Abstract
The mechanisms through which dietary restriction enhances health and longevity in diverse species are unclear. The transsulfuration pathway (TSP) is a highly conserved mechanism for metabolizing the sulfur-containing amino acids, methionine and cysteine. Here we show that Drosophila cystathionine β-synthase (dCBS), which catalyzes the rate-determining step in the TSP, is a positive regulator of lifespan in Drosophila and that the pathway is required for the effects of diet restriction on animal physiology and lifespan. dCBS activity was up-regulated in flies exposed to reduced nutrient conditions, and ubiquitous or neuron-specific transgenic overexpression of dCBS enhanced longevity in fully fed animals. Inhibition of the TSP abrogated the changes in lifespan, adiposity, and protein content that normally accompany diet restriction. RNAi-mediated knockdown of dCBS also limited lifespan extension by diet. Diet restriction reduced levels of protein translation in Drosophila, and we show that this is largely caused by increased metabolic commitment of methionine cycle intermediates to transsulfuration. However, dietary supplementation of methionine restored normal levels of protein synthesis to restricted animals without affecting lifespan, indicating that global reductions in translation alone are not required for diet-restriction longevity. Our results indicate a mechanism by which dietary restriction influences physiology and aging.
Keywords: hydrogen sulfide, essential amino acids, metabolism, healthspan
For a broad range of taxonomically diverse organisms, the quality of their diet acts as a powerful modulator of health and longevity through molecular mechanisms that are largely unknown. Lifespan is extended, for example, when food is restricted to an extent that falls short of inducing starvation. In mammals, this manipulation, which is often called dietary restriction (DR), not only increases lifespan but also imparts a broad-spectrum improvement in health during aging. In humans, for example, DR reduces risk factors for diabetes, cardiovascular disease, and cancer (1).
Transsulfuration is an evolutionarily ancient metabolic process that involves a network of enzymes responsible for the metabolism of sulfur-containing amino acids. The transsulfuration pathway (TSP) has been studied extensively in mammals, in which it has been shown to direct the conversion of homocysteine to cysteine following the breakdown of methionine, an essential amino acid. Flux through the TSP is known to affect overall cellular metabolism by directly influencing cysteine and methionine levels. Methionine availability affects protein synthesis and methylation, and it has been implicated in murine aging (2). Cysteine availability controls the synthesis of glutathione (GSH), which is the chief regulator of cellular redox homeostasis and an important agent in xenobiotic detoxification (Fig. 1A). Patients with genetic defects in the TSP are characterized by high levels of homocysteine, low levels of GSH, and increased incidence of age-related pathologies. Elevated homocysteine level is also associated with neurodegenerative disorders such as Parkinson disease and Alzheimer's disease (3–5).
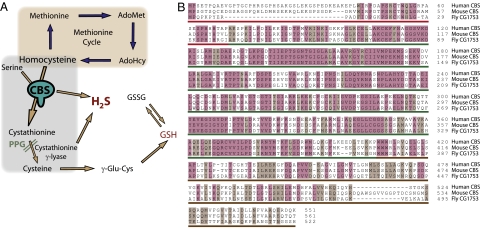
Fig. 1.
CG1753 is the D. melanogaster CBS. (A) Schematic representation of the TSP and associated relationships. Components of the TSP are shown within the gray box. (B) Multiple sequence alignment of human, mouse, and fly CBS proteins shows a high degree of evolutionary conservation. Three regions containing important domains in human CBS are indicated by underlining: the heme binding domain (40% sequence identity; red), a catalytic domain (64% sequence identity; green), and two CBS domains (28% sequence identity; brown).
The central metabolic and regulatory functions of the TSP, together with its connection to age-related diseases, led us to examine whether it plays a causal role in aging or in diet-dependent longevity extension in the fruit fly, Drosophila melanogaster. We identified key components of the TSP in Drosophila and found that DR results in an increase in gene expression, protein level, and endogenous activity of the rate-limiting enzyme of the pathway. We also found that transgene-mediated increases in gene expression and enzyme activity of Drosophila cystathionine β-synthase (dCBS) are sufficient to increase fly lifespan and that inhibition of the TSP effectively blocks the lifespan extension normally observed in diet-restricted animals. Modulation of the TSP was also found to underlie the impact of diet on overall levels of protein translation, as well as on physiological homeostasis, suggesting that it is a key mechanism by which multiple aspects of organism biology respond to changes in nutrient availability.
