Abstract
The autoregulatory loops of the circadian clock consist of feedback regulation of transcription/translation circuits but also require finely coordinated cytoplasmic and nuclear proteostasis. Although protein degradation is important to establish steady-state levels, maturation into their active conformation also factors into protein homeostasis. HSP90 facilitates the maturation of a wide range of client proteins, and studies in metazoan clocks implicate HSP90 as an integrator of input or output. Here we show that the Arabidopsis circadian clock-associated F-box protein ZEITLUPE (ZTL) is a unique client for cytoplasmic HSP90. The HSP90-specific inhibitor geldanamycin and RNAi-mediated depletion of cytoplasmic HSP90 reduces levels of ZTL and lengthens circadian period, consistent with ztl loss-of-function alleles. Transient transfection of artificial microRNA targeting cytoplasmic HSP90 genes similarly lengthens period. Proteolytic targets of SCFZTL, TOC1 and PRR5, are stabilized in geldanamycin-treated seedlings, whereas the levels of closely related clock proteins, PRR3 and PRR7, are unchanged. An in vitro holdase assay, typically used to demonstrate chaperone activity, shows that ZTL can be effectively bound, and aggregation prevented, by HSP90. GIGANTEA, a unique stabilizer of ZTL, may act in the same pathway as HSP90, possibly linking these two proteins to a similar mechanism. Our findings establish maturation of ZTL by HSP90 as essential for proper function of the Arabidopsis circadian clock. Unlike metazoan systems, HSP90 functions here within the core oscillator. Additionally, F-box proteins as clients may place HSP90 in a unique and more central role in proteostasis.
Keywords: protein maturation, proteolysis
The circadian clock system sustains 24-h rhythmicity at the molecular and physiological level in most organisms that have been examined. A key function of the clock is to appropriately phase many essential physiological and biochemical processes in anticipation of the light/dark and temperature changes that occur each day. In both plants and animals impaired operation of the circadian clock results in reduced function and fitness, leading to increased propensity for disease and poor growth and reproductive success (1–5).
The underlying basis of circadian clocks involves autoregulatory feedback loops that consist of positive activating and negative repressing elements that control gene transcription and protein activity and localization (6–8). The Arabidopsis circadian system consists of at least three interlocked feedback loops. Although more than 20 different genes are associated with circadian timing in plants, only a small subset has been incorporated into coherent interaction schemes (9, 10). Current models are based largely on transcriptional relationships, but increasingly posttranslational processes, such as regulated proteolysis, have been found to be critical for proper clock function (11–17). In Arabidopsis, the F-box protein ZEITLUPE (ZTL) is an evening-phased clock component responsible for the proteasome-dependent degradation of TIMING OF CAB EXPRESION 1 (TOC1) and PSEUDO-RESPONSE REGULATOR 5 (PRR5). ztl mutants are long period and PRR5 and TOC1 protein damp to high levels in these backgrounds (18–20). ZTL is constitutively transcribed, but ZTL protein oscillates in part through phase-specific proteasome-dependent degradation (12). Uniquely, ZTL and related family members possess a light sensing domain [LIGHT, OXYGEN, VOLTAGE (LOV)] at the N terminus that confers increased stability in blue light (21, 22). This feature provides a unique point of light input into the plant circadian system.
GIGANTEA (GI) is a plant-specific single-copy gene that acts as a posttranslational stabilizer of ZTL. In gi mutants ZTL mRNA levels are unaffected but ZTL protein is constitutively low (22). Originally identified as a regulator of flowering time, GI is increasingly found as a factor in controlling a wide range of plant processes (23–25). In the circadian clock, transcriptional cycling of GI mRNA drives an evening-phased peak in GI protein abundance rhythm. The GI–ZTL interaction is mediated through blue light absorbance by the ZTL LOV domain, which helps create and sustain a posttranslational rhythm of ZTL abundance that is in phase with GI through phase-specific proteasome-dependent degradation (12, 22). This ZTL rhythm in turn contributes to the maintenance of high-amplitude oscillations of TOC1 and PRR5 (18, 22).
The effects of GI deficiencies are highly pleiotropic, and the molecular mechanism of GI action is unknown, suggesting that other components contribute to the posttranslational stabilization of ZTL. The molecular chaperone HSP90 is an abundant and central cellular element essential to the maturation and stabilization of numerous regulatory proteins involved in signaling pathways (26, 27). HSP90 acts as a dimer and in an ATPase-dependent cycle alternately complexes with and separates from additional factors and cochaperones to effect a kinetically dynamic process of client protein maturation. In plants, HSP90 is best characterized as associating with the cochaperone SGT1 to stabilize NLR proteins, which mediate plant defense mechanisms (28–30). Additionally, HSP90 is important in phenotypic plasticity, developmental stability, and buffering of genetic variation (31–33).
Here we establish the maturation of ZTL by HSP90 as essential for proper function of the circadian clock. These results also demonstrate a unique role for HSP90 in the direct control of proteolysis and protein homeostasis through F-box protein maturation. In addition, we find that the GI acts in the same pathway as HSP90, linking these two proteins to the same stabilizing mechanism governing the posttranslational regulation of ZTL.
Results
HSP90 Depletion Lengthens Circadian Period.
