Abstract
Melanoma is a malignancy with increasing incidence. Although primary tumors that are localized to the skin can be successfully treated by surgical removal, there is no satisfactory treatment for metastatic melanoma, a condition that has currently an estimated 5-year survival of just 6%. During the last decade, β- or α-emitter-radiolabeled peptides that bind to different receptors on a variety of tumors have been investigated as potential therapeutic agents in both the preclinical and clinical settings with encouraging results. A recent study demonstrated that 188-Rhenium (188Re)-labeled, via HYNIC ligand, fungal melanin-binding decapeptide 4B4 was effective against experimental MNT1 human melanoma and was safe to normal melanized tissues. The availability of radiolanthanides with diverse nuclear emission schemes and half-lives provides an opportunity to expand the repertoire of peptides for radionuclide therapy of melanoma. The melanin-binding decapeptide 4B4 was radiolabeled with 177Lu, 166Ho, and 153Sm via a DO3A chelate. The stability studies of Ln*-DO3A-4B4 in phosphate-buffered saline, serum, and a hydroxyapatite assay demonstrated that 177Lu-labeled peptide was more stable than 166Ho- and 153Sm-labeled peptides, most likely because of the smallest ionic radius of the former allowing for better complexation with DO3A. Binding of Ln*-DO3A-4B4 to the lysed highly melanized MNT1 melanoma cells demonstrated the specificity of peptides binding to melanin. In vivo biodistribution data for 177Lu-DO3A-4B4 given by intraperitoneal administration to lightly pigmented human metastatic A2058 melanoma-bearing mice demonstrated very high uptake in the kidneys and low tumor uptake. Intravenous administration did not improve the tumor uptake. The plausible explanation of low tumor uptake of 177Lu-DO3A-4B4 could be its decreased ability to bind to melanin during in vitro binding studies in comparison with 188Re-HYNIC-4B4, exacerbated by the very fast clearance from the blood and the kidneys “sink” effect.
Key words: cancer, melanoma, radioimmunotherapy, radiopharmaceuticals, targeted therapy
Introduction
Melanoma is a malignancy with increasing incidence that affects ∼60,000 new patients each year in the United States and an estimated 132,000 worldwide.1 Melanoma is particularly notable as an important cause of cancer among individuals 30–50 years of age, which imposes economic losses to society that further compound the human loss and suffering caused by this disease. Although primary tumors that are localized to the skin can be successfully treated by surgical removal, there is no satisfactory treatment for metastatic melanoma, a condition that has currently an estimated 5-year survival of just 6%.2 Unfortunately, there has been little improvement in the prognosis for metastatic melanoma in the past 25 years.
Most melanomas are pigmented by the presence of melanin. Some melanomas are called “amelanotic,” because they are not black or darkly pigmented. However, even amelanotic melanomas contain some melanin,3,4 which makes this pigment a convenient target for development of radionuclide therapy of metastatic melanoma. Several years ago the feasibility of targeting melanin, an intracellular melanocyte pigment, to deliver cytotoxic radiation to human melanoma cells in vivo has been demonstrated using a fungal melanin-binding monoclonal antibody (mAb 6D2) with promising therapeutic results.5 This approach is currently being tested in a clinical trial in patients with metastatic melanoma.6
Another approach to specifically deliver cytocidal radiation to the tumors is peptide–receptor radionuclide therapy (PRRT), which has an established role in the therapy of some tumors.7 A recent study expanded PRRT to melanoma treatment by performing therapy of experimental melanoma with a beta-emitter 188-Rhenium (188Re)-labeled melanin-binding deca- or heptapeptides.8,9 The results indicated that 188Re-labeled melanin-binding peptides had activity against melanoma and that these reagents could be potentially useful against this tumor, especially if the efficacy of the treatment could be improved.
Beta-emitting radioisotopes of rare earth elements (so called radiolanthanides) are of interest for the development of the antibody- or peptide-directed radionuclide therapy of melanoma for several reasons. First, a selection of half-lives, particle energies, and ranges in tissues is available, thus allowing a match with the tumor size and density and residence time of the targeting vector.10–13 Imaging and dosimetry are facilitated by the γ rays, which all radiolanthanides have in their nuclear decay schemes. Second, all of the lanthanides exhibit stability in macrocyclic and acyclic aminocarboxylate bifunctional ligands; thus, the radiolanthanide can be easily varied. Third, the technology for conjugation of the bifunctional ligands to peptides and antibodies is mature and radiolabeling and formation of a stable chelate should be straightforward. The present study describes the results of the radiolabeling of melanin-binding decapeptide 4B4 with three different radiolanthanides—177Lu, 166Ho and 153Sm—and the in vitro and in vivo evaluation of radiolabeled peptides with the purpose of improving peptide-targeted radiotherapy of metastatic melanoma.
Materials and Methods
General
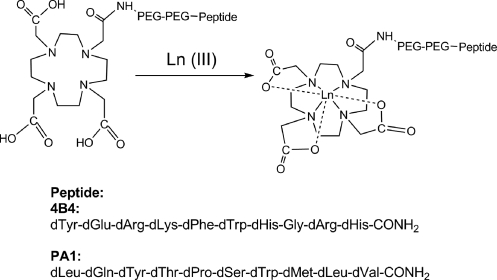
The radiolanthanides were obtained as 153Sm, 166Ho, and 177Lu in 0.05 M HCl from the University of Missouri Research Reactor. The specific activities range from 3.06 Ci/mg for 166Ho to 25.28 Ci/mg for 177Lu on the day of shipping. 4B4 peptide and the irrelevant control decapeptide, PA1,14 were synthesized from D-amino acids with DO3A at the N-terminus. Two PEG, 8-amino-3,6,-dioxaoctanoic acid, groups were used as a linker to attach the DO3A moiety to the peptide. DO3A was incorporated into the peptide sequence, reacting a DO3A(tri-tert-butyl)-CH2COOH with the PEG-PEG linker appended to the N-terminus. The DO3A ligand provides three carboxylate groups and an amide group to bind to the lanthanide ion. The peptides were synthesized using standard solid-phase peptide synthesis by Fmoc procedures from Peptide International. The formulations of the peptides are shown in Figure 1. The DO3A-4B4 melanin-binding peptide and the irrelevant control peptide, DO3A-PA1, were received as trifluoroacetate salts in powdered form, with analyzed purity (HPLC) of 96.6% and 88.5% for 4B4 and PA1, respectively. ES-MS analyses performed by Peptide International were in agreement with the calculated formulations. Both peptide samples were used as received without any further purification. The peptides will be denoted as DO3A-4B4 or DO3A-PA1, for convenience.
FIG. 1.
The peptide constructs employed in this study. DO3A was coupled to the melanin-binding 4B4 peptide and the irrelevant PA1 control using standard solid-phase peptide synthesis techniques. Both peptide constructs were radiolabeled with natLu, 177Lu, 153Sm, and 166Ho. PEG, 8-amino-3,6-dioxaoctanoic acid.
