Abstract
This study was performed to determine the maximum tolerated dose (MTD) and therapeutic effects of rhenium-186 (186Re)-labeled liposomal doxorubicin (Doxil), investigate associated toxicities, and calculate radiation absorbed dose in head and neck tumor xenografts and normal organs. Doxil and control polyethylene glycol (PEG)-liposomes were labeled using 186Re-N,N-bis(2-mercaptoethyl)-N′,N′-diethylethylenediamine (BMEDA) method. Tumor-bearing rats received either no therapy (n=6), intravenous Doxil (n=4), or escalating radioactivity of 186Re-Doxil (185–925 MBq/kg) or 186Re-PEG-liposomes (1110–1665 MBq/kg) and were monitored for 28 days. Based on body weight loss and systemic toxicity, MTD for 186Re-Doxil and 186Re-PEG-liposomes were established at injected radioactivity/body weight of 740 and 1480 MBq/kg, respectively. 186Re-injected radioactivity/body weight for therapy studies was determined to be 555 MBq/kg for 186Re-Doxil and 1295 MBq/kg for 186Re-PEG-liposomes. All groups recovered from their body weight loss, leucopenia, and thrombocytopenia by 28 days postinjection. Normalized radiation absorbed dose to tumor was significantly higher for 186Re-Doxil (0.299±0.109 Gy/MBq) compared with 186Re-PEG-liposomes (0.096±0.120 Gy/MBq) (p<0.05). In a separate therapy study, tumor volumes were significantly smaller for 186Re-Doxil (555 MBq/kg) compared with 186Re-PEG-liposomes (1295 MBq/kg) (p<0.01) at 42 days postinjection. In conclusion, combination chemoradionuclide therapy with 186Re-Doxil has promising potential, because good tumor control was achieved with limited associated toxicity.
Key words: 186Re, liposomal doxorubicin, MTD, head and neck cancer, radionuclide therapy, imaging
Introduction
Squamous cell carcinoma of the head and neck (HNSCC) accounts for nearly 5% of all cancers diagnosed in the United States and Europe.1 Following diagnosis of HNSCC, the choice of therapy depends on the site and stage of the cancer and also the overall health of the patient.2 Surgery and radiation therapy are the primary modalities used to control local cancer (stage I/II) and these patients have a good prognosis. Despite improvements in locoregional treatment by surgery and/or radiotherapy for stages III and IV (70%), the failure rate for either local recurrence or distant metastases is still high.3–5 Chemotherapy is generally not effective as an adjuvant systemic treatment for the local tumor therapy of solid tumors because of inhomogeneous distribution of the drug in the tumor leading to decreased therapeutic effects of the anticancer drug.6 Combined treatment modality approaches have been implemented to improve locoregional tumor control, reduce distant metastases, preserve function, and maintain anatomic structure. For head and neck cancer, a significant increase in therapeutic benefit has been reported when radiotherapy is combined with chemotherapy in an effort to increase local tumor control and reduce chances of recurrence and distant metastases.7–9 Unfortunately, this improved efficacy is accompanied by increased acute local and systemic toxicity.8 Hence, an effective systemic therapy that provides better local tumor control with reduced systemic toxicity would be beneficial.
Management of advanced stage disease can be improved by delivering high concentrations of drug to the local tumor while reducing normal tissue toxicity through use of a drug delivery system. Nanocarriers, especially liposomes, have been developed and studied for targeted delivery of diagnostic and therapeutic agents.10 Liposomes are small spherical colloidal systems of dimensions 20–1000 nm and are naturally formed when lipids or phospholipids are dispersed in an aqueous solution.11,12 They have the ability to carry therapeutic agents in different ways: bound to the outer membrane, integrated into the bilayer, dissolved within the bilayer, or entrapped in the interior.11,12 Encapsulation of anticancer drugs in liposomes alters their spatial and temporal distribution in the body, thereby reducing the unwanted side-effects and increasing treatment efficacy.11,13 Liposomes coated with polyethylene glycol (PEG) overcome the rapid reticuloendothelial system clearance and thus have long circulation times and significantly increased nonspecific accumulation in tumors when compared with non–PEG-coated liposomes.11,12 Clinical trials with pegylated liposomal doxorubicin (Doxil) have been conducted with many solid tumors such as that of breast, prostate, and head and neck.14,15 Studies have shown that Doxil enhances the effect of radiotherapy in treating head and neck cancer.16,17
Another attractive option of liposomes is that they can serve as delivery vehicles for therapeutic radionuclides.18,19 Rhenium isotopes, rhenium-186 (186Re) and rhenium-188 (188Re), are β-emitting radionuclides that allow for treatment of tumor cells isotropically within a radius of 2–4 mm.20 Phase I studies with 186Re-labeled antibodies for radioimmunotherapy of head and neck cancer in patients have shown some promise,21–23 but this technology remains limited by low tumor-to-normal tissue radioactivity ratio because of the slow penetration rate of the antibody into the tumor.24,25 Theoretical studies have shown that intravenously administered liposomes have significant advantages as carriers of therapeutic radionuclides, particularly those coated with PEG and carrying 188Re.26,27 A significant theoretical advantage was the low dose delivered to the bone marrow for radiolabeled liposomes compared with radiolabeled antibodies.26,27 Preclinical studies of 188Re-liposomes have also shown the acute toxicity28 and potential of this therapy in colon carcinoma.29–32 A simple method of encapsulating rhenium radionuclides into liposomes and Doxil using N,N-bis(2-mercaptoethyl)-N′,N′-diethylethylenediamine (BMEDA) with high labeling efficiency and good stability has been reported.33,34
The labeling efficiency, in vitro stability, pharmacokinetics, and biodistribution of 186Re-Doxil and 186Re-PEG-liposomes have been previously evaluated.35 Control PEG-liposomes were prepared with similar lipid composition, ammonium sulfate gradient, and diameter as Doxil. The results of the study revealed that 186Re-Doxil and 186Re-PEG-liposomes had similar in vitro stability but 186Re-Doxil had a circulation time of 28.2 hours and 186Re-PEG-liposomes were rapidly cleared from circulation by liver and spleen. Biodistribution at the end of 5 days postinjection revealed that 186Re-Doxil had 20-fold increased accumulation in tumor in comparison to 186Re-PEG-liposomes.
The present study determines the effectiveness of tumor therapy and associated normal tissue toxicity for escalating activities of 186Re-Doxil compared with 186Re-PEG-liposomes or Doxil alone. The hypothesis of the present study was that 186Re-Doxil would have an increased therapeutic efficacy over 186Re-PEG-liposomes or Doxil alone, because the 2 mm pathlength of isotropically emitted β-particle offers the potential of treating cancer cells not reached by Doxil alone. In addition, the long circulation time of 186Re-Doxil would have a favorable effect on the tumor accumulation and hence the radiation absorbed dose in the tumor.
Materials and Methods
Preparation of PEG-liposomes
Doxil, a commercially available liposomal doxorubicin formulation manufactured by Ben Venue Laboratories, Inc. for Johnson & Johnson was purchased from Oak Hills Pharmacy. Doxil contains 2 mg/mL of doxorubicin, 9.58 mg/mL of fully hydrogenated soy phosphatidylcholine, 3.19 mg/mL of N-(carbonyl-methoxypolyethylene glycol 2000)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine sodium salt, and 3.19 mg/mL of cholesterol. Control PEG-liposomes containing ammonium (pH) gradient and similar lipid composition and diameter as Doxil were prepared. Liposomes containing 1,2-distearoyl-sn-glycero-phosphatidylcholine (Avanti Polar Lipids), 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol) 2000] (Avanti Polar Lipids), and cholesterol (Calbiochem) (weight ratio: 3:1:1) were manufactured using a modified protocol.33 Lipid mixture was dissolved in a chloroform/methanol mixture (2:1, v/v) (Fisher Scientific),36 dried to form a lipid thin film by rotary evaporation, and desiccated overnight. The lipid film was rehydrated with 300 mM sucrose (Ferro Pfanstiehl Laboratories) in sterile water for injection, warmed to 60°C for complete suspension of lipids, and lyophilized overnight. The dried lipid–sucrose mixture was rehydrated with 240 mM ammonium sulfate (Sigma) in sterile water and then subjected to five freeze–thaw cycles at 60°C followed by extrusion through a series of polycarbonate filters (2 μm, 400 nm, and 200 nm, 2 passes each; 100 nm, 5 passes; 50 nm, 10 passes) (Lipex Extruder and Whatman Nucleopore Filters; Northern Lipids). The extruded liposome solution was stored at 4°C until needed.
