Abstract
Objective
At what age are children with an autism spectrum disorder (ASD) identified by community providers? What factors influence the timing of when children are identified with ASDs? This study examined the timing of when children with ASDs are identified.
Method
Data came from 13 sites participating in the Centers for Disease Control and Prevention’s 2002 multisite, ongoing autism surveillance program, the Autism and Developmental Disabilities Monitoring Network. Survival analysis was used to examine factors that influence the timing of community-based identification and diagnosis.
Result
Data from health and education records reveal that the median age of identification was 5.7 years (SE 0.08). Parametric survival models revealed that several factors were associated with a younger age of identification: being male, having IQ ≤ 70, and having experienced developmental regression. Significant differences in the age of identification among the 13 sites were also discovered.
Conclusions
The large gap between the age at which children can be identified and when they actually are identified suggests a critical need for further research, innovation, and improvement in this area of clinical practice.
MeSH KEYWORDS: Pervasive Child Development Disorders, Autism, Diagnosis, Epidemiology, Survival Analysis
Timely community-based identification of children with an autism spectrum disorder (ASD) has important implications for individual development, clinical practice, and policy decisions. Identification is a broad construct that includes both clinical diagnoses of ASD and eligibility-related designations of ASD for public services, including early intervention and special education. The American Academy of Pediatrics (AAP) recently emphasized the importance of early identification of ASDs, and recommended close developmental observation at every well-child visit and screening for autism with a standardized instrument at 18 and 24 months of age.1 Previous studies have established that impairments associated with ASDs can be modified by intervention.2 Timely start of intervention is contingent on early identification. Early and accurate diagnosis of ASDs also enables families to learn about their child’s developmental challenges, cope with caregiving demands, seek appropriate services, and obtain genetic counseling.3
Experienced clinicians can reliably diagnose autistic disorder in children as young as 2 years of age, whereas accurate diagnosis of Asperger’s disorder and Pervasive Developmental Disorder-Not Otherwise Specified (PDD-NOS) often cannot reliably be obtained until 4–5 years of age.4 Previous research conducted in the United States has consistently documented a gap between the age at which children with ASDs can be identified and the age at which they are identified. Public health systems and policies vary widely among nations. We focus on previous U.S. studies in this paper, as their findings are most relevant to the data examined and we do not want suggest that our findings generalize to health care systems in other countries. These U.S. studies have varied by study year, catchment area, specific diagnoses examined, and the study design.3, 5–7 For example, a mail survey of Pennsylvania families found the mean age of diagnosis was 3.1 years for children with autistic disorder, 3.9 years for PDD-NOS, and 7.2 years for Asperger’s disorder.6 A study of children enrolled in Medicaid in Philadelphia, Pennsylvania, found that the mean age of ASD diagnosis was 7.4 years, although no information was available on diagnostic subtypes or clinical presentation.3 Data from a multisite network monitoring the prevalence of ASDs in 8-year-old children using a record review method (the same data used in this report) found the median age of ASD diagnosis ranged from 4.1 to 5.5 years, depending on surveillance site.5 Using similar methods, a population-based study of children in the Atlanta area found the mean age of first ASD diagnosis was 5.1 years.7
A limitation of these studies is that they excluded censored cases (i.e., those meeting case criteria for surveillance but not yet identified as autistic by community practitioners) when they computed the mean age of identification, creating the potential for downwardly biased estimates. The simple mean age of identification among all children meeting ASD case criteria would be calculable only if they were all followed forward in time until the age of community identification were known for each. In contrast, our study uses survival analysis methods, which allow censored cases to be included in estimates of the timing of identification.
Various factors have been associated with later ASD diagnosis, including race and ethnicity, level of child impairment, and family income. Studies have found contradictory results regarding the role of race and ethnicity; some studies suggest that certain minority groups are diagnosed later, whereas other studies suggest comparable ages of diagnosis across racial and ethnic groups.3, 6, 7 Children with more severe impairments tend to be diagnosed at younger ages 6, 7 and children who live in moderate poverty tend to be diagnosed at an older age.6
Baseline estimates of the timing of ASD identification are necessary for evaluating public health initiatives to improve screening and diagnostic practices. Examining the correlates of timing can reveal disparities and subpopulations at risk for late identification. This information can, in turn, guide clinical and community interventions focused on improvement.
The objective of this study was to examine the timing of community identification among children with ASDs using data from a national public health surveillance study. We hypothesized that younger age of identification would be associated with evidence of early developmental regression, cognitive impairment (having IQ ≤ 70), and higher family socioeconomic status. In this paper, developmental regression refers to the phenomenon in which a period of apparently typical development in the first 1–2 years of life is followed by a marked loss of previously acquired skills and subsequent diagnosis of autism.8, 9 We also predicted there would be significant variability among sites in the timing of identification because of differences in community screening and diagnostic practices, and access to surveillance data. Site-specific estimates can serve as baselines for evaluating local efforts to improve early identification.
