Abstract
The gene encoding the receptor for hyaluronan-mediated motility (RHAMM) is overexpressed in many human cancers. However, it is unclear whether RHAMM plays a causal role in tumor initiation or progression. Using somatic gene transfer in a mouse model of islet cell tumorigenesis, we demonstrate that RHAMM isoform B (RHAMMB) promotes tumor growth and metastases to lymph nodes and the liver. The propensity of RHAMMB-expressing cells to metastasize to the liver was confirmed using an experimental metastasis assay in which cells were injected into the tail vein of immunodeficient mice. However, RHAMMB did not increase cell migration or proliferation in culture. In initial efforts to identify signaling pathways activated by RHAMMB, we found that RHAMMB induced phosphorylation of epidermal growth factor receptor (EGFR), Erk1/2, and STAT3 and conferred susceptibility to apoptosis after treatment with an EGFR inhibitor, gefitinib. Taken together, the results indicate that RHAMMB promotes hepatic metastasis by islet tumor cells, perhaps through growth factor receptor-mediated signaling.
We have previously reported a bitransgenic mouse model, RIP-Tag; RIP-tva, in which the rat insulin promoter (RIP) drives production of both the SV40 T antigen (RIP-Tag) and the receptor for subgroup A avian leukosis virus (RIP-tva) in pancreatic β cells (1). Coding domains of genes suspected of contributing to tumor progression can be introduced into premalignant lesions by infection with the avian retroviral vector, RCASBP, after intracardiac injection. RIP-Tag transgenic mice develop islet tumors through well-defined stages that resemble the progression of several kinds of human cancers; for this reason, we and others have used these mice, with or without additional transgenes, to identify and validate mechanisms of tumorigenesis that may operate in multiple tissues. For instance, we have used RIP-Tag; RIP-tva mice to show that RCASBP-mediated delivery of Bcl-xL or E-cadherin, factors implicated in various neoplasms, promotes tumorigenesis and invasion in islet cells (1).
High-throughput genomic technologies have identified many genes that may be critical in tumor initiation and progression. However, it remains difficult to distinguish causative and passenger mutations and to assign specific biological functions to altered genes in human cancers, and the RIP-Tag; RIP-tva mouse model of multistage tumorigenesis offers an opportunity to address such issues. To that end, we have assessed the oncogenic functions of a small number of incompletely characterized genes that are up-regulated in human hepatocellular carcinomas (HCC) and during mouse liver regeneration (2). One of the candidate genes, a gene encoding a receptor for hyaluronan-mediated motility (RHAMM) is overexpressed in many types of human cancers, including pancreatic ductal carcinoma, hepatocellular carcinoma, multiple myeloma, breast cancer, gliomas, colon cancer, and prostate cancer (2–7); but the functions of at least four proteins encoded by its alternatively spliced messenger RNAs and their roles, if any, in tumorigenesis are unclear.
Using the RIP-Tag; RIP-tva model, we find that isoform B of RHAMM (RHAMMB) enhances the growth of mouse islet tumors and promotes metastasis exclusively to the liver and local lymph nodes. Furthermore, we show that mouse pancreatic islet tumor cells programmed to express RHAMMB form hepatic metastasis when injected into the tail vein of mice in a traditional assay for metastasis. The cells also show evidence that RHAMM has enhanced signaling via the epidermal growth factor receptor (EGFR). These observations and others suggest that RHAMMB may be an important factor in tumor growth and progression and that a better understanding of the RHAMM gene might offer insights into the organotropism of metastatic cancer cells.
Results
RHAMMB Promotes Tumor Growth and Metastasis to Pancreatic Lymph Nodes and the Liver in a Mouse Model of Pancreatic Islet Tumors.
To evaluate the malignant potential of genes reported to be up-regulated both in HCC and during mouse liver regeneration (2), we examined five candidate genes, including RHAMMB, paternally expressed 10 (PEG10), FLJ10540, FLJ11252, and FLJ11164. FLJ stands for the “full-length long Japan” collection of human cDNAs (8).
The cDNAs of the candidates were cloned into avian retroviral vector, RCASBP, with a FLAG epitope tag added to the N terminus of PEG10, FLJ10540, and FLJ11252. We injected high titer virus stocks (0.1 mL; >108 infectious units per milliliter) into 7-wk-old RIP-Tag; RIP-tva mice by the intracardiac route. At this point, many islets show evidence of hyperplasia, allowing infection with oncoretrovirus vectors, which are dependent on cell division for successful infection (1). RCASBP–ALPP (Alkaline Phosphatase) and RCASBP–Bcl-xL were chosen as controls. ALPP encodes a protein unlikely to contribute to tumorigenesis, serving as a negative control for effects of viral infection. We have previously shown that infection with RCASBP–Bcl-xL promotes tumor growth and lymph node metastasis in RIP-Tag; RIP-tva mice (1), so infection with this virus provided a positive control.
