Abstract
Miraculin (MCL) is a homodimeric protein isolated from the red berries of Richadella dulcifica. MCL, although flat in taste at neutral pH, has taste-modifying activity to convert sour stimuli to sweetness. Once MCL is held on the tongue, strong sweetness is sensed over 1 h each time we taste a sour solution. Nevertheless, no molecular mechanism underlying the taste-modifying activity has been clarified. In this study, we succeeded in quantitatively evaluating the acid-induced sweetness of MCL using a cell-based assay system and found that MCL activated hT1R2-hT1R3 pH-dependently as the pH decreased from 6.5 to 4.8, and that the receptor activation occurred every time an acid solution was applied. Although MCL per se is sensory-inactive at pH 6.7 or higher, it suppressed the response of hT1R2-hT1R3 to other sweeteners at neutral pH and enhanced the response at weakly acidic pH. Using human/mouse chimeric receptors and molecular modeling, we revealed that the amino-terminal domain of hT1R2 is required for the response to MCL. Our data suggest that MCL binds hT1R2-hT1R3 as an antagonist at neutral pH and functionally changes into an agonist at acidic pH, and we conclude this may cause its taste-modifying activity.
Keywords: G protein-coupled receptor, positive allosteric modulator, taste-modifying protein, calcium imaging
Humans recognize structurally diverse sweeteners, such as sugars, d-amino acids, peptides, and sweet-tasting proteins. Miraculin (MCL), a protein found in the fruit of a West-African plant, Richadella dulcifica, is famous for its unique sweet sensation (1, 2), which is a homodimeric glycosylated protein composed of 191 amino acid residues (3). Although flat in taste at neutral pH, MCL has taste-modifying activity to convert sour stimuli to sweetness. Once MCL is held on the tongue, humans can sense strong sweetness each time they taste a sour solution and this activity lasts for 1 to 2 h (4).
Sweeteners are received by the human sweet taste receptor, hT1R2-hT1R3, which belongs to class C G protein-coupled receptors (GPCRs) (5, 6). Although MCL has been well-characterized from a biochemical point of view, no data on its interaction with taste receptors have been reported. This lack of knowledge is mainly because of the absence of objective assay systems for measuring the acid-induced sweetness of MCL.
Neoculin (NCL) is another taste-modifying protein that converts sourness to sweetness. Unlike MCL, NCL per se tastes sweet, but tastes much sweeter at acidic pH (7, 8). We have recently revealed that NCL is functionally in pH-dependent equilibrium between the active and inactive forms because it activates hT1R2-hT1R3 at acidic pH but inhibits the activation of the receptor at neutral pH (9, 10). Because MCL and NCL have no amino acid sequence similarity and share no functional motifs, it is an intriguing question as to whether both share the same molecular mechanism of hT1R2-hT1R3 activation. This study aimed to elucidate the taste-modification mechanism of MCL at the molecular level.
Results
In Vitro Evaluation of the pH-Dependent Acid-Induced Sweetness of MCL.
It has been reported that the acid-induced sweetness of MCL is diminished by a sweet taste inhibitor, lactisole (11), which inhibits hT1R2-hT1R3 by interacting with the transmembrane domain (TMD) of the hT1R3 subunit (12). This finding strongly indicates that MCL acts through the hT1R2-hT1R3. However, no experimental evidence for the interaction between MCL and hT1R2-hT1R3 has been obtained. To evaluate the acid-induced sweetness of MCL in vitro, we first carried out a calcium imaging analysis using HEK293T cells transiently expressing hT1R2-hT1R3 together with G15Gi3 and DsRed2.
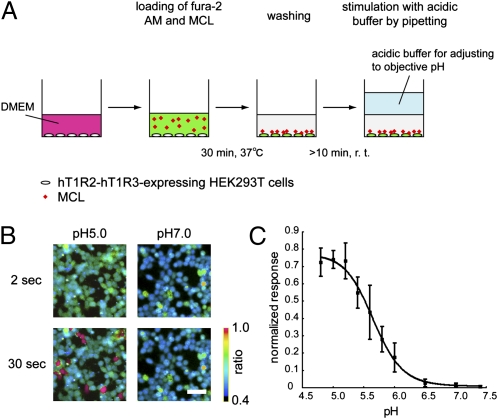
Because the taste-modifying activity of MCL can be detected for more than 1 h in sensory tests (4), we hypothesized that MCL strongly adheres to taste-receptor cells on the tongue. To mimic the conditions of sensory tests using a cell-based assay, MCL (30 nM) was preincubated with the hT1R2-hT1R3–expressing cells together with the fluorescent calcium indicator [fura-2 acetoxymethyl ester (AM)] before acid stimulation. After washing with assay buffer, the cells were stimulated by the addition of the assay buffer adjusted at different pH (Fig. 1A). When the MCL-preincubated cells were stimulated with acidic buffer (final pH: 5.0), the number of responding cells was significantly greater than that after the addition of neutral buffer (final pH: 7.0), in which few cells responded (Fig. 1B). As control, we also confirmed that the cell response after acid application was not detectable without either MCL preincubation or the expression of hT1R2-hT1R3 (Fig. S1). It should be noted that when aspartame (10 mM) was used as a control, the number of responding cells was similar between both the acidic and neutral conditions, as described previously (9).
Fig. 1.
