Abstract
Although most self-reactive T cells are eliminated in the thymus, mechanisms to inactivate or control T cells specific for extrathymic antigens are required and exist in the periphery. By investigating the site in which autoreactive T cells are tolerized, we identify a unique mechanism of peripheral deletion in which naïve autoreactive CD8 T cells are rapidly eliminated in the liver after intrahepatic activation. T cells actively invade hepatocytes, enter endosomal/lysosomal compartments, and are degraded. Blockade of this process leads to accumulation of autoreactive CD8 T cells in the liver and breach of tolerance, with the development of autoimmune hepatitis. Cell into cell invasion, or emperipolesis, is a long-observed phenomenon for which a physiological role has not been previously demonstrated. We propose that this “suicidal emperipolesis” is a unique mechanism of autoreactive T-cell deletion, a process critical for the maintenance of tolerance.
Keywords: transgenic mice transplantation, hepatitis C virus, cell-in cell, cannibalism, entosis
Most self-reactive T cells are deleted in the thymus after recognition of self-antigen (1). However, not all self-antigens are expressed in this organ (2). Furthermore, food- and innocuous environmental antigens (e.g., gut microflora) are not presented intrathymically. T cells specific for these extrathymic antigens are silenced in the periphery by other mechanisms including deletion, anergy, immune deviation, and regulation (3). This control is critical for maintaining tolerance and preventing autoimmunity.
Peripheral deletion is a major mechanism of self-reactive T-cell silencing. The molecular mechanisms leading to peripheral deletion and/or exhaustion of autoreactive CD8 T cells have been dissected, and the role of immature dendritic cells in lymphoid tissues described (reviewed in refs. 3 and 4). However, it is not clear whether other sites contribute to peripheral deletion, playing a role in removal of autoreactive T cells. Naïve CD8 T-cell recirculation is restricted to the blood and lymph, and primary activation of naive T cells is limited to lymphoid organs [lymph nodes (LN), spleen, and bone marrow (5)] and liver (6, 7). It is thus likely that tolerization of self-reactive T cells in the periphery is determined in these compartments.
In investigating the fate of autoreactive naïve CD8 T cells transferred into mice ubiquitously expressing their cognate antigen, we demonstrate here that the lack of autoimmunity was associated with the preferential accumulation of most autoreactive T cells within the livers of recipients, despite ubiquitous antigen expression in these animals. Surprisingly, 80–90% of T cells undergoing primary activation within the liver were rapidly deleted by a nonapoptotic mechanism as a result of their invading hepatocytes, leading to their rapid destruction in endosomal/lysosomal compartments. Blockade of this phenomenon in vivo led to accumulation of autoreactive T cells within the livers of recipient animals and breach of tolerance, with development of immune-mediated hepatitis.
Invasion of a cell into another cell, also known as “emperipolesis,” has been observed since the 1920s, but its physiological role has not been elucidated. By showing that recently activated CD8 T cells undergo emperipolesis in hepatocytes expressing their target antigen and are rapidly destroyed before they expand and acquire cytotoxic function, this study suggests that suicidal emperipolesis plays a physiological role in maintaining tolerance.
Results
Self-Reactive T Cells Did Not Induce Generalized Autoimmunity in Mice Ubiquitously Expressing Cognate Antigen.
It has been known for some time that allogeneic T cells do not cause overt graft versus host disease in unirradiated recipients (8, 9). To examine whether lack of immune mediated injury was also observed with transfer of transgenic (Tg) populations of T cells entirely specific for the host, we adoptively transferred Des CD8 T cells (which express a Tg TCR specific for a self-peptide presented in the context of H-2Kb) into C57BL/6 (B6) mice, which ubiquitously express H-2Kb. Surprisingly, despite the high frequency of autoreactive CD8 T cells transferred, host mice never developed immune-mediated pathology, as assessed by measuring alanine aminotransferase (ALT) levels for hepatitis (Fig. 1A), diarrhea for colitis, and inspection of the skin for dermatitis, suggesting that T cells had been silenced. Even injecting 108 Des LN cells did not lead to autoimmune hepatitis or systemic autoimmunity (Fig. S1A), indicating that the mechanism responsible for silencing donor T cells was very efficient and could not easily be saturated. This mechanism resulting in the lack of immunopathology was restricted to naïve T cells. When in vitro-generated effector T cells were adoptively transferred, they induced severe hepatitis in B6 but not non-antigen–expressing B10.BR recipients (Fig. S1B), suggesting that effector T cells behaved differently to naive T cells undergoing activation following transfer T cells that never mediated pathology.
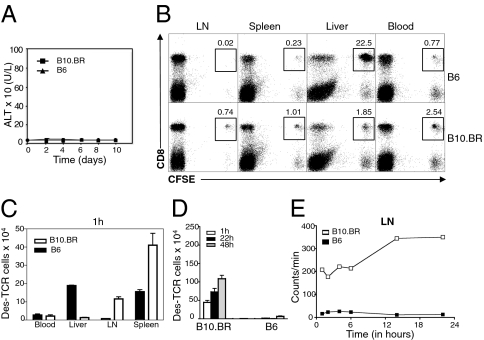
Fig. 1.
