Abstract
Glucocorticoids (GCs) are used to treat pregnant women at risk for preterm delivery; however, prenatal exposure to GCs may trigger adverse neurological side effects due to reduced neural progenitor cell (NPC) proliferation. Whereas many established cell-cycle regulators impact NPC proliferation, other signaling molecules, such as the gap junction protein connexin-43 (Cx43), also influence proliferation. Gap junction intercellular communication (GJIC) is influenced by GCs in some cells, but such hormone effects have not been examined in coupled stem cells. We found that both continuous and transient exposure of embryonic day 14.5 mouse neurosphere cultures to dexamethasone (DEX) limits proliferation of coupled NPCs, which is manifested by both a reduction in S-phase progression and enhanced cell-cycle exit. A short (i.e., 1-h) DEX treatment also reduced GJIC as measured by live-cell fluorescence recovery after photobleaching, and altered the synchrony of spontaneous calcium transients in coupled NPCs. GC effects on GJIC in NPCs are transcription-independent and mediated through plasma membrane glucocorticoid receptors (GRs). This nongenomic pathway operates through lipid raft-associated GRs via a site-specific, MAPK-dependent phosphorylation of Cx43, which is linked to GR via caveolin-1 (Cav-1) and c-src. Cav-1 is essential for this nongenomic action of GR, as DEX effects on GJIC, Cx43 phosphorylation, and MAPK activation are not observed in Cav-1 knockout NPCs. As transient pharmacologic inhibition of GJIC triggers reduced S-phase progression but not enhanced cell-cycle exit, the nongenomic GR signaling pathway may operate via distinct downstream effectors to alter the proliferative capacity of NPCs.
Glucocorticoid hormones (GCs) mediate a wide array of physiological actions following their binding to the glucocorticoid receptor (GR). The principal effects of GCs are mediated by transcriptional responses (i.e., activation or repression) that follow either direct binding of a GR–ligand complex to glucocorticoid response elements contained within target genes, or the indirect association of the receptor with other DNA elements or DNA-bound transcription factors (1). However, the GR may also act via nongenomic mechanisms to mediate rapid cellular responses to GCs in the absence of measurable alterations in gene expression (1–3).
Although many studies of nongenomic GR signaling have focused on rapid alterations of synaptic activity by GCs (4–6), the possibility that this pathway operates in neural progenitor cells (NPCs) before synaptogenesis has not been examined. The inhibition of NPC proliferation by GCs may be responsible in part for the detrimental effects of stress within select adult and embryonic brain regions (7, 8) and putative neurological deficiencies observed following prenatal treatment with glucocorticoids. Whereas some GR gene targets have been identified in NPCs that mediate hormone effects on proliferation (9, 10), the contribution of nongenomic GC action has not been examined.
Gap junctions contain assemblies of connexin proteins that form intercellular channels between adjacent cells for passage of ions and molecules less than 1 kDa (11) and participate in the regulation of NPC proliferation (12, 13). Connexin-43 (Cx43) is the most abundant gap junction protein in the developing CNS as well as in NPC cultures (12, 14). It contains many phosphorylation sites that are targeted by kinases that influence gap junction intercellular communication (GJIC) (15). GRs regulate connexin gene expression in some cells (16), but the breadth of their effects on gap junction function has not been evaluated.
Using neurosphere cultures of embryonic murine NPCs, we uncovered a mechanism for rapid, nongenomic GR regulation of GJIC. This caveolin-1 (Cav-1)-dependent process is mediated by site-specific phosphorylation of Cx43 with subsequent effects on the synchrony of spontaneous calcium transients and NPC proliferation.
Results
Inhibition of GJIC in NPCs Following Brief Exposure to GCs.
NPCs from embryonic day 14.5 (E14.5) mice express Cx43-containing gap junctions (12, 17) and were used between passages 2 and 6 to ensure enrichment of the progenitor cells (18). NPCs were treated for 1 h with dexamethasone (DEX), a cell-impermeable DEX conjugate (DEX–BSA), and/or the GR antagonist RU-486. GJIC was quantified using a fluorescence recovery after photobleaching (FRAP) assay. This gap-FRAP assay is a well-documented means of measuring GJIC that has high temporal resolution, is noninvasive, and uniquely allows for precise determination of GJIC kinetics (19). The use of carbenoxolone (Cbx), a gap junction inhibitor, confirmed that fluorescence recovery was specifically assessing GJIC (Fig. 1A).
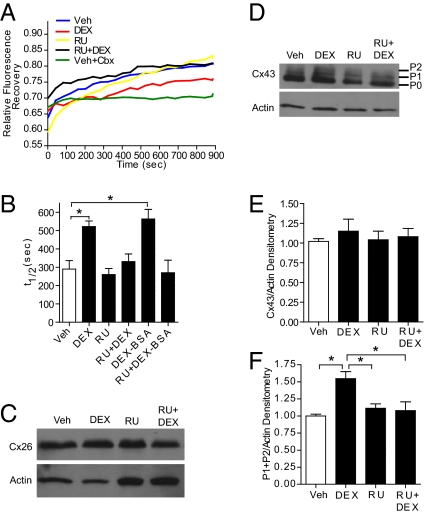
Fig. 1.