Results
To determine whether the TSP is important for the DR response, we first asked whether key components of the pathway are conserved in Drosophila. Because mammalian cystathionine β-synthase (CBS) catalyzes the first and rate-determining step in the TSP, which involves the pyridoxal 5′-phosphate–dependent condensation of serine and homocysteine to form cystathionine (6), we focused on the CBS enzyme (Fig. 1A). By using the human CBS protein sequence to search for the Drosophila gene, we confirmed previous indications that D. melanogaster has a single potential homologue, CG1753 (6). As with human CBS, CG1753 contains a catalytic domain, a regulatory heme domain (6, 7), and two CBS domains that have been shown to function in AMP/ATP sensing in other proteins (8). CG1753 showed high similarity to human CBS in all these putative functional domains, including 64% identity in the catalytic domain (Fig. 1B). We measured CBS activity of purified CG1753 by using radiolabeling methods, and found it to be equally or more active than human CBS, which is in agreement with the high sequence identity (9, 10). The heme spectrum of CG1753 protein is also identical to human CBS (Fig. S1). Together, these results indicate that CG1753 is the single fly orthologue of human CBS, and we hereafter refer to it as dCBS.
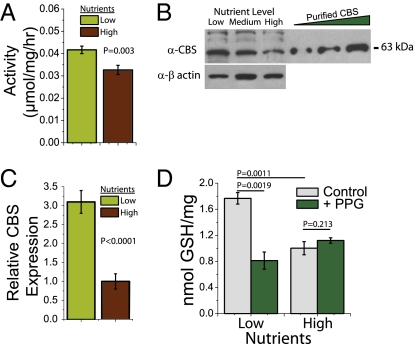
We next asked whether diet alters dCBS activity or activity of the TSP in vivo. We found that endogenous dCBS activity was increased in extracts prepared from diet-restricted flies compared with extracts from fully fed animals (Fig. 2A). We observed a concomitant increase in dCBS protein (Fig. 2B) and dCBS mRNA (Fig. 2C), which indicates significant transcriptional up-regulation. Notably, GSH is a downstream metabolite of the TSP; its synthesis is dependent on the availability of cysteine. GSH levels were increased in diet-restricted flies (Fig. 2D, gray bars). These data indicate that the significant increases in dCBS mRNA and protein levels in low-nutrient conditions led to an increase in overall TSP activity.
Fig. 2.
DR activates dCBS and transsulfuration in Drosophila. (A) Endogenous dCBS enzyme activity in whole-fly homogenates is increased upon DR, indicating increased cystathionine production and TSP activity. (B) dCBS protein abundance is higher in reduced-nutrient conditions. β-Actin was used as a loading control. (C) Food restriction results in an approximately threefold increase in dCBS mRNA level. (D) DR results in an increased concentration of the reduced form of GSH, which is reversed by PPG.
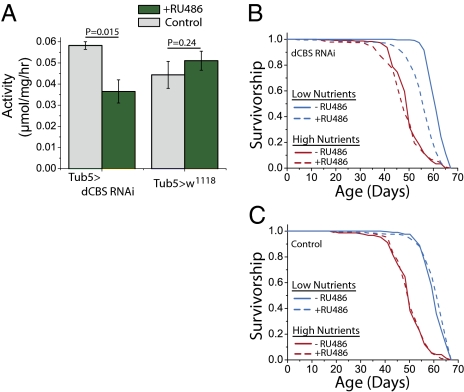
If DR involves up-regulation of dCBS and the TSP, we would expect that inhibition of the pathway should abrogate any diet-dependent changes in lifespan. CBS−/− KO mice exhibit severe growth retardation and death by 5 wk of age (11), and we found that strong, constitutive expression of dCBS RNAi resulted in death during development. We therefore targeted RNAi-mediated knock-down of dCBS to the adult stage by using the Gene-switch inducible expression system (12, 13). Using this system, transgenic expression is promoted when flies are exposed to the drug mifepristone (RU486), which is administered by placing it in the adult food (200 μM). Ubiquitous expression of dCBS RNAi using a Geneswitch GAL4 driver under control of the α-tubulin84B promoter was ineffective at 25 °C; we found no detectable change in dCBS mRNA or CBS enzyme activity in whole-animal homogenates from 10-d-old flies that were exposed to RU486 for 1 wk, suggesting inefficient knockdown. As expected, RNAi transgene expression at 25 °C also had no effect on lifespan. Geneswitch drivers often result in weaker transgene expression compared with traditional GAL4 drivers, which likely explains the observed differences in larval vs. adult effects of dCBS RNAi. To enhance the potential for effective knockdown in the adult only, we coexpressed dicer2 by using the Tubulin Geneswitch driver and observed a partial reduction in dCBS activity in fly homogenates from diet-restricted animals (Fig. 3A). When flies were aged at 29 °C to further enhance RNAi expression, we found that dCBS RNAi partially abrogated increased lifespan by DR (Fig. 3B) but had no effect on fully fed animals (Fig. 3 B and C). These results are consistent with a requirement for dCBS up-regulation for increased lifespan under low-nutrient conditions.
Fig. 3.
