Abstract
Biodegradable polyurethane urea (PUU) elastomers are ideal candidates for fabricating tissue engineering scaffolds with mechanical properties akin to strong and resilient soft tissues. PUU with a crystalline poly(ε-caprolactone) (PCL) macrodiol soft segment (SS) showed good elasticity and resilience at small strains (<50%), but showed poor resilience under large strains due to stress-induced crystallization of the PCL segments, with a permanent set of 677±30% after tensile failure. To obtain softer and more resilient PUUs, noncrystalline poly(trimethylene carbonate) (PTMC) or poly(δ-valerolactone-co-ε-caprolactone) (PVLCL) macrodiols of different molecular weights were used as SSs that were reacted with 1, 4-diisocyanatobutane and chain extended with 1, 4-diaminobutane. Mechanical properties of the PUUs were characterized by tensile testing with static or cyclic loading and dynamic mechanical analysis. All the PUUs synthesized showed large elongations at break (800–1400%) and high tensile strength (30–60 MPa). PUUs with non-crystalline SSs all showed improved elasticity and resilience relative to the crystalline PCL-based PUU, especially for the PUUs with high molecular weight SSs (PTMC 5400 Mn and PVLCL 6000 Mn), of which the permanent deformation after tensile failure was only 12±7% and 39±4%, respectively. The SS molecular weight also influenced the tensile modulus in an inverse fashion. Accelerated degradation studies in PBS containing 100 U/mL lipase showed significantly greater mass loss for the two polyester-based PUUs versus the polycarbonate-based PUU, and for PVLCL versus PCL polyester PUUs. Basic cytocompatibility was demonstrated with primary vascular smooth muscle cell culture. The synthesized families of PUUs showed variable elastomeric behavior that could be explained in terms of the underlying molecular design and crystalline behavior. Depending upon the application target of interest, these materials may provide options or guidance for soft tissue scaffold development.
Keywords: Polyurethane urea, Biodegradable elastomer, poly(trimethylene carbonate) (PTMC), poly(δ-valerolactone-co-ε-caprolactone) (PVLCL)
Introduction
Scaffolds utilized in tissue engineering and regenerative medicine applications generally should have mechanical properties resembling those of the healthy target tissue to avoid producing adverse mechanical stimuli at the interface with host tissues or stress-shielding cells within the scaffolds. Modulus mismatch between biomaterials and nearby tissue can stimulate unwanted inflammatory reactions and exacerbate the foreign body response, which is detrimental to the tissue regeneration process. 1 For example, modulus mismatch between vascular graft materials and the native arterial wall may induce negative effects such as intimal hyperplasia and thrombosis around or downstream from the anastomosis.2,3
Soft tissues generally are strong and resilient, with nonlinear stress–strain behavior characterized by a very steep rise in stress as the limiting elastic strain is approached.4 Mechanical properties of natural tissues vary significantly, with elastic moduli ranging over several orders of magnitude, from ~1 kPa for brain and ~10 kPa for muscle to 100–1000 kPa for cartilage and bone tissue.5 It is therefore desirable to develop a broad array of mechanically appropriate elastomers for various tissue regeneration applications. Although most tissues experience relatively small passive deformations under normal physiological conditions (cardiac muscle<30% 4, blood vessel <25% 6, bladder <30%7, esophagus <200% 8), an implant capable of being resilient over a larger deformation range (0–400%) would be beneficial to minimize the possibility of rupture of the implant under abnormally increased stress or fatigue failure under dynamic cyclic loading.
Design and development of synthetic biodegradable elastomers with properties appropriate for soft tissue applications has been of keen interest in the biomaterials community 9–11. Different types of biodegradable elastomers have been synthesized, including amorphous polyesters12, polyhydroxyalkanoates 13, polyamides 14, polycarbonates 15 and their random 12,16,17 or block copolymers 18, thermoset polyester networks, 9,11,12,19 polyurethanes (PUs) 20–30 and polyurethane ureas (PUUs), 31–39 elastin-like peptides 40,41 and hydrogels 42. Segmented PUs and PUUs with hydrolytically labile polyester 20–26, 30–33 or polycarbonate 27–28, 34–36, 43 blocks in their soft segments provide biodegradable elastomers with robust mechanical properties. The utilization of peptides (AAK 33, YIGSR 44 or RGD 45) as chain extenders in the PU/PUU, has provided a means of introducing specific biological functionality such as enzyme-liability 33 or enhanced cell-adhesion 44–45. With a two-step method, we have previously synthesized a series of biodegradable poly(ester urethane) ureas (PEUUs) based on a poly(caprolactone) soft segment 31–35. Faster or slower degradation speed were obtained by including poly(ethylene glycol) or poly(hexamethyl carbonate) in the soft segment, respectively. 32, 34–35. A fast degrading PUU with enzymatic liability was synthesized by introducing an elastase-specific peptide in the molecule 33.
The goal of this work was to synthesize PUUs using SSs with varying chemical structures, crystallinity and molecular weights and to study the effects of these varied SSs on PUU mechanical and degradation behavior. In addition to the crystalline poly(ε-caprolactone) (PCL) diol, amorphous biodegradable15–17 poly(trimethylene carbonate) PTMC diols and poly(δ-valerolactone-co-ε-caprolactone) (PVLCL) diols with different molecular weights were used as SSs in PUU synthesis. The synthesized families of PUUs showed variable elastomeric behavior that could be explained in terms of the underlying molecular design and crystalline behavior. Depending upon the application target of interest, these materials may provide options or guidance for soft tissue scaffold development.
Materials and Methods
Materials
All chemicals were obtained from Sigma-Aldrich unless otherwise stated. ε-caprolactone (CL), δ-valerolactone (VL), diethylene glycol (DEG), 1, 4-diisocyanatobutane (BDI) and putrescine (1, 4-diaminobutane) were purified by vacuum distillation before use. Stannous octoate (Sn(Oct)2), trimethylene carbonate (TMC, Boehringer-Ingelheim) and polycaprolactone diol (PCL diol, Mn 2000) were used as received.
Synthesis of macrodiols
PTMC diol and PVLCL diol were synthesized with Sn(Oct)2 catalyzed ring opening polymerization with DEG as an initiator (Scheme 1). In a typical process, monomers and DEG (with variable molar ratios to control molecular weight) were mixed and heated to 140°C under argon protection, to which Sn(Oct)2 (0.05 wt% with respect to the monomer) was added under stirring. The reaction lasted for 24 hrs. Product was precipitated in methanol from a dichloromethane solution and vacuum dried. In PVLCL diol synthesis, the molar ratio of VL to CL was set at 1:1. Yields of the products were > 95%. In Table 1 all of the synthesized macrodiols and their number average molecular weights (Mn, as determined by 1H-NMR spectrum) are reflected in the polymer nomenclature (e.g. PUU-PCL2000 reflects a PUU with a PCL soft segment of 2000).