The diameter of Doxil and control PEG-liposomes were measured with 488-nm laser light scattering instrument (Brookhaven Instruments) and found to be 87.3±8.5 and 91.3±11.8 nm, respectively. Phospholipid content was measured for the control PEG-liposomes using Stewart assay37 as 17.47 mg/mL. The concentration of PEG 2000 was determined as 4.71 mg/mL.38 The cholesterol concentration was assumed to be the same as PEG 2000 for a total lipid concentration of 26.89 mg/mL. Control PEG-liposomes were checked for bacterial growth and pyrogenicity (University Hospital Pathology Laboratory). No bacterial growth was detected within 14-day culture and endotoxin level was <5 EU/mL.
Preparation of 186Re-Doxil/186Re-PEG-liposomes
To a vial containing 50 mg glucoheptonate (GH) (Sigma Aldrich) and 3.0 μL BMEDA (prepared in-house), 2.0 mL of nitrogen-degassed saline was added.33,35 The mixture was mixed by magnetic stirring for 20 min followed by the addition of 240 μL of freshly prepared stannous chloride solution (15 mg/mL) (Aldrich Chemical Co.). An aliquot of 1.0 mL of the GH-BMEDA-stannous chloride mixture was placed in a new vial after adjusting the pH of the mixture to 5.0. The vial was flushed with nitrogen and sealed. 186Re-perrhenate solution (4.44–5.18 GBq [120–140 mCi]; University of Missouri Research Reactor) was added to the mixture in the vial and incubated at 80°C for 1 hour. After incubation, the 186Re-BMEDA solution was cooled to room temperature before adjusting the pH of the solution to 7.0. Immediately before radiolabeling, Doxil/PEG-liposomes were eluted with PBS (pH 7.4) through a PD-10 column (GE Healthcare) to create the ammonium (pH) gradient by removing free ammonium sulfate from liposome exterior. Eluted Doxil/PEG-liposomes were added to 186Re-BMEDA solution and incubated at 37°C for 1 hour. Finally, labeled 186Re-Doxil/PEG-liposomes were separated from free 186Re-BMEDA by eluting through PD-10 columns with PBS (pH 7.4). The labeling efficiency of 186Re-Doxil and 186Re-PEG-liposomes were 45%–50% and 60%–65%, respectively.
Animal model
All animal experiments were conducted with the prior approval of the authors' Institutional Animal Care Committee and according to the National Institutes of Health Animal Use Guidelines. All experimental procedures were conducted after the animals were anesthetized with 1%–3% isoflurane (Vedco) in 100% oxygen using an anesthesia inhalation unit (Bickford). A head and neck squamous cell carcinoma model was set up in nude rats by the subcutaneous inoculation of 5×106 human tongue squamous cell carcinoma (SCC-4) cells (ATCC) in 0.2 mL saline on the dorsum of each male rnu/rnu rat (Harlan, 3–4 weeks old, 75–100 g) at the level of the scapulae. This tumor xenograft model has been previously characterized.39 Tumor volume was determined by measuring the length (l), width (w), and depth (d) of each tumor using calipers. The tumor volume was calculated using the following ellipsoid volume formula: V=(π/6)lwd.39 The animals were used for the study when the tumor volume was ∼1.7–2.0 cm3, which typically occurred between 15 and 16 days.
Maximum tolerated dose study groups
Fifty (50) male, nude rats with tumor xenograft were used for this study. Forty (40) of these rats were injected i.v. with escalating activities of 186Re-Doxil or 186Re-PEG-liposomes, calculated for each group as MBq/kg. Four (4) rats were injected i.v. with Doxil only and 6 rats were used as control (Table 1). All injections were via tail vein. The total lipid dose of Doxil/liposomes was maintained at 52 mg/kg for all therapy groups. Doxorubicin dose of 6.5 mg/kg was maintained for 186Re-Doxil and Doxil groups.
Table 1.
Experimental Groups Intravenously Injected with Escalating Radioactivities in MBq/kg of 186Re-Doxil and 186Re-Polyethylene Glycol-Liposomes and the Control Groups
| Therapeutic agent | Number of rats | Injected radioactivity MBq/kg | Injected lipid dose mg/kg | Injected doxorubicin dose mg/kg | Mean±SD of body weight loss (% of body weight compared with day 0) |
|---|---|---|---|---|---|
| Control | 6 | 0 | 0 | 0 | |
| Doxil | 4 | 0 | 52 | 6.5 | 7.6±1.9 |
| 186Re-Doxil | 2 | 925 | 52 | 6.5 | 19. 9±6.6 |
| 186Re-Doxil | 4 | 740 | 52 | 6.5 | 13.8±5.2 |
| 186Re-Doxil | 4 | 555 | 52 | 6.5 | 11.8±4.7 |
| 186Re-Doxil | 4 | 370 | 52 | 6.5 | 11.1±4.3 |
| 186Re-Doxil | 2 | 185 | 52 | 6.5 | 10.9±0.8 |
| 186Re-PEG-liposomes | 4 | 1665 | 52 | 0 | 19.8±4.9 |
| 186Re-PEG-liposomes | 4 | 1480 | 52 | 0 | 13.8±7.0 |
| 186Re-PEG-liposomes | 4 | 1295 | 52 | 0 | 11.3±8.8 |
| 186Re-PEG-liposomes | 4 | 1110 | 52 | 0 | 9.5±4.9 |
| 186Re-PEG-liposomes | 4 | 925 | 52 | 0 | 9.4±6.1 |
| 186Re-PEG-liposomes | 4 | 740 | 52 | 0 | 6.9±2.7 |
The decrease in body weight on day 5 postinjection of the different treated groups compared with their body weight on day 0. Mean±SD are shown.
PEG, polyethylene glycol.
Microsingle-photon emission computed tomography imaging protocol
186Re has a penetrative 137 keV γ-emission, which allows for monitoring of the biodistribution of labeled liposomes and absorbed dose calculation using gamma scintigraphy. High-resolution parallel hole collimators were used to acquire planar gamma camera images and single-photon emission computed tomography (SPECT) images with a microSPECT scanner (FLEX SPECT/CT/PET; Gamma Medica). Static planar images were acquired in two views, anterior–posterior and lateral at baseline, 4, 22, 46, 70, 94, and 118 hours after 186Re-Doxil/PEG-liposome injection. A standard source of 186Re-Doxil/PEG-liposomes of ∼0.26 MBq (∼70 μCi) was placed in the field of view but outside the position of the rat during static planar image acquisition for image quantification. Tomographic images were also acquired at the same time points as the planar static images. SPECT images reconstructed using the Lumagen® processing software available with the system had an image size of 56×56×56 and isotropic pixel dimension of 2.23 mm.
Post-therapy follow-up and hematology
Body weight of all rats was measured on alternate days. To monitor therapy-induced toxicity, blood (400 μL) was collected from the tail vein in a tube containing acid-citrate-dextrose anticoagulant before therapy and every 3–4 days post-therapy until day 28. Complete blood counts with differential white blood cell (WBC) count were determined by automated counting at the University Hospital Pathology Laboratory. Control group was followed to day 16 and then euthanized by cervical dislocation under deep anesthesia because of excessive tumor mass. Therapy groups were followed to day 28 and then euthanized. Maximum tolerated dose (MTD) was defined as the highest radioactivity that allows for 100% survival of all animals with <20% loss in body weight and no clinical signs of toxicity such as infection or bleeding.
Histopathological examination
After euthanization, the tumor, liver, spleen, kidney, and femur (bone marrow) were dissected, fixed in 10% formalin, and embedded in paraffin. Four (4)-micrometer-thick sections were then stained with hematoxylin and eosin (H&E) for histopathologic examination under a light microscope. Tumor sections were stained for Ki67 (MIB-1) to determine degree of proliferation (n=12). Microscopic images were captured using a Nikon Eclipse 80i light microscope equipped with a high-resolution digital camera (Nikon DS-Fi1) interfaced to a personal computer equipped with NIS-Elements software (Nikon). Individual tumor microscope slides were scanned using a Model CS ScanScope® system (Aperio Technologies, Inc.) and digitally captured using ImageScope™ software (version 7.1.22.1025; Aperio) for display.