METHOD
Sample and surveillance methodology
Data are from 13 sites (listed in Table 1) participating in the Centers for Disease Control and Prevention’s (CDC’s) multisite, ongoing public health surveillance program, the Autism and Developmental Disabilities Monitoring (ADDM) Network. The study sample included all 8-year-old children meeting criteria for ASD case status as defined by the ADDM Network in the 2002 study year (N = 2,568). A detailed description of the surveillance methodology can be found elsewhere.5 Briefly, the surveillance protocol entails defining a geographic catchment area for each site and then contacting health care and education providers in each given area. As a public health surveillance study, the aim is to identify every 8-year old child with an ASD in each catchment area in order to produce valid population-based estimates of prevalence. Health and education records of children who were 8-years old in the target study year and identified as having a variety of behavioral, development, and psychiatric diagnoses (not just ASD) were systematically abstracted and entered into a secure database. Records from multiple sources were linked, combined, and then reviewed by trained clinicians to determine case status using a highly structured scoring protocol based on the Diagnostic and Statistical Manual of Mental Disorders-4th edition-Text Revision.10 Previous analyses found that 98% of children with a documented diagnosis of ASD met surveillance case criteria;11 therefore, partial records were abstracted for some children with a previously documented health or educational ASD diagnosis and no contradicting indicators (a practice called “streamlining”).12 Inter-rater reliability was established before surveillance began and was continuously monitored. Percent agreement for final case determination ranged from 79% to 100% (Kappa = 0.55 to 1.00) across sites. The ADDM protocol was approved by the institutional review board at each surveillance site.
Table 1.
Variable Distributions and Unadjusted Kaplan-Meier Estimates of the Median Age of Autism Identification for Children Who Were 8-Years-Old in 2002 and Met the Autism Case Criteria of the U.S. Autism and Developmental Disabilities Monitoring (ADDM) Network Surveillance System.a
| Variable Distributions | Kaplan-Meier Estimates | |||
|---|---|---|---|---|
| Variables | N | % | Median age of identification | χ2 (df), P |
| All cases | 2,568 | 100% | 5.7 | |
| Child and Family Characteristics | ||||
| Sex | 4.9 (1), .027 | |||
| Female | 491 | 19.1% | 6.1 | |
| Male | 2,077 | 80.9% | 5.6 | |
| Race/ethnicity | 18.2 (5), .003 | |||
| White, non-Hispanic | 1,620 | 63.1% | 5.7 | |
| Black, non-Hispanic | 589 | 22.9% | 5.3 | |
| Asian or Pacific | 57 | 2.2% | ||
| Islander, non-Hispanic | 5.2 | |||
| Other, non-Hispanic | 44 | 1.7% | 6.7 | |
| Hispanic | 194 | 7.6% | 6.2 | |
| Race missing | 64 | 2.5% | 7.0 | |
| Cognitive Status | 71.3 (2), <.001 | |||
| IQ > 70 | 1,063 | 41.4% | 6.6 | |
| IQ ≤ 70 | 859 | 33.5% | 5.1 | |
| IQ missing | 646 | 25.2% | 5.3 | |
| Developmental Regression | 172.7 (1), <.001 | |||
| No | 2,087 | 81.3% | 6.2 | |
| Yes | 481 | 18.7% | 4.2 | |
| Maternal Age at Birth | 9.1 (3), .028 | |||
| 13–19 | 151 | 5.9% | 6.6 | |
| 20–29 | 896 | 34.9% | 5.7 | |
| 30–46 | 758 | 29.5% | 5.4 | |
| Missing age | 763 | 29.7% | 5.8 | |
| Maternal Education at Birth | 15.4 (5), .009 | |||
| < 12 years | 275 | 10.7% | 6.3 | |
| 12 years | 607 | 23.6% | 5.7 | |
| 1–3 years college | 400 | 15.6% | 5.5 | |
| 4 years college | 336 | 13.1% | 5.3 | |
| > 5 years college | 146 | 5.7% | 5.3 | |
| Missing education | 804 | 31.3% | 5.8 | |
| Surveillance-Related Variables | ||||
| Source of Records Reviewed | 99.8 (2), <.001 | |||
| Health-only | 898 | 35.0% | 5.8 | |
| Education-only | 829 | 32.3% | 6.6 | |
| Both | 841 | 32.7% | 5.1 | |
| Case Classification | 812.4 (2), <.001 | |||
| Autistic disorder | 967 | 37.7% | 7.3 | |
| ASD-NOS | 555 | 21.6% | 8.8 | |
| Streamlined | 1,046 | 40.7% | 4.7 | |
| Sites | 83.2 (12), <.001 | |||
| Alabama | 116 | 4.5% | 7.0 | |
| Arkansas | 251 | 9.8% | 5.5 | |
| Arizona | 281 | 10.9% | 7.3 | |
| Colorado | 119 | 4.6% | 6.5 | |
| Georgia | 337 | 13.1% | 5.2 | |
| Maryland | 199 | 7.7% | 5.2 | |
| Missouri & Illinois | 227 | 8.8% | 6.3 | |
| North Carolina | 135 | 5.3% | 5.4 | |
| New Jersey | 318 | 12.4% | 5.3 | |
| Pennsylvania | 111 | 4.3% | 5.4 | |
| South Carolina | 140 | 5.5% | 6.3 | |
| Wisconsin | 181 | 7.0% | 5.4 | |
| West Virginia | 153 | 6.0% | 5.1 | |
Variables with nonsignificant differences not reported (e.g., birth weight, gestational age, maternal marital status at birth, whether a site had access to education records are not reported). ASD-NOS = Autism Spectrum Disorder-Not otherwise specified
Variables