Nine weeks after infection, the pancreas and other organs were harvested for histological staging and grading of the lesions. Human RHAMMB significantly increased pancreatic tumor burden in 8 of 12 mice, but not all, compared with mice infected with RCASBP–ALPP (Fig. 1, P = 0.0097, Wilcoxon rank sum test). RCASBP–Bcl-xL induced a small increase in pancreatic tumor burden (P = 0.0087), whereas none of the other vectors (RCASBP–FLAG-PEG10, RCASBP–FLAG-FLJ10540, RCASBP–FLAG-FLJ11252, or RCASBP–FLJ11164) caused any significant increase in tumor burden (Table 1 and Table S1).
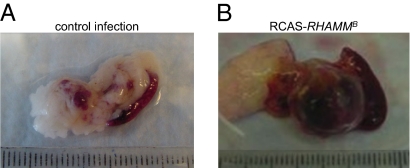
Fig. 1.
Significantly increased pancreatic islet tumor burden in 67% of RIP-Tag; RIP-tva mice infected with RCASBP–RHAMMB (n = 12). Representative pancreas and spleen from RIP-Tag; RIP-tva mice infected with RCASBP–ALPP (A) or RCASBP–RHAMMB (B). Mouse islet tumors are red due to angiogenesis.
Table 1.
Impact of candidate genes on tumorigenesis in vivo
| RCASBP– | Age (wk) | Tumor burden (mm3) | Lymph node metastasis (%) | Liver metastasis (%) |
| ALPP | 12 | 8.1 ± 4.5 | 0/5 mice (0) | 0/5 mice (0) |
| ALPP | 16 | 99.7 ± 19.4 | 0/10 mice (0) | 0/10 mice (0) |
| RHAMMB | 12 | 16.2 ± 8.9 | 0/6 mice (0) | 0/6 mice (0) |
| RHAMMB | 16 | 298.3 ± 121.3 | 8/11 mice (73) | 8/12 mice (67) |
| Bcl-xL | 16 | 150.5 ± 23 | 7/15 mice (46) | 0/6 mice (0) |
| FLAG-PEG10 | 16 | 57.8 ± 26.8 | 2/8 mice (25) | 0/8 mice (0) |
| FLAG-FLJ10540 | 16 | 126.1 ± 54.3 | 4/7 mice (57) | 2/7 mice (28) |
| FLAG-FLJ11252 | 16 | 41.2 ± 30.9 | 0/7 mice (0) | 0/7 mice (0) |
| FLJ11164 | 16 | 43.8 ± 25.1 | 1/9 mice (11) | 0/6 mice (0)* |
RCASBP retroviruses carrying indicated cDNAs were introduced to RIP-Tag; RIP-tva mice through intracardiac injection at 7 wk of age. Mice were euthanized at 12 or 16 wk of age for measurement of tumor burden and for metastasis survey. A standard formula for tumor volume was applied (volume [mm3] = 0.52 × width2 × length). Tumor burden is the sum of the tumor volume per mouse.
*Mice having micrometastases with fewer than five cells were excluded.
To aid the search for metastasis of islet tumors in RIP-Tag; RIP-tva mice, tissue sections were subjected to immunohistochemical staining for synaptophysin, a neuroendocrine marker, and for insulin, a β-cell marker. Local lymph node metastases were detected in mice infected with RCASBP–RHAMMB (8 of 11 mice, P = 0.001, Fisher's exact test), RCASBP–Bcl-xL (7 of 15 mice, P = 0.013), RCASBP–FLAG-PEG10 (2 of 8 mice, P = 0.183), RCASBP–FLAG-FLJ10540 (4 of 7 mice, P = 0.015), and RCASBP–FLJ11164 (1 of 9 mice, P = 0.474) (Fig. 2, Table 1, and Table S1). The sizes of lymph node metastases in mice infected with RCASBP–FLAG-PEG10, RCASBP–FLAG-FLJ10540, and RCASBP–FLJ11164 were small and were not easily detected by hematoxylin and eosin staining, unlike those in mice infected with RCASBP–RHAMMB and RCASBP–Bcl-xL. No lymph node metastases were found in mice receiving the negative control virus or RCASBP–FLAG-FLJ11252 (Table 1 and Table S1).
