Abstract
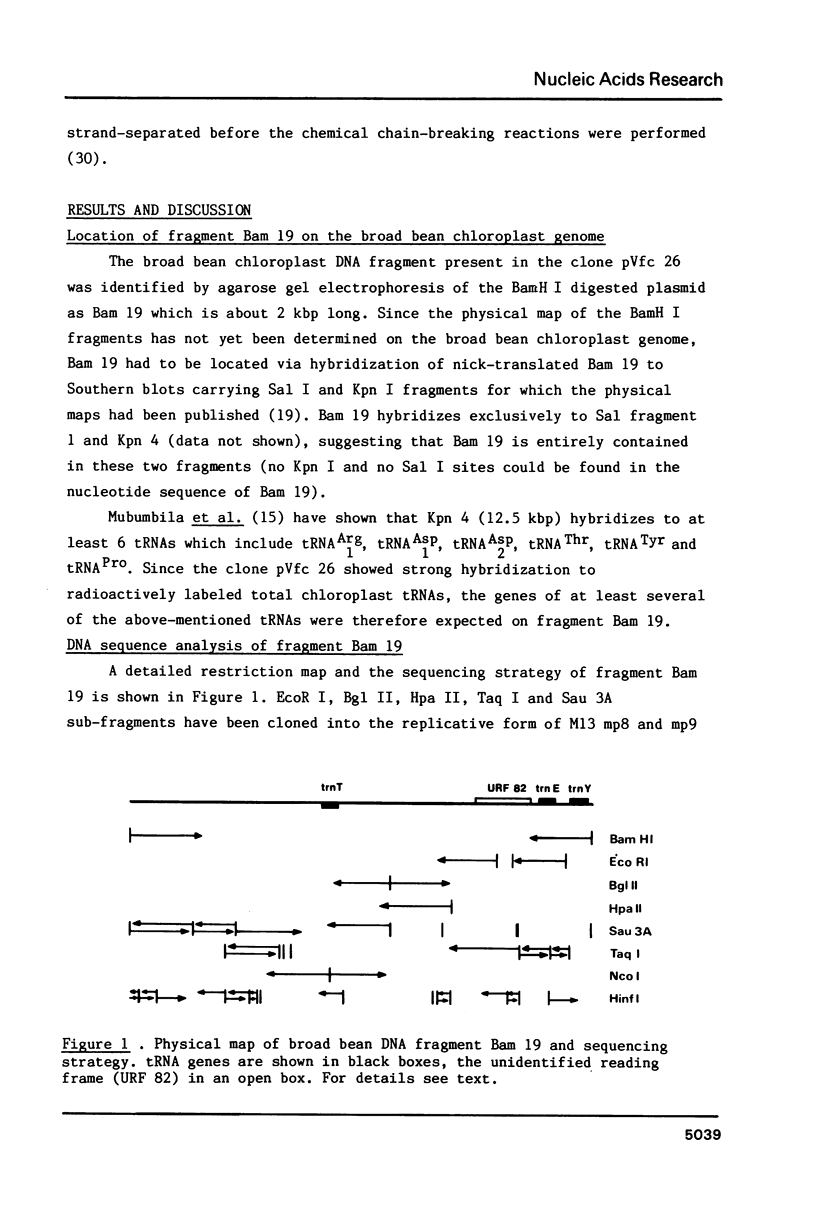
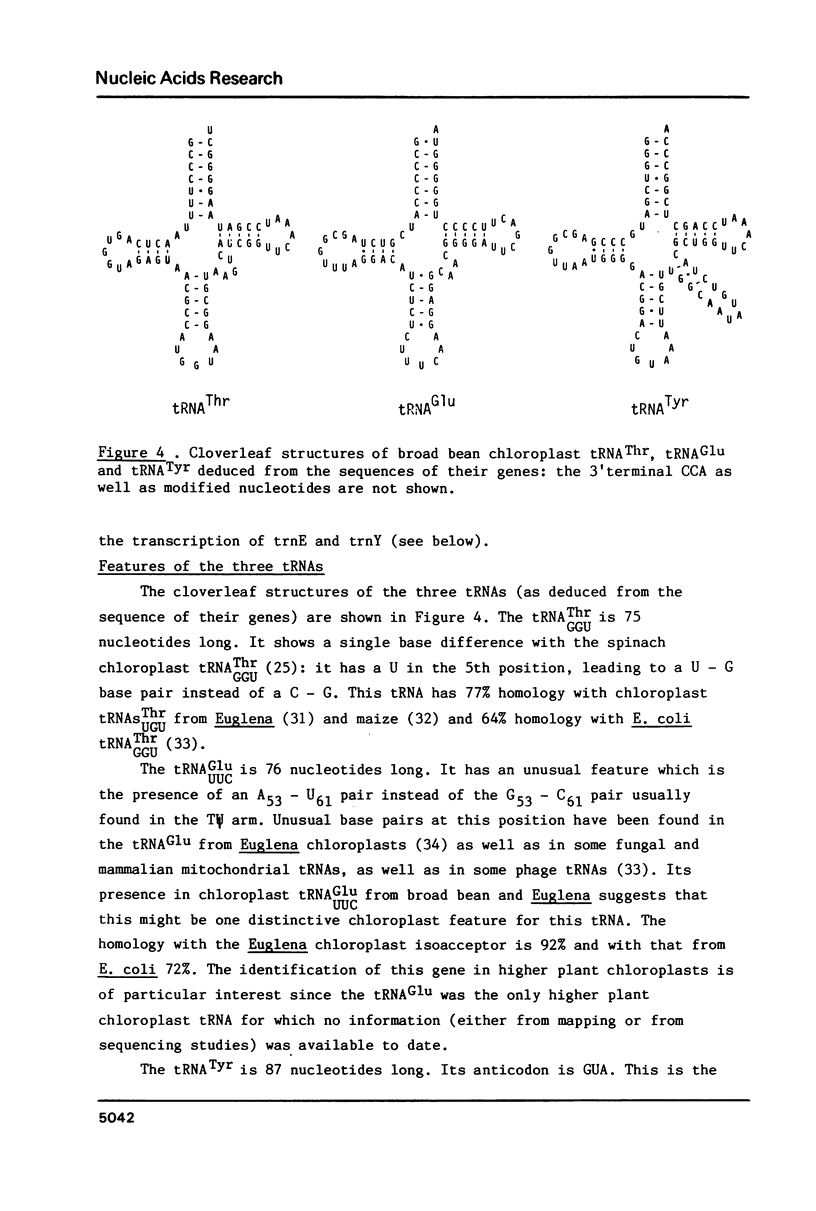
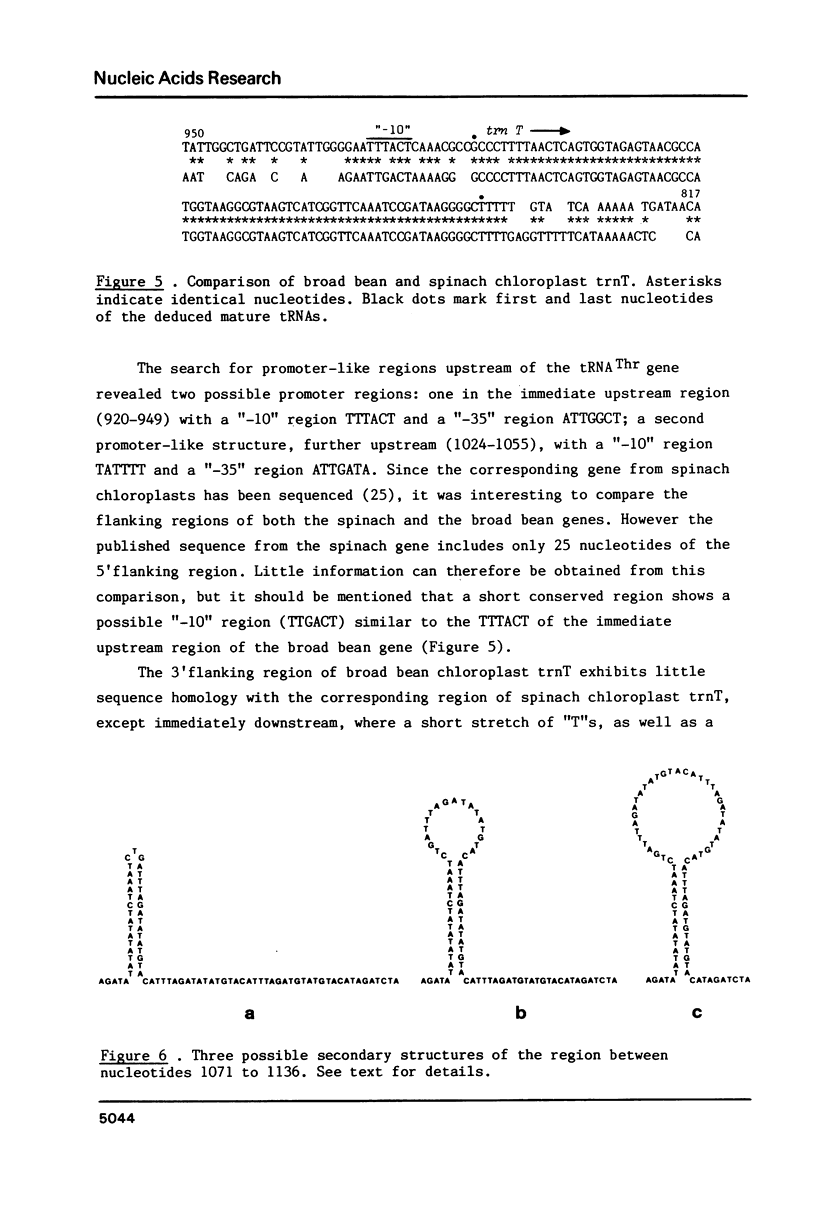
The entire nucleotide sequence of a 2014 bp BamH I fragment from broad bean (Vicia faba) chloroplast DNA containing the genes for tRNAThr (trnT), tRNAGlu (trnE) and tRNATyr (trnY) has been determined. The tRNAGlu and tRNATyr genes are separated by only 60 bp and are probably part of the same transcriptional unit. The tRNAThr gene is located on the complementary strand, 876 bp away from the tRNAGlu gene. This fragment also contains an open reading frame of 82 codons, as well as a series of AT-rich, direct and inverted repeats.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beier H., Barciszewska M., Krupp G., Mitnacht R., Gross H. J. UAG readthrough during TMV RNA translation: isolation and sequence of two tRNAs with suppressor activity from tobacco plants. EMBO J. 1984 Feb;3(2):351–356. doi: 10.1002/j.1460-2075.1984.tb01810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque D. P., Wildman S. G. Evidence that nuclear genes code for several chloroplast ribosomal proteins. Biochem Biophys Res Commun. 1973 Jan 23;50(2):532–537. doi: 10.1016/0006-291x(73)90872-3. [DOI] [PubMed] [Google Scholar]
- Chu N. M., Oishi K. K., Tewari K. K. Physical mapping of the pea chloroplast DNA and localization of the ribosomal RNA genes. Plasmid. 1981 Nov;6(3):279–292. doi: 10.1016/0147-619x(81)90036-6. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F. H. Codon--anticodon pairing: the wobble hypothesis. J Mol Biol. 1966 Aug;19(2):548–555. doi: 10.1016/s0022-2836(66)80022-0. [DOI] [PubMed] [Google Scholar]