Determination of microscopic viability factor and viable tumor volume
To evaluate the effect of therapy, the H&E-stained images from the laser scanner were analyzed using ImageJ (NIH, Bethesda, MD) (http://rsbweb.nih.gov/ij/docs/examples/stained-sections/index.html). All H&E-stained tumor slides were scanned at the same time at 1200 dpi resolution using a high-resolution laser scanner (Hewlett-Packard) to eliminate any scan errors when using the images to determine microscopic viability factor. Individual tumor images were opened as red-blue-green (RGB) stack in ImageJ and the images were displayed in the blue channel to obtain the best distinction between the H&E-stained areas.40 The images were manually adjusted using “threshold” to highlight only the hematoxylin-stained area. Next, the threshold was manually adjusted to highlight the entire tumor section and the area measured. The ratio of the measured hematoxylin-stained area to entire tumor section area was calculated as microscopic viability factor, as hematoxylin stains viable cells. This microscopic viability factor for each tumor was then applied to the corresponding tumor volume determined at necropsy to calculate the viable tumor volume. To ensure that the determined hematoxylin area contained viable and proliferating cells, corresponding H&E sections were compared with Ki67 staining to localize and correlate the hematoxylin- and Ki67-stained areas. Ki67-stained tumor sections (n=12) were used to determine the proliferative area using the described procedure. The proliferative area determined from Ki67 staining was correlated to viable area determined from corresponding H&E-stained tumor sections to validate this method.
Image analysis and radiation absorbed dose calculation
Planar images were used to determine the radioactive counts in each organ. Anterior–posterior images were used to determine radioactive counts in heart, lungs, liver, spleen, and kidneys and lateral planar images were used for tumor. Region of interest (ROI) (10 pixels) was drawn over the standard source and the counts in the region were obtained. This count value was divided by the known activity of the standard source to obtain a count-to-activity (mCi) conversion factor. The 186Re activity in tumor at each time point was determined by drawing ROI (10 pixels) over tumor of each rat to obtain the counts at each time point and using the conversion factor. The activity in tumor and normal organs such as liver, spleen, and kidney were similarly calculated by drawing ROI over the entire organ. The time–activity curve was obtained for each organ after decay correction, and the area under the curve from 0 to 118 hours was determined to obtain the cumulative radioactivity (Ã; mCi-h) in each organ for radiation absorbed dose calculation.
186Re has a nonpenetrative β-emission of 362 keV on average, with a mean range of 1.8 mm in tissue and a high nonpenetrative-to-penetrative (photons) energy ratio of 16.5.20 This results in a high local radiation dose deposition. The radiation absorbed dose was calculated assuming that the radiation absorbed dose in each organ is contributed by β-radiation, neglecting the contribution of γ-emission. Rat scale phantom studies have shown that the absorbed fraction for 140 keV energy is about 5%. As 186Re has less than 10% γ-emission of 137 keV energy, there would not be a significant dose from these emissions.41 It was also assumed that the radiation absorbed dose in an organ is due to the locally deposited nonpenetrative β-energies and there was no “cross-fire” β-energy deposition from surrounding organs. As the organ and tumor dimensions in the rat are of the order of 1 cm, most of the β-emissions would be absorbed within the organ as their range is only 2–4 mm in tissue.42–44 Based on this dose calculation model, the average radiation absorbed dose in each normal organ and tumor was calculated using the following equation42,45,46:
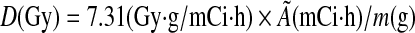 |
In this equation, D is the average radiation absorbed dose in Gy, Ã is cumulative radioactivity in mCi·h calculated as described earlier, and m is the weight of the organ in g. To calculate radiation absorbed dose for tumor, tumor weight was calculated from the average of the measured tumor volume from day 0 to 5, assuming the tumor density is 1 g/cm3. Normal organ weight for heart (0.76±0.08 g), lungs (1.15±0.07 g), liver (10.67±1.42 g), spleen (0.61±0.09 g), and kidneys (1.06±0.09 g) was obtained from previous 99mTc-liposomes organ biodistribution studies (n=12) (unpublished data).
Therapy study with therapeutic dose
To determine the therapeutic efficacy of the cancer treatment, five groups of rats (n=6/group) were followed for 42 days post-therapy and their tumor growth trend was monitored. The rats were treated with either 6.5 mg/kg of Doxil, 555 MBq/kg of 186Re-Doxil, or 1295 MBq/kg of 186Re-PEG-liposomes. The unlabeled PEG-liposome group was injected with the same lipid concentration as the therapy groups. The control group did not receive any treatment. Rats were euthanized before 42 days if the tumor volume reached 7 cm3.
Statistical analysis
Groups were statistically compared by one-way ANOVA using Origin Pro 8.0 statistical software (Origin Lab). A value of p<0.05 was considered statistically significant.
Results
Body weight and hematology for treatment toxicity assessment
To assess the treatment toxicity and determine the MTD, body weight loss and change in blood parameters were monitored. Table 1 shows the % body weight loss for escalating doses of 186Re-Doxil and 186Re-PEG-liposomes. All treated animals lost weight postinjection, reaching a nadir on day 5. Rats receiving 925 MBq/kg of 186Re-Doxil and 1665 MBq/kg of 186Re-PEG-liposomes had the highest average body weight loss of 19.9%±6.6% and 19.8%±4.9%, respectively. MTD was defined as the highest radioactivity that allows for 100% survival with<20% body weight loss and no signs of infection or bleeding. In the group receiving 925 MBq/kg of 186Re-Doxil, 50% of the animals exceeded the 20% weight loss threshold. For the group receiving 1665 MBq/kg of 186Re-PEG-liposomes, 25% of the rats exceeded the 20% body weight loss threshold. Thus, the above two levels of radioactivity doses were toxic. Also, all rats that received 925 MBq/kg of 186Re-Doxil died on day 12 postinjection because of gastrointestinal toxicity. There was no mortality in any of the other treated groups. All injected rats gained weight after day 5 at the same rate as the control animals. Rats that received intravenous Doxil alone had a weight loss of only 7.6%. Comparison of body weight loss for the same dose of 925 MBq/kg of 186Re-Doxil and 186Re-PEG-liposomes supports the idea of a combination effect of 186Re and doxorubicin in rats receiving 186Re-Doxil.
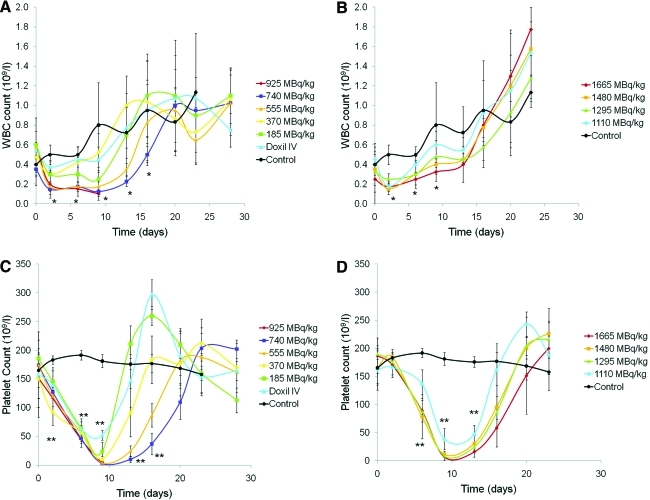
The 186Re-Doxil and 186Re-PEG-liposomes therapy as well as the control groups showed no signs of bleeding or infection. A decrease in WBC was observed on day 2 postinjection for all 186Re therapy groups (Fig. 1). Decrease in WBC count for 186Re-Doxil group treated with 740 MBq/kg (days 2–16) and 555 MBq/kg (days 2–13) was statistically significant compared with the control group (p<0.05). Animals receiving 740 MBq/kg of 186Re-Doxil recovered to their initial value by day 20 (Fig. 1A), whereas animals receiving 1665 MBq/kg of 186Re-PEG-liposomes recovered to baseline value by day 9 (Fig. 1B). Decrease in WBC count for 186Re-PEG-liposomes group treated with 1665 MBq/kg (days 2–9) and 1480 MBq/kg (days 2–6) was statistically significant compared with the control group (p<0.05). No appreciable change was seen in the RBC counts for both 186Re therapy groups. Platelet counts showed a decline postinjection for all 186Re therapy groups, reaching a nadir on day 9 (p<0.01, compared with the control group) (Fig. 1). A clear dose–response relationship was observed regarding the recovery in platelet counts. Platelet counts had recovered to their initial value by day 20 for 186Re-PEG-liposomes (Fig. 1D) and day 23 for 186Re-Doxil therapy groups (Fig. 1C). Animals treated with 186Re-Doxil exhibited mild skin rash on their abdomen, paws, and feet by week 2, but the skin condition cleared with no external treatment by week 3.
FIG. 1.