Scheme 1.
Synthesis of the utilized macrodiols (HO-PTMC-OH, HO-PVLCL-OH) and PUUs.
Table 1.
Synthesized PUU physical and mechanical properties
| Intrinsic viscosity (dL/g) | Tensile strength at break (MPa) | Initial modulus (MPa) | Strain at break (%) | Permanent set (%) | Storage modulus(E′), 25–55°C | Loss modulus (E″), 25–55°C | |
|---|---|---|---|---|---|---|---|
| PUU-PCL2000 | 2.17±0.01 | 58.0±4.7 | 12.1±2.5 | 1192±102 | 677±30 | -* | -* |
|
| |||||||
| PUU-PTMC1500 | 0.90±0.02 | 29.8±6.1 | 21.1±0.1 | 617±104 | 131±31 | 12.2 | 1.0 |
| PUU-PTMC2500 | 1.74±0.02 | 47.0±7.8 | 12.2±1.2 | 831±75 | 149±12 | 8.6 | 0.7 |
| PUU-PTMC5400 | 2.45±0.03 | 39.2±7.7 | 5.6±1.3 | 778±34 | 12±7 | 2.4 | 0.2 |
|
| |||||||
| PUU-PVLCL2250 | 1.76±0.01 | 35.0±6.2 | 11.5±3.8 | 1295±150 | 132±1 | 5.2 | 0.4 |
| PUU-PVLCL6000 | 2.75±0.01 | 29.3±5.2 | 2.8±1.3 | 1354±226 | 39±4 | 2.5 | 0.13 |
Data are mean +/− standard deviation
refer to Fig. 8a
PUU synthesis
PUUs were synthesized from the macrodiols (PCL, PTMC or PVLCL) and BDI using putrescine as a chain extender by a two-step method 31 as shown in Scheme 1, with the macrodiol:BDI:putrescine molar ratio being 1:2:1. In a typical process, the macrodiol in a 3-neck flask was dried by azeotropic distillation in toluene. Anhydrous DMSO was then added to the flask to dissolve the macrodiol, and BDI was added, followed by the addition of Sn(Oct)2 (0.05 wt% with respect to the macrodiols). The reaction was carried out for 3 hrs at 70°C, and then cooled at room temperature. A putrescine/DMSO solution was added drop-wise into the agitated solution. Argon protection was applied throughout the synthesis process. The product was precipitated in deionized water and then dried in a vacuum at 60°C for 3 d. The yields for all the PUUs were >95%.
Characterization
1H-NMR spectra of the macrodiols and the PUUs were recorded with a 300 MHz Bruker spectrometer using CD3Cl or DMSO-d6 as a solvent. Fourier transform infrared (FTIR) spectra of the PUUs were obtained with a Nicolet FTIR spectrometer, with samples prepared by coating a 1% copolymer solution onto a NaCl transmission crystal window. The inherent viscosities of PUUs at 25 °C were measured (n=4) with 1, 1, 1, 3, 3, 3-hexafluoroisopropanol (HFIP, Oakwood Products) as a solvent using an Ubbelohde viscometer as described previously.36
PUU films (~150 μm thick) were prepared by casting PUU solutions in HFIP onto a polytetrafluoroethylene plate followed by solvent evaporation. Differential scanning calorimetry (DSC) of PUU films was conducted on a DSC-60 (Shimadzu) instrument at a rate of 10°C/min with a nitrogen flow. The temperature interval of the DSC analysis was 0.2°C. One dimensional x-ray diffraction (1-D XRD) experiments were carried out on PUU films using a Bruker D8 Discover XRD instrument with a CuKα source. Two dimensional XRD (2-D XRD) experiments were carried out on a Bruker Smart Apex CCD diffractometer with a MoKα radiation.
For tensile testing, dumbbell-shaped PUU samples (2.5×20 mm, n=4) were cut from the PUU films using a punch and were tested on an MTS Tytron 250 MicroForce Testing Workstation at room temperature, with crosshead speed of 10 mm/min and an initial grip separation of 10 mm. Tests were carried out in accordance with ASTM D638M-89. Permanent deformation (PD) was measured after 30 min of failure of the samples which were pre-marked at both ends of the original length of L0. The length (L1) between the two marked ends after tensile failure was measured and the PD was calculated as (L1-L0)/L0×100%. Initial modulus was calculated as the slope of the initial linear region (strain <30%) of the tensile curves.
For cyclic tensile testing, dumbbell shaped specimens (2.5×20 mm) were stretched to the maximum strain of 50% or 400% and retracted back to the initial length for 10 cycles at a constant rate of 2.5 mm/sec. The test was conducted on a custom designed uniaxial cyclic tensile test system as described previously46 and modified with an MDB-25 25 lb load cell and TMO-2 load cell amplifier (Transducer Techniques), LabVIEW 7.1 software and DAQ card (model PCI-MIO-16XE-10, National Instruments). The system was calibrated to ASTM standards to measure applied force and displacement synchronously.
Dynamic mechanical analysis (DMA) was performed on a TA Q800 Dynamic Mechanical Analysis instrument. Dumbbell-shaped PUU film samples (2.5×20 mm) were mounted on the DMA analyzer with an initial grip separation of 10 mm and strained to 30% at a frequency of 1 Hz, with temperature scanned from 30 to 60 °C.
Polymer degradation
Enzyme accelerated degradation testing was performed in PBS (Lonza) containing 100 U/mL lipase (Sigma, from thermomyces lanuginosus) at 37°C. 47,48 Briefly, weighed samples (W0) of PUU films were incubated in 2 mL PBS containing 100 U/mL lipase (lipase buffer) which was changed twice per week. No pH value change was observed during the degradation. The samples were removed at 1, 2 and 3 weeks for PUU-PCL2000 and PUU-PVLCL2250 and 1, 2, 3 and 4 weeks for PUU-PTMC2500, rinsed using deionized water (3 times), dried at 60°C in a vacuum oven, and then weighed (W1). The mass remaining was calculated as W1/W0*100%.