Radiolabeled peptide samples were purified and analyzed on a Rainin Dynamax HPLC (Model SD-200 pump) equipped with a Dynamax UV-1/UV-vis detector and a NaI(Tl) scintillation detector (Ortec 905-3). HPLC chromatograms were analyzed using Varian Pro STAR software (Version 6.4). The mobile phase was comprised of H2O (A) and acetonitrile (B), both containing 0.1% TFA. The method developed for evaluation of radiolabeled product utilized an initial concentration of 5% B with a linear gradient to 55% B over a period of 25 minutes with a flow rate of 1 mL/minute. Following the analysis, the column was equilibrated with 5% Solvent B for a period of 10 minutes prior to the next injection. Gamma counting was performed on an automated Perkin Elmer Wizard® Model 1470-015 5-well detector run in an open window mode.
Synthesis of natLu DO3A-4B4
The DO3A-4B4 peptide was dissolved in 500 μL of ammonium acetate buffer (0.15 M, pH 6.0) to give a final concentration of 5.0 mg/mL. One hundred microliters of the peptide solution was diluted with 50 μL of ammonium acetate buffer (pH 5), and 20 μL of LuCl3 (0.024 M) in 0.05 M HClaq stock solution was added. This provided an Lu-to-peptide ratio of 2:1. The solution was adjusted to a pH of 5.5 using 10 μL of 0.05 M HClaq. The reaction was heated to 90°C for 4 hours in a heating block. This solution was used for the mass spectral analysis and the HPLC coelution studies.
Mass spectral analysis of natLu DO3A-4B4
LC-MS analysis was performed on an Agilent Technologies G6520A high resolution Q-TOF mass spectrometer attached to an Agilent Technologies 1200 Capillary HPLC system. Samples were ionized by electrospray ionization. The instrument was controlled with Agilent MassHunter Workstation Acquisition software (Version B.02.00) and data were analyzed using Agilent MassHunter Workstation Qualitative Analysis software (Version B.03.01) with Agilent's BioConfirm software installed.
Radiolabeling of Ln* peptides (Ln*=177Lu, 166Ho, or 153Sm) and purification
The DO3A-4B4 peptide was dissolved in 500 μL of ammonium acetate buffer (0.15 M, pH 6.0) to give a final concentration of 5.0 mg/mL. Ten microliters of the peptide solution was diluted with 50 μL of ammonium acetate buffer (0.15 M, pH 6) and 20 μL of Ln*-chloride (Ln*=177Lu, 166Ho, or 153Sm) stock solution was added. The reaction was heated to 90°C for 1 hour in a heating block. Following incubation, 50 μL of 1 mM EDTA was added to quench the reaction and scavenge any unreacted Ln*. For coelution studies, a spike of the macroscopic natLu-DO3A-4B4 (50 μL) was added prior to injection on the HPLC. Radiolabeling of the DO3A-PA1 control peptide followed the same procedure as that used for the 4B4 analog. The Ln*DO3A-4B4 and Ln*DO3A-PA1 were purified before in vitro and in vivo assays as follows: the appropriate radio peak was collected and 500 μL of NH4OAc was added to the sample to prevent acid degradation. The sample was lyophilized overnight. Following lyophilization, samples were redissolved in 500 μL of phosphate-buffered saline (PBS) and used for the in vitro assays.
In vitro stability
PBS assay
For PBS stability, DO3A-4B4 and DO3A-PA1 were radiolabeled and purified as described earlier. Following lyophilization, samples were redissolved in 500 μL of PBS (pH 7.4) and incubated at 37°C in a heating block. At each time point, a 100 μL sample was removed and injected on the HPLC to determine complex stability.
Hydroxyapatite assay
A modification to the hydroxyapatite (HA) assay procedure previously reported15 was followed. Samples were analyzed at 24-hour time intervals from 0 to 5 days. To 5-mL scintillation vials, 0.125 g of 40% (by weight) HA solution and 2 mL of 50 mM TRIS with 0.01 mM CaCl2 (pH 7.5) were added. Subsequently, 25 μL of purified radiolabeled peptide in PBS was added to each sample. To separate the liquid phase, an Acrodisc® (0.45 μm) was attached to a 20-mL syringe, and the contents of the scintillation vial was put into the syringe and pushed through the Acrodisc. The sides of the scintillation vial were washed with 8 mL of water and the wash water was pushed through the syringe. To separate the solid phase, 10 mL of 1.3 N HNO3 was added to digest the solid material and pushed through the Acrodisc. Samples were counted using a Perkin-Elmer NaI gamma scintillation counter. Each time point consisted of five samples; each sample was counted for a period of 1 minute. All samples were counted following a period of 120 hours to eliminate the need for decay corrections.
Serum stability assay
The 177Lu DO3A-4B4 peptide was evaluated over a period of 72 hours using a modification to the procedure previously reported.16 Briefly, 100 μL of the lyophilized radiolabeled peptide was added to 400 μL of human serum and incubated at 37°C. A 100 μL aliquot was removed for each time point, diluted with 200 μL of PBS (pH 7.4), and filtered using a 0.45-μm filter, after which, 100 μL injections of the samples were analyzed using an SE-HPLC equipped with a TosoHaas size exclusion column (TSKGel® G4000 SW 30 cm×7.5 mm). The mobile phase consisted of 1.0 mM PBS (pH 7.4) at a flow rate of 1.0 mL/minute. The percentage of intact complex was determined by the total area under the radiopeak at the appropriate retention time compared with the total of the spectra.
In vitro binding assay
The Ln*-DO3A-Peptide (Peptide=4B4 or PA1) samples were prepared and purified as described earlier and the appropriate radiopeak was collected and lyophilized overnight and reconstituted in 500 μL of PBS (1.5 mM, pH 7.4). Following reconstitution in PBS, a 55 μL aliquot was removed from each sample to perform a QC analysis using RP-HPLC. The final volume of the samples was 445 μL. Peptide concentrations were estimated using the UV absorbance of the species at 260 nm from the HPLC trace and a previously prepared standard curve. The specific activities of the purified radiolabeled peptides are given in Table 1.
Table 1.
Specific Activities of Purified Radiolabeled Peptides
| |
Lu-177 |
Sm-153 |
Ho-166 |
|||
|---|---|---|---|---|---|---|
| DO3A-PA1 | DO3A-4B4 | DO3A-PA1 | DO3A-4B4 | DO3A-PA1 | DO3A-4B4 | |
| Total activity (mCi) | 1.16 | 1.2 | 1.49 | 1.68 | 1.58 | 1.71 |
| Concentration (mCi/mL) | 2.522 | 2.609 | 2.98 | 3.36 | 3.435 | 3.717 |
| SA (mCi/mg) | 42.757 | 32.38 | 13.817 | 20.923 | 16.846 | 20.487 |
The melanin-binding peptide is DO3A-4B4 and the control peptides are DO3A-PA1.
