Abstract
Increasing evidence suggests that regulatory T cell (Treg) function is impaired in chronic inflammatory diseases such as rheumatoid arthritis (RA). Here we demonstrate that Tregs are unable to modulate the spontaneous production of TNF-α from RA synovial cells cultured from the diseased synovium site. Cytokine (IL-2, IL-6, TNF-α) activated T cells (Tck), cells we previously demonstrated to mimic the effector function of pathogenic RA synovial T cells, contained Tregs that survived and divided in this cytokine environment; however, the up-regulation of key molecules associated with Treg function (CTLA-4 and LFA-1) was impaired. Furthermore, Tregs were unable to suppress the function of Tcks, including contact-dependent induction of TNF-α from macrophages, supporting the concept that impaired Treg function/responsiveness contributes to chronicity of RA. However, ectopic foxp3 expression in both Tcks and pathogenic RA synovial T cells attenuated their cytokine production and function, including contact-dependent activation of macrophages. This diminished response to cytokine activation after ectopic foxp3 expression involved inhibited NF-κB activity and differed mechanistically from that displayed endogenously in conventional Tregs. These results suggest that diseases such as RA may perpetuate owing to the inability of Tregs to control cytokine-activated T-cell function. Understanding the mechanism whereby foxp3 attenuates the pathogenic function of synovial T cells may provide insight into the mechanisms of chronicity in inflammatory disease and potentially reveal new therapeutic candidates.
Keywords: immunoregulation, autoimmunity
Rheumatoid arthritis (RA) is a chronic autoimmune inflammatory disease affecting ≈1% of the world's population. The genetic predisposition to RA is dominated by the major histocompatibility complex HLA-DR, referred to as the “shared epitope” (1), and because the function of HLA-DR is to present peptides to CD4+ T cells, a role for antigen-activated T cells in at least the initiation of RA is implied. However, despite the abundance of T cells in the active synovium [approximately 20% of total mononuclear cells (MNC)], analysis of T-cell receptor (TCR) sequences has not shown dominant clones (2), and autoantigen reactive T cells have been elusive (3). T cells can also be activated by cytokines released by other leukocytes (e.g., IL-2, IL-6, and TNF-α). This leads to transcription of cytokine genes, up-regulation of cell surface activation markers, and acquisition of the ability to induce Ig production from B cells (4) and induce monocytes, macrophages, and synovial fibroblasts to produce proinflammatory cytokines (5–7). Because synovial T cells are predominantly of the CD4+ effector memory phenotype, the population most responsive to antigen-independent activation (4, 8–10), yet are paradoxically hyporesponsive to TCR stimulation, we hypothesized that “bystander-activated” T cells may be important in the perpetuation of RA inflammation. Indeed, we previously reported that cytokine (IL-2, IL-6, TNF-α) activated “bystander” T cells (abbreviated Tck) induce signaling pathways in monocytes that were similar to those induced by RA synovial T cells but contrasted with those activated by antigen-activated T cells (6, 11). Thus, the majority of T cells isolated from a chronic active RA synovium resemble Tcks. Alternatively, inflammatory processes within the RA synovium can be modeled by culturing the synovial MNCs ex vivo, and in these cultures TNF-α acts as a “master regulator,” controlling the production of other proinflammatory cytokines, including IL-6 and IL-1β (12, 13). This finding was pivotal for first suggesting TNF-α as a therapeutic target, helped establish TNF-α blockade as part of the mainstream therapy of RA, and thus has been validated as a predictive model of RA (14).
There has also been much interest in examining the function and therapeutic potential of Tregs in RA. Although foxp3+ Tregs are present at equivalent proportions in RA patients and are enriched within the synovium (15, 16), several reports have indicated that proinflammatory cytokines (including TNF-α) attenuate Treg suppressiveness (17–21). Therefore, we determined whether Tregs were capable of suppressing the spontaneous production of TNF-α from RA synovial MNCs in these cultures. Moreover, we investigated whether Tregs were present and functional in populations of Tck cells. We hypothesized that if Tck are the critical phenotype of T cells responsible for perpetuating inflammation in the joint, then they might not be suppressible by Tregs. Although we found that indeed both RA synovial T-cell and Tck effector function were not “suppressible” by Tregs, we did observe that ectopic foxp3 expression delivered by lentiviral transduction directly into these pathogenic effector cells was effective in diminishing their function.
Results
RA MNC Production of TNF-α Is Not Modulated by Tregs.
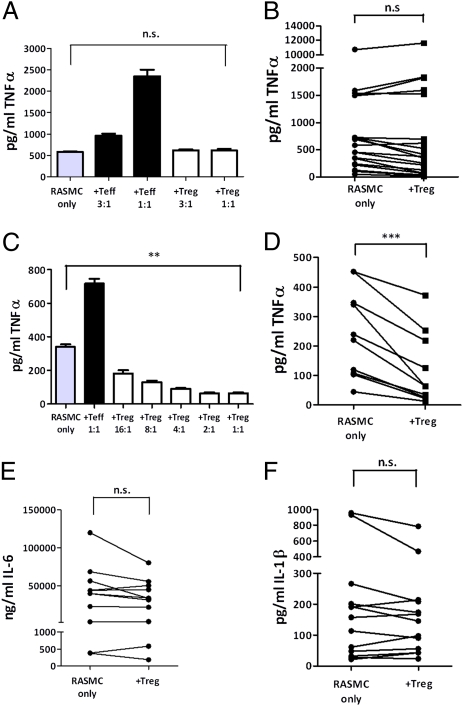
Peripheral blood Tregs did not suppress the spontaneous production of TNF-α, IL-6, or IL-1β from the majority of ex vivo RA synovial MNC cultures (Fig. 1 A–D), despite being able to suppress the proliferation of allogenic peripheral blood T effector cells (Teffs) in conventional Treg suppressor assays (Fig. S1). In keeping with several studies, we were not able to detect IFN-γ within these cultures (22, 23). However, in those RA MNC cultures (Fig. 1 C and D) in which production of TNF-α was “modest” (<500 pg/mL), Tregs were able to suppress TNF-α production by ≈46%. Furthermore, in these RA MNC cultures, we also observed a down-regulation in the expression of HLA-DR and CD86 on the synovial macrophages in all three donors tested, whereas expression of CD80 was unaffected (Fig. S1). In all experiments the addition of Teffs enhanced the production of TNF-α by RA synovial MNC cultures in the presence of anti-CD3 (Fig. 1 A and C).