To investigate the molecular weight change of the PUUs during degradation, samples were removed from the lipase buffer solution after one week, rinsed with deionized water 3 times and dried at 60°C in a vacuum oven. The intrinsic viscosity for each sample was measured (n=4) at 25 °C with HFIP as a solvent using an Ubbelohde viscometer as described previously. 36
Cytocompatibility
The cytocompatibility of the PUUs was assessed by evaluating cell mitochondrial activity on the PUU films and utilizing tissue culture polystyrene (TCPS) as control. Rat vascular smooth muscle cells (RSMCs)49 were seeded onto PUU(PCL2000), PUU(PTMC25000 and PUU(PVLCL2250) films (diameter 9 mm) at a seeding density of 7,000/cm2. Total cell metabolic activity was measured (n=3 independent samples) using an MTS assay kit (Promega CellTiter 96® Cell Proliferation Assay) to quantify mitochondrial activity. For qualitative verification that mitochondrial activity results were related to increased cell numbers on the polymer films, cells were also observed under confocal microscopy (Fluoview 500, Olympus) after live/dead staining with a Promokine® Live/Dead Cell Staining Kit.
Statistics
Data are expressed as means with the standard deviation. Statistical analyses were performed by one-way ANOVA followed by Tukey’s post-hoc testing with SPSS software (SPSS Inc, Chicago IL). Comparisons between two groups were assessed by a student’s t-test. Statistical significance was considered to exist at p<0.05.
Results
NMR
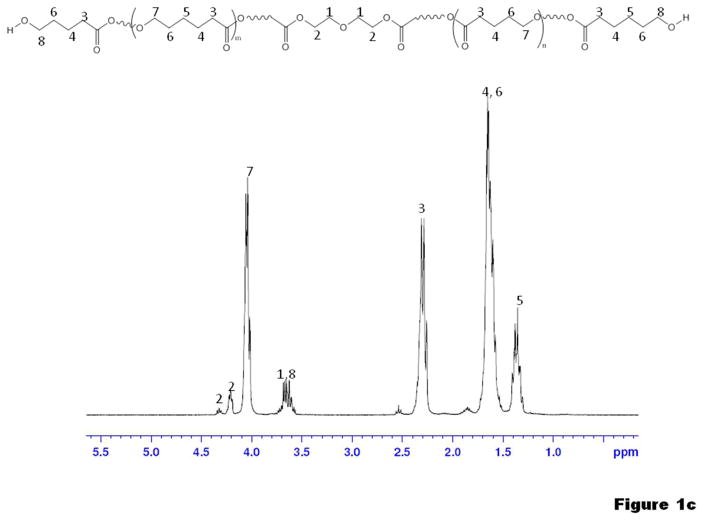
1H-NMR confirmed the molecular structure of PCL2000 (Sigma) and the synthesized macrodiols PTMC2500 and PVLCL2250 as shown in Figure 1a–c. In the spectrum of the PVLCL2250 diol (Figure 1c), the molar ratio of the VL to the CL was determined as 1:1 by the ratio (2:1) of peak 3 (from both VL and CL) and peak 5 (from only CL). The same ratio of VL to CL was also found for PVLCL6000 diol. Number average molecular weights (Mn) of the macrodiols were calculated with the NMR spectra, through the ratio between integrals of peaks characteristic of the main chain and those of the chain terminals ending with hydroxyl groups (Figure 1a–c). Successful PUU synthesis was confirmed by 1H-NMR spectra with typical results for PUUs with PCL, PTMC and PVLCL soft segments shown in Figure 2. In addition to the 1H peaks characteristic of the soft segments (Figure 1), characteristic peaks from urea and urethane groups of the hard segment are seen in Figure 2. Intrinsic viscosities of the PUUs are summarized in Table 1. Both the intrinsic viscosities and the tensile strengths observed for the PUUs were relatively high (Table 1) compared with those reported in the literature, 31–36 indicating high PUU molecular weights.
Figure 1.
1H-NMR spectra of (a) PCL2000 diol, (b) PTMC2500 diol and (c) PVLCL2250 diol
Figure 2.
1H-NMR spectra of (a) PUU-PCL2000, (b) PUU-PTMC2500 and (c) PUU-PVLCL2250
FTIR
In Figure 3 the FTIR spectra of the PUUs is presented with absorption peaks assigned according to the literature. 50–52 All of the spectra demonstrated strong C=O stretch vibration peaks from the ester groups in PCL and PVL at ~1735 cm−1 or the carbonate group in PTMC at ~1750 cm−1, in which the small shoulder peaks at ~1700 cm−1 could be attributed to the urethane group. The broad absorption peaks between 2800–3000 cm−1 are due to the –CH2– groups which are abundant mainly in the SSs. For PUUs containing PTMC, a pronounced peak at 1260 cm−1 characteristic of the carbonate group (–O–C(=O)–O–) was found. 28 The spectra of the PUUs also showed peaks from the urethane and urea groups in the hard segments (HSs) at 3200–3500 cm−1 (N-H stretch), 1700 cm−1 (C=O stretch of C=O···H–N in the urethane group), 1630 cm−1 (C=O stretch of ordered C=O···H–N in the urea group), 1580 cm−1 (C-N stretch) and 1530 cm−1 (N-H in plane bending). The intensity ratio between the area of the peaks characteristic of the SS (2800–3000 cm−1) and the HS (3200–3500 cm−1) qualitatively reflects the ratio of content of the SS and HS in the polymers (Figure 3). A PUU with a higher molecular weight SS will have relatively lower absorptions peaks at 3200–3500 cm−1 because of its lower HS content.
Figure 3.
FTIR spectra of the synthesized PUUs.
Further analysis of the FTIR spectra of the PUUs (Figure 3) gives insights into the physical structure of the HS domains. Absorption peaks of the urethane and urea groups provide information on hydrogen bond formation and crystallization.50–52 The infrared bands at 3420 and 3320 cm−1 were assigned to the stretching modes of the “free” and hydrogen bonded N-H groups, respectively, depending on whether there was hydrogen bonding between the N-H and carbonyl oxygen (C=O). 50 In the FTIR spectra of all the PUUs the N-H peaks were mainly centered at 3320 cm−1, with only a small shoulder peak at 3420 cm−1, indicating that most of the N-H groups formed hydrogen bonds with carbonyl oxygens. Hydrogen bond formation in the HS was also supported by the appearance of the peak (1630 cm−1) of the urea C=O groups hydrogen bonded with H–N, while “free” C=O groups of urea should have a peak at 1700 cm−1. The urea (C=O) peak at 1630 cm−1 also implies formation of ordered hydrogen bonds in the HS and therefore a mainly crystalline HS structure, because the disordered hydrogen bonded C=O peak of urea (1660–1670 cm−1) 51 was not found in the spectra.