Rapid inhibition of GJIC in NPCs by GCs is associated with Cx43 phosphorylation. (A and B) NPCs preloaded with Calcein AM were subjected to 1-h treatments with ethanol vehicle (Veh), 100 nM DEX (±1 μM RU-486), RU-486 alone, DEX–BSA (±RU-486), and 100 μM carbenoxolone (Cbx). Fluorescence recovery within individual bleached cells in a representative experiment is shown (A) along mean values for t1/2 ± SEM of recovery (B; n = 4 independent experiments; one-way ANOVA, P < 0.0001; post hoc Tukey's multiple comparison test, P < 0.05). Representative Western blots are shown that measure Cx26 and Cx43 levels under analogous treatment conditions (C and D) along with mean ± SEM of densitometric scans of multiple blots (E and F) (n = 4; F: P = 0.0042; post hoc test, *P < 0.05). Stripped blots were probed with an anti-actin antibody. Positions of unphosphorylated (P0) and phosphorylated forms (P1, P2) of Cx43 are indicated (D).
A 1-h DEX treatment of NPCs resulted in a significant increase in the t1/2 of fluorescence recovery (Fig. 1 A and B), which reflects an inhibition of GJIC. Cotreatment with 1 μM GR antagonist RU-486 prevented the DEX-mediated reduction in GJIC (Fig. 1 A and B), indicating that the rapid inhibitory effect of GCs on GJIC in NPCs is GR-dependent. All t1/2 values were calculated by fitting a decaying exponential to the FRAP recovery curves (Fig. 1A). By measuring the fluorescence loss in all cells adjacent to the photobleached NPC, the number of NPCs connected to the photobleached NPCs can be tabulated. The t1/2 values were normalized to this number (Fig. S1A). The average number of cells connected to NPCs that recovered from photobleaching did not differ significantly between vehicle- and hormone-treated groups (Fig. S1B).
Because most GR-dependent nongenomic signaling mechanisms originate from activation of plasma membrane GR, a cell-impermeable BSA-conjugated DEX (DEX–BSA) was used (1). A 1-h DEX–BSA treatment of NPCs led to a significant loss in GJIC that was comparable to that observed with DEX exposure. In addition, cotreatment with 1 μM RU-486 prevented the DEX–BSA-mediated inhibition of GJIC (Fig. 1B). Therefore, a GR-dependent nongenomic signaling mechanism contributes to GC inhibition of GJIC in NPCs.
Brief GC Exposure Leads to Increased Cx43 Phosphorylation.
A reduction in GJIC may result from phosphorylation (15, 20) of connexins and/or from a change in connexin gene expression. GC induces connexin-26 (Cx26) expression in cultured rat hepatocytes (16). However, short-term DEX exposure did not alter expression of total Cx26 or Cx43 protein, two of the major connexin subtypes expressed in developing neuronal cells (Fig. 1 C–E). Because the activity of Cx43 in gap junctions, but not Cx26, is regulated by its phosphorylation at multiple sites, Western blot analysis was used to examine DEX effects on overall Cx43 phosphorylation. As shown in Fig. 1 D and F, a 1-h DEX treatment of NPCs led to increased expression of the slower-migrating phosphorylated forms of Cx43 (i.e., P1 and P2) (15). In accordance with the results of gap-FRAP experiments (Fig. 1 A and B), the DEX-mediated increase in overall Cx43 phosphorylation was prevented by cotreatment with RU-486 and is therefore GR-dependent. In addition, Triton fractionation experiments examining the proportion of membrane Cx43 and nonmembrane Cx43 did not reveal any significant differences following a 1-h DEX treatment (Fig. S2 A and B).
Brief GC Exposure Leads to Rapid Phosphorylation of ERK-1/2, Site-Specific Phosphorylation of Cx43, and Inhibition of GJIC.
Cx43 activity is differentially regulated by a variety of kinases. For example, phosphorylation of Cx43 at serines 279 and 282 by the mitogen-activated protein kinase (MAPK) extracellular signal-regulated kinase-1/2 (ERK-1/2) leads to an inhibition of GJIC (21). MAPKs have been previously implicated in rapid GR and estrogen receptor (ER) nongenomic signaling (3, 22, 23). A 1-h DEX exposure of NPCs led to a GR-dependent activation of ERK-1/2, as measured by Western blot analysis using a phospho-specific ERK-1/2 antibody (Fig. 2 A and B). A detailed time-course analysis revealed a rapid and biphasic increase in pERK-1/2, with peaks of activation within 2 min and 1 h following DEX treatment (Fig. S2 C and D).
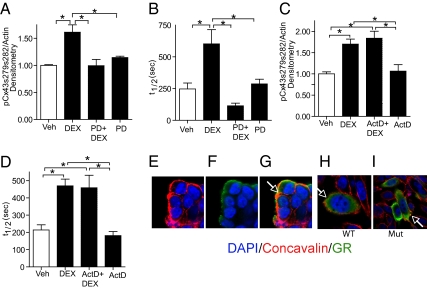
Fig. 2.
Rapid activation of ERK-1/2 and site-specific phosphorylation of Cx43 by GCs in NPCs. Western blot analysis was used to measure phosphorylated ERK-1/2 (pERK-1/2) and total ERK-1/2 (tERK-1/2) (A and B) and phosphorylated Cx43 at serines 279 and 282 (pCx43s279s282) (C and D) following a 1-h treatment of NPCs with 100 nM DEX and/or 1 μM RU-486. Representative Western blots are shown (A and C) along with densitometric scans ±SEM of multiple blots (B and D). DEX treatment significantly enhanced pERK-1/2 (B) (n = 4; P = 0.0085; post hoc test, *P < 0.05) and pCx43s279s282 levels (D) (n = 3; P = 0.0023; post hoc test, *P < 0.05).