RNAi-mediated knockdown of dCBS partially reverses DR-mediated lifespan extension. (A) Transgenic expression of dCBS RNAi in diet-restricted flies by exposure to RU486 decreases CBS enzyme activity; however, there is no change in the control genotype following exposure to RU486. (B) Flies exposed to RNAi-mediated knockdown of dCBS exhibit a partial rescue of DR-mediated lifespan extension (P < 1 × 10−10). dCBS RNAi has no significant effect on lifespan in high-nutrient conditions (P = 0.22); n = 239 and n = 240 for RU486+ and RU486− (low nutrients); n = 238 and n = 240 for RU486+ and RU486− (high nutrients). (C) RU486 has no effect on lifespan in control genotypes consisting of Geneswitch tubulin (i.e., Tub5) and UAS-Dicer alone (no UAS-RNA); n = 240 and n = 240 for RU486+ and RU486− (low nutrients); n = 239 and n = 240 for RU486+ and RU486− (high nutrients).
The difficulty of effective manipulation of dCBS by RNAi in adult flies led us to investigate pharmacological methods to alter TSP activity. We used propargylglycine (PPG) to inhibit the second enzyme of the TSP, γ-cystathionase (Fig. 1). PPG is a specific suicidal inhibitor of γ-cystathionase, and it has been used extensively in cell culture and mouse model studies as an efficient and specific tool for modulation of TSP activity (14, 15). To determine whether PPG targeted and inhibited the TSP in Drosophila, we measured its effect on the levels of GSH, which we have shown previously to be affected by diet. We confirmed that GSH levels were decreased by PPG administration in flies subjected to DR, whereas there was no effect of the inhibitor on fully fed animals (Fig. 2D). These results confirm that PPG targets and inhibits the TSP in flies as it does in other organisms, and they support the notion that it effectively blocks increased pathway activity following DR.
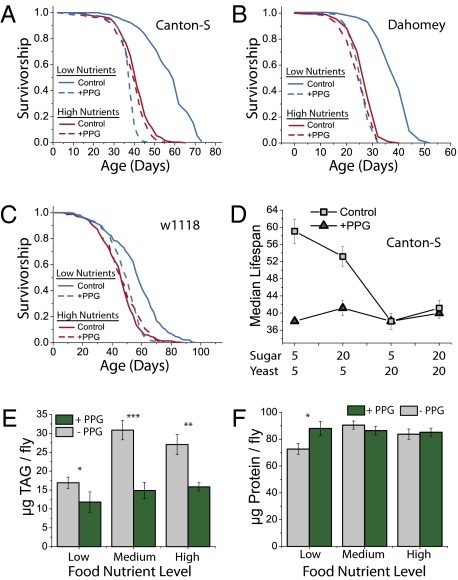
Consistent with our results from dCBS RNAi, we observed a robust suppression of DR lifespan extension upon administration of PPG. Among female flies that were administered the inhibitor, survival in low- and high-nutrient conditions was statistically indistinguishable (Fig. 4A). Furthermore, longevity of fully fed flies was not affected by the addition of PPG, indicating that its effects are specific to diet restriction. We repeated this test using two additional wild-type lines (in two laboratories) and in all cases observed a complete suppression of lifespan extension (Fig. 4 B and C). We also observed that PPG abrogated the changes in lifespan that are normally observed when flies are maintained in different dietary concentrations and compositions (Fig. 4D). For unknown reasons, the response of male flies to DR is known to be muted in comparison with females, and we find similar, but generally lesser, effects of PPG in this sex (Fig. S2).
Fig. 4.
Inhibition of the TSP abrogates diet-mediated changes in longevity and physiology. (A–C) Survivorship of female flies from three different genetic backgrounds diet-restricted or fully fed, with or without the specific TSP inhibitor PPG (Materials and Methods provides diet recipes). Inhibition of the TSP using PPG reverses DR lifespan extension. There is no effect of PPG on fully fed flies. (A) Canton S background: P < 1 × 10−10; n = 280 and n = 253 for control and +PPG, respectively, under diet restriction; and P = 0.003; n = 278 and n = 279 for control and +PPG, respectively, in fully fed conditions. (B) Dahomey background: n = 181 and n = 182 for control and +PPG, respectively, under diet restriction; and n = 181 and n = 205 for control and +PPG in fully fed flies. (C) w1118 background: P < 1 × 10−10; n = 234 and n = 237 for control and +PPG, respectively, under diet restriction; and P = 0.286; n = 237 and n = 239 for fully fed control and +PPG, respectively. (D) Median lifespans of Canton-S female flies across a range of dietary compositions with and without PPG. Error bars represent 95% confidence intervals of the median. Sample sizes are as follows: 5S/5Y, n = 280 (control), n = 270 (+PPG); 5S/20Y, n = 284 (control), n = 276 (+PPG); 20S/5Y, n = 275 (control), n = 275 (+PPG); and 20S/20Y, n = 278 (control), n = 279 (+PPG). (E) Inhibition of transsulfuration reduces triglyceride levels and abrogates changes normally induced by diet. (F) Inhibition of transsulfuration increases total protein content in diet-restricted flies and abrogates changes normally induced by diet. Similar data are presented for males in Fig. S2 (*P < 0.10; **P ≤ 0.001; ***P ≤ 0.0003).