MCL activates hT1R2-hT1R3 in a pH-dependent manner. (A) Schematic illustration of the MCL preincubation method. Illustration of a single well of a 96-well plate. (B) Representative ratiometric images of fura-2–loaded and MCL (100 nM)-preincubated hT1R2-hT1R3– and G15Gi3-expressing cells in response to acidic buffer (pH 5.0, Left) and neutral buffer (pH 7.0, Right). The pH values of the ligand solution were adjusted with citric acid such that the pH values after adding the ligands to the cells were as indicated. The Upper and Lower rows show the representative cell images obtained 2 and 30 s after acidic buffer application, respectively. The color scale indicates the F340/F380 ratio as a pseudocolor. (Scale bar, 50 μm.) (C) Responses of hT1R2-hT1R3 to MCL (30 nM) under different pH conditions. The number of responding cells was normalized relative to the response to 10 mM aspartame at pH 7.4. Each point represents the mean ± SE (n = 3–6).
Next, we evaluated the relationship between the pH value and the response of MCL-preincubated cells. At a fixed MCL concentration (30 nM), the responses of MCL-preincubated cells were evaluated in the range of pH 4.8 to 7.4, and the number of the responding cells was normalized relative to the response to 10 mM aspartame at pH 7.4. Between pH 4.8 and 6.5, the relative response increased in a pH-dependent manner as pH value decreased, whereas little response was observed at pH 6.5 to 7.4. At pH 5.7, the cells were half as responsive as they were at pH 4.8 (Fig. 1C). This pH-dependent curve correlated well with the data obtained from human sensory tests (11). Thus, our method using hT1R2-hT1R3–expressing cells is appropriate for evaluating the pH dependency of the acid-induced sweetness of MCL.
Effect of the Concentration of Preincubated MCL on the Response of hT1R2-hT1R3–Expressing Cells.
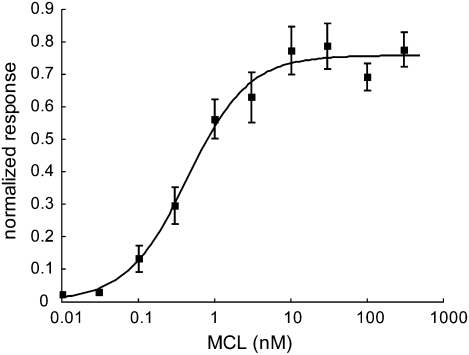
Next, we evaluated the dose–response relationship of MCL. Cells transiently expressing hT1R2-hT1R3 were preincubated with different concentrations of MCL and subsequently stimulated by acidic buffer adjusted to pH 5.0 after application. When cells were preincubated with 0.01 to 300 nM MCL, a dose-dependent response was clearly observed (Fig. 2). The cellular response to the acidic buffer (pH 5.0) increased as MCL concentration increased, leading to an EC50 value of ∼0.44 nM. In contrast, cells did not respond to neutral buffer (pH 7.0), even after preincubation with high concentration (100 nM) of MCL (Fig. 1B). In previous sensory tests, the taste-modifying activity of MCL was noted at 3 nM and the half-maximal sweetness occurs at about 10 nM (13). The activity of MCL measured using the cell-based assay is in agreement with the sensory evaluation data. Because the EC50 values for most sweeteners are of the micromolar or millimolar order (14), our data suggest that MCL binds to hT1R2-hT1R3 with higher affinity than other conventional sweeteners.
Fig. 2.
hT1R2-hT1R3 responds to MCL in a dose-dependent manner. Concentrations of MCL during preincubation are indicated. Cells expressing hT1R2-hT1R3 and G15Gi3 were preincubated with different concentrations of MCL and were stimulated with acidic buffer adjusted to be pH 5.0 after application. The number of responding cells was normalized relative to the response to 10 mM aspartame at pH 7.4. Each point represents the mean ± SE (n = 5–7).
Response of MCL-Preincubated Cells Expressing hT1R2-hT1R3 to Repetitive Acid Stimulation.
In sensory tests, once MCL is held on the tongue, strong sweetness can be detected each time humans taste a sour solution. Moreover, this activity lasts for more than 1 h, although the sweet intensity decreases with time (4). To determine whether responses of cultured cells expressing hT1R2-hT1R3 directly parallel the psychophysical responses, hT1R2-hT1R3–expressing cells were preincubated with 100 nM MCL. After the MCL solution was washed out, the cells were repeatedly stimulated with the perfusion of acidic buffer (pH 5.0) (Fig. 3). In this perfusion assay system, among the aspartame-responding cells, nearly half of the cells (19 ± 6.4 of 51 ± 15 cells) responded to the first stimulation with pH 5.0 buffer and part of them repeatedly responded to repetitive stimulation [11 ± 1.2 and 5.3 ± 0.33 of 51 ± 15 cells (mean ± SE) in the second and third stimulations, respectively; n = 3] (Fig. 3A). As control, we checked that cells did not respond to acidic stimulation without MCL preincubation (Fig. 3B). These results strongly support the hypothesis that MCL remains bound to hT1R2-hT1R3 expressed on the membranes of cultured cells under neutral condition, and that MCL is able to activate the receptor repeatedly. These observations led us to conclude that the continuous taste-modifying activity of MCL is caused by its direct binding to hT1R2-hT1R3. However, we cannot exclude the possibility that MCL is rapidly released from the receptor after the first acid stimulation, but modifies the receptor function posttranslationally to respond to the acid solution repeatedly.
Fig. 3.