Failure to develop graft versus host disease was associated with antigen-specific intrahepatic retention of CD8 Des T cells. (A) ALT levels in B6 and B10.BR mice after transfer of 1.5 × 106 cells isolated from LN of Des mice. (B) FACS profiles of leukocytes purified from LN, spleen, liver, and blood 1 h after transfer of CFSE-labeled Des cells. (C) Distribution of total numbers of Des T cells in different organs 1 h after transfer of CFSE-labeled Des cells (identified as CD8+ CFSE+). Results in the blood are expressed per milliliter. (D) Donor T-cell counts at 1, 22 and 48 h in the LN of B6 and B10.BR recipients after transfer of 1.5 × 106 LN CFSE-labeled LN Des T cells. (E) Radioactivity in LN at various time-points after transfer of 51Cr-labeled CD8 Des T cells into B6 and B10.BR mice. Error bars represent SEM of three mice per group. Data are representative of at least two independent experiments.
Lack of Autoimmunity in B6 Recipients Was Associated with Lack of Donor T-Cell Migration into LN After Avid Intrahepatic Retention.
To understand the basis for the lack of immune-mediated pathology in B6 recipients, we harvested leukocytes at a very early time point (1 h after transfer) and analyzed the number and phenotype of donor CD8 Des T cells in blood, LN, spleen, and liver. As expected, in nonantigen-expressing B10.BR control recipients, the transferred donor T cells remained naïve as assessed by lack of CD69 expression at 5 h post T cell transfer (Fig. S1C) and migrated predominantly to LN and spleen (Fig. 1 B and C). In B6 recipients, however, donor T cells were very rarely found in the LN of recipient animals, despite antigen expression within this compartment (Fig. 1 B and C). The spleen and blood of B6 mice also contained a lower proportion of donor T cells compared with B10.BR mice (Fig. 1 B and C). At 24 h and 48 h after transfer, donor T cells were still not detected in LN of B6 recipients (Fig. 1D), suggesting that lack of LN homing was maintained. Transfer of radiolabeled donor T cells confirmed that donor T cells never reached the LN (Fig. 1E).
Contrasting with the lack of migration to LN, most donor T cells were contained in the livers of B6 mice at 1 h (Fig. 1 B and C) and expressed high levels of CD69 (Fig. S1C), suggesting that most T cells entering the liver were specifically retained after in situ activation.
A High Proportion of Donor T Cells Retained in the Liver Was Lost Within the First 22 h After Transfer.
Although a very high number of potentially harmful T cells accumulated in the B6 liver, they never caused autoimmune hepatitis, which prompted us to explore their fate. The liver is recognized as a site of primary CD8 T-cell activation leading to tolerance (6, 10). We have shown that naïve CD8 T cells activated intrahepatically died prematurely by apoptosis in a Bim-dependent manner at 72 h after transfer (11). Donor Des T cells recovered from the B6 liver at 48 h also expressed high levels of Bim (Fig. S1D). If Bim-dependent apoptosis was the predominant mechanism responsible for silencing Des T cells in B6 animals, we would expect donor T-cell numbers to start dropping after 48 h, when Bim is up-regulated. Surprisingly, the numbers of CFSE-labeled Des T cells recovered from the liver had already dropped 80–90% by 22 h after transfer, just before T cells started to divide and before Bim up-regulation (Fig. 2 A and B), suggesting that the mechanism responsible for the silencing of the majority of T cells was not Bim-dependent. A similar fall between 1 and 22 h was also observed when Bim-deficient Des T cells were transferred into B6 recipients (Fig. S1E), definitively ruling out a role for Bim in early T cell loss. This cell loss was seen regardless of the method of cell extraction (Fig. S1F). Consistent with our initial experiments (Fig. 1 B and C), there was no associated increase of T cells in LN, blood, or spleen (Fig. 2 A and B) at this time point, suggesting that loss of donor T cells from the liver was not due to migration to these compartments. Kinetic studies revealed that up to 75% of T cells disappeared within 6 h after transfer, suggesting that T-cell loss was very rapid (Fig. 2C). Liver histology confirmed that the number of CFSE+ donor T cells detected in the liver decreased between 1 and 22 h after transfer (Fig. 2 D and E). Interestingly, this process occurred just as efficiently when higher Des T-cell numbers were transferred (Fig. S1G).
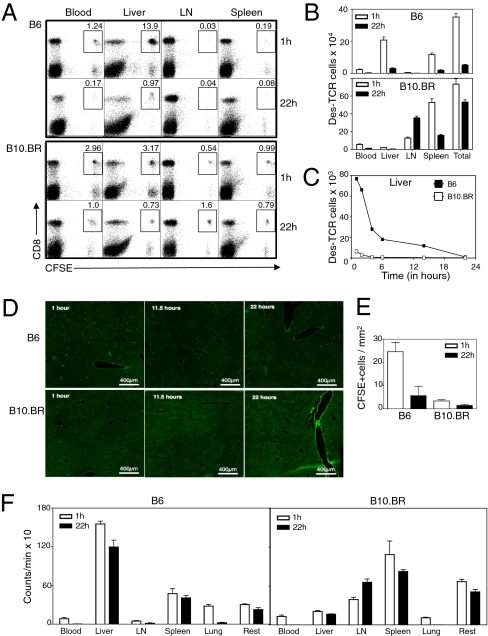
Fig. 2.
CD8 T cells initially retained in the liver were not recovered at 22 h. (A) FACS profiles of live CFSE-labeled Des cells isolated from blood, liver, LN, and spleen of B6 and B10.BR recipients at 1 h and 22 h after transfer. (B) Quantification of CFSE+ CD8+ T-cell numbers in B6 and B10.BR recipient mice at 1 and 22 h confirming early retention of donor T cells in B6 liver within 1 h after transfer and loss of 90% of donor T cells at 22 h. (C) Time course showing that 75% of donor Des cells recovered from B6 livers were lost within the first 6 h after transfer. (D) Livers sections of B6 and B10.BR recipient mice transfered with purified Oregon-green-labeled donor CD8 Des T cells at different time points. (E) Quantification of cell numbers in similar experiment to D but using CFSE labeled T cells instead of Oregon green. CFSE-labeled T cells were quantified in liver sections by manual cell counting of 10 fields by using a 10× objective. (F) Distribution of radioactivity at 1 and 22 h after transfer of 51Cr radiolabeled naïve Des cells into B6 and B10.BR mice. Results expressed as mean ± SEM of three mice per group. All data, except for D and E (two independent experiments), are representative of at least three independent experiments.