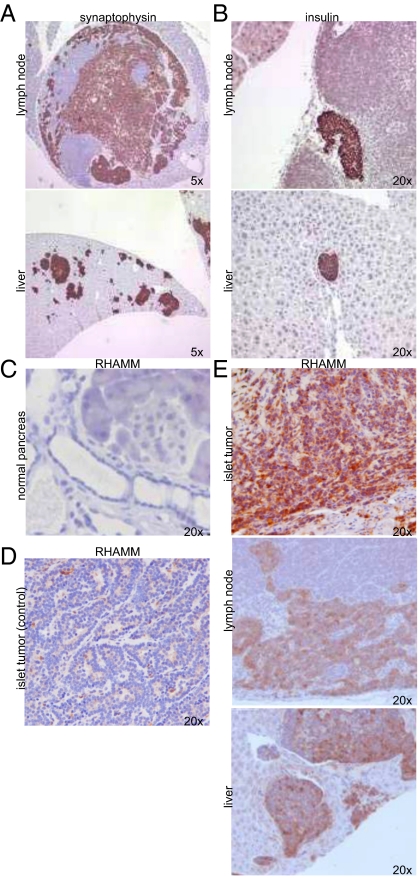
Fig. 2.
Detection of metastases in RIP-Tag; RIP-tva mice infected with RCASBP–RHAMMB. (A and B) Representative lymph node and liver metastases found in RCASBP–RHAMMB infected mice. Immunohistochemical staining of synaptophysin (A) and insulin (B) is shown. (C–E) Immunohistochemical staining of RHAMM in pancreas from wild-type mouse (C), islet tumor from a control RIP-Tag; RIP-tva mice (D), and islet tumor, lymph node metastases, and liver metastases found in RCASBP–RHAMMB infected mice (E).
Importantly, liver metastases were found in 8 of 12 RCASBP–RHAMMB infected mice (Fig. 2, Table 1, and Table S1, P = 0.002, Fisher's exact test), and the appearance of metastasis was not closely associated with the aggregated size of primary tumors. Several RIP-Tag; RIP-tva mice infected with RCASBP–RHAMMB with less or similar pancreatic tumor burden than control mice still developed metastasis (Table S1), suggesting that RHAMMB does not merely promote metastasis as a direct consequence of promoting tumor growth. In addition, 2 of 7 mice infected with RCASBP–FLAG-FLJ10540 developed small liver metastases, and 3 of 9 mice infected with RCASBP–FLJ11164 developed liver micrometastases with fewer than five tumor cells on the histological sections (Table 1 and Table S1). These findings suggest that PEG10, FLJ10540, and FLJ11164 have a modest effect on the promotion of metastasis in this model, but only RHAMMB had a dramatic effect on both tumor size and metastasis to lymph nodes and the liver. No metastases were found at other sites, including lungs, heart, thymus, kidney, and spleen.
In view of the profound effects of RHAMMB on tumor size and metastasis 9 wk after infection, we asked whether such effects could be detected at earlier times. RIP-Tag; RIP-tva mice were euthanized 5 wk after being infected with RCASBP–RHAMMB at 7 wk of age. Although these RIP-Tag; RIP-tva mice had twofold more tumor burden than did the mice infected with RCASBP–ALPP at the same age, no metastases were found after microscopic examination of tissue slices stained with reagents that detect insulin and synaptophysin (Table 1), indicating that more than 5 wk were required to observe any effects on metastasis.
To verify that RHAMM was indeed produced in the tumors and metastases of RCASBP–RHAMMB infected mice, we generated rabbit polyclonal antibodies against a region entirely conserved among human, mouse, and rat RHAMM proteins, and present in all four human isoforms (Table S2 and Fig. S1). Some islet tumors and the metastases in pancreatic lymph nodes and the livers in RIP-Tag; RIP-tva mice infected with RCASBP–RHAMMB stained positive for RHAMM (Fig. 2E). Although RHAMM is negatively regulated by p53 in cell lines (9) and T-antigen represses p53 transactivation in RIP-Tag; RIP-tva mice, endogenous mouse RHAMM was not detectable by immunohistochemical staining in most of the pancreatic islets and islet tumors from uninfected RIP-Tag; RIP-tva mice (Fig. 2 C and D). Taken together, our results suggest that ectopic expression of human RHAMMB increases tumor burden and independently promotes metastasis to pancreatic lymph nodes and the liver in our model of mouse pancreatic islet tumors.
RHAMMB Promotes Liver-Specific Metastasis in a Tail Vein Assay of Cancer Metastasis.
The portal vein brings blood from the pancreas, spleen, stomach, duodenum, and colon to the liver. Thus, it seemed possible that RHAMMB promotes metastasis of pancreatic islet tumor cells to the liver because it promotes metastasis in general and the liver is the first target organ in the path of blood drainage from the primary tumors. To distinguish more rigorously between enhancement of liver-specific versus generalized metastasis, we asked whether mouse islet tumor cell lines expressing RHAMMB metastasized preferentially to the liver after introduction into the general circulation. For this experiment, we injected established cell lines into the tail vein of recipient mice. Cells entering the venous circulation will first encounter the capillaries of the lungs. If they are able to pass through the lungs, they will then enter the arterial system and eventually pass through the portal circulation. Assuming that promotion of metastasis by RHAMMB affects steps following intravasation, this approach should distinguish between liver-specific tropism and dependency on the circulatory route as an explanation for the observed liver metastases.