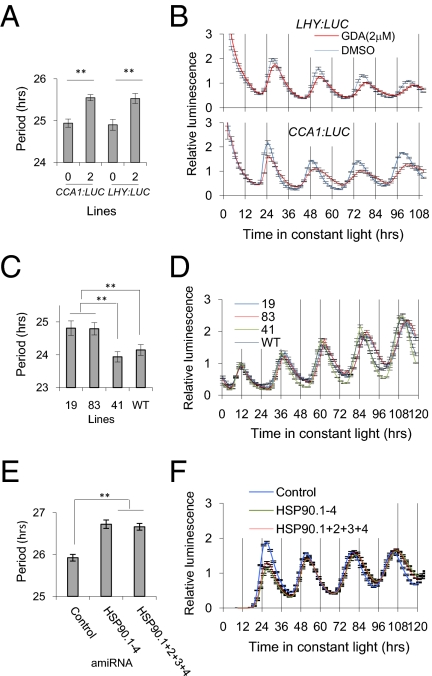
Previous reports demonstrating the importance of protein stability to clock function (11, 13–15, 22) led us to test whether protein maturation factors, such as HSP90, may also affect the circadian oscillator. We tested the effects of reduced levels of HSP90 activity on free-running period by treating young seedlings with geldanamycin (GDA), a specific inhibitor of HSP90 (34). Plants expressing luciferase driven by the promoters of the morning-phased core clock reporter genes LHY:luciferase (LHY:LUC) and CCA1:luciferase (CCA1:LUC) were treated with 2 μM GDA (or DMSO vehicle alone) and monitored for rhythmicity under continuous red light. GDA significantly lengthened the free-running period as measured by both reporters by 0.6 h, relative to the control (one-way ANOVA, P < 0.001 for CCA1:LUC and P = 0.002 for LHY:LUC) (Fig. 1 A and B and Table S1).
Fig. 1.
Reduction in HSP90 lengthens period in Arabidopsis. (A and B) Seedlings were grown in 12 h white light/12 h dark for 5–7 d before release into constant red light (17 μmol m−2 s−1) and imaged for LHY:LUC or CCA1:LUC activity for the indicated time. Free-running period of Arabidopsis seedlings expressing the CCA1:LUC (n = 12) or LHY:LUC (n = 12) reporters treated with 2 μM GDA or DMSO (0) during the imaging. **P < 0.01. (C and D) An RNAi-mediated HSP90-reduced line was crossed with CCR2:LUC, and F3 plants expressing the reporter and positive for the transgene were imaged for CCR2:LUC activity. Lines 19 (n = 46) and 83 (n = 52) displayed characteristic features of HSP90 reduction (multiple branches), whereas line 41 (n = 74) and WT (n = 71) did not. Seedlings grown and imaged as in A and B. **P < 0.01 between indicated comparisons according to Hsu's MCB (multiple comparisons with the best) (Fig. S2A). (E and F) Free-running period under constant red light of CCA1:LUC in Arabidopsis protoplasts simultaneously transiently transfected with CCA1:LUC and amiRNA designed to reduce HSP90 expression. Cytosolic HSP90 expression (HSP90.1–4) was reduced by one (HSP90.1–4) or four simultaneously transfected constructs (HSP90.1+2+3+4). Protoplasts imaged in constant red light for the time indicated. **P < 0.01 between indicated comparisons according to Hsu's MCB (Fig. S2B).
In Arabidopsis HSP90 homologs are encoded by three organellar (HSP90.5–7) and four cytosolic (HSP90.1–4) genes (31). Because GDA treatment cannot distinguish among the seven types of HSP90 proteins, a stably transformed HSP90 RNAi line (RNAi-C) targeting cytosolic HSP90 genes was characterized for its circadian phenotype (32). This RNAi-C line (35) was crossed into a CCR2:luciferase (CCR2:LUC) reporter background, and only segregants demonstrating HSP90 reduction (loss of apical dominance; Fig. S1) showed a significantly longer period (0.7 h) (Fig. 1C and D, Fig. S2A, and Table S2). These results are consistent with the effects of GDA and support the notion that cytosolic HSP90 is required for normal clock function in Arabidopsis.
As a final test we used transient transfection of gene-specific amiRNAs to avoid potential developmental effects of long-term HSP90 reduction. Arabidopsis leaf mesophyll protoplasts were cotransfected with the CCA1:LUC reporter plasmid along with either an artificial microRNA (amiRNA) targeting all four cytosolic HSP90 genes (amiRNA HSP90.1–4) or four individual amiRNA plasmids (amiRNA HSP90.1+2+3+4) designed to specifically target each of the cytosolic HSP90 genes (Table S3). Transcript levels of the four cytosolic HSP90 genes were reduced to between 25% and 40% of their endogenous levels (Fig. S3). Both approaches lengthened period by 0.7–1 h, consistent with a role for cytosolic HSP90 in circadian control (Fig. 1 E and F, Fig. S2B, and Table S4).
ZTL Levels Are Diminished by HSP90 Depletion.
HSP90 acts on a functionally diverse set of clients, including transcription factors and kinases (26). In Arabidopsis, a number of the cytosolic HSP90 genes oscillate with an evening phase under light/dark (LD) cycles (36) (Fig. S4). This, together with the long period of HSP90-depleted plants, led us to consider the F-box protein ZTL as a candidate HSP90 client protein.
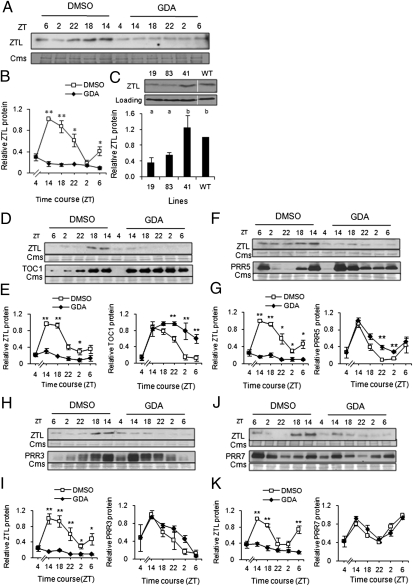
Seedlings were treated with GDA or vehicle (DMSO) at Zeitgeber time (ZT) 4 and harvested over an LD cycle. After DMSO treatment the normal oscillation in ZTL protein was observed, whereas GDA caused a significant and sustained reduction in ZTL levels (Fig. 2 A and B). The steady-state level of detectable ZTL in GDA-treated plants was similar to the minimum amount observed in controls (ZT 2–4). ZTL levels in the two long period RNAi-C lines (lines 19 and 83) were reduced to less than 50% of WT levels, whereas line 41 was as WT (Fig. 2C). Taken together these results link HSP90-dependent stabilization of ZTL to the control of circadian period.
Fig. 2.
