Abstract
Numerous pathogens of humans, animals, and plants are transmitted by specific arthropod vectors. However, understanding the mechanisms governing these pathogen–vector interactions is hampered, in part, by the lack of easy-to-use analytical tools. We investigated whitefly transmission of Lettuce infectious yellows virus (LIYV) by using a unique immunofluorescent localization approach in which we fed virions or recombinant virus capsid components to whiteflies, followed by feeding them antibodies to the virions or capsid components, respectively. Fluorescent signals, indicating the retention of virions, were localized in the anterior foregut or cibarium of a whitefly vector biotype but not within those of a whitefly nonvector biotype. Retention of virions in these locations strongly corresponded with the whitefly vector transmission of LIYV. When four recombinant LIYV capsid components were individually fed to whitefly vectors, significantly more whiteflies retained the recombinant minor coat protein (CPm). As demonstrated previously and in the present study, whitefly vectors failed to transmit virions preincubated with anti-CPm antibodies but transmitted virions preincubated with antibodies recognizing the major coat protein (CP). Correspondingly, the number of insects that specifically retained virions preincubated with anti-CPm antibodies were significantly reduced compared with those that specifically retained virions preincubated with anti-CP antibodies. Notably, a transmission-defective CPm mutant was deficient in specific virion retention, whereas the CPm-restored virus showed WT levels of specific virion retention and transmission. These data provide strong evidence that transmission of LIYV is determined by a CPm-mediated virion retention mechanism in the anterior foregut or cibarium of whitefly vectors.
Keywords: arthropod vector transmission, crinivirus, noncirculative transmission, semipersistent transmission, Bemisia tabaci
Transmission by arthropod (insect) vectors is essential to the infection cycle of many viruses, including those that cause diseases in humans, animals, and plants (1–3), and is mediated by critical but poorly understood processes that vary across phases of virus acquisition, retention, and inoculation. With few exceptions, viral determinants and vector sites mediating transmission of vector-specific viruses remain poorly investigated for numerous plant viruses, many of which cause serious diseases that constrain crop and fiber production worldwide (1, 4–6).
Members of the genus Crinivirus (family Closteroviridae) are emerging viruses affecting many different crop plants (7). The genomes of criniviruses are among the largest and most complex of the single-stranded positive-sense RNA viruses and, based on sequence information and biological data, are organized into two separate components. RNA 1 encodes several functions including virus replication and synergism, whereas RNA 2 contains as many as 10 ORFs involved in cytopathology, virion assembly, and vector transmission (8–14). Criniviruses occur in low titer and are restricted to the phloem of infected plants. They cannot be transmitted by leaf-rub inoculation but are readily transmitted in a noncirculative, semipersistent manner by specific whitefly vectors in the insect order Hemiptera. Molecular mechanisms underlying this mode of transmission are poorly understood, but several biological features are known or inferred. First, the vector penetrates a phloem sieve element with its stylets and acquires virions by ingesting phloem sap. Acquisition success increases with prolonged ingestion of phloem sap (from minutes to hours). When they have been acquired, virions are retained in the vector for hours to days and are lost if the insect molts. Second, virions do not circulate through the insect and invade the salivary glands, in contrast to viruses that are transmitted in a circulative, persistent manner; nor do they replicate in the vector (7).
During acquisition, virus-laden phloem sap enters the opening at the tip of the maxillary stylets into a short lumen where the maxillary food and salivary canals merge. From this location, infected sap moves up the maxillary food canal into a region referred to as the precibarium before entering the cibarium, which functions as a sucking pump. Sap is then pumped out of the cibarium into the anterior foregut, the pharynx, followed by the esophagus. In a few early studies, transmission EM of viruliferous leafhoppers and aphids have provided some evidence that the foregut may be where virions of some semipersistently transmitted viruses are retained, and from where they are eventually released and inoculated into the plant (15–17). However, some uncertainties exist whether viruses retained in the foregut are related to transmission (15, 16). In addition, the viral determinants mediating virion retention in the foregut have not been identified for any of these viruses. Cauliflower mosaic virus (CaMV) transmission by aphids is noncirculative and generally regarded as semipersistent. A recent study localized CaMV virion-like particles and a virus-encoded protein within the tip of the maxillary stylets, rather than the foregut. Thus, there appears to be significant variation in retention site among different semipersistently transmitted plant viruses (18). Interestingly, retention of plant virus virions in the stylets and foreguts of insect vectors appears to be analogous to a feature occasionally exhibited by certain animal viruses. This feature, referred to as mechanical transmission (not to be confused with “mechanical leaf rub inoculation” coined by plant virologists), is thought to occur when virions of viruses that do not replicate in arthropod vectors are nonspecifically harbored in their mouthparts (and even in the foreguts, as suggested in the case of retroviruses) and get transmitted from one vertebrate to another during blood meals taken by the vectors (19–21). For example, Chihota et al. (22) showed that Lumpy skin disease virus can survive in mosquitoes without replication for at least 6 d and still remains transmissible, and Smith et al. (23) demonstrated that Vesicular stomatitis New Jersey virus can be mechanically transmitted among domestic swine by biting flies after minutes of acquisition and inoculation feeding. However, as will be inferred from our study, the interactions mediating virion retention associated with mechanical transmission of animal viruses may be more sophisticated than currently appreciated.