Change in the WBC and platelet counts over a period of 28 days after injection of escalating radioactivities of 186Re-Doxil (A and C) and 186Re-PEG-liposomes (B and D), respectively. The WBC count decreased on day 2 post-therapy and then gradually recovered to baseline value by day 15 (A and B). Platelet counts reached their nadir on day 9 postinjection for all treated groups. The platelets showed a dose–response relationship for their recovery with higher radioactivities, requiring more days to return to baseline value (C and D). *p<0.05, **p<0.01: control vs. 740 MBq/kg of 186Re-Doxil and control vs. 1480 MBq/kg of 186Re-PEG-liposomes. WBC, white blood cell. Color images available online at www.liebertonline.com/cbr
Based on the body weight loss and recovery of blood parameters, the MTD was determined as 740 MBq/kg for 186Re-Doxil and 1480 MBq/kg for 186Re-PEG-liposomes.
Distribution of 186Re-Doxil and 186Re-PEG-liposomes
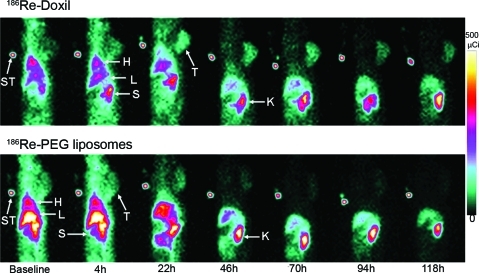
The distribution of 186Re-Doxil and 186Re-PEG-liposomes at their respective MTD in xenograft-bearing rats were observed in the parallel-hole planar images acquired at various time points (Fig. 2). The images revealed the uptake of radioactivity over time in liver, spleen, and tumor. The longer circulation time and reduced clearance through liver and spleen of 186Re-Doxil in comparison to 186Re-PEG-liposomes could be observed in the images. In the images acquired at 46 hours and later, kidneys are seen prominently, suggesting that the 186Re-BMEDA released from metabolized liposomes is cleared through the kidney. Qualitative analysis of the images showed that 186Re-Doxil has a higher uptake of radioactivity in the tumor than 186Re-PEG-liposomes from 22 to 118 hours.
FIG. 2.
Planar images of the distribution of 186Re-Doxil (740 MBq/kg) (upper panel) and 186Re-PEG-liposomes (1480 Mbq/kg) (lower panel) acquired at various time points postinjection. Accumulation of radioactivity in tumor, liver, spleen, and kidney is seen. 186Re-Doxil had higher tumor accumulation in comparison to 186Re-PEG-liposomes at each time point. ST, standard; T, tumor; H, heart; L, liver; S, spleen; K, kidney. Color images available online at www.liebertonline.com/cbr
Radiation absorbed dose in tumor and normal organs
Table 2 depicts the total radiation absorbed dose and normalized radiation absorbed dose (Gy/MBq) for normal organs and tumor for the different injected radioactivity levels of 186Re-Doxil and 186Re-PEG-liposomes. The radiation absorbed dose in the tumor was 46.8±21.3 Gy for 740 MBq/kg of 186Re-Doxil and 31.6±7.0 Gy for 1480 MBq/kg of 186Re-PEG-liposomes. Even though twice the amount of radioactivity was injected for the 186Re-PEG-liposome group, the radiation absorbed dose was similar to that of 186Re-Doxil (740 MBq/kg) (p>0.05). The faster clearance of 186Re-PEG-liposomes from circulation did not allow for optimum accumulation of 186Re-PEG-liposomes in the tumor for effective cell killing. The total radiation absorbed dose in lungs, liver, and kidneys for 740 MBq/kg of 186Re-Doxil were 68.0±5.7, 37.7±4.3, and 288.0±17.8 Gy, respectively. The corresponding radiation absorbed doses for 1480 MBq/kg of 186Re-PEG-liposomes were 57.1±2.3, 71.3±14.5, and 425.3±71.9 Gy (p<0.01 for liver, p<0.05 for lungs and kidneys, compared with 740 MBq/kg of 186Re-Doxil).
Table 2.
Radiation Absorbed Dose (in Gy and Gy/MBq) by Tumor and Normal Organs for the Different Injected Doses of 186Re-Doxil and 186Re-Polyethylene Glycol-Liposomes
| |
|
Total radiation absorbed dose (Gy) |
Normalized radiation absorbed dose (Gy/MBq) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Therapy | Injected activity (MBq/kg) | Tumor | Liver | Spleen | Left Kidney | Right Kidney | Heart | Lungs | Tumor | Liver | Spleen | Left kidney | Right kidney | Heart | Lungs |
| 186Re-Doxil | 925 | 54.4±2.7 | 48.4±21.3 | 163.3±11.6 | 198.1±3.3 | 168. 5±1.5 | 32.9±5.3 | 84.9±14.7 | 0.265±0.029 | 0.235±0.001 | 0.792±0.01 | 0.962±0.04 | 0.819±0.04 | 0.159±0.016 | 0.411±0.048 |
| 186Re-Doxil | 740 | 46.8±21.3 | 37.7±4.3** | 136.1±21.9 | 150.8±12.3* | 137.2±5.5* | 26.3±3.0 | 68.0±5.7* | 0.299±0.109* | 0.250±0.046 | 0.895±0.146** | 0.994±0.115* | 0.905±0.094* | 0.170±0.019** | 0.44±0.037** |
| 186Re-Doxil | 555 | 32.2±8.7 | 30.3±5.1 | 122.3±26.7 | 128.6±27.2 | 116.9±23.0 | 20.2±3.4 | 52.6±11.5 | 0.273±0.099 | 0.248±0.033 | 0.991±0.052 | 1.045±0.077 | 0.952±0.063 | 0.130±0.022 | 0.340±0.074 |
| 186Re-Doxil | 370 | 30.4±5.1 | 22.2±3.8 | 84.0±16.0 | 97.1±0.5 | 90.9±0.3 | 15.9±1.9 | 43.4±0.2 | 0.377±0.071 | 0.273±0.022 | 1.032±0.122 | 1.201±0.063 | 1.124±0.096 | 0.103±0.012 | 0.281±0.04 |
| 186Re-Doxil | 185 | 14.5±1.0 | 11.9±1.7 | 56.3±0.8 | 44.7±0.3 | 40.7±0.4 | 8.0±0 | 22.7±0.9 | 0.334±0.062 | 0.270±0.006 | 1.280±0.019 | 1.013±0.022 | 0.925±0.032 | 0.118±0.022 | 0.277±0.081 |
| 186Re-PEG-liposomes | 1665 | 38.5±5.9 | 86.2±20.7 | 203.0±94.4 | 239.6±40.4 | 241.1±38.4 | 29.2±6.4 | 75.8±2.5 | 0.120±0.008 | 0.266±0.037 | 0.617±0.225 | 0.744±0.054 | 0.754±0.118 | 0.098±0.022 | 0.255±0.042 |
| 186Re-PEG-liposomes | 1480 | 31.6±7.0 | 71.3±14.5 | 139.2±33.9 | 214.4±41.4 | 210.9±31.5 | 22.5±3.3 | 57.1±0.3 | 0.120±0.038 | 0.262±0.021 | 0.510±0.066 | 0.790±0.076 | 0.781±0.072 | 0.076±0.011 | 0.192±0.018 |
| 186Re-PEG-liposomes | 1295 | 28.5±3.7 | 68.9±18.8 | 119.9±8.5 | 188.4±13.4 | 185.1±17.4 | 20.0±3.8 | 49.2±0.3 | 0.110±0.0003 | 0.264±0.044 | 0.467±0.056 | 0.731±0.048 | 0.716±0.039 | 0.067±0.013 | 0.166±0.035 |
| 186Re-PEG-liposomes | 1110 | 23.0±2.4 | 52.0±3.4 | 106.8±11.1 | 146.8±8.5 | 144.7±4.0 | 17.6±0.9 | 45.0±0.8 | 0.119±0.010 | 0.270±0.025 | 0.552±0.056 | 0.760±0.052 | 0.749±0.032 | 0.059±0.003 | 0.152±0.016 |
Values are presented as mean±SD. *p<0.05, **p<0.01: 740 MBq/kg of 186Re-Doxil compared with 1480 MBq/kg of 186Re-PEG-liposomes.