Reduction in HSP90 diminishes ZTL levels and stabilizes only SCFZTL targets. (A and B) Seedlings were grown 7–10 d in 12 h light/12 h dark (LD) cycles then treated at ZT 4 with 5 μM GDA or vehicle (DMSO), treated again 10 h later at ZT 14, and sampled every 4 h at the indicated times over an LD cycle. Lights on at ZT 0 and lights off at ZT 12 in all experiments. Immunoblot is representative of at least three trials; error bars in B represent ±SEM. Quantitation relative to Coomassie (Cms)-stained portion of gel and normalized to maximum expression level. *0.01 < P ≤ 0.05; **P ≤ 0.01. (C) Representative ZTL immunoblot (Upper) and average ZTL levels in three RNAi-mediated HSP90-reduced lines and untransformed WT (Lower). Samples were harvested at ZT 14 after 3 d of entrainment under LD from the seedlings used in Fig. 1 C and D. Identical letters above each line indicate not significantly different (Tukey's test). Quantitation relative to Cms-stained portion of gel and normalized to maximum expression level (in B) or nonspecific bands near the target band (in C). (D–K) Seedlings expressing TOC1:TOC1-YFP or PRRn:PRRn-GFP in four separate transgenic lines were grown 7–10 d in LD cycles then treated at ZT 4 with 5 μM GDA or vehicle (DMSO) and further treated and processed as described in A and B. (D, F, H, J) LD time course of ZTL and TOC1 or PRRn protein abundance in the corresponding backgrounds and treatment. (E, G, I, K) Quantitation of ZTL (Left) and TOC1 or PRRn protein (Right) for the time course shown. *0.01 < P ≤ 0.05; **P ≤ 0.01. All immunoblots are representative of at least three trials; error bars in all panels indicate ±SEM. Quantitation of the protein levels relative to Cms-stained portion of gel and normalized to maximum expression level.
When similar experiments were performed in a ZTL protein null background (ztl-3), period was further lengthened by up to 4 h (Fig. S5). These results are consistent with HSP90-dependent stabilization of ZTL family members FKF1 and LKP2: free-running period in the ztl lkp2 fkf1 background is significantly longer than in the ztl mutant (37). Experiments performed with radicicol, an additional HSP90 inhibitor, confirm the reduction of both ZTL and FKF1-TAP protein, supporting this notion (Fig. S6). These results do not exclude the possibility of other HSP90 client proteins in the context of the circadian clock.
Molecular Phenotype of HSP90-Reduced Lines Phenocopy ztl Mutants.
Because TOC1 and PRR5 protein levels are strongly stabilized in ztl mutants, whereas PRR3 and PRR7 levels are unchanged (18–20), we tested whether depletion of HSP90 could phenocopy the ztl mutant phenotype. Within 10 h of GDA application endogenous ZTL levels were reduced to between 20% and 40% of the DMSO controls, and over the time course levels continued to decline to ≈10% of the peak value (ZT 14) (ZTL panel in Fig. 2 E, G, I, and K). In contrast, TOC1 levels in GDA-treated seedlings continued to increase over the time course and remained high during the period when TOC1 normally declines (Fig. 2 D and E). PRR5 also showed a strongly reduced rhythm in the HSP90-depleted seedlings due to significantly higher PRR5 levels at trough time points (Fig. 2 F and G). In contrast, the levels of PRR3 and PRR7 were essentially identical to the controls at all time points (Fig. 2 H and I, J and K). Taken together, the diurnal pattern of protein abundance of the PRR family members (TOC1, PRR5, PRR3, and PRR7) in the HSP90-depleted seedlings was very similar to that observed in ztl mutants (18). Examination of the mRNA expression pattern of all five genes (ZTL, TOC1, PRR5, PRR3, and PRR7) under the same conditions showed that GDA and DMSO treatments were identical (Fig. S7), indicating that the changes in TOC1 and PRR5 protein occurred posttranscriptionally.
HSP90 Interacts with ZTL and Exhibits Holdase Activity in Vitro.
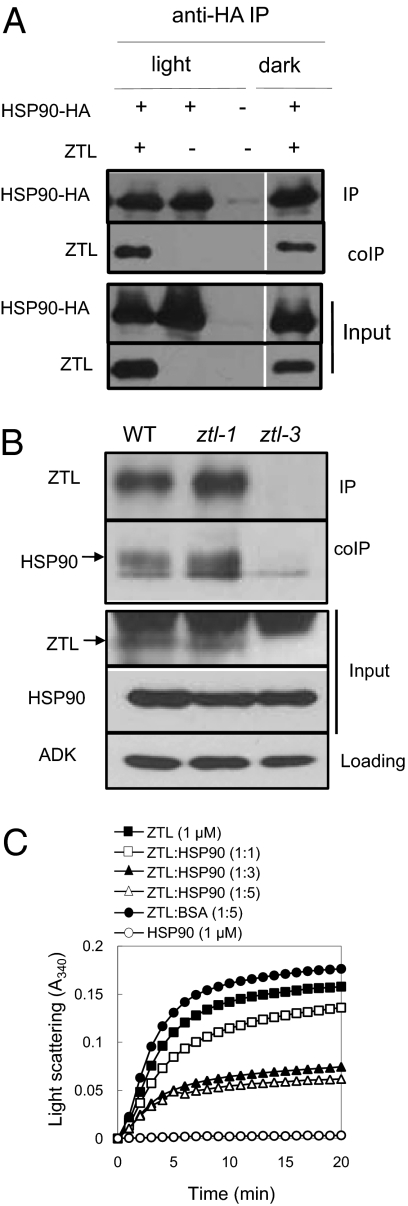
We next tested whether ZTL interacts with HSP90 in vivo by performing coimmunoprecipitations (co-IPs) from transiently coexpressed ZTL and HSP90-HA in Nicotiana benthamiana leaves. ZTL was successfully detected in HSP90-HA immunoprecipitates from both light- and dark-treated tissues, indicating that a ZTL–HSP90 complex can form in vivo independent of light conditions (Fig. 3A). Immunoprecipitations performed using an anti-ZTL antibody on extracts from WT Arabidopsis seedlings detected HSP90 in the immunoprecipitates (Fig. 3B). No detectable HSP90 was co-IPed from a ztl-3 null background, but HSP90 was detected in co-IPs using the protein-positive allele ztl-1 (Fig. 3B). These results show that ZTL and HSP90 interact in vivo at endogenous levels and support the notion that ZTL is a client of HSP90. Yeast two-hybrid interaction tests confirm this result and suggest the interaction does not require additional plant-specific factors (Fig. S8).