MCL remains bound onto hT1R2-hT1R3, having the ability to activate it. (A) The traces show each Δratio of the fluorescence intensities (F340/F380) of the MCL (100 nM)-preincubated hT1R2-hT1R3– and G15Gi3- expressing cells that responded to 10 mM aspartame. The results obtained from one experiment are shown here. A perfusion assay system was used for the application of acidic buffer and ligands. Acidic buffer (pH 5.0) and 10 mM aspartame were applied to the cells for 16 s, and the duration of each stimulation is indicated by red and blue squares, respectively. (B) The traces describe each Δratio of the fluorescence intensities (F340/F380) of the non-MCL–preincubated hT1R2-hT1R3– and G15Gi3-expressing cells that responded to 10 mM aspartame.
In contrast, when cells were preincubated with NCL, no response was observed when they were stimulated with acidic buffer (pH 5.0) (Fig. S2). This finding suggests that MCL interacts with hT1R2-hT1R3 more strongly than NCL does; this may reflect the fact that NCL and MCL act at micromolar and nanomolar levels, respectively, in sensory tests (13, 15).
MCL Acts as a Potential Inhibitor of hT1R2-hT1R3 at Neutral pH and as a Positive Allosteric Modulator at Weakly Acidic pH.
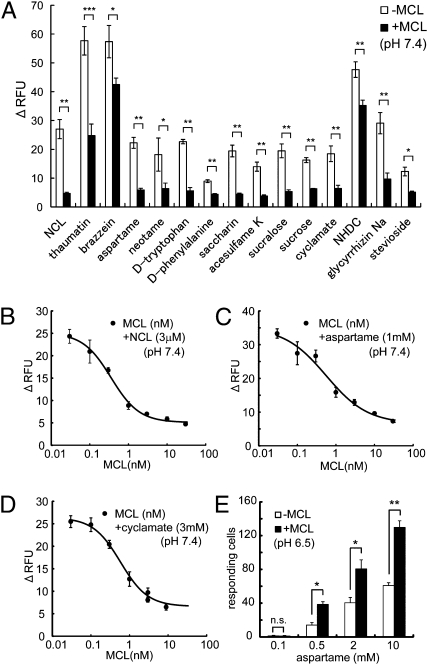
We previously found that at neutral pH, NCL is an hT1R2-hT1R3 antagonist, but at acidic pH, it functionally changes into an agonist (9). Because MCL is also an hT1R2-hT1R3 agonist at acidic pH (Fig. 1), we investigated whether it could behave as an hT1R2-hT1R3 antagonist at neutral pH. The cellular response to NCL was significantly suppressed after preincubating stable cell lines expressing hT1R2, hT1R3, and G16gust44 (16) with 30 nM MCL (Fig. 4A). This result is in agreement with our psychophysical observation that NCL did not taste sweet of its own when we tasted it after coating our tongues with MCL (Fig. S3). These results suggest that the binding of MCL to hT1R2-hT1R3 inhibits the interaction between hT1R2-hT1R3 and NCL. It is interesting to note that in the cell-based assay, this inhibitory effect of MCL was observed not only for NCL but also for other sweet proteins (thaumatin and brazzein) and small sweet molecules, such as aspartame, saccharin, and sucrose (Fig. 4A). Among the sweeteners, we chose NCL, aspartame, and cyclamate, which requires different regions for activation, the amino-terminal domain (ATD) of hT1R3, the ATD of hT1R2, and the TMD of hT1R3, respectively, and examined the dose-relationship of inhibition. As a result, MCL inhibited these sweeteners in a dose-dependent manner (Fig. 4 B–D). The IC50 value of MCL for NCL (3 μM), aspartame (1 mM), and cyclamate (3 mM) were 0.35, 0.56, and 0.58 nM, respectively (Fig. 4 B–D). These results indicate that MCL could potentially act as an inhibitor of other sweetener-induced hT1R2-hT1R3 activations under neutral conditions. Therefore, this finding suggests that MCL and NCL act as an hT1R2-hT1R3 antagonist and an agonist at neutral and acidic pH, respectively.
Fig. 4.
MCL inhibits the hT1R2-hT1R3 activation induced by other sweeteners at pH 7.4 and enhances it at pH 6.5. (A) The response of stable cell lines expressing hT1R2, hT1R3, and G16gust44 to each sweetener in the absence (white bars) or presence (black bars) of preincubated MCL (30 nM) at neutral pH (pH 7.4). The final concentrations of each sweetener are as follows: NCL, 3 μM; thaumatin, 0.1%; brazzein, 0.02%; aspartame, 1 mM; neotame, 5 μM; d-tryptophan, 3 mM; d-phenylalanine, 15 mM; saccharin, 300 μM; acesulfame K, 1 mM; sucralose, 100 μM; sucrose, 100 mM; cyclamate, 3 mM; NHDC, 2 mM; glycyrrhizic acid, 300 μM; and stevioside, 100 μM. Each bar represents the mean ± SE (n = 4–5). *P < 0.05, **P < 0.01, and ***P < 0.001 (Student t test). RFU, relative fluorescence units. (B–D) Dose-dependent inhibition of NCL-induced hT1R2-hT1R3 activation by MCL at pH 7.4. The responses of stable cell lines expressing hT1R2, hT1R3, and G16gust44 to NCL (3 μM) (B), aspartame (1 mM) (C), and cyclamate (3 mM) (D) in the presence of different concentration of preincubated MCL. Each point represents the mean ± SE (n = 3). (E) The response of the HEK293T cells expressing hT1R2, hT1R3, and G15Gi3 to different concentrations of aspartame in the absence (−MCL) or presence (+MCL) of preincubated MCL (100 nM) at pH 6.5. Each bar represents the mean ± SE (n = 3). n.s., not significant. *P < 0.05 and **P < 0.01 (Student t test).
