Abstract
Light is critical for supplying carbon to the energetically expensive, nitrogen-fixing symbiosis between legumes and rhizobia. Here, we show that phytochrome B (phyB) is part of the monitoring system to detect suboptimal light conditions, which normally suppress Lotus japonicus nodule development after Mesorhizobium loti inoculation. We found that the number of nodules produced by L. japonicus phyB mutants is significantly reduced compared with the number produced of WT Miyakojima MG20. To explore causes other than photoassimilate production, the possibility that local control by the root genotype occurred was investigated by grafting experiments. The results showed that the shoot and not the root genotype is responsible for root nodule formation. To explore systemic control mechanisms exclusive of photoassimilation, we moved WT MG20 plants from white light to conditions that differed in their ratios of low or high red/far red (R/FR) light. In low R/FR light, the number of MG20 root nodules dramatically decreased compared with plants grown in high R/FR, although photoassimilate content was higher for plants grown under low R/FR. Also, the expression of jasmonic acid (JA) -responsive genes decreased in both low R/FR light-grown WT and white light-grown phyB mutant plants, and it correlated with decreased jasmonoyl-isoleucine content in the phyB mutant. Moreover, both infection thread formation and root nodule formation were positively influenced by JA treatment of WT plants grown in low R/FR light and white light-grown phyB mutants. Together, these results indicate that root nodule formation is photomorphogenetically controlled by sensing the R/FR ratio through JA signaling.
Keywords: symbiotic nitrogen fixation, shade avoidance syndrome
Many leguminous plants establish a symbiosis with nitrogen-fixing soil bacteria called rhizobia. Inside the root nodules, the rhizobia differentiate into nitrogen-fixing bacteroids, which convert atmospheric nitrogen into ammonia, a source of fixed nitrogen for the host plant. In return, the host plant supplies photosynthetic products to be used as carbon sources to the rhizobia (1–3). Root nodule development requires considerable photosynthetic energy. Energy must not only be consumed to drive rhizobial nitrogen fixation but also, to transport the fixed nitrogen to other tissues (4). However, excessive root nodule formation can be detrimental to the growth of the host plant. For example, many hypernodulation mutants, such as har1 (5–7), sunn (8), sym29 (9), nts1 (10, 11), and klavier (12) grow poorly. Therefore, legumes have developed a negative regulation mechanism, termed autoregulation of nodulation, in which earlier formed nodules inhibit additional root nodule formation (13–15).
It is well-known that plants require light for photosynthesis and monitor both light quality and quantity for optimal survival. Because assimilates from photosynthesis provide energy to fuel the symbiosis between legumes and rhizobia, the light conditions under which host plants grow are very important. Plants have photoreceptors that sense the presence of their neighbors by monitoring the ratio of red (R), which is absorbed by chlorophyll, and far red (FR), which is not absorbed by chlorophyll, light. A low R/FR ratio indicates the presence of neighbors that may compete for photosynthetically active radiation (PAR) and initiates the shade avoidance syndrome (SAS), where plant crowding causes plants to grow taller or bend to the light to avoid shade (16–19). These low R/FR light conditions are suboptimal for root nodule formation. Earlier studies showed that light quality influences legume root nodule formation, implicating the involvement of phytochrome (20, 21). To test the hypothesis that shade avoidance is important for root nodulation, we compared Lotus japonicus phytochrome B (phyB) mutants with WT Miyakojima MG20 plants to evaluate the link between root nodule formation and light quality and quantity.
Results and Discussion
Reduced Root Nodule Formation in Putative phyB Mutants.
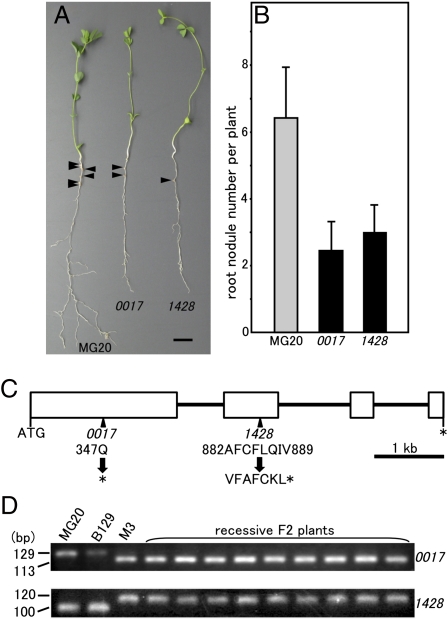
Two L. japonicus mutants (01–0017 and 01–14280) that exhibit a long hypocotyl phenotype under R light were obtained from ethylmethane sulfonate mutant lines of the National BioResouce Project Lotus/Glycine; 3-d-old germinated MG20 and the putative phyB mutant seedlings were transplanted into vermiculite-filled pots watered with Broughton & Dilworth (B&D) medium and inoculated with Mesorhizobium loti. The plants were grown for 28 d under a 16 h white light and 8 h dark cycle. Fig. 1A shows the difference in appearance of MG20 WT and 01–0017 and 01–1428 mutant plants. Although the mutants were almost as tall or taller than the MG20 plant (Fig. 1A and Fig. S1A), root growth was noticeably reduced (Fig. 1A and Fig. S1B). Also, shoot and root fresh weights were significantly lower in both mutants compared with MG20 (Fig. S1 C and D). The mutants were paler than MG20 and contained much less chlorophyll a and b (Fig. S1E). In addition, the number of root nodules of 01–0017 or 01–1428 plants was reduced to about 37% or about 46% compared with MG20, respectively (Fig. 1B). We examined the response of the mutants during germination to various light qualities, including continuous R, FR, and blue (B) light as well as continuous darkness. As shown in Fig. S2, the responses of both mutants were indistinguishable from MG20 under FR, B, and darkness; only WT MG20 plants responded to R light, showing reduced hypocotyl length and expanded cotyledons. In contrast, hypocotyl length was increased, and the angle between the cotyledons was reduced in the mutants grown under R light compared with WT MG20. Thus, the mutant plants’ responses phenocopied previously reported phyB mutant responses (22, 23).