Tensile testing
Figure 4 shows representative stress–strain curves of the PUUs, with calculated mechanical parameters summarized in Table 1. The polymer tensile strengths ranged from 30 to 60 MPa and elongations at break from 800 to 1300%. Varying the PUU soft segment impacted the mechanical properties in terms of initial modulus and permanent deformation. PUUs with crystalline PCL SS had much higher permanent deformation than those with non-crystalline SS of PTMC or PVLCL (Figure 5). Initial moduli were mainly dependent on SS molecular weight, with higher SS associated with a lower initial modulus.
Figure 4.
Tensile response curves of the synthesized PUUs. The portion of the curves at low strains is magnified in the inset with the same units used for both axes.
Figure 5.
(a) Typical image showing the large elongation of the PUUs before break. (b) A large permanent deformation was found for PUU-PCL2000. (c) PUU-PTMCs showed relatively small permanent deformation.
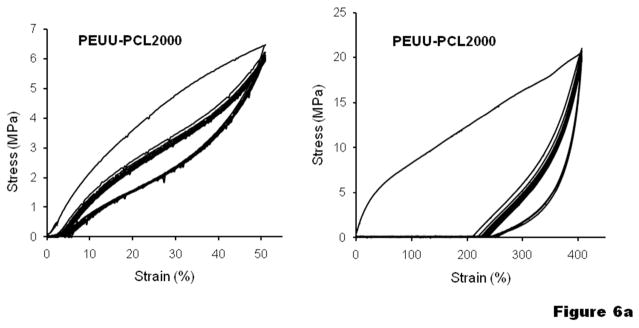
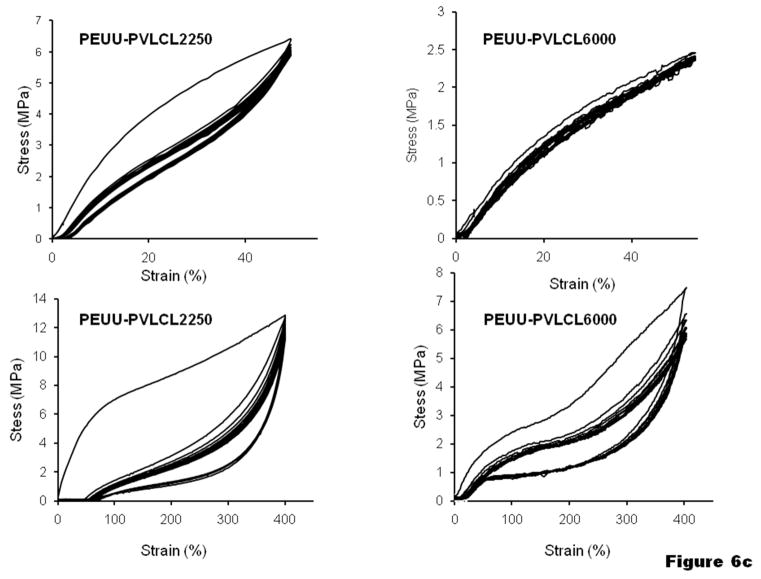
To further understand the elasticity of the materials, cyclic tensile loading was performed with a maximum strain of 50% or 400 %, as shown in Figure 6. Most of the PUUs exhibited a large hysteresis loop in the first cycle, followed by much smaller hysteresis loops in the next 9 cycles. With a maximum strain of 50%, most of the samples showed a small unrecoverable deformation (<5%), while unrecoverable deformation for PUU-PTMC5400 and PUU-PVLCL6000 were almost negligible (Figure 6b, c). With an increased maximum strain of 400%, the unrecoverable deformations became much more appreciable (typically 50–80%) for all the PUU samples, especially for PUU-PCL2000, which was higher than 200% (Figure 6a).
Figure 6.
Cyclic tensile response curves at low and high deformations for (a) PUU-PCL2000, (b) PUU-PTMCs and (c) PUU-PVLCLs.
DSC
DSC analysis provided PUU crystalline and glass transition properties, as shown in Figure 7. Although subtle, glass transitions around −60°C for the PUU-PCL2000, PUU-PVLCL2250 and PUU-PVLCL6000 were detected. For PUU-PCL2000, a melting peak was found at Tm=31°C (ΔHm=27.8 J/g) (Figure 7a). For PUUs with PTMC as SS, a sharp glass transition at −17°C was observed (Figure 7a), as reported for pure PTMC in the literature. 36, 53 No melting peaks were found for the PTMC segment since it is a well know amorphous polymer.54 PUU-PVLCL2250 and 6000 showed Tm at −5°C (ΔHm=12.4J/g) and 8°C (ΔHm=21.4J/g) respectively (Figure 7a). DSC analysis was also conducted for stretched PUU-PCL2000 samples (Figure 7b) which were stretched until broken in the tensile testing, possessing a permanent deformation of 677%. Analyzed with a heating-cooling-heating program, the stretched PUU-PCL2000 showed a Tm of 50°C (sharp) in the 1st heating, a Tc of 0°C (broad) in the cooling process, and a Tm at 30°C (broad) in the 2nd heating process, respectively.
Figure 7.
DSC analysis of (a) PUUs and (b) stretched PUU-PCL2000 (permanent deformation of 677%) with temperature change.
DMA
DMA analysis of PUUs indicated the effect of temperature on elastic mechanical properties. The DMA spectrum of PUU-PCL2000 (Figure 8a) showed a pronounced transition at 40°C corresponding to the melting peak (Tm=31°C) of the PCL crystal (Figure 7a), while other PUUs showed stable storage modulus (E′) and loss modulus (E″) (summarized in Table 1) between room temperature (25°C) and 55°C, as representatively shown in Figure 8b, d for PUU-PTMC2500 and PUU-PVLCL6000, respectively. DMA analysis of PUU(PTMC2500) with temperature scanning up to 200°C was conducted (Figure 8c). Both the E′ and the E″ decreased with the increasing temperature due to the partial breaking of hydrogen bonds in the HS, while the E″/E′ ratio remained relatively constant, indicating that the material was still a solid network physically crosslinked by the HS domains.
Figure 8.
DMA analysis of (a) PUU-PCL2000, (b, c) PUU-PTMC2500 and (d) PUU-PVLCL6000. Frequency = 1Hz.