To determine whether Cx43 phosphorylation at the ERK-1/2 target sites occurs in response to GC exposure, Western blot analysis was performed using an antibody directed against Cx43 phosphorylated at serines 279 and 282 (pCx43s279s282). As shown in Fig. 2 C and D, a 1-h DEX treatment led to a significant increase in pCx43s279s282. This increase was not present in NPCs treated with DEX and RU-486 (Fig. 2 C and D). To determine whether GR effects on Cx43 phosphorylation and function are dependent on ERK-1/2, Western blot analysis of pCx43s279s282 and gap-FRAP were performed in the presence of the MAPK kinase MEK-1/2 inhibitor PD98059 (PD). A dose of 40 μM PD inhibited DEX-mediated ERK-1/2 activation (Fig. S3A). As shown in Fig. 3A, a 40 μM PD cotreatment of NPCs prevented the increase in pCx43s279s282 following 1-h DEX exposure (a representative blot is in Fig. S3B). Similar effects were observed following cotreatment with 10 μM alternative MEK-1/2 inhibitor U0126 (Fig. S3 C–E). In addition, 40 μM PD treatment also prevented the DEX-mediated decrease in GJIC (Fig. 3B). Phosphorylation of Cx43 at serine 255, the remaining ERK-1/2 consensus target on Cx43, was not affected by DEX (Fig. S3 F and G) (21).
Fig. 3.
GC site-specific phosphorylation of Cx43 and inhibition of GJIC is ERK-1/2-dependent and occurs by a nongenomic GR signaling pathway. Western blot analysis was used to measure pCx43s279s282 following a 1-h treatment with 100 nM DEX and/or 40 μM PD98059 (A) or a 1-h pretreatment with 100 ng/mL ActD before a 1-h DEX treatment (C). Results of mean ± SEM of densitometric scans of multiple blots are shown (A: n = 4; P = 0.0012; post hoc test, *P < 0.05; C: n = 4; P = 0.0009; post hoc test, *P < 0.05). Representative blots are in Figs. S3 A and B and S4B. U0126 pretreatment (10 μM) had a similar effect as PD treatment (Fig. S3 C–E). NPCs preloaded with Calcein AM were subjected to analogous treatments [i.e., DEX ± PD (B) or ActD (D)] and mean values were measured for t1/2 ± SEM of recovery (B: n = 5 independent experiments, P = 0.0002; post hoc test, *P < 0.05; D: n = 4 independent experiments, P < 0.0001; post hoc test, *P < 0.05). (E–I) Untransfected WT NPCs or CHO cells transiently transfected with an alanine-to-cysteine mutant (Mut) or WT human GR were stained as live cells with the fluorescent-tagged lectin membrane marker concavalin-A, followed by fixation and IIF staining for GR. In the merged images (G–I), an arrow indicates plasma membrane GR localization. Ninety-seven percent (34/35 from four separate fields) of WT GR and 96% (48/50 from five separate fields) of mutant GR-transfected cells displayed plasma membrane costaining of GR and concavalin-A.
De Novo Gene Transcription Is Not Necessary for GC Effects on Cx43 Phosphorylation or GC Inhibition of GJIC.
DEX-exposed NPCs were pretreated for 1 h with 100 ng/mL of the transcriptional inhibitor Actinomycin D (ActD). Quantitative RT-PCR indicated that 1-h ActD pretreatment effectively inhibited DEX induction of the GC responsive gene glucocorticoid-induced leucine zipper (GILZ) (Fig. S4A). However, ActD pretreatment had no effect on the induction of pCx43s279s282 following 1-h DEX treatment (Fig. 3C and Fig. S4B). Similarly, gap-FRAP experiments reveal that ActD pretreatment had no effect on inhibition of GJIC following 1-h DEX exposure (Fig. 3D). As shown by confocal microscopic analysis in Fig. 3 E–G, a fraction of GR is present within the plasma membrane of NPCs (i.e., colocalized with a fluorescent-tagged cell-impermeable lectin). Human GR (in transiently transfected CHO cells) containing an alanine substitution at cysteine 665 also exhibits plasma membrane localization comparable to that seen with wild-type GR (Fig. 3 H and I). Cysteine 665 of human GR is contained within a palmitoylation motif highly conserved among steroid receptors (Fig. S5A) and required for palmitoylation and plasma membrane localization of androgen, estrogen, and progesterone receptors (24). Therefore, GRs along with mineralocorticoid receptors (MRs), which lacks this essential cysteine, may utilize a distinct mechanism for plasma membrane localization (24, 25).
GR Is Associated with Cav-1 in Lipid Rafts of NPCs.
Caveolae are specialized membrane invaginations localized to sphingolipid-rich lipid raft domains (26). Cav-1 is a major protein component of caveolae, and has been implicated in membrane GR signaling and in facilitating Cx43-dependent GJIC (26, 27). As shown in Fig. 4A, GR is detected within a Cav-1-enriched membrane fraction in sucrose gradient analysis in both untreated and 1-h 100 nM DEX-treated NPCs (i.e., fractions 4–6; Fig. 4A). Furthermore, GR–Cav-1 complexes can be isolated from untreated or DEX-treated NPCs by coimmunoprecipitation using either anti-GR or anti-Cav-1 antibodies for immune enrichment (Fig. 4 B and C).