To determine whether donor T cells had migrated to other organs, donor cells were labeled with 51Cr before transfer, and all organs, including the carcass, were harvested at 1 and 22 h (Fig. 2F). Consistent with migration of CFSE+ donor T cells to the liver, up to 70% of the total radioactivity recovered from B6 animals was contained within the livers at 1 h. However, in contrast to CFSE labeling studies, which indicated a marked loss of donor T cells from the liver between 1 and 22 h, radioactivity distribution remained almost unchanged at 22 h, suggesting that the donor cell content was retained within the livers of B6 animals, despite failure to recover viable CFSE+ cells.
Early T-Cell Loss Required Recognition of Cognate Antigen in the Liver and Was Not Mediated by Recipient Lymphocytes.
Des T cells also disappear after transfer into RAG-1−/− B6 mice (Fig. S2A), suggesting recipient B cells, T cells, and natural killer (NK) T cells did not contribute to this phenomenon. Des T cells also disappeared when transferred into syngeneic 178.3 recipient mice (B10.BR mice expressing an H-2Kb transgene under the control of an MHC class I promoter) and H-2kb F1 mice (Fig. S2 B and C), indicating that T-cell loss was not mediated by recipient NK cells or alloreactive recipient T cells. Further excluding a role for NK cells, similar results were observed when OT-I CD8 T cells expressing a Tg TCR specific for the ovalbumin peptide SIINFEKL (12) were transferred into syngeneic mice injected with SIINFEKL (Fig. S2D). Interestingly, CFSE-labeled Des-TCR adoptively transferred into 178.3 bone marrow chimeras, in which H-2Kb expression was restricted to bone marrow-derived cells (13), also disappeared after initial retention in the liver (Fig. S2E), suggesting that T cell loss also occurred when T cells were activated by liver bone marrow-derived cells (mostly Kupffer cells).
Collectively, these results demonstrated that loss of antigen-specific T cells was associated with recognition of cognate antigen in the liver and was independent of NK cells and host lymphocytes.
Liver-Activated T-Cell Deletion Was Independent of Apoptosis and Phagocytic Cells.
Annexin V staining of donor CD8 T cells was not increased at 6 h, a time when most T cells had disappeared (Fig. S3A), confirming that T-cell apoptosis could not account for the massive T-cell loss occurring during this time. This was consistent with the finding that Bim−/− Des T cells transferred into B6 mice also disappeared within 22 h (Fig. S1E). In addition, efficient depletion of macrophages/Kupffer cells using clodronate liposomes and blocking potential exposure of phosphatidylserine on the T-cell surface using a dimer of its ligand, Annexin V, both failed to prevent T-cell deletion (Fig. S3 B–D).
Taken together, these results suggested that deletion of donor T cells in the liver was not mediated by apoptosis and did not require phagocytic cells.
Naïve T Cells Activated Within the Liver Invaded Hepatocytes and Were Degraded in Lysosomes.
To further investigate T-cell loss after intrahepatic activation, we performed ultrastructural studies by using electron microscopy (EM) and confocal microscopy (CM) at 3–6 h after transfer when donor T-cell loss was apparent. Surprisingly, we observed lymphocytes invading or within hepatocytes (Fig. 3 A and B). To confirm that cells contained within vesicles were donor T cells, hepatocytes were purified from B6 recipient livers within the first 12 h after transfer of CFSE-labeled donor T cells and analyzed by CM. CFSE+ T cells were detected inside purified hepatocytes (Fig. 3C and Movie S1). Other T cells had cytoplasmic extensions protruding into hepatocytes, suggesting that they were in the process of invading (Fig. 3D). CFSE+ DAPI+ T cells in hepatocytes could not be detected at the same frequency at later time-points after transfer, suggesting that the CFSE fluorescence, DNA, and T-cell content were rapidly destroyed after entry into hepatocytes. To detect potential intermediate remnants of donor T cells, we used an antibody specific for FITC that cross-reacts with CFSE. Donor T-cell remnants were identified within vesicles coated with the lysosomal marker LAMP-1 (Fig. 3E and Movie S2). Importantly, DAPI staining was inversely correlated with LAMP-1 staining, suggesting that the DNA of donor T cells was rapidly broken down in a late endosomal/lysosomal compartment before complete degradation of the T cell. Similar results were obtained in B6 mice injected with SIINFEKL followed by syngeneic OT-I Tg T cells (Fig. 3F), extending the observations to a syngeneic model. This phenomenon was also observed when H-2b T cells were injected into allogeneic H-2dk and H-2d recipient mice (Fig. S4), albeit at a much lower frequency, consistent with the lower precursor frequency of T cells specific for alloantigen that would be expected in this wild-type response. T cells were not detected in hepatocytes of control nonantigen-expressing mice in any of the experimental systems examined.