XRD
Wide angle XRD was performed on the non-stretched and stretched PUU-PCL2000 to investigate stretch-induced molecular orientation and re-crystallization. Figure 9 shows 1-D XRD spectrum (CuKα) for the two samples. Peaks at 2θ=21.6° and 24° corresponding to the diffraction of the 110 and 200 lattice plane 55,56 of the orthorhombic crystalline PCL were observed, while in the stretched samples the peaks become much stronger due to the stretch-enhanced crystallization. In the 2-D XRD patterns (Figure 9, recorded with MoKα) of the non-stretched PUU-PCL2000 a broad circle corresponding with 2θ= 9.8° and 10.9° (110 and 200 lattice planes of PCL) was observed. In the 2-D XRD patterns of the stretched PUU-PCL2000, the circular-shaped patterns transferred into discontinuous arcs distributed symmetrically with the stretch axis, indicative of a stretch-induced molecular orientation in the stretched samples. 55,56
Figure 9.

XRD analysis of non-stretched and stretched PUU-PCL2000. The double arrow in the 2-D XRD image indicates stretch direction.
Polymer degradation
Three different PUUs with similar soft segment molecular weight, PUU-PCL2000, PUU-PTMC2500 and PUU-PVLCL2250, were chosen for accelerated degradation studies in lipase buffer (Figure 10). The mass loss over the measurement period from the two polyester-based PUU-PCL2000 and PUU-PVLCL2250 was markedly higher (p<0.05) than for the polycarbonate-based PUU-PTMC2500. The two polyester-based PUUs also exhibited differences from one another in terms of mass loss with PUU-PVLCL2250 losing greater mass than PUU-PCL2000 at day 21 (p<0.05).
Figure 10.
Degradation curves of PUUs in PBS solution containing 100 U/ml lipase at 37°C.
The intrinsic viscosities of three samples, PUU-PCL2000, PUU-PTMC2500 and PUU-PVLCL2250, significantly decreased after one week in lipase buffer (2.17±0.01 to 0.48±0.13, 1.74±0.02 to 0.66±0.06 and 1.76±0.01 to 0.40±0.02 dL/g, respectively, p<0.01). Although PUU-PTMC2500 experienced relatively less viscosity loss than the other two polymers, the viscosity loss was in greater proportion than one might expect based on the measured mass loss (Figure 10).
Cytocompatibility
In Figure 11a the mitochondrial activity of the RSMCs at days 1 and 5 suggests cell adherence and proliferation on the PUU films as well as the control TCPS surface. At day 1, no significant difference was found between PUUs and TCPS (P>0.05) except for PUU-PVLCL2250 was significantly higher than PUU-PTMC2500. At day 5, each PUU material was significantly lower than TCPS (P<0.05) while there were no significant differences between the three PUU groups. Fluorescent live/dead staining of RSMCs in Figure 11b qualitatively confirmed that the increased mitochondrial activity corresponded with increased cell numbers. Negligible dead cells (stained red) were seen over several culture wells for all of the samples.
Figure 11.
(a) Mitochondrial activity and (b) fluorescent micrographs of RSMCs cultured on different PUUs and tissue culture polystyrene (TCPS) after live/dead staining.
Discussion
Capable of forming families of robust elastomers, biodegradable segmented PUs or PUUs have been synthesized with various SSs, diisocyanates and chain extenders. 20–36, 57–60 Compared with PUs, the highly polar urea groups in PUUs provide enhanced hydrogen bonding in the hard segments and thus decreased creep under long term loading. 51, 61. We have previously synthesized PUUs using BDI as a diisocyanate and putrescine as a chain extender and evaluated these materials for biomedical applications since this system provides a biocompatible HS structure of which the final putative hydrolysis product (putrescine) is a naturally occurring metabolite.31
With the same HS structure, PUUs with SSs of variable crystallinity and molecular weight were synthesized in this work. In addition to the crystalline PCL, a biodegradable amorphous polymer, PTMC, 15–17, 36, 62–65 was used as a SS. Another noncrystalline SS was synthesized by copolymerizing CL with VL at an equal molar ratio. All the PUUs synthesized were robust mechanically as shown in Table 1. PUUs with the three different SS chemical structures were all biodegradable in lipase buffer (Figure 10) and exhibited basic RSMC cytocompatibility (Figure 11). Polyester based PUUs (PUU-PCL2000 and PUU-PVLCL2250) degraded faster than the polycarbonate-based PUU (PUU-PTMC2500), in agreement with previous studies 35 and earlier reports suggesting that the carbonate group in PUs is less hydrolytically susceptible than ester groups. 43 The slow degradation of PUU(PTMC2500) (mass remaining 88.3% at 4 weeks) was also in accordance with a previous study which showed a mass remaining of 94.6% of PTMC (Mw 6.9k) in lipase buffer at 3 weeks. 47,62–65 The faster mass loss of PUU-PVLCL2250 than PUU-PCL2000 can be explained by the former’s non-crystalline soft domain and thus easier water/enzyme diffusion inside the material, in agreement with previous reports that soft segment crystallinity in PUs may contribute to reduce enzymatic degradation. 43 Although the CL has one more -CH2- group than the VL therefore the PUU-PCL2000 should be slightly more hydrophobic than PUU-PVLCL2250, this is not considered to be the major factor for the faster degradation of the latter.
PUUs with crystalline SS of PCL
PCL is a popular crystalline biodegradable polyester used for degradable PU or PUU synthesis.20–21,23,25, 31–35 PCL has a Tm at ~55°C, while PUs or PUUs containing PCL segments usually show Tm at lower temperatures of 30–40°C, 31–35 due to the hindrance effects on the crystallization of PCL SS by the physical crosslinks of the HS domain. Although the Tm of PUU-PCL2000 is 31°C by DSC analysis (Figure 7a), the material still exhibited semi-crystalline behavior at body temperature (37°C) by DMA analysis (Figure 8a), where an energy loss (tan delta) peak corresponding to crystallization over a temperature range of 37–45°C was identified. The crystallinity and melting temperature of the PUU-PCL2000 was greatly affected by the deformation history of the sample (Figure 7d). For the sample stretched with a permanent set of 677%, the stretching gave rise to higher crystallinity and a sharper Tm peak (50°C) than the original sample, while after the crystals were melted and the sample cooled under no stress, the Tm went back again to 31°C. The increased crystallinity and molecular orientation in the stretched PUU-PCL2000 was further verified by the stronger crystal peaks in 1-D XRD and an asymmetrically distributed diffraction pattern in 2-D XRD analysis (Figure 9).