We infected a β-cell tumor cell line βTC-N134, (N134 for brevity) (1) derived from an islet tumor in a RIP-Tag; RIP-tva mouse with RCASBP–Luciferase, RCASBP–RHAMMB, or RCASBP–Bcl-xL in vitro. To verify the expression of human RHAMMB in N134 cells infected with RCASBP–RHAMMB, we performed reverse transcription–PCR using primers specific for exon 16 of human RHAMM and readily detected human RHAMM mRNA in cells infected with RCASBP–RHAMMB, but not in uninfected cells (Fig. 3A).
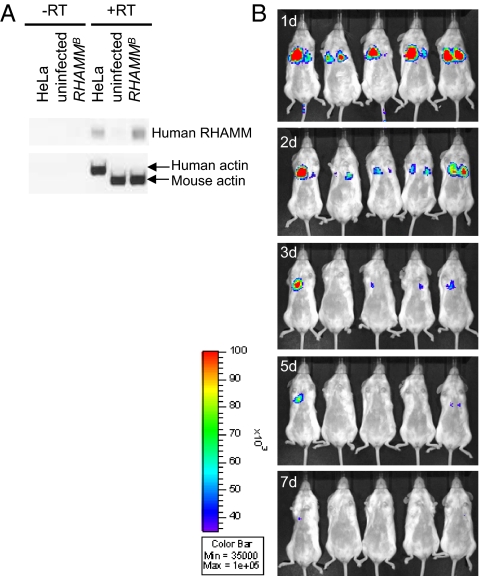
Fig. 3.
Detection of human RHAMM mRNA or luciferase activity in the mouse islet tumor cell line infected with RCASBP–RHAMMB or RCASBP–Luciferase. (A) The expression of human RHAMMB in N134 cells infected with RCASBP–RHAMMB was confirmed by reverse transcription–PCR. mRNAs from HeLa cell, N134 cells (uninfected), and N134 cells infected with RCASBP–RHAMMB (RHAMMB) were isolated for RT-PCR. Specific primers for human RHAMM (exon 16) and human/mouse β-actin (as a control) were used. (B) A total of 1 × 106 N134 cells infected with RCASBP–Luciferase were injected into the tail vein of five recipient mice. The locations of tumor cells overexpressing luciferase were monitored by in vivo bioluminescent imaging 1, 2, 3, 5, and 7 d after injection. RT, reverse transcriptase.
A total of 1 × 106 tumor cells were introduced into the tail vein of immunodeficient mice, NOD/scid-lL2Rgc knockout (NSG). The mice receiving tumor cells infected with RCASBP–Luciferase were monitored by in vivo bioluminescence imaging (Fig. 3B). We observed signals from the thoracic cavity during the first few days postinjection and the signals gradually became undetectable within a week, suggesting that the tumor cells are initially trapped in the capillary beds of the lungs.
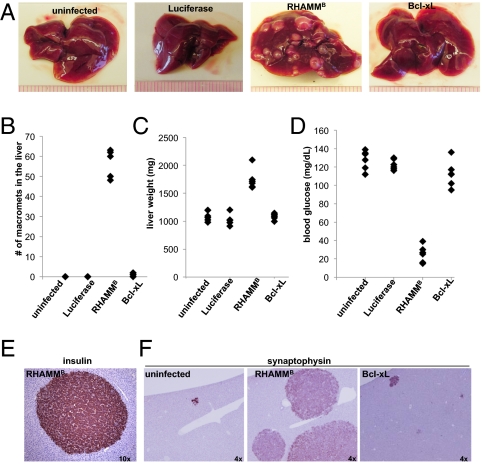
After 5 wk, organs of the recipient mice were harvested to survey for metastases. The findings were dramatic: all five mice receiving RHAMMB-expressing tumor cells exhibited macrometastases in the livers, with an average of 57 macrometastases per mouse (Fig. 4 A and B). In contrast, three of five mice receiving Bcl-xL–expressing tumor cells had liver macrometastases, with an average of one macrometastases per mouse. No macrometastases were found in mice receiving uninfected tumor cells or Luciferase-expressing tumor cells (Fig. 4 A and B). The liver weight was increased about twofold in the mice receiving RHAMMB-expressing tumor cells compared with that in other mice (Fig. 4C). Moreover, the liver metastases in recipients of RHAMMB-expressing tumor cells continued to express insulin as indicated by low blood glucose levels and by immunohistochemical staining for insulin (Fig. 4 D and E). To survey for micrometastases, immunohistochemical staining for synaptophysin was performed. Mice receiving uninfected tumor cells had 1.8 ± 0.8 liver micrometastases and mice receiving Bcl-xL–expressing tumor cells had 26.6 ± 5 liver micrometastases (Fig. 4F). Immunohistochemical staining for synaptophysin and insulin in sections of liver, lung, heart, thymus, brain, pancreas, spleen, kidney, bone marrow, and mammary gland revealed that the liver is the only organ with metastases (Fig. 4E). Our results indicate that overexpression of RHAMMB significantly promotes the pancreatic islet tumor cells to establish large liver-specific metastases after injection into the general venous circulation.