Nutrient manipulation in flies and in other organisms influences a range of phenotypes that are associated with general health, including fat deposition and overall energy balance (16). Similar to mammals, Drosophila store energy from surplus calories in the form of triglycerides in specialized lipid droplet-containing cells (17). In flies that were exposed to diet-restriction, inhibition of the TSP significantly reduced levels of triglyceride and significantly increased levels of protein (Fig. 4 E and F), effectively eliminating the effects of diet on these physiological phenotypes. Triglyceride levels were significantly reduced in flies from all diets. Total protein levels, however, were specifically increased under DR conditions to the levels observed for fully fed flies. These results suggest that, in addition to its effects on lifespan, increased TSP activity in response to DR is associated with a metabolic shift that favors lipid storage and limits protein synthesis.
We next tested whether enhanced dCBS levels and TSP activity are sufficient to increase lifespan under fully fed conditions. We generated transgenic animals with dCBS cDNA fused downstream of the yeast upstream activating sequence for use in the Drosophila GAL4/UAS bipartite expression system. We took advantage of the ϕC31/PACMAN system to eliminate variation caused by insertional mutations by targeting constructs into known genomic locations (18). To maintain background homogeneity, transgenic and control lines were obtained directly, without the use of chromosome balancer stocks. We confirmed effective and inducible transgene expression in adult flies (driven by the ubiquitous Geneswitch tubulin driver) by observing increases in dCBS mRNA and in CBS enzyme activity in whole-animal extracts (Fig. 5A).
Fig. 5.
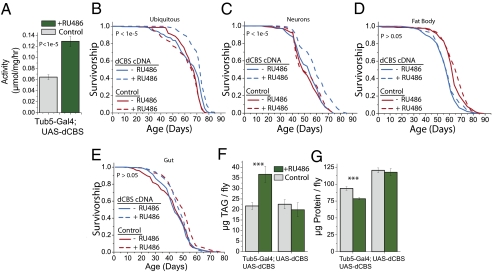
dCBS overexpression increases lifespan and alters physiology. (A) Endogenous dCBS enzyme activity is increased by transgenic expression of dCBS cDNA under control of a ubiquitous (tubulin, i.e., Tub5) Geneswitch promoter, establishing successful integration of the plasmid into the ϕC31 integration site and functional dCBS gene expression. mRNA levels are also increased. (B) Overexpression of dCBS cDNA under the control of a ubiquitous (tubulin) Geneswitch promoter increases fly lifespan in fully fed conditions (15% sugar/yeast media). Transgenic expression is induced by exposure of flies to the drug RU486. We found no effect of RU486 on lifespan in control lines, further confirming that longevity extension results from dCBS overexpression. At least three independent replicate experiments revealed ubiquitous dCBS overexpression lifespan extension ranging from 12% to 43% (Table S1). (C) dCBS overexpression under the control of a neuronal driver (Elav) modestly extends lifespan. (D) dCBS overexpression predominantly in abdominal fat body, using the Geneswitch S1-106 driver, did not affect lifespan. (E) Overexpression of dCBS in gut, using the Geneswitch TIGS-2 driver, also had no effect on lifespan. (F and G) dCBS overexpression increases levels of triglycerides (TAG) (F) and decreases total protein (G); ***P ≤ 0.003. Relevant statistics for replicate longevity experiments are presented in Table S1.
We compared lifespans and physiology of fully fed transgenic and control flies in the presence and absence of RU486. We found in independent experiments (Table S1) that adult-specific ubiquitous dCBS expression was sufficient to increase female longevity from 12% to 43% (Fig. 5B and Table S1). Males, whose lifespan is relatively less affected by DR, exhibited a smaller, but significant, increase in lifespan upon dCBS overexpression (Fig. S3 and Table S1). No such difference was observed in the empty-vector control lines, which rules out genomic-position effects and nonspecific effects of RU486 as causes for longevity extension. We then asked whether dCBS overexpression in key tissues that are known to be involved in the DR response in flies and other model systems is sufficient to affect lifespan. We found that neuronal overexpression also increased lifespan (Fig. 5C), albeit modestly (approximately 12%), whereas overexpression in the fat body and the gut had no effect (Fig. 5 D and E). Finally, overexpression mirrored the effect of TSP inhibition on physiology, supporting the idea that dCBS and TSP activation potentiate TAG storage and limit protein synthesis (Fig. 5 F and G).
We next sought to define the underlying mechanism(s) through which dCBS and the TSP modulate lifespan and physiology in response to diet. We first investigated how the TSP and dCBS are activated under reduced-nutrient conditions. dCBS has significant homology to human CBS in two putative “CBS domains,” which are found in otherwise unrelated proteins where they function as energy sensors (Fig. 1B). AMPK, for example, which is required for certain methods of diet restriction to increase Caenorhabditis elegans lifespan, contains two pairs of CBS domains that bind AMP directly to mediate its activity (19, 20). We therefore investigated whether dCBS activity was affected by changes in the ATP/AMP ratio. Having cloned and purified dCBS protein, we measured the activity of the recombinant protein using an in vitro assay to determine the sensitivity of dCBS activity to the presence/absence of ATP and AMP. We found no effect of these nucleotides on activity (Fig. S4). Thus, we find no evidence that in vitro dCBS activity is sensitive to ATP or AMP levels.