Fig. 4.
GR is associated with Cav-1 in lipid rafts of NPCs, and Cav-1 along with c-src are necessary for rapid GC signaling. (A) NPC extracts were subjected to sucrose gradient fractionation to enrich for lipid rafts (i.e., fractions 4–6) and analyzed for GR and Cav-1 expression using Western blots (n = 2). The Golgi-associated protein ARF-1 was used to assess effective partitioning of the fractions. (B and C) Triton-soluble extracts were subjected to a coimmunoprecipitation (co-IP) assay with subsequent Western blots to reveal an association between GR and Cav-1. A nonimmune IgG was used in control co-IPs (n = 3). In both sucrose gradient fractionation and co-IP, NPCs were subjected to 1-h vehicle or 1-h 100 nM DEX. Western blot analysis was used to measure pERK-1/2 and tERK-1/2 (D) following pretreatment with PP2 for 30 min and a 1-h exposure to 100 nM DEX. Significant effects of DEX were revealed in results of the mean ± SEM ratio of pERK:tERK from densitometric scans (n = 6; P = 0.0001; post hoc test, Bonferroni, *P < 0.05). A representative blot is in Fig. S6A. Analogous results were seen for pCx43s279s282 following PP2 pretreatment (Fig. S6 B and C). (E) Western blot shows lack of Cav-1 in NPCs prepared from Cav-1 KO mice. Western blot analysis was used to measure pERK-1/2 and tERK-1/2 (F: n = 3; a representative blot is in Fig. S6D) and pCx43s279s282 (G: n = 3; a representative blot is in Fig. S6E) following 1-h treatments of Cav-1 KO NPCs with vehicle, 100 nM DEX (±1 μM RU-486), or RU-486 alone. No significant effects of DEX exposure were observed. (H) Cav-1 KO and WT and Cav-1 KO NPCs preloaded with Calcein AM were subjected to treatments with vehicle or 100 nM DEX. Mean values for t1/2 ± SEM of recovery were obtained by fitting a decaying exponential to individual fluorescence recovery curves. No effects of DEX exposure were observed on GJIC in Cav-1 KO NPCs (n = 4 independent experiments; P = 0.0027; post hoc test, P < 0.05).
C-Src Inhibition Prevents GC Activation of pERK-1/2.
GRs and the nonreceptor tyrosine kinase c-src localize to Cav-1-enriched membrane fractions (27). A 30-min pretreatment of NPCs with 10 μM src family inhibitor PP2 followed by a 1-h DEX exposure prevented the DEX-mediated increase in pERK-1/2 and pCx43s279s282 (Fig. 4D and Fig. S6 A–C), showing that c-src activation is coupled to GR-dependent ERK-1/2 activation and Cx43 phosphorylation.
Cav-1 Is Necessary for Rapid GC-Mediated ERK-1/2 Phosphorylation, Cx43 Phosphorylation, and Reduction of GJIC.
A 1-h DEX treatment of Cav-1 knockout (KO) NPCs (Fig. 4E) did not trigger ERK-1/2 activation (Fig. 4F and Fig. S6D) or alter Cx43s279s282 phosphorylation (Fig. 4G and Fig. S6E). Furthermore, no significant effects on GJIC were observed from a 1-h DEX exposure of Cav-1 KO NPCs (Fig. 4H). Therefore, Cav-1 is required for rapid GC-mediated signaling that results in ERK-1/2-mediated Cx43 phosphorylation at s279/s282 and subsequent reductions in NPC GJIC.
Transient GC Exposure Is Sufficient to Reduce S-Phase Progression in NPCs and Enhance Cell-Cycle Exit.
A prolonged (i.e., 24-h) DEX treatment reduced NPC proliferation (Fig. 5 A and B and Fig. S7A) in a GR-dependent manner (i.e., blocked by a simultaneous 24-h treatment with RU-486), in accordance with results obtained in NPCs derived from other brain regions and ages (28, 29). In these assays, a 1-h BrdU pulse immediately preceding harvest was used to identify cells progressing through S phase [i.e., BrdU+ staining by indirect immunofluorescence (IIF) analysis]. Furthermore, IIF was also used to detect NPCs positive for Ki67, which is expressed in cells actively progressing through the cell cycle (i.e., G1, S, G2/M).
Fig. 5.