- Driesel A. J., Crouse E. J., Gordon K., Bohnert H. J., Herrmann R. G., Steinmetz A., Mubumbila M., Keller M., Burkard G., Weil J. H. Fractionation and identification of spinach chloroplast transfer RNAs and mapping of their genes on the restriction map of chloroplast DNA. Gene. 1979 Aug;6(4):285–306. doi: 10.1016/0378-1119(79)90070-2. [DOI] [PubMed] [Google Scholar]
- Fox L., Erion J., Tarnowski J., Spremulli L., Brot N., Weissbach H. Euglena gracilis chloroplast EF-Ts. Evidence that it is a nuclear-coded gene product. J Biol Chem. 1980 Jul 10;255(13):6018–6019. [PubMed] [Google Scholar]
- Gauss D. H., Sprinzl M. Compilation of tRNA sequences. Nucleic Acids Res. 1983 Jan 11;11(1):r1–53. [PMC free article] [PubMed] [Google Scholar]
- Hecker L. I., Egan J., Reynolds R. J., Nix C. E., Schiff J. A., Barnett W. E. The sites of transcription and translation for Euglena chloroplastic aminoacyl-tRNA synthetases. Proc Natl Acad Sci U S A. 1974 May;71(5):1910–1914. doi: 10.1073/pnas.71.5.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth M. J., Hallick R. B. Euglena gracilis chloroplast transfer RNA transcription units. Nucleotide sequence analysis of a tRNATyr-tRNAHis-tRNAMet-tRNATrp-tRNAGlu-tRNAGly gene cluster. J Biol Chem. 1982 Nov 10;257(21):12795–12799. [PubMed] [Google Scholar]