Histopathology
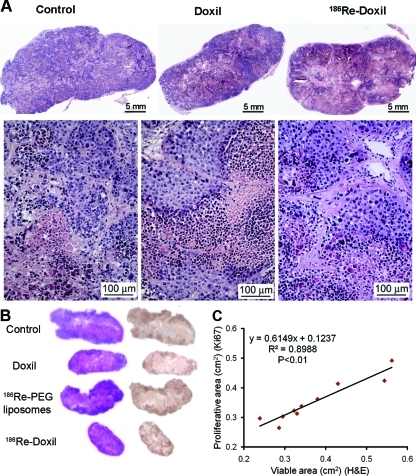
Examination of the H&E-stained tumor sections for control group (Fig. 3A) showed lobules of pleomorphic cancer cells with round to oval nuclei separated by extracellular matrix and collagen. Each lobule had a central region of necrosis surrounded by viable cells. The histopathological examination of 186Re-Doxil (740 and 555 MBq/kg), 186Re-PEG-liposomes (1480 and 1295 MBq/kg), and Doxil therapy groups showed similar architecture of the tumor cells as controls (Fig. 3A). As the tumor volume and microscopic examination of 186Re-PEG-liposomes–treated tumors were similar to that of the control group, the microscopic images are not shown. To validate that the hematoxylin area determined by image analysis of H&E-stained tumor sections contain viable and proliferative cells, images of corresponding H&E- and Ki67-stained sections were individually analyzed to determine viable and proliferative area, respectively. The localization and area of hematoxylin staining were correlated to that of Ki67 staining (Fig. 3B, C). There was very good correlation between the viable area determined from H&E images and the proliferative area determined from Ki67 images (p<0.01; Fig. 3C).
FIG. 3.
The scanned H&E-stained tumor sections from control rats and rats treated with intravenously injected Doxil or 186Re-Doxil show the presence of viable tumor cells stained with hematoxylin (A). Control tumor was collected on day 20 post–therapy, whereas tumors from the intravenous Doxil- or 186Re-Doxil-treated groups were collected on day 28 post-therapy. Viable tumor cells with regions of necrosis are present in the tumors of control and treated rats. H&E-stained tumor sections and corresponding Ki67-stained tumor sections were compared to correlate localization of staining (B) and area measurement (C). H&E, hematoxylin and eosin. Color images available online at www.liebertonline.com/cbr
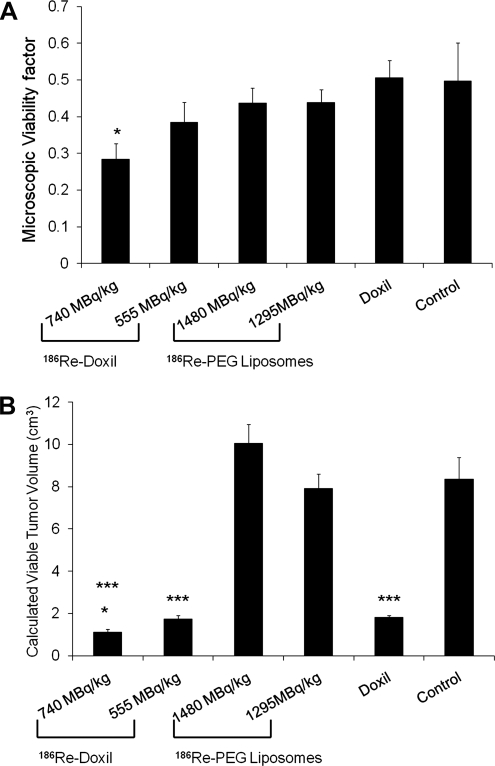
To evaluate the differences in tumors from rats treated with 186Re-Doxil, Doxil, or 186Re-PEG-liposomes, compared with control rats, analysis of the H&E-stained images was performed to determine the viability factor (Fig. 4A). Analysis revealed that even though tumors receiving Doxil alone had a final tumor volume smaller than the control group, 50% of the microscopic tumor section consisted of viable tumor cells. In contrast, for the tumors treated with 186Re-Doxil (740 MBq/kg), only 28% of the tumor section area was comprised of viable tumor cells. The calculated viable tumor volumes (Fig. 4B) were significantly different for comparisons between control and 555 MBq/kg 186Re-Doxil (p<0.001), and control and Doxil (p<0.001). The calculated viable tumor volume for 186Re-Doxil (740 MBq/kg)-treated group (Fig. 4B) was significantly different from control and Doxil-treated rats (p<0.001 and p<0.05, respectively). No differences were seen between the 186Re-PEG-liposome–treated and control groups.
FIG. 4.
The area of tumor microscopic section that was stained with hematoxylin, representing viable tumor cells as a function of the entire tumor microscopic specimen area (microscopic viability factor), was determined for treated groups and control (A) (*p<0.05, control vs. 740 MBq/kg of 186Re-Doxil) and applied to the tumor volume on the day of necropsy to calculate viable tumor volume (B). Animals treated with 186Re-Doxil and Doxil had a smaller viable tumor volume than rats treated with 186Re-PEG-liposomes and control rats. *p<0.05, Doxil vs. 740 MBq/kg of 186Re-Doxil; ***p<0.001, control vs. 740 MBq/kg of 186Re-Doxil and control vs. 555 MBq/kg of 186Re-Doxil.
Cancer treatment effect with therapeutic dose
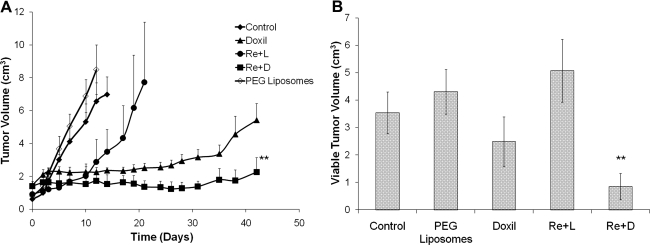
Tumor growth trend indicated a statistically smaller tumor volume for the 186Re-Doxil group (2.261±0.89 cm3) (p<0.01) compared with the Doxil group (5.426±1.02 cm3) (Fig. 5A). Though follow-up for the 186Re-PEG-liposome group was terminated at day 19 as tumor volumes reached 7 cm3, treatment with 186Re-PEG-liposomes significantly extended the time needed to reach 7 cm3, compared with the control group (day 12) (p<0.05). Histopathological examination of the tumors obtained at necropsy for each group indicated a significantly smaller viable tumor volume for 186Re-Doxil (0.85±0.48 cm3) (p<0.01) compared with Doxil (2.48±0.91 cm3) (Fig. 5B).
FIG. 5.
Measured tumor volumes (A) and calculated viable tumor volumes (B) of 186Re-Doxil (Re+D) (555 MBq/kg, 6.5 mg/kg doxorubicin) compared with 186Re-PEG-liposomes (Re+L) (1295 MBq/kg), unlabeled Doxil (6.5 mg/kg doxorubicin), and unlabeled PEG-liposomes for 42 days postinjection. The data in B are from histopathological analysis of the tumors obtained at necropsy, which was day 42 for the 186Re-Doxil and Doxil groups, day 10 for the unlabeled PEG-liposome and control groups, and day 19 for the 186Re-PEG-liposome group (**p<0.01, 186Re-Doxil vs. Doxil).
Discussion
Approximately two-thirds of all HNSCC patients have advanced-stage disease (stage III/IV).4 Despite improvements in locoregional treatment modalities such as aggressive postsurgery radiotherapy and/or chemotherapy, incidence of local recurrence and distant metastases remains high.3–5 Hence, an effective systemic treatment is required to improve survival in this group.
Radionuclides with β-emission such as 186Re have been considered for the treatment of tumors from “inside-out,” thereby avoiding the associated side-effects of external beam radiotherapy. 186Re has been used for radioimmunotherapy of solid tumors in HNSCC patients, with treatment of small tumors being more successful than treatment of larger tumors.21–23,47,48 The simultaneous use of β-emitting radionuclide and chemotherapy agents for the treatment of large solid tumors holds appeal, as the inhomogeneous distribution of the chemotherapy agent is offset by the penetrative ability of the 2 mm pathlength of the β-particle in tumor. The β-emitting radionuclides also offer the potential to treat hypoxia regions in the tumor where chemotherapeutic agents alone may have reduced efficacy. Systemic toxicity associated with intravenous injection of radionuclide and chemotherapeutic agents can be minimized by encapsulating them in liposomes.10
Liposomes have been used for the delivery of chemotherapeutic agents,49 genes,50 and radionuclides.10,33 These are viable options for the treatment of HNSCC; however, the complicated release mechanism of the chemical and biological therapeutic agents to reach the cancer cells limits the efficacy of liposomal chemotherapy and gene therapy in the treatment of solid tumors.27 A homogeneous tumor distribution is not required by liposomal radionuclides, because β-emissions from the radionuclide can cause killing of cancer cells in the pathlength of radiation dose deposition. Also, imaging techniques can be used to systemically monitor the biodistribution of liposomal radionuclides.