Fig. 1.
Phenotypes of the phyB mutants of L. japonicus. (A and B) Symbiotic and growth phenotypes of the WT MG20 and 01–0017 and 01–1428 mutant plants. Plants are shown 28 DAI. The arrows indicate nodules. (Scale bar: 1 cm.) (B) Root nodule number per plant at 28 DAI. Values are means of 30 (MG20), 30 (01–0017), and 30 (01–1428) plants. Error bars represent SE. (C) Genomic structure of phyB gene in L. japonicus. Exons are enclosed in boxes, and introns are represented by black lines. The amino acid sequences in the mutated site, indicated by arrows, are shown. Stop codons are marked by asterisks. The numbers refer to the position of the amino acids. (D) Agarose gel electrophoresis for dCAPS analysis using recessive F2 plants. DNA fragments were separated on a 3.5% agarose gel, subjected to electrophoresis, and visualized by ethidium bromide staining. Genomic DNA of MG20, L. japonicus Gifu B129, and M3 plant of phyB mutant were also used for dCAPS analysis.
Genetic Analysis of Putative phyB Mutants.
The phenotypes of 01–0017 and 01–1428 were stably inherited in the M4 and M5 generations. To confirm that the described phenotypes were because of a mutation in a single gene, both mutants were crossed to the parental WT MG20. The F1 plants derived from both mutants were found to exhibit a WT phenotype. Segregation analyses of F2 progenies derived from self-pollinated F1 plants showed that F2 plants segregated at 308:101 (x2 = 0.02, P = 0.89) or 284:92 (x2 = 0.06, P = 0.81) for WT and mutant phenotypes for 01–0017 or 01–1428, respectively. These results closely fit the expected value of 3:1. Moreover, all of the phenotypes of F1 progeny prepared by crossing 01–0017 and 01–1428 were mutant. Thus, we conclude that the mutation for both 01–0017 and 01–1428 is monogenic and recessive and that the mutations in both mutant plants are allelic.
To verify that the 01–0017 and 01–1428 phenotypes were caused by a mutation in phyB, we compared the mutants’ nucleotide sequences with the sequences of MG20. Substitution of a single nucleotide from C to T, which results in an amino acid conversion of Q into a stop codon, was found in exon 1 of the 01–0017 mutant. We also found that a deletion of a single C in phyB exon 2 of the 01–1428 mutant converted AFCFLQIV into VFAFCKL; the frame shift resulted in a gene truncation because of a stop codon (Fig. 1C).
Next, to confirm genetic linkage between the mutants and the LjPHYB gene, a derived cleaved amplified polymorphic sequence (dCAPS) technique was carried out using dCAPS analysis primers (Table S1). For the 01–0017 mutant, 110 recessive plants from the F2 population generated by crossing 01–0017 and MG20 were examined; all amplified fragments tested were digested by HindIII. For the 01–1428 mutant, HaeII did not digest any of the amplified fragments. These results indicated that LjPHYB gene and the mutant phenotypes were tightly linked (nine examples are shown in Fig. 1D). We conclude that the gene responsible for the 01–0017 and 01–1428 phenotypes is a mutated LjPHYB.
Shoot Genotype Is Responsible for Reduced Nodule Development in phyB Mutants.
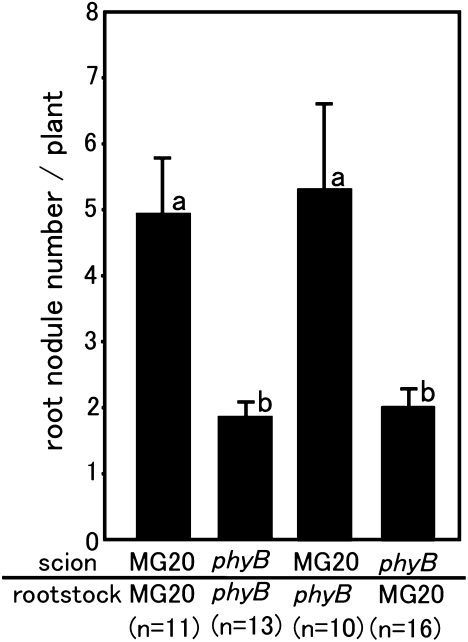
Earlier studies suggested that reduced photoassimilate production influences nodule development (20, 21). Photoassimilates are produced in leaves and mobilized to developing nodules that house nitrogen-fixing bacteria. Because phyB is important for the development of the photosynthetic apparatus (24), this finding may be one explanation of why the two mutants exhibit reduced nodule formation. However, other factors in addition to photoassimilate production may be involved in nodule development. First, the possibility that local control by the root genotype occurred was investigated by grafting experiments between phyB mutant and MG20 plants. As shown in Fig. 2, when phyB was the scion, the number of nodules decreased regardless of the rootstock genotype in contrast to using MG20 as a scion. This result indicates that the phyB shoot and not the root genotype controls nodule number.