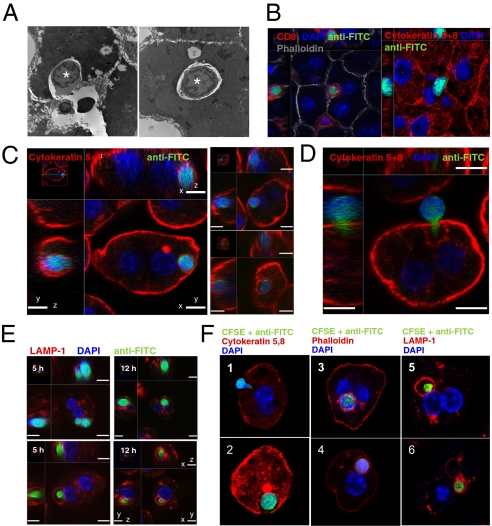
Fig. 3.
T cells invaded hepatocytes and were destroyed in lysosomal compartments. (A) TEM of a liver section showing a lymphocyte invading a hepatocyte 3 h after transfer of Des cells into an H-2 Kb+ recipient (Left). Right shows a lymphocyte contained within a giant vesicle inside a hepatocyte. Original magnification ×4,000. (B) CM of B6 liver sections show CFSE-labeled Des cells invading hepatocytes in vivo 6 h after transfer. Donor CFSE was revealed by using an anti-FITC Alexa Fluor-488 antibody. Hepatocytes express Cytokeratin5,8 and are stained in red. (C) CM of purified hepatocytes confirming that CFSE-labeled Des cells were contained within B6 hepatocytes. Right displays more examples of these cell-in-cell structures. (Scale bar: 7 μm.) (D) CFSE-labeled Des T cell with a cytoplasmic protrusion into a B6 hepatocyte suggesting active invasion. (Scale bar: 7 μm.) (E) CM showing B6 hepatocytes containing either whole DAPI+ CD8 Des T cells or a DAPI− donor T-cell remnant surrounded by LAMP-1. Hepatocytes were purified from recipient B6 mice at 5 and 12 h after transfer of CFSE-labeled T cells. (Scale bar: 7 μm.) (F) CM of CFSE-labeled OT-I T cells invading and inside hepatocytes at 4–6 h after transfer into SIINFEKL-treated B6 mice. 1, an OT-I T cell is invading a SIINFEKL-loaded hepatocyte. 2–4, a CFSE labeled OT-I T-cell is contained within a hepatocyte vesicle. 5 and 6, T-cell remnants are contained within LAMP-1+ vesicles.
Collectively, these experiments suggested that T cells undergoing intrahepatic activation entered hepatocytes and were rapidly destroyed within lysosomes. Using radiolabeled donor T cells, up to 65% of liver radioactivity was found in B6 hepatocytes (Fig. S5), suggesting that this process was responsible for most of donor T cell loss observed between 1 and 22 h.
T Cells Actively Invaded Hepatocytes.
To investigate the molecular mechanisms of this process, we determined whether T cells entered hepatocytes in vitro. Naïve CD8 Des T cells were cocultured with purified B6 and B10.BR hepatocytes and analyzed by CM and EM. At 4 h, T cells podosomal protusions extended into antigen-expressing but not control B10.BR hepatocytes (Fig. 4 A and B). After 6 h, T cells were completely contained within large vesicles inside hepatocytes (Fig. 4 A–C). Fixed or heat-inactivated T cells were not internalized (Fig. S6), indicating that T cells were not passively engulfed by phagocytosis but needed to be metabolically active to enter hepatocytes. This model was consistent with the lack of phagocytic cups around T cells (Fig. 4C) as well as live-cell imaging experiments showing that T cells were active and mobile in invading the more inert hepatocytes (Movie S3 and Movie S4).
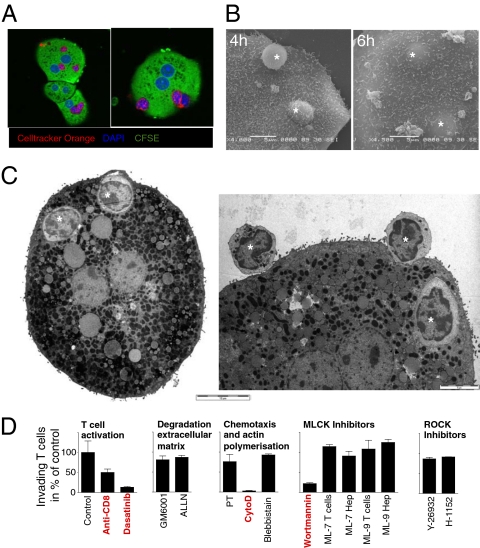
Fig. 4.
Wortmannin inhibited T-cell invasion into hepatocytes in vitro. Naïve Cell Tracker Orange-labeled CD8 Des T cells were cocultured with CFSE-labeled H-2Kb+ B6 hepatocytes for 4–6 h. T-cell invasion was visualized by using CM (A), SEM (B) and TEM (C). One, and often several, T cells (* in B and C) were contained in giant vesicles inside hepatocytes. (Scale bars in B and C: 5 μm except Left in C: 10 μm.) (D) CM-based in vitro assay was used to quantify Des cell invasion of hepatocytes in the presence or absence of specific inhibitors and antibodies. Vehicle controls (ethanol, DMSO) had no effect at the dilutions used (in all cases <0.5%). All data are representative of at least three independent experiments.
T-Cell Invasion into Hepatocytes Depended on T-Cell Activation, Cytoskeletal Rearrangement, and Wortmannin-Sensitive Kinases.