Based on our observations that TSP inhibition and dCBS overexpression affected total protein levels (Figs. 4F and 5G), we considered the possibility that the TSP might directly impact aging by modulation of global mRNA translation. Activation of the TSP would be predicted to drain metabolites from the methionine cycle and limit availability of this amino acid for protein synthesis. Reduction of general translation is associated with DR longevity in Saccharomyces cerevisiae and C. elegans (21–23), perhaps through a mechanism whereby key mRNA are preferentially translated (21, 24). We found that, as in yeast and nematodes, DR in flies resulted in a nearly 50% decrease in global levels of protein synthesis and in total protein levels (Fig. 6A and Fig. S5). Surprisingly, inhibition of the TSP reversed these effects and had no effect on translation rates in fully fed animals (Fig. 6A and Fig. S5). These data indicate that a significant portion of available methionine is used to support transsulfuration under low-nutrient conditions and that increased commitment of methionine to transsulfuration contributes to the decreased rate of overall translation.
Fig. 6.
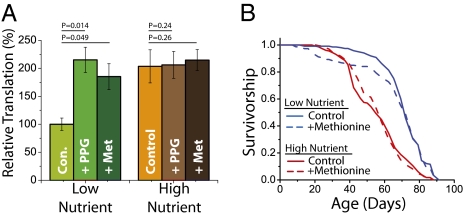
The TSP modulates levels of protein translation. (A) Global translation is reduced in female flies under low-nutrient conditions (P = 0.004 for low- v. high-nutrient control). Inhibition of the TSP by PPG reverses the diet effect (P = 0.29 for flies maintained on a low-nutrient, PPG-supplemented diet vs. flies maintained on a high-nutrient diet), suggesting that activation of the TSP under DR may reduce methionine availability for translation. The reduction of global translation rates under DR can also be rescued by supplementing the food with methionine, confirming its limitation under these conditions. [14C]Serine is used for labeling, and P values are obtained from standard t test. (B) Longevity extension is not affected by methionine supplementation (1.5 mM), indicating that global changes in translation are not required for increased lifespan under DR. Sample sizes: n = 244 and n = 240 for diet restriction and diet restriction plus methionine respectively (P = 0.32); n = 240 and n = 241 for fully fed and fully fed plus methionine, respectively (P = 0.54).
Because TSP inhibition reversed diet-mediated changes in lifespan and protein translation, we explored the hypothesis that these two processes are causally linked. Under the assumption that TSP activity was affecting protein synthesis by modulating the availability of methionine, we supplemented the low-nutrient diet with free methionine to bring the dietary concentration of this amino acid up to the level found in nutrient-rich conditions, at which it is not limiting (25). Our goal was to uncouple protein synthesis from TSP activity by ensuring sufficient levels of available methionine even in conditions of DR. The treatment was effective; methionine supplementation was sufficient to restore significantly higher levels of protein synthesis to diet-restricted animals (Fig. 6A and Fig. S5B). Fecundity was also increased, as expected (25). Lifespan, however, was unaffected, indicating that broad-spectrum reductions in protein translation are not required for DR longevity (Fig. 6B).
Discussion
We have shown that the TSP, which controls the metabolism of sulfur-containing amino acids and is conserved across taxa, acts on nutritional information to modulate aging and physiology in an invertebrate model system. Inhibition of the TSP blocked nutrient-dependent changes in lifespan, triglyceride levels, and total protein. On the contrary, overexpression of the rate-limiting enzyme in the TSP, dCBS, was sufficient to enhance fat storage and mimic important aspects of DR, including reduced total protein levels and enhanced longevity. Our data therefore suggest a model whereby DR induces up-regulation of dCBS and the TSP to promote fat storage, inhibit protein synthesis, and increase lifespan. Thus, we establish a conserved regulatory function for the TSP in aging and a requirement of the pathway for multiple nutrient-dependent phenotypes in flies.
We found that up-regulation of the TSP upon diet restriction results in conditions whereby methionine becomes limiting for protein translation. Perturbing key components of the protein translation machinery modulates lifespan in yeast, worm, and fly (26), and it has been suggested that reduced translation is an important mechanism underlying DR longevity (21, 24). By supplementing methionine to diet-restricted animals, however, we were able to restore translation to levels normally observed in fully fed animals without influencing lifespan, indicating that reduced translation per se is not the cause of enhanced longevity. Methionine was also recently used to uncouple diet-dependent reproductive output and longevity (25). Thus, although our data do not rule out the possibility that a global reduction in protein translation is sufficient to increase lifespan, perhaps through a mechanism in common with DR, they do establish that this is not required.