Fig. 2.
Root nodule formation in grafted plants. Root nodule number per plant of grafted plants. Error bars represent SE, and the significance of differences among the four groups was determined by the two-tailed multiple t test with Bonferroni correction using ANOVA (six comparisons in four groups). Means denoted by the same letter did not differ significantly at P < 0.05.
Cause of Negative Regulation of Root Nodule Development in phyB Mutants.
phyB signaling regulates responses to photoperiodism, end of day FR (25), and R/FR ratios in bright light-grown plants. To test the involvement of photoperiodism, we examined whether a critical day length was needed for root nodulation. As shown in Fig. S3, the number of nodules 28 days after inoculation (DAI) decreased steadily as the day length shortened from 16 to 8 h, suggesting that a gradual decrease in the supply of photoassimilates through photosynthesis occurred. For day lengths shorter than 8 h, it is likely that not enough photosynthesis took place to support the high carbon costs of nodule formation.
Next, we examined the nodulation response to changing R/FR ratios. Because SAS is a well-known response on plant perception of R/FR ratios, root nodulation tests were carried out under conditions where SAS was likely to occur. Fig. 3A shows the experimental design of root nodulation tests under different R/FR conditions; 13-d-old plants were moved from continuous white light to either continuous high R/FR or low R/FR light, and 2 d later, M. loti MAFF303099 cells carrying plasmid pFAJPcycA were inoculated onto the roots. The number of nodules and nodule primordia were counted 14 DAI. The intensity of the PAR of the R light-emitting diode (LED) remained constant under both treatments to maintain photosynthesis. As shown, no statistically significant difference was observed for either root length or shoot fresh weight of phyB mutant or MG20 plants, respectively, grown in high R/FR and low R/FR light (Fig. 3 C and E). However, a statistically significant difference was observed in the fresh weight of shoots in high vs. low R/FR-grown phyB plants and root length of the MG20 plants grown in high vs. low R/FR (Fig. 3 B and F). More importantly, very few nodules developed on phyB mutant roots under either light regime (Fig. 3D). Moreover, although nodules developed on MG20 plants grown in high R/FR light, very few nodules developed on the roots of the low R/FR-grown plants (Fig. 3G).
Fig. 3.
Root nodule formation under different R/FR ratios. (A) Experimental design of root nodulation tests under different R/FR conditions. (B–D) Growth and symbiotic phenotypes of a phyB mutant (01–0017) 14 DAI under different R/FR light conditions. (E–G) Growth and symbiotic phenotypes of MG20 14 DAI under different R/FR light ratios. Error bars represent SE, and a statistically significant difference is indicated by asterisks (**P < 0.01 by Student t test).
The sucrose content of the roots of high and low R/FR-grown MG20 plants was measured at 570.7 ± 42.9 and 763.3 ± 60.1 μg/g fresh weight, respectively (mean ± SE, n ≥ 18). A small quantity of PAR wavelength generated by the FR LED (MIL-IF18; Sanyo) may be responsible for the increase in the sucrose content of the low R/FR-grown plants. Nevertheless, the fact that nodule number was reduced in the low R/FR-grown plants despite a higher sucrose content strongly suggests that the sensing of phyB of the R/FR ratio has a significant influence on nodulation. We cannot eliminate the possibility, however, that photoassimilate production contributes to some extent to nodule formation under these conditions.
Expression Analysis of Jasmonic Acid-Responsive Genes and Endogenous Concentrations of Jasmonic Acid and Jasmonoyl-Isoleucine.
Because the number of root nodules of both low R/FR-grown MG20 plants and white light-grown phyB mutants declined, we hypothesized that the observed reduction in root nodule number should be controlled by the same mechanism in both plant genotypes and furthermore, that it would be mediated by phyB. Several reports suggested a link between jasmonic acid (JA) and light signaling (26). For example, in low R/FR light, phyB signaling suppressed both JA-mediated gene expression and JA-dependent defenses against insect herbivory (27). By contrast, mutations in either HY1 or HY2, which encode a phytochromobillin synthase, enhanced JA production and sensitivity (28). For root nodule formation, JA has been reported as a negative regulator. In L. japonicus, foliar application of 1 μM or more methyl jasmonate (MeJA) inhibited nodule development (29). In Medicago truncatula, JA addition to the growth medium also suppressed root nodule development, even at a lower concentration (0.1 μM) (30). Thus, we predicted that JA production and/or sensitivity would be enhanced in white light-grown phyB mutants and low R/FR-grown MG20 plants.
