Abstract
Ipilimumab, a monoclonal antibody against cytotoxic T lymphocyte antigen 4 (CTLA-4), has been shown to improve survival in patients with advanced metastatic melanoma. It also enhances immunity to NY-ESO-1, a cancer/testis antigen expressed in a subset of patients with melanoma. To characterize the association between immune response and clinical outcome, we first analyzed NY-ESO-1 serum antibody by ELISA in 144 ipilimumab-treated patients with melanoma and found 22 of 140 (16%) seropositive at baseline and 31 of 144 (22%) seropositive following treatment. These NY-ESO-1–seropositive patients had a greater likelihood of experiencing clinical benefit 24 wk after ipilimumab treatment than NY-ESO-1–seronegative patients (P = 0.02, relative risk = 1.8, two-tailed Fisher test). To understand why some patients with NY-ESO-1 antibody failed to experience clinical benefit, we analyzed NY-ESO-1–specific CD4+ and CD8+ T-cell responses by intracellular multicytokine staining in 20 NY-ESO-1–seropositive patients and found a surprising dissociation between NY-ESO-1 antibody and CD8 responses in some patients. NY-ESO-1–seropositive patients with associated CD8+ T cells experienced more frequent clinical benefit (10 of 13; 77%) than those with undetectable CD8+ T-cell response (one of seven; 14%; P = 0.02; relative risk = 5.4, two-tailed Fisher test), as well as a significant survival advantage (P = 0.01; hazard ratio = 0.2, time-dependent Cox model). Together, our data suggest that integrated NY-ESO-1 immune responses may have predictive value for ipilimumab treatment and argue for prospective studies in patients with established NY-ESO-1 immunity. The current findings provide a strong rationale for the clinical use of modulators of immunosuppression with concurrent approaches to favor tumor antigen-specific immune responses, such as vaccines or adoptive transfer, in patients with cancer.
Metastatic melanoma has been shown to be an immunogenic malignancy, associated with spontaneous immunity to a variety of antigens that include differentiation antigens gp100, tyrosinase, and Melan-A/MART-1, as well as cancer/testis antigens MAGE-3 or NY-ESO-1 (1–4). The development of immunotherapies to target these antigens, whether in the form of active immunization (5–10) or adoptive transfer of T cells (11, 12), has led to multiple clinical trials. Cancer vaccines in melanoma and other tumor types have taught us that strong immune responses could be induced (13–17), although only a small number of objective clinical responses have been observed to date. In contrast, adoptive transfer of T cells has been shown to lead to regression of large established melanoma tumors, as well as other tumor types (12, 18).
We chose to study NY-ESO-1, an intracellular antigen expressed in 30% to 40% of stage III and IV melanoma (4, 19–22) but transcriptionally silenced in normal adult tissues except testis and placenta, because of its capacity to spontaneously induce antibody responses in as many as 50% of patients with NY-ESO-1–expressing tumors (4, 23). With regard to T-cell responses, we have previously shown that naturally occurring antibody responses to NY-ESO-1 were consistently associated with the simultaneous presence of circulating NY-ESO-1–specific CD4+ and CD8+ T-cell responses detectable from peripheral blood lymphocytes (4, 24–26). Furthermore, cytotoxic CD8+ T lymphocytes specific for NY-ESO-1 from patients seropositive for NY-ESO-1 are able to recognize melanoma tumor cells expressing NY-ESO-1 in vitro (24). Vaccines targeting NY-ESO-1 have been shown to induce integrated antibody, CD4+ and CD8+ T-cell antigen–specific immune responses (4, 6, 27–29). Adoptive transfer of NY-ESO-1–specific T cells has induced objective clinical responses in patients with advanced melanoma (12, 18). Because of these characteristics, NY-ESO-1 is considered an excellent vaccine target as well as a good surrogate for precise measurement of immune response to antigens specifically expressed in tumor cells.
With the growing recognition that cancer can exert a profound suppressive effect on the immune response, recent efforts to increase the efficacy of melanoma immunotherapy have focused on the development of potent and specific immunomodulators. Blockade of cytotoxic T lymphocyte antigen 4 (CTLA-4), a coinhibitory molecule on activated and regulatory T cells, is the most advanced of these efforts. CTLA-4 plays a critical role in natural immune homeostasis and tolerance to self (30–32). The safety and clinical efficacy of ipilimumab, a fully human IgG1 monoclonal antibody targeting CTLA-4, has been evaluated in numerous phase I/II trials, with recent phase III evidence of significantly improved overall survival in patients with metastatic melanoma (33–37). This is particularly notable as it was the first therapy ever to show prolongation of overall survival in this disease. Changes in humoral and cellular NY-ESO-1–specific immune responses have been reported in patients with metastatic melanoma and ovarian and prostate cancer treated with ipilimumab therapy, although an association with clinical response has not yet been clearly established (38–41).
To gain a better understanding of NY-ESO-1–specific immunity following CTLA-4 blockade, we monitored antibody and T-cell responses in a cohort of patients with metastatic melanoma who received ipilimumab in several clinical trials at Memorial Sloan–Kettering Cancer Center (MSKCC) and Yale University, characterizing these immune responses and associating them with clinical outcome and survival.
Results
NY-ESO-1 Antibody Response Following Ipilimumab.
Based on our earlier observations in a small number of patients with melanoma in which we found that antibody, CD4+, and CD8+ T-cell responses against NY-ESO-1 increased following ipilimumab treatment, we decided to extend the analysis of NY-ESO-1 immunity to a larger cohort of patients with ipilimumab-treated advanced melanoma in an attempt to investigate correlation with clinical benefit. A total of 144 patients with metastatic melanoma at MSKCC or Yale University were analyzed for an NY-ESO-1 antibody response.