Fig. 1.
RA MNC cytokine production is not modulated by Tregs. CD4+CD25+ blood Tregs from healthy donors were cocultured with RA synovial MNCs with soluble anti-CD3, and supernatants were harvested at 24 h for TNF-α by ELISA. A representative titratable experiment (A) and pooled data (B) (n = 19) are shown; and where RA MNCs spontaneously produced <500 pg/mL of TNF-α a representative titratable experiment (C) and pooled data (D) (n = 9) are illustrated. Similarly IL-6 (E) and IL-1β (F) levels were determined in RA MNC cultures with or without CD4+CD25+ Tregs. IFN-γ was not detectable in supernatants/cell lysates. **P < 0.01, ***P < 0.001. n.s., not significant.
Tregs Are Sustained in Cytokine-Activated Populations.
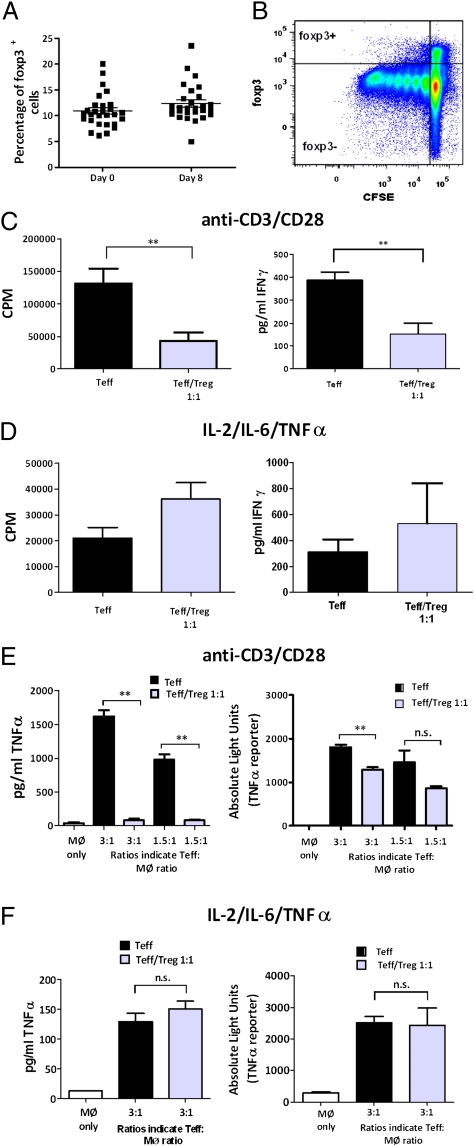
The proportion of Tregs in Tck cultures was determined by assessing the percentage of CD25+foxp3+ cells within the CD4+CD45RO+ population. Although cytokine stimulation up-regulated foxp3 in Teffs (Fig. S2A), the foxp3hi Treg subset remained a discernible population that was unable to produce IFN-γ or IL-2 after phorbol 12-myrisate 13-acetate (PMA)/ionomycin stimulation (Fig. S2 B and C). Treg proportions in day-8 Tck cultures were similar to those measured in freshly isolated CD4+CD45RO+ cells (Fig. 2A), although they divided significantly less than the foxp3− cells in these cultures (Fig. 2B).
Fig. 2.
Tregs do not suppress Teffs in cytokine-activated cultures. (A) Proportion of cells expressing foxp3 (mean ± SEM; n = 28) was determined before and after (8 d) stimulation with the Tck cytokine mixture by flow cytometry. (B) Carboxyfluorescein succinimidyl ester (CFSE) content of foxp3+ and foxp3− populations of CD4+CD45RO+ cells at day 8 (n = 3). CD4+CD45RO+CD25− (Teff) cells were cultured in the presence (gray bars) or absence (black bars) of CD4+CD45RO+CD25+ (Treg) cells at a 1:1 ratio and stimulated with either (C) anti-CD3 (1 μg/mL)/anti-CD28 (2.5 μg/mL) or (D) the Tck cytokine mixture. Proliferation was assessed by 3H-thymidine incorporation at 102–120 h. IFN-γ production was determined after 120 h (anti-CD3/anti-CD28) or 8 d (Tck cytokine mixture) (n > 3). Cells were stimulated for (E) 3 d with anti-CD3/anti-CD28 or (F) 8 d with Tck cytokine mixture, then cocultured for 8 h with autologous macrophages transduced with a TNF-α–luciferase reporter. Representative experiments of TNF-α levels (ELISA) and expression of TNF-α reporter are shown. **P < 0.01. n.s., not significant.
Treg Do Not Suppress Cytokine-Activated Teff Function.