Fig. 4.
RHAMMB greatly promotes liver metastasis of pancreatic islet tumor cells in the tail vein metastasis assay. A total of 1 × 106 parental mouse pancreatic islet tumor N134 cells and tumor cells overexpressing Luciferase, RHAMMB, or Bcl-xL were injected into the tail vein of recipient mice. Five weeks later, the recipient mice (n = 5 for each group) were euthanized to survey for metastatic sites and incidence (A and B), to record liver weight (C), and to measure the blood glucose (D). (E) The liver metastases in mice receiving RHAMMB-expressing cells continued to express insulin. Liver sections from recipient mice with N134 tumor cells or tumor cells overexpressing RHAMMB or Bcl-xL were subjected to immunohistochemical staining of synaptophysin (F) to reveal the presence of metastases. Original magnification is indicated.
In addition, we examined histological sections of several organs for the existence of islet tumor cells at earlier time points (i.e., 1.5 h and 1, 3, 5, 7, and 14 d after tail vein injection). Consistent with in vivo bioluminescent results using Luciferase-expressing N134 cells in Fig. 3B, parental and RHAMMB-expressing N134 cells in clusters of various sizes were found within pulmonary vessels associated with fresh fibrin during the first few days. Over the course of 7 d, we observed increasing organization of fibrin, infiltrated neutrophils centered on pulmonary vessels, and gradual clearance of tumor cells. By 14 d, tumor cells were no longer found in pulmonary vessels. Immunohistochemical staining for synaptophysin in liver sections revealed a few positive cells. Thus, RHAMMB did not appear to enhance infiltration of the lung by circulating tumor cells or by outgrowth of micrometastasis in the liver at these early time points.
RHAMMB Does Not Enhance Cell Migration in a Transwell Assay or Proliferation in Vitro.
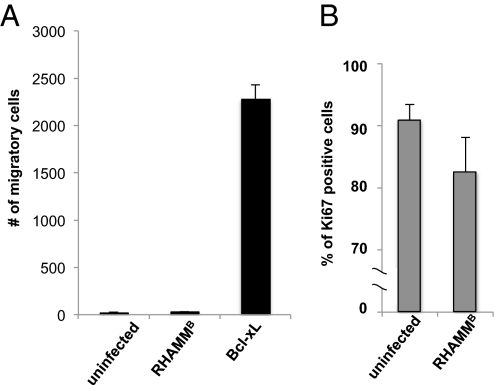
To examine whether RHAMMB enhanced the ability of mouse pancreatic tumor cells to migrate in vitro, we performed a two-chamber cell migration assay. Uninfected N134 cells, RHAMMB-expressing tumor cells, or Bcl-xL–expressing tumor cells were seeded in the upper chambers of Transwell inserts. Bcl-xL–expressing tumor cells was used as a positive control, as we have previously demonstrated that these cells have greater ability to migrate in the two-chamber migration assay than do parental cells (1). We measured cell migration along a serum gradient through the membrane to the bottom of the chambers. Although RHAMMB, but not Bcl-xL, strongly promoted liver metastasis of mouse islet tumors in mice, the number of migratory RHAMMB-expressing tumor cells was similar to that of uninfected cells (Fig. 5A), suggesting that expression of RHAMMB does not affect migration in vitro. This result indicates that the effect of RHAMMB on hepatic metastasis is unrelated mechanistically to cell migration or that the Transwell assay does not mimic the in vivo microenvironment sufficiently well to demonstrate an effect of RHAMMB on migration.
Fig. 5.
RHAMMB does not promote migration in a Transwell assay in vitro. (A) Uninfected N134 cells and N134 cells infected with RCASBP–RHAMMB or RCASBP–Bcl-xL were plated in the upper chambers of Transwell plates. After 72 h, cells were counted in the lower chambers. Data are presented as the mean numbers of cells in five fields under 20× magnification, and are representative of three independent experiments. (B) Proliferative indices of the two cell lines. Cultures grown on chamber slides from uninfected N134 cells and N134 cells infected with RCASBP–RHAMMB were stained with antisera against Ki67. Data shown are the mean percentage ± SD from triplicate experiments.