Highly pigmented human melanoma cells MNT1 (a kind gift from Dr. V. Hearing, NIH, Bethesda, MD) were grown in MEM/20% fetal bovine serum medium. The binding of Ln*-DO3A-4B4 and irrelevant control peptide Ln*-DO3A-PA1 to MNT1 cells was evaluated by incubating (20 ng/mL) with 0.25–2.0×106 osmotically lysed cells. After incubation for 1 hour at 37°C with gentle shaking, the cells were collected by centrifugation, the supernatant was removed, the cell pellet was washed with PBS, and the pellet and the supernatant were counted in a gamma counter to calculate the percentage of peptide binding to the cells. As an additional control for proving the specificity of Ln*-DO3A-4B4 peptide binding, the MNT1 cells were also preincubated with an excess (50 μg/mL) of unlabeled DO3A-4B4 for 1 hour before the addition of Ln*-DO3A-4B4.
In vivo biodistribution
All animal studies were carried out in accordance with the guidelines of the Institute for Animal Studies at the Albert Einstein College of Medicine. For the purpose of biodistribution, female nude mice were inoculated with ∼8×106 A2058 human melanoma cells by a subcutaneous injection (100 μL of the cell growth medium) into the right flank. Tumors were allowed to grow for 10 days and reached 0.3–0.7 cm in diameter. To determine the in vivo fate of the melanin-binding peptide, 48 mice were divided into 2 groups of 24 mice, with 1 group being injected intraperitoneally with 100 μL 177Lu-DO3A-4B4 and the other with 100 μL 177Lu-DO3A-PA1 intraperitoneally. Mice were sacrificed at 1, 3, 24, 48, 120, and 168 hours, with 4 mice per time point. Organs and tumors were collected, weighed, and counted on a gamma counter and the percentage of injected dose per gram of tissue (%ID/g) was calculated.
To compare the intraperitoneal (i.p.) injection with intravenous (i.v.) administration, a subsequent biodistribution study was performed using similar methods to those described above substituting i.v. injection, administered via the tail vein, in place of the i.p. injection. A total of 15 mice were used, divided into 4 groups, one receiving an i.v. injection of 177Lu-DO3A-4B4, one receiving an i.v. injection of 177Lu-DO3A-PA1, one receiving an i.p. injection of 177Lu-DO3A-4B4, and the other receiving an i.p. injection of 177Lu-DO3A-PA1. Mice were sacrificed at 3 and 72 hours. Organs and tumors were collected and counted on a gamma counter and the %ID/g was calculated.
Results
Synthesis
The peptide constructs and the schematic of the lanthanide complexes are shown in Figure 1. ESI-MS of the melanin-binding peptide DO3A-4B4 showed a single peak with m/z of 2090.26 (calcd.: 2090.05) and the control peptide DO3A-PA1 of 1884.01 (calcd.: 1883.90), verifying the integrity of these peptides. Amino acid sequencing was consistent with the formulation of the peptides.
The natLu-DO3A-4B4 construct was prepared by heating a 2:1 natLuCl3.3H2O:DO3A-4B4 solution, pH 5.5, at 90°C. The high-resolution ESI-MS data for natLu-DO3A-4B4 support the molecular formula for the structure shown in Figure 1. Supplementary Figure S1 (Supplementary Data are available online at www.liebertonline.com/cbr) shows the overall mass spectral data as well as the individual +4, +3, and +2 charge state regions. For each charge state, the observed m/z values show excellent agreement with the calculated m/z values (Supplementary Table S1). This construct was used for coelution studies.
Radiolabeling
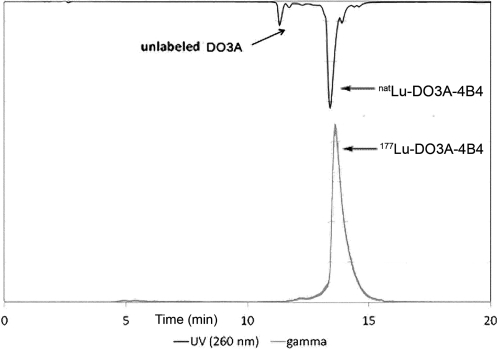
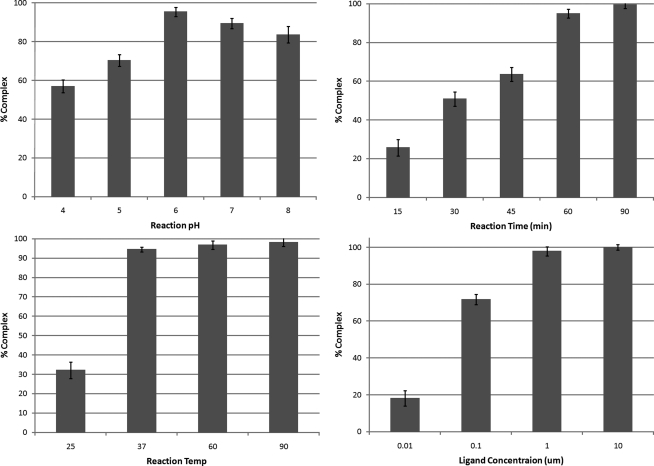
Initial radiolabeling was performed with 177Lu and the radiochemical yields were determined by radio-HPLC (Fig. 2). Radiolabeling yields were optimized for pH, temperature, incubation time, and peptide concentration. The results of these experiments are illustrated in Figure 3. The details of the optimization experiments are given in Supplementary Table S2. First, the pH was varied at constant concentration (10 μM), temperature (37°C), and time (1 hour). A pH of 6 was chosen and the reaction temperature was varied (concentration: 10 μM; time: 1 hour). The ligand concentration was varied next at constant pH 6, temperature 37°C, and 1 hour time. Finally, the reaction time was varied as a function of temperature 37°C, pH 6, and 1 μM ligand concentration. Reaction temperatures of 37°C or greater were found to achieve 90+% radiolabeling yield. The labeling yield was quite sensitive to ligand concentration and time. For example, the DO3A-4B4 ligand concentration of 0.1 μM resulted in 70% radiolabeling yield, whereas a 10-fold higher ligand concentration resulted in 98% yield. At least 1 hour was necessary to achieve >90% labeling at 37°C. The final conditions were pH 6, 90°C, 1 hour time, and 1 μM ligand concentration.
FIG. 2.
HPLC trace of natLnDO3A-4B4 coinjected with 177Lu-DO3A-4B4. The coelution of the 177Lu and natLn peptide complexes demonstrates that the 177Lu complex possesses the same chemical structure of the characterized natLnDO3A-4B4.
FIG. 3.
Optimization of the radiochemical yield for labeling reactions of DO3A-4B4 with 177Lu. The radiochemical yield was determined by counts under the radiopeak of 177Lu-DO3A-4B4 divided by the counts in the total spectra.
In vitro stability
To probe the stability of the radiolabeled complex in vitro, samples were evaluated using PBS, HA, and human serum.