Because pharamacological properties of MCL are opposite between neutral and acidic pHs, we carried out experiments to look at whether MCL acts as a positive allosteric modulator (PAM) for hT1R2-hT1R3 under acidic conditions. The hT1R2-hT1R3– and G15Gi3-expressing cells were first incubated with or without MCL at 7.4, and then stimulated by aspartame at the various concentrations, 0.1, 0.5, 2.0, and 10 mM, at pH 6.5 and pH 5.7, before counting the responding cells. As a result, at pH 6.5, where almost no response was observed with MCL preincubation (Fig. 1C), the response to aspartame was enhanced (Fig. 4E). On the other hand, at pH 5.7, where half the maximum response to MCL occurred (Fig. 1C), we cannot define MCL as a PAM at this pH because an increase in response under MCL preincubation may be caused as an additive effect by the activation by MCL itself but not by any PAM effect (Fig. S4). These results suggest that MCL acts as a PAM for hT1R2-hT1R3 at weakly acidic pH.
Requirement of the ATD of hT1R2 for the Responsiveness to MCL.
T1R is composed of three domains: an ATD, a cysteine-rich domain (CRD), and a TMD and it is known that hT1R2-hT1R3 possesses multiple ligand-binding sites for different sweeteners (12). Here, we identified which part of hT1R2-hT1R3 is involved in the response to MCL.
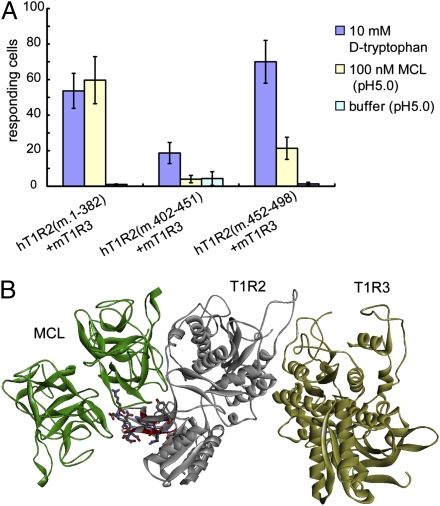
Animal species exhibit differences in their sensitivity to the acid-induced sweetness of MCL (17–19). Although MCL elicits sweetness in humans under acidic conditions, electrophysiological experiments show that rodents are unable to detect the sweetness induced by sour solutions (20). We examined whether this differential responsiveness to MCL is caused by differences in the amino acid sequences between hT1R2-hT1R3 and its mouse homolog, mT1R2-mT1R3. To determine whether one or both hT1R2 and hT1R3 are necessary for the response to MCL, we transiently expressed the following combinations in HEK293T cells together with G15Gi3 and DsRed2: hT1R2-hT1R3, mT1R2-mT1R3, hT1R2-mT1R3, and mT1R2-hT1R3. We then monitored receptor activation by using the preincubation method described above (Fig. 1A). Two ligands were used as positive controls: d-tryptophan (d-Trp), which tastes sweet both to humans and rodents, and aspartame, which is a human-specific sweetener that interacts with the ATD of hT1R2 (12). As expected, mT1R2-mT1R3 did not respond to acidic stimulation after MCL preincubation, whereas hT1R2-hT1R3 did (Fig. 5A). In addition, hT1R2-mT1R3 responded to MCL at pH 5.0, as well as to aspartame or d-Trp at pH 7.4 (Fig. 5 A and B). mT1R2-hT1R3 did not respond to sweeteners, including aspartame and d-Trp, as Xu et al. (21) reported. These results suggest that there is a human-specific region in hT1R2 that is necessary for receiving MCL. Next, we designed human/mouse chimeric T1R2 receptors: h/h/m T1R2, h/m/m T1R2, and m/m/h T1R2 (Fig. 5C). The receptor h/h/m T1R2 is composed of hT1R2 ATD and CRD (ATD: 1–494 aa, corresponding to 1–498 aa in mT1R2 numbering; CRD: 495–564 aa, corresponding to 499–568 aa in mT1R2 numbering), and mT1R2 TMD (569–843 aa, corresponding to 565–839 aa in hT1R2 numbering). Because the cells expressing each chimeric T1R2 and hT1R3 did not respond well in general (Fig. S5), we evaluated the function of the chimeric T1R2 by expressing it in conjunction with mT1R3 (Fig. 5C). The cells expressing h/h/m T1R2 and mT1R3, and h/m/m T1R2 and mT1R3 responded to acidic buffer after MCL preincubation. However, although the cells expressing m/m/h T1R2 and mT1R3 responded to d-Trp, they did not respond to MCL (Fig. 5C). These results show that the ATD of hT1R2 is required for hT1R2-hT1R3 responsiveness to MCL.
Fig. 5.
The ATD of hT1R2 is required for hT1R2-hT1R3 to respond to MCL. (A) Representative ratiometric images of MCL (100 nM)-preincubated cells that were transfected with four different combinations of human and mouse T1R2-T1R3 together with G15Gi3 in response to acidic buffer (pH 5.0). The Upper and Lower rows show the representative cell images obtained 2 and 30 s after acidic buffer application, respectively. The color scale indicates the F340/F380 ratio as a pseudocolor. (Scale bar, 50 μm.) (B) Responses of the cells expressing hT1R2-hT1R3, mT1R2-mT1R3, hT1R2-mT1R3, and mT1R2-hT1R3, together with G15Gi3. Cells expressing mT1R2-hT1R3 did not respond to any sweetener. Without MCL preincubation, few responses were observed after acidic buffer application when each combination of T1R2-T1R3 was expressed [see buffer (pH 5.0)]. Data are expressed as the mean ± SE (n = 3) numbers of responding cells. (C) Responses of the cells expressing each human/mouse chimeric T1R2 together with mT1R3 and G15Gi3. Data are expressed as the mean ± SE (n = 3) numbers of responding cells.