To investigate whether Tregs inhibited Tck effector function, Teffs were cultured with and without Tregs at a 1:1 ratio (Fig. 2). Tregs suppressed the proliferation and IFN-γ production of Teffs when cells were stimulated with anti-CD3/anti-CD28 (P < 0.01) (Fig. 2C) but not when the Teffs were stimulated with IL-2/IL-6/TNF-α (Fig. 2D). Similarly, Tregs potently suppressed both the expression of a TNF-α–luciferase reporter gene and TNF-α protein production in Teff:macrophage cocultures when the Teff were stimulated using anti-CD3/anti-CD28 (P < 0.01) (Fig. 2E) but not when Teffs were stimulated with IL-2/IL-6/TNF-α (Fig. 2F). Using the TNF-α–luciferase reporter transduced into macrophages confirmed that Tregs modulate TNF-α production from macrophages in these cocultures (Figs. 2 E and F). In conventional (antigen-dependent) Treg assays, suppressor function is not induced unless the Tregs can respond to the cognate antigen (i.e., they require activation). Thus, our data may infer that the cytokine mixture is insufficient to induce Treg activation. However, it is also possible that cytokine-activated Teffs may be resistant to suppression. To determine whether Tregs could suppress cytokine-activated cells in the presence of TCR stimulation, we cocultured Tregs with Teffs in the presence of IL-2, IL-6, TNF-α, or a combination thereof (Fig. S3). As previously reported by others, IL-2 was found to abrogate Treg-mediated suppression (24). However, neither TNF-α nor IL-6 affected the ability of Tregs to suppress the proliferation of Teffs. Although TNF-α has been reported to abrogate Treg-mediated suppression (21), our results are in line with others using this concentration (25 ng/mL) of TNF-α (25). Although IL-6 has been implicated in rendering Teffs resistant to Treg-mediated suppression (19), it has subsequently been suggested that additional cytokines may be needed in combination with IL-6 to induce this effect (26–28).
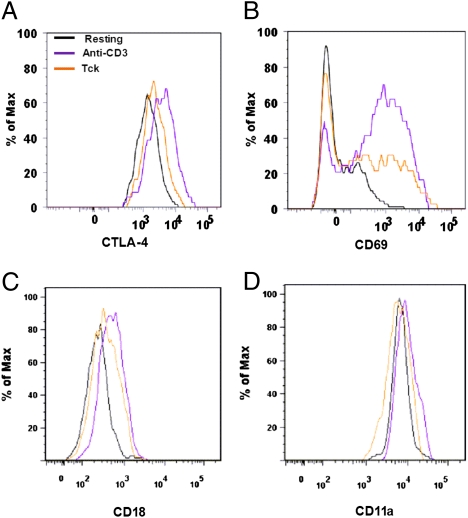
Cytokine-Activated Tregs Do Not Up-Regulate CTLA-4 and LFA-1.
Fig. 3 illustrates the molecules associated with Treg activity (29, 30) after stimulation with either anti-CD3 or the cytokine mixture with analysis restricted to the foxp3hi subset of activated cells (Fig. S2). CTLA-4, LFA-1 (CD11a, CD18), and CD69 (a marker of T-cell activation) were up-regulated on Tregs after activation with anti-CD3 but not IL-2/IL-6/TNF-α (Fig. 3 A–D). This is in contrast to Teffs, which up-regulated CTLA-4 and LFA-1 to a similar extent with either anti-CD3 or the cytokine mixture (Table S1). Therefore, because CTLA-4 and LFA-1 are critical for Treg function (29–31), failure to up-regulate LFA-1 and CTLA-4 may contribute to the compromised suppressive function of Tregs in the RA synovium. Indeed, restoration of CTLA-4 expression has been shown to reverse the defect in function reported in Tregs derived from RA patients (32). These results indicate that cytokine stimulation is sufficient to activate Teffs but not Tregs and thus is likely to contribute to the lack of Treg function observed in Tck cultures (Fig. 2 D and F).
Fig. 3.
Cytokine stimulation does not induce fully activated Treg phenotype. CD4+CD45RO+ cells were isolated from PBLs by negative selection (5) and activated with either anti-CD3 for 48 h or Tck cytokine mixture for 8 d and expression of intracellular CTLA-4 (A), CD69 (B), CD18 (LFA-1 β-chain) (C), and CD11a (LFA-1 α-chain) (D) on foxp3hi gated cells determined by flow cytometry (n = 4).
Pathogenic Effector Function of CD4+ Cells Is Attenuated by Ectopic foxp3 Expression.
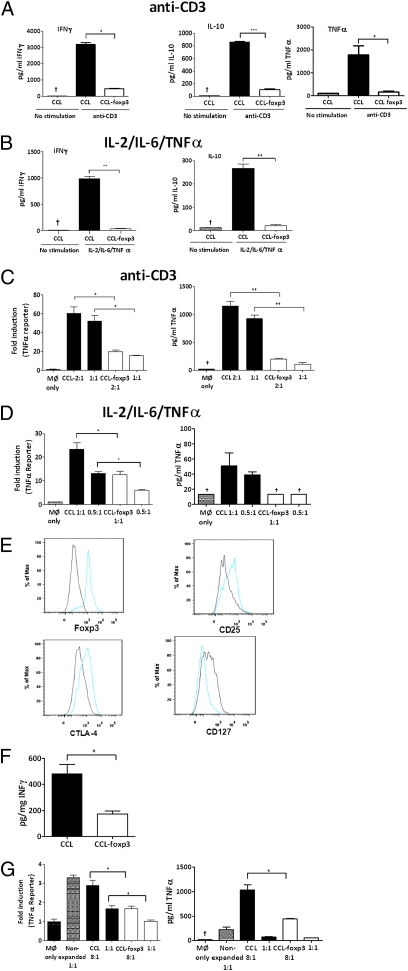
We next investigated whether ectopic foxp3 [using foxp3-expressing lentivirus previously used to generate suppressive foxp3+ cells (33)] transduced directly into RA CD4+ lymphocytes or the CD4+ T cells used to generate Tck cells could modulate their proinflammatory nature. Ectopic foxp3 expression (Fig. S4 A and B) induced the up-regulation of CD25, CTLA-4, and the down-regulation of CD127 (Fig. S4C) and attenuated cytokine production after stimulation with either anti-CD3 (Fig. 4A) or IL-2/IL-6/TNF-α (Fig. 4B). Furthermore, ectopic foxp3 expression in T cells resulted in attenuated TNF-α expression in Teff:macrophage cocultures, where transduced T cells were stimulated with either anti-CD3 (Fig. 4C) or IL-2/IL-6/TNF-α (Fig. 4D). These results indicate that pathogenic Teff function driven by proinflammatory cytokines at sites of inflammation can be attenuated by ectopic expression of foxp3.