The dependent construct of interest was the age at which a child was first identified by a health, education, or other community service provider as having an ASD. The construct of “identification” was operationalized as meeting one or more of the following criteria: 1) a clinical diagnosis noted in an abstracted evaluation, including reference to a diagnosis from a previous evaluation that was not available for abstraction; 2) eligibility for special education services under an ASD category, as noted in an abstracted evaluation or by the student’s primary classification at age 8; or 3) an International Classification of Diseases, 9th Edition (ICD-9) code for an ASD (299.0 or 299.8). Using these criteria, we found that 1,873 of the 2,568 (72.9%) children in our sample had been previously identified as having an ASD. Of the 1,873 children with an ASD classification noted in their records, the age of identification was clearly specified in records for 1,655. For the remaining 218 meeting identification criteria, the age at identification was not clearly specified in the records. These 218 cases were compared to the 1,655 cases with an age at identification using the following variables: sex, race, cognitive status, ASD classification, special education eligibility, and ICD-9 code status. These 218 cases were no different in terms of sex or cognitive status. Significant differences were found on the other variables. Rather than treating these 218 children (8.5% of all cases) as missing, we used predicted values from a regression model to impute age at identification on the basis of the following variables: site, ASD classification, cognitive status, and whether the child experienced developmental regression. The remaining 695 children (27.1% of all cases) with no mention of ever having been identified with an ASD were considered to be unidentified through age 8 years. Because these children met ADDM case criteria, they are at risk for diagnosis and can be incorporated into a survival analysis approach as censored observations.
Independent variables were grouped into three blocks: child and family characteristics, surveillance-related variables, and sites. Child characteristics included sex, race/ethnicity, cognitive status, and whether developmental regression was mentioned in a child’s records. Surveillance records were matched with birth records, when available, to obtain birth weight (grams), gestational age (weeks), maternal age at birth (years), maternal education at birth (years), and maternal marital status at birth.
Variables related to the process of conducting ADDM surveillance included an indicator of the sources for reviewed records, coded as health-only, education-only, or both health and education. As described elsewhere,5, 12 cases were assigned one of three classification codes on the basis of the presence of prior diagnoses, the number of autistic symptoms reported in evaluation records, and the quantity of information about autistic behaviors present in records. Cases were described as “streamlined” when there was an existing ASD diagnosis and records did not contain information contradicting an ASD diagnosis. A code for “autistic disorder” was assigned to cases in which enough behavioral data were available in records for clinician reviewers to make case determinations for autistic disorder on the basis of the DSM-IV TR criteria. The ASD-NOS code was assigned to children whose records included enough behavioral data for clinician reviewers to make case determinations on the basis of DSM-IV TR criteria for PDD-NOS or Asperger’s disorder. Classifying children into one of these three categories depended on the severity and number of symptoms described in available records. In turn, the likelihood of symptoms being recorded was associated with the quantity and quality of detailed behavioral information in each child’s records. Therefore, this variable measures both symptom severity and the density of information in the records.
We handled missing data by creating a missing category for each variable with missing values. The sample would have reduced to 1,317 if we only used cases with complete data on every variable used in analysis. We experimented with more complex approaches for handling missing data, but the substantive findings were not altered. The advantage of the chosen categorical approach is that it is simple, easily interpreted, and allows for an informal assessment of the impact of missingness.
Surveillance methodology varies somewhat across sites.5 Nine sites had full access to education records. Three sites did not have access to education records, and one site had partial access. We created a dichotomous variable to distinguish cases from sites with full access from sites with no or reduced access to education records. We combined Missouri and Illinois because the surveillance catchment area was the St. Louis metropolitan area, which straddles the states’ common border, and surveillance for this area was conducted by one investigative team.
DATA ANALYSIS
We used two different forms of survival analysis to examine the timing of identification. Survival analysis is a general class of analytic techniques appropriate for research questions about the timing of event occurrence in samples, including situations where not every subject has experienced the event by the end of follow-up. In our study, childre who did experience identification through age 8 were in fact included in the analyses, as they were still considered part of the population at risk for identification. Thus, survival analysis allowed us to include all 2,568 children in the analysis and treat the fact some have not yet been identified as meaningful information, not as cause for deletion from analysis.