Most of our knowledge on the whitefly transmission of criniviruses originates from studies of Lettuce infectious yellows virus (LIYV). Four LIYV RNA 2-encoded proteins—a heat shock protein 70 homologue, HSP70h; a 59-kDa protein, P59; the major coat protein, CP; and the minor coat protein, CPm—are components of the long flexuous rod shape virion (11). Purified virions of LIYV can be transmitted by the whitefly Bemisia tabaci biotype A via membrane feeding, a procedure in which insects acquire virions in artificial liquid diet trapped between a pair of stretched and closely spaced parafilm sheet. Thus, LIYV, unlike the potyviruses or CaMV, does not require a nonvirion virus protein “helper” component for acquisition and transmission by the insect vector (7, 11, 24). Recent studies have revealed that the CPm is essential for LIYV transmission by B. tabaci biotype A (10, 11), but much crucial information needed to understand the virus–vector interactions involved in LIYV transmission is still missing.
Here, we used a unique membrane feeding and immunofluorescent localization system to provide unique insights on the mechanism of whitefly transmission of LIYV by demonstrating the correspondence between the specific retention of virions within the whitefly vector and successful transmission of LIYV. We also provide biochemical and molecular evidence that vector-specific retention and transmission of virions is mediated by the LIYV CPm. These results are significant not only from a plant virus–insect vector biology standpoint, but also from an evolutionary context given the parallels between noncirculative plant virus transmission and the mechanical transmission of animal viruses (20, 25).
Results
LIYV Virions Are Retained in Anterior Foregut or Cibarium of Whitefly Vectors.
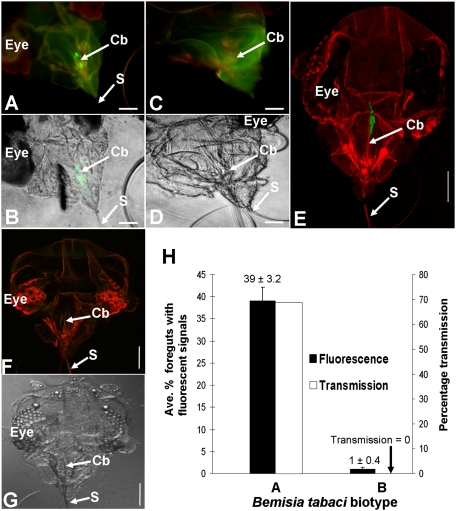
To determine the retention site of LIYV in its whitefly vector, we developed a membrane feeding and immunofluorescent localization assay in which caged whiteflies were given sequential access to basal artificial liquid diet containing (i) purified LIYV virions, (ii) anti-LIYV virion IgG, and (iii) goat anti-rabbit IgG conjugated with Alexa Fluor 488. Afterward, the heads were dissected from individual whiteflies and analyzed by fluorescence microscopy. To first validate the assay, two groups of whitefly vectors, B. tabaci biotype A, were compared: in step 1 above, one group fed on diet containing WT LIYV virions whereas the other group fed on diet alone (i.e., no virions). Results from three independent experiments revealed the presence of bright green fluorescent signals distributed in the anterior foregut or cibarium of virion-fed vectors, representative images of which are shown in Fig. 1 A, B, and E and Movie S1. Two virion concentrations, 0.01 μg/μL and 0.1 μg/μL, were used, both of which were readily detectable in vitro under nondenaturing conditions by double antibody-sandwiched (DAS) ELISA (Table S1). In the first two experiments, when 0.01 μg/μL of virions were fed to the vectors, fluorescent signals were observed in the anterior foregut or cibarium of six of 10 and 17 of 65 individuals, respectively. Conversely, a much weaker signal was observed in the same regions of only one of 20 diet-fed (i.e., no virions) vectors in the first experiment (i.e., 19 of 20 showed no signal), whereas, in the second experiment, none of 65 diet-fed vectors showed a signal in these regions. Representative images of a diet-fed vector that displayed no fluorescent signal in the specified regions are shown in Fig. 1 C and D. In the third experiment, when the virion concentration was increased to 0.1 μg/μL, fluorescent signals were seen in the specified regions of 18 of 48 vectors that fed on virion augmented diet, whereas none of 37 diet-fed vectors showed any signal. In all cases, no signal was observed anywhere on the maxillary stylets of virion- or diet-fed vectors (Fig. 1 A, B, and E), and only very rarely was an extremely weak signal observed in the precibarium. To verify that the signals in LIYV virion-fed vectors were not a result of nonspecific virion binding, we used a similar immunofluorescent localization assay to analyze B. tabaci biotype A, except that they were fed on a diet containing 0.5 μg/μL or 1 μg/μL of the aphid transmitted Cucumber mosaic virus (CMV) virions in step 1, and on a diet containing an anti-CMV antiserum in step 2. No signal was observed in the cibarium, foregut, or maxillary stylets (including the tips) of all 183 CMV-fed whiteflies we examined (Fig. S1 A and B), even though the virion concentrations used in the feeding were readily detectable in vitro under nondenaturing conditions by triple antibody-sandwiched (TAS) ELISA (Table S1).
Fig. 1.
Retention of LIYV virions in the anterior foregut or cibarium of whiteflies and transmissibility of LIYV by whiteflies B. tabaci biotypes A and B after sequential membrane feeding of the following solutions: (i) diet alone or diet containing virions, (ii) diet containing anti-LIYV IgG, and (iii) diet containing a goat anti-rabbit IgG conjugated with Alexa Fluor 488. The presence or absence of fluorescent signals in the dissected whitefly heads was analyzed by using fluorescence microscopy. (A) Widefield fluorescence microscopy micrograph of the head of B. tabaci biotype A fed diet containing LIYV virions, with background transmitted light blocked. (B) The image in A with background transmitted light unblocked. (C and D) The same as A and B, except that B. tabaci biotype A was fed diet without virions. (E) Confocal laser scanning microscopy micrograph of the head of a B. tabaci biotype A fed diet containing LIYV virions (Movie S1). (F) Confocal laser scanning microscopy micrograph of the head of a B. tabaci biotype B fed diet containing LIYV virions. (G) Transmitted light view of F. The eye, cibarium (Cb), and stylets (S) are indicated. (Scale bars, 45 μm.) (H) The average percentage of anterior foreguts or cibarium with fluorescent signals between virion-fed biotypes A and B and the corresponding percentage of LIYV transmissibility; the data were pooled from three independent experiments (Table S2). Error bars represent SE.