Fig. 4.
Ectopic foxp3 expression attenuates CD4+ effector function. CD4+ lymphocytes isolated from either blood of healthy donors (A–D) or RA synovial MNCs (E–G) were transduced with either pCCL or pCCL-foxp3 lentivirus. NGFR+ cells were restimulated with either anti-CD3 (5 μg/mL) for 24 h or the Tck cytokine mixture for 5 d. RA synovial CD4+ lymphocytes were restimulated with the Tck cytokine mixture. Ratios indicate T cell:macrophage proportions. Representative experiments of >n = 3 are shown and illustrate cytokine production from anti-CD3 activated (A) and cytokine-stimulated (B) T lymphocytes. TNF-α reporter gene and TNF-α protein levels are shown for cocultures with anti-CD3 (C) and cytokine-stimulated (D) T lymphocytes. (E) Phenotype of synovial CD4+ lymphocytes was determined after transduction with pCCL (black) or pCCL-foxp3 (blue) lentivirus. (F) IFN-γ production from RA CD4+ lymphocytes and (G) TNF-α reporter gene activity and protein levels from RA CD4+ lymphocyte:macrophage cocultures after transduction with pCCL-foxp3. *P < 0.05, **P < 0.01, ***P < 0.001, †cytokine concentration below limits of ELISA.
Ectopic foxp3 Expression in RA Synovial T Cells Abrogates Effector Function.
Ectopic foxp3 expression in pathogenic RA synovial CD4+ cells also resulted in up-regulated CD25 and CTLA-4 and down-regulated CD127 (Fig. 4E) and attenuated the production of IFN-γ (P < 0.05) by these cells when they were stimulated with cytokines (Fig. 4F). RA synovial T cells (without further activation) induce the production of TNF-α from resting monocytes in a contact-dependent manner (11). However, ectopic expression of foxp3 in these synovial CD4+ lymphocytes down-modulated both TNF-α luciferase reporter gene expression (P < 0.05) and TNF-α protein production (P < 0.05) in macrophages (Fig. 4G).
Ectopic foxp3 Attenuates NF-κB Activity in Activated CD4+ Cells.
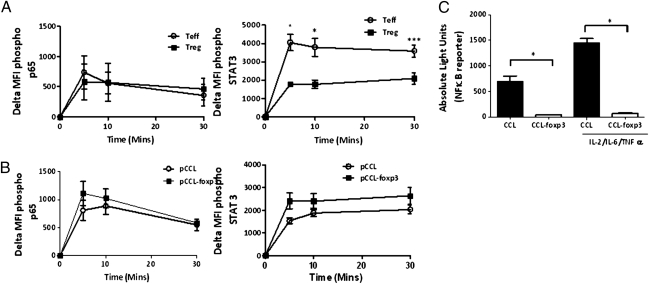
To investigate the mechanism whereby ectopic foxp3 expression resulted in “anergy” of pathogenic CD4+ T cells, cytokine receptor expression and downstream signaling moieties were compared between pCCL-foxp3 and pCCL transduced cells. Ectopic foxp3 expression increased CD25 expression (Fig. S4C) but not the expression of TNF-RII, CD126 (IL-6R α-subunit), or CD130 (IL-6R β-subunit) (Fig. S5A), in contrast to Tregs, which express elevated levels of CD25 and TNF-RII, equivalent amounts of CD126, and less CD130 than Teff counterparts (Fig. S5B) (21, 34, 35). Because TNF-α and IL-6 are known to activate NF-κB–p65 and STAT3, we determined whether foxp3 modulated their phosphorylation after stimulation with the cytokine mixture. p65 phosphorylation kinetics were similar between both Teffs and Tregs (Fig. 5A) and control/foxp3 transduced T cells (Fig. 5B). However, there was a distinct reduction (P < 0.05) in STAT3 phosphorylation in the nTregs compared with Teffs (Fig. 5A), but no difference in the phosphorylation of this substrate was found after ectopic foxp3 overexpression (Fig. 5B). Moreover, ectopic foxp3 expression did not affect the phosphorylation of STAT5 (Fig. S6). These results suggested that foxp3 may act downstream of p65/STAT3/STAT5 to modulate cytokine activation. To investigate this further we used an NF-κB–luciferase reporter and found that ectopic foxp3 expression attenuated this NF-κB reporter gene activity (P < 0.05) both in the resting phase and after cytokine stimulation (Fig. 5C). It has been reported that foxp3 binds to p65 and inhibits NF-κB transcriptional activity in murine cells stimulated with anti-CD3/anti-CD28 (36), which may explain our observations using cytokine-activated cells.
Fig. 5.