There are several apparent candidate mechanisms that may be responsible for the effects of transsulfuration on fly lifespan and physiology. One possibility invokes oxidative stress. A heme-binding domain is absent from the yeast and protozoan CBS orthologues, but it is present in dCBS. Therefore, similar to mammals, dCBS activity in flies may be modulated by redox state to direct the synthesis of GSH and to influence xenobiotic and antioxidant responses. Diet-restricted flies do not exhibit significant changes in traditional antioxidant enzymes, suggesting that small molecular weight antioxidants may be influential (27). However, whether these effects are directly related to increased lifespan remains to be determined. Indeed, the role of oxidative stress resistance in aging is controversial, and the data presented here may provide new avenues for determining whether there is a causal link between the two (28, 29). Irrespective of the role of oxidative stress resistance in aging, alterations in redox homeostasis alone can impact cell-signaling pathways in many ways (30, 31).
The position of the TSP at the nexus of several important biosynthetic pathways implicates other candidate mechanisms for its role in aging. Increased transsulfuration would promote increased cysteine biosynthesis and may allow its use in other pathways (32, 33). We find that DR increases dCBS protein more than it does enzyme activity, suggesting that components of the TSP, along with metabolites involved in one-carbon metabolism, might be regulated posttranslationally through modification by sumoylation or allosteric regulation by AdoMet (10, 34). Furthermore, the TSP is the primary source of hydrogen sulfide production in the cell (35–37). Recent years have seen the identification of H2S as an important gaseous signaling molecule with physiological roles in nervous, vascular, and intestinal systems (38–40). Notably, exposure of C. elegans to increased concentrations of H2S resulted in enhanced thermotolerance and increased lifespan (41).
Regardless of the mechanisms involved, there are several indications that our results may be directly relevant to understanding human biology. Although it remains to be determined whether the TSP affects mammalian aging, it is promising that similar patterns of CBS regulation are seen in mammals, in which analysis of published and unpublished gene expression data revealed that CBS was up-regulated in adipose tissue of diet-restricted rats (42). Furthermore, manipulation of the pathway in Drosophila affects development and fat deposition in a manner that mirrors effects seen in mice. We find that dCBS and TSP activity promote fat deposition in flies. This is reminiscent of the metabolic consequences of transsulfuration in mammals, in which hepatic CBS enzyme activity was significantly reduced when mice were fed a high-fat/high-sucrose diet (43) and CBS deficiency induced dysregulation of genes involved in hepatic lipid homeostasis (44, 45).
It is notable that most metazoans rely on their diet to provide key sulfur-containing metabolites necessary for metabolic and regulatory functions. This requirement, together with the work described here, suggest that the TSP may be an essential component of the mechanism by which dietary intake influences organism health and longevity in animals ranging from fly to human.
Materials and Methods
Below we provide a brief overview of the methods used for experiments presented in this article. For further details, please see SI Materials and Methods.
Lifespan Studies.
Following controlled larval rearing, same-age adult flies were allowed to mate and then were sorted by sex under light CO2 anesthesia into vials for lifespan analysis. Flies were transferred to fresh food every other day, and dead flies were removed and recorded. For experiments involving inhibition of the transsulfuration pathway, proparglyglycine (Sigma) was added directly to the food during cooling (at 55 °C) to give a final concentration of 2 mM.
Molecular Biology.
Protocols for cloning, glutathione measurement, western blotting, qPCR, identification of dCBS and measurement of its activity, metabolic labeling, and creation of expression plasmids used in this study can be found in SI Materials and Methods.
Fly Husbandry.
Detailed media recipes are provided in Table S2.
Supplementary Material
Acknowledgments
We thank Azra Dervisefendic and the S.D.P. laboratory for help with Drosophila husbandry and critical comments on the manuscript; and Vadim Gladyshev and Marjorie Lou for sharing their reagents. This work was supported by National Institutes of Health (NIH) Grants R01AG030593 (to S.D.P.), R01AG023166 (to S.D.P.), and R01DK074136 (to L.G.H.) and NIH Award HL58984 (to R.B.); the Glenn Foundation (S.D.P.), American Federation for Aging Research (S.D.P.), Ellison Medical Foundation (S.D.P.), and Nebraska Gateway for Nutritional Genomics (L.G.H.); and an American Federation for Aging Research Postdoctoral Fellowship (to H.K.). This work used the Drosophila Aging Core of the Nathan Shock Center of Excellence in the Biology of Aging funded by National Institute on Aging Grant P30-AG-013283.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1102008108/-/DCSupplemental.