To gain further insights into the molecular process regulating T-cell invasion, we used this in vitro system to quantify T-cell invasion of hepatocytes. Hepatocytes and T cells were cocultured in the presence of inhibitors of molecular pathways potentially playing a role in invasion, including TCR signaling, degradation of extracellular matrix (MMP), G protein-coupled chemotaxis, cell adhesion and podosome formation (calpains), and polymerization/contraction of the actin/myosin cytoskeleton (actin, myosin light chain). The role of myosin light chain kinase (MLCK) and rho-associated protein kinase 1 (ROCK), two predominant kinases regulating cell movement (14) and transendothelial migration (15), were also assessed by using specific inhibitors (ML-7, ML-9, and wortmannin for MLCK; Y-27632 and H-1152 for ROCK) (Fig. 4D). Only inhibitors of T-cell activation (Dasatinib, anti-CD8 antibody), filamentous actin reorganization (cytochalasin D), and wortmannin were able to inhibit T-cell invasion in vitro (Fig. 4D). In addition to being an inhibitor of MLCK, wortmannin is a known irreversible inhibitor of several kinases including PI3 kinase, suggesting an essential role for a non-MLCK kinase in T-cell invasion.
Wortmannin Treatment Increased T-Cell Numbers in the Blood and Liver, and Led to Breach of Tolerance in B6 Mice.
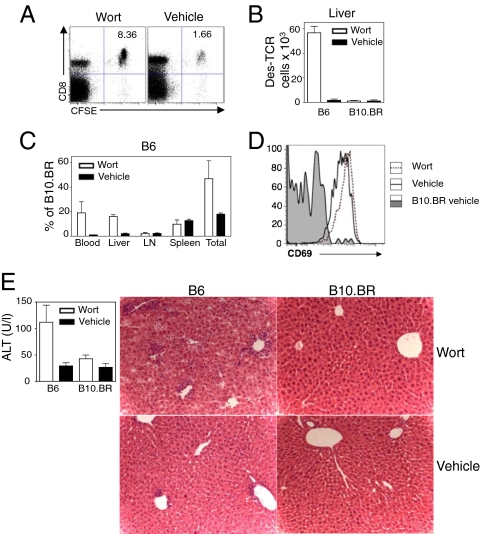
To explore whether wortmannin was also effective in inhibiting T-cell invasion into hepatocytes in vivo, B6 mice were treated with this inhibitor before transfer of Des T cells. Wortmannin has been described to be toxic in vivo (16), and we noted a general decrease in total leukocyte numbers in all treated recipients (Fig. S7A). For this reason, the number of transferred T cells in B6 mice was always compared with the number of cells in wortmannin-treated B10.BR H-2Kb- control mice, instead of untreated B6 mice alone. Wortmannin dramatically increased the percentage and number of donor T cells isolated from the blood and livers of treated B6 mice (Fig. 5 A–C). T cells from wortmannin-treated recipients were activated in a similar manner to untreated B6 mice (Fig. 5D), indicating that wortmannin inhibited a critical step in T-cell clearance rather than affecting T-cell activation. Overall, treatment of B6 mice with wortmannin increased the proportion of transferred T cells recovered at 22 h from 20% to 50% compared with B10.BR controls (Fig. 5C). Using the same quantification strategy as described above, we observed that wortmannin decreased the radioactivity in the hepatocyte fraction by 40%, confirming a direct effect of wortmannin on T-cell invasion into hepatocytes (Fig. S7B). Interestingly, T cells rescued with wortmannin treatment were able to mediate hepatitis at day 3 (Fig. 5E) in B6 mice, whereas wortmannin-treated control B10.BR and untreated B6 mice did not develop pathology (Fig. 5E).
Fig. 5.
Wortmannin inhibited Des T-cell deletion in vivo and broke tolerance in B6 mice. (A) FACS plot of leukocytes purified from the liver of wortmannin and vehicle control treated mice 22 h after transfer. (B) Bar graph showing that the number of donor T cells recovered from the livers of wortmannin treated B6 animals increased in comparison with vehicle treated B6 controls. (C) Relative proportions of Des cells from wortmannin or vehicle control treated B6 mice in relation to B10.BR control animals showing that up to 50% of T cells were rescued at 22 h compared with 20% of vehicle treated animals. (D) Des cells in B6 livers expressed similar levels of CD69 at 22 h after transfer in the presence or absence of wortmannin. (E) After Des cell transfer into untreated and wortmannin-treated animals, wortmannin treatment broke tolerance in B6 animals and induced immune-mediated hepatitis at day 3 as shown by increased ALT levels and histological liver damage (original magnification 200×). Plots indicate mean ± SEM of three mice per group. Experiment was performed at least three times for A–D and twice for E with similar results.
These experiments suggested that wortmannin inhibited a critical step in T-cell clearance and its administration broke tolerance in B6 recipient mice.
Discussion
Invasion of a cell into another cell was first reported in 1925 (17). In 1956, Humble et al. termed this process “emperipolesis” to describe a cell “inside round-about wandering” (18). The classical form of emperipolesis describes a process whereby a visiting cell enters a host cell, wanders within it, and may then depart without any physical alteration of either cell. Cell-in-cell structures have been reported in many settings (reviewed in ref. 19). Emperipolesis of lymphocytes inside hepatocytes has also been observed in liver sections from patients infected with hepatitis B, hepatitis C, and Epstein–Barr viruses, and in patients with autoimmune hepatitis (20–22). However, the significance of these structures has remained obscure.
Here, we provide physiological evidence of emperipolesis of CD8 T cells after antigen-specific primary activation in the liver. This process leads to the nonapoptotic destruction of these CD8 T cells after degradation by lysosomal proteolytic enzymes. To better describe the invasion event that leads to the death of T cells, and to distinguish it from other forms of emperipolesis, we propose to term this process “suicidal emperipolesis” (SE).