To further identify the region required to respond to MCL within ATD, we performed a docking simulation between MCL and hT1R2-hT1R3 models. Based on the docking models, we further constructed human/mouse chimeric T1R2s, in which parts of hT1R2 ATD were replaced with the corresponding parts of mT1R2 (Fig. 6A). We found the chimera with mouse-type residues in its N terminal 378 aa (1–382 aa in mT1R2 numbering) retained the ability to respond to d-Trp and MCL. The chimera carrying the mouse-type residues in 398 to 447 aa (402–451 aa in mT1R2 numbering) severely lost response to both the ligands. In contrast, the replacement of 448 to 494 aa (452–498 aa in mT1R2 numbering) significantly reduced the ability to respond to MCL with keeping the response to d-Trp (Fig. 6A). Consequently, the 448 to 494 aa in hT1R2 ATD are required to respond to MCL. This result suggests that MCL binds to T1R2 at this region. Fig. 6B shows a docking model in which the intermolecular interactions occur at this region. Although the interface on hT1R2 probably extends outside of this region and some conserved residues between human and mouse may be involved in the binding and the activation, the MCL binding site is clearly different from the hinge region of hT1R2 ATD, to where representative low molecular-weight sweeteners are bound (12).
Fig. 6.
The 448 to 494 aa residues are required for hT1R2-hT1R3 to respond to MCL. (A) Responses of the cells expressing each of hT1R2(m.1–382), hT1R2(m.402–451), and hT1R2(m.452–498) together with mT1R3 and G15Gi3. Data are expressed as the mean ± SE (n = 3) numbers of responding cells. (B) Ribbon representation of a docking model in which the intermolecular interactions occur at the region of 448 to 494 aa of hT1R2. MCL, T1R2, and T1R3 are colored green, gray, and yellow, respectively. The residues that are different between human and mouse in this region are colored red. Their side-chain atoms are shown with stick model. Carbon, nitrogen, and oxygen atoms are colored gray, blue, and red, respectively.
Discussion
The perception of MCL at the neural level in both peripheral and central nervous systems has been previously reported. Electrophysiological experiments showed that nerve fibers responding to sweeteners are also activated, in addition to acid-responding fibers in monkey (22) and chimpanzee (23) chorda tympani nerves after treating their tongues with acidic solution after MCL preincubation; however, this is not observed in rodents (20). In humans, a magnetoencephalography study showed that the response latency and the across-region response pattern of the cerebral cortex with citric acid application after tasting MCL are very similar to those with sucrose application. This finding suggests that the sourness component of citric acid is greatly diminished at the level of subcortical relays, and mainly sweetness information reaches the cortical primary taste area (24). However, the mechanism of MCL interaction with taste receptors remains unknown. To our knowledge, the present study is unique in elucidating the relationship between MCL and the human sweet taste receptor.
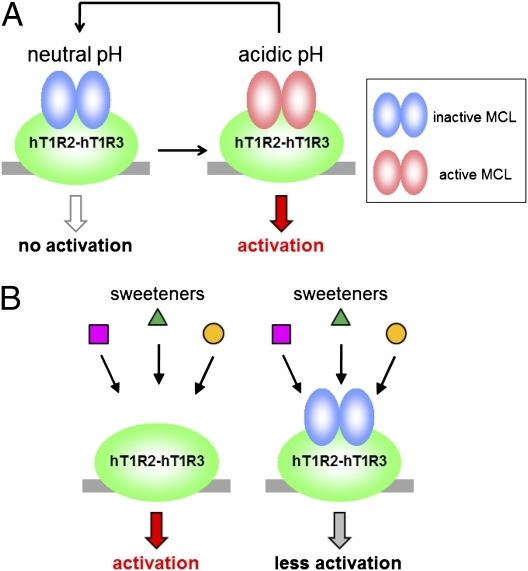
We previously proposed that NCL is in equilibrium between its active and inactive forms and that the taste-modifying activity of NCL is caused by this equilibrium (9). In our assay, MCL activated hT1R2-hT1R3 in a pH-dependent manner at acidic pH (Fig. 1C) and repetitive activation was observed by stimulation with acidic buffer (Fig. 3). Moreover, MCL preincubation led to the decrease of other sweetener-induced hT1R2-hT1R3 activation at neutral pH (Fig. 4). Taking these results into consideration, MCL is also likely to be in equilibrium between being an agonist and an antagonist at acidic and neutral pH, respectively. Because we also confirmed that MCL preincubation hardly affected the form of hT1R protein expressed in cultured cells, such as deglycosylation or proteolysis judging from the result of Western blotting (Fig. S6), we propose the following as most probable molecular mechanisms that were suitable for our experimental results. Once humans taste MCL, it is held on hT1R2-hT1R3 and the bound MCL subsequently becomes an agonist every time a sour solution is tasted (Fig. 7A). MCL becomes an antagonist when the pH reverts to neutral and inhibits the activation of the receptor by other sweeteners (Fig. 7B). Our previous study has shown that replacement of the histidine residues in MCL, His30 and His60, with alanine abolishes its taste-modifying activity (11). Because histidines are protonated below their pKa values (about 6.0), our result strongly indicates that the protonations of these histidine residues are a key step for MCL to act as an hT1R2-hT1R3 agonist. Considering that MCL presumably stays bound to the receptor at neutral pH after acidic pH stimulation and can reactivate it (Figs. 3 and 7), the protonations probably do not affect MCL's affinity to the receptor largely, but changes MCL's conformation so that the receptor adopts the active conformation. Although there is no amino acid sequence similarity and no similar functional motifs between MCL and NCL, it is hypothesized that their taste-modifying activities are both caused by their pH-dependent equilibrium between being agonists and antagonists of hT1R2-hT1R3.