The stretch induced crystallization process also affects mechanical behavior of the PUU-PCL2000 under cyclic loading. Since the amount of the PCL crystal domain is inadequate to form a continuous rigid network, PUU(PCL2000) is a resilient elastic material at small deformation (<50%) (Figure 6a). However, the material showed poor resilience under large strain (>400%) (Figure 6a), indicated by the large first hysteresis loop in the cyclic tensile testing, which can be attributed to the fact that the new PCL crystalline domains formed by stretching help to “freeze” the deformation of the sample.
PUUs with non-crystalline SS of PTMC or PVLCL
Since the PUU with crystalline PCL SS is susceptible to the stretch-induced crystallization under large strain, non-crystalline and biodegradable PTMC or PVLCL were used as SSs to obtain more resilient PUUs. PTMC is an amorphous biodegradable elastomer of a relatively soft nature with a tensile modulus of 2.9 MPa 61 and a Tg of −17°C.53,54 PUU with a PTMC SS has previously been synthesized using water vapor as a chain extending agent.36,65 Also, in previous work a PUU was synthesized with a two-step method using a triblock SS containing a pluronic L31 block with two flanking PTMC blocks.34 The other noncrystalline SS used in this work was PVLCL, a random copolymer synthesized by ring-opening copolymerization of CL and VL. VL is a similar lactone which has one less -CH2- group than CL. Although both PCL and PVL are crystalline polymers with similar Tg (−63 and −72°C, respectively) and Tm (61 and 56°C, respectively), 66,67 random copolymerization of the two different monomers gives decreased regularity in the polymer chains to adversely affect the crystallization. To achieve the maximum randomness in the polymer chain structure, a 50:50 molar ratio of CL to VL was used. The DSC spectra of the PUU-PVLCL2500 and PUU-PVLCL6000 showed much lower Tm (−5 and 8°C, respectively, Figure 7a) than those of the PCL or PVL. Since crystalline domains do not exist between room and body temperature, there was no effect on the elasticity of the material as demonstrated by the DMA analysis of the PUU-PVLCL6000 (Figure 8d). The PUUs with PTMC or PVLCL SSs had remarkably higher resilience than the PUU-PCL2000, as evidenced by their smaller permanent deformation after tensile failure (Table 1 and Figure 5).
Cyclic tensile testing of the PUU-PTMCs and PUU-PVLCLs (Figure 6b-c) provides information on the stretch-induced material structural changes. When the cyclic tensile test was performed with a maximum strain of 50%, the first large hysteresis loop of the PUU samples is mainly caused by the stretch-induced dissociation of weak physical interactions, such as the H-bonds formed sporadically between the N-H groups from the HSs and the ester carbonyl (C=O) from the SSs. Reestablishment of such weak interactions may not occur in a short time and are therefore not observed in the following next 9 cycles. When the maximum strain was increased to 400%, the major reason for the large first hysteresis loop is speculated to be stretch-induced reorganization of the HS domain, in which the H-bonds of the HS domains were dissociated and the molecular chains reorganized to form new HS domain structures. Such structural change leads to unrecoverable deformation of the PUU samples. This would also explain the permanent deformation of the PUU-PTMC and PUU-PVLCL samples after their tensile failure (Table 1). PUU-PTMC1500 showed a larger hysteresis loop in the first cycle (Figure 6b) likely due to the shorter SS molecular chain and higher HS content in the material leading to a higher degree of reorganization of the HS domains.
Comparing the different materials shown in Table 1 and Figure 6, it can be seen that the most resilient materials are PUUs with high molecular weight noncrystalline SSs, e.g. PTMC 5400 and PVLCL 6000, which showed the lowest permanent deformation after tensile failure (Table 1). In Figure 6b-c, the PUU-PTMC5400 and PUU-PVLCL6000 showed almost no hysteresis and permanent deformation in their cyclic tensile test with 50% maximum strain presumably because the non-crystalline and high molecular weight SS made the material very soft (low modulus) and the lower stress did not substantially reorganize HS domains. The SS molecular weight also impacted the modulus of PUU-PTMC and PUU-PVLCL, with a longer SS chain leading to a lower modulus of the PUU (Table 1), in agreement with thermodynamic theory of rubbers stating that the initial modulus of an elastomer is inversely proportional to its average molecular weight between crosslink points.68
Several limitations in the current study should be noted. First, many of the mechanical property characterizations were performed at room temperature and in a dry state, while biomedical applications would generally be at body temperature in an aqueous environment. The PUU-PTMCs and PUU-PVLCLs that are amorphous at room temperature should not show remarkably different mechanical behavior at 37°C, as implied by DMA with temperature ramping between room and body temperature (Figure 8). For PUU-PCL2000 some temperature effect was noted (Figure 8a). Water uptake by the PUUs in vivo should be considered in future work because the interaction of water molecules with both the HS and SS could potentially interrupt some of the hydrogen bonding that is relevant for PUU properties.43 As the materials degrade, this effect would likely be enhanced. Cytocompatibility was investigated with a primary cell type relevant for some applications, but cell types appropriate for a given application, and ideally in vivo evaluations in an appropriate animal model would provide the logical next steps for evaluating material biocompatibility. Such experimentation might be performed in a manner that allows comparisons between similar PUUs with varying SS structure, particularly of interest would be the effect of SS length and crystallinity on cell adhesion and behavior.
Conclusions
Strong, biodegradable and cell-compatible PUU biodegradable elastomers with variable mechanical properties were synthesized by choosing SSs of different chemical structure and molecular weight. PUUs with non-crystalline SS (PTMC or PVLCL) showed improved elasticity and resilience compared with the PUUs with crystalline SS (PCL). PUUs with different moduli were obtained by manipulating the molecular weight of the SS, with a longer SS giving a softer and more resilient material. The relationship between these mechanical properties and the chemical-physical structures of the PUUs was revealed by NMR, FTIR, DSC, XRD, DMA analysis and tensile testing under static or cyclic loads. These results offer useful approaches in the molecular design and synthesis of biodegradable linear PUU tissue engineering scaffolds with tailored mechanical properties to satisfy the design objectives for the engineering of a variety of soft tissues.
Acknowledgments
This work was supported by the National Institutes of Health (NIH), grant #HL069368, the McGowan Foundation and the Commonwealth of Pennsylvania. Mr. Nelson was supported by NIH training grant #T32-HL076124. Dr. Hong was supported by the NSF (Award #0812348).