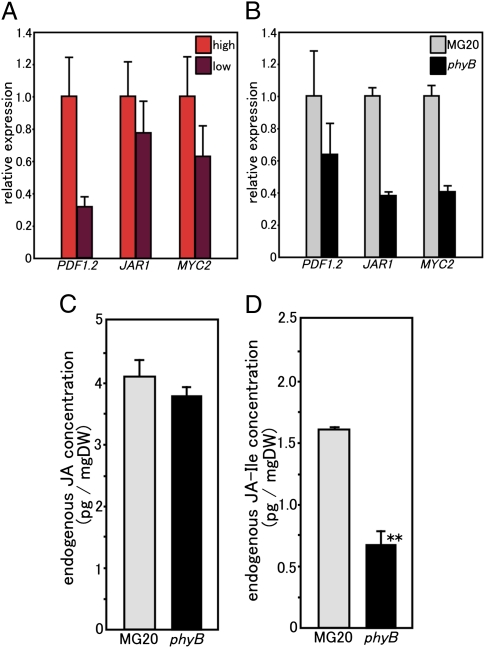
We first selected JA-responsive genes for analyzing JA production and/or sensitivity in L. japonicus by investigating gene expression responses to MeJA treatment. Through a database search, L. japonicus genes that were homologous to genes reported as members of JA signaling pathways or JA-responsive in other plants were chosen (27). Expression levels of L. japonicus genes with similarity to PDF1.2, JAR1, and MYC2 increased more than three times in response to a 50 μM MeJA addition (Fig. S4). The PDF1.2 gene encodes a plant defensin that is implicated in JA-dependent defense responses (31), whereas JAR1 codes for an enzyme that conjugates JA with amino acids to produce the active JA derivative [most likely jasmonoyl-isoleucine (JA-Ile)] (32). The MYC2 gene encodes the key transcriptional activator of JA responses (33, 34). The expression levels of these three genes decreased in low R/FR-grown MG20 plants compared with those plants grown in high R/FR (Fig. 4A). Moreover, their expression levels declined in white light-grown, 22-d-old phyB mutants (7 DAI) in contrast to WT MG20 plants (Fig. 4B). These results suggested that JA production and/or sensitivity decreased, which was contrary to our expectations. To expand this study, we measured the endogenous concentrations of JA and JA-Ile in the roots of white light-grown, 22-d-old plants (at the same growth stage). Interestingly, no significant difference was observed in JA concentration between MG20 and phyB mutant plants (Fig. 4C). However, the endogenous concentration of JA-Ile (the active JA derivative) was significantly decreased in the root of phyB mutants (Fig. 4D). This trend was obvious even at an earlier stage (day 10) (Fig. S5A), especially for JA-Ile (Fig. S5B). These results suggested that the conversion of JA to JA-Ile was suppressed because of the decreased level of JAR1 gene expression in the phyB mutants. JA-Ile production is likely decreased by the inhibition of the step from JA to JA-Ile in the phyB mutant, because JAR1 catalyzes the conjugation of JA with isoleucine to form JA-Ile. Hence, the lower concentration of JA-Ile in the phyB mutants may be a consequence of the decreased amount of transcription from the JAR1 gene.
Fig. 4.
Expression levels of JA-responsive genes and endogenous concentration of JA and JA-Ile. (A) Comparison of transcript amounts of JA-responsive genes between high R/FR light-grown and low R/FR light-grown MG20 plants. (B) Comparison of transcript amounts of JA-responsive genes between white light-grown MG20 and phyB plants (01–0017). (A and B) Transcript amounts were normalized against ATP synthase (internal control) transcripts. The mean value of expression in MG20 grown in high R/FR light (A) and MG20 (B) was set as one. The data represent the averages ± SE of three independent experiments using roots derived from six different plants. (C and D) Fifteen-day-old plants were inoculated with M. loti. After 7 d, endogenous JA and JA-Ile concentrations were measured. The data represent the averages ± SE of three independent experiments using roots derived from six different plants. (C) Comparison of endogenous JA concentration between white light-grown WT MG20 and phyB mutant (01–0017) plants. (D) Comparison of endogenous JA-Ile concentration between white light-grown MG20 and phyB plants (01–0017).
Positive Regulation of JA on Root Nodule Formation in L. japonicus.
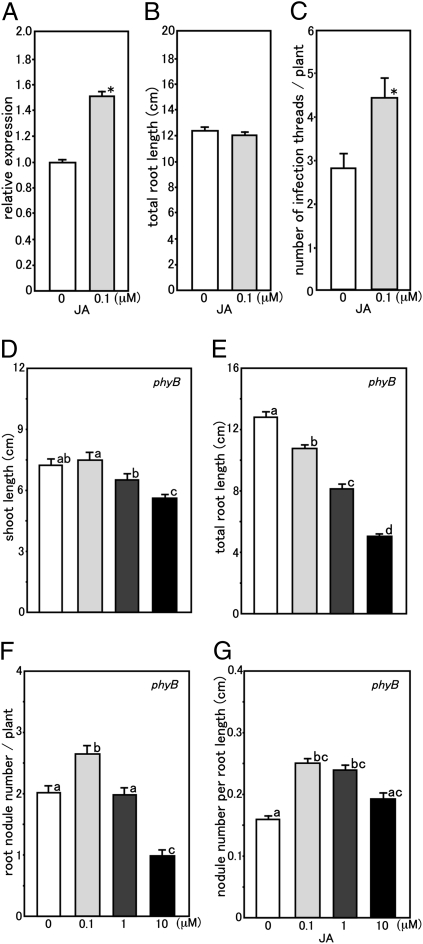
To confirm the effect of JA on nodule formation, JA was added to the plant growth medium. We found that MG20 shoot and root growth measured 28 DAI decreased as the concentration of JA increased (Fig. S6 A and B). Although root nodule number was reduced with 10 μM JA addition, the number per plant showed a statistically significant increase over the untreated control in the 0.1 μM JA treatment, and it was unaffected by adding 1 μM JA (Fig. S6C). Furthermore, nodule number per unit root length increased over the untreated control at all JA concentrations tested (Fig. S6D). Our results differ from those results reported for M. truncatula, where nodulation and its early stages are repressed by a low level of JA treatment (0.1 μM) (30).