Fig. 3.
HSP90 interacts with ZTL in vivo and exhibits holdase activity in vitro. (A) ZTL and HSP90 interact independent of light conditions. N. benthamiana were grown in LD, and protein extracts from leaves transiently and ectopically expressing ZTL and AtHSP90.1-HA were harvested at ZT 8 (light) and at the equivalent time in dark-adapted leaves (dark) and cross-linked with 1% formaldehyde. Anti-HA immunoprecipitates (IP) were probed with anti-ZTL polyclonal antibody (coIP). (B) ZTL and HSP90 interact in Arabidopsis at endogenous levels. Seedlings were grown 7–10 d in LD cycles, harvested at ZT 13, and cross-linked with 1% formaldehyde. ZTL immune complexes from protein extracts were probed for endogenous ZTL (IP) and coimmunopreciptated endogenous HSP90 (coIP). Adenosine kinase (ADK) was used as loading control. ztl-3 was used as negative control; ztl-1 is a ZTL protein-positive mutant allele. Arrows indicate migration position of ZTL or HSP90. (C) ZTL is stabilized in the presence of AtHsp90.2 under thermal denaturing conditions. Bacterial-expressed and HPLC-purified MBP-ZTL (1 μM) was tested in the absence or presence of AtHSP90.2 in various molar ratios in a chaperone holdase assay (denaturating conditions: 26.6 mM guanidine·HCl and 45 °C). Absorbance was measured over a time course of heat treatment. BSA was used as a negative control.
To assess whether HSP90 can associate with ZTL in vitro and exhibit holdase activity, we performed a turbidity assay using ZTL as a substrate. This assay is commonly used to test for the first necessary step between a chaperone and client protein, which is a sustained and protective interaction under denaturing conditions (38, 39). When ZTL alone, or in the presence of BSA, was denatured the level of light-scattering aggregates increased (Fig. 3C). When ZTL was coincubated with increasing levels of HSP90, absorbance levels dropped, indicating the ability of HSP90 to associate with ZTL and protect against denaturation.
HSP90 and GI Are Linked in the Posttranslational Regulation of ZTL Protein.
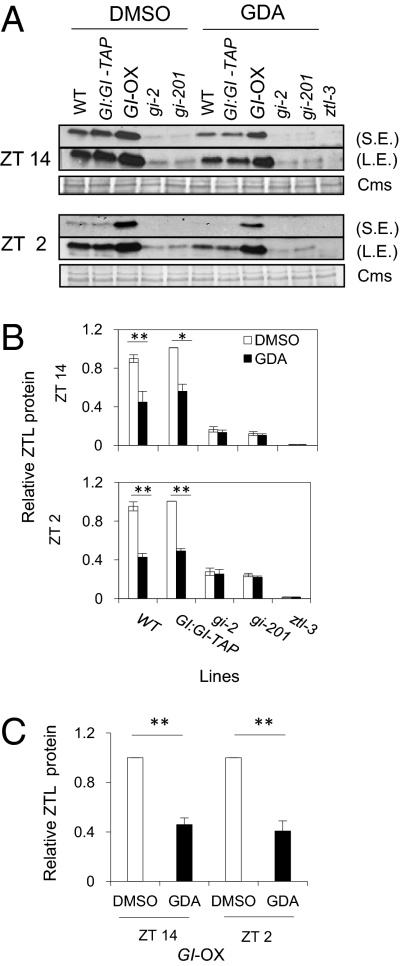
Although reduction in HSP90 levels triggers acute loss of ZTL, GI is also a key regulator of ZTL protein stability, with levels significantly lower in gi mutants (22). Therefore, we tested whether GDA-mediated ZTL reduction resulted indirectly from an effect of HSP90 depletion on GI levels. We performed the same HSP90 depletion assay and time course sampling on GI:GI-TAP transgenic lines as described for the PRR experiments. Whereas ZTL levels were strongly reduced, neither GI mRNA levels nor GI protein levels were significantly affected by GDA treatment (Fig. S9). These data support the notion that the effects of HSP90 depletion on PRR protein levels are mediated through a direct effect on ZTL, independent of GI.
We next tested whether GI and HSP90 function in the same ZTL stabilization pathway. GDA treatment significantly reduced ZTL to 40–60% of DMSO-treated WT levels at ZT 2 and 14 (Fig. 4 A and B). Detectable ZTL was also diminished by GDA treatment to a similar extent at both time points when GI is mildly overexpressed (GI:GI-TAP) and in the strong ectopically expressing GI-OX background (Fig. 4 B and C). These results indicate that reducing HSP90 can limit ZTL accumulation even at abnormally high GI levels, just as GI is limiting at endogenously high HSP90 levels [i.e., gi mutants (22)].
Fig. 4.
HSP90 and GI are linked in the posttranscriptional regulation of ZTL protein. (A) Seedlings of the indicated genotype were grown 7–10 d in LD cycles then treated with 5 μM GDA or DMSO 10 h before harvest at ZT 14. A second addition was immediately applied to the remaining tissues subsequently harvested at ZT 2. ZTL protein levels from WT, GI:GI-TAP, GI-OX, gi-2, gi-201, and ztl-3 lines under GDA or vehicle (DMSO) treatment at ZT 14 (Upper) and ZT 2 (Lower) were detected by immunoblot. Shorter (S.E.) or longer (L.E.) exposures are shown to visualize the wide range of signal intensities. (B) ZTL quantitations are based on L.E. at both time points in WT, GI:GI-TAP, gi-2, gi-201, and ztl-3 lines. (C) ZTL protein quantification based on S.E. at both time points for GI-OX. *0.01 < P ≤ 0.05; **P ≤ 0.01. GDA treatment significantly reduced ZTL abundance in all genotypes except in the gi-2 and gi-201 backgrounds (ZTL is undetectable in ztl-3). ZTL levels expressed relative to Coomassie and normalized to the maximum level at each time point. Mean is derived from at least three trials; error bars represent ±SEM.