Ectopic foxp3 expression attenuates NF-κB transcription. Kinetics of p65 and STAT-3 phosphorylation were determined in Teffs and nTregs (A) and pCCL and pCCL-foxp3 (B) transduced cytokine-stimulated Teffs by flow cytometry (mean ± SEM of > n = 3). (C) CD4+ cells transduced with NF-κB-luciferase reporter lentivirus and pCCL/pCCLfo×p3 were stimulated with the Tck cytokine mixture for 2 h and luciferase activity determined (mean ± SEM, n = 4; *P < 0.05).
Discussion
In this study we addressed two questions. First, can regulatory T cells modulate the effector function of T cells in a chronic inflammatory environment such as RA synovial tissue? Second, could the effector function of these pathogenic T cells be “switched” off by ectopic expression of foxp3, the transcription factor essential for natural Treg function? Although Tregs have received much attention because of their potential therapeutic use in autoimmune diseases, evidence suggests this will only be effective if the Treg population is specific to the relevant antigen (37–41). This is a problem in chronic inflammatory diseases such as RA, for which the autoantigen(s) driving disease perpetuation are not understood. Furthermore, our studies (5, 6, 11, 42) demonstrated that in established RA disease synovial T-cell effector function resembles that of “bystander” or cytokine-activated T cells and not that of T cells stimulated through the TCR. Moreover, although Tregs isolated from the RA synovium are “functional” in vitro, this is counterbalanced by an increased resistance of RA synovial Teffs to Treg-mediated suppression (43). The mechanism responsible is not fully understood, but it has been noted that the proinflammatory cytokines present within the synovial environment (including TNF-α, IL-6, IL-1β, and IL-21) attenuate the suppressive function of Tregs in vitro (18–21). Therefore, the potential for Tregs to modulate ongoing inflammation in RA remains equivocal.
First, we observed that Tregs enriched from the blood of normal donors and subsequently activated through the TCR were not capable of modulating the spontaneous production of TNF-α in the majority of the RA MNC cultures tested. We did, however, observe in the less “active” RA MNC cultures that Tregs induced some inhibition of TNF-α production. The inability of Tregs to modulate TNF-α production in “active” RA synovial MNC cultures maybe be related to the ability of TNF-α itself to attenuate Treg function (21). Interestingly, anti–TNF-α therapy in RA patients enhances Treg function, which may relate to our current observations (25, 44). Tregs were also unable to modulate the effector function of Tcks, the “surrogate model” of RA synovial T cells. This is not surprising given that cytokine activation was not sufficient to activate Tregs, as reflected by impaired up-regulation of key molecules (LFA-1 and CTLA-4) involved in Treg function (29–31). Moreover, Tck cells were clearly resistant to Tregs even in the presence of TCR stimulation.
Taken together, our results have clear implications for the therapeutic potential of Tregs in RA. First, given that Tregs are less amenable to cytokine activation, the capacity of transferred Tregs to be active within the synovium is questionable. Second, because Tregs activated through the TCR were also unable to modulate the most active RA synovial cultures, cotherapies that reduce the inflammatory environment of the synovium may be required for Tregs to be effective. Importantly, however, we noted that although cytokine-stimulated Tregs were not fully functional, they did not acquire a “pathogenic” phenotype, as has been described by others using an alternative combination of proinflammatory cytokines (45). We considered that because foxp3 converts T cells into a Treg-like phenotype in response to TCR activation (33, 46), pathogenic T cells themselves might be rendered inert by constitutive foxp3 expression.
Foxp3-transduced cells have several advantages over Tregs with regard to potential cellular therapy, including less limitation on cell numbers and less risk of conversion to pathogenic effector cells (e.g., TH17) (45, 47, 48). Foxp3-transduced cells have been shown to be an effective treatment in antigen-dependent models of inflammation, including collagen-induced arthritis (37, 49, 50) and nonobese diabetic mice (51). These studies have shown that antigen-specific foxp3-transduced cells are significantly more effective therapeutics than non–antigen-specific populations. However, because the autoantigen(s) in RA and other autoimmune diseases are not well defined, a strategy to generate antigen-specific Tregs is required. One possibility is to cotransduce CD4+foxp3+ lymphocytes with a TCR that recognizes an antigen expressed at the site of inflammation (52); an alternative is to use lymphocytes isolated from the synovium because these cells are likely to be enriched for autoantigen-specific clones. However, the question arises as to whether ectopic foxp3 expression in synovial effector T cells is sufficient to reverse their pathological function. Our studies clearly indicate that ectopic foxp3 expression in human effector T cells isolated from a site of inflammation was indeed sufficient to convert them to a Treg phenotype and abrogate their pathological effector function. Further, this was achieved despite using CD4+ memory lymphocytes, which are predominant in this tissue (53), whereas it is the CD4+CD45RA+ population that is most amenable to adopting Treg function after ectopic foxp3 expression (54). These findings complement the observations made by Andersen et al. (49), who reported that the conversion of Teffs into Tregs via the induction of constitutive foxp3 expression in vivo was more effective than conventional foxp3-transduced T cells in treating collagen-induced arthritis.
Thus, the data presented here document that nTregs are unable to inhibit the proinflammatory cytokine production of rheumatoid synoviocytes, potentially limiting the scope for Treg therapy in this disease. However, pathogenic effector function of RA synovial T lymphocytes was abrogated by ectopic expression of foxp3, opening up the possibility of therapy with ectopic foxp3-transduced cells.
Methods
Reagents.
IL-6 (Novartis Pharmaceutical Ltd., Surrey, UK), IL-2 (National Institutes of Health, Bethesda, MD), and TNF-α (Boehringer Ingelheim Ltd., Berkshire, UK) were gifts. Antibodies for flow cytometry were purchased from BD Pharmingen. Media and sera were tested for endotoxin using the Limulus amebocyte lysate test (Lonza).