Fig. 7.
Possible model for the taste-modifying activity of MCL. (A) MCL is held on hT1R2-hT1R3 at neutral pH (Left) and activates it under acidic pH (Right). When the pH reverts to neutral, MCL turns into its inactive form on the receptor (Left). (B) A variety of sweeteners activate hT1R2-hT1R3 (Left), but MCL inhibits this activation at neutral pH (Right).
However, there are two differences between the actions of MCL and NCL. First, although our previous study shows that NCL activates the receptor in a pH-dependent manner between 4.7 and 7.6 (9), MCL did not activate hT1R2-hT1R3 at pH values higher than 6.5 (Fig. 1C). These results suggest that there is a pH shift in agonist-antagonist equilibrium between these two taste-modifying proteins. In sensory tests, MCL does not taste sweet by itself, unlike NCL; furthermore, MCL does not make water sweet but NCL does (25). The shift observed in in vitro assays could correspond to the difference in sweetness of MCL and NCL at neutral pH. Second, MCL and NCL need different regions of hT1R2-hT1R3 for activation. Here, we demonstrated that the ATD of hT1R2 is required for the responsiveness to MCL (Fig. 5), but the ATD of hT1R3 is required for the responsiveness to NCL (26). This finding suggests that these two proteins require different specific sites on hT1R2-hT1R3, despite the fact that they have the same taste-modifying activity.
MCL suppressed the activation of hT1R2-hT1R3 induced by all of the other sweeteners tested (Fig. 4). These sweeteners are known to interact with different regions of hT1R2-hT1R3: aspartame and d-Trp interact with hT1R2 ATD, NCL interacts with hT1R3 ATD, brazzein interacts with hT1R3 CRD, and cyclamate and neohesperidin dihydrochalcone (NHDC) interact with hT1R3 TMD (12, 26). It is to be noted that the region required for the response to MCL, the 448 to 494 aa in hT1R2 ATD is clearly different from the hinge regions of ATD that binds low-molecular sweeteners (Fig. 6). In addition, the dose-dependent inhibition curve was observed for NCL, aspartame, and cyclamate and IC50 values (Fig. 4 B–D) were similar to EC50 values at pH 5.0 (Fig. 2). Taking these results into consideration, it is not likely that MCL acts as a competitive antagonist for the sweeteners, but that MCL acts as an noncompetitive antagonist. It is known that lactisole, which interacts with hT1R3 TMD, is an inverse agonist and blocks the activation of hT1R2-hT1R3 induced by all of the sweeteners (27). Although the degree of suppression is weaker than lactisole, MCL at neutral pH inhibited the receptor activation. Thus, MCL would be a partial inverse agonist of hT1R2-hT1R3. It is intriguing that MCL, which requires hT1R2 ATD for response, inhibits the activation of hT1R2-hT1R3 induced by other sweeteners.
In addition, it is interesting that MCL acts as PAM at pH 6.5 (Fig. 4E). Although MCL negatively controlled the activation of human sweet taste receptor at pH 7.4 (Fig. 4A), it potentiated the receptor activation by working as its agonist at pH 6.5. PAMs have already been known for some members of class C GPCRs, such as GABAB receptor, calcium-sensing receptor, and several subtypes of the mGluR family (28). Those substances do not activate the receptor on its own, whereas they enhance the activation of the receptor in the presence of those agonists. As for taste receptors, PAMs for the human sweet taste receptor (SE-1, -2, and -3) have also been identified recently, which enhance the sweet taste of sucralose and/or sucrose (29). Moreover, the umami taste intensity of glutamate is significantly enhanced by purine ribonucleotides, such as inosine-5′-monophosphate and guanosine-5′-monophosphate through the umami taste receptor, T1R1-T1R3 (5, 30). In contrast to PAMs for many GPCRs, which in general bind to the TMD (28), these taste enhancers have been found to bind adjacent to the agonists in the hinge region of the ATD, and they further stabilize the closed and active conformation of the receptor by coordinating the positively charged amino acid residues via their negatively charged moieties (31, 32). However, there has never been report on a protein that acts as PAM, and this article is unique in reporting on the presence of any protein to act as PAM for GPCRs. Because MCL bind not to the TMD but to the nonhinge region of the ATD of the receptor (Fig. 6), there may be another allosteric modulating mechanism not seen in the other class C GPCRs. Elucidation of this mechanism will provide new insights into the activation/inhibition mechanisms of class C GPCRs in general.
Materials and Methods
Tastants.
MCL and NCL were isolated from the fruits of R. dulcifica and Curculigo latifolia, respectively, and purified as described previously (7, 11). Recombinant brazzein has been expressed using the yeast Pichia pastoris. The recombinant protein was secreted into the extracellular medium using the α-factor preprosequence peptide of Saccharomyces cerevisiae and purified using ion-exchange chromatography. Their purities were found to be more than 95% by SDS/PAGE. Other sweeteners were purchased from Wako Pure Chemical Industries, Ltd. or Sigma-Aldrich.
Construction of Expression Plasmids for Human and Mouse Sweet Taste Receptors.