We used Kaplan-Meier survival curves to estimate the cumulative probability of reaching a given age without being identified as having an ASD. The resulting median age of identification is the age by which 50% of cases in the sample were identified as having an ASD. The related Tarone-Ware chi-squared test was used to test whether groups of survival curves were significantly different. It is preferable to the log-rank test in situations where the hazard functions do not vary proportionally and is less susceptible to differences in censoring patterns between groups than the Wilcoxon test.13
We used multivariable log-logistic parametric survival models to examine the effects of independent variables on the timing of identification. A Cox proportional hazards survival model would have been inappropriate for these data because several predictors violated the proportionality assumption. Using a stratified Cox model would have prevented us from directly examining the adjusted effects of these substantively important predictors. Furthermore, examination of the unadjusted kernel smoothed 14 hazard curve for our data showed that the shape of the hazard function rose through the early years of development and then fell in later childhood. The log-logistic parametric model is appropriate for examining data with this type of underlying hazard function.15–17 A plot of the log-odds of survival beyond t against log t confirmed the appropriateness of this model specification.15–17 An advantage of this accelerated failure time method is that the exponentiated coefficients can be interpreted as ratios to the timing of identification (denoted “time ratios” in statistical literature and in the Stata software package used for analyses). Time ratios greater than 1 indicate an effect that delays the timing of identification; whereas, time ratios less than 1 indicate an accelerating effect on the timing of identification.18 For instance, a time ratio of 2 would mean a 1-unit change in the covariate is associated with a doubling of the median age of identification, controlling for other variables in the model.16, 19
All variables with significant differences in Kaplan-Meier median ages of identification were included in the multivariable modeling. Hierarchical sets of variables were entered sequentially (Table 2). Model 1 included child and family characteristics. Model 2 added surveillance-related factors. Model 3 added sites. The adjusted median age of identification is reported for each model. This adjusted median age was produced using Stata’s “adjust, exp” post-estimation command which computes the average estimated prediction using the mean of each predictor variable. Thus, the adjusted median age is not the predicted median age defined by which predictor categories are omitted, as would be the case interpreting the intercept of a linear regression model as the predicted value when all predictors equal 0. Instead, the adjusted median age represents the expected age of identification for a hypothetical child who is “average” with respect to all the independent variables in the model. Likelihood ratio tests (LRTs) were conducted to compare models.
Table 2.
Age of Autism Identification Parametric Log-Logistic Survival Models for Children Who Were 8-Years-Old in 2002 and Met the Autism Case Criteria of the U.S. Autism and Developmental Disabilities Monitoring (ADDM) Network Surveillance System.
| Time Ratios | |||
|---|---|---|---|
| Variables | Model 1 | Model 2 | Model 3 |
| Child and Family Characteristics | |||
| Sex | |||
| Female | - | - | - |
| Male | 0.92** | 0.94* | 0.95* |
| Race/ethnicity | |||
| White, non-Hispanic | - | - | - |
| Black, non-Hispanic | 1.01 | 1.03 | 0.99 |
| Asian or Pacific | 1.05 | 1.06 | 1.07 |
| Islander, non- Hispanic | |||
| Other, non-Hispanic | 1.20* | 1.10 | 1.07 |
| Hispanic | 1.11* | 1.09* | 1.04 |
| Race missing | 1.22** | 1.07 | 1.09 |
| Cognitive Status | |||
| IQ > 70 | - | - | - |
| IQ ≤ 70 | 0.82*** | 0.88*** | 0.90*** |
| IQ missing | 0.83*** | 0.90*** | 0.92** |
| Developmental Regression Ever Mentioned | |||
| No | - | - | - |
| Yes | 0.71*** | 0.78*** | 0.79*** |
| Maternal Age at Birth | |||
| 13–19 | 1.10 | 1.10 | 1.09 |
| 20–29 | 1.02 | 1.00 | 1.02 |
| 30–46 | - | - | - |
| Missing age | 0.85 | 0.92 | 0.93 |
| Maternal Years of Education at Birth | |||
| < 12 | 1.15** | 1.03 | 1.05 |
| 12 years | 1.09* | 1.01 | 1.03 |
| 1–3 years college | 1.07 | 1.03 | 1.04 |
| 4 years college | - | - | - |
| > 5 years college | 1.01 | 1.03 | 1.02 |
| Missing education | 1.24* | 1.10 | 1.11 |
| Surveillance Related Variables | |||
| Source of Records Reviewed | |||
| Health-only | 1.07** | 1.00 | |
| Education-only | 1.24*** | 1.21*** | |
| Both | - | - | |
| Case Classification | |||
| Autistic disorder | - | - | |
| ASD-NOS | 1.15*** | 1.21*** | |
| Streamlined | 0.63** | 0.60*** | |
| Sitesa | |||
| Alabama | 1.19*** | ||
| Arkansas | -a | ||
| Arizona | 1.07* | ||
| Colorado | 1.03 | ||
| Georgia | 1.08** | ||
| Maryland | 1.01 | ||
| Missouri & Illinois | 1.16*** | ||
| North Carolina | 0.93 | ||
| New Jersey | 1.02 | ||
| Pennsylvania | 1.10* | ||
| South Carolina | 0.96 | ||
| Wisconsin | 0.87** | ||
| West Virginia | 0.66*** | ||
| Log likelihood | −2,195 | −1,858 | −1,783 |
| Likelihood ratio test vs. preceding model: χ2 (df) | 674.4*** (4) | 149.1*** (12) | |
| Adjusted median age of identificationb | 5.96 | 6.09 | 6.08 |
P < .05
P < .01
P < .001
Based on effect coding, the time ratios for sites are relative to the grand mean across all states rather than being relative to a specific site (as in regular dummy coding).