A recent study has reported the nonapoptotic death of malignant cells after their invasion into other tumor cells in vitro (23). This phenomenon (termed by the investigators “entosis”) bears similarities to the phenomenon described in this study, because invading cells died within host cells in their in vitro system. Although Overholtzer et al. (23) speculated that cell-in-cell structures increase tumor growth by providing nutrients for the survival of malignant cells lacking appropriate vascular access, it is unclear whether entosis occurs between non-tumoural cells and whether it plays a physiological role. Furthermore, unlike SE, entosis is ROCK-dependent, suggesting either that the two phenomena are distinct or that different cell types might use distinct intracellular pathways. NK and CD8 T cells have also been reported to be internalized and die by apoptosis inside tumoural cell lines (24) and metastatic melanomas (25), a process thought to provide nutrients to the tumor. Although the end result is a cell in cell structure, the cell cannibalism described in these reports might be different to entosis and SE. First, these cells seem to be internalized by the tumoral cell via a process resembling phagocytosis rather than actively invading the host cell. Second, unlike SE and entosis, most of the engulfed cells died by apoptosis after internalization. Finally, NK cell invasion resulted in the death of the internalizing tumoural cell (24). Unlike mature NK cells, recently activated CD8 T cells do not express cytolytic molecules such as granzymes and are thus unlikely to induce damage after invading hepatocytes.
We have shown that the liver, unlike other peripheral organs, supports activation of naïve T cells outside lymphoid tissues followed by deletion (6). Our current study suggests that SE is a significant mechanism by which naïve CD8 T cells activated in the liver die. This process occurs within the first few hours before T cells are able to divide and expand. Notably, it is an extremely efficient mechanism able to rapidly inactivate T cells without being saturated. Further reinforcing the role of SE in the maintenance of tolerance, inhibition of this process by wortmannin treatment in vivo was associated with breach of tolerance, with the development of immune-mediated liver damage.
Although T cells rescued by wortmannin treatment caused autoimmunity at day 3, they were unable to induce sustained damage after this time point consistent with the existence of other mechanisms silencing T cells surviving SE. Indeed, we have demonstrated that T cells activated in the liver developed poor CTL function and subsequently died by Bim-dependent apoptosis (11). We thus propose that Bim-dependent apoptosis is a fail-safe mechanism that leads to deletion of the minority of autoreactive T cells escaping degradation in hepatocytes following primary intrahepatic activation.
SE was observed in both allogeneic and syngeneic transfer systems, suggesting that it is a general mechanism by which naïve CD8 T cells recognizing their cognate antigen in the liver are deleted. The reasons why this process has evolved is not clear at this stage. Because the liver receives direct blood supply from the intestine, SE may contribute to tolerance to food antigens (oral tolerance). Additionally, the liver produces and secretes a range of proteins including albumin, clotting, and complement factors that might not be presented in the thymus and, therefore, could be potential targets of autoimmunity. By presenting peptides derived from these proteins directly to naïve T cells, the liver might be an important immunological filter, screening autoreactive T cells escaping thymic censorship.
It is interesting to note that emperipolesis has also been described between T cells and another type of epithelial cell, thymic nurse cells, which can contain up to 200 thymocytes (26). The role of thymic nurse cells and the fate of thymocytes within these cells is unclear (27). In light of our results, it is tempting to speculate that SE regulates both the deletion of autoreactive T cells in the thymus and in the periphery, in the liver, to promote immune tolerance.
In summary, this study describes a unique mechanism that removes CD8 T cells activated in the liver. Although emperipolesis has been described as early as the 1920s, to our knowledge, a physiological role has never been ascribed to this process. By showing that T cells undergoing emperipolesis are rapidly degraded in hepatocytes, our study reveals an important physiological role for emperipolesis in the immune system. This nonapoptotic mechanism is very efficient and able to inactivate large numbers of autoreactive CD8 T cells before they start to proliferate and acquire cytotoxic function. Degradation in lysosomes might be an energy-efficient process allowing rapid recycling of cell content, possibly by autophagy. We propose that SE plays a dominant role in the rapid clearance of T cells specific for antigens presented in the liver, including self- and food-derived antigens, contributing to the tolerogenic properties of liver transplants (28–30) and the susceptibility of this organ to persistent viral infections (31).
Methods
Mice.
Des, Des RAG-1−/−, Des Bim−/−, OT-I, and 178.3 Tg mice have been described (11, 12) and were bred in-house in an SPF environment. C57BL/6, CBA/CaH × C57BL/6 F1, and B10.BR were purchased from the Animal Resources Centre. All experimental procedures were approved by the University of Sydney Animal Ethics Committee.
Antibodies and Reagents.
All primary antibodies, including anti-LAMP-1 and anti-cytokeratin 5/8 antibodies, were either purified from hybridomas or purchased from BD Pharmingen. Alexa Fluor-488-anti-FITC antibody was purchased from Invitrogen; CFSE from Molecular Probes; anti-rat IgG FITC, DAPI, PI, wortmannin, Y-27632, and ML-7 from Sigma Aldrich; ALLN, Blebbistatin, Cytochalasin D (CytoD), Pertussis toxin (PT), GM6001, H-1152, and ML-9 from Calbiochem; Dasatinib from LC Laboratories, 51Cr sodium chromate from MP Biomedicals; and anti-FITC microbeads from Miltenyi Biotec. Diannexin has been described (32), and clodronate liposomes (Roche Diagnostics) and control PBS liposomes were prepared as reported (33).
Cell Tracking.