Radionuclide therapy for the treatment of solid tumors greater than 1 cm has been generally associated with poor tumor response rate of relatively short duration.25,51 In this study, HNSCC was simultaneously treated with radionuclide and chemotherapy agent, with the long-term goal of increasing the therapeutic efficacy. Liposomal doxorubicin was labeled with 186Re and injected at escalating radioactivity levels into rats with head and neck xenograft. The therapeutic efficacy and systemic toxicity were monitored over a period of 28 days. 186Re-labeled empty liposomes were also injected into rats at various activities for comparison of treatment efficiency and systemic toxicity with 186Re-Doxil therapy. In the present study, the MTD was determined as 740 MBq/kg for 186Re-Doxil and 1480 MBq/kg for 186Re-liposomes.
The injection of 1480 MBq/kg of 186Re-PEG-liposomes produced the same radiation absorbed dose in tumor as 740 MBq/kg of 186Re-Doxil because of the faster clearance time of 186Re-PEG-liposomes. The radiation absorbed dose in tumor was 46.84±21.29 Gy for 740 MBq/kg of 186Re-Doxil and 31.58±7.02 Gy for 1480 MBq/kg of 186Re-PEG-liposomes. The final tumor volumes for the 186Re-Doxil group and the 186Re-PEG-liposome group at their respective MTD were 1.84±0.09-fold and 9.82±1.59-fold their initial volume. The better tumor control achieved with 186Re-Doxil in comparison to 186Re-liposomes was probably due to the combination effect of 186Re and doxorubicin. The combination effect could be due to G2/M phase arrest of the cancer cells by doxorubicin, thereby sensitizing them to radiation from 186Re.52–54 Studies have shown that doxorubicin causes DNA unwinding and DNA double-strand breaks, which lead to radiosensitization of the tumor cells.55–57 Another possible reason for better therapeutic efficacy with 186Re-Doxil could be due to the increased blood circulation time, which allowed for better accumulation and distribution of the agent in the tumor.35 Although 186Re-PEG-liposomes were less effective in the treatment of large solid tumors in this study, they could be potentially used for the treatment of small tumors, as an inverse correlation between tumor size and accumulation of radioactivity has been shown in preclinical47,48 and clinical studies.58 Also, 186Re-PEG-liposomes could be used for the treatment of large intact solid tumors or partially resected tumors via intratumoral injection of the therapeutic agent.46,59,60
The therapeutic effect of 186Re-Doxil, 186Re-PEG-liposomes, and intravenous Doxil on tumors is shown by histopathologic examination. Control tumors and 186Re-liposome-treated tumors showed lobules of tumor cells separated by extracellular matrix. Image analysis of digital images of histological slides is being increasingly utilized today for histopathological assessment.61 Quantitative image analysis of stained tissue sections using RGB image profiling is the most commonly accepted technique performed using commercially available software.40,62 In the present study, the freeware ImageJ was used to analyze the H&E-stained tumor sections to determine the hematoxylin-stained area (viability factor). The use of microscopic viability factor to determine the differences in therapeutic effect was validated by using Ki67 staining to confirm the proliferating activity of viable area on H&E tumor sections. Image analysis of the tumor microscopic sections revealed a smaller viable tumor volume for 186Re-Doxil (740 MBq/kg) in comparison to Doxil (p<0.05). This long-term therapeutic efficacy study showed that tumors treated with intravenous Doxil grew rapidly past the 4 weeks time point, reaching ∼4-fold their initial tumor volume by 6 weeks. For rats treated with 186Re-Doxil, the tumors stayed as stable disease at ∼1.5-fold their initial volume at 6 weeks. Histopathological analysis at 6 weeks indicated a significantly smaller viable tumor volume for 186Re-Doxil. These data confirm that 186Re-Doxil therapy had a better tumor therapeutic response over a longer duration than intravenous Doxil alone. The establishment of the pharmacokinetic landscape of this chemoradionuclide agent could help assess its dosimetric effectiveness in patients in future investigations.63
Combination chemoradionuclide therapy cannot be considered feasible if the associated systemic toxicity is unacceptable. No long-term side-effects or complications were observed in this study. All animals in the therapy group showed weight loss until day 5 postinjection, after which they recovered to reach weights similar to that of control. At approximately the end of week 2, the animals treated with 186Re-Doxil developed skin rash on their abdomen, paws, and feet similar to the palmar-plantar erythrodysesthesia condition seen in patients treated with Doxil.64 This condition was resolved by the end of week 3 without any topical or external treatment. The animals treated with 186Re showed a dose–response relationship for the recovery of platelet counts. By the end of 28 days, all treated animals had recovered their WBC and platelet counts to that of the control group. Bone marrow is the dose-limiting organ in most radionuclide therapy, because the rapidly proliferating cells are sensitive to radiation. Although the radiation dose to bone marrow was not calculated, the decrease seen in the WBC and platelets indicates a radiation effect for 186Re-Doxil in addition to toxicity from doxorubicin. Recovery of WBC and platelets to baseline values within the study period of 28 days indicates an acute effect. Delayed bone marrow failure would have to be evaluated with longer follow-up periods.
The histopathologic examination of the normal organs did not show any difference between the treated and control animals despite the large radiation dose deposited in the liver and kidneys, which could have potentially caused acute toxicity. The toxicity observed was similar to that reported for 188Re-BMEDA-liposomes.28 Kidneys are radiosensitive organs and have to be closely monitored, because they are the main mode of excretion of the radioactivity. Liver and kidney are radiosensitive late-responding critical organs.65 The follow-up period of 4–6 weeks may not be enough to evaluate the long-term radiation effect to the liver and kidneys. Studies with longer follow-up period of at least 6 months to a year would be needed. It is becoming increasingly clear that dose-rate considerations should be taken into account during radionuclide dosimetry.66,67 Biological effective dose relates absorbed dose and dose rate with radiosensitivity and radiation damage repair using the linear quadratic model and could be investigated in the future as a method for comparing the differences in dose–response and dose–toxicity between 186Re-Doxil and 186Re-PEG–liposomes, as they would have different dose rates because of their significantly different circulation time.
Imaging plays an important role in radionuclide therapy for noninvasive radiation dose calculation by quantifying the activity in the target organ using planar and/or tomographic imaging methods. In the present study, planar images were used to quantify the activity in the tumor and normal organs for radiation absorbed dose estimation. Planar imaging method has the limitation that it represents a 3D distribution of radioactivity in a 2D display, causing difficulty in accurate localization and errors in radiotracer quantification.68,69 The high radiation absorbed dose calculated for the different organs in the present study could be due to the low resolution of the planar images leading to overestimation of the radiotracer activity in the target organ. Also, the high radiation absorbed dose calculated in heart and lungs may be due to the blood pool in these organs for which no correction was applied during image quantification. As this was the authors' first study looking at the toxicity and dosimetry of 186Re-Doxil, the planar images provided a reasonable profile and estimate of the radiation absorbed dose in the different organs and also helped identify the dose-limiting organs. Studies have shown that SPECT and SPECT/CT provide a more accurate localization and quantification of radioactivity in animals.68,70 Future studies with 186Re-Doxil could be imaged using SPECT/CT to better estimate the radiation absorbed dose to organs.
Conclusions
A potential systemic chemoradionuclide therapy for the treatment of HNSCC was shown in this study. Combination radionuclide therapy and chemotherapy provided adequate local tumor control. Systemic 186Re-Doxil therapy could be used for the simultaneous treatment of solid tumors. Chemoradionuclide therapy with 186Re-Doxil has potential for translation to clinical use in the treatment of solid tumors.
Acknowledgments
This study was funded by National Institutes of Health 5P30CA054174 supplement grant. The authors thank Cristina A. Santoyo and Ricardo Perez, III, for help with animal care and imaging experiments.
Disclosure Statement
No potential conflict of interest or financial interest exists.