The average estimated prediction based on using the mean of each predictor variable.
We used deviation effect coding for the sites because there was no logical choice of reference category. The category effects for these dummy variables are relative to the grand mean effect across all sites rather than to a specific site. This approach still requires omitting a category, as in regular dummy coding, but the interpretation of the effect is not in comparison to the omitted category. Instead, these time ratios represent a given site’s deviation from the mean value among sites. The omitted site (Arkansas) had a Kaplan-Meier median age of identification (5.5 years) closest to the overall unadjusted sample estimate (5.7 years).
Stata SE 9.1 software was used for all analyses.
RESULTS
Sample description
Table 1 reports descriptive statistics for the sample and all variables used in analysis. The male:female ratio was 4.2:1. Nearly two-thirds (63.1%) were white. One-third had cognitive impairment (IQ ≤ 70).
Median age of identification
Table 1 also reports the Kaplan-Meier unadjusted median age of identification estimates and related tests for equality of survival functions. The median age of identification for the entire sample was 5.7 years.
The median age of identification for females (6.1 years) was significantly older than for males (5.6 years). Prior research has established cognitive impairment (IQ ≤ 70) to be more prevalent among females with ASD 5. Females in our sample were identified at a later age despite a tendency to be more cognitively impaired, which prompted us to examine the distribution of cognitive ability stratified by sex. Of children with available IQ scores, 53.7% of females had IQ scores <= 70, compared with 42.8% of males, χ2(1, N = 1,922) = 13.4, P < .001. We then stratified the median age of identification by sex and IQ. Within the IQ > 70 strata, the median age of identification was 7.1 years (95% confidence interval: 6.4, 8.3) for females and 6.5 years (95% confidence interval: 6.2, 6.8) for males. Within the IQ <= 70 strata, the median age of identification was 5.5 years (95% confidence interval: 5.0, 6.3) for females and 5.1 years (95% confidence interval: 4.9, 5.3) for males.
The overall test for race and ethnicity was statistically significant. Non-Hispanic black and Asian children had the lowest median ages of identification (5.3 and 5.2, respectively). Other non-Hispanic children and those for whom information on race was missing had the highest median ages (6.7 and 7.0, respectively). White non-Hispanic children had a median age of identification of 5.7 years and Hispanic children had a median age of identification of 6.2 years.
Two factors indicating greater severity of autism were significantly associated with a younger median age of identification. Those with cognitive impairment were identified earlier (5.1 years) than for those without such impairment (6.6 years). Children with a record of developmental regression had a median age of identification younger than those without a history of regression (4.2 and 6.2 years, respectively).
Younger maternal age at birth and lower maternal educational attainment were significantly associated with older ages of ASD identification, but maternal marital status at birth, child’s birth weight, and gestational age were not.
Cases in which records were obtained from both education and health sources had a younger median age of identification (5.1 years) than cases with records from education-only sources (6.6 years) and cases with records from health-only sources (5.8 years). With respect to different groups of ASD classification, streamlined cases had the youngest median age of identification (4.7 years), children classified ASD-NOS had the oldest age (8.8 years), and those classified with autistic disorder were in the middle (7.3 years).
The median age of identification varied significantly across sites, ranging from a low of 5.1 years in West Virginia to a high of 7.3 years in Arizona (Table 1). We considered the possibility that some of this variation might be because of differences among sites in access to education records. Investigators in Wisconsin, Missouri-Illinois, and Alabama were not able to access education records, and those in Pennsylvania only had access to education records in some of the catchment area. The difference in median age of identification for sites with full access to education records (5.6 years) and sites with reduced or no access to education records (6.0 years) was not statistically significant, χ2(1, N = 2,568) = 0.3, p = .61.
Multivariable time ratios
Model 1 examined the effects of child and family characteristics (Table 2). Significantly younger age of identification was associated with being male, IQ ≤ 70, and indication of developmental regression. Significantly older age at identification was associated with “other” race and Hispanic ethnicity (vs. white), and 12 years or less of maternal educational attainment. There was no significant interaction between sex and IQ in an exploratory model; therefore, we did not include an interaction term in the final models. The time ratio of 1.11 for Hispanic children indicates their median age of identification was 11% higher than for white children. The time ratio for noted presence of developmental regression, the covariate with the largest effect size, was 0.71 — indicating that the adjusted median age of identification for these cases was 29% younger than cases without noted regression. The adjusted median age of identification for this model was 5.96 years.