Cells were prepared as described (11). For adoptive transfer experiments, unless otherwise stated, 1.5 × 107 of lymphocytes from pooled LN of Des mice, equivalent to 5 × 106 CD8 Des T cells, were labeled with CFSE, CellTracker Orange, or 51Cr as described (34). When a pure population of CD8 T cells was required (radioactivity and microscopy experiments), CD8 Des T cells were either purified from LN suspensions of Des mice by negative selection as described (34) or from the LN of Des RAG-1−/− mice. Flow cytometry was performed by using a FACSCanto (BD Pharmingen) or LSR II (BD Pharmingen) cytometer and analyzed using FlowJo software (TreeStar) on a Macintosh computer. For radiolabeling experiments, organs were collected and counted by using a Wallac Wizard 1480 3-inch gamma-counter (PerkinElmer Wallac).
Microscopy.
To visualize T cells inside hepatocytes, hepatocytes were purified as described (13) followed by fixation in 70% ethanol and incubation with anti-Lamp-1, anti-cytokeratin 5/8, anti-rat-Alexa Fluor 594, anti-mouse-Alexa Fluor 647, and anti-FITC-Alexa Fluor 488 antibodies. CM was performed on a Nikon Eclipse C1 confocal microscope (Nikon) or Leica SP5 (Leica Microsystems) and analyzed by using ImageJ (National Institutes of Health), VGStudio Max (Volumegraphics), or Volocity (Improvision) software. Electron microscopy was performed as described (35).
In Vitro Assay of Cell Invasion.
Purified hepatocytes were labeled with CFSE, and 105 cells were cultured overnight on glass coverslips. After 14 h of coculture with 2.5 × 105 Cell Tracker Orange labeled CD8 Des T cells, cells were fixed in ice-cold acetone and analyzed by CM. Lack of overlap of orange and green fluorescence on CM sections identified T cells that had invaded hepatocytes. The number of invading T cells per hepatocyte surface area was determined using ImageJ software. Inhibitors were either added together with the T cells, or T cells and hepatocytes were preincubated for 1 h followed by two washes before the T cells were added. Inhibitors were tested at different concentrations, and any toxic effect on hepatocytes and T cells was excluded by flow cytometry for T cells or by microscopy for hepatocytes.
Inhibitors.
Pertussis toxin (preincubation of T cells for 3 h by using 100 ng/mL), Y-27632 (10 μM), and H-1152 (1 μM) were prepared in H2O, GM6001 (10 μM), ALLN (5 μM), wortmannin (500 nM), ML-7 (50 μM), and ML-9 (50 μM) were dissolved in ethanol, whereas blebbistatin (20 μM), cytochalasin D (5 μM), and dasatinib (10 nM) were dissolved in DMSO. For ML-7 and ML-9, T cells and hepatocytes were preincubated for 1 h separately because of the toxicity on the cells if the inhibitor was left in the assay. For in vivo treatment, wortmannin (1 mg/kg) was administered at 1 h before, then 7 h and 14 h after, T-cell transfer.
Supplementary Material
Acknowledgments
We thank the Centenary Institute animal and flow cytometry facilities for their technical support; D. Lovejoy for help in performing the radiolabeling experiments; B. Roediger, R. Whan, and E. Kable for help in confocal imaging; A. Strasser, P. Bouillet, and L. O'Reilly for the Bim−/− mice and the anti-Bim antibody; M. Vadas and W. Weninger for helpful comments on the manuscript; and A. Demetris for reminding us of the literature on emperipolesis. This work was supported by the National Health and Medical Research Council of Australia (Program Grant 571408) and the Ageing and Alzheimer's Research Foundation. V.B. was supported by a Deutsche Forschungsgemeinschaft Postgraduate Research Scholarship (BE 3256/1-2). P.B. was supported by a National Health and Medical Research Council Senior Research Fellowship.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1112251108/-/DCSupplemental.
References
- 1.Sprent J, Kishimoto H. The thymus and negative selection. Immunol Rev. 2002;185:126–135. doi: 10.1034/j.1600-065x.2002.18512.x. [DOI] [PubMed] [Google Scholar]
- 2.Derbinski J, et al. Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J Exp Med. 2005;202:33–45. doi: 10.1084/jem.20050471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Srinivasan M, Frauwirth KA. Peripheral tolerance in CD8+ T cells. Cytokine. 2009;46:147–159. doi: 10.1016/j.cyto.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Redmond WL, Sherman LA. Peripheral tolerance of CD8 T lymphocytes. Immunity. 2005;22:275–284. doi: 10.1016/j.immuni.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Feuerer M, et al. Bone marrow as a priming site for T-cell responses to blood-borne antigen. Nat Med. 2003;9:1151–1157. doi: 10.1038/nm914. [DOI] [PubMed] [Google Scholar]
- 6.Bertolino P, Bowen DG, McCaughan GW, Fazekas de St Groth B. Antigen-specific primary activation of CD8+ T cells within the liver. J Immunol. 2001;166:5430–5438. doi: 10.4049/jimmunol.166.9.5430. [DOI] [PubMed] [Google Scholar]
- 7.Klein I, Crispe IN. Complete differentiation of CD8+ T cells activated locally within the transplanted liver. J Exp Med. 2006;203:437–447. doi: 10.1084/jem.20051775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L. The fate of adoptively transferred antigen-specific T cells in vivo. Eur J Immunol. 1996;26:2208–2214. doi: 10.1002/eji.1830260937. [DOI] [PubMed] [Google Scholar]