Transient GC exposure is sufficient to reduce NPC proliferation and enhance cell-cycle exit, whereas transient inhibition of GJIC limits S-phase progression but does not affect cell-cycle exit. (A and B) NPCs subjected to 24-h treatments with vehicle, 100 nM DEX (±1 μM RU-486), RU-486 alone, or a 1-h DEX pretreatment followed by a 23-h RU-486 exposure (PreDEX+RU) were treated with a 10 μM BrdU pulse during the final hour of treatment. Mean values for BrdU+/Ki67+ cells ±SEM obtained following IIF show a significant reduction in NPCs actively in S phase of the cell cycle (A: n = 3; four random fields per image; P = 0.0024; post hoc test, *P < 0.05), whereas analysis of Ki67-immunostained cells alone indicated a significant reduction in NPCs actively engaged in the cell cycle (i.e., G1-S-G2/M) (B: n = 4; four random fields per image; P < 0.0001; post hoc test, *P < 0.05). A representative image is in Fig. S7A. (C) Proliferation assays performed as described above reveal a significant effect of a limited (i.e., 1-h) 3 mM 1-heptanol exposure on NPCs actively progressing through S phase of the cell cycle measured 23 h following 1-heptanol removal and wash (1hr Hept/23hrWash) (mean number ± SEM of BrdU+/Ki67+ cells, n = 4; four random fields per image; P < 0.0001; post hoc test, *P < 0.05). (D) Analysis of mean ± SEM of Ki67-only labeled cells reveals no significant effect of 1-heptanol exposure on NPCs exiting the cell cycle (n = 4). FRAP results indicated rapid and reversible inhibition of NPC GJIC by 1-heptanol (Fig. S7B). (E) NPCs treated with vehicle, 1-h 100 nM DEX, or 1-h 3 mM 1-heptanol were preloaded with the ratiometric calcium indicator Fura-2 AM and then subjected to live-cell calcium imaging. Although vehicle-treated NPCs show highly synchronized Ca2+ bursts in coupled NPCs as determined by the ratio of Fura-2 excitation at 340:380 nm, both the synchronicity and bursting behavior is reduced in DEX- and 1-heptanol-exposed NPC pairs. (F) Analysis of Ca2+ bursts in 10 pairs of coupled NPCs indicated a significant reduction in the correlation of Ca2+ transients as measured by the Pearson correlation coefficient (r2) (n = 10 pairs; P = 0.0056; post hoc test, *P < 0.05).
To limit the duration of GR activity, RU-486 was added to neurosphere cultures following a 1-h preexposure to DEX (preDEX+RU). As shown in Fig. 5 A and B, a 1-h DEX “pulse” followed by an additional 23-h incubation was sufficient to reduce BrdU incorporation and Ki67 staining in NPCs, demonstrating that a transient GC exposure is sufficient to both limit S-phase entry of NPCs and enhance their exit from the cell cycle.
Transient Inhibition of GJIC Is Sufficient to Reduce S-Phase Progression in NPCs but Does Not Trigger Cell-Cycle Exit.
Treatment of neurosphere cultures with the reversible GJIC inhibitor 1-heptanol led to a loss in GJIC in NPCs that was sustained for 1 h but could be rapidly reversed (Fig. S7B). NPC proliferation was therefore examined after exposing cultures to 3 mM 1-heptanol for only 1 h followed by a 23-h incubation. As shown in Fig. 5C, a 1-h 1-heptanol pulse led to a significant reduction in BrdU-positive NPCs, but did not alter the number of Ki67-positive cells (Fig. 5D). Therefore, whereas either transient GR activation or GJIC inhibition is sufficient to limit NPC entry into S phase, cell-cycle exit requires additional actions of the GR that extend beyond its nongenomic effects limiting GJIC.
Loss of Synchronous, Spontaneous Calcium Bursts Following GC Treatment of NPCs.
To ascertain proximal events linking nongenomic GR effects on GJIC to regulation of proliferation, we examined an effect of gap junction and hemichannel activity on an established regulator of NPC proliferation, that is, coupled calcium waves (13). As shown in Fig. 5E, NPCs in neurosphere cultures generate spontaneous intracellular calcium bursts that are highly synchronous between coupled cells. In contrast, the spontaneous calcium bursts in coupled NPCs following 1-h DEX or 1-heptanol treatment are asynchronous and fewer in number (Fig. 5E and Fig. S8 A–C). Statistical analysis of Ca2+ synchrony reveals a significant reduction in correlation between paired NPCs following either 1-h DEX or 1-heptanol exposure (Fig. 5F).
Discussion
Glucocorticoids are used to reduce respiratory distress in premature babies and as antenatal therapy for women at risk for delivery of a baby with virilizing congenital adrenal hyperplasia (30, 31). However, this therapy is associated with detrimental outcomes on neurological development due to GC effects on NPC proliferation and function (30–32). In this report, we identify a nongenomic GR signaling pathway that impacts NPC proliferation in vitro through inhibitory effects on GJIC. Phosphorylation of specific connexin proteins regulates GJIC in other systems, and we provide evidence for rapid activation of ERK-1/2 by GCs that triggers site-specific phosphorylation of Cx43, a major component of NPC gap junctions. This phosphorylation event, in turn, limits GJIC but does not influence Cx43 protein expression or subcellular trafficking. Rapid GR-dependent activation of ERK-1/2 requires a c-src family member, and may be initiated by a signaling complex assembled at the plasma membrane through GR interactions in lipid rafts with Cav-1. Our studies corroborate the role for Cav-1 in mediating the antiproliferative effects of GCs that was established in mouse embryonic fibroblasts from Cav-1 knockout mice (33). In addition, we identify a unique downstream target of this signaling, GJIC, in a progenitor cell population that uses GJIC and/or connexins to maintain synchronous progression through the cell cycle.