References
- 1.Ratner BD. Polym Int. 2007;56:1183–1185. [Google Scholar]
- 2.Stewart SF, Lyman DJ. J Biomech. 1992;25:297–310. doi: 10.1016/0021-9290(92)90027-x. [DOI] [PubMed] [Google Scholar]
- 3.Salacinski HJ, Goldner S, Giudiceandrea A, Hamilton G, Seifalian AM, Edwards A, Carson RJ. J Biomater Appl. 2001;15:241–278. doi: 10.1106/NA5T-J57A-JTDD-FD04. [DOI] [PubMed] [Google Scholar]
- 4.Hunter PJ, McCulloch AD, ter Keurs HE. Prog Biophys Mol Biol. 1998;69:289–331. doi: 10.1016/s0079-6107(98)00013-3. [DOI] [PubMed] [Google Scholar]
- 5.Engler AJ, Sen S, Sweeney HL, Discher DE. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 6.Soletti L, Hong Y, Guan J, Stankus JJ, El-Kurdi MS, Wagner WR, Vorp DA. Acta Biomater. 2010;6:110–122. doi: 10.1016/j.actbio.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gloeckner DC, Sacks MS, Fraser MO, Somogyi GT, De Groat WC, Chancellor MB. J Urol. 2002;167:2247–2252. [PubMed] [Google Scholar]
- 8.Goyal RK, Biancani P, Ppmuas A, Spiro HM. J Clin Invest. 1971;50:1456–1465. doi: 10.1172/JCI106630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Ameer GA, Sheppard BJ, Langer R. Nat Biotechnol. 2002;20:602–606. doi: 10.1038/nbt0602-602. [DOI] [PubMed] [Google Scholar]
- 10.Dey J, Xu H, Shen J, Thevenot P, Gondi SR, Nguyen KT, Sumerlin BS, Tang L, Yang J. Biomaterials. 2008;29:4637–4649. doi: 10.1016/j.biomaterials.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen QZ, Bismarck A, Hansen U, Junaid S, Tran MQ, Harding SE, Ali NN, Boccaccini AR. Biomaterials. 2008;29:47–57. doi: 10.1016/j.biomaterials.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Webb AR, Yang J, Ameer GA. Expert Opin Biol Ther. 2004;4:801–812. doi: 10.1517/14712598.4.6.801. [DOI] [PubMed] [Google Scholar]
- 13.Poirier Y, Nawrath C, Somerville C. Biotechnology (NY) 1995;13:142–150. doi: 10.1038/nbt0295-142. [DOI] [PubMed] [Google Scholar]
- 14.Bettinger CJ, Bruggeman JP, Borenstein JT, Langer RS. Biomaterials. 2008;29:2315–2325. doi: 10.1016/j.biomaterials.2008.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andronova N, Albertsson AC. Biomacromolecules. 2006;7:1489–1495. doi: 10.1021/bm060081c. [DOI] [PubMed] [Google Scholar]
- 16.Pego AP, Poot AA, Grijpma DW, Feijen J. J Biomater Sci Polym Ed. 2001;12:35–53. doi: 10.1163/156856201744434. [DOI] [PubMed] [Google Scholar]
- 17.Pego AP, Poot AA, Grijpma DW, Feijen J. J Mater Sci Mater Med. 2003;14:767–773. doi: 10.1023/a:1025084304766. [DOI] [PubMed] [Google Scholar]
- 18.Cohn D, Salomon AH. Biomaterials. 2005;26:2297–2305. doi: 10.1016/j.biomaterials.2004.07.052. [DOI] [PubMed] [Google Scholar]
- 19.Yang J, Webb AR, Pickerill SJ, Hageman G, Ameer GA. Biomaterials. 2006;27:1889–1898. doi: 10.1016/j.biomaterials.2005.05.106. [DOI] [PubMed] [Google Scholar]
- 20.Spaans CJ, De Groot JH, Belgraver VW, Pennings AJ. J Mater Sci Mater Med. 1998;9:675–678. doi: 10.1023/a:1008922128455. [DOI] [PubMed] [Google Scholar]
- 21.Gogolewski S. Colloid Polym Sci. 1989;267:757–785. [Google Scholar]
- 22.Storey RF, Hickey TP. Polymer. 1994;35:830–838. [Google Scholar]
- 23.Guelcher SA. Tissue Eng Part B Rev. 2008;14:3–17. doi: 10.1089/teb.2007.0133. [DOI] [PubMed] [Google Scholar]
- 24.Santerre JP, Woodhouse K, Laroche G, Labow RS. Biomaterials. 2005;26:7457–7470. doi: 10.1016/j.biomaterials.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 25.Wang W, Ping P, Yu H, Chen X, Jing X. J Polym Sci Part A: Polym Chem. 2006;44:5505–5512. [Google Scholar]
- 26.Li X, Loh XJ, Wang K, He C, Li J. Biomacromolecules. 2005;6:2740–2747. doi: 10.1021/bm050234g. [DOI] [PubMed] [Google Scholar]
- 27.Eceiza A, Martin MD, DelaCaba K, Kortaberria G, Gabilondo N, Corcuera MA, Mondragon I. Polym Eng Sci. 2008;48:297–306. [Google Scholar]
- 28.Szelest-Lewandowska A, Masiulanis B, Szymonowicz M, Pielka S, Paluch D. J Biomed Mater Res A. 2007;82:509–520. doi: 10.1002/jbm.a.31357. [DOI] [PubMed] [Google Scholar]
- 29.Kylma J, Seppala JV. Macromolecules. 1997;30:2876–2882. [Google Scholar]
- 30.Skarja GA, Woodhouse KA. J Biomater Sci Polym Edn. 1998;9:271–295. doi: 10.1163/156856298x00659. [DOI] [PubMed] [Google Scholar]
- 31.Guan J, Sacks MS, Beckman EJ, Wagner WR. J Biomed Mater Res. 2002;61:493–503. doi: 10.1002/jbm.10204. [DOI] [PubMed] [Google Scholar]
- 32.Guan J, Sacks MS, Beckman EJ, Wagner WR. Biomaterials. 2004;25:85–96. doi: 10.1016/s0142-9612(03)00476-9. [DOI] [PubMed] [Google Scholar]