Lymphocyte Separation and Stimulation.
Human peripheral blood mononuclear cells (PBMCs) were isolated from plateletphoresis residues (North London Blood Transfusion Service) by Ficoll-Hypaque centrifugation. Peripheral blood lymphocytes (PBLs) and monocytes were isolated by centrifugal elutriation. CD4+CD45RO+ cells were isolated from PBLs by negative selection (5). Tregs were separated from CD4+CD45RO+ cells by FACS sorting (CD25+CD127lo-) or CD25 microbeads (Miltenyi). CD4+ lymphocytes or CD4+CD25+ Tregs were isolated using the Regulatory T Cell Isolation Kit (Miltenyi). T lymphocyte populations were resuspended in RPMI 1650 plus 10% heat-inactivated AB+ human serum (Sigma). Lymphocytes were stimulated with either IL-2 (25 ng/mL), TNF-α (25 ng/mL), and IL-6 (100 ng/mL) or anti-CD3 (OKT3- Insight Biotechnology) and anti-CD28 (BD Pharmingen) as indicated.
Isolation of RA Mononuclear Cells and Rheumatoid CD3+ Lymphocytes.
RA MNCs were obtained as waste tissue from synovial tissue specimens from patients fulfilling the American College of Rheumatology criteria for RA (55) (demographics in Table S2) after joint replacement surgery as approved by the Riverside Research ethics committee (RREC 07/HO7068/81). RA MNCs were extracted as described in ref. 11, and CD3+ cells were isolated using Dynabeads FlowComp Human CD3 Kit (Invitrogen) and fixed (11) for coculture with macrophages or not fixed for transduction with lentivirus, as per PBLs. Four days after lentiviral transduction CD8+ cells were removed from the cultures using CD4 Dynabeads (Invitrogen).
Cocultures of Activated Lymphocytes with Macrophages.
Autologous monocytes were cultured in RPMI 1650 (PAA) plus 5% FCS (Gibco) and 100 ng/mL macrophage colony-stimulating factor (Peprotech) for 2–6 d, dissociated from plates, and cultured overnight in 5% FCS RPMI in a 96-well plate, 1 × 105 per well. Macrophages were transduced at a multiplicity of infection (MOI) of 50 with adenovirus encoding a TNF-α–luciferase reporter as previously described (56). After 8 h coculture with activated lymphocytes, supernatants were collected and luciferase activity determined as previously described (56). For cocultures involving CD4+ cells isolated and fixed with glutaldehyde/glycine from RA patients, nonautologous macrophages derived from healthy donors were used.
Lentiviral Transduction of Human Cells.
Lentiviral particles were generated using the psPAX and pMD2.G packaging vectors and pCCL-foxp3 (or pCCL control) transfer vectors expressing truncated (signaling deficient) nerve growth factor receptor (ΔNGFR) marker gene (33). HEK293T/17 cells were cotransfected with 10 μg psPAX, 30 μg pMD2.G, and 40 μg of pCCL transfer vector using calcium phosphate precipitation (57). Virus-containing supernatants were collected 24 and 48 h later, then filtered and concentrated by ultracentrifugation. Lentivirus encoding the NF-κB–luciferase reporter was purchased from Qiagen (Cignal lenti NF-κB reporter). CD4+CD25− lymphocytes isolated from PBMCs or RA T cells were transduced as previously described (33). Cells were cultured in X-VIVO 15 (Lonza) supplemented with 10% heat-inactivated human AB serum, 100 ng/mL IL-2, and 15 ng/mL IL-7 (expansion media) and stimulated for 48 h with 1 μg/mL plate-bound anti-CD3 and 1 μg/mL soluble anti-CD28. Cells were transduced at an MOI of 50 in 6 μg/mL polybrene and centrifuged at 387 × g for 2 h. After overnight culture, lentivirus was removed and cells maintained at 1 × 106/mL in the expansion media. Eight days after transduction, cells expressing the NGFR marker gene were isolated by immunomagnetic bead sorting using anti-CD271 (NGFR) microbeads (Miltenyi). NGFR+ cells were cultured in 100 ng/mL IL-2 for 4 d.
Flow Cytometry.
Foxp3 staining was conducted using the foxp3 fix/perm buffer set (Biolegend) and p65 and STAT3 phosphorylation carried out after permeabilizing the cells with BD Cytofix and BD Phosflow perm buffer II.
Supplementary Material
Acknowledgments
We thank Prof. M. Levings for pCCL-foxp3 vector; Prof. D. Trono for pMD2.G and psPAX vectors; the late Prof. Brian Foxwell for adenoviral TNF-α reporter; surgeons at Charing Cross, Epsom, and Royal United Hospitals; D. Ennis and H. Thompson for the procurement of rheumatoid arthritis synovial tissue; and Ms. Sandra Lock for expert assistance in compiling this manuscript. IL-2 was provided by the AIDS Research and Reference Reagent Program, National Institutes of Health, from Fisher BioServices.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1112722108/-/DCSupplemental.