Plasma membrane targeting of the GR in NPCs does not use a mechanism shared by other steroid receptors that requires palmitoylation at an essential cysteine within a highly conserved motif (24). Although we cannot exclude the possibility that GR palmitoylation is required for plasma membrane targeting in other cell types, in NPCs it may share a membrane-targeting mechanism with its closest relative within the nuclear receptor superfamily, the MR, which lacks the essential cysteine but shares a high degree of homology, like the GR, with functional palmitoylation motifs (25).
Because the interaction between Cav-1 and the GR was not altered by DEX, hormone effects on NPC GJIC and proliferation may be mediated by conformational changes in the GR that alter receptor interactions with components of the MAPK pathway. Nongenomic signaling initiated by plasma membrane-associated ERs in breast cancer cells use an analogous pathway, with Cav-1-associated membrane ER activation also leading to c-src-dependent ERK-1/2 activation (34, 35). However, in contrast to our results in NPCs, ER-mediated ERK-1/2 activation leads to increased proliferation (34, 35). Whereas ERK-1/2 is also rapidly activated by a nongenomic steroid hormone signaling pathway in NPCs, its site-specific phosphorylation of Cx43 leads to reduced GJIC and subsequently decreased proliferation. Thus, rapid activation of GR alters ERK-1/2 target selection in NPCs and directs this kinase to targets (Cx43) that negatively impact cellular processes (e.g., synchronous calcium waves) that promote proliferation. The neurosphere culture system that we used maintains coupling between NPCs as observed in vivo and enabled us to reveal a unique pathway for nongenomic GR regulation of the synchrony of spontaneous calcium bursts, which overrides signals that may otherwise direct the proliferation of NPCs.
Our results also suggest that the rapid activation of both nongenomic and genomic signaling pathways by the GR accounts for the decrease in NPC proliferation and increased cell-cycle exit brought about by a transient (1-h) DEX exposure. A transient inhibition of GJIC by 1-heptanol, in the absence of GR activation, also reduced S-phase progression of NPCs but did not affect cell-cycle exit. Nongenomic GC effects mediated by loss of GJIC may lead to a decrease in the rate of cell cycling in S phase, whereas genomic effects, including some that have been previously characterized, may force NPCs to exit the cell cycle entirely (28).
Gap junction-dependent spontaneous Ca2+ waves have previously been shown to be essential for the maintenance of NPC proliferation in the developing neocortex (13). Moreover, recent findings suggest that even transient GC exposure can influence the periodic (circadian and/or ultradian) expression of certain GR target genes (36, 37). A select subset of these circadian genes (e.g., per1 and per2) also regulate proliferation in various cell types, including NPCs (38, 39). In fact, per2 gene expression is sensitive to intracellular Ca2+ concentrations, suggesting that per2 expression may be influenced by alterations in GJIC (40). Given the observations from the present study, this raises the intriguing possibility that a combination of genomic and nongenomic effects on circadian genes may influence the loss in NPC proliferation that occurs from even a transient GC exposure.
Our work supports the view that both genomic and nongenomic pathways mobilized by steroid receptors can act in concert to bring about changes in cell physiology (1, 41). Dissection of the mechanisms operating in genomic and nongenomic action of steroid receptors may reinvigorate the search for novel ligands that preferentially activate one pathway or otherwise provide potential targets that allow for more selective actions of steroid hormones (42).
Materials and Methods
Details of specific methods beyond what is described below are provided in SI Materials and Methods.
Mouse NPC Culture.
Mouse NPCs were prepared from E14.5 embryonic cortex according to the technical manual provided by StemCell Technologies. Cells were used between passages 2 and 6.
Western Blot Analysis.
NPC protein lysates used in Western blot analysis included Triton-soluble and -insoluble fractions, sucrose gradient fractions to enrich for lipid rafts, and material following coimmunoprecipitation assay with GR or Cav-1 antibodies. Specific antibodies can be found in SI Materials and Methods. Images were quantified (densitometry) using National Institutes of Health (NIH) ImageJ software (http://rsbweb.nih.gov/ij).
Gap-FRAP.
NPCs were loaded with 1 μg/mL of Calcein AM (Invitrogen) 30 min before FRAP analysis, which was conducted on an Olympus IX81 confocal microscope equipped with FluoView data collection software. FRAP recovery curves were fit to an exponential decay equation present in the GraphPad menu, and t1/2 was tabulated by the software from this fit.
Live-Cell Intracellular Ca2+ Imaging.
NPCs were loaded with 500 nM Fura-2 AM for 30 min before Ca2+ imaging, and alternatively illuminated with 340 and 380 nm light for Fura-2 using a Leica HC N PLAN BD 40× oil immersion objective (Leica). Data were collected and analyzed in SimplePCI software (Compix) as the 340:380 ratio.
NPC Proliferation Assays.
NPCs were exposed to 10 μM BrdU 1 h before harvesting and 23 h after initiating various treatments. Cells were fixed, permeabilized, and processed for IIF analysis to detect BrdU and Ki67 staining.
Statistical Analysis.
Statistical significance was determined by one-way ANOVA followed by a post hoc Tukey's multiple comparison test to determine within-group differences, unless otherwise noted.
Supplementary Material
Acknowledgments
We thank Rebecca Hughey, Carol Lynn Truschel, Melanie Warnes, and Michael Palladino for technical assistance with the human GR studies and John Cidlowski for providing the human GR expression plasmid. We thank J. Timothy Greenamyre for support of R.D.M. Finally, we thank Vanessa Franco, Max Horowitz, and Jamey Maniscalco for critical reading of the manuscript and Nuria Pator-Soler for advice regarding plasma membrane GR staining. This project was supported in part by National Institutes of Health Grants T32GM008424-16 and R01DK078394.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1102821108/-/DCSupplemental.