Model 2 included the surveillance-related variables indicating the source of records reviewed and case classification. The likelihood ratio test indicated this model fit the data significantly better than Model 1. The adjusted median age of identification was 6.09 years. Significantly younger age at identification was associated with being a streamlined case (vs. an autistic disorder case). Significantly older age at identification was associated with having health-only and education-only records (vs. both types) and being coded as ASD-NOS (vs. autistic disorder). The largest effect sizes were for education-only source records and streamlined case classification (time ratios of 1.24 and 0.63, respectively). The low maternal education time ratios decreased 7%–10% when compared to Model 1, and were no longer significant. The time ratios for other race, missing race, and missing education decreased 8%, 12%, and 11%, respectively, and were no longer significant. All other significant variables from Model 1 remained significant in Model 2 and changed less than 10% (male, Hispanic, IQ ≤ 70, IQ missing, developmental regression).
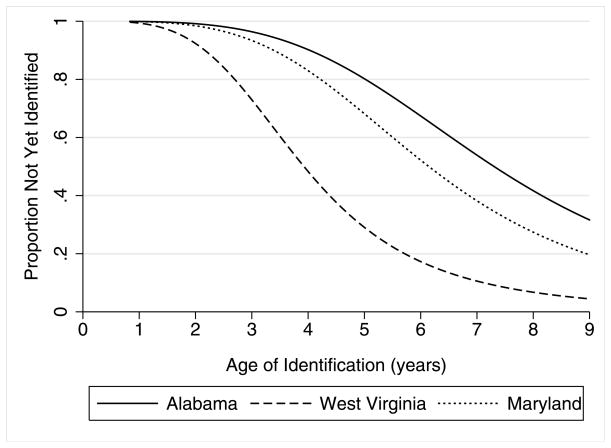
Model 3 included the dummy indicators for sites. Recall that deviation coding means the site time ratios are interpreted in relation to the mean effect across sites. The likelihood ratio test indicated Model 3 fits the data significantly better than Model 2. The adjusted median age of identification was 6.08 years. The adjusted age of identification was significantly younger in Wisconsin and West Virginia, with time ratios of 0.87 and 0.66, respectively. The adjusted age of identification was significantly older in Alabama (time ratio = 1.19), Arizona (time ratio = 1.07), Georgia (time ratio = 1.08), Missouri-Illinois (time ratio = 1.16), and Pennsylvania (time ratio = 1.10). The time ratios for the remaining seven sites were not significantly different from the mean across all sites. The range of variability among sites is depicted in Figure 1, which displays 3 sites’ adjusted survival curves. Maryland had the time ratio closest to 1, indicating the closest to average adjusted timing of identification curve across all the sites. West Virginia and Alabama are the youngest and oldest age of identification curves, respectively.
Figure 1.
Proportion of Cases Not Yet Identified with Autism in Alabama, Maryland, and West Virginia for Children Who Were 8-Years-Old in 2002 and Met Autism Case Criteria as Part of the U.S. Autism and Developmental Disabilities Network Surveillance System.a
a Adjusted for all variables listed under Model 3 of Table 2.
DISCUSSION
This paper presented findings on the timing of identification in ASDs and its correlates using data from the largest U.S. population-based effort to date. The unadjusted median age of identification at each site in this study was older than the median ages in a prior report5 based on the same data. This discrepancy exists because the present study used survival analysis to include the 695 censored cases (27.1% of all cases) that met ASD surveillance case criteria, but had no previous record or documentation of an ASD diagnosis. The prior report computed the simple median age of identification using only cases with a known age of identification.
The variability among sites was notable, with seven sites having adjusted ages of identification significantly higher or lower than average. Geographic variability in educational practices, health care access, and use of services is not uncommon, and such variability has significant implications for decisions about the allocation of finite resources. Screening and diagnostic practices in these communities could be examined in future research to search for factors that facilitate or impede early identification.
Two findings especially relevant to policy are the unadjusted median age of identification (5.7 years) and the proportion of cases that had not been identified as having an ASD through age 8 (27.1%). Given that experienced clinicians can generally diagnose autism between the ages of 2 and 3, the gap between potential and actual age of identification (for those identified) is in the range of 2.7–3.7 years. Combined with the fact that more than one-quarter of cases were never identified as having ASD through age 8, this gap indicates significant weaknesses in the overall system of community screening and identification for ASD. Future research should examine the developmental, family, and societal consequences of late identification. Researchers also should conduct studies that help the public health community develop interventions to improve the timing of identification.
Several current efforts aim to improve the timing of identification of ASDs among young children. The American Academy of Pediatrics has published specific recommendations for ASD identification and evaluation.1 The “Learn the Signs. Act Early” campaign is a partnership between CDC and other groups focused on increasing awareness about early identification and diagnosis of autism among parents and health care providers.20 The National Medical Home Autism Initiative promotes the application of the medical home concept to children with ASDs.21 Little is known about the effect of these campaigns on appropriate and timely identification. The fact that many children are not identified until after age 5 suggests future efforts should include an emphasis on recognition and diagnosis among school-age children, not just among very young children.