PBS stability assays
For analysis of stability in PBS solution, RP-HPLC was utilized, and the percentage of intact complex was determined by the total area under the peak at the appropriate retention time compared with the total area of the HPLC. These data, presented in Table 2, show that the Ln* DO3A-4B4 and 177Lu-DO3A-PA1 complexes are stable over 72 hours. There was no statistical difference in the complex stability of 177Lu- or 166Ho-labeled peptides through 72-hour time period. However, the 153Sm analog showed a marked decrease in stability when compared with the other two isotopes.
Table 2.
Stability of the Radiolabeled DO3A-4B4 and DO3A-PA1 in Phosphate-Buffered Saline Assays
| |
177Lu-DO3A-4B4 |
177Lu-DO3A-PA1 |
166Ho-DO3A-4B4 |
153Sm-DO3A-4B4 |
||||
|---|---|---|---|---|---|---|---|---|
| Time (hours) | Average | Standard deviation | Average | Standard deviation | Average | Standard deviation | Average | Standard deviation |
| 24 | 99.56 | 0.53 | 95.63 | 2.84 | 99.08 | 1.01 | 91.61 | 2.33 |
| 48 | 99.05 | 0.74 | 82.94 | 2.39 | 96.17 | 3.17 | 87.22 | 7.29 |
| 72 | 96.23 | 6.21 | 84.21 | 2.13 | 93.11 | 5.83 | 76.42 | 3.00 |
| 96 | 92.27 | 2.43 | ||||||
The time points and the percentage of intact radiolanthanide complex as determined by RP-HPLC are shown.
HA stability assays
Table 3 shows the results of the HA assays. The 177Lu/153Sm/166Ho DO3A-4B4 shows 85%–90% complex stability in solution at 24 hours and decreases to around 78%–80% complex stability in solution at 120 hours. There was no statistical difference between the three isotopes. The 177Lu-DO3A-PA1 showed a higher stability to HA from 24 to 96 hours.
Table 3.
Radiolanthanide DO3A-Peptide Stability: Hydroxyapatite Stability Assays
|
177Lu-DO3A-4B4 |
177Lu-DO3A-PA1 |
153Sm-DO3A-4B4 |
166Ho-DO3A-4B4 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Time (hours) | % intact | Standard deviation | Time (hours) | % intact | Standard deviation | Time (hours) | % intact | Standard deviation | Time (hours) | % intact | Standard deviation |
| 0 | 100.00 | — | 0 | 100.00 | — | 0 | 100.00 | — | 0 | 100.00 | — |
| 24 | 85.16 | 3.12 | 24 | 97.00 | 2.35 | 24 | 86.33 | 5.02 | 24 | 91.84 | 6.11 |
| 48 | 84.14 | 2.97 | 48 | 95.77 | 3.61 | 48 | 86.13 | 4.55 | 48 | 87.67 | 8.01 |
| 72 | 83.89 | 3.03 | 72 | 95.33 | 3.29 | 72 | 85.76 | 5.42 | 72 | 86.52 | 6.53 |
| 96 | 79.73 | 2.96 | 96 | 92.03 | 3.27 | 96 | 79.20 | 5.97 | 96 | 83.67 | 5.02 |
| 120 | 79.77 | 2.79 | 120 | 85.01 | 6.09 | 120 | 78.77 | 6.23 | 120 | 80.47 | 5.58 |
| n=10 | n=5 | n=5 | n=5 | ||||||||
The time points and the activity remaining in solution when contacted with hydroxyapatite solid are shown. The % intact indicates the activity remaining in solution compared with the total activity (solution plus solid hydroxyapatite).
Serum stability assay
For the purpose of the serum stability studies, 177Lu was used for radiolabeling. The serum stability assay for the 177Lu-DO3A-4B4 is illustrated in Table 4. The time points and the % of intact 177Lu-DO3A-4B4, as determined from SE-HPLC, are shown. The % intact radiolabeled complex slightly decreases with time when incubated in human serum over a period of 72 hours.
Table 4.
Radiolanthanide DO3A-Peptide Stability: Serum Stability
| Time (hours) | Average | Standard deviation |
|---|---|---|
| 0 | 99.31 | 0.64 |
| 24 | 93.62 | 3.20 |
| 48 | 85.08 | 5.39 |
| 72 | 80.31 | 4.85 |
The time points and the average % activity of the 177Lu DO3A-4B4 upon incubation in serum are shown (n=3).
In vitro binding assay
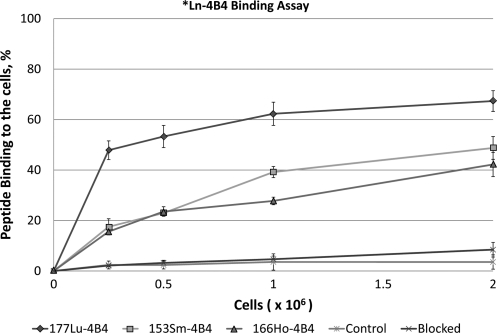
In vitro binding was performed with highly melanized MNT1 melanoma cells for the reason that the abundant melanin in MNT1 cells facilitates the experimental procedure by distinguishing the melanin pellet from cellular debris after cell lysis. Figure 4 illustrates the binding of the Ln-DO3A-4B4 complexes to the MNT1 cells. The data are presented in Supplementary Table S3. Peptide binding to melanin was evaluated using highly pigmented MNT1 cells, which were lysed prior to addition of the radiolabeled peptides. The 177Lu-DO3A-4B4 shows a rapid binding to the melanized cells and reaches saturation after a cell concentration of 1×106. Both 166Ho and 153Sm exhibit a similar binding profile though the initial specific activity of the radiolanthanide is lower than that of 177Lu. The control peptides, 177Lu, 166Ho, and 153Sm DO3A-PA1, show no binding to the cells and experiments adding a blocking peptide DO3A-4B4 peptide prior to the radiolabeled peptides also show little or no binding to the cells.
FIG. 4.
Cell binding assay of *Ln-DO3A-4B4 in highly pigmented MNT1 cells. Cells were osmotically lysed prior to addition of the radiolabeled complex. For the blocking experiment, 50 μg/mL DO3A-4B4 peptide was used before addition of radiolabeled peptides. Data are given in Supplementary Table S3.
In vivo biodistribution
In vivo biodistribution data were obtained with A2058 tumors, because the MNT1 cell line has lost its tumorigenecity in nude mice several generations ago and thus cannot be utilized for tumor induction in mice, whereas A2058 has a 100% tumor take in nude mice. The A2058 tumors become visibly necrotic at ∼20 days postinduction, which means that there are multiple isles of necrosis within the tumors long before that which makes melanin in nonviable necrotic cells available for peptide binding.17 In the present study, the same in vivo model was utilized for 188Re-labeled peptides biodistribution.8,9
The biodistribution of 177Lu-DO3A-4B4 and 177Lu-DO3A-PA1, administered by i.p. injection is shown in Table 5. Data for 177Lu-DO3A-4B4 show high uptake by the kidneys. By comparison, the activity within the bone is very low, which indicates that the 177Lu remains in the chelate in vivo. The PA1 analog shows low retention within all organs and no discernable tumor uptake was observed. The lower than expected uptake within the tumor of the 177Lu-DO3A-4B4 complex is most probably due to rapid removal from the blood pool by the kidneys.