Positive Effects of JA on Root Nodule Development in Low R/FR-Grown MG20.
If reduced nodule formation in low R/FR-grown MG20 and white light-grown phyB mutants is because of an inhibition of JA-Ile production and/or reduced sensitivity, we predicted that nodule development would be enhanced by JA or MeJA application. We found that the addition of MeJA enhanced JAR1 gene expression levels (Fig. S4). To further assess this possibility, the effect of JA treatment on root nodule development was analyzed. When 0.1 μM JA was added to MG20 plants, the number of nodules and nodule primordia was slightly increased (P = 0.099) over the controls (Fig. S7). We also followed the expression in response to JA or null treatment of the marker gene NIN (35), which is required for infection thread formation and nodule primordium initiation. When 0.1 μM JA was added to MG20 plants, NIN gene expression was significantly augmented (Fig. 5A). Although JA addition did not affect the total root length of MG20 plants compared with the untreated controls (Fig. 5B), the number of infection threads per unit root length was significantly increased (Fig. 5C). These results strongly suggest that JA functions as a positive regulator for root nodule development in L. japonicus.
Fig. 5.
Positive effects of JA on root nodule formation. (A–C) Effect of JA treatment on LjNIN gene expression (A), root length (B), and infection thread formation (C) in inoculated low R/FR light-grown MG20 plants. (A) The mean value of expression in untreated MG20 was set as one. The data represent averages ± SE of three independent experiments using roots derived from six different plants. (B and C) Values are the means of 62 (0 μM JA) and 56 (0.1 μM JA) plants. Error bars represent SE. Statistical significance is indicated by asterisks (*P < 0.05 by Student t test). (D–G) Effect of JA treatment on shoot length (D), total root length (E), nodule number per plant (F), and nodule number per unit root length (G) in white light-grown phyB mutant plants 28 DAI. Values are means of 46 (0 μM JA), 46 (0.1 μM JA), 48 (1 μM JA), and 47 (10 μM JA) plants. Error bars represent SE, and the significance of differences among the four groups was determined by the two-tailed multiple t test with Bonferroni correction using ANOVA (six comparisons in four groups). Means denoted by the same letter did not differ significantly at P < 0.05.
Positive Effects of JA on Root Nodule Development in White Light-Grown phyB Mutants.
With 0.1 μM JA addition to phyB mutants, shoot length was unaffected, and root growth decreased (Fig. 5 D and E); however, nodule number per plant significantly increased (Fig. 5F). Moreover, the nodule number per unit root length significantly increased with 0.1 and 1 μM JA treatments (Fig. 5G). Based on the responses of JA treatment to shoot length (Fig. 5D and Fig. S6A), total root length (Fig. 5E and Fig. S6B), and root nodule number (Fig. 5 F and G and Fig. S6 C and D) in MG20 and the phyB mutant plants, sensitivity to JA is not likely to be significantly different between them. Taken together, these results indicate that the cause of reduced root nodule formation in low R/FR-grown MG20 plants and the white light-grown phyB mutants is because of the inhibition of JA-Ile production.
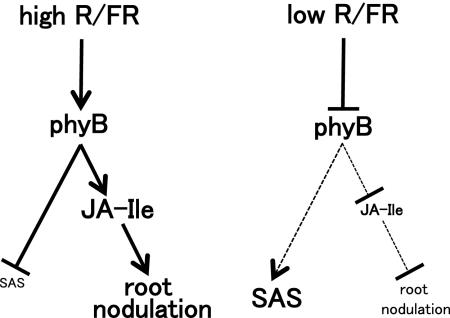
A model representing the proposed mechanism of JA and phyB signaling for shade perception and root nodule formation is depicted in Fig. 6. In high R/FR light, phyB suppresses SAS and enhances root nodule formation through increased concentration of JA-Ile. In contrast, in low R/FR light, SAS is restored by the inactivation of phyB and results in suppressing root nodule formation through a reduced concentration of JA-Ile. Our data show that root nodule formation involves the perception of the R/FR ratio and requires signaling through phyB and JA. Although root nodule development is initiated in the soil under limited light, these low R/FR light conditions are suboptimal for sustaining nodule function. Thus, the legume host, like other plants, initiates a shade avoidance response and modifies its growth to obtain sufficient light for maximizing photosynthesis. Moreover, the host plants suppress root nodule development under such conditions to prevent wasting energy. We conclude that this shade avoidance syndrome for root nodule formation is required for L. japonicus nodule development. In conclusion, monitoring both light quality and quantity is essential for establishing and maintaining a successful nitrogen-fixing symbiosis.
