-J., Galvin, J. J.III. (2004). “The number of spectral channels required for speech recognition depends on the difficulty of the listening situation,” Acta Otolaryngol. Suppl. 552, 1–5. [DOI] [PubMed] [Google Scholar]
- Srinivasan, A. G., Landsberger, D. M., and Shannon, R. V. (2010). “Current focusing sharpens local peaks of excitation in cochlear implant stimulation,” Hear. Res. 270, 89–100. 10.1016/j.heares.2010.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]