To determine whether RHAMMB promoted cell proliferation in vitro, we performed immunocytochemistry using antisera against a proliferation marker, Ki67. The frequency of Ki67-positive cells was slightly reduced in RHAMMB-expressing tumor cells compared with the uninfected cells (Fig. 5B). These findings indicate that RHAMMB does not stimulate cell proliferation in vitro and may even have a slight inhibitory effect.
RHAMMB Increases Phosphorylation of EGFR, Erk, and STAT3.
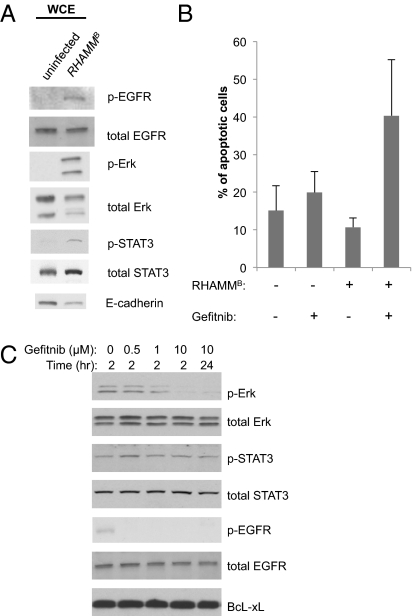
Little is known about the biochemical properties of the RHAMMB. It has been shown that Erk1/2 activity is aberrant in RHAMM−/− fibroblasts (10) and activation of EGFR is required for wounding-induced hyaluronan synthesis (11). As an initial effect to explain the physiological consequences of overexpression of RHAMMB, we measured the relative amounts and phosphorylation status of EGFR, Erk1/2, and STAT3 by Western blot analysis using antibodies against total and phosphorylated EGFR, Erk, and STAT3. We observed increased phosphorylation of EGFR (on Tyr1068), Erk1/2 (on Thr202/Tyr204), and STAT3 (on Ser727) in RHAMMB-expressing tumor cells compared with uninfected N134 cells (Fig. 6A), suggesting that overexpression of RHAMMB activates signaling pathways that include EGFR, Erk1/2, and STAT3. In addition, we observed slightly reduced levels of the cell adhesion molecule E-cadherin in RHAMMB-expressing tumor cells; reduced E-cadherin could contribute to invasiveness of the tumor cells (12).
Fig. 6.
Overexpression of RHAMMB activates EGFR in pancreatic tumor cells, and EGFR small molecule inhibitor induced apoptosis of mouse pancreatic islet tumor cells in vitro. (A) Western blot analysis revealed the elevated phosphorylated EGFR (p-EGFR), phosphorylated Erk (p-Erk), and phosphorylated STAT3 (p-STAT3), and decreased E-cadherin in N134 cells overexpressing RHAMMB (lane 2) compared with uninfected N134 cells (lane 1). (B) N134 cells and N134 cells overexpressing RHAMMB were cultured in the presence of DMSO (vehicle control) or 10 μM gefitnib. Two days later, cells were harvested and labeled with Alexa Fluor 488 annexin. The apoptotic cells were distinguished using a flow cytometer with a 488-nm laser to excite the dye. Data shown are the mean percentage ± SD from triplicate experiments. (C) N134 cells overexpressing RHAMMB were cultured in the presence of DMSO, 0.5, 1, or 10 μM gefitnib, and whole cell extracts were prepared 2 or 24 h later for Western blot analysis of indicated antibodies. WCE, whole cell extracts.
To gauge whether the EGFR signaling pathway might be required for the survival of N134 cells overexpressing RHAMMB, we treated cells with gefitnib, a small molecule inhibitor of the EGFR kinase (13). Two days later, gefitnib-treated and untreated cells were labeled with Alexa Fluor 488 annexin V and propidium iodide and then subjected to flow cytometry to identify apoptotic cells. We found that (i) overexpression of RHAMMB in untreated tumor cells provided modest protection against apoptosis, and (ii) gefitnib induced an approximately fourfold increase in apoptosis of RHAMMB-expressing cells, but did not induce significant apoptosis in parental N134 cells in vitro (Fig. 6B). Gefitinib treatment also reduced phosphorylation of both Erk and EGFR, without significant changes in phosphorylation of STAT3 or Bcl-xL protein levels, in RHAMMB-expressing tumor cells (Fig. 6C). These results suggest that RHAMM uses the EGFR signaling pathway to promote tumor cell survival, although this effect cannot explain the specific increase in hepatotropic metastasis.