Table 5.
Biodistribution of 177Lu DO3A-4B4 and Irrelevant Control 177Lu DO3A-PA1 in A2058 Tumor-Bearing Nude Mice
| |
177Lu-DO3A-4B4 (%ID/G) |
177Lu-DO3A-PA1 (%ID/G) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Organ | 1 hour | 3 hours | 24 hours | 48 hours | 120 hours | 168 hours | 1 hour | 3 hours | 24 hours | 48 hours | 120 hours | 168 hours |
| Blood | 0.02±0.02 | 0.07±0.03 | 0.02±0.001 | 0.07±0.08 | 0.02±0.002 | 0.03±0.002 | 0.14±0.15 | 0.10±0.03 | 0.09±0.03 | 0.04±0.02 | 0.02±0.01 | 0.02±0.01 |
| Lung | 0.07±0.08 | 0.14±0.10 | 0.06±0.04 | 0.05±0.02 | 0.07±0.05 | 0.05±0.004 | 0.29±0.31 | 0.12±0.02 | 0.08±0.03 | 0.07±0.02 | 0.03±0.01 | 0.04±0.01 |
| Heart | 0.05±0.01 | 0.08±0.03 | 0.04±0.01 | 0.05±0.01 | 0.05±0.01 | 0.10±0.03 | 0.18±0.19 | 0.10±0.01 | 0.07±0.02 | 0.06±0.02 | 0.04±0.002 | 0.04±0.01 |
| Spleen | 0.33±0.21 | 0.82±0.51 | 0.55±0.28 | 0.73±0.29 | 0.33±0.11 | 0.20±0.14 | 2.63±03.66 | 0.27±0.12 | 0.12±0.07 | 0.26±0.19 | 0.06±0.03 | 0.13±0.03 |
| Liver | 2.07±1.88 | 4.18±2.48 | 4.10±1.08 | 4.37±1.47 | 2.07±0.08 | 2.13±2.00 | 1.43±2.01 | 0.54±0.02 | 0.21±0.17 | 0.18±0.02 | 0.05±0.08 | 0.06±0.02 |
| Kidney | 16.89±14.47 | 57.18±19.92 | 37.89±7.89 | 35.23±13.06 | 16.89±8.37 | 10.44±6.45 | 0.03±0.002 | 3.00±0.57 | 0.90±0.57 | 1.04±0.42 | 0.14±0.20 | 0.31±0.17 |
| Bone | 0.16±0.07 | 0.35±0.12 | 0.15±0.05 | 0.22±0.03 | 0.16±0.01 | 0.18±0.08 | 0.40±0.35 | 0.17±0.12 | 0.18±0.01 | 0.09±0.02 | 0.10±0.06 | 0.09±0.01 |
| Tumor | 0.05±0.06 | 0.19±0.05 | 0.10±0.02 | 0.08±0.01 | 0.05±0.01 | 0.03±0.003 | 0.74±0.05 | 0.23±0.10 | 0.07±0.06 | 0.12±0.04 | 0.08±0.05 | 0.07±0.07 |
| Muscle | 0.02±0.01 | 0.09±0.08 | 0.01±0.001 | 0.04±0.01 | 0.02±0.01 | 0.03±0.01 | 0.04±0.05 | 0.05±0.02 | 0.05±0.02 | 0.03±0.01 | 0.02±0.01 | 0.02±0.003 |
The radiolabeled peptides were administered by i.p. injection.
%ID/G, percent injected dose per gram of tissue; i.p., intraperitoneal.
The comparison of average %ID/g for both i.p. and i.v. injection methods is given in Table 6. The 4B4 analogs show clearance through the kidney in both i.p. and i.v. models, although the i.v. administration shows about 50% less uptake in the kidney at 3 hours and about 33% less uptake at 72 hours. Generally, all organs show lower uptake for all time points in the i.v. model. The PA1 shows similar uptakes in organs and tumors in both injection models.
Table 6.
Comparison of Intravenous and Intraperitoneal Injection Methods for Biodistribution in A 2058 Human Metastatic Melanoma Tumor-Bearing Nude Mice
| |
177Lu-DOTA-4B4 (%ID/G) |
177Lu-DOTA-PA1 (%ID/G) |
||||||
|---|---|---|---|---|---|---|---|---|
| Organ | 3 hours (i.v.) | 3 hours (i.p.) | 72 hours (i.v.) | 72 hours (i.p.) | 3 hours (i.v.) | 3 hours (i.p.) | 72 hours (i.v.) | 72 hours (i.p.) |
| Blood | 0.06±0.003 | 0.08±0.04 | 0.03±0.01 | 0.04±0.03 | 0.11±0.03 | 0.13±0.02 | 0.01±0.002 | 0.06±0.03 |
| Lung | 0.33±0.11 | 0.25±0.13 | 0.06±0.01 | 0.20±0.11 | 0.18±0.10 | 0.14±0.03 | 0.17±0.01 | 0.07±0.02 |
| Heart | 0.06±0.02 | 0.11±0.04 | 0.04±0.001 | 0.06±0.002 | 0.10±0.01 | 0.11±0.02 | 0.04±0.01 | 0.07±0.02 |
| Spleen | 0.43±0.3 | 1.41±0.05 | 0.32±0.2 | 0.82±0.05 | 0.25±0.30 | 0.27±0.03 | 0.16±0.02 | 0.20±0.06 |
| Liver | 2.49±0.9 | 3.57±2.3 | 1.00±0.15 | 2.55±0.51 | 0.56±0.57 | 0.57±0.04 | 0.27±0.03 | 0.19±0.002 |
| Kidney | 39.56±1.7 | 68.05±36.4 | 7.02±0.30 | 21.32±4.1 | 1.66±0.002 | 2.83±0.21 | 0.35±0.03 | 0.49±0.05 |
| Bone | 0.23±0.01 | 0.43±0.08 | 0.91±0.83 | 0.59±0.30 | 0.26±0.05 | 0.30±0.03 | 0.53±0.05 | 0.78±0.59 |
| Tumor | 0.15±0.03 | 0.20±0.1 | 0.21±0.14 | 0.12±0.04 | 2.28±2.8 | 0.23±0.03 | 0.09±0.03 | 0.18±0.02 |
| Muscle | 0.03±0.04 | 0.06±0.002 | 0.06±0.006 | 0.05±0.003 | 0.06±0.008 | 0.07±0.03 | 0.06±0.01 | 0.08±0.007 |
Injection mode: i.v., intravenous; i.p., intraperitoneal.