If HSP90 and GI act via independent but convergent pathways we would expect simultaneous reduction of both to act additively. When GDA was applied to gi mutants the low levels of ZTL in that background were not significantly reduced at either time point, relative to the DMSO controls (Fig. 4B). Taken together, these results suggest that HSP90 and GI are tightly connected in the same ZTL-stabilization pathway.
Discussion
HSP90 Is Required for Proper Period Regulation of the Circadian Clock System.
Different and overlapping approaches to reduce endogenous levels of cytosolic HSP90 mutually support the notion that HSP90 is necessary to maintain normal function of the circadian oscillator in Arabidopsis. Although RNAi effectively reduced HSP90 cytosolic levels, this sustained loss over the entire early growth of the plant means that circadian dysfunction might arise from the indirect effects of developmental or physiological defects. Transient amiRNA transfection in protoplasts avoided this concern, and the lengthened period supports results obtained from whole plants. The maintenance of very robust cycling in this assay also suggests that only a discrete set of clock factors are affected by HSP90. GDA treatments were able to reduce ZTL levels to 20–40% of peak (ZT 14), and RNAi-mediated reduction of HSP90 diminished ZTL to ≈40–50% of endogenous levels. If this reduction in HSP90 even partially affected a wide range of circadian factors, we might expect a more acute loss of robustness, similar to that observed in Drosophila (see below). Even weak alleles of certain circadian factors, such as ELF3, can severely diminish the robustness of oscillations (40, 41). Similarly, the absence of an effect of GDA treatment on PRR3 and PRR7 levels (Fig. 2) implies that HSP90 is not involved in the regulation of these proteins, and their stabilization and turnover must occur through another mechanism.
Given the wide-ranging role of HSP90 in the cell, it is not surprising that HSP90 affects some aspect of the circadian system. However, previous tests of the effects of HSP90 depletion on the clock in animals have shown little to no effect on period. In Drosophila, pharmacologically or genetically reduced HSP90 levels greatly diminished robustness of circadian locomotor activity and slightly lengthened period in certain strains (42). This group concluded that HSP90 acts primarily downstream of the oscillator, affecting output pathway(s) that translate molecular oscillations into behavioral rhythms. In mouse, a systems-level analysis of an assortment of microarray data implicated HSP90 in a circadian gene regulatory network, primarily in the role of integrating regulatory inputs from a variety of environmental signals (43). This is consistent with recent results linking HSF1, a client of HSP90 (44), to temperature entrainment in peripheral oscillators in mouse (45, 46). Hence, in these metazoan studies HSP90 has been implicated as an integrator of processes leading to (input) or from (output) the central oscillator. These previous studies have not linked HSP90 to a specific client protein within the core oscillator, as reported here.
GDA application to the single-cell alga Ostreococcus tauri also lengthens period (47). Although these results are similar to Arabidopsis, no ZTL homolog has been identified in this species (48), although a recent report suggests that O. tauri possesses a ZTL-like protein lacking the blue light-sensing LOV domain (49). The circadian oscillator in this alga seems to be quite simple, consisting of a TOC1-CCA1–based loop, although other components may still be identified. Because a GI homolog is also lacking (48), it is likely that regulation of TOC1 differs in this species.
Under normal conditions HSP90 levels can be quite high, accounting for up to 1% of total cellular protein (27). In Arabidopsis, HSP90.1 gene expression is stress-induced, whereas HSP90.2–4 have been described as constitutively expressed (50, 51). However, recent data from the DIURNAL Project microarray repository (36) show robust diurnal and circadian oscillations of transcripts of some HSP90 transcripts (Fig. S4). Because antibodies are lacking that are capable of distinguishing between these highly similar isoforms, the significance of these cycles remains unclear. We were unable to detect reproducible variations in total cytosolic HSP90 levels in Arabidopsis under diurnal cycles or under constant light after entrainment.
Alternatively, HSP90 activity in the context of the clock may be regulated posttranslationally. Recent reports have identified key phosphorylation sites on HSP90 that affect its ability to interact with a select subset of cochaperones, and consequently, client proteins (52, 53). These findings clearly reveal that HSP90 abundance alone is not the sole determinant of its activity, because the phosphorylation of HSP90 by the yeast cell cycle-regulated kinase Swe1 implies a time- or development-specific window of HSP90 activation (53). Phase-specific differences in the phosphorylation state of key proteins are important in the control of the clock (54–56), and the possibility of similar regulation extending to HSP90 bears examination.
HSP90/GI Pathway.
GI and HSP90 are both essential to the stabilization of ZTL. GI also shares with HSP90 the distinction of affecting a wide range of processes in plant development and physiology. Deficiencies in GI affect the circadian system (ZTL stability and temperature compensation) and flowering time but also lead to excess starch accumulation, alterations to sucrose metabolism, and altered sensitivity to light and oxidative stress (22–25, 57–59). Our tests indicate that GI and HSP90 likely act in the same pathway in the control of ZTL levels.
Specificity of HSP90 action resides in part with the different cochaperones that associate with the HSP90 complex during the maturation cycle (27, 60). This can be a complex process, with the order of entry and exit from the cycle being specific to the particular client protein, and in most cases the specific cochaperone(s) are unknown. If found to directly participate in the HSP90 complex responsible for ZTL maturation, the late-phase oscillation of GI may be an example of how the circadian system can impose rhythmic regulation on otherwise constitutively present process. Additionally, because both gi mutants and HSP90 depletion act pleiotropically this unique pairing of two previously unconnected proteins opens up wider possibilities for their functioning together in other aspects of plant development.
F-Box Proteins as Unique HSP90 Clients.