Discussion
Metastasis is the major cause of cancer mortality and appears to occur in an orderly sequence of general steps: local invasion, intravasation, survival in the circulation, extravasation, and colonization. Two in vivo assays, “spontaneous metastasis” in RIP-Tag; RIP-tva mouse model and “tail vein assay of cancer metastasis,” provide complementary information to dissect the specific metastatic steps. Using the avian retroviral vector to deliver five candidate genes that are up-regulated in human HCC into mouse pancreatic hyperplastic cells in RIP-Tag; RIP-tva mice, we have shown that RHAMMB among the five genes dramatically promotes metastasis in an organ-specific pattern, with the appearance of liver metastasis regardless of whether tumor cells enter the portal circulation endogenously in a mouse model or whether they are injected into a tail vein to enter the major venous circulation.
In addition to RHAMMB, we found that FLJ10540 promotes metastasis to lymph nodes and the liver, but the sizes and numbers of metastases are less profound than RHAMMB. PEG10, FLJ11252, and FLJ11164 were not able to promote liver metastasis in the RIP-Tag; RIP-tva model. FLJ10540 has been shown to be up-regulated in HCC and oral cavity squamous cell carcinoma and is associated with poor survival (14, 15). Overexpression of FLJ10540 in 3T3 cells was also shown to promote survival in soft agar and in low serum medium and induces tumor formation in nude mice (14).
The functions and subcellular localizations of the various products of the RHAMM locus are controversial (16). RHAMM proteins have been implicated in multiple functions due to their association with hyaluronan, BRCA1, BARD1, CD44, Erk1/2, mitotic spindle, microtubules, and microfilaments (17). They have been reported to act as cell-surface receptors but have also been reported in the cytoplasm or the cell nucleus. These observations may be attributed to the existence of different isoforms generated by alternative splicing, as it is not clear which isoform(s) was examined in many reports due to the uncertain specificity of the antibodies and nucleic acid probes. Even though RHAMM proteins have been shown to be overexpressed in various cancer cells, their oncogenic potentials and growth promoting signals have not been rigorously demonstrated either in vitro or in vivo. One possible explanation is that the oncogenic activity of RHAMMB is too modest to be observed in conventional cell transformation assays but becomes evident in a more sensitive context, such as our RIP-Tag; RIP-tva mouse model. Further investigation is needed to decipher the mechanism by which metastasis is enhanced and to determine whether other RHAMM isoforms have similar effects.
Because hepatotropic metastasis promoted by RHAMMB is also observed with tail vein injection, the effect cannot be mediated directly through intravasation or be dependent on access to the liver through the portal vein. The effect is not a consequence of size of the primary tumors, because abundant hepatic metastases developed even in RCASBP–RHAMMB infected RIP-Tag; RIP-tva mice that had relatively small tumors. Increased proliferation also appears not to explain the enhanced hepatic metastasis, because RCASBP–RHAMMB infected N134 cells had a lower Ki67 index than uninfected cells. Furthermore, the effect is unlikely to be attributable to enhanced migration, because RHAMMB does not promote tumor cell migration in a two-chamber Transwell assay. Our findings suggest that RHAMMB may allow islet tumor cells to invade and grow specifically in the microenvironment of the liver. It will be important to determine the cell specificity of this phenomenon and to identify the factor(s) in the liver that might be recognized by hepatotropic cancer cells and thus serve as targets for interventions that prevent metastasis.
In a preliminary effort to characterize the biochemical effects of RHAMMB on pancreatic islet tumor cells, we showed that it enhances phosphorylation of EGFR, Erk1/2, and STAT3, implying activation of signaling pathways that are often affected in cancers. Furthermore, treatment with an inhibitor of EGFR signaling, gefitnib, induced apoptosis in RHAMMB-expressing cells, although we cannot exclude the possibility that this effect may be mediated by inhibition of other uncharacterized kinases. Additionally, it has been shown that EGFR signaling contributes to tumorigenesis in RIP-Tag mice (18). The RHAMM full-length isoform, RHAMMA, enhances serum-induced Erk1/2 phosphorylation in embryonic fibroblasts from RHAMM knockout mice (10), and here we showed that overexpression of RHAMMB phosphorylates Erk1/2 in mouse pancreatic islet tumor cells. Thus, Erk1/2 seem to be common targets of two RHAMM isoforms. Conditional STAT3 knockout mice have been used to show that STAT3 is important for the function of interleukin-6 (IL-6) in liver regeneration (19). Because IL-6 is enriched in the liver, tumor cells with activated STAT3 may have a growth advantage in the liver.