The sex disparity is especially relevant to clinical practice. Females are identified at a later age despite a greater likelihood of cognitive impairment. This disparity could conceivably stem from sex differences in the clinical presentation of ASDs. However, a recent study that matched males and females with autism by age, IQ, and diagnosis found no sex differences in core autistic symptoms, but did find that females had more significant social, attention, and thought problems.22 Another recent study found higher social-competence ratings among boys with autism.23 That females are identified later despite tending to be more severely affected across a range of indicators suggests the possibility of sex bias in cultural expectations of children’s behavior or in clinical practices for screening, referral, and diagnosis. Different cultural expectations of what constitutes normative behavior in boys and girls may result in different thresholds for recognition of behavioral deviance. For instance, research has shown that shyness in girls is more socially acceptable than in boys.24
As predicted, earlier age of identification was associated with greater severity of cognitive impairment. The younger age of identification among children with early developmental regression is possibly related to this phenomenon, which is typically observable by age 2.8 The clinical presentation is relatively unambiguous, and some research indicates that autistic children with this course of development tend to be more severely impaired relative to those without this course of development.25
Children with evaluation records from both education and health sources had a significantly younger adjusted age of identification compared with those with education-only sources of records. We examined the possibility that this might be a result of differential access to education records across sites. However, no significant difference existed in the timing of identification between sites with full access to education records compared with those without this access. Another possible explanation for this finding is that greater access to multiple systems of care is an enabling factor that can facilitate earlier identification.
Significant racial differences were noted in the unadjusted Kaplan-Meier estimates and in the first two multivariable models. However, these differences were not significant in the final adjusted model when dummy indicators for sites were added. The black-white differences were negligible across all analyses. Hispanic children had later ages of identification than white children in the unadjusted analysis and the first two adjusted analyses. The time ratio for Hispanic children was 6 % lower in Model 1 (1.11) than in Model 3 (1.4). Although no large racial differences existed in the timing of identification, there were significant racial differences in whether children who meet ASD case criteria are ever identified as such by health and education professionals. In adjusted analyses from another report using a subset of these data, black children (odds ratio [OR] = 0.78; 95% confidence interval: 0.64, 0.96), Hispanic (OR = 0.75; 0.55, 0.99) or “other” ethnicity (OR = 0.65; 0.44, 0.97) were less likely than white children to have a documented ASD.26 This disparity was concentrated among children with IQ ≤ 70.
This study has some limitations. The range and quality of independent variables measuring autism symptoms, child health, family socioeconomic status, and access to health care were limited because of the use of record abstraction for the surveillance protocol. The lack of in-person contact with children also means we had no way of validating ASD diagnosis using a standard clinical assessment. Nor was there any way to directly confirm the age of identification. Because of the record abstraction methodology, information about the diagnostic history of a given child was possibly incomplete, and/or the record of a child with an earlier diagnosis was not able to be located.
This study also has several strengths. The population-based nature of the sample reduces the influence of volunteer biases found in community surveys. The size and demographic diversity of the sample allowed us to test for demographic disparities with adequate statistical power. The linkage with birth records allowed us to test hypotheses that would have been impossible otherwise.
In summary, this study examined the timing of identification among children with autism using a population-based sample from an ongoing surveillance effort. The large gap between the age at which children can be identified on the basis of best available practices and when they actually are identified suggests the substantial need for further research, innovation, and improvement in this area of practice.
Acknowledgments
This work was supported by the Centers for Disease Control and Prevention, Cooperative Agreements UR3/CCU523235 and UR3/DD000078, the University of Wisconsin’s Waisman Center (T32 HD07489), and the Washington University Center for Mental Health Services Research (P30 MH068579). Additional funding for graduate student support for data analysis was provided by the University of Wisconsin.
Footnotes
Disclosure: Dr. Rice serves as a trainer for professionals in the assessment of Autism Spectrum Disorders using the Autism Diagnostic Observation Scale. The other authors report no conflicts of interest.