Correspondence Between Virion Retention in Anterior Foregut or Cibarium of Whiteflies and LIYV Transmission.
The above results led us to hypothesize that LIYV virions retained in the anterior foregut or cibarium are those that the whitefly vector transmits. To determine if retention of virions in the foregut or cibarium corresponded to transmission success, we used the membrane feeding and immunofluorescent localization technique in which whiteflies [with ∼100 vector (B. tabaci biotype A) or nonvector (B. tabaci biotype B) whiteflies per cage] were fed a diet containing 0.4 μg/μL of LIYV virions or a diet without virions (Table S2). Then, half (∼50) of the whiteflies from each cage were transferred to a noninfected lettuce plant for overnight inoculation access feeding whereas the other half of the whiteflies from the same cage were subjected to the subsequent steps of the membrane feeding and immunofluorescent localization assay as described earlier. In three independent experiments comparing vector whiteflies (biotype A) fed a diet with or without virions, strong green fluorescent signals were seen in the anterior foregut or cibarium of 16% to 63% (average, 39%) of virion-fed vectors (Fig. 1H), whereas faint signals were seen in the same regions of only 0% to 2% of diet-fed vectors (Table S2, experiments 1–3). This was consistent with results of the LIYV virion acquisition experiments detailed earlier. The corresponding LIYV transmission success by the other half of the virion-fed whiteflies that were given an inoculation access to lettuce plants was 13 of 19 test plants infected (68%; Fig. 1H). Similarly handled diet-fed whitefly vectors did not result in any LIYV transmission (Table S2, experiments 1–3). The difference in transmission success was highly significant (P < 0.0001; Fisher exact test).
In contrast, fluorescent signal in the anterior foregut or cibarium was absent in almost all (99%) of the virion-fed nonvectors (biotype B; Fig. 1 F–H and Table S2, experiments 4–6). In a few cases, weak signals were seen in the same regions of only one or two nonvectors fed on virus augmented diet, and no signal was observed in the same regions of all diet-fed nonvectors (Table S2, experiments 4–6). Correspondingly, for the half of the nonvector whiteflies that were transferred to lettuce plants after feeding on virus-spiked diet or diet alone, no transmission was observed (Fig. 1H and Table S2, experiments 4–6).
Recombinant LIYV CPm Is Localized in Anterior Foregut or Cibarium of Whitefly Vectors Following Acquisition.
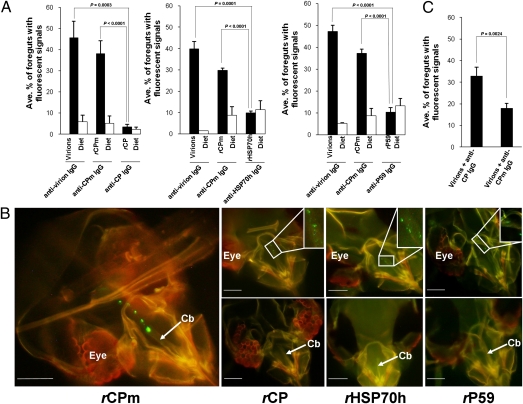
The above data indicate that LIYV virions are retained specifically in the anterior foregut or cibarium of the vector but not the nonvector whitefly; this suggests that LIYV encodes determinants that function in the specific recognition of retention sites within its whitefly vector. Consequently, we conducted three experiments to determine which of the four capsid components are involved in retention in the vector B. tabaci biotype A. Each of the experiments had three pairs of treatments (Fig. 2A): (i) unaugmented diet and diet augmented with 0.4 μg/μL of virions, each followed by diet augmented with anti-LIYV IgG; (ii) unaugmented diet and diet augmented with purified Escherichia coli expressed recombinant LIYV CPm (rCPm; Fig. S2A), each followed by diet augmented with anti-CPm IgG; and (iii) unaugmented diet and diet augmented with one of the following purified E. coli expressed recombinant capsid components—rCP, rHSP70h, or rP59 (Fig. S2A)—each followed by diet augmented with the corresponding anticapsid protein IgG (anti-CP, anti-HSP70h, or anti-P59, respectively). The concentrations of the recombinant capsid components used in membrane feeding are indicated in Tables S3–S5. Whiteflies in all treatments were then fed diet augmented with goat anti-rabbit IgG conjugated with Alexa Fluor 488. Fluorescent signals were observed in the anterior foregut or cibarium of 40% to 47% of virion-fed vectors and 30% to 38% of rCPm-fed vectors (Fig. 2A and Tables S3–S5). The percentages of virion-fed whiteflies and rCPm-fed whiteflies differed significantly from their respective diet-fed controls in all three experiments (P < 0.0001; Student t test; Fig. 2A). In contrast, only 4% of rCP-fed whiteflies, 10% of rHSP70h-fed whiteflies, and 10% of rP59-fed whiteflies showed positive signals, and none of these differed significantly from their respective diet-fed controls (P > 0.05; Student t test; Fig. 2A and Tables S3–S5). The percentages of rCP-fed, rHSP70h-fed, and rP59-fed whiteflies with signals in their anterior foregut or cibarium were all significantly lower than in virion-fed and rCPm-fed whiteflies (P ≤ 0.0003; Student t test). Overall, although the average percentages of virion-fed and rCPm-fed whiteflies with signals in their anterior foregut or cibarium were relatively high, they were statistically different from each other (P = 0.0228; Student t test; data pooled over all three experiments; Fig. 2A). In most cases, the signals seen in rCPm-fed vectors occurred more in the form of multiple punctate spots as opposed to a larger, more continuous area of fluorescence as was typically observed in virion-fed vectors (compare Fig. 2B vs. Fig. 1 A and E and Movie S1). Similar punctate patterns were also seen in anterior foregut or cibarium of the few rCP-, rHSP70h-, rP59-, and diet-fed vectors that showed fluorescent signals (Fig. 2B).