The involvement of HSP90 in the maturation of ZTL suggests a unique role in protein turnover. Previous work in plant defense has linked HSP90 to Cullin1-based Skp1-Cul-F-box protein (SCF) complexes through SGT1, a cochaperone that associates with both HSP90 and the F-box protein Skp1 (29, 61). Here the exact role of the HSP90–SGT1 association is unclear, but one possibility is to allow a target protein to associate with either HSP90 as a client or with Skp1 as an SCF substrate, depending on cellular conditions (61). In yeast and humans the SGT1–HSP90 interaction with Skp1 is important to kinetechore assembly, suggesting a primary role in the proper assembly of multiprotein complexes (62, 63).
Our results relating HSP90 to SCFZTL expand on this latter notion. The assembly and disassembly of the SCF ubiquitin ligase complex is still poorly understood but involves modification of the CUL1 subunit by the addition of the ubiquitin-related protein RUB/NEDD8. This step facilitates assembly of a functional SCF complex. Later disassembly occurs as the COP9 signalosome removes RUB/NEDD8, freeing CUL1 to interact with CAND1, which inhibits the SKP1-Cul1 interaction (64, 65). Our findings now suggest that HSP90 is directly involved in the regulation of the assembly of a functional SCF E3 ligase through an additional mechanism, that of the maturation of the essential F-box protein component of the ligase complex. With nearly 700 F-box proteins in plants (66), specific cochaperones or other factors could associate with HSP90 in a cell-, tissue-, or development-specific manner and position F-box protein maturation as a finely tuneable step in the control SCF complex function. If found to be more widespread, this would place HSP90 in a unique and more central role in the regulation of proteolysis.
Materials and Methods
Plant Materials and Growth Conditions.
Further information on experimental growth conditions, GDA treatments, plasmid constructions, and rhythm analyses used in this study are provided in SI Materials and Methods.
Protein Analysis.
Details of the immunoblot procedures, protein crosslinking protocol, and the chaperone holdase assay are provided in SI Materials and Methods.
PCR Techniques.
Details of semiquantitative and quantitative PCR techniques are included in SI Materials and Methods.
Luminescence Assay Using amiRNA.
Details describing protoplast isolation, DNA transfection, and luciferase imaging are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Christine Queitsch for generously sharing the HSP90 RNAi lines and advice in the early stages of this work, David Bisaro for the adenosine kinase antibody, Jelena Brkljacic for helpful suggestions, Sung Suk Suh for assistance with radicicol experiments, and Sun Bin Kang for assistance with yeast two-hybrid experiments. This work was funded by National Science Foundation Grant 0748749 and National Institutes of Health Grant GM093285 (to D.E.S.), and the Rural Development Administration for the Next-Generation BioGreen 21 Program (Systems and Synthetic Agrobiotech Center, no. PJ008025) and by the Ministry of Education, Science and Technology for the World Class University (WCU) program (R32-10148) (to W.Y.K.). S.F. was supported in part by the Yamada Science Foundation (Support for Long-Term Visit).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1110406108/-/DCSupplemental.
References
- 1.Michael TP, et al. Enhanced fitness conferred by naturally occurring variation in the circadian clock. Science. 2003;302:1049–1053. doi: 10.1126/science.1082971. [DOI] [PubMed] [Google Scholar]
- 2.Dodd AN, et al. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science. 2005;309:630–633. doi: 10.1126/science.1115581. [DOI] [PubMed] [Google Scholar]
- 3.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: Implications for physiology and disease. Nat Rev Genet. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yerushalmi S, Yakir E, Green RM. Circadian clocks and adaptation in Arabidopsis. Mol Ecol. 2011;20:1155–1165. doi: 10.1111/j.1365-294X.2010.04962.x. [DOI] [PubMed] [Google Scholar]
- 6.Benito J, Zheng H, Ng FS, Hardin PE. Transcriptional feedback loop regulation, function, and ontogeny in Drosophila. Cold Spring Harb Symp Quant Biol. 2007;72:437–444. doi: 10.1101/sqb.2007.72.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harmer SL. The circadian system in higher plants. Annu Rev Plant Biol. 2009; 60:357–377. doi: 10.1146/annurev.arplant.043008.092054. [DOI] [PubMed] [Google Scholar]
- 8.Zhang EE, Kay SA. Clocks not winding down: Unravelling circadian networks. Nat Rev Mol Cell Biol. 2010;11:764–776. doi: 10.1038/nrm2995. [DOI] [PubMed] [Google Scholar]
- 9.Pokhilko A, et al. Data assimilation constrains new connections and components in a complex, eukaryotic circadian clock model. Mol Syst Biol. 2010;6:416. doi: 10.1038/msb.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeilinger MN, Farré EM, Taylor SR, Kay SA, Doyle FJ., 3rd A novel computational model of the circadian clock in Arabidopsis that incorporates PRR7 and PRR9. Mol Syst Biol. 2006;2:58. doi: 10.1038/msb4100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Godinho SI, et al. The after-hours mutant reveals a role for Fbxl3 in determining mammalian circadian period. Science. 2007;316:897–900. doi: 10.1126/science.1141138. [DOI] [PubMed] [Google Scholar]
- 12.Kim WY, Geng R, Somers DE. Circadian phase-specific degradation of the F-box protein ZTL is mediated by the proteasome. Proc Natl Acad Sci USA. 2003;100:4933–4938. doi: 10.1073/pnas.0736949100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He Q, Liu Y. Degradation of the Neurospora circadian clock protein FREQUENCY through the ubiquitin-proteasome pathway. Biochem Soc Trans. 2005;33:953–956. doi: 10.1042/BST20050953. [DOI] [PubMed] [Google Scholar]