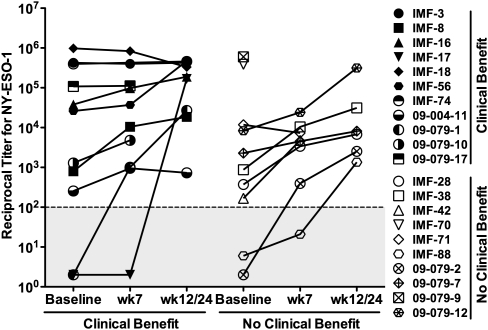
Of 99 evaluable patients with melanoma treated with ipilimumab at MSKCC, 17 (17%) had preexisting serum antibodies to NY-ESO-1, with titers ranging from 1/150 to 1/1,000,000 (Fig. 1). These numbers are within the expected range of NY-ESO-1 seropositivity in advanced melanoma, in which 30% to 40% of patients show expression of NY-ESO-1 in the tumor (4). Several NY-ESO-1–seropositive patients showed significant titer increases (>5×) during early time points of anti–CTLA-4 treatment (week 12 or 24 of treatment), followed by a plateau or gradual decrease over later time points up to 83 wk following onset of treatment (Fig. 1). Another four patients showed seroconversion to NY-ESO-1 during ipilimumab treatment at week 7 or 12 (Fig. 1). Of note, patient IMF-17, who was initially treated at dose level 0.3 mg/kg, displayed a striking NY-ESO-1 seroconversion at week 12 when the patient was switched to a higher dose of ipilimumab (10 mg/kg). Together, this represents 21 patients (21%) with antibody responses to NY-ESO-1 following anti–CTLA-4 treatment (Fig. 1).
Fig. 1.
Titers in NY-ESO-1–seropositive patients from MSKCC. Reciprocal antibody titers to NY-ESO-1 throughout CTLA-4 blockade treatment in patients experiencing clinical benefit (closed symbols, Left; n = 11) or no clinical benefit (open symbols, Right; n = 10) among 99 patients treated with ipilimumab at MSKCC. The remaining 78 patients did not show significant Ab reactivity against NY-ESO-1 at any time point tested and were considered seronegative (i.e., titers <100). Each symbol represents a patient (Right) at baseline, week 7, and week 12 or 24.
To confirm these observations, we analyzed a comparable cohort of 45 patients with melanoma treated with ipilimumab at Yale University for presence of antibodies to NY-ESO-1, in a blinded fashion. We found NY-ESO-1 seropositivity in 10 patients (22%) treated with anti–CTLA-4, with a similar range of titers to what had been observed in the MSKCC cohort. Of the 10 ipilimumab-treated patients with evidence of serum antibodies to NY-ESO-1, two cases were the result of seroconversion during treatment and two patients had no prestudy sample available. Additionally, among patients without evidence of NY-ESO-1 antibody, two did not have a prestudy sample.
We analyzed NY-ESO-1 antigen expression by immunohistochemistry in a subset of 13 patients who had available tumor tissues from their surgical resections. We confirmed that patients IMF-3, -8, -16, -38, 09-079-01, 09-079-07, and 09-079-10, who had or developed NY-ESO-1 antibodies, showed expression of NY-ESO-1 in tumor before or after ipilimumab treatment. NY-ESO-1 antigen expression by RT-PCR or IHC is shown in Table S1 and Fig. S1.
Correlation of NY-ESO-1 Antibodies with Clinical Benefit from Ipilimumab.
We then asked whether the presence of NY-ESO-1 serum antibodies before treatment correlated with eventual clinical benefit from ipilimumab according to immune-related response criteria. When analyzing the combined clinical data from MSKCC and Yale, we found that a majority of patients (12 of 22; 55%) who were seropositive for NY-ESO-1 had evidence of clinical benefit after 24 wk of ipilimumab treatment [one complete response (CR), four partial responses (PRs), and seven cases of stable disease (SD); Table 1]. In contrast, baseline seronegative patients for NY-ESO-1 had a lower frequency of clinical benefit (36 of 118; 31%; Table 1). This difference was found to be significant [P = 0.0481, relative risk (RR) = 1.8 (95% confidence interval: 1.1–2.9), two-tailed Fisher test], with an RR of 1.8 of experiencing clinical benefit when comparing NY-ESO-1–seropositive patients with NY-ESO-1–seronegative patients. Separate analyses of MSKCC and Yale cohorts showed very comparable frequencies with similar trends.
Table 1.
Correlation of NY-ESO-1 antibody at baseline (before treatment) with clinical course following anti–CTLA-4 treatment
| Response at wk 24 | Total (%) | NY-ESO-1 seronegative | NY-ESO-1 seropositive |
| Clinical benefit | 48 (34.3) | 36 (30.5) | 12 (54.6) |
| Complete response | 4 (2.9) | 3 | 1 |
| Partial response | 14 (10.0) | 10 | 4 |
| Stable disease | 30 (21.4) | 23 | 7 |
| No clinical benefit | 92 (65.7) | 82 (69.5) | 10 (45.4) |
| Total | 140 | 118 | 22 |
Patients seropositive for NY-ESO-1 are more likely to experience disease control than seronegative patients [P = 0.0481, RR = 1.8 (1.1–2.9), two-tailed Fisher test].