The hT1R2 (GenBank accession no. BK000151) and hT1R3 (BK000152) were prepared as reported previously (26). mT1R2 (NM_031873) and mT1R3 (NM_031872) were subcloned into the pEAK10 expression vector (Edge Biosystems).
Construction of Human/Mouse Chimeric Receptors.
Human/mouse T1R2 chimeras were constructed by PCR using overlapping primers. All chimeras were subcloned into the pEAK10 expression vector at the AscI-NotI site, and all clones were verified by sequencing.
Calcium Imaging.
cDNAs of T1R2, T1R3, and G15Gi3 were transfected into HEK293T cells together with DsRed2 and transfected cells were used for calcium imaging, as previously described (33). For MCL assay, MCL was added to the cells when fura-2 AM was loaded. After rinsing and incubation in 100 μL of assay buffer for more than 10 min at room temperature, cells were challenged by addition of 100 μL of acidic buffer for adjusting to objective pH. The pH values after stimulation are indicated in the text and figures. The pH was adjusted with 500 mM citric acid. For the perfusion assay, the cells were transferred onto a glass-bottom dish (AGC Techno Glass Co.) 6 h after transfection. After 40 h, the cells were loaded with 5 μM fura-2 AM and 100 nM MCL or 10 μM NCL, and incubated for 30 min at 37 °C. After rinsing and incubation in the assay buffer for 10 min, the cells were subsequently perfused under gravity at a flow rate of 8 mL/min and stimulated with the application of acidic buffer (pH 5.0) or 10 mM aspartame. Each solution was applied to the cells for a period of 16 s.
Fluorescence images were recorded at 3- or 4-s intervals and analyzed using MetaFluor software (Molecular Devices). Cells were regarded as responsive when the increase in the F340/F380 was above 0.15. The result was quantitatively represented as the count of the responding cells among ∼1,000 cells observed in the microscopic field.
Measurement of Cellular Responses with Cell-Based Assays.
The hT1R2-hT1R3 stable cell lines (16) were trypsinized and seeded at a density of 80,000 cells per well into 96-well black-wall CellBIND surface plates (Corning). Twenty-four hours later, they were washed with assay buffer before loading with a calcium indicator dye from the FLIPR Calcium 4 Assay Kit (Molecular Devices) diluted with assay buffer in the absence or presence of MCL (30 nM). The cells were incubated for 45 min at 27 °C, and measurements were made using FlexStation 3 (Molecular Devices). A 100-mL aliquot of assay buffer supplemented with 2× ligands was added at 20 s, and scanning continued for an additional 100 s. Dose–response curves were fitted using Hill's equations.
Docking Simulation.
Docking was performed for all possible pairs of two MCL models (11) and four human T1R2-T1R3 models (10) using the ZDOCK program (34). Two-thousand models were generated for each pair. Models with large interaction surface areas were used in the analysis to define the possible interaction sites on the surface of T1R2.
Supplementary Material
Acknowledgments
This study was supported in part by a grant from the Research and Development Program for New Bio-industry Initiatives of the Bio-oriented Technology Research Advancement Institution; the Japan Society for the Promotion of Science Research Fellowship for Young Scientists (to A.K.); Grants-in-Aid for Scientific Research 21880015 (to K.-i.N.), 19300248 (to T.A.), 20688015 and 21658046 (to T.M.), and 20380183 (to K.A.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; and Funding Program for Next Generation World-Leading Researchers from the Japan Society for the Promotion of Science Grant LS037 (to T.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016644108/-/DCSupplemental.
References
- 1.Brouwer JN, van der Wel H, Francke A, Henning GJ. Mieraculin, the sweetness-inducing protein from miracle fruit. Nature. 1968;220:373–374. doi: 10.1038/220373a0. [DOI] [PubMed] [Google Scholar]
- 2.Kurihara K, Beidler LM. Taste-modifying protein from miracle fruit. Science. 1968;161:1241–1243. doi: 10.1126/science.161.3847.1241. [DOI] [PubMed] [Google Scholar]
- 3.Theerasilp S, et al. Complete amino acid sequence and structure characterization of the taste-modifying protein, miraculin. J Biol Chem. 1989;264:6655–6659. [PubMed] [Google Scholar]
- 4.Kurihara K, Beidler LM. Mechanism of the action of taste-modifying protein. Nature. 1969;222:1176–1179. doi: 10.1038/2221176a0. [DOI] [PubMed] [Google Scholar]
- 5.Li X, et al. Human receptors for sweet and umami taste. Proc Natl Acad Sci USA. 2002;99:4692–4696. doi: 10.1073/pnas.072090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson G, et al. Mammalian sweet taste receptors. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- 7.Shirasuka Y, et al. Neoculin as a new taste-modifying protein occurring in the fruit of Curculigo latifolia. Biosci Biotechnol Biochem. 2004;68:1403–1407. doi: 10.1271/bbb.68.1403. [DOI] [PubMed] [Google Scholar]
- 8.Yamashita H, Akabane T, Kurihara Y. Activity and stability of a new sweet protein with taste-modifying action, curculin. Chem Senses. 1995;20:239–243. doi: 10.1093/chemse/20.2.239. [DOI] [PubMed] [Google Scholar]