Discussion
During the last decade, β- or α-emitter-radiolabeled peptides that bind to different receptors on a variety of tumors have been investigated as potential therapeutic agents in both the preclinical and clinical settings with encouraging results.7,18 Preclinical studies of the radiolabeled peptides in melanoma treatment have concentrated on melanin or melanin synthesis-associated substances as targets for PRRT. For example, the metal-cyclized alpha-melanocyte-stimulating hormone (MSH) peptide analogs such as Re-(Arg11)CCMSH and Re-CCMSH are suitable for the incorporation of Re isotopes such as 188Re or 186Re, radioiodination, or attachments of various radiometals, for example, 177Lu or 212Bi via bifunctional chelating agents such as DOTA (reviewed by Miao and Quinn19). The authors of the present study have recently demonstrated that 188Re-labeled, via HYNIC ligand, fungal melanin-binding decapeptide 4B4 was effective against experimental MNT1 human melanoma and was safe to normal melanized tissues.8 This study was followed by the evaluation of human tumoral melanin 188Re-labeled shorter heptapeptides as potential PRRT agents.9 The availability of radiolanthanides with diverse nuclear emission schemes and half-lives provides an opportunity to expand PRRT of melanoma armamentarium by radiolabeling melanin-binding peptides with a variety of radiolanthanides.
The peptides 4B4 and PA1 are bound to the DO3A ligand via an amide bond to two PEG units as spacers before the peptides. The DO3A-CH2CO-chelator (often called the generic DOTA) binds to lanthanide metals and has been used as the chelator for 177Lu-AMBA, a selective 177Lu-labeled GRP-R agonist for systemic radiotherapy of prostate cancer.20,21 This ligand is formally a heptadentate DO3A ligand, although it is possible for the carbonyl that is on the arm linking the DO3A moiety to the PEG linker to weakly bind to the radiolanthanide.
Radiolabeling yields for the formation of 177Lu-DO3A-4B4 were optimized by considering the variables such as pH, reaction time, reaction temperature, and ligand concentration. These variables were tested each independently while holding the other three constant. It was found that the pH of 5.5–6 was optimal for this reaction. Above 6, the labeling yields decreased slightly, possibly because of competition between the 177Lu binding into the DO3A ligand and formation of Lu colloids. The labeling yield was dependent on reaction temperature, with temperatures above 37°C required for >90% labeling. Because of the fact that DOTA, with four carboxylate arms, complexes of lanthanides possess slow kinetics of formation,22 radiolabeling was routinely done at 90°C to ensure full formation of the thermodynamically stable complex. The time of the reaction and ligand concentration were also factors in the labeling yield. Sixty minutes of reaction time was required for high labeling yield, with the ligand concentration being 1 μM. No degradation of the peptide was observed at the elevated temperatures. The optimal radiolabeling yields were achieved using relatively facile conditions of pH 6, reaction time of 90 minutes, incubation temperature of 90°C, and ligand concentration of 1 μM.
The synthesis of the natLu DO3A-4B4 employed an excess of lutetium chloride; the reaction solution was heated to 90°C for 4 hours. The reaction solution was used for mass spectral analysis; the observed and calculated m/z values for the +2, +3, and +4 charge clusters are in excellent agreement for the formulation shown in Figure 1, where the lutetium(III) ion is bound into the DO3A ligand.
The characterized natLu DO3A-4B4 was employed for the HPLC coelution study (Fig. 2). This experiment showed that the 177Lu-DO3A-4B4 coeluted with the characterized natLu DO3A-4B4 complex. Therefore, it can be deduced that the labeled 177Lu conjugate and natLu conjugate are similar in chemical structure.
The stability studies of 177Ln-DO3A-4B4 in PBS and HA assay demonstrated that 177Lu-labeled peptide showed some trend toward being more stable than 166Ho- and 153Sm-labeled peptides, most likely because of the smaller ionic radius of the former allowing for better complexation with DO3A. The same observation was made by Miao and Quinn19 when the stability studies of 166Ho-, 149Pm-, and 177Lu-labeled DO3A-conjugated mAb cc49 were performed. Interestingly, at later time points, the stability of 166Ho-DO3A-cc49 and 177Lu-DO3A-cc49 antibodies in the above assays, reported by Miao and Quinn,19 was ∼10% higher than that of 166Ho-DO3A-4B4 and 177Lu-DO3A-4B4 peptides in the present study, although the form of the DO3A ligand in both studies was the same—one carboxyl group sacrificed for the attachment to the mAb or the peptide. A possible explanation could be the steric stabilization of Ln*-DO3A complexes attached to the mAb by secondary interactions with various functionalities on the mAb. Alternatively, as 4B4 peptide carries a strong positive charge, the radiolabeled peptide could bind to some extent via electrostatic interaction to the negatively charged protein or HA components, creating the impression of instability.
The biological half-life of unmodified 4B4 has not been determined. However, for these and previous studies using 188Re-HYNIC-4B4 and other melanin-binding peptides, the constructs have been synthesized entirely from D-amino acids.8,9 The synthesis of the peptides using D-amino acids is a technique that is known to extend the biological half-lives of peptides from minutes to several hours.23 This and the introduction of the DO3A moiety may have contributed to the serum stability of the 177Lu-DO3A-4B4, which was comparable to the 188Re-HYNIC-4B4,8 for which at 72 hours, 80% of the 177Lu activity was still associated with the peptides and not with plasma proteins (Table 4).
Binding of Ln*-DO3A-4B4 to the lysed highly melanized MNT1 melanoma cells demonstrated the specificity of peptides binding to melanin, with 177Lu-DO3A-4B4 binding ∼50% better than 166Ho-DO3A-4B4 and 153Sm-DO3A-4B4 most likely because of the higher specific activity of the 177Lu-DO3A-4B4. Overall, the binding of Ln*-DO3A-4B4 to the lysed MNT1 cells was on average twofold lower than the binding of 188Re-HYNIC-4B4 to these cells, which might be due to the much higher specific activity of the latter. 188Re is carrier-free, whereas the radiolanthanides used in this study possess carrier.
The cell binding data were obtained in the presence of equimolar amounts of peptides, 20 ng/mL for every sample in the assay. Thus, the approximately threefold higher specific activity of 177Lu-labeled peptide translated into three times higher binding of this peptide to the cells in comparison with 166Ho- and 153Sm-labeled peptides. This is consistent with the differences in binding due to the differences in specific activities. In addition, an Ln*-DOTA complex, which is attached to 4B4 by a flexible PEG-PEG linker and is much larger than the 188Re-HYNIC complex, might interfere with the binding to melanin. Hence, in the present study, it was chosen to move forward with in vivo biodistribution for the 177Lu-DO3A-4B4 that exhibited the highest binding to MNT1 melanoma cells among the radiolanthanides tested.