When considering NY-ESO-1 antibody responses at any time point (i.e., not just baseline) during the study, including patients who showed seroconversion or had missing pretherapy samples, the frequency of patients with clinical benefit was 17 of 31 (55%) of NY-ESO-1–seropositive patients compared with 34 of 113 (30%) of NY-ESO-1–seronegative patients (Table S2). The association between greater clinical benefit and NY-ESO-1–seropositive status was still significant [P = 0.02, RR = 1.8 (1.2–2.8), two-tailed Fisher test], indicating that these additional seroconversions did not affect the observation using baseline serostatus.
Interestingly, NY-ESO-1–seropositive patients appeared to have proportionally more objective responses (two CRs and six PRs among 31 seropositive patients) than seronegative patients (two CRs and nine PRs among 113 seronegative patients). Of note, although NY-ESO-1–seropositive patient IMF-38 had complete disappearance of right pleural masses and stabilization of lung metastases, this patient was classified as showing disease progression because of progressive retroperitoneal, gluteal, and brain metastases.
NY-ESO-1–Specific T-Cell Response Following Ipilimumab.
Based on the serologic results detailed here, there remained a number of patients who showed disease progression or died during ipilimumab treatment despite having serum antibodies to NY-ESO-1 (14 of 31; Table S2). To ask whether the lack of clinical benefit could be attributed to differences in other facets of NY-ESO-1 immunity, we analyzed peripheral blood mononuclear cell (PBMC) samples from NY-ESO-1–seropositive patients treated at MSKCC for CD4+ and CD8+ T-cell responses against NY-ESO-1. The generation of NY-ESO-1–specific CD4+ and CD8+ T-cell responses was evaluated based on the availability of PBMC samples in 20 NY-ESO-1–seropositive patients as well as in 11 seronegative patients. Following a 10-d in vitro stimulation with NY-ESO-1 overlapping peptides and subsequent restimulation, PBMCs from baseline and posttherapy time points were assessed for the production of IFN-γ in T-cell populations by intracellular cytokine staining (ICS).
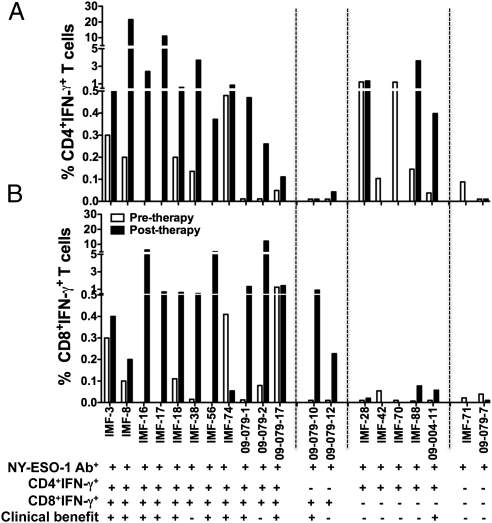
A low frequency of NY-ESO-1–specific CD4+ T-cell response was detected in five of 11 NY-ESO-1–seronegative patients. As expected from patients without serum antibody to NY-ESO-1, CD8+ T-cell responses against NY-ESO-1 were not detectable in 10 of 11 patients seronegative for NY-ESO-1, except in patient IMF-11, who received previous NY-ESO-1 protein vaccine (Table S3 and Fig. S2) (42, 43). In contrast, the majority of patients who were seropositive for NY-ESO-1 showed evidence of NY-ESO-1–specific CD4+ and/or CD8+ T-cell response (Table 2). Of 20 patients with NY-ESO-1 antibody, 17 pretherapy PBMC samples were available, with eight (47.1%) and seven (41.1%) patients having a spontaneous NY-ESO-1–specific CD4+ and CD8+ T-cell response, respectively. With regard to posttherapy samples, 18 were available, with 15 (83.3%) and 13 (72.2%) patients having an NY-ESO-1–specific CD4+ or CD8+ T-cell response, respectively (Table 2). Overall, an NY-ESO-1–specific CD4+ or CD8+ T-cell response was observed in 16 of 20 (80%) and 13 of 20 (65%) of the NY-ESO-1–seropositive patients, respectively, at any time point. Representative dot plots for patients with both NY-ESO-1–specific CD4+ and CD8+ T cells, with either CD4+ T cells or CD8+ T cells alone, or with no specific T-cell response, are shown in Fig. 2. Overall, 11 of 20 patients had both CD4+ and CD8+ T-cell responses detected; two of 20 patients had a CD8+ T-cell response alone; five of 20 patients had only a CD4+ T-cell response; whereas two of 20 did not have any quantifiable T-cell response (Fig. 3). These four patterns of cellular immune response to NY-ESO-1 in NY-ESO-1–seropositive patients are summarized in Table S4.
Table 2.
NY-ESO-1–specific response in patients treated with anti–CTLA-4 antibody
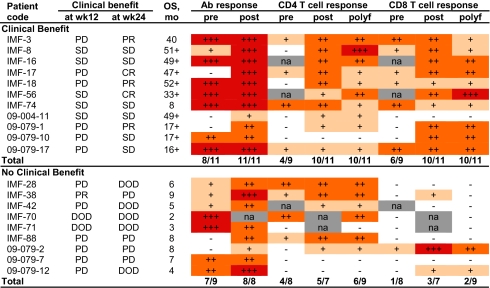 |
NY-ESO-1 Ab titer: −, negative; +, 100∼1,000; ++, 1,000∼10,000; +++, >10,000. NY-ESO-1 T-cell response: −, <0.1%; +, 0.1∼0.5%; ++, 0.5∼5%; +++, >5%. NA, not available. No availability of pretherapy PBMCs for patients IMF-16, 42, and 56. No posttherapy PBMC sample was available for patient IMF-70, 71. Polyfunctional T-cell response was defined as T cells producing double functions for IFN-γ, TNF-α, MIP-1β, and CD107a; and the value ≥0.1%. OS, overall survival; DOD, disease of death; PD, progressive disease; polyf, polyfunctionality of T cells.