References
- 1.Gregersen PK, Silver J, Winchester RJ. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987;30:1205–1213. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- 2.Wagner UG, Koetz K, Weyand CM, Goronzy JJ. Perturbation of the T cell repertoire in rheumatoid arthritis. Proc Natl Acad Sci USA. 1998;95:14447–14452. doi: 10.1073/pnas.95.24.14447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brennan FM, et al. Heterogeneity of T cell receptor idiotypes in rheumatoid arthritis. Clin Exp Immunol. 1988;73:417–423. [PMC free article] [PubMed] [Google Scholar]
- 4.Unutmaz D, Pileri P, Abrignani S. Antigen-independent activation of naive and memory resting T cells by a cytokine combination. J Exp Med. 1994;180:1159–1164. doi: 10.1084/jem.180.3.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brennan FM, et al. Resting CD4+ effector memory T cells are precursors of bystander-activated effectors: A surrogate model of rheumatoid arthritis synovial T-cell function. Arthritis Res Ther. 2008;10:R36. doi: 10.1186/ar2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sebbag M, Parry SL, Brennan FM, Feldmann M. Cytokine stimulation of T lymphocytes regulates their capacity to induce monocyte production of tumor necrosis factor-alpha, but not interleukin-10: Possible relevance to pathophysiology of rheumatoid arthritis. Eur J Immunol. 1997;27:624–632. doi: 10.1002/eji.1830270308. [DOI] [PubMed] [Google Scholar]
- 7.Tran CN, et al. Molecular interactions between T cells and fibroblast-like synoviocytes: Role of membrane tumor necrosis factor-alpha on cytokine-activated T cells. Am J Pathol. 2007;171:1588–1598. doi: 10.2353/ajpath.2007.070004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bangs SC, et al. Human CD4+ memory T cells are preferential targets for bystander activation and apoptosis. J Immunol. 2009;182:1962–1971. doi: 10.4049/jimmunol.0802596. [DOI] [PubMed] [Google Scholar]
- 9.Eberl G, Brawand P, MacDonald HR. Selective bystander proliferation of memory CD4+ and CD8+ T cells upon NK T or T cell activation. J Immunol. 2000;165:4305–4311. doi: 10.4049/jimmunol.165.8.4305. [DOI] [PubMed] [Google Scholar]
- 10.Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4(+) T cells. J Exp Med. 2001;194:1711–1719. doi: 10.1084/jem.194.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brennan FM, et al. Evidence that rheumatoid arthritis synovial T cells are similar to cytokine-activated T cells: Involvement of phosphatidylinositol 3-kinase and nuclear factor kappaB pathways in tumor necrosis factor alpha production in rheumatoid arthritis. Arthritis Rheum. 2002;46:31–41. doi: 10.1002/1529-0131(200201)46:1<31::AID-ART10029>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 12.Brennan FM, Chantry D, Jackson A, Maini R, Feldmann M. Inhibitory effect of TNF alpha antibodies on synovial cell interleukin-1 production in rheumatoid arthritis. Lancet. 1989;2:244–247. doi: 10.1016/s0140-6736(89)90430-3. [DOI] [PubMed] [Google Scholar]
- 13.Butler DM, Maini RN, Feldmann M, Brennan FM. Modulation of proinflammatory cytokine release in rheumatoid synovial membrane cell cultures. Comparison of monoclonal anti TNF-alpha antibody with the interleukin-1 receptor antagonist. Eur Cytokine Netw. 1995;6:225–230. [PubMed] [Google Scholar]
- 14.Feldmann M, Maini RN. Lasker Clinical Medical Research Award. TNF defined as a therapeutic target for rheumatoid arthritis and other autoimmune diseases. Nat Med. 2003;9:1245–1250. doi: 10.1038/nm939. [DOI] [PubMed] [Google Scholar]
- 15.Jiao Z, et al. Accumulation of FoxP3-expressing CD4+CD25+ T cells with distinct chemokine receptors in synovial fluid of patients with active rheumatoid arthritis. Scand J Rheumatol. 2007;36:428–433. doi: 10.1080/03009740701482800. [DOI] [PubMed] [Google Scholar]
- 16.Lin SC, et al. The quantitative analysis of peripheral blood FOXP3-expressing T cells in systemic lupus erythematosus and rheumatoid arthritis patients. Eur J Clin Invest. 2007;37:987–996. doi: 10.1111/j.1365-2362.2007.01882.x. [DOI] [PubMed] [Google Scholar]
- 17.Clough LE, et al. Release from regulatory T cell-mediated suppression during the onset of tissue-specific autoimmunity is associated with elevated IL-21. J Immunol. 2008;180:5393–5401. doi: 10.4049/jimmunol.180.8.5393. [DOI] [PubMed] [Google Scholar]
- 18.O'Sullivan BJ, et al. IL-1 beta breaks tolerance through expansion of CD25+ effector T cells. J Immunol. 2006;176:7278–7287. doi: 10.4049/jimmunol.176.12.7278. [DOI] [PubMed] [Google Scholar]
- 19.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 20.Peluso I, et al. IL-21 counteracts the regulatory T cell-mediated suppression of human CD4+ T lymphocytes. J Immunol. 2007;178:732–739. doi: 10.4049/jimmunol.178.2.732. [DOI] [PubMed] [Google Scholar]
- 21.Valencia X, et al. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 2006;108:253–261. doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steiner G, et al. Cytokine production by synovial T cells in rheumatoid arthritis. Rheumatology (Oxford) 1999;38:202–213. doi: 10.1093/rheumatology/38.3.202. [DOI] [PubMed] [Google Scholar]
- 23.Firestein GS, Alvaro-Gracia JM, Maki R. Quantitative analysis of cytokine gene expression in rheumatoid arthritis. J Immunol. 1990;144:3347–3353. [PubMed] [Google Scholar]
- 24.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ehrenstein MR, et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004;200:277–285. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Amelsfort JM, et al. Proinflammatory mediator-induced reversal of CD4+,CD25+ regulatory T cell-mediated suppression in rheumatoid arthritis. Arthritis Rheum. 2007;56:732–742. doi: 10.1002/art.22414. [DOI] [PubMed] [Google Scholar]