References
- 1.Parkin DM. Bray F. Ferlay J, et al. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.Al-Sarraf M. Treatment of locally advanced head and neck cancer: Historical and critical review. Cancer Control. 2002;9:387. doi: 10.1177/107327480200900504. [DOI] [PubMed] [Google Scholar]
- 3.Dennington ML. Carter DR. Meyers AD. Distant metastases in head and neck epidermoid carcinoma. Laryngoscope. 1980;90:196. doi: 10.1288/00005537-198002000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Vokes EE. Weichselbaum RR. Lippman SM, et al. Head and neck cancer. N Engl J Med. 1993;328:184. doi: 10.1056/NEJM199301213280306. [DOI] [PubMed] [Google Scholar]
- 5.Zbaren P. Lehmann W. Frequency and sites of distant metastases in head and neck squamous cell carcinoma. An analysis of 101 cases at autopsy. Arch Otolaryngol Head Neck Surg. 1987;113:762. doi: 10.1001/archotol.1987.01860070076020. [DOI] [PubMed] [Google Scholar]
- 6.Minchinton AI. Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 7.Vokes E. Current treatments and promising investigations in a multidisciplinary setting. Ann Oncol. 2005;16(Suppl 6):vi25. doi: 10.1093/annonc/mdi455. [DOI] [PubMed] [Google Scholar]
- 8.Brizel DM. Radiotherapy and concurrent chemotherapy for the treatment of locally advanced head and neck squamous cell carcinoma. Semin Radiat Oncol. 1998;8:237. doi: 10.1016/s1053-4296(98)80021-0. [DOI] [PubMed] [Google Scholar]
- 9.Dimery IW. Hong WK. Overview of combined modality therapies for head and neck cancer. J Natl Cancer Inst. 1993;85:95. doi: 10.1093/jnci/85.2.95. [DOI] [PubMed] [Google Scholar]
- 10.Mitra A. Nan A. Line BR. Ghandehari H. Nanocarriers for nuclear imaging and radiotherapy of cancer. Curr Pharm Des. 2006;12:4729. doi: 10.2174/138161206779026317. [DOI] [PubMed] [Google Scholar]
- 11.Lasic DD. Novel applications of liposomes. Trends Biotechnol. 1998;16:307. doi: 10.1016/s0167-7799(98)01220-7. [DOI] [PubMed] [Google Scholar]
- 12.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 13.Torchilin VP. Targeted pharmaceutical nanocarriers for cancer therapy and imaging. AAPS J. 2007;9:E128. doi: 10.1208/aapsj0902015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gabizon A. Shmeeda H. Barenholz Y. Pharmacokinetics of pegylated liposomal Doxorubicin: Review of animal and human studies. Clin Pharmacokinet. 2003;42:419. doi: 10.2165/00003088-200342050-00002. [DOI] [PubMed] [Google Scholar]
- 15.Gabizon AA. Pegylated liposomal doxorubicin: Metamorphosis of an old drug into a new form of chemotherapy. Cancer Invest. 2001;19:424. doi: 10.1081/cnv-100103136. [DOI] [PubMed] [Google Scholar]
- 16.Faivre S. Alsabe H. Djafari L, et al. Locoregional effects of pegylated liposomal doxorubicin (Caelyx) in irradiated area: A phase I-II study in patients with recurrent squamous cell carcinoma of the head and neck. Eur J Cancer. 2004;40:1517. doi: 10.1016/j.ejca.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Harrington KJ. Rowlinson-Busza G. Syrigos KN, et al. Pegylated liposome-encapsulated doxorubicin and cisplatin enhance the effect of radiotherapy in a tumor xenograft model. Clin Cancer Res. 2000;6:4939. [PubMed] [Google Scholar]
- 18.Carlsson J. Forssell Aronsson E. Hietala SO, et al. Tumour therapy with radionuclides: Assessment of progress and problems. Radiother Oncol. 2003;66:107. doi: 10.1016/s0167-8140(02)00374-2. [DOI] [PubMed] [Google Scholar]
- 19.Hamoudeh M. Kamleh MA. Diab R, et al. Radionuclides delivery systems for nuclear imaging and radiotherapy of cancer. Adv Drug Deliv Rev. 2008;60:1329. doi: 10.1016/j.addr.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 20.Zweit J. Radionuclides and carrier molecules for therapy. Phys Med Biol. 1996;41:1905. doi: 10.1088/0031-9155/41/10/004. [DOI] [PubMed] [Google Scholar]
- 21.Borjesson PK. Postema EJ. Roos JC, et al. Phase I therapy study with (186)Re-labeled humanized monoclonal antibody BIWA 4 (bivatuzumab) in patients with head and neck squamous cell carcinoma. Clin Cancer Res. 2003;9(10 Pt 2):3961S. [PubMed] [Google Scholar]
- 22.Colnot DR. Quak JJ. Roos JC, et al. Phase I therapy study of 186Re-labeled chimeric monoclonal antibody U36 in patients with squamous cell carcinoma of the head and neck. J Nucl Med. 2000;41:1999. [PubMed] [Google Scholar]
- 23.Postema EJ. Borjesson PK. Buijs WC, et al. Dosimetric analysis of radioimmunotherapy with 186Re-labeled bivatuzumab in patients with head and neck cancer. J Nucl Med. 2003;44:1690. [PubMed] [Google Scholar]
- 24.DeNardo SJ. Denardo GL. Targeted radionuclide therapy for solid tumors: An overview. Int J Radiat Oncol Biol Phys. 2006;66(2 Suppl):S89. doi: 10.1016/j.ijrobp.2006.03.066. [DOI] [PubMed] [Google Scholar]
- 25.Srivastava S. Dadachova E. Recent advances in radionuclide therapy. Semin Nucl Med. 2001;31:330. doi: 10.1053/snuc.2001.27043. [DOI] [PubMed] [Google Scholar]
- 26.Emfietzoglou D. Kostarelos K. Sgouros G. An analytic dosimetry study for the use of radionuclide-liposome conjugates in internal radiotherapy. J Nucl Med. 2001;42:499. [PubMed] [Google Scholar]
- 27.Kostarelos K. Emfietzoglou D. Tissue dosimetry of liposome-radionuclide complexes for internal radiotherapy: Toward liposome-targeted therapeutic radiopharmaceuticals. Anticancer Res. 2000;20:3339. [PubMed] [Google Scholar]
- 28.Liu CM. Chang CH. Chang YJ, et al. Preliminary evaluation of acute toxicity of (188) Re-BMEDA-liposome in rats. J Appl Toxicol. 2010;30:680. doi: 10.1002/jat.1541. [DOI] [PubMed] [Google Scholar]
- 29.Chang YJ. Chang CH. Chang TJ, et al. Biodistribution, pharmacokinetics and microSPECT/CT imaging of 188Re-bMEDA-liposome in a C26 murine colon carcinoma solid tumor animal model. Anticancer Res. 2007;27:2217. [PubMed] [Google Scholar]
- 30.Chang YJ. Chang CH. Yu CY, et al. Therapeutic efficacy and microSPECT/CT imaging of 188Re-DXR-liposome in a C26 murine colon carcinoma solid tumor model. Nucl Med Biol. 2010;37:95. doi: 10.1016/j.nucmedbio.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Chen LC. Chang CH. Yu CY, et al. Biodistribution, pharmacokinetics and imaging of (188)Re-BMEDA-labeled pegylated liposomes after intraperitoneal injection in a C26 colon carcinoma ascites mouse model. Nucl Med Biol. 2007;34:415. doi: 10.1016/j.nucmedbio.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Chen MH. Chang CH. Chang YJ, et al. MicroSPECT/CT imaging and pharmacokinetics of 188Re-(DXR)-liposome in human colorectal adenocarcinoma-bearing mice. Anticancer Res. 2010;30:65. [PubMed] [Google Scholar]
- 33.Bao A. Goins B. Klipper R, et al. 186Re-liposome labeling using 186Re-SNS/S complexes: In vitro stability, imaging, and biodistribution in rats. J Nucl Med. 2003;44:1992. [PubMed] [Google Scholar]
- 34.Bao A. Goins B. Klipper R, et al. Direct 99mTc labeling of pegylated liposomal doxorubicin (Doxil) for pharmacokinetic and non-invasive imaging studies. J Pharmacol Exp Ther. 2004;308:419. doi: 10.1124/jpet.103.059535. [DOI] [PubMed] [Google Scholar]
- 35.Soundararajan A. Bao A. Phillips WT, et al. [(186)Re]Liposomal doxorubicin (Doxil): In vitro stability, pharmacokinetics, imaging and biodistribution in a head and neck squamous cell carcinoma xenograft model. Nucl Med Biol. 2009;36:515. doi: 10.1016/j.nucmedbio.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gabizon A. Shiota R. Papahadjopoulos D. Pharmacokinetics and tissue distribution of doxorubicin encapsulated in stable liposomes with long circulation times. J Natl Cancer Inst. 1989;81:1484. doi: 10.1093/jnci/81.19.1484. [DOI] [PubMed] [Google Scholar]
- 37.Stewart JC. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal Biochem. 1980;104:10. doi: 10.1016/0003-2697(80)90269-9. [DOI] [PubMed] [Google Scholar]