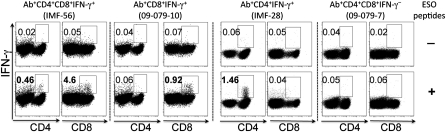
Fig. 2.
NY-ESO-1–specific CD4+ and CD8+ T-cell responses were induced after CTLA-4 blockade. Representative dot plots from four patients (IMF-56, 09–079-10, IMF-28, and 09–079-7) with or without NY-ESO-1 overlapping peptide stimulation. Patient IMF-56 had both NY-ESO-1–specific CD4+ and CD8+ T-cell response; patients 09–079-10 and IMF-28 had only NY-ESO-1–specific CD8+ T-cell response or CD4+ T-cell response, respectively; patient 09–079-7 had neither NY-ESO-1–specific CD4+ nor CD8+ T-cell response.
Fig. 3.
Four types of NY-ESO-1 immunity integration were detected during ipilimumab treatment. (A) NY-ESO-1–specific CD4+ T-cell response before and after CTLA-4 blockade. (B) NY-ESO-1–specific CD8+ T-cell response before and after CTLA-4 blockade. Category I, 11 of 20 patients had NY-ESO-1–specific CD4+ and CD8+ T-cell response; category II, two of 20 patients had CD8 T-cell response; category III, five of 20 patients had CD4+ T-cell response; and category IV, two of 20 patients had neither CD4+ nor CD8 T-cell response.
To further evaluate the functionality of these antigen-specific responses, all IFN-γ+ CD4+ and CD8+ T cells were analyzed for polyfunctional responses by using antibodies detecting MIP-1β, CD107a, and TNF-α. CTLA-4 blockade induced NY-ESO-1–specific IFN-γ+MIP1β+ and IFN-γ+TNFα+ CD4+ and CD8+ T-cell responses, consistent with our earlier findings (40). Patients with clinical benefit had more frequent evidence of double functionality by NY-ESO-1–specific CD8+ T cells than patients without clinical benefit, although these numbers were too low to establish statistical significance (Fig. S3). Of the 20 NY-ESO-1–seropositive patients, 16 (80%) and 12 (60%) patients showed the generation of a polyfunctional CD4+ or CD8+ T-cell response, respectively, defined as producing IFN-γ and at least one other function.
Correlation of NY-ESO-1–Specific CD4+ and CD8+ T-Cell Response with Clinical Benefit Using Immune-Related Response Criteria.
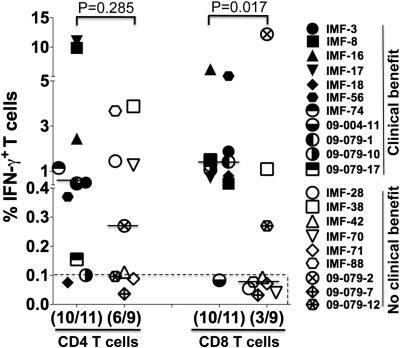
We next asked whether the presence of NY-ESO-1–specific CD4+ and CD8+ T-cell responses in patients with evidence of NY-ESO-1 antibody was correlated with clinical benefit, and results are summarized in Table 2, Fig. 4, and Table S4. With regards to NY-ESO-1–specific CD8+ T cells in these seropositive patients, pretherapy PBMC samples were available from 17 patients, and seven showed a NY-ESO-1–specific CD8+ T-cell response and 10 did not. Spontaneous CD8+ T-cell responses to NY-ESO-1 were found in six of nine seropositive patients who experienced clinical benefit, compared with one of eight seropositive patients who did not experience clinical benefit, and this difference was statistically significant [P = 0.0498, RR = 2.9 (1.1–7.7), two-tailed Fisher test; Table 2]. When considering CD8+ T-cell responses at any time point during the treatment (i.e., not just baseline), 13 of 20 available patients had a detectable CD8+ T-cell response and seven did not (Fig. 4). Among these, CD8+ T-cell responses to NY-ESO-1 were found in 10 of 11 seropositive patients who experienced clinical benefit compared with three of nine seropositive patients who did not experience clinical benefit, and this was found significant [P = 0.017, RR = 5.4 (0.9–33.9), two-tailed Fisher test; Fig. 4]. With regard to NY-ESO-1–specific CD4+ T-cell responses at any time point during the study, these were found in 10 of 11 seropositive patients who experienced clinical benefit compared with six of nine seropositive patients who did not experience clinical benefit, but this difference did not reach statistical significance (P = 0.285; Fig. 4). Overall, nine of the 11 patients (81.8%) with both NY-ESO-1–specific CD4+ and CD8+ T-cell responses had clinical benefit (Table 2, Fig. 4, and Table S4). Single functions of TNF-α, MIP-1β, and CD107a were not associated with patient clinical benefit.
Fig. 4.
NY-ESO-1 antigen-specific CD8+ T-cell responses are associated with clinical benefit. Maximal percentage of NY-ESO-1–specific CD4+ IFN-γ+ and CD8+IFN-γ+ T cell at any time point during CTLA-4 blockade treatment in NY-ESO-1–seropositive patients treated with ipilimumab at MSKCC who experienced clinical benefit (closed symbols; n = 11) or no clinical benefit (open symbols; n = 9). Responses were considered positive if at least 0.1%. Each symbol represents a patient (Right). Although the frequency of CD4+IFN-γ+ T-cell responses did not significantly differ in patients with or without clinical benefit (P = 0.285), CD8+IFN-γ+ T-cell response were significantly more frequent in patients who experienced clinical benefit (10 of 11) compared with patients who did not experience clinical benefit [three of nine; P = 0.017, RR = 5.4 (0.9–33.9), two-tailed Fisher test].