In vivo biodistribution data for 177Lu-DO3A-4B4 given intraperitoneally to lightly pigmented human metastatic A2058 melanoma-bearing mice demonstrated very high uptake in the kidneys and low uptake in the tumor; the uptake in the tumor was not melanin-specific when compared with the biodistribution of the control peptide 177Lu-DO3A-PA1. When the i.v. route was investigated, the kidney uptake decreased almost twofold to the values observed for 188Re-HYNIC-4B4 in the same melanoma model; however, this change in the route of administration did not improve the tumor uptake of 177Lu-DO3A-4B4, which remained low (0.15% ID/g) at 3 hours p.i. in comparison with ∼1% ID/g for 188Re-HYNIC-4B4.8 The plausible explanation for low tumor uptake of 177Lu-DO3A-4B4 could be its decreased ability to bind to melanin in comparison with 188Re-HYNIC-4B4 as seen during in vitro binding studies exacerbated by the kidneys “sink” effect. In the present study, it was, however, not attempted to block the kidneys with positively charged amino acids such as d-lysine, as such attempts were unsuccessful when 188Re-HYNIC human tumor melanin-binding peptides were used.9 It has been recently convincingly demonstrated that high uptake of many peptides used in PRRT in the kidney is due to the multiplicity of mechanisms and is not simply due to electrostatic interactions.24
There are potentially four reasons for the differences in biodistribution comparing the 188Re-HYNIC-4B4 and 177Lu-DO3A-4B4. Very likely, stability is not an issue as there was very little uptake in the bone or liver for both constructs. The issues that likely impact biodistribution lie with (1) the structures and sizes of the chelates, (2) charges of the chelates, (3) the moieties linking the chelate to the B4B peptide, and (4) the half-life of the radionuclide.
The structures of 188Re-HYNIC-4B4 and 177Lu-DO3A-4B4 conjugates are quite different and charges are likely different. Although chemically robust, the 99mTc and Re-HYNIC coligand species are poorly characterized at the tracer level.25 It is considered that multiple species exist under physiological conditions. The HYNIC moiety can coordinate as a monodentate or bidentate ligand, and if HYNIC coordinates in a 1:1 stoichiometry (as demonstrated by mass spectroscopic data), a “coligand” is required to complete the coordination sphere. In the case of 188Re-HYNIC-4B4, the coligand is gluconate. It is possible that the 188Re (V) is coordinated to HYNIC and gluconate without an oxo or halide ligand.26 The coordination and the charge are not well defined. The degree of deprotonation of the coligand remains unsolved under physiological conditions, and therefore, the charge of the Re-HYNIC-gluconate complex is not known. Very likely, the 188Re-HYNIC complex is a fairly compact structure. Unlike the 177Lu-DO3A-4B4, there are no PEG units as linkers in the 188Re-HYNIC-4B4.
On the other hand, the structures of lanthanide DO3A complexes and lanthanide DOTA complexes are fairly well known, including their stereochemistry.27–30 In this case, the 177Lu-DO3A is likely heptacoordinate with the DO3A ligand bound to the 177Lu(III) center and likely a secondary interaction to the amide of the linker linking to the PEG. The charge of the 177Lu-DO3A complex is neutral. The complex is linked to the 4B4 peptide via two PEG units. In summary, the charge of the complex (unknown for 188Re-HYNIC and neutral for 177Lu-DO3A), their size, and their hydrophilicities (that are higher for the 177Lu-DO3A because of the PEG units) may account for the differences in biodistribution.
A salient difference between the 188Re-HYNIC-4B4 and 177Lu-DO3A-4B4 constructs is the PEG-PEG linker. This linker may be too hydrophilic, resulting in fast clearance. There are several examples wherein the linker can have a drastic impact on peptide affinity. The impact of linkers have been observed in thorough studies on the development of Cu-64 bombesin constructs.31,32 It may be important in future studies to identify different linker technologies that offer more hydrophobicity to the construct to keep them in the blood stream longer, to potentially increase tumor uptake. Also, different ligands that may impart a different overall charge to the complex may be examined, for example, the DOTA and DTPA ligands.
Finally, the half-life is likely very important in realization of therapeutic efficacy.11–13177Lu was chosen because of the higher melanin-binding ability compared with 166Ho and 153Sm. However, the half-life of 166Ho (26 hours) is closest to that of 188Re (16.9 hours) and may have the optimal half-life to match the residence time of the 4B4 peptide.
In conclusion, this study is an initial examination of radiolanthanide peptide constructs for therapy of melanoma. The constructs show binding to melanin that is dependent on the specific activity. Further optimization of the chelate, linker, and radiolanthanide is required to achieve therapeutic applications.
Supplementary Material
Acknowledgments
The authors acknowledge the NIH (NCI 5 SC1 CA138177) for support of this work. The research infrastructure at Hunter College was partially supported by NIH (Research Centers in Minority Institutions Grant RR03037-08). Funding for the LC-MS instrument system was provided by NIH Shared Instrumentation Grant 1S10RR022649-01 and the CUNY Instrumentation Fund.
Disclosure Statement
All authors of this article do not have institutional or commercial affiliations that might pose a conflict of interest regarding the publication of this article. E. Dadachova is a co-inventor on the granted U.S. patent on radioimmunotherapy of melanoma with melanin-binding antibodies.
References
- 1.Linos E. Swetter SM. Cockburn MG, et al. Increasing burden of melanoma in the United States. J Investig Dermatol. 2009;129:1666. doi: 10.1038/jid.2008.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun W. Schuchter LM. Metastatic melanoma. Curr Treat Options Oncol. 2001;2:193. doi: 10.1007/s11864-001-0033-5. [DOI] [PubMed] [Google Scholar]
- 3.Busam KJ. Hester K. Charles C, et al. New York, NY: Department of Pathology, Memorial Sloan-Kettering Cancer Center; 2001. Detection of Clinically Amelanotic Malignant Melanoma and Assessment of Its Margins by In Vivo Confocal Scanning Laser Microscopy. [PubMed] [Google Scholar]
- 4.Cohen-Solal KA. Crespo-Carbone SM. Namkoong J, et al. Progressive appearance of pigmentation in amelanotic melanoma lesions. Pigment Cell Res. 2002;15:282. doi: 10.1034/j.1600-0749.2002.02024.x. [DOI] [PubMed] [Google Scholar]