- 9.Nakajima K, et al. Acid-induced sweetness of neoculin is ascribed to its pH-dependent agonistic-antagonistic interaction with human sweet taste receptor. FASEB J. 2008;22:2323–2330. doi: 10.1096/fj.07-100289. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu-Ibuka A, et al. Crystal structure of neoculin: Insights into its sweetness and taste-modifying activity. J Mol Biol. 2006;359(1):148–158. doi: 10.1016/j.jmb.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 11.Ito K, et al. Microbial production of sensory-active miraculin. Biochem Biophys Res Commun. 2007;360:407–411. doi: 10.1016/j.bbrc.2007.06.064. [DOI] [PubMed] [Google Scholar]
- 12.Behrens M, Meyerhof W, Hellfritsch C, Hofmann T. Sweet and umami taste: Natural products, their chemosensory targets, and beyond. Angew Chem Int Ed Engl. 2011;50:2220–2242. doi: 10.1002/anie.201002094. [DOI] [PubMed] [Google Scholar]
- 13.Kurihara Y, Terasaki S. Isolation and chemical properties of multiple active principles from miracle fruit. Biochim Biophys Acta. 1982;719:444–449. [Google Scholar]
- 14.Li X, Servant G. Functional characterization of the human sweet taste receptor: High-throughput screening assay development and structural function relation. ACS Symposium Series. 2008;979:368–385. [Google Scholar]
- 15.Nakajima K, et al. Neoculin, a taste-modifying protein, is recognized by human sweet taste receptor. Neuroreport. 2006;17:1241–1244. doi: 10.1097/01.wnr.0000230513.01339.3b. [DOI] [PubMed] [Google Scholar]
- 16.Imada T, et al. Amiloride reduces the sweet taste intensity by inhibiting the human sweet taste receptor. Biochem Biophys Res Commun. 2010;397:220–225. doi: 10.1016/j.bbrc.2010.05.088. [DOI] [PubMed] [Google Scholar]
- 17.Hellekant G. On the gustatory effects of gymnemic acid and miraculin in dog, pig and rabbit. Chem Senses. 1976;2(1):85–95. [Google Scholar]
- 18.Hellekant G, Glaser D, Brouwer JN, van der Wel H. Gustatory effects of miraculin, monellin and thaumatin in the Saguinus midas tamarin monkey studied with electrophysiological and behavioural techniques. Acta Physiol Scand. 1976;97:241–250. doi: 10.1111/j.1748-1716.1976.tb10257.x. [DOI] [PubMed] [Google Scholar]
- 19.Hellekant G, Hagstrom EC, Kasahara Y, Zotterma Y. On the gustatory effects of miraculin and gymnemic acid in monkey. Chem Senses. 1974;1(2):137–145. [Google Scholar]
- 20.Diamant H, Hellekant G, Zotterman Y. The effect of miraculin on the taste buds of man, monkey and rat. In: Schneider D, editor. Olfaction and Taste IV. Germany: Wissenschaftliche Verlagsgesellschaft MBH; 1972. pp. 241–244. [Google Scholar]
- 21.Xu H, et al. Different functional roles of T1R subunits in the heteromeric taste receptors. Proc Natl Acad Sci USA. 2004;101:14258–14263. doi: 10.1073/pnas.0404384101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brouwer JN, et al. The sweetness-inducing effect of miraculin; behavioural and neurophysiological experiments in the rhesus monkey Macaca mulatta. J Physiol. 1983;337:221–240. doi: 10.1113/jphysiol.1983.sp014621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hellekant G, Ninomiya Y, Danilova V. Taste in chimpanzees. III: Labeled-line coding in sweet taste. Physiol Behav. 1998;65:191–200. doi: 10.1016/s0031-9384(97)00532-5. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto C, et al. Cortical representation of taste-modifying action of miracle fruit in humans. Neuroimage. 2006;33:1145–1151. doi: 10.1016/j.neuroimage.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Kurihara Y. Characteristics of antisweet substances, sweet proteins, and sweetness-inducing proteins. Crit Rev Food Sci Nutr. 1992;32:231–252. doi: 10.1080/10408399209527598. [DOI] [PubMed] [Google Scholar]
- 26.Koizumi A, et al. Taste-modifying sweet protein, neoculin, is received at human T1R3 amino terminal domain. Biochem Biophys Res Commun. 2007;358:585–589. doi: 10.1016/j.bbrc.2007.04.171. [DOI] [PubMed] [Google Scholar]
- 27.Galindo-Cuspinera V, Winnig M, Bufe B, Meyerhof W, Breslin PA. A TAS1R receptor-based explanation of sweet ‘water-taste’. Nature. 2006;441:354–357. doi: 10.1038/nature04765. [DOI] [PubMed] [Google Scholar]
- 28.Urwyler S. Allosteric modulation of family C G-protein-coupled receptors: From molecular insights to therapeutic perspectives. Pharmacol Rev. 2011;63(1):59–126. doi: 10.1124/pr.109.002501. [DOI] [PubMed] [Google Scholar]
- 29.Servant G, et al. Positive allosteric modulators of the human sweet taste receptor enhance sweet taste. Proc Natl Acad Sci USA. 2010;107:4746–4751. doi: 10.1073/pnas.0911670107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson G, et al. An amino-acid taste receptor. Nature. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- 31.Zhang F, et al. Molecular mechanism of the sweet taste enhancers. Proc Natl Acad Sci USA. 2010;107:4752–4757. doi: 10.1073/pnas.0911660107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang F, et al. Molecular mechanism for the umami taste synergism. Proc Natl Acad Sci USA. 2008;105:20930–20934. doi: 10.1073/pnas.0810174106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakajima K, et al. Identification and modulation of the key amino acid residue responsible for the pH sensitivity of neoculin, a taste-modifying protein. PLoS ONE. 2011;6:e19448. doi: 10.1371/journal.pone.0019448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mintseris J, et al. Integrating statistical pair potentials into protein complex prediction. Proteins. 2007;69:511–520. doi: 10.1002/prot.21502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.