Fig. 2.
Retention of recombinant LIYV capsid proteins in the anterior foregut or cibarium of whitefly vectors. Individual rLIYV capsid proteins, rCPm, rCP, rHSP70h, and rP59 produced from an E. coli expression system were purified, concentrated, and compared with LIYV virions for specific retention in B. tabaci biotype A. (A) Average percentage of whiteflies with their anterior foregut or cibarium labeled with fluorescent signals among virion-, rCPm-, rCP-, rHSP70h-, rP59-, and diet-fed whiteflies that subsequently fed on diet containing the specific antibodies indicated beneath the columns. Error bars represent SE; P values determined by Student t test from two or three independent experiments. (B) Left: Widefield fluorescence microscopy image of the dissected head of a whitefly that had fed on diet containing rCPm, followed sequentially by diet containing an anti-LIYV CPm IgG, and diet containing a goat anti-rabbit IgG conjugated with Alexa Fluor 488. Right: Images of heads dissected from whiteflies that had fed on diet containing rCP, rHSP70h, or rP59, followed sequentially by diet containing an anti-LIYV CP, anti-LIYV HSP70h, or anti-LIYV P59 IgG, respectively, and diet containing a goat anti-rabbit IgG conjugated with Alexa Fluor 488. Foregut regions with (Top) and without (Bottom) fluorescent signals are compared. Punctate fluorescent signals in the insets (Top) are enlarged. The positions of the eye and cibarium (Cb) are indicated. (Scale bars, 45 μm.) (C) Average percentage of anterior foregut or cibarium with fluorescent signals for B. tabaci biotype A that had fed on diet containing virions preincubated with anti-CP and anti-CPm IgGs. Error bars represent SE; P values determined by Student t test from two independent experiments.
The above data are consistent with those of serological infectivity neutralization (SIN) experiments that implicated a role of the LIYV CPm in whitefly transmission (11). In that study, preincubation of virions with antibodies specific to the CPm before membrane feeding by B. tabaci biotype A successfully blocked the transmission of LIYV, whereas preincubation of virions with antibodies specific to the CP, HSP70h, and P59 did not. Here, we used SIN and immunofluorescent localization assays to determine the basis for CPm's involvement in LIYV transmission. When SIN was performed by using anti-CP antibodies, 34% (118 of 350 observed) of the vectors showed fluorescent signals in their anterior foregut or cibarium (Fig. 2C). Correspondingly, consistent with the results presented in the work by Tian et al. (11), transmission was observed in two of six plants. In contrast, when SIN was performed by using anti-CPm antibodies, 18% (72 of 403 observed), i.e., a nearly twofold reduction (P = 0.0024; Student t test) in percentage, of vectors had fluorescent signals in their anterior foregut or cibarium (Fig. 2C). Correspondingly, no transmission was observed in all six plants tested, which was also consistent with the previous study (11).
A Transmission-Defective Mutant Exhibits Altered Virion Retention in Foregut or Cibarium of Whitefly Vectors.
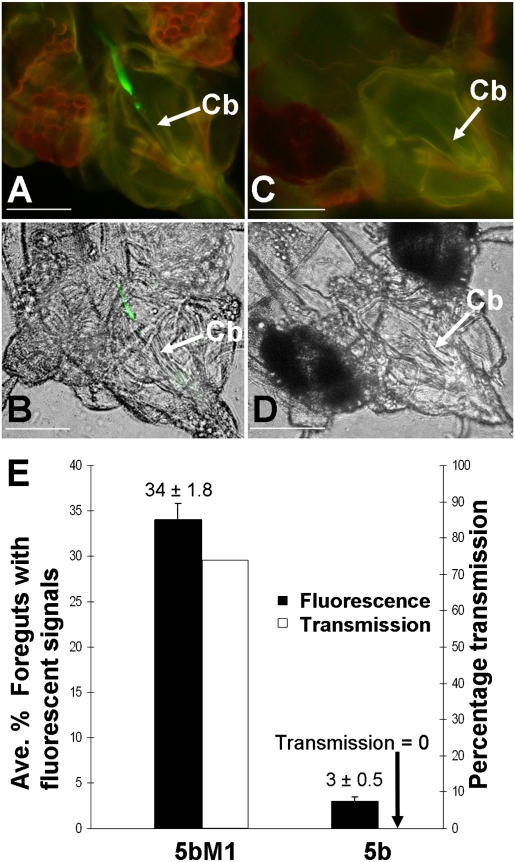
As a stringent test of the hypothesis that the CPm plays an essential role for virion retention in the anterior foregut or cibarium of whitefly vectors and determines whitefly transmissibility of LIYV, another strategy was to compare WT and mutant LIYV virions for differences in virion retention and transmission. Previously, we characterized a transmission-defective mutant, which contains a single nucleotide deletion in the CPm ORF that is predicted to result in a frameshift and premature termination of the protein (26). The cDNA sequence corresponding to this mutant CPm ORF (p1-5b CPm) and one in which an engineered mutation was rendered to restore the CPm ORF (p1-5bM1 CPm) were each swapped into a WT LIYV RNA 2 binary vector construct, agro-pR6, a construct that was engineered for the agrobacterium-mediated inoculation of plants. Insertion of these cDNA sequences generated the agro-pR6-5b and agro-pR6-5bM1 constructs, respectively (10). Consistent with our previous results, immunoblot analysis using antibodies produced against LIYV virions showed that both virions contained the approximately 28-kDa CP (Fig. S2B) (10). Both virions also reacted positively with antibodies produced against the LIYV CP in immunogold-labeling transmission EM, but only agro-pR6-5bM1 virions were recognized by antibodies to the LIYV CPm (Fig. S2C) (10). In immunofluorescent localization and transmission experiments, fluorescent signals were observed in the anterior foregut or cibarium of 34% of vectors that had fed on agro-pR6-5bM1 virions (Fig. 3 A, B, and E) but only 3% of vectors that had fed on agro-pR6-5b virions (Fig. 3 C–E and Table S6). Transmission using half of the vectors that had acquired virions from the same membrane feeding cages as those that were subjected to immunofluorescent localization resulted in 11 infected among 15 test plants for vectors that had fed on agro-pR6-5bM1 virions, and none infected among 19 test plants for vectors that had fed on agro-pR6-5b virions (Fig. 3E and Table S6). The difference between the two transmission scores was highly significant (P < 0.0001; Fisher exact test).