References
- 1.Fontana L, Partridge L, Longo VD. Extending healthy life span—from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller RA, et al. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell. 2005;4:119–125. doi: 10.1111/j.1474-9726.2005.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beyer K, et al. Cystathionine beta synthase as a risk factor for Alzheimer disease. Curr Alzheimer Res. 2004;1:127–133. doi: 10.2174/1567205043332243. [DOI] [PubMed] [Google Scholar]
- 4.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isobe C, Murata T, Sato C, Terayama Y. Increase of total homocysteine concentration in cerebrospinal fluid in patients with Alzheimer's disease and Parkinson's disease. Life Sci. 2005;77:1836–1843. doi: 10.1016/j.lfs.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Evande R, Ojha S, Banerjee R. Visualization of PLP-bound intermediates in hemeless variants of human cystathionine beta-synthase: evidence that lysine 119 is a general base. Arch Biochem Biophys. 2004;427:188–196. doi: 10.1016/j.abb.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 7.Kabil O, Toaka S, LoBrutto R, Shoemaker R, Banerjee R. Pyridoxal phosphate binding sites are similar in human heme-dependent and yeast heme-independent cystathionine beta-synthases. Evidence from 31P NMR and pulsed EPR spectroscopy that heme and PLP cofactors are not proximal in the human enzyme. J Biol Chem. 2001;276:19350–19355. doi: 10.1074/jbc.M100029200. [DOI] [PubMed] [Google Scholar]
- 8.Scott JW, et al. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J Clin Invest. 2004;113:274–284. doi: 10.1172/JCI19874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kabil O, Banerjee R. Deletion of the regulatory domain in the pyridoxal phosphate-dependent heme protein cystathionine beta-synthase alleviates the defect observed in a catalytic site mutant. J Biol Chem. 1999;274:31256–31260. doi: 10.1074/jbc.274.44.31256. [DOI] [PubMed] [Google Scholar]
- 10.Koutmos M, Kabil O, Smith JL, Banerjee R. Structural basis for substrate activation and regulation by cystathionine beta-synthase (CBS) domains in cystathionine beta-synthase. Proc Natl Acad Sci USA. 2010;107:20958–20963. doi: 10.1073/pnas.1011448107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe M, et al. Mice deficient in cystathionine beta-synthase: Animal models for mild and severe homocyst(e)inemia. Proc Natl Acad Sci USA. 1995;92:1585–1589. doi: 10.1073/pnas.92.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roman G, Endo K, Zong L, Davis RL. P[Switch], a system for spatial and temporal control of gene expression in Drosophila melanogaster. Proc Natl Acad Sci USA. 2001;98:12602–12607. doi: 10.1073/pnas.221303998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osterwalder T, Yoon KS, White BH, Keshishian H. A conditional tissue-specific transgene expression system using inducible GAL4. Proc Natl Acad Sci USA. 2001;98:12596–12601. doi: 10.1073/pnas.221303298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abeles RH, Walsh CT. Acetylenic enzyme inactivators. Inactivation of gamma-cystathionase, in vitro and in vivo, by propargylglycine. J Am Chem Soc. 1973;95:6124–6125. doi: 10.1021/ja00799a053. [DOI] [PubMed] [Google Scholar]
- 15.Beatty PW, Reed DJ. Involvement of the cystathionine pathway in the biosynthesis of glutathione by isolated rat hepatocytes. Arch Biochem Biophys. 1980;204:80–87. doi: 10.1016/0003-9861(80)90009-0. [DOI] [PubMed] [Google Scholar]
- 16.Skorupa DA, Dervisefendic A, Zwiener J, Pletcher SD. Dietary composition specifies consumption, obesity and lifespan in Drosophila melanogaster. Aging Cell. 2008;7:478–490. doi: 10.1111/j.1474-9726.2008.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Horst DJ, van Hoof D, van Marrewijk WJ, Rodenburg KW. Alternative lipid mobilization: The insect shuttle system. Mol Cell Biochem. 2002;239:113–119. [PubMed] [Google Scholar]
- 18.Venken KJ, He Y, Hoskins RA, Bellen HJ. P[acman]: A BAC transgenic platform for targeted insertion of large DNA fragments in D. melanogaster. Science. 2006;314:1747–1751. doi: 10.1126/science.1134426. [DOI] [PubMed] [Google Scholar]
- 19.Greer EL, et al. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr Biol. 2007;17:1646–1656. doi: 10.1016/j.cub.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Day P, et al. Structure of a CBS-domain pair from the regulatory gamma1 subunit of human AMPK in complex with AMP and ZMP. Acta Crystallogr D Biol Crystallogr. 2007;63:587–596. doi: 10.1107/S0907444907009110. [DOI] [PubMed] [Google Scholar]
- 21.Steffen KK, et al. Yeast life span extension by depletion of 60s ribosomal subunits is mediated by Gcn4. Cell. 2008;133:292–302. doi: 10.1016/j.cell.2008.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powers RW, 3rd, Kaeberlein M, Caldwell SD, Kennedy BK, Fields S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 2006;20:174–184. doi: 10.1101/gad.1381406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen M, et al. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell. 2007;6:95–110. doi: 10.1111/j.1474-9726.2006.00267.x. [DOI] [PubMed] [Google Scholar]