- 5.Dadachova E. Nosanchuk JD. Shi L, et al. Dead cells in melanoma tumors provide abundant antigen for targeted delivery of ionizing radiation by a mAb to melanin. Proc Natl Acad Sci U S A. 2004;101:14865. doi: 10.1073/pnas.0406180101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein M. Shibli N. Friedmann N, et al. Imaging of metastatic melanoma (MM) with a 188Rhenium (188Re)-labeled melanin binding antibody. J Nucl Med Meeting Abstracts. 2008;49:52P. [Google Scholar]
- 7.Pool Stefan E. Krenning Eric P. Koning Gerben A, et al. Preclinical and clinical studies of peptide receptor radionuclide therapy. Semin Nucl Med. 2010;40:209. doi: 10.1053/j.semnuclmed.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Dadachova E. Moadel T. Schweitzer AD, et al. Radiolabeled melanin-binding peptides are safe and effective in treatment of human pigmented melanoma in a mouse model of disease. Cancer Biother Radiopharm. 2006;21:117. doi: 10.1089/cbr.2006.21.117. [DOI] [PubMed] [Google Scholar]
- 9.Howell RC. Revskaya E. Pazo V, et al. Phage display library derived peptides that bind to human tumor melanin as potential vehicles for targeted radionuclide therapy of metastatic melanoma. Bioconjug Chem. 2007;18:1739. doi: 10.1021/bc060330u. [DOI] [PubMed] [Google Scholar]
- 10.Lang L. Jagoda E. Wu C, et al. Factors influencing the in vivo pharmacokinetics of peptides and antibody fragments: the pharmacokinetics of two PET-labeled low molecular weight proteins. Q J Nucl Med. 1997;41(2):53–61. [PubMed] [Google Scholar]
- 11.Eckelman WC. Unparalleled contribution of Technetium-99m to Medicine over 5 decades. J. Am. Coll. Cardiol. Img. 2009;2:364–368. doi: 10.1016/j.jcmg.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Ter-Pogossian MM. Wagner HN., Jr A new look at the cyclotron for making short-lived isotopes. Nucleonics. 1966;24:50–62. doi: 10.1016/s0001-2998(98)80026-3. [DOI] [PubMed] [Google Scholar]
- 13.Ter-Pogossian MM. Wagner HN., Jr A new look at the cyclotron for making short-lived isotopes (first printed in 1966) Seminars in nuclear medicine. 1998;28(3):202–212. doi: 10.1016/s0001-2998(98)80026-3. [DOI] [PubMed] [Google Scholar]
- 14.Nosanchuk JD. Veladon P. Feldmesser M, et al. Melanization of Cryptococcus neoformans in murine infection. Mol Cell Biol. 1999;19:745. doi: 10.1128/mcb.19.1.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li WP. Ma DS. Higginbotham C, et al. Development of an in vitro model for assessing the in vivo stability of lanthanide chelates. Nucl Med Biol. 2001;28:145. doi: 10.1016/s0969-8051(00)00196-7. [DOI] [PubMed] [Google Scholar]
- 16.Mohsin H. Jia F. Sivaguru G, et al. Radiolanthanide-labeled monoclonal antibody CC49 for radioimmunotherapy of cancer: Biological comparison of DOTA conjugates of 149Pm, 166Ho, 177Lu. Bioconjug Chem. 2006;17:485. doi: 10.1021/bc0502356. [DOI] [PubMed] [Google Scholar]
- 17.Revskaya E. Jongco AM. Sellers RS, et al. Radioimmunotherapy of experimental human metastatic melanoma with melanin-binding antibodies and in combination with dacarbazine. Clin. Cancer Res. 2009;15(7):2373–2379. doi: 10.1158/1078-0432.CCR-08-2376. [DOI] [PubMed] [Google Scholar]
- 18.Dadachova E. Cancer therapy with alpha-emitters labeled peptides. Semin Nucl Med. 2010;40:204. doi: 10.1053/j.semnuclmed.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Miao Y. Quinn TP. Peptide-targeted radionuclide therapy for melanoma. Crit Rev Oncol Hematol. 2008;67:213. doi: 10.1016/j.critrevonc.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lantry LE. Cappelletti E. Maddalena ME, et al. 177Lu-AMBA: Synthesis and characterization of a selective 177Lu-labeled GRP-R agonist for systemic radiotherapy of prostate cancer. J Nucl Med. 2006;47:1144. [PubMed] [Google Scholar]
- 21.Chen J. Linder KE. Cagnolini A, et al. Synthesis, stabilization and formulation of [177Lu]Lu-AMBA, a systemic radiotherapeutic agent for gastrin releasing peptide receptor positive tumors. Appl Radiat Isotopes. 2008;66:497. doi: 10.1016/j.apradiso.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Moreau J. Guillon E. Pierrard J-C, et al. Complexing mechanism of the lanthanide cations Eu3+, Gd3+, and Tb3+ with 1,4,7,10-tetrakis(carboxymethyl)-1,4,7,10-tetraazacyclododecane (dota)—characterization of three successive complexing phases: Study of the thermodynamic and structural properties of the complexes by potentiometry, luminescence spectroscopy, and EXAFS. Chem Eur J. 2004;10:5218. doi: 10.1002/chem.200400006. [DOI] [PubMed] [Google Scholar]
- 23.Pless J. The history of somatostatin analogs. J Endocrinol Invest. 2005;28(11 Suppl International):1–4. [PubMed] [Google Scholar]
- 24.Vegt E. de Jong M. Wetzels JFM, et al. Renal toxicity of radiolabeled peptides and antibody fragments: Mechanisms, impact on radionuclide therapy, and strategies for prevention. J Nucl Med. 2010;51:1049. doi: 10.2967/jnumed.110.075101. [DOI] [PubMed] [Google Scholar]
- 25.Banerjee SR. Francesconi L. Valliant J. Babich JW. Zubieta J. New directions in the coordination chemistry of 99mTc: a reflection on technetium core structures and a strategy for new chelate design. Nucl. Med. Biol. 2005:1–20. doi: 10.1016/j.nucmedbio.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 26.King RC. Surfraz M. Biagini SCG. Blower PJ. Mather SJ. How do HYNIC-conjugated peptides bind technetium? Insights from LC-MS and stability studies. Dalton Trans. 2007;43:4998–5007. doi: 10.1039/b705111e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar K. Chang CA. Francesconi LC, et al. Synthesis, Stability, and Structure of Gadolinium(III) and Yttrium(III)Macrocyclic Poly(amino carboxylates) Inorg. Chem. 1994:33. [Google Scholar]
- 28.Regueiro-Figueroa M. Esteban-Gomez D. de Blas A. Rodriguez-Blas T. Structure and Dynamics of Lanthanide(III) Complexes with an N-Alkylated do3a Ligand (H3do3a=1,4,7,10-Tetraazacyclododecane-1,4,7-triacetic Acid): A Combined Experimental and DFT Study. Eur. J. Inorg. Chem. 2010:3586–3595. [Google Scholar]
- 29.Faulkner S. Burton-Pye BP. pH Dependent self-assembly of dimetallic lanthanide complexes. Chem. Commun. 2005 doi: 10.1039/b412329h. [DOI] [PubMed] [Google Scholar]
- 30.Caravan P. Ellison JJ. McMurry TJ. Lauffer RB. Gadolinium(III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications. Chem. Rev. 1999;99:2293–2352. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 31.Hoffman T. Smith CJ. True radiotracers: Cu-64 targeting vectors based upon bombesin peptide. Nucl. Med. Biol. 2009;36:579–585. doi: 10.1016/j.nucmedbio.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 32.Lane SR. Nandaa P. Rold TL, et al. Optimization, biological evaluation and microPET imaging of copper-64-labeled bombesin agonists, [64Cu-NO2A-(X)-BBN(7–14)NH2], in a prostate tumor xenografted mouse model. Nucl. Med. Biol. 2010;37:751–761. doi: 10.1016/j.nucmedbio.2010.04.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.