Fig. 3.
Retention of virions of a CPm mutant LIYV (agro-pR6-5b) and a CPm-restored LIYV (agro-pR6-5bM1) in the anterior foregut or cibarium of the whitefly vector and the corresponding virus transmissibility. Whiteflies (B. tabaci biotype A) were sequentially fed the following components: (i) diet containing virions, (ii) diet containing anti-LIYV virion IgG, and (iii) diet containing a goat anti-rabbit IgG conjugated with Alexa Fluor 488. (A) Widefield fluorescence microscopy micrograph of the head of B. tabaci biotype A fed diet containing agro-pR6-5bM1 virions, with background transmitted light blocked. (B) Image in A with background transmitted light unblocked. (C and D) Same as A and B except that B. tabaci biotype A was fed diet containing agro-pR6-5b virions. The cibarium (Cb) is indicated. (Scale bars, 45 μm.) (E) The average percentage (error bars represent SEs) of whiteflies with their anterior foregut or cibarium labeled with fluorescent signals for agro-pR6-5bM1 (5bM1) virion- and agro-pR6-5b (5b) virion-fed whiteflies and the corresponding LIYV transmissibility after half the virion-fed whiteflies from each feeding cage (containing whiteflies that subsequently fed on components ii and iii) were transferred to a noninfected lettuce plant. Values were determined from three or four independent experiments.
Discussion
Acquisition of a virus by an insect vector marks the beginning of an intimate relationship between the virus and the vector—a relationship that necessitates the virus to equip itself with the means to be retained at specific locations within the vector and eventually to be released and inoculated into a suitable plant host. Viral determinants and/or vector sites that participate in virion retention and transmission have been keenly studied for a number of noncirculatively transmitted viruses, including CMV, viruses in the genus Potyvirus, and CaMV (7, 27, 28). Virions of these viruses are noncirculative and are retained or, in the case of CMV, thought to be retained on the stylets, but they differ in other regards. CMV and potyviruses are nonpersistently transmitted, with transmission occurring in seconds to minutes after acquisition (7), whereas CaMV is considered to be semipersistently transmitted, with transmission continuing over hours or days after acquisition (29). In contrast, all other semipersistently transmitted viruses whose retention sites have been investigated previously are retained in the foreguts of their vectors—the aphid-transmitted Anthriscus yellows virus (AYV) and Parsnip yellow fleck virus (PYFV) (17), and the leafhopper-transmitted Maize chlorotic dwarf virus (MCDV) (15, 16). Although the retention sites of these viruses have been known for more than 20 years, there has been little progress in dissecting the transmission mechanisms. Viral molecular determinants of foregut retention of virions have not been identified previously. The limited knowledge is, in part, attributable to the lack of progress in development of reliable and user-friendly tools for in situ localization of virions within the vectors. Criniviruses, by virtue of their semipersistent transmission characteristics, have been postulated to be retained in the foreguts of their whitefly vectors. However, as with AYV, PYFV, and MCDV, molecular mechanisms underlying the retention of crinivirus virions had not been elucidated previously. This gap in knowledge can now be bridged with the membrane feeding and immunofluorescent localization assay, which has greatly facilitated routine large-scale analysis of differently treated whiteflies.
All the fluorescent signals seen in the whitefly vectors in the present study were observed within the anterior foregut or cibarium. In no instance were signals seen in the maxillary stylets or the alimentary tract preceding the cibarium (only a very weak signal was occasionally seen in the precibarium). These observations are clearly in agreement with the classical paradigm that semipersistently transmitted viruses are retained within the foregut regions of their insect vectors (7). However, they appeared to conflict with the results presented in the contemporary study by Uzest et al. (18), who demonstrated that putative receptors of the semipersistently transmitted CaMV are located at the tip of the maxillary stylets of several aphid species. Although CaMV is considered to be a semipersistently transmitted virus, it has several characteristics that differ from other semipersistently transmitted viruses. First, the optimum acquisition access period for the successful transmission of CaMV by aphid vectors varies in a bi- or multiphasic manner, with transmission rate peaking at several minutes and again at several hours of access feeding time (30). Second, unlike other semipersistently transmitted viruses, CaMV is transmissible by leaf-rub inoculation and can invade both mesophyll and phloem cells, and is therefore able to be acquired relatively more quickly than typical semipersistently transmitted viruses that apparently must reach the phloem to infect and be acquired (31). Moreover, aphid transmission of CaMV requires the participation of additional viral-encoded helper proteins (27), whereas the whitefly transmission of LIYV does not. Taken together, these characteristics suggest that different semipersistently transmitted viruses may not necessarily “behave” similarly within their respective insect vectors, and may even differ in vector retention sites. Thus, as reviewed by Hogenhout et al. (32), knowledge of the biological transmission features cannot be used to assume a specific vector retention site for semipersistently transmitted viruses.