- 33.Guan J, Wagner WR. Biomacromolecules. 2005;6:2833–2842. doi: 10.1021/bm0503322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang F, Li Z, Lannutti JL, Wagner WR, Guan J Acta Biomater. 2009;5:2901–2912. doi: 10.1016/j.actbio.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 35.Hong Y, Guan J, Fujimoto KL, Hashizume R, Pelinescu AL, Wagner WR. Biomaterials. 2010;31:4249–4258. doi: 10.1016/j.biomaterials.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asplund JO, Bowden T, Mathisen T, Hilborn J. Biomacromolecules. 2007;8:905–911. doi: 10.1021/bm061058u. [DOI] [PubMed] [Google Scholar]
- 37.Elliott SL, Fromstein JD, Santerre JP, Woodhous KA. J Biomater Sci Polym Ed. 2002;13:691–711. doi: 10.1163/156856202320269166. [DOI] [PubMed] [Google Scholar]
- 38.Caracciolo PC, Buffa F, Abraham GA. J Mater Sci Mater Med. 2009;20:145–155. doi: 10.1007/s10856-008-3561-8. [DOI] [PubMed] [Google Scholar]
- 39.Caracciolo PC, Thomas V, Vohra YK, Buffa F, Abraham GA. J Mater Sci Mater Med. 2009;20:2129–2137. doi: 10.1007/s10856-009-3768-3. [DOI] [PubMed] [Google Scholar]
- 40.Urry DW, Pattanaik A, Xu J, Woods TC, McPherson DT, Parker TM. J Biomater Sci, Polym Ed. 1998;9:1015–1048. doi: 10.1163/156856298x00316. [DOI] [PubMed] [Google Scholar]
- 41.MacEwan SR, Chilkoti A. Biopolymers. 2010;94:60–77. doi: 10.1002/bip.21327. [DOI] [PubMed] [Google Scholar]
- 42.Tibbitt MW, Anseth KS. Biotechnol Bioeng. 2009;103:655–663. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang YW, Labow RS, Santerre JP. J Biomed Mater Res. 2001;56:516–528. doi: 10.1002/1097-4636(20010915)56:4<516::aid-jbm1123>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 44.Jun HW, West JL. J Biomed Mater Res Part B: Appl Biomater. 2005;72B:131–139. doi: 10.1002/jbm.b.30135. [DOI] [PubMed] [Google Scholar]
- 45.Ho SP, Britton HO. Advanced Materials. 1994;6:130–132. [Google Scholar]
- 46.Raghavan ML, Webster MW, Vorp DA. Ann Biomed Eng. 1996;24:573–582. doi: 10.1007/BF02684226. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Z, Kuijer R, Bulstrab SK, Grijpma DW, Feijen J. Biomaterials. 2006;27:1741–1748. doi: 10.1016/j.biomaterials.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 48.Nelson DM, Baraniak PR, Ma Z, Guan J, Mason NS, Wagner WR. Pharm Res. doi: 10.1007/s11095-011-0391-z. Available online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ray JL, Leach R, Herbert JM, Benson M. Methods Cell Sci. 2001;23:185–188. doi: 10.1023/a:1016357510143. [DOI] [PubMed] [Google Scholar]
- 50.Wang W, Wang WS, Chen X, Jin X, Su Z. J Polym Sci Part B: Polym Phys. 2009;47:685–695. [Google Scholar]
- 51.Yilgor E, Yilgor I, Yurtsever E. Polymer. 2002;43:6551–6559. [Google Scholar]
- 52.Marcos-Fernández A, Lozano AE, González L, Rodríguez A. Macromolecules. 1997;30:3584–3592. [Google Scholar]
- 53.Pego AP, Poot AA, Grijpma DW, Feijen J. Macromol Biosci. 2002;2:411–419. [Google Scholar]
- 54.Bat E, Plantinga JA, Harmsen MC, van Luyn MJ, Zhang Z, Grijpma DW, Feijen J. Biomacromolecules. 2008;9:3208–3215. doi: 10.1021/bm8007988. [DOI] [PubMed] [Google Scholar]
- 55.Wong SC, Baji A, Leng S. Polymer. 2008;49:4713–4722. [Google Scholar]
- 56.Bittiger H, Marchessault RH, Niegisch WD. Acta Cryst. 1970;B26:1923–1927. [Google Scholar]
- 57.Tang YW, Labow RS, Santerre JP. J Biomed Mater Res. 2001;57:597–611. doi: 10.1002/1097-4636(20011215)57:4<597::aid-jbm1207>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 58.Guelcher SA, Gallagher KM, Didier JE, Klinedinst DB, Doctor JS, Goldstein AS, Wilkes GL, Beckman EJ, Hollinger JO. Acta Biomater. 2005;1:471–484. doi: 10.1016/j.actbio.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 59.Guelcher SA, Srinivasan A, Dumas JE, Didier JE, McBride S, Hollinger JO. Biomaterials. 2008;29:1762–1775. doi: 10.1016/j.biomaterials.2007.12.046. [DOI] [PubMed] [Google Scholar]
- 60.Fontaine L, Menard L, Cayuela O, Brosse JC, Sennyey G, Senet JP. Macromolecular Symposia. 1997;122:287–290. [Google Scholar]
- 61.Bras W, Derbyshire GE, Bogg D, Cooke J, Elwell MJ, Komanschek BU, Naylor S, Ryan AJ. Science. 1995;267:996–999. doi: 10.1126/science.267.5200.996. [DOI] [PubMed] [Google Scholar]
- 62.Zhu KJ, Hendren RW, Jensen K, Pitt CG. Macromolecules. 1991;24:1736–1740. [Google Scholar]
- 63.Pego AP, van Luyn MJA, Brouwer LA, van Wachem PB, Poot AA, Grijpma DW, Feijen J. J Biomed Mater Res A. 2003;67A:1044–1054. doi: 10.1002/jbm.a.10121. [DOI] [PubMed] [Google Scholar]
- 64.Storey RF, Hickey TP. Polymer. 1994;35:830–838. [Google Scholar]
- 65.Asplund B, Aulin C, Bowden T, Eriksson N, Mathisen T, Bjursten LM, Hilborn J. J Biomed Mater Res B. 2008;86B:45–55. doi: 10.1002/jbm.b.30986. [DOI] [PubMed] [Google Scholar]
- 66.Yang J, Jia L, Yin L, Yu J, Shi Z, Fang Q, Cao A. Macromol Biosci. 2004;4:1092–1104. doi: 10.1002/mabi.200400128. [DOI] [PubMed] [Google Scholar]
- 67.Wang J, Cheung MK, Mi Y. Polymer. 2002;43:1357–1364. [Google Scholar]
- 68.Kloczkowski A. Polymer. 2002;43:1503–1525. [Google Scholar]