- Holschuh K., Bottomley W., Whitfeld P. R. Sequence of the genes for tRNACys and tRNAAsp from spinach chloroplasts. Nucleic Acids Res. 1983 Dec 20;11(24):8547–8554. doi: 10.1093/nar/11.24.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabin G. D., Hallick R. B. Euglena gracilis chloroplast transfer RNA transcription units. Nucleotide sequence analysis of a tRNAThr-tRNAGly-tRNAMet-tRNASer-tRNAGln gene cluster. J Biol Chem. 1983 May 10;258(9):5512–5518. [PubMed] [Google Scholar]
- Kashdan M. A., Dudock B. S. Structure of a spinach chloroplast threonine tRNA gene. J Biol Chem. 1982 Feb 10;257(3):1114–1116. [PubMed] [Google Scholar]
- Kashdan M. A., Dudock B. S. The gene for a spinach chloroplast isoleucine tRNA has a methionine anticodon. J Biol Chem. 1982 Oct 10;257(19):11191–11194. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Montandon P. E., Stutz E. Nucleotide sequence of a Euglena gracilis chloroplast genome region coding for the elongation factor Tu; evidence for a spliced mRNA. Nucleic Acids Res. 1983 Sep 10;11(17):5877–5892. doi: 10.1093/nar/11.17.5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mubumbila M., Gordon K. H., Crouse E. J., Burkard G., Weil J. H. Construction of the physical map of the chloroplast DNA of Phaseolus vulgaris and localization of ribosomal and transfer RNA genes. Gene. 1983 Mar;21(3):257–266. doi: 10.1016/0378-1119(83)90009-4. [DOI] [PubMed] [Google Scholar]