Fig. 6.
Model representing the proposed mechanism of JA and phyB signaling for shade perception and root nodule formation. In high R/FR light, phyB suppresses SAS and enhances root nodule formation through increased concentration of JA-Ile. In low R/FR light, SAS is restored by the inactivation of phyB and suppresses root nodule formation by the reduced concentration of JA-Ile. Small letters and dotted lines mean inactivation and suppression, respectively.
Materials and Methods
Plant Material, Inoculation, and Growth Conditions.
Mutants 01–0017 and 01–1428 were derived from L. japonicus accession MG20 by mutagenesis with ethylmethane sulfonate. M. loti strains MAFF303099 and MAFF303099 carrying a GUS gene (36) were used for inoculation. For B, R, and FR light irradiation, LEDs (Sanyo) were used. For the analysis of root nodule formation using pots, the swollen seeds were sown on vermiculite watered with B&D medium (nitrogen-deficient) (37) and inoculated with M. loti (1.0 × 107 cells mL−1). The plants were grown at 24 °C under continuous white light at a light intensity of 150 μmol m−2 s−1. For the analysis of root nodule formation using square plates (8.3 cm width × 23.5 cm length × 1.8 cm depth), seeds were sown on 0.8% (wt/vol) agar medium, and the plates were placed vertically at 24 °C in the dark. After 3 d, the germinated seedlings were transplanted onto another set of square plates containing −N B&D medium solidified with 1.5% (wt/vol) agar. Because light irradiation to roots prevents root nodule formation, the root areas of the square plates were shaded with black paper. However, even if this type of shading was used, small quantities of light reached the root area but did not seriously affect root nodule formation. The plates were placed vertically, and the plants were cultivated under the indicated conditions. The square plate method was used for the analysis of root nodule formation under high or low R/FR light despite the low number of nodules formed, because the method generated a reproducible number of nodules (Fig. 3 D and G). In contrast, when vermiculite-filled pots were used, the interruption of light irradiation by the periodic addition of water to the pot resulted in a variation in nodule number such that consistent measurements could not be obtained from experiment to experiment. Germinated seedlings were transplanted onto 1.5% agar-solidified B&D medium and incubated under continuous white light at a light intensity of 100 μmol m−2s−1. After 10 d (day 13), the plants were moved to high R/FR light (R/FR = ∞; R LED intensity = 10 μmol m−2s−1; FR LED intensity = 0 μmol m−2s−1) or low R/FR light (R/FR = 0.1; R LED intensity = 10 μmol m−2s−1; FR LED intensity = 100 μmol m−2s−1). This FR intensity was almost maximum in the available LEDs; thus, the R intensity needed to be 10 μmol m−2s−1 to generate a value of 0.1 for the R/FR ratio; 2 d later (day 15), M. loti MAFF303099 cells carrying plasmid GUS were inoculated at a concentration of 1.0 × 107 cells per plant (36). After 14 d (day 29), GUS staining was carried out to observe nodule and nodule primordia (Fig. 3A) (38). Root nodule formation or JA-responsive gene expression was analyzed (39) at the indicated DAI. For analysis of the effect of JA on root nodule formation or infection thread formation, B&D agar medium with or without JA was used. Detailed information is provided in SI Materials and Methods.
Sequencing LjPHYB.
Detailed information is provided in SI Materials and Methods.
Expression Analysis.
To quantify the relative amount of transcripts of JA-related genes, real-time RT-PCR was performed in the one-step SYBR Primescript RT-PCR Kit (TaKaRa). Detailed information is provided in SI Materials and Methods.
dCAPS Analysis.
Genomic DNA of the F2 population was used for dCAPS analysis with dCAPS primers. After amplification, digested fragments were separated and visualized by ethidium bromide staining. Detailed information is provided in SI Materials and Methods.
Grafting Experiments.
Three-week-old MG20 and phyB mutant (01–0017) plants were severed at the hypocotyls, and the shoots were grafted either to self or reciprocal roots using small plastic tubes as splints. Grafted plants were grown in vermiculite watered with B&D medium for 3 wk at 24 °C under 16 h light and 8 h dark conditions, and then, they were inoculated with M. loti. After 4 wk, root nodules were counted.
Measurement of Endogenous Concentration of JA and JA-Ile.
Three-day-old seedlings were transplanted onto B&D agar medium and incubated under continuous white light. After 12 d, plants were inoculated with M. loti (1.0 × 107 cells/plant), and then, plants were quick frozen in liquid N2 7 DAI (day 22). The endogenous concentration of JA and JA-Ile was measured by the method previously reported (40).
Supplementary Material
Acknowledgments
L. japonicus Miyakojima MG20 seeds were provided by the National BioResource Project of the Ministry of Education, Culture, Sports, Science and Technology, Japan. This work was supported by Japan Society for the Promotion of Science Challenging Exploratory Research Grant-in-Aid 21657017 (to A.S.) and the Kato Memorial Bioscience Foundation Grant (to A.S.).