References
- 1.Haller J, Mikics E, Makara GB. The effects of non-genomic glucocorticoid mechanisms on bodily functions and the central neural system. A critical evaluation of findings. Front Neuroendocrinol. 2008;29:273–291. doi: 10.1016/j.yfrne.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Pasricha N, Joëls M, Karst H. Rapid effects of corticosterone in the mouse dentate gyrus via a nongenomic pathway. J Neuroendocrinol. 2011;23:143–147. doi: 10.1111/j.1365-2826.2010.02091.x. [DOI] [PubMed] [Google Scholar]
- 3.Qiu J, et al. Rapid activation of ERK1/2 mitogen-activated protein kinase by corticosterone in PC12 cells. Biochem Biophys Res Commun. 2001;287:1017–1024. doi: 10.1006/bbrc.2001.5691. [DOI] [PubMed] [Google Scholar]
- 4.Karst H, et al. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci USA. 2005;102:19204–19207. doi: 10.1073/pnas.0507572102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ffrench-Mullen JM. Cortisol inhibition of calcium currents in guinea pig hippocampal CA1 neurons via G-protein-coupled activation of protein kinase C. J Neurosci. 1995;15:903–911. doi: 10.1523/JNEUROSCI.15-01-00903.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olijslagers JE, et al. Rapid changes in hippocampal CA1 pyramidal cell function via pre- as well as postsynaptic membrane mineralocorticoid receptors. Eur J Neurosci. 2008;27:2542–2550. doi: 10.1111/j.1460-9568.2008.06220.x. [DOI] [PubMed] [Google Scholar]
- 7.McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mirescu C, Gould E. Stress and adult neurogenesis. Hippocampus. 2006;16:233–238. doi: 10.1002/hipo.20155. [DOI] [PubMed] [Google Scholar]
- 9.Ayroldi E, Riccardi C. Glucocorticoid-induced leucine zipper (GILZ): A new important mediator of glucocorticoid action. FASEB J. 2009;23:3649–3658. doi: 10.1096/fj.09-134684. [DOI] [PubMed] [Google Scholar]
- 10.Rogatsky I, Trowbridge JM, Garabedian MJ. Glucocorticoid receptor-mediated cell cycle arrest is achieved through distinct cell-specific transcriptional regulatory mechanisms. Mol Cell Biol. 1997;17:3181–3193. doi: 10.1128/mcb.17.6.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruzzone R, Dermietzel R. Structure and function of gap junctions in the developing brain. Cell Tissue Res. 2006;326:239–248. doi: 10.1007/s00441-006-0287-0. [DOI] [PubMed] [Google Scholar]
- 12.Cheng A, et al. Gap junctional communication is required to maintain mouse cortical neural progenitor cells in a proliferative state. Dev Biol. 2004;272:203–216. doi: 10.1016/j.ydbio.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 13.Weissman TA, Riquelme PA, Ivic L, Flint AC, Kriegstein AR. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron. 2004;43:647–661. doi: 10.1016/j.neuron.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 14.Nadarajah B, Jones AM, Evans WH, Parnavelas JG. Differential expression of connexins during neocortical development and neuronal circuit formation. J Neurosci. 1997;17:3096–3111. doi: 10.1523/JNEUROSCI.17-09-03096.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solan JL, Lampe PD. Connexin43 phosphorylation: Structural changes and biological effects. Biochem J. 2009;419:261–272. doi: 10.1042/BJ20082319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kojima T, Mitaka T, Shibata Y, Mochizuki Y. Induction and regulation of connexin26 by glucagon in primary cultures of adult rat hepatocytes. J Cell Sci. 1995;108:2771–2780. doi: 10.1242/jcs.108.8.2771. [DOI] [PubMed] [Google Scholar]
- 17.Viti J, Gulacsi A, Lillien L. Wnt regulation of progenitor maturation in the cortex depends on Shh or fibroblast growth factor 2. J Neurosci. 2003;23:5919–5927. doi: 10.1523/JNEUROSCI.23-13-05919.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen JB, Parmar M. Strengths and limitations of the neurosphere culture system. Mol Neurobiol. 2006;34:153–161. doi: 10.1385/MN:34:3:153. [DOI] [PubMed] [Google Scholar]
- 19.Abbaci M, Barberi-Heyob M, Blondel W, Guillemin F, Didelon J. Advantages and limitations of commonly used methods to assay the molecular permeability of gap junctional intercellular communication. Biotechniques. 2008;45:33–52, 56–62. doi: 10.2144/000112810. [DOI] [PubMed] [Google Scholar]
- 20.Moreno AP, Lau AF. Gap junction channel gating modulated through protein phosphorylation. Prog Biophys Mol Biol. 2007;94:107–119. doi: 10.1016/j.pbiomolbio.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warn-Cramer BJ, Cottrell GT, Burt JM, Lau AF. Regulation of connexin-43 gap junctional intercellular communication by mitogen-activated protein kinase. J Biol Chem. 1998;273:9188–9196. doi: 10.1074/jbc.273.15.9188. [DOI] [PubMed] [Google Scholar]