All five patients with objective responses (IMF-3, -17, -18, -56, and 09-079-1) had a detectable polyfunctional NY-ESO-1–specific CD8+ T-cell response. As we had observed before in a smaller cohort, polyfunctional NY-ESO-1–specific CD8+ T cells were induced following ipilimumab treatment in patients who showed clinical benefit (40). There was no evidence of polyfunctionality of NY-ESO-1–specific CD8+ T cells for the majority of patients without clinical benefit, except for patients 09-079-2 and 09-079-12 (Table 2).
Correlation of NY-ESO-1 Immunity with Overall Survival.
In addition to demonstrating the importance of NY-ESO-1 immunity in terms of disease control to ipilimumab, we asked whether the relationship between NY-ESO-1 antibody response alone or in conjunction with a CD4+ or CD8+ NY-ESO-1–specific T-cell response was also reflected in overall survival for the 144 patients treated at MSKCC and Yale University. By using the Kaplan–Meier method or a time-dependent Cox regression model, we found a trend between having a measurable NY-ESO-1 antibody response and experiencing improved overall survival, although this did not reach statistical significance (P = 0.10 and P = 0.06, respectively; Fig. S4 A and B). However, among patients at MSKCC, those patients who were seropositive for NY-ESO-1 and also had a detectable NY-ESO-1–specific CD8+ T-cell response showed a significant survival advantage compared with seropositive patients without detectable CD8+ T-cell response [P = 0.01, hazard ratio (HR) = 0.18 (0.05–0.71), time-dependent Cox model]. There was no statistical significant association between CD4+ T-cell response and survival in patients who were seropositive for NY-ESO-1 [P = 0.24, HR = 0.42 (0.1–1.79), time-dependent Cox model; Fig. S4C].
Discussion
In this report, we present results from the immunological monitoring of a cohort of 144 ipilimumab-treated patients with advanced melanoma tested for presence of antibody to NY-ESO-1, as well as for associated T-cell responses in a subset of 20 NY-ESO-1–seropositive individuals. Consistent with previous studies of spontaneous immunity to NY-ESO-1, integrated NY-ESO-1–specific antibody and T-cell responses (both CD4+ and CD8+) was the general pattern observed in these patients. We show that the presence of serum antibody to NY-ESO-1 is associated with clinical benefit in patients with advanced melanoma treated with anti–CTLA-4. Patients with preexisting antibody responses to NY-ESO-1 or who show a seroconversion to NY-ESO-1 during ipilimumab treatment were nearly twice as likely to experience clinical benefit compared with NY-ESO-1–seronegative patients. Furthermore, in NY-ESO-1–seropositive patients, the presence of peripheral CD8+ T-cell responses to NY-ESO-1 was highly correlated with clinical benefit to ipilimumab. Together, the presence of an integrated antibody and CD8+ T-cell immune response to NY-ESO-1 was associated with a significant survival advantage.
What influence does a strong spontaneous immune response to NY-ESO-1 have on the natural history of NY-ESO-1–expressing tumors? As this immune response is seen in patients with progressive tumor growth, the assumption is that NY-ESO-1 immunity by itself is insufficient to inhibit tumor growth, with the proviso that any influence on tumor initiation, rate of tumor growth, or frequency of metastases would be very difficult to assess. From a range of experimental tumor systems, we know that an immune response to tumors does not necessarily translate into protection against tumor growth. The reasons for this are multiple, but what has become clear is that intrinsic T-cell immunosuppressive mechanisms and extrinsic tumor-related T-cell immunosuppression are two major factors in the successful growth of tumors in the face of an immune response. Intrinsic T-cell mechanisms include various inhibitory molecules, such as TIM-3, LAG-3, and PD-1, up-regulated on T cells that inhibit their function (44–46); extrinsic factors mediating this immunosuppression are multiple, and include regulatory T cells, myeloid-derived suppressor cells, cytokines, and chemokines (47). This collective immunosuppression and the profound inhibition of CD8+ T cells in tumors would account for the failure of NY-ESO-1 immunity alone to have a clear antitumor effect. These same considerations are directly relevant to the situation with vaccine-induced NY-ESO-1 immunity, in which, despite the generation of strong humoral and T-cell immunity, tumor responses have been rare. Clearly, the development of maximally effective cancer immunotherapies will require the integration of modulators of immunosuppression, such as anti–CTLA-4, into the treatment strategy. Our results support the notion that the full potential for NY-ESO-1 immunity to control tumor growth will not be realized until our understanding of tumor-related immunosuppression is more advanced.