- Palmer J. D., Thompson W. F. Chloroplast DNA rearrangements are more frequent when a large inverted repeat sequence is lost. Cell. 1982 Jun;29(2):537–550. doi: 10.1016/0092-8674(82)90170-2. [DOI] [PubMed] [Google Scholar]
- Parthier B. Cytoplasmic site synthesis of chloroplast aminoacyl-tRNA synthetases in Euglena gracilis. FEBS Lett. 1973 Dec 15;38(1):70–74. doi: 10.1016/0014-5793(73)80516-2. [DOI] [PubMed] [Google Scholar]
- Passavant C. W., Stiegler G. L., Hallick R. B. Location of the single gene for elongation factor Tu on the Euglena gracilis chloroplast chromosome. J Biol Chem. 1983 Jan 25;258(2):693–695. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Siebenlist U., Simpson R. B., Gilbert W. E. coli RNA polymerase interacts homologously with two different promoters. Cell. 1980 Jun;20(2):269–281. doi: 10.1016/0092-8674(80)90613-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steinmetz A. A., Krebbers E. T., Schwarz Z., Gubbins E. J., Bogorad L. Nucleotide sequences of five maize chloroplast transfer RNA genes and their flanking regions. J Biol Chem. 1983 May 10;258(9):5503–5511. [PubMed] [Google Scholar]
- Steinmetz A., Gubbins E. J., Bogorad L. The anticodon of the maize chloroplast gene for tRNA Leu UAA is split by a large intron. Nucleic Acids Res. 1982 May 25;10(10):3027–3037. doi: 10.1093/nar/10.10.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari K. K., Wildman S. G. Information content in the chloroplast DNA. Symp Soc Exp Biol. 1970;24:147–179. [PubMed] [Google Scholar]