Our immunofluorescent localization and whitefly transmission data strongly indicate that LIYV virions transmitted by whitefly vectors are specifically those that are retained in their anterior foregut or cibarium. The absence of fluorescent signals in 99% of individuals of the virion-fed nonvector B. tabaci biotype B, and their inability to transmit LIYV, could not be a result of the lack of ingestion of virions from the artificial diet, as we were able to consistently detect LIYV by RT-PCR at the whole-insect level after they were given access to virions by membrane feeding (Fig. S3). Thus, the likeliest explanation why LIYV virions are not transmitted by B. tabaci biotype B is that they are not retained in the foregut or cibarium of this insect.
Our results are unambiguous in determining that CPm plays an essential role in mediating the binding of virions to the vector's anterior foregut or cibarium: (i) the rCPm but not the other three recombinant capsid components specifically binds at these locations; and (ii) virions derived from the transmission-defective mutant, agro-pR6-5b, which exhibits a truncated CPm genotype (10), are not retained in the anterior foregut or cibarium, whereas virions derived from the transmission-restored agro-pR6-5bM1 are retained. Although the other three recombinant capsid components do not bind specifically to the whitefly vector, we cannot rule out the possibility that, in the context of the assembled virion, they may have some yet unknown functions associated with virus transmission. Because the CPm, CP, HSP70h, and P59 are interacting capsid components (11, 33, 34), it is intriguing that in the absence of a complete CPm, the agro-pR6-5b mutant is still capable of systemic plant infection and encapsidation, perhaps by one or more of the other three components compensating for the truncated CPm (10). However, it is clear from our data that virion retention and transmission by vectors are dependent on the presence of a complete CPm and cannot be substituted or compensated for.
The distinction among the fluorescent signals observed is qualitative (i.e., they are meant to indicate the presence or absence of virions or recombinant capsid proteins), not quantitative. Therefore, the signals could not be used to estimate the amount of virions or proteins present, nor could they be used to determine the retention affinity between the vector and the virion/capsid protein. Qualitative differences in signals were distinguishable between the rCPm-fed vectors and the virion-fed vectors, with the former appearing more frequently as punctate spots and those of the latter occupying a more continuous and larger area in the anterior foregut or cibarium. Several possibilities could account for the differences in distribution between whole virion and expressed proteins. For example, CPms in intact virions are specifically organized at one end of the filamentous LIYV virion; in this configuration, the proteins may present multiple copies of their vector binding surfaces. In contrast, the expressed CPm by itself may be self-associating into more random oligomers, causing the punctate appearance. Alternatively, an assembled virion is a much bigger entity than a capsid component alone and clearly presents more opportunities for interaction with recognizing antibodies than do retained recombinant capsid components. The latter explanation also could account for the slightly but statistically lower percentage of whiteflies showing positive signals following rCPm feeding vs. virion feeding.
Our findings support the ingestion–egestion hypothesis for transmission of LIYV, which proposes that virions are acquired during ingestion, are retained at a binding site in the alimentary canal, and then are dislodged and inoculated when the vector egests (i.e., regurgitates) into a previously uninfected plant (35). Virions that are retained at the tip of the maxillary stylets, such as the case for CaMV, in which the food and salivary canals are confluent, could potentially be dislodged and inoculated into a plant by salivation or egestion. However, a retention site in the cibarium or foregut is not in the pathway of saliva secretion, and virions retained there could be dislodged and inoculated only by egestion. Consequently, ingestion–egestion is the most likely mechanism for transmission of noncirculative viruses that are retained in the cibarium or foregut.
In summary, our results provide unequivocal evidence that (i) LIYV virions are retained within the anterior foregut or cibarium of its whitefly vector, (ii) retention of virions at these sites correlates with whitefly transmission of the virus, and (iii) binding of the virus to this retention site is mediated by the CPm. These results offer insights into the transmission mechanism of this and other criniviruses and virus–vector interactions in general. These results also warrant a reexamination of the concept of mechanical transmission by arthropod vectors of animal viruses, which is believed to be mediated by a nonspecific interaction between the mouthparts of vectors and viruses they transmit, especially for those viruses that remain viable for extended periods during their association with the vectors (22).
Materials and Methods
Virions at the retention sites of whitefly vectors were fluorescently labeled by the following procedure: adult whiteflies were placed in membrane feeding cages (9, 11), with approximately 100 whiteflies per cage. The caged whiteflies then were fed one of the following two components: (i) basal artificial liquid diet [15% sucrose and 1% BSA in 1× TE (10 mM Tris-HCl, 1 mM EDTA, pH 7.4)] or (ii) basal artificial liquid diet augmented with purified LIYV virions (0.01 or 0.1 μg/μL) or CMV-Fny virions (1 or 0.5 μg/μL; the CMV-Fny virion augmented diet was used as a control for potential nonspecific virion retention). Afterward, the whiteflies were fed basal artificial liquid diet containing anti-LIYV polyclonal IgG raised in rabbit (1/833-fold dilution of 2.3 mg/mL) or anti–CMV-Fny polyclonal antiserum raised in rabbit (diluted 1/800 fold), followed by basal artificial liquid diet containing a 1/200-fold dilution of goat anti-rabbit IgG conjugated with Alexa Fluor 488 (Invitrogen). Whiteflies were given a 10- to 12-h acquisition access period for each of the aforementioned solutions. To remove unbound or nonspecifically bound virions or antibodies present in the ingested solutions, the food canals and foreguts of whiteflies were cleared or flushed out by feeding whiteflies a basal artificial diet for several hours after the acquisition feeding of the first and third solutions.