- 9.Dey B, et al. The fate of donor T-cell receptor transgenic T cells with known host antigen specificity in a graft-versus-host disease model. Transplantation. 1999;68:141–149. doi: 10.1097/00007890-199907150-00026. [DOI] [PubMed] [Google Scholar]
- 10.Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol. 2003;3:51–62. doi: 10.1038/nri981. [DOI] [PubMed] [Google Scholar]
- 11.Holz LE, et al. Intrahepatic murine CD8 T-cell activation associates with a distinct phenotype leading to Bim-dependent death. Gastroenterology. 2008;135:989–997. doi: 10.1053/j.gastro.2008.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hogquist KA, et al. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 13.Bowen DG, et al. Cytokine-dependent bystander hepatitis due to intrahepatic murine CD8 T-cell activation by bone marrow-derived cells. Gastroenterology. 2002;123:1252–1264. doi: 10.1053/gast.2002.36058. [DOI] [PubMed] [Google Scholar]
- 14.Totsukawa G, et al. Distinct roles of MLCK and ROCK in the regulation of membrane protrusions and focal adhesion dynamics during cell migration of fibroblasts. J Cell Biol. 2004;164:427–439. doi: 10.1083/jcb.200306172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muller WA. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003;24:327–334. doi: 10.1016/s1471-4906(03)00117-0. [DOI] [PubMed] [Google Scholar]
- 16.Norman BH, et al. Studies on the mechanism of phosphatidylinositol 3-kinase inhibition by wortmannin and related analogs. J Med Chem. 1996;39:1106–1111. doi: 10.1021/jm950619p. [DOI] [PubMed] [Google Scholar]
- 17.Lewis WH. The engulfment of living blood cells by others of the same type. Anat Rec. 1925;31:43–49. [Google Scholar]
- 18.Humble JG, Jayne WH, Pulvertaft RJ. Biological interaction between lymphocytes and other cells. Br J Haematol. 1956;2:283–294. doi: 10.1111/j.1365-2141.1956.tb06700.x. [DOI] [PubMed] [Google Scholar]
- 19.Overholtzer M, Brugge JS. The cell biology of cell-in-cell structures. Nat Rev Mol Cell Biol. 2008;9:796–809. doi: 10.1038/nrm2504. [DOI] [PubMed] [Google Scholar]
- 20.Dienes HP. Viral and autoimmune hepatitis. Morphologic and pathogenetic aspects of cell damage in hepatitis with potential chronicity. Veroff Pathol. 1989;132:1–107. [PubMed] [Google Scholar]
- 21.Meyer zum Büschenfelde KH, Dienes HP. Autoimmune hepatitis. Definition—classification—histopathology—immunopathogenesis. Virchows Arch. 1996;429:1–12. doi: 10.1007/BF00196814. [DOI] [PubMed] [Google Scholar]
- 22.Ilić G, et al. The electron-microscopic findings on the liver in chronic abuse of heroin. Facta Universitatis. Series. Igaku To Seibutsugaku. 2006;13:6–10. [Google Scholar]
- 23.Overholtzer M, et al. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell. 2007;131:966–979. doi: 10.1016/j.cell.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 24.Wang S, et al. Internalization of NK cells into tumor cells requires ezrin and leads to programmed cell-in-cell death. Cell Res. 2009;19:1350–1362. doi: 10.1038/cr.2009.114. [DOI] [PubMed] [Google Scholar]
- 25.Lugini L, et al. Cannibalism of live lymphocytes by human metastatic but not primary melanoma cells. Cancer Res. 2006;66:3629–3638. doi: 10.1158/0008-5472.CAN-05-3204. [DOI] [PubMed] [Google Scholar]
- 26.Wekerle H, Ketelsen UP, Ernst M. Thymic nurse cells. Lymphoepithelial cell complexes in murine thymuses: Morphological and serological characterization. J Exp Med. 1980;151:925–944. doi: 10.1084/jem.151.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aguilar LK, Aguilar-Cordova E, Cartwright J, Jr, Belmont JW. Thymic nurse cells are sites of thymocyte apoptosis. J Immunol. 1994;152:2645–2651. [PubMed] [Google Scholar]
- 28.Calne RY, et al. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;223:472–476. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]
- 29.Kamada N, Brons G, Davies HS. Fully allogeneic liver grafting in rats induces a state of systemic nonreactivity to donor transplantation antigens. Transplantation. 1980;29:429–431. doi: 10.1097/00007890-198005000-00021. [DOI] [PubMed] [Google Scholar]
- 30.Benseler V, et al. The liver: A special case in transplantation tolerance. Semin Liver Dis. 2007;27:194–213. doi: 10.1055/s-2007-979471. [DOI] [PubMed] [Google Scholar]
- 31.Bowen DG, Walker CM. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature. 2005;436:946–952. doi: 10.1038/nature04079. [DOI] [PubMed] [Google Scholar]
- 32.Kuypers FA, Larkin SK, Emeis JJ, Allison AC. Interaction of an annexin V homodimer (Diannexin) with phosphatidylserine on cell surfaces and consequent antithrombotic activity. Thromb Haemost. 2007;97:478–486. [PubMed] [Google Scholar]
- 33.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: Mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 34.Bertolino P, et al. Early intrahepatic antigen-specific retention of naïve CD8+ T cells is predominantly ICAM-1/LFA-1 dependent in mice. Hepatology. 2005;42:1063–1071. doi: 10.1002/hep.20885. [DOI] [PubMed] [Google Scholar]
- 35.Warren A, et al. T lymphocytes interact with hepatocytes through fenestrations in murine liver sinusoidal endothelial cells. Hepatology. 2006;44:1182–1190. doi: 10.1002/hep.21378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.