- 38.Shimada K. Matsuo S. Sadzuka Y, et al. Determination of incorporated amounts of poly(ethylene glycol)-derivatized lipids in liposomes for the physicochemical characterization of stealth liposomes. Int J Pharm. 2000;203:255. doi: 10.1016/s0378-5173(00)00466-x. [DOI] [PubMed] [Google Scholar]
- 39.Bao A. Phillips WT. Goins B, et al. Setup and characterization of a human head and neck squamous cell carcinoma xenograft model in nude rats. Otolaryngol Head Neck Surg. 2006;135:853. doi: 10.1016/j.otohns.2006.06.1257. [DOI] [PubMed] [Google Scholar]
- 40.Vrekoussis T. Chaniotis V. Navrozoglou I, et al. Image analysis of breast cancer immunohistochemistry-stained sections using ImageJ: An RGB-based model. Anticancer Res. 2009;29:4995. [PubMed] [Google Scholar]
- 41.Funk T. Sun M. Hasegawa BH. Radiation dose estimate in small animal SPECT and PET. Med Phys. 2004;31:2680. doi: 10.1118/1.1781553. [DOI] [PubMed] [Google Scholar]
- 42.Bao A. Zhao X. Phillips WT, et al. Theoretical study of the influence of a heterogeneous activity distribution on intratumoral absorbed dose distribution. Med Phys. 2005;32:200. doi: 10.1118/1.1833151. [DOI] [PubMed] [Google Scholar]
- 43.Prestwich WV. Nunes J. Kwok CS. Beta dose point kernels for radionuclides of potential use in radioimmunotherapy. J Nucl Med. 1989;30:1036. [PubMed] [Google Scholar]
- 44.Simpkin DJ. Mackie TR. EGS4 Monte Carlo determination of the beta dose kernel in water. Med Phys. 1990;17:179. doi: 10.1118/1.596565. [DOI] [PubMed] [Google Scholar]
- 45.Kievit E. van Gog FB. Schluper HM, et al. Comparison of the biodistribution and the efficacy of monoclonal antibody 323/A3 labeled with either 131I or 186Re in human ovarian cancer xenografts. Int J Radiat Oncol Biol Phys. 1997;38:813. doi: 10.1016/s0360-3016(97)00007-2. [DOI] [PubMed] [Google Scholar]
- 46.Wang SX. Bao A. Herrera SJ, et al. Intraoperative 186Re-liposome radionuclide therapy in a head and neck squamous cell carcinoma xenograft positive surgical margin model. Clin Cancer Res. 2008;14:3975. doi: 10.1158/1078-0432.CCR-07-4149. [DOI] [PubMed] [Google Scholar]
- 47.Gerretsen M. Visser GW. Brakenhoff RH, et al. Complete ablation of small squamous cell carcinoma xenografts with 186Re-labeled monoclonal antibody E48. Cell Biophys. 1994;24–25:135. doi: 10.1007/BF02789224. [DOI] [PubMed] [Google Scholar]
- 48.Hagan PL. Halpern SE. Dillman RO, et al. Tumor size: Effect on monoclonal antibody uptake in tumor models. J Nucl Med. 1986;27:422. [PubMed] [Google Scholar]
- 49.Gabizon AA. Liposomal drug carrier systems in cancer chemotherapy: Current status and future prospects. J Drug Target. 2002;10:535. doi: 10.1080/1061186021000043061. [DOI] [PubMed] [Google Scholar]
- 50.Fenske DB. Cullis PR. Liposomal nanomedicines. Expert Opin Drug Deliv. 2008;5:25. doi: 10.1517/17425247.5.1.25. [DOI] [PubMed] [Google Scholar]
- 51.Knox SJ. Meredith RF. Clinical radioimmunotherapy. Semin Radiat Oncol. 2000;10:73. doi: 10.1016/s1053-4296(00)80045-4. [DOI] [PubMed] [Google Scholar]
- 52.Aytac U. Claret FX. Ho L, et al. Expression of CD26 and its associated dipeptidyl peptidase IV enzyme activity enhances sensitivity to doxorubicin-induced cell cycle arrest at the G(2)/M checkpoint. Cancer Res. 2001;61:7204. [PubMed] [Google Scholar]
- 53.Ling YH. el-Naggar AK. Priebe W, et al. Cell cycle-dependent cytotoxicity, G2/M phase arrest, and disruption of p34cdc2/cyclin B1 activity induced by doxorubicin in synchronized P388 cells. Mol Pharmacol. 1996;49:832. [PubMed] [Google Scholar]
- 54.Milas L. Milas MM. Mason KA. Combination of taxanes with radiation: Preclinical studies. Semin Radiat Oncol. 1999;9(2 Suppl 1):12. [PubMed] [Google Scholar]
- 55.Fornari FA., Jr. Jarvis WD. Grant S, et al. Induction of differentiation and growth arrest associated with nascent (nonoligosomal) DNA fragmentation and reduced c-myc expression in MCF-7 human breast tumor cells after continuous exposure to a sublethal concentration of doxorubicin. Cell Growth Differ. 1994;5:723. [PubMed] [Google Scholar]
- 56.Fornari FA. Randolph JK. Yalowich JC, et al. Interference by doxorubicin with DNA unwinding in MCF-7 breast tumor cells. Mol Pharmacol. 1994;45:649. [PubMed] [Google Scholar]
- 57.Supiot S. Gouard S. Charrier J, et al. Mechanisms of cell sensitization to alpha radioimmunotherapy by doxorubicin or paclitaxel in multiple myeloma cell lines. Clin Cancer Res. 2005;11(19 Pt 2):7047s. doi: 10.1158/1078-0432.CCR-1004-0021. [DOI] [PubMed] [Google Scholar]
- 58.Chatal JF. Saccavini JC. Gestin JF, et al. Biodistribution of indium-111-labeled OC 125 monoclonal antibody intraperitoneally injected into patients operated on for ovarian carcinomas. Cancer Res. 1989;49:3087. [PubMed] [Google Scholar]
- 59.Bao A. Phillips WT. Goins B, et al. Potential use of drug carried-liposomes for cancer therapy via direct intratumoral injection. Int J Pharm. 2006;316:162. doi: 10.1016/j.ijpharm.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 60.French JT. Goins B. Saenz M, et al. Interventional therapy of head and neck cancer with lipid nanoparticle-carried rhenium 186 radionuclide. J Vasc Interv Radiol. 2010;21:1271. doi: 10.1016/j.jvir.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mulrane L. Rexhepaj E. Penney S, et al. Automated image analysis in histopathology: A valuable tool in medical diagnostics. Expert Rev Mol Diagn. 2008;8:707. doi: 10.1586/14737159.8.6.707. [DOI] [PubMed] [Google Scholar]
- 62.Lehr HA. Mankoff DA. Corwin D, et al. Application of photoshop-based image analysis to quantification of hormone receptor expression in breast cancer. J Histochem Cytochem. 1997;45:1559. doi: 10.1177/002215549704501112. [DOI] [PubMed] [Google Scholar]
- 63.Grudzinski JJ. Burnette RR. Weichert JP, et al. Dosimetric effectiveness of targeted radionuclide therapy based on a pharmacokinetic landscape. Cancer Biother Radiopharm. 2010;25:417. doi: 10.1089/cbr.2009.0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lorusso D. Di Stefano A. Carone V, et al. Pegylated liposomal doxorubicin-related palmar-plantar erythrodysesthesia (“hand-foot” syndrome) Ann Oncol. 2007;18:1159. doi: 10.1093/annonc/mdl477. [DOI] [PubMed] [Google Scholar]
- 65.Brush J. Lipnick SL. Phillips T, et al. Molecular mechanisms of late normal tissue injury. Semin Radiat Oncol. 2007;17:121. doi: 10.1016/j.semradonc.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 66.Baechler S. Hobbs RF. Prideaux AR, et al. Extension of the biological effective dose to the MIRD schema and possible implications in radionuclide therapy dosimetry. Med Phys. 2008;35:1123. doi: 10.1118/1.2836421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grudzinski JJ. Tome W. Weichert JP, et al. The biological effectiveness of targeted radionuclide therapy based on a whole-body pharmacokinetic model. Phys Med Biol. 2010;55:5723. doi: 10.1088/0031-9155/55/19/007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carlson SK. Classic KL. Hadac EM, et al. In vivo quantitation of intratumoral radioisotope uptake using micro-single photon emission computed tomography/computed tomography. Mol Imaging Biol. 2006;8:324. doi: 10.1007/s11307-006-0058-z. [DOI] [PubMed] [Google Scholar]
- 69.Pereira JM. Stabin MG. Lima FR, et al. Image quantification for radiation dose calculations—limitations and uncertainties. Health Phys. 2010;99:688. doi: 10.1097/HP.0b013e3181e28cdb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hasegawa BH. Wong KH. Iwata K, et al. Dual-modality imaging of cancer with SPECT/CT. Technol Cancer Res Treat. 2002;1:449. doi: 10.1177/153303460200100605. [DOI] [PubMed] [Google Scholar]