These data were collected under a cooperative agreement funded by the Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Johnson CP, Myers SM Council on Children with Disabilities. Identification and evaluation of children with autism spectrum disorders. Pediatrics. 2007;120(5):1183–1215. doi: 10.1542/peds.2007-2361. [DOI] [PubMed] [Google Scholar]
- 2.National Research Council. Educating children with autism. Washington, D.C: National Academy Press; 2001. [Google Scholar]
- 3.Mandell DS, Listerud J, Levy SE, Pinto-Martin JA. Race differences in the age at diagnosis among Medicaid-eligible children with autism. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41(12):1447–1453. doi: 10.1097/00004583-200212000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Lord C, Spence S. Autism spectrum disorders: Phenotype and diagnosis. In: Moldin SO, Rubenstein JLR, editors. Understanding autism: From basic neuroscience to treatment. CRC Taylor and Francis; 2006. pp. 1–23. [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC) Prevalence of autism spectrum disorders - Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2002. Morbidity and Mortality Weekly Report. 2007;56(SS-1):12–28. [PubMed] [Google Scholar]
- 6.Mandell DS, Novak MM, Zubritsky CD. Factors associated with age of diagnosis among children with autism spectrum disorders. Pediatrics. 2005;116:1480–1486. doi: 10.1542/peds.2005-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiggins LD, Baio J, Rice C. Examination of the time between first evaluation and first autism spectrum diagnosis in a population-based sample. Developmental and Behavioral Pediatrics. 2006;27(2):s79–s87. doi: 10.1097/00004703-200604002-00005. [DOI] [PubMed] [Google Scholar]
- 8.Lord C, Shulman C, DiLavore PC. Regression and word loss in autistic spectrum disorders. Journal of Child Psychology and Psychiatry. 2004;45(5):936–955. doi: 10.1111/j.1469-7610.2004.t01-1-00287.x. [DOI] [PubMed] [Google Scholar]
- 9.Stoelb M, Yarnal R, Miles J, Takahashi T, Farmer J, McCathren R. Predicting responsiveness to treatment of children with autism: A retrospective study of the importance of physical dysmorphology. Focus on Autism and Other Developmental Disabilities. 2004;19(2):66–77. [Google Scholar]
- 10.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, D.C: American Psychiatric Association; 2000. TR. [Google Scholar]
- 11.Yeargin-Allsopp M, Rice C, Karapurkar T, Doernberg N, Boyle C, Murphy C. Prevalence of autism in a US metropolitan area. JAMA: Journal of the American Medical Association. 2003;289(1):49–55. doi: 10.1001/jama.289.1.49. [DOI] [PubMed] [Google Scholar]
- 12.Van Naarden Braun K, Pettygrove S, Daniels J, et al. Evaluation of a methodology for a collaborative multiple source surveillance network for autism spectrum disorders -Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2002. Morbidity and Mortality Weekly Report. 2007;56(SS-1):29–40. [PubMed] [Google Scholar]
- 13.StataCorp. Stata Survival Analysis and Epidemiological Tables Reference Manual Release 9. College Station: Stata Press; 2005. [Google Scholar]
- 14.Klein JP, Moeschbrger ML. Survival Analysis Techniques for Censored and Truncated Data. 2. New York: Springer; 2003. [Google Scholar]
- 15.Marubini E, Valsecchi MG. Analysing survival data from clinical trials and observational studies. Chichester, England: John Wiley & Sons; 1995. [Google Scholar]
- 16.Kleinbaum DG, Klein M. Survival Analysis: A Self-Learning Text. 2. New York: Springer; 2005. [Google Scholar]
- 17.Collett D. Modelling Survival Data in Medical Research. 2. Boca Raton: Chapman & Hall; 2003. [Google Scholar]
- 18.Bradburn MJ, Clark TG, Love SB, Altman DG. Survival analysis part II: Multivariate data analysis - an introduction to concepts and methods. Br J Cancer. 2003;89:431–436. doi: 10.1038/sj.bjc.6601119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel K, Kay R, Rowell L. Comparing proportional hazards and accelerated failure time models: An application in influenza. Pharmaceutical Statistics. 2006;5:213–224. doi: 10.1002/pst.213. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Learn the Signs. Act Early. [Accessed May 16, 2006.];Early Identification of Children with Autism or other Developmental Disorders Awareness Campaign. http://www.cdc.gov/ncbddd/autism/actearly/
- 21.Waisman Center. [Accessed May 16, 2006.];National Medical Home Autism Initiative. http://www.waisman.wisc.edu/cedd/NMHAI/DESCRIPTION.HTML.
- 22.Holtmann M, Bolte S, Poustka Autism spectrum disorders: Sex differences in autistic behaviour domains and coexisting psychopathology. Dev Med Child Neurol. 2007;49:361–366. doi: 10.1111/j.1469-8749.2007.00361.x. [DOI] [PubMed] [Google Scholar]
- 23.Carter AS, Black DO, Tewani S, Connolly CE, Kadlec MB, Tager-Flusber H. Sex differences in toddlers with autism spectrum disorders. J Autism Dev Disord. 2007;37:86–97. doi: 10.1007/s10803-006-0331-7. [DOI] [PubMed] [Google Scholar]
- 24.Shiner RL. Temperament and personality in childhood. In: Mroczek DK, Little TD, editors. Handbook of Personality Development. Mahwah, NJ: Lawrence Erlbaum Associates; 2006. pp. 213–231. [Google Scholar]
- 25.Rogers SJ. Developmental regression in autism spectrum disorders. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10:139–143. doi: 10.1002/mrdd.20027. [DOI] [PubMed] [Google Scholar]
- 26.Mandell DS, Wiggins L, Carpenter LA, et al. Racial and ethnic disparities in the identification of children with autism spectrum disorders. American Journal of Public Health. doi: 10.2105/AJPH.2007.131243. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]