The antitumor effect of anti–CTLA-4 presumably involves the amplification of preexisting or induced immune responses against the tumor. Given the large number of antigenic targets in human cancer, it may appear surprising that immunity to one antigen, NY-ESO-1, is associated with antitumor responses. Searching for other antigens associated with favorable response to CTLA-4 is of obvious importance, because a majority of patients who show a response to CTLA-4 blockade show no evidence of NY-ESO-1 immunity. However, several characteristics could account for the importance of NY-ESO-1 immunity in CTLA-4 blockade. In the extensive immunological analysis of human tumors to date, NY-ESO-1 has emerged as uniquely immunogenic in comparison with other cancer/testis antigens and with other categories of tumor antigens (1–4). This immunogenicity generally results in an integrated immune response involving the generation of NY-ESO-1–specific antibodies and CD4+ and CD8+ T cells. The high immunogenicity of NY-ESO-1 may be related to its unique antigen presentation modalities (48, 49). In the present study, a majority of patients with integrated immune responses to NY-ESO-1 experienced a favorable clinical course after ipilimumab treatment. However, a subset of patients did not show detectable NY-ESO-1–specific CD8+ T cells, even though they were seropositive for NY-ESO-1, and most of them did not experience clinical benefit. The reason for the dissociation between antibody responses and antigen-specific CD8+ T-cell responses in these patients is unknown. Such a profile of split T-cell tolerance is uncommon for NY-ESO-1, and is more frequently observed with other tumor antigens, in which there is a dissociation between a strong antibody and/or CD4+ T-cell response and a weak or absent CD8+ T-cell response (50, 51). CD8+ T cells with specificity for NY-ESO-1 recognize naturally processed NY-ESO-1 on tumor cells in vitro, and in clinical trials with adoptive CD8+ T-cell transfer, NY-ESO-1–reactive CD8+ T cells have strong antitumor activity (18). This provides compelling evidence that NY-ESO-1, in addition to its strong immunogenicity, also functions as a protective tumor rejection antigen. In addition to a direct role of NY-ESO-1 immunity in mediating anti–CTLA-4 action, another possibility is that the strengthening or induction of NY-ESO-1 immunity by anti–CTLA-4 leads to activation of immunity to other tumor antigens (i.e., antigen spreading), and we are seeking evidence for this possibility by analyzing humoral immune responses by ELISA and protein arrays, and CD4+ and CD8+ T-cell responses to known melanoma antigens.
Disease stabilization, rather than complete or partial tumor regression, was the most frequent tumor response seen in anti–CTLA-4–treated melanoma. However, a number of patients showed heterogeneous tumor responses, with tumor regression, stabilization, and progressive growth occurring simultaneously in different tumor metastases in the same patient. This distinctive behavior of individual tumors can be viewed within the framework of the tumor immunoediting concept, with the three phases (“three Es”) of elimination, equilibrium, and escape having their clinical counterparts in tumor regression, stabilization, or progressive growth (52, 53). Why should stabilization rather than regression be the most frequent therapeutic response in anti–CTLA-4–treated patients? One explanation is that compensatory immunosuppressive circuits that replace CTLA-4 action are activated or strengthened in anti–CTLA-4–treated patients. In line with these multiple and distinct pathways of cancer immunosuppression, combination treatment with anti–CTLA-4 and other modulators of immune suppression, such as anti–PD-1 or anti-GITR, is now being pursued in both the laboratory and clinic (54, 55). In addition, a careful study of the changes in the gene-expression profile of tumors before, during, and after anti–CTLA-4 therapy should be very revealing, particularly with emphasis on changes in the expression pattern of cytokines, chemokines, MHC molecules, tumor antigens, and other immune-related factors. In addition, it will be essential to characterize changes in the resident and tumor infiltrating lymphocyte populations and other cell types accompanying anti–CTLA-4 therapy. The outcome of this detailed analysis may give insight into how to transform stabilization into tumor regression.
Recently in a letter to the editor, Rosenberg and coworkers reported no association between spontaneous NY-ESO-1 antibody and anti–CTLA-4 benefit in a cohort of 46 patients with advanced melanoma treated with CTLA-4 blockade at the National Cancer Institute (41). As many key factors distinguish this study from the present study, e.g., use of Response Evaluation Criteria In Solid Tumors rather than immune-related response criteria, variations in dose of antibody, and a smaller heterogeneous patient population, valid comparisons are challenging.
Together, our data suggest that antibody and CD8+ T-cell responses to NY-ESO-1 may be important predictive markers for clinical response to anti–CTLA-4 treatment. NY-ESO-1 vaccines have been shown to generate antigen-specific CD8+ T cells, along with antibody and CD4+ T cells, but these NY-ESO-1–specific immune reactions have not shown a clear impact on clinical outcome. Incorporating anti–CTLA-4 and other modulators of immune suppression into our NY-ESO-1 vaccine strategies is a pivotal next step. In addition, it will be important to extend our findings with anti–CTLA-4 in melanoma to other tumor types expressing NY-ESO-1 and eliciting NY-ESO-1 spontaneous immunity.
Materials and Methods
Patient Eligibility and Selection.
All subjects in the present study had advanced metastatic melanoma and were enrolled on several multicenter Bristol Myers-Squibb–sponsored trials of ipilimumab at MSKCC or Yale University under institutional review board-approved protocols for treatment and collection of correlative biological specimens for immunologic analysis. Further details are provided in SI Materials and Methods.
Antibody Responses and in Vitro T-Cell Stimulation Against NY-ESO-1.
Analysis of serum samples for NY-ESO-1 by ELISA was performed in a blinded fashion to the knowledge of clinical results by using a method described previously (56). T-cell culture for NY-ESO-1 in vitro was performed for 10 d, based on a well validated protocol (40, 57). SI Materials and Methods provides further details and information regarding data analysis and statistical methods.
Supplementary Material
Acknowledgments
The authors thank Dr. Arvin Yang for comments on the manuscript. This work was supported by National Institutes of Health Grant P01CA33049, an RC2 grant, Swim Across America, the Experimental Therapeutics Center of Memorial Sloan-Kettering Cancer Center, the Cancer Vaccine Collaborative of the Cancer Research Institute, the Ludwig Trust, a Damon Runyon–Lilly Clinical Investigator Award (to J.D.W.), the Melanoma Research Alliance (J.D.W.), and the Yale Specialized Program of Research Excellence (SPORE) in Skin Cancer funded by National Cancer Institute Grant 1 P50 CA121974 (to R.H.).