- 24.Zid BM, et al. 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila. Cell. 2009;139:149–160. doi: 10.1016/j.cell.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grandison RC, Piper MD, Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature. 2009;462:1061–1064. doi: 10.1038/nature08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaeberlein M, Kennedy BK. Protein translation, 2008. Aging Cell. 2008;7:777–782. doi: 10.1111/j.1474-9726.2008.00439.x. [DOI] [PubMed] [Google Scholar]
- 27.Kabil H, Partridge L, Harshman LG. Superoxide dismutase activities in long-lived Drosophila melanogaster females: Chico1 genotypes and dietary dilution. Biogerontology. 2007;8:201–208. doi: 10.1007/s10522-006-9065-3. [DOI] [PubMed] [Google Scholar]
- 28.Van Raamsdonk JM, Hekimi S. Reactive oxygen species and aging in caenorhabditis elegans: Causal or casual relationship? Antioxid Redox Signal. 2010;13:1911–1953. doi: 10.1089/ars.2010.3215. [DOI] [PubMed] [Google Scholar]
- 29.Salmon AB, Richardson A, Pérez VI. Update on the oxidative stress theory of aging: Does oxidative stress play a role in aging or healthy aging? Free Radic Biol Med. 2010;48:642–655. doi: 10.1016/j.freeradbiomed.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fratelli M, et al. Gene expression profiling reveals a signaling role of glutathione in redox regulation. Proc Natl Acad Sci USA. 2005;102:13998–14003. doi: 10.1073/pnas.0504398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orr WC, et al. Overexpression of glutamate-cysteine ligase extends life span in Drosophila melanogaster. J Biol Chem. 2005;280:37331–37338. doi: 10.1074/jbc.M508272200. [DOI] [PubMed] [Google Scholar]
- 32.Kim HM, Do CH, Lee DH. Taurine reduces ER stress in C. elegans. J Biomed Sci. 2010;17(suppl 1):S26. doi: 10.1186/1423-0127-17-S1-S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El Idrissi A. Taurine improves learning and retention in aged mice. Neurosci Lett. 2008;436:19–22. doi: 10.1016/j.neulet.2008.02.070. [DOI] [PubMed] [Google Scholar]
- 34.Kabil O, Zhou Y, Banerjee R. Human cystathionine beta-synthase is a target for sumoylation. Biochemistry. 2006;45:13528–13536. doi: 10.1021/bi0615644. [DOI] [PubMed] [Google Scholar]
- 35.Chiku T, et al. H2S biogenesis by human cystathionine gamma-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J Biol Chem. 2009;284:11601–11612. doi: 10.1074/jbc.M808026200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kabil O, Banerjee R. The redox biochemistry of hydrogen sulfide. J Biol Chem. 2010;285:21903–21907. doi: 10.1074/jbc.R110.128363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh S, Padovani D, Leslie RA, Chiku T, Banerjee R. Relative contributions of cystathionine beta-synthase and gamma-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. J Biol Chem. 2009;284:22457–22466. doi: 10.1074/jbc.M109.010868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li L, Bhatia M, Moore PK. Hydrogen sulphide—a novel mediator of inflammation? Curr Opin Pharmacol. 2006;6:125–129. doi: 10.1016/j.coph.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 39.Moore PK, Bhatia M, Moochhala S. Hydrogen sulfide: From the smell of the past to the mediator of the future? Trends Pharmacol Sci. 2003;24:609–611. doi: 10.1016/j.tips.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 40.Wang R. Two's company, three's a crowd: Can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- 41.Miller DL, Roth MB. Hydrogen sulfide increases thermotolerance and lifespan in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2007;104:20618–20622. doi: 10.1073/pnas.0710191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Linford NJ, et al. Transcriptional response to aging and caloric restriction in heart and adipose tissue. Aging Cell. 2007;6:673–688. doi: 10.1111/j.1474-9726.2007.00319.x. [DOI] [PubMed] [Google Scholar]
- 43.Fonseca V, et al. Effects of a high-fat-sucrose diet on enzymes in homocysteine metabolism in the rat. Metabolism. 2000;49:736–741. doi: 10.1053/meta.2000.6256. [DOI] [PubMed] [Google Scholar]
- 44.Namekata K, et al. Abnormal lipid metabolism in cystathionine beta-synthase-deficient mice, an animal model for hyperhomocysteinemia. J Biol Chem. 2004;279:52961–52969. doi: 10.1074/jbc.M406820200. [DOI] [PubMed] [Google Scholar]
- 45.Hamelet J, Demuth K, Paul JL, Delabar JM, Janel N. Hyperhomocysteinemia due to cystathionine beta synthase deficiency induces dysregulation of genes involved in hepatic lipid homeostasis in mice. J Hepatol. 2007;46:151–159. doi: 10.1016/j.jhep.2006.07.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.