To determine which of the LIYV capsid proteins (CP, CPm, HSP70h, or P59) bind to the retention site with the whitefly vector, whiteflies were membrane-fed the same series of solutions as described earlier except that the first solution fed to the whiteflies was basal artificial liquid diet augmented with each of the specific recombinant capsid proteins rather than augmented with purified LIYV virions, and the second solution was basal artificial liquid diet augmented with the corresponding antibodies to these proteins. Another exception was that clearing was conducted for 1 h following the acquisition feeding of the third solution. The construction of expression vectors for the E. coli expression of the recombinant capsid proteins, as well as their expression, postexpression processing, and concentration estimation are included in SI Materials and Methods. Antibodies (rabbit-raised polyclonal IgGs) corresponding to the respective capsid proteins were used at the following dilutions: anti-CPm IgG (1/250-fold of 2.5 mg/mL), anti-CP IgG (1/500-fold of 1.2 mg/mL), anti-HSP70h IgG (1/250-fold of 1.9 mg/mL), and anti-P59 IgG (1/250-fold of 2 mg/mL). SIN assays using anti-CP and anti-CPm antisera were as previously described (11). To verify that fluorescent signals observed were not caused by nonspecific binding to the insect by individual anticapsid protein IgGs, diet-fed whiteflies were given access to basal artificial liquid diet containing each of these IgGs.
After the final membrane feeding, heads were removed from the whiteflies and examined under widefield fluorescence microscopy (bandpass filter, 450–490/long-pass filter, 520) with a Zeiss Axioskop microscope using a 40×/0.65 NA objective, and images were taken using a Coolpix 995 digital camera (Nikon). Confocal laser scanning microscopy of dissected whitefly heads was performed with an SP2 microscope (Leica Microsystems). Alexa Fluor 488 was imaged with a 20×/0.7 NA water objective using the 488-nm line of an Argon ion laser, with a detection range set between 500 and 600 nm. Three-dimensional rendered confocal images were reconstructed with Imaris software (Bitplane).
Materials and methods used for whitefly and virus maintenance, whitefly transmission, agroinoculation, virion preparation, virion quantification, ELISA, statistical analyses, and RT-PCR detection of LIYV in whiteflies are included in SI Materials and Methods.
Supplementary Material
Acknowledgments
The authors thank Tom Smith for helpful discussions; Katherine Hadikusumo, Chawin Mongkolsiriwattana, Eric Oduca, and Tongyan Tian for technical assistance; and Shou-wei Ding and A. L. N. Rao for editorial comments. Funding was provided by startup funds from the University of California, Riverside (UCR), College of Natural and Agricultural Sciences and by a grant from the Los Alamos National Laboratory–UCR Collaborative Program in Pathogen-Induced Plant Infectious Disease (to J.C.K.N.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1109384108/-/DCSupplemental.
References
- 1.Pennisi E. Armed and dangerous. Science. 2010;327:804–805. doi: 10.1126/science.327.5967.804. [DOI] [PubMed] [Google Scholar]
- 2.Weaver SC, Reisen WK. Present and future arboviral threats. Antiviral Res. 2010;85:328–345. doi: 10.1016/j.antiviral.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dedryver CA, Le Ralec A, Fabre F. The conflicting relationships between aphids and men: A review of aphid damage and control strategies. C R Biol. 2010;333:539–553. doi: 10.1016/j.crvi.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Costa AS. Whitefly-transmitted plant diseases. Annu Rev Phytopathol. 1976;14:429–449. [Google Scholar]
- 5.Czosnek H, et al. Whiteflies: Vectors, and victims (?), of geminiviruses. Adv Virus Res. 2001;57:291–322. doi: 10.1016/s0065-3527(01)57006-2. [DOI] [PubMed] [Google Scholar]
- 6.Wisler GC, Duffus JE. Transmission properties of whitefly-borne criniviruses and their impact on virus epidemiology. In: Harris KE, Smith OP, Duffus JE, editors. Virus-Insect-Plant Interactions. San Diego: Academic; 2001. pp. 293–307. [Google Scholar]
- 7.Ng JCK, Falk BW. Virus-vector interactions mediating nonpersistent and semipersistent transmission of plant viruses. Annu Rev Phytopathol. 2006;44:183–212. doi: 10.1146/annurev.phyto.44.070505.143325. [DOI] [PubMed] [Google Scholar]
- 8.Cuellar WJ, et al. Elimination of antiviral defense by viral RNase III. Proc Natl Acad Sci USA. 2009;106:10354–10358. doi: 10.1073/pnas.0806042106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ng JCK, Tian T, Falk BW. Quantitative parameters determining whitefly (Bemisia tabaci) transmission of Lettuce infectious yellows virus and an engineered defective RNA. J Gen Virol. 2004;85:2697–2707. doi: 10.1099/vir.0.80189-0. [DOI] [PubMed] [Google Scholar]
- 10.Stewart LR, et al. A mutation in the Lettuce infectious yellows virus minor coat protein disrupts whitefly transmission but not in planta systemic movement. J Virol. 2010;84:12165–12173. doi: 10.1128/JVI.01192-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian T, Rubio L, Yeh HH, Crawford B, Falk BW. Lettuce infectious yellows virus: In vitro acquisition analysis using partially purified virions and the whitefly Bemisia tabaci. J Gen Virol. 1999;80:1111–1117. doi: 10.1099/0022-1317-80-5-1111. [DOI] [PubMed] [Google Scholar]