- 22.Cato AC, Nestl A, Mink S. Rapid actions of steroid receptors in cellular signaling pathways. Sci STKE. 2002;2002:re9. doi: 10.1126/stke.2002.138.re9. [DOI] [PubMed] [Google Scholar]
- 23.Moriarty K, Kim KH, Bender JR. Estrogen receptor-mediated rapid signaling. Endocrinology. 2006;147:5557–5563. doi: 10.1210/en.2006-0729. [DOI] [PubMed] [Google Scholar]
- 24.Pedram A, et al. A conserved mechanism for steroid receptor translocation to the plasma membrane. J Biol Chem. 2007;282:22278–22288. doi: 10.1074/jbc.M611877200. [DOI] [PubMed] [Google Scholar]
- 25.Grossmann C, Freudinger R, Mildenberger S, Husse B, Gekle M. EF domains are sufficient for nongenomic mineralocorticoid receptor actions. J Biol Chem. 2008;283:7109–7116. doi: 10.1074/jbc.M708751200. [DOI] [PubMed] [Google Scholar]
- 26.Langlois S, Cowan KN, Shao Q, Cowan BJ, Laird DW. Caveolin-1 and -2 interact with connexin43 and regulate gap junctional intercellular communication in keratinocytes. Mol Biol Cell. 2008;19:912–928. doi: 10.1091/mbc.E07-06-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matthews L, et al. Caveolin mediates rapid glucocorticoid effects and couples glucocorticoid action to the antiproliferative program. Mol Endocrinol. 2008;22:1320–1330. doi: 10.1210/me.2007-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sundberg M, Savola S, Hienola A, Korhonen L, Lindholm D. Glucocorticoid hormones decrease proliferation of embryonic neural stem cells through ubiquitin-mediated degradation of cyclin D1. J Neurosci. 2006;26:5402–5410. doi: 10.1523/JNEUROSCI.4906-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu S, et al. Depletion of the neural precursor cell pool by glucocorticoids. Ann Neurol. 2010;67:21–30. doi: 10.1002/ana.21812. [DOI] [PubMed] [Google Scholar]
- 30.Hirvikoski T, et al. Cognitive functions in children at risk for congenital adrenal hyperplasia treated prenatally with dexamethasone. J Clin Endocrinol Metab. 2007;92:542–548. doi: 10.1210/jc.2006-1340. [DOI] [PubMed] [Google Scholar]
- 31.Yeh TF, et al. Outcomes at school age after postnatal dexamethasone therapy for lung disease of prematurity. N Engl J Med. 2004;350:1304–1313. doi: 10.1056/NEJMoa032089. [DOI] [PubMed] [Google Scholar]
- 32.Modi N, et al. The effects of repeated antenatal glucocorticoid therapy on the developing brain. Pediatr Res. 2001;50:581–585. doi: 10.1203/00006450-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Jasmin JF, Yang M, Iacovitti L, Lisanti MP. Genetic ablation of caveolin-1 increases neural stem cell proliferation in the subventricular zone (SVZ) of the adult mouse brain. Cell Cycle. 2009;8:3978–3983. doi: 10.4161/cc.8.23.10206. [DOI] [PubMed] [Google Scholar]
- 34.Evinger AJ, III, Levin ER. Requirements for estrogen receptor α membrane localization and function. Steroids. 2005;70:361–363. doi: 10.1016/j.steroids.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 35.Razandi M, Pedram A, Rosen EM, Levin ER. BRCA1 inhibits membrane estrogen and growth factor receptor signaling to cell proliferation in breast cancer. Mol Cell Biol. 2004;24:5900–5913. doi: 10.1128/MCB.24.13.5900-5913.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Segall LA, Milet A, Tronche F, Amir S. Brain glucocorticoid receptors are necessary for the rhythmic expression of the clock protein, PERIOD2, in the central extended amygdala in mice. Neurosci Lett. 2009;457:58–60. doi: 10.1016/j.neulet.2009.03.083. [DOI] [PubMed] [Google Scholar]
- 37.So AY, Bernal TU, Pillsbury ML, Yamamoto KR, Feldman BJ. Glucocorticoid regulation of the circadian clock modulates glucose homeostasis. Proc Natl Acad Sci USA. 2009;106:17582–17587. doi: 10.1073/pnas.0909733106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borgs L, et al. Period 2 regulates neural stem/progenitor cell proliferation in the adult hippocampus. BMC Neurosci. 2009;10:30. doi: 10.1186/1471-2202-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee S, Donehower LA, Herron AJ, Moore DD, Fu L. Disrupting circadian homeostasis of sympathetic signaling promotes tumor development in mice. PLoS One. 2010;5:e10995. doi: 10.1371/journal.pone.0010995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takashima N, et al. Gq/11-induced intracellular calcium mobilization mediates Per2 acute induction in Rat-1 fibroblasts. Genes Cells. 2006;11:1039–1049. doi: 10.1111/j.1365-2443.2006.00999.x. [DOI] [PubMed] [Google Scholar]
- 41.Levin ER. Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol. 2005;19:1951–1959. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chambliss KL, et al. Non-nuclear estrogen receptor α signaling promotes cardiovascular protection but not uterine or breast cancer growth in mice. J Clin Invest. 2010;120:2319–2330. doi: 10.1172/JCI38291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.