- 14.Siepka SM, et al. Circadian mutant Overtime reveals F-box protein FBXL3 regulation of cryptochrome and period gene expression. Cell. 2007;129:1011–1023. doi: 10.1016/j.cell.2007.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koh K, Zheng X, Sehgal A. JETLAG resets the Drosophila circadian clock by promoting light-induced degradation of TIMELESS. Science. 2006;312:1809–1812. doi: 10.1126/science.1124951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Somers DE, Schultz TF, Milnamow M, Kay SA. ZEITLUPE encodes a novel clock-associated PAS protein from Arabidopsis. Cell. 2000;101:319–329. doi: 10.1016/s0092-8674(00)80841-7. [DOI] [PubMed] [Google Scholar]
- 17.Lamaze A, et al. The E3 ubiquitin ligase CTRIP controls CLOCK levels and PERIOD oscillations in Drosophila. EMBO Rep. 2011;12:549–557. doi: 10.1038/embor.2011.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujiwara S, et al. Post-translational regulation of the Arabidopsis circadian clock through selective proteolysis and phosphorylation of pseudo-response regulator proteins. J Biol Chem. 2008;283:23073–23083. doi: 10.1074/jbc.M803471200. [DOI] [PubMed] [Google Scholar]
- 19.Más P, Kim WY, Somers DE, Kay SA. Targeted degradation of TOC1 by ZTL modulates circadian function in Arabidopsis thaliana. Nature. 2003;426:567–570. doi: 10.1038/nature02163. [DOI] [PubMed] [Google Scholar]
- 20.Kiba T, Henriques R, Sakakibara H, Chua NH. Targeted degradation of PSEUDO-RESPONSE REGULATOR5 by an SCFZTL complex regulates clock function and photomorphogenesis in Arabidopsis thaliana. Plant Cell. 2007;19:2516–2530. doi: 10.1105/tpc.107.053033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fornara F, et al. Arabidopsis DOF transcription factors act redundantly to reduce CONSTANS expression and are essential for a photoperiodic flowering response. Dev Cell. 2009;17:75–86. doi: 10.1016/j.devcel.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 22.Kim WY, et al. ZEITLUPE is a circadian photoreceptor stabilized by GIGANTEA in blue light. Nature. 2007;449:356–360. doi: 10.1038/nature06132. [DOI] [PubMed] [Google Scholar]
- 23.Park DH, et al. Control of circadian rhythms and photoperiodic flowering by the Arabidopsis GIGANTEA gene. Science. 1999;285:1579–1582. doi: 10.1126/science.285.5433.1579. [DOI] [PubMed] [Google Scholar]
- 24.Fowler S, et al. GIGANTEA: A circadian clock-controlled gene that regulates photoperiodic flowering in Arabidopsis and encodes a protein with several possible membrane-spanning domains. EMBO J. 1999;18:4679–4688. doi: 10.1093/emboj/18.17.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huq E, Tepperman JM, Quail PH. GIGANTEA is a nuclear protein involved in phytochrome signaling in Arabidopsis. Proc Natl Acad Sci USA. 2000;97:9789–9794. doi: 10.1073/pnas.170283997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearl LH, Prodromou C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu Rev Biochem. 2006;75:271–294. doi: 10.1146/annurev.biochem.75.103004.142738. [DOI] [PubMed] [Google Scholar]
- 27.Taipale M, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: Emerging mechanistic insights. Nat Rev Mol Cell Biol. 2010;11:515–528. doi: 10.1038/nrm2918. [DOI] [PubMed] [Google Scholar]
- 28.Kadota Y, Shirasu K, Guerois R. NLR sensors meet at the SGT1-HSP90 crossroad. Trends Biochem Sci. 2010;35:199–207. doi: 10.1016/j.tibs.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Shirasu K. The HSP90-SGT1 chaperone complex for NLR immune sensors. Annu Rev Plant Biol. 2009;60:139–164. doi: 10.1146/annurev.arplant.59.032607.092906. [DOI] [PubMed] [Google Scholar]
- 30.Hubert DA, He Y, McNulty BC, Tornero P, Dangl JL. Specific Arabidopsis HSP90.2 alleles recapitulate RAR1 cochaperone function in plant NB-LRR disease resistance protein regulation. Proc Natl Acad Sci USA. 2009;106:9556–9563. doi: 10.1073/pnas.0904877106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sangster TA, Queitsch C. The HSP90 chaperone complex, an emerging force in plant development and phenotypic plasticity. Curr Opin Plant Biol. 2005;8:86–92. doi: 10.1016/j.pbi.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 32.Sangster TA, et al. Phenotypic diversity and altered environmental plasticity in Arabidopsis thaliana with reduced Hsp90 levels. PLoS ONE. 2007;2:e648. doi: 10.1371/journal.pone.0000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sangster TA, et al. HSP90-buffered genetic variation is common in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2008;105:2969–2974. doi: 10.1073/pnas.0712210105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taldone T, Sun W, Chiosis G. Discovery and development of heat shock protein 90 inhibitors. Bioorg Med Chem. 2009;17:2225–2235. doi: 10.1016/j.bmc.2008.10.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sangster TA, et al. HSP90 affects the expression of genetic variation and developmental stability in quantitative traits. Proc Natl Acad Sci USA. 2008;105:2963–2968. doi: 10.1073/pnas.0712200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mockler TC, et al. The DIURNAL project: DIURNAL and circadian expression profiling, model-based pattern matching, and promoter analysis. Cold Spring Harb Symp Quant Biol. 2007;72:353–363. doi: 10.1101/sqb.2007.72.006. [DOI] [PubMed] [Google Scholar]
- 37.Baudry A, et al. F-box proteins FKF1 and LKP2 act in concert with ZEITLUPE to control Arabidopsis clock progression. Plant Cell. 2010;22:606–622. doi: 10.1105/tpc.109.072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jang HH, et al. Two enzymes in one; two yeast peroxiredoxins display oxidative stress-dependent switching from a peroxidase to a molecular chaperone function. Cell. 2004;117:625–635. doi: 10.1016/j.cell.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Jakob U, Lilie H, Meyer I, Buchner J. Transient interaction of Hsp90 with early unfolding intermediates of citrate synthase. Implications for heat shock in vivo. J Biol Chem. 1995;270:7288–7294. doi: 10.1074/jbc.270.13.7288. [DOI] [PubMed] [Google Scholar]
- 40.McWatters HG, Bastow RM, Hall A, Millar AJ. The ELF3 zeitnehmer regulates light signalling to the circadian clock. Nature. 2000;408:716–720. doi: 10.1038/35047079. [DOI] [PubMed] [Google Scholar]