- 27.Ruprecht CR, et al. Coexpression of CD25 and CD27 identifies FoxP3+ regulatory T cells in inflamed synovia. J Exp Med. 2005;201:1793–1803. doi: 10.1084/jem.20050085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng G, et al. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005;309:1380–1384. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- 29.Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Natl Acad Sci USA. 2008;105:10113–10118. doi: 10.1073/pnas.0711106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wing K, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 31.Tran DQ, et al. Analysis of adhesion molecules, target cells, and role of IL-2 in human FOXP3+ regulatory T cell suppressor function. J Immunol. 2009;182:2929–2938. doi: 10.4049/jimmunol.0803827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flores-Borja F, Jury EC, Mauri C, Ehrenstein MR. Defects in CTLA-4 are associated with abnormal regulatory T cell function in rheumatoid arthritis. Proc Natl Acad Sci USA. 2008;105:19396–19401. doi: 10.1073/pnas.0806855105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allan SE, et al. Generation of potent and stable human CD4+ T regulatory cells by activation-independent expression of FOXP3. Mol Ther. 2008;16:194–202. doi: 10.1038/sj.mt.6300341. [DOI] [PubMed] [Google Scholar]
- 34.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 35.Oberg HH, Wesch D, Grüssel S, Rose-John S, Kabelitz D. Differential expression of CD126 and CD130 mediates different STAT-3 phosphorylation in CD4+CD25- and CD25high regulatory T cells. Int Immunol. 2006;18:555–563. doi: 10.1093/intimm/dxh396. [DOI] [PubMed] [Google Scholar]
- 36.Bettelli E, Dastrange M, Oukka M. Foxp3 interacts with nuclear factor of activated T cells and NF-kappa B to repress cytokine gene expression and effector functions of T helper cells. Proc Natl Acad Sci USA. 2005;102:5138–5143. doi: 10.1073/pnas.0501675102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujio K, et al. Gene therapy of arthritis with TCR isolated from the inflamed paw. J Immunol. 2006;177:8140–8147. doi: 10.4049/jimmunol.177.11.8140. [DOI] [PubMed] [Google Scholar]
- 38.Hori S, Haury M, Coutinho A, Demengeot J. Specificity requirements for selection and effector functions of CD25+4+ regulatory T cells in anti-myelin basic protein T cell receptor transgenic mice. Proc Natl Acad Sci USA. 2002;99:8213–8218. doi: 10.1073/pnas.122224799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang Q, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tarbell KV, et al. Dendritic cell-expanded, islet-specific CD4+ CD25+ CD62L+ regulatory T cells restore normoglycemia in diabetic NOD mice. J Exp Med. 2007;204:191–201. doi: 10.1084/jem.20061631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tarbell KV, Yamazaki S, Olson K, Toy P, Steinman RM. CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J Exp Med. 2004;199:1467–1477. doi: 10.1084/jem.20040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beech JT, et al. T-cell contact-dependent regulation of CC and CXC chemokine production in monocytes through differential involvement of NFkappaB: Implications for rheumatoid arthritis. Arthritis Res Ther. 2006;8:R168. doi: 10.1186/ar2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Amelsfort JM, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS. CD4(+)CD25(+) regulatory T cells in rheumatoid arthritis: Differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 2004;50:2775–2785. doi: 10.1002/art.20499. [DOI] [PubMed] [Google Scholar]
- 44.Nadkarni S, Mauri C, Ehrenstein MR. Anti-TNF-alpha therapy induces a distinct regulatory T cell population in patients with rheumatoid arthritis via TGF-beta. J Exp Med. 2007;204:33–39. doi: 10.1084/jem.20061531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Voo KS, et al. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci USA. 2009;106:4793–4798. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yagi H, et al. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int Immunol. 2004;16:1643–1656. doi: 10.1093/intimm/dxh165. [DOI] [PubMed] [Google Scholar]
- 47.Lal G, et al. Epigenetic regulation of Foxp3 expression in regulatory T cells by DNA methylation. J Immunol. 2009;182:259–273. doi: 10.4049/jimmunol.182.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang XO, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andersen KG, Butcher T, Betz AG. Specific immunosuppression with inducible Foxp3-transduced polyclonal T cells. PLoS Biol. 2008;6:e276. doi: 10.1371/journal.pbio.0060276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohata J, et al. Enhanced efficacy of regulatory T cell transfer against increasing resistance, by elevated Foxp3 expression induced in arthritic murine hosts. Arthritis Rheum. 2007;56:2947–2956. doi: 10.1002/art.22846. [DOI] [PubMed] [Google Scholar]
- 51.Jaeckel E, von Boehmer H, Manns MP. Antigen-specific FoxP3-transduced T-cells can control established type 1 diabetes. Diabetes. 2005;54:306–310. doi: 10.2337/diabetes.54.2.306. [DOI] [PubMed] [Google Scholar]
- 52.Wright GP, et al. Adoptive therapy with redirected primary regulatory T cells results in antigen-specific suppression of arthritis. Proc Natl Acad Sci USA. 2009;106:19078–19083. doi: 10.1073/pnas.0907396106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salmon M, et al. Inhibition of T cell apoptosis in the rheumatoid synovium. J Clin Invest. 1997;99:439–446. doi: 10.1172/JCI119178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Allan SE, et al. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19:345–354. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 55.Arnett FC, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 56.Denys A, et al. Evidence for a dual mechanism for IL-10 suppression of TNF-alpha production that does not involve inhibition of p38 mitogen-activated protein kinase or NF-kappa B in primary human macrophages. J Immunol. 2002;168:4837–4845. doi: 10.4049/jimmunol.168.10.4837. [DOI] [PubMed] [Google Scholar]
- 57.Dull T, et al. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.