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequences of gene identification reported in this paper have been deposited in the L. japonicus genome database (miyakogusa.jp: http://www.kazusa.or.jp/lotus/) [gene codes chr6.CM0139.1610.r2.m. (LjPHYB), chr6.CM1613.110.r2.m (LjERF1a), chr5.CM0341.90.r2.m (LjERF1b), chr5.LjT29J15.50.r2.m (LjHELa), LjSGA_078238.1 (LjHELb), chr2.CM0177.810.r2.m (LjMYC2), chr1.CM0105.550.r2.d (LjJAR1), LjT09F12.40.r2.d (LjCOI1a), chr1.CM0269.1230.r2.a (LjCOI1b), LjSGA_023148.1 (PDF1.2), chr2.CM0065.740.r2.d (LjVSP1a), and chr3.CM0634.190.r2.m (LjVSP1b)].
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105892108/-/DCSupplemental.
References
- 1.Bethlenfalvay GJ, Norris RF, Phillips DA. Effect of bentazon, a hill reaction inhibitor, on symbiotic nitrogen-fixing capacity and apparent photosynthesis. Plant Physiol. 1979;63:213–215. doi: 10.1104/pp.63.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finn GA, Brun WA. Effect of atmospheric CO(2) enrichment on growth, nonstructural carbohydrate content, and root nodule activity in soybean. Plant Physiol. 1982;69:327–331. doi: 10.1104/pp.69.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bethlenfalvay GJ, Phillips DA. Effect of light intensity on efficiency of carbon dioxide and nitrogen reduction in Pisum sativum L. Plant Physiol. 1977;60:868–871. doi: 10.1104/pp.60.6.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshida C, Funayama-Noguchi S, Kawaguchi M. plenty, a novel hypernodulation mutant in Lotus japonicus. Plant Cell Physiol. 2010;51:1425–1435. doi: 10.1093/pcp/pcq115. [DOI] [PubMed] [Google Scholar]
- 5.Wopereis J, et al. Short root mutant of Lotus japonicus with a dramatically altered symbiotic phenotype. Plant J. 2000;23:97–114. doi: 10.1046/j.1365-313x.2000.00799.x. [DOI] [PubMed] [Google Scholar]
- 6.Krusell L, et al. Shoot control of root development and nodulation is mediated by a receptor-like kinase. Nature. 2002;420:422–426. doi: 10.1038/nature01207. [DOI] [PubMed] [Google Scholar]
- 7.Nishimura R, et al. HAR1 mediates systemic regulation of symbiotic organ development. Nature. 2002;420:426–429. doi: 10.1038/nature01231. [DOI] [PubMed] [Google Scholar]
- 8.Schnabel E, Journet EP, de Carvalho-Niebel F, Duc G, Frugoli J. The Medicago truncatula SUNN gene encodes a CLV1-like leucine-rich repeat receptor kinase that regulates nodule number and root length. Plant Mol Biol. 2005;58:809–822. doi: 10.1007/s11103-005-8102-y. [DOI] [PubMed] [Google Scholar]
- 9.Sagan M, Duc G. Sym28 and Sym29, two new genes involved in regulation of nodulation in pea (Pisum sativum L.) Symbiosis. 1996;20:229–245. [Google Scholar]
- 10.Carroll BJ, McNeil DL, Gresshoff PM. Isolation and properties of soybean [Glycine max (L.) Merr.] mutants that nodulate in the presence of high nitrate concentrations. Proc Natl Acad Sci USA. 1985;82:4162–4166. doi: 10.1073/pnas.82.12.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carroll BJ, McNeil DL, Gresshoff PM. A supernodulation and nitrate-tolerant symbiosis (nts) soybean mutant. Plant Physiol. 1985;78:34–40. doi: 10.1104/pp.78.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oka-Kira E, et al. klavier (klv), a novel hypernodulation mutant of Lotus japonicus affected in vascular tissue organization and floral induction. Plant J. 2005;44:505–515. doi: 10.1111/j.1365-313X.2005.02543.x. [DOI] [PubMed] [Google Scholar]
- 13.Kosslak RM, Bohlool BB. Suppression of nodule development of one side of a split-root system of soybeans caused by prior inoculation of the other side. Plant Physiol. 1984;75:125–130. doi: 10.1104/pp.75.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Brussel AAN, Tak T, Boot KJM, Kijne JW. Autoregulation of root nodule formation: Signals of both symbiotic partners studied in a split-root system of Vicia sativa subsp. nigra. Mol Plant Microbe Interact. 2002;15:341–349. doi: 10.1094/MPMI.2002.15.4.341. [DOI] [PubMed] [Google Scholar]
- 15.Okamoto S, et al. Nod factor/nitrate-induced CLE genes that drive HAR1-mediated systemic regulation of nodulation. Plant Cell Physiol. 2009;50:67–77. doi: 10.1093/pcp/pcn194. [DOI] [PubMed] [Google Scholar]
- 16.Franklin KA, Quail PH. Phytochrome functions in Arabidopsis development. J Exp Bot. 2010;61:11–24. doi: 10.1093/jxb/erp304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith H, Whitelam GC. The shade avoidance syndrome: Multiple responses mediated by multiple phytochromes. Plant Cell Environ. 1997;20:840–844. [Google Scholar]
- 18.Neff MM, Fankhauser C, Chory J. Light: An indicator of time and place. Genes Dev. 2000;14:257–271. [PubMed] [Google Scholar]