- 41.Reed JW, et al. Independent action of ELF3 and phyB to control hypocotyl elongation and flowering time. Plant Physiol. 2000;122:1149–1160. doi: 10.1104/pp.122.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hung HC, Kay SA, Weber F. HSP90, a capacitor of behavioral variation. J Biol Rhythms. 2009;24:183–192. doi: 10.1177/0748730409333171. [DOI] [PubMed] [Google Scholar]
- 43.Yan J, Wang H, Liu Y, Shao C. Analysis of gene regulatory networks in the mammalian circadian rhythm. PLOS Comput Biol. 2008;4:e1000193. doi: 10.1371/journal.pcbi.1000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Voellmy R. On mechanisms that control heat shock transcription factor activity in metazoan cells. Cell Stress Chaperones. 2004;9:122–133. doi: 10.1379/CSC-14R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buhr ED, Yoo SH, Takahashi JS. Temperature as a universal resetting cue for mammalian circadian oscillators. Science. 2010;330:379–385. doi: 10.1126/science.1195262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reinke H, et al. Differential display of DNA-binding proteins reveals heat-shock factor 1 as a circadian transcription factor. Genes Dev. 2008;22:331–345. doi: 10.1101/gad.453808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O'Neill JS, et al. Circadian rhythms persist without transcription in a eukaryote. Nature. 2011;469:554–558. doi: 10.1038/nature09654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Corellou F, et al. Clocks in the green lineage: Comparative functional analysis of the circadian architecture of the picoeukaryote ostreococcus. Plant Cell. 2009;21:3436–3449. doi: 10.1105/tpc.109.068825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Ooijen G, Dixon LE, Troein C, Millar AJ. Proteasome function is required for biological timing throughout the twenty-four hour cycle. Curr Biol. 2011;21:869–875. doi: 10.1016/j.cub.2011.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yabe N, Takahashi T, Komeda Y. Analysis of tissue-specific expression of Arabidopsis thaliana HSP90-family gene HSP81. Plant Cell Physiol. 1994;35:1207–1219. doi: 10.1093/oxfordjournals.pcp.a078715. [DOI] [PubMed] [Google Scholar]
- 51.Krishna P, Gloor G. The Hsp90 family of proteins in Arabidopsis thaliana. Cell Stress Chaperones. 2001;6:238–246. doi: 10.1379/1466-1268(2001)006<0238:thfopi>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mollapour M, et al. Threonine 22 phosphorylation attenuates Hsp90 interaction with cochaperones and affects its chaperone activity. Mol Cell. 2011;41:672–681. doi: 10.1016/j.molcel.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mollapour M, et al. Swe1Wee1-dependent tyrosine phosphorylation of Hsp90 regulates distinct facets of chaperone function. Mol Cell. 2010;37:333–343. doi: 10.1016/j.molcel.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chiu JC, Vanselow JT, Kramer A, Edery I. The phospho-occupancy of an atypical SLIMB-binding site on PERIOD that is phosphorylated by DOUBLETIME controls the pace of the clock. Genes Dev. 2008;22:1758–1772. doi: 10.1101/gad.1682708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vanselow K, Kramer A. Role of phosphorylation in the mammalian circadian clock. Cold Spring Harb Symp Quant Biol. 2007;72:167–176. doi: 10.1101/sqb.2007.72.036. [DOI] [PubMed] [Google Scholar]
- 56.Gallego M, Virshup DM. Post-translational modifications regulate the ticking of the circadian clock. Nat Rev Mol Cell Biol. 2007;8:139–148. doi: 10.1038/nrm2106. [DOI] [PubMed] [Google Scholar]
- 57.Edwards J, et al. GIGANTEA is a component of a regulatory pathway determining wall ingrowth deposition in phloem parenchyma transfer cells of Arabidopsis thaliana. Plant J. 2010;63:651–661. doi: 10.1111/j.1365-313X.2010.04269.x. [DOI] [PubMed] [Google Scholar]
- 58.Gould PD, et al. The molecular basis of temperature compensation in the Arabidopsis circadian clock. Plant Cell. 2006;18:1177–1187. doi: 10.1105/tpc.105.039990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dalchau N, et al. The circadian oscillator gene GIGANTEA mediates a long-term response of the Arabidopsis thaliana circadian clock to sucrose. Proc Natl Acad Sci USA. 2011;108:5104–5109. doi: 10.1073/pnas.1015452108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zuehlke A, Johnson JL. Hsp90 and co-chaperones twist the functions of diverse client proteins. Biopolymers. 2010;93:211–217. doi: 10.1002/bip.21292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang M, et al. Structural and functional coupling of Hsp90- and Sgt1-centred multi-protein complexes. EMBO J. 2008;27:2789–2798. doi: 10.1038/emboj.2008.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davies AE, Kaplan KB. Hsp90-Sgt1 and Skp1 target human Mis12 complexes to ensure efficient formation of kinetochore-microtubule binding sites. J Cell Biol. 2010;189:261–274. doi: 10.1083/jcb.200910036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lingelbach LB, Kaplan KB. The interaction between Sgt1p and Skp1p is regulated by HSP90 chaperones and is required for proper CBF3 assembly. Mol Cell Biol. 2004;24:8938–8950. doi: 10.1128/MCB.24.20.8938-8950.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goldenberg SJ, et al. Structure of the Cand1-Cul1-Roc1 complex reveals regulatory mechanisms for the assembly of the multisubunit cullin-dependent ubiquitin ligases. Cell. 2004;119:517–528. doi: 10.1016/j.cell.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 65.Deshaies RJ, Joazeiro CA. RING domain E3 ubiquitin ligases. Annu Rev Biochem. 2009;78:399–434. doi: 10.1146/annurev.biochem.78.101807.093809. [DOI] [PubMed] [Google Scholar]
- 66.Gagne JM, Downes BP, Shiu SH, Durski AM, Vierstra RD. The F-box subunit of the SCF E3 complex is encoded by a diverse superfamily of genes in Arabidopsis. Proc Natl Acad Sci USA. 2002;99:11519–11524. doi: 10.1073/pnas.162339999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.