- 12.Yeh HH, Tian T, Rubio L, Crawford B, Falk BW. Asynchronous accumulation of Lettuce infectious yellows virus RNAs 1 and 2 and identification of an RNA 1 trans enhancer of RNA 2 accumulation. J Virol. 2000;74:5762–5768. doi: 10.1128/jvi.74.13.5762-5768.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Yeh HH, Falk BW. cis preferential replication of Lettuce infectious yellows virus (LIYV) RNA 1: The initial step in the asynchronous replication of the LIYV genomic RNAs. Virology. 2009;386:217–223. doi: 10.1016/j.virol.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Stewart LR, Kiss Z, Falk BW. Lettuce infectious yellows virus (LIYV) RNA 1-encoded P34 is an RNA-binding protein and exhibits perinuclear localization. Virology. 2010;403:67–77. doi: 10.1016/j.virol.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Ammar ED, Nault LR. Maize chlorotic dwarf virus-like particles associated with the foregut in vector and non-vector leafhopper species. Phytopathology. 1991;81:444–448. [Google Scholar]
- 16.Childress SA, Harris KF. Localization of virus-like particles in the foreguts of viruliferous Graminella nigrifrons leafhoppers carrying the semi-persistent Maize chlorotic dwarf virus. J Gen Virol. 1989;70:247–251. [Google Scholar]
- 17.Murant AF, Roberts IM, Elnagar S. Association of virus-like particles with foregut of aphid Cavariella aegopodii transmitting semi-persistent viruses Anthriscus yellows and Parsnip yellow fleck. J Gen Virol. 1976;31:47–57. [Google Scholar]
- 18.Uzest M, et al. A protein key to plant virus transmission at the tip of the insect vector stylet. Proc Natl Acad Sci USA. 2007;104:17959–17964. doi: 10.1073/pnas.0706608104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carn VM. The role of dipterous insects in the mechanical transmission of animal viruses. Br Vet J. 1996;152:377–393. doi: 10.1016/s0007-1935(96)80033-9. [DOI] [PubMed] [Google Scholar]
- 20.Kuno G, Chang GJ. Biological transmission of arboviruses: Reexamination of and new insights into components, mechanisms, and unique traits as well as their evolutionary trends. Clin Microbiol Rev. 2005;18:608–637. doi: 10.1128/CMR.18.4.608-637.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kloft WJ. Radioisotopes in vector research. Adv Dis Vector Res. 1992;9:41–66. [Google Scholar]
- 22.Chihota CM, Rennie LF, Kitching RP, Mellor PS. Mechanical transmission of lumpy skin disease virus by Aedes aegypti (Diptera: Culicidae) Epidemiol Infect. 2001;126:317–321. doi: 10.1017/s0950268801005179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith PF, et al. Mechanical transmission of vesicular stomatitis New Jersey virus by Simulium vittatum (Diptera: Simuliidae) to domestic swine (Sus scrofa) J Med Entomol. 2009;46:1537–1540. doi: 10.1603/033.046.0643. [DOI] [PubMed] [Google Scholar]
- 24.Blanc S, Hébrard E, Drucker M, Froissart R. Molecular basis of vector transmission: caulimoviruses. In: Harris K, Smith O, Duffus J, editors. Virus-Insect-Plant Interactions. San Diego: Academic; 2001. pp. 143–166. [Google Scholar]
- 25.Gray SM, Banerjee N. Mechanisms of arthropod transmission of plant and animal viruses. Microbiol Mol Biol Rev. 1999;63:128–148. doi: 10.1128/mmbr.63.1.128-148.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ng JCK, Falk BW. Bemisia tabaci transmission of specific Lettuce infectious yellows virus genotypes derived from in vitro synthesized transcript-inoculated protoplasts. Virology. 2006;352:209–215. doi: 10.1016/j.virol.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 27.Brault V, Uzest M, Monsion B, Jacquot E, Blanc S. Aphids as transport devices for plant viruses. C R Biol. 2010;333:524–538. doi: 10.1016/j.crvi.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Pirone TP, Blanc S. Helper-dependent vector transmission of plant viruses. Annu Rev Phytopathol. 1996;34:227–247. doi: 10.1146/annurev.phyto.34.1.227. [DOI] [PubMed] [Google Scholar]
- 29.Markham PG, Pinner MS, Raccah B, Hull R. The acquisition of a caulimovirus by different aphid species: Comparison with a potyvirus. Ann Appl Biol. 1987;111:571–587. [Google Scholar]
- 30.Bouchery Y, Givord L, Monestiez P. Comparison of short- and long-feed transmission of the Cauliflower mosaic virus Cabb-S strain and S delta II hybrid by two species of aphid: Myzus persicae (Sulzer) and Brevicoryne brassicae (L.) Res Virol. 1990;141:677–683. doi: 10.1016/0923-2516(90)90040-p. [DOI] [PubMed] [Google Scholar]
- 31.Palacios I, et al. Cauliflower mosaic virus is preferentially acquired from the phloem by its aphid vectors. J Gen Virol. 2002;83:3163–3171. doi: 10.1099/0022-1317-83-12-3163. [DOI] [PubMed] [Google Scholar]
- 32.Hogenhout SA, Ammar D, Whitfield AE, Redinbaugh MG. Insect vector interactions with persistently transmitted viruses. Annu Rev Phytopathol. 2008;46:327–359. doi: 10.1146/annurev.phyto.022508.092135. [DOI] [PubMed] [Google Scholar]
- 33.Peremyslov VV, et al. Complex molecular architecture of beet yellows virus particles. Proc Natl Acad Sci USA. 2004;101:5030–5035. doi: 10.1073/pnas.0400303101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Satyanarayana T, Gowda S, Ayllón MA, Dawson WO. Closterovirus bipolar virion: evidence for initiation of assembly by minor coat protein and its restriction to the genomic RNA 5′ region. Proc Natl Acad Sci USA. 2004;101:799–804. doi: 10.1073/pnas.0307747100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harris KF. An ingestion-egestion hypothesis of noncirculative virus transmission. In: Harris K, Maramorosch K, editors. Aphids as Virus Vectors. New York: Academic; 1977. pp. 165–219. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.