Footnotes
Conflict of interest statement: J.P.A. and J.D.W. are paid consultants to Bristol-Myers Squibb. J.P.A. is the primary inventor on the patent “Blockade of T lymphocyte down-regulation associated with CTLA-4 signaling.”
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1110814108/-/DCSupplemental.
References
- 1.Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- 2.Wang RF, Rosenberg SA. Human tumor antigens for cancer vaccine development. Immunol Rev. 1999;170:85–100. doi: 10.1111/j.1600-065x.1999.tb01331.x. [DOI] [PubMed] [Google Scholar]
- 3.Preuss KD, Zwick C, Bormann C, Neumann F, Pfreundschuh M. Analysis of the B-cell repertoire against antigens expressed by human neoplasms. Immunol Rev. 2002;188:43–50. doi: 10.1034/j.1600-065x.2002.18805.x. [DOI] [PubMed] [Google Scholar]
- 4.Gnjatic S, et al. NY-ESO-1: Review of an immunogenic tumor antigen. Adv Cancer Res. 2006;95:1–30. doi: 10.1016/S0065-230X(06)95001-5. [DOI] [PubMed] [Google Scholar]
- 5.Old LJ. Cancer vaccines: An overview. Cancer Immun. 2008;8(suppl 1):1. [PubMed] [Google Scholar]
- 6.Jäger E, et al. Induction of primary NY-ESO-1 immunity: CD8+ T lymphocyte and antibody responses in peptide-vaccinated patients with NY-ESO-1+ cancers. Proc Natl Acad Sci USA. 2000;97:12198–12203. doi: 10.1073/pnas.220413497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan J, et al. Safety and immunogenicity of a human and mouse gp100 DNA vaccine in a phase I trial of patients with melanoma. Cancer Immun. 2009;9:5. [PMC free article] [PubMed] [Google Scholar]
- 8.Wolchok JD, et al. Safety and immunogenicity of tyrosinase DNA vaccines in patients with melanoma. Mol Ther. 2007;15:2044–2050. doi: 10.1038/sj.mt.6300290. [DOI] [PubMed] [Google Scholar]
- 9.Speiser DE, et al. Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J Clin Invest. 2005;115:739–746. doi: 10.1172/JCI23373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palucka K, Ueno H, Banchereau J. Recent developments in cancer vaccines. J Immunol. 2011;186:1325–1331. doi: 10.4049/jimmunol.0902539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21:233–240. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunder NN, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358:2698–2703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higano CS, et al. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115:3670–3679. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- 14.Bendandi M, et al. Complete molecular remissions induced by patient-specific vaccination plus granulocyte-monocyte colony-stimulating factor against lymphoma. Nat Med. 1999;5:1171–1177. doi: 10.1038/13928. [DOI] [PubMed] [Google Scholar]
- 15.Kantoff PW, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jäger E, et al. Recombinant vaccinia/fowlpox NY-ESO-1 vaccines induce both humoral and cellular NY-ESO-1-specific immune responses in cancer patients. Proc Natl Acad Sci USA. 2006;103:14453–14458. doi: 10.1073/pnas.0606512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steele JC, et al. Phase I/II trial of a dendritic cell vaccine transfected with DNA encoding melan A and gp100 for patients with metastatic melanoma. Gene Ther. 2011;18:584–593. doi: 10.1038/gt.2011.1. [DOI] [PubMed] [Google Scholar]
- 18.Robbins PF, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2011;29:917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen YT, et al. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci USA. 1997;94:1914–1918. doi: 10.1073/pnas.94.5.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jungbluth AA, et al. Immunohistochemical analysis of NY-ESO-1 antigen expression in normal and malignant human tissues. Int J Cancer. 2001;92:856–860. doi: 10.1002/ijc.1282. [DOI] [PubMed] [Google Scholar]
- 21.Barrow C, et al. Tumor antigen expression in melanoma varies according to antigen and stage. Clin Cancer Res. 2006;12:764–771. doi: 10.1158/1078-0432.CCR-05-1544. [DOI] [PubMed] [Google Scholar]
- 22.Velazquez EF, et al. Expression of the cancer/testis antigen NY-ESO-1 in primary and metastatic malignant melanoma (MM)—correlation with prognostic factors. Cancer Immun. 2007;7:11. [PMC free article] [PubMed] [Google Scholar]
- 23.Stockert E, et al. A survey of the humoral immune response of cancer patients to a panel of human tumor antigens. J Exp Med. 1998;187:1349–1354. doi: 10.1084/jem.187.8.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jäger E, et al. Simultaneous humoral and cellular immune response against cancer-testis antigen NY-ESO-1: definition of human histocompatibility leukocyte antigen (HLA)-A2-binding peptide epitopes. J Exp Med. 1998;187:265–270. doi: 10.1084/jem.187.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jäger E, et al. Monitoring CD8 T cell responses to NY-ESO-1: correlation of humoral and cellular immune responses. Proc Natl Acad Sci USA. 2000;97:4760–4765. doi: 10.1073/pnas.97.9.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gnjatic S, et al. Survey of naturally occurring CD4+ T cell responses against NY-ESO-1 in cancer patients: correlation with antibody responses. Proc Natl Acad Sci USA. 2003;100:8862–8867. doi: 10.1073/pnas.1133324100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis ID, et al. Recombinant NY-ESO-1 protein with ISCOMATRIX adjuvant induces broad integrated antibody and CD4(+) and CD8(+) T cell responses in humans. Proc Natl Acad Sci USA. 2004;101:10697–10702. doi: 10.1073/pnas.0403572101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valmori D, et al. Vaccination with NY-ESO-1 protein and CpG in Montanide induces integrated antibody/Th1 responses and CD8 T cells through cross-priming. Proc Natl Acad Sci USA. 2007;104:8947–8952. doi: 10.1073/pnas.0703395104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karbach J, et al. Tumor-reactive CD8+ T-cell responses after vaccination with NY-ESO-1 peptide, CpG 7909 and Montanide ISA-51: association with survival. Int J Cancer. 2010;126:909–918. doi: 10.1002/ijc.24850. [DOI] [PubMed] [Google Scholar]