- 19.Franklin KA. Shade avoidance. New Phytol. 2008;179:930–944. doi: 10.1111/j.1469-8137.2008.02507.x. [DOI] [PubMed] [Google Scholar]
- 20.Kasperbauer MJ, Hunt PG. Shoot/root assimilate allocation and nodulation of Vigna unguiculata seedlings as influenced by shoot light environment. Plant Soil. 1994;161:97–101. [Google Scholar]
- 21.Lie TA. Symbiotic nitrogen fixation under stress conditions. Plant Soil. 1971;35:117–127. [Google Scholar]
- 22.Koornneef M, Rolff E, Spruit CJP. Genetic control of light-inhibited hypocotyl elongation in Arabidopsis thaliana L. Heynh. Z Planzenphysiol. 1980;100:147–160. [Google Scholar]
- 23.Kendrick RE, Nagatani A. Phytochrome mutants. Plant J. 1991;1:133–139. [Google Scholar]
- 24.López-Juez E, et al. The cucumber long hypocotyl mutant lacks a light-stable PHYB-like phytochrome. Plant Cell. 1992;4:241–251. [PMC free article] [PubMed] [Google Scholar]
- 25.Franklin KA, et al. Phytochromes B, D, and E act redundantly to control multiple physiological responses in Arabidopsis. Plant Physiol. 2003;131:1340–1346. doi: 10.1104/pp.102.015487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robson F, et al. Jasmonate and phytochrome A signaling in Arabidopsis wound and shade responses are integrated through JAZ1 stability. Plant Cell. 2010;22:1143–1160. doi: 10.1105/tpc.109.067728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moreno JE, Tao Y, Chory J, Ballaré CL. Ecological modulation of plant defense via phytochrome control of jasmonate sensitivity. Proc Natl Acad Sci USA. 2009;106:4935–4940. doi: 10.1073/pnas.0900701106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhai QZ, et al. Phytochrome chromophore deficiency leads to overproduction of jasmonic acid and elevated expression of jasmonate-responsive genes in Arabidopsis. Plant Cell Physiol. 2007;48:1061–1071. doi: 10.1093/pcp/pcm076. [DOI] [PubMed] [Google Scholar]
- 29.Nakagawa T, Kawaguchi M. Shoot-applied MeJA suppresses root nodulation in Lotus japonicus. Plant Cell Physiol. 2006;47:176–180. doi: 10.1093/pcp/pci222. [DOI] [PubMed] [Google Scholar]
- 30.Sun J, et al. Crosstalk between jasmonic acid, ethylene and Nod factor signaling allows integration of diverse inputs for regulation of nodulation. Plant J. 2006;46:961–970. doi: 10.1111/j.1365-313X.2006.02751.x. [DOI] [PubMed] [Google Scholar]
- 31.Brown RL, Kazan K, McGrath KC, Maclean DJ, Manners JM. A role for the GCC-box in jasmonate-mediated activation of the PDF1.2 gene of Arabidopsis. Plant Physiol. 2003;132:1020–1032. doi: 10.1104/pp.102.017814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Staswick PE, Tiryaki I. The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell. 2004;16:2117–2127. doi: 10.1105/tpc.104.023549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boter M, Ruíz-Rivero O, Abdeen A, Prat S. Conserved MYC transcription factors play a key role in jasmonate signaling both in tomato and Arabidopsis. Genes Dev. 2004;18:1577–1591. doi: 10.1101/gad.297704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lorenzo O, Chico JM, Sánchez-Serrano JJ, Solano R. JASMONATE-INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. Plant Cell. 2004;16:1938–1950. doi: 10.1105/tpc.022319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schauser L, Roussis A, Stiller J, Stougaard J. A plant regulator controlling development of symbiotic root nodules. Nature. 1999;402:191–195. doi: 10.1038/46058. [DOI] [PubMed] [Google Scholar]
- 36.Okazaki S, Hattori Y, Saeki K. The Mesorhizobium loti purB gene is involved in infection thread formation and nodule development in Lotus japonicus. J Bacteriol. 2007;189:8347–8352. doi: 10.1128/JB.00788-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Broughton WJ, Dilworth MJ. Control of leghaemoglobin synthesis in snake beans. Biochem J. 1971;125:1075–1080. doi: 10.1042/bj1251075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suzuki A, et al. Split-root study of autoregulation of nodulation in the model legume Lotus japonicus. J Plant Res. 2008;121:245–249. doi: 10.1007/s10265-007-0145-5. [DOI] [PubMed] [Google Scholar]
- 39.Tominaga A, et al. Enhanced nodulation and nitrogen fixation in the abscisic acid low-sensitive mutant enhanced nitrogen fixation1 of Lotus japonicus. Plant Physiol. 2009;151:1965–1976. doi: 10.1104/pp.109.142638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohkama-Ohtsu N, et al. 12-oxo-phytodienoic acid-glutathione conjugate is transported into the vacuole in Arabidopsis. Plant Cell Physiol. 2011;52:205–209. doi: 10.1093/pcp/pcq181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.