- 30.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brunner MC, et al. CTLA-4-mediated inhibition of early events of T cell proliferation. J Immunol. 1999;162:5813–5820. [PubMed] [Google Scholar]
- 32.Karandikar NJ, Vanderlugt CL, Walunas TL, Miller SD, Bluestone JA. CTLA-4: A negative regulator of autoimmune disease. J Exp Med. 1996;184:783–788. doi: 10.1084/jem.184.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weber JS, et al. Phase I/II study of ipilimumab for patients with metastatic melanoma. J Clin Oncol. 2008;26:5950–5956. doi: 10.1200/JCO.2008.16.1927. [DOI] [PubMed] [Google Scholar]
- 35.Weber J, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15:5591–5598. doi: 10.1158/1078-0432.CCR-09-1024. [DOI] [PubMed] [Google Scholar]
- 36.Wolchok JD, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 37.Robert C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 38.Hodi FS, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci USA. 2008;105:3005–3010. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fong L, et al. Potentiating endogenous antitumor immunity to prostate cancer through combination immunotherapy with CTLA4 blockade and GM-CSF. Cancer Res. 2009;69:609–615. doi: 10.1158/0008-5472.CAN-08-3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan J, et al. CTLA-4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit. Proc Natl Acad Sci USA. 2008;105:20410–20415. doi: 10.1073/pnas.0810114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goff SL, Robbins PF, El-Gamil M, Rosenberg SA. No correlation between clinical response to CTLA-4 blockade and presence of NY-ESO-1 antibody in patients with metastatic melanoma. J Immunother. 2009;32:884–885. doi: 10.1097/CJI.0b013e3181affbf0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adams S, et al. Immunization of malignant melanoma patients with full-length NY-ESO-1 protein using TLR7 agonist imiquimod as vaccine adjuvant. J Immunol. 2008;181:776–784. doi: 10.4049/jimmunol.181.1.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuan J, et al. CTLA-4 blockade increases antigen-specific CD8(+) T cells in prevaccinated patients with melanoma: Three cases. Cancer Immunol Immunother. 2011;60:1137–1146. doi: 10.1007/s00262-011-1011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fourcade J, et al. PD-1 is a regulator of NY-ESO-1-specific CD8+ T cell expansion in melanoma patients. J Immunol. 2009;182:5240–5249. doi: 10.4049/jimmunol.0803245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsuzaki J, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci USA. 2010;107:7875–7880. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fourcade J, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nagata Y, et al. Differential presentation of a soluble exogenous tumor antigen, NY-ESO-1, by distinct human dendritic cell populations. Proc Natl Acad Sci USA. 2002;99:10629–10634. doi: 10.1073/pnas.112331099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeng G, et al. Dendritic cell surface calreticulin is a receptor for NY-ESO-1: direct interactions between tumor-associated antigen and the innate immune system. J Immunol. 2006;177:3582–3589. doi: 10.4049/jimmunol.177.6.3582. [DOI] [PubMed] [Google Scholar]
- 50.Gnjatic S, et al. NY-CO-58/KIF2C is overexpressed in a variety of solid tumors and induces frequent T cell responses in patients with colorectal cancer. Int J Cancer. 2010;127:381–393. doi: 10.1002/ijc.25058. [DOI] [PubMed] [Google Scholar]
- 51.Tsuji T, et al. Split T cell tolerance against a self/tumor antigen: Spontaneous CD4 but not CD8 T cell responses against p53 in cancer patients and healthy donors. PLoS ONE. 2011;6:e23651. doi: 10.1371/journal.pone.0023651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 53.Yuan J, et al. Correlation of clinical and immunological data in a metastatic melanoma patient with heterogeneous tumor responses to ipilimumab therapy. Cancer Immun. 2010;10:1. [PMC free article] [PubMed] [Google Scholar]
- 54.Mitsui J, et al. Two distinct mechanisms of augmented antitumor activity by modulation of immunostimulatory/inhibitory signals. Clin Cancer Res. 2010;16:2781–2791. doi: 10.1158/1078-0432.CCR-09-3243. [DOI] [PubMed] [Google Scholar]
- 55.Cohen AD, et al. Agonist anti-GITR monoclonal antibody induces melanoma tumor immunity in mice by altering regulatory T cell stability and intra-tumor accumulation. PLoS ONE. 2010;5:e10436. doi: 10.1371/journal.pone.0010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gnjatic S, Old LJ, Chen YT. Autoantibodies against cancer antigens. Methods Mol Biol. 2009;520:11–19. doi: 10.1007/978-1-60327-811-9_2. [DOI] [PubMed] [Google Scholar]
- 57.Lin Y, et al. Optimization and validation of a robust human T-cell culture method for monitoring phenotypic and polyfunctional antigen-specific CD4 and CD8 T-cell responses. Cytotherapy. 2009;11:912–922. doi: 10.3109/14653240903136987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.