We previously showed that normal, untransformed mammary cells, when introduced to the tail vein of the recipient mice, colonize to the lungs, a major organ site for breast cancer metastasis (20). Although RHAMMB promotes hepatotropic metastasis of islet tumor cells in the tail vein assay, it remains possible that the hepatotropism is an inherent property of the islet tumor cells rather than determined by RHAMM protein. Further studies are required to determine whether uninfected pancreatic islet tumor cells also reach the liver, but remain dormant or are unable to survive and/or grow. Although RHAMMB does not provide a proliferation advantage in vitro, the slight protection against apoptosis observed in RHAMMB-expressing tumor cells might help them survive better in ectopic and hemodynamically stressful sites, such as the liver.
The liver is the most common organ for the metastases in human pancreatic neuroendocrine tumors and cancers of the intestines, and almost all patients will succumb to liver failure from the metastases. Because RIP-Tag mice (21) develop islet tumors that do not metastasize, whereas such tumors in RIP-Tag; RIP-tva mice infected with vectors encoding genes like RHAMMB form liver metastases readily, the model described here should be useful for preclinical studies of cancers that preferentially metastasize to the liver.
Numerous microarray-based screens and immunohistochemical screens have been performed to identify genes differentially expressed in human tumors and normal cells. Some have reported overexpression of RHAMM in various tumors and a negative prognostic significance for its expression in breast cancer (4), multiple myeloma (22), and colon cancer (7). Our study provides support for these observations by demonstrating a causal role for RHAMMB in liver metastasis in a mouse model of multistep tumorigenesis.
Materials and Methods
Generation of RIP-Tag; RIP-tva mice and N134 cell line has been described (1). NSG mice were generated by The Jackson Laboratory. All mice were housed in accordance with institutional guidelines. All procedures involving mice were approved by the institutional animal care and use committee. RCASBP is a replication-competent avian leukosis virus with a splice acceptor and the Bryan-RSV pol gene. RCASBP–ALPP has been described previously (23). RCASBP–Luciferase was generated by Yi Li in the Varmus laboratory. RCASBP–RHAMMB, RCASBP–FLAG-PEG10, RCASBP–FLAG-FLJ10540, RCASBP–FLAG-FLJ11252, and RCASBP–FLJ11164 were generated in the Chou laboratory. Viral propagation and titer determination were described (1). Intracardiac injection was performed as described (24).
Tail Vein Injection of Tumor Cells and in Vivo Bioluminescent Imaging.
Single cell suspension of tumor cells was prepared before tail vein injection. Mice were i.v. injected in the tail vein using insulin syringes with 1 × 106 tumor cells in 150 μL PBS. Mice were subjected to in vivo bioluminescent imaging as described previously (25). All other experimental details are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank members in the H.V. laboratory, especially Jennifer Demers, Mary Ann Melnick, Andreas Giannakou, Gabriela Sanchez, and Raymond Dematteo for technical assistance; Katrina Podsypanina and Romel Somwar for insightful discussions; Levi Beverly for 293T cell line and reagents. We thank Danny Huang for mouse database design; and Irina Linkov for protocols. We thank Leigh Selesner in the Y.-C.N.D. laboratory for technical assistance; Hua-Chien Chen at Chang-Gung University for RHAMM expression data from HCC samples; Mouse Genetics Core Facility and Research Animal Resource Center for foster service and animal husbandry; and Memorial Sloan-Kettering Cancer Center's Molecular Cytology, Flow Cytometry, and Small-Animal Imaging Cores for technical assistance. This work was funded in part by a grant from the National Institutes of Health (5P01CA094060).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1114022108/-/DCSupplemental.
References
- 1.Du YC, Lewis BC, Hanahan D, Varmus H. Assessing tumor progression factors by somatic gene transfer into a mouse model: Bcl-xL promotes islet tumor cell invasion. PLoS Biol. 2007;5:e276. doi: 10.1371/journal.pbio.0050276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang CW, et al. Integrative genomics based identification of potential human hepatocarcinogenesis-associated cell cycle regulators: RHAMM as an example. Biochem Biophys Res Commun. 2005;330:489–497. doi: 10.1016/j.bbrc.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Akiyama Y, et al. Hyaluronate receptors mediating glioma cell migration and proliferation. J Neurooncol. 2001;53:115–127. doi: 10.1023/a:1012297132047. [DOI] [PubMed] [Google Scholar]
- 4.Assmann V, et al. The pattern of expression of the microtubule-binding protein RHAMM/IHABP in mammary carcinoma suggests a role in the invasive behaviour of tumour cells. J Pathol. 2001;195:191–196. doi: 10.1002/path.941. [DOI] [PubMed] [Google Scholar]
- 5.Grützmann R, et al. Gene expression profiling of microdissected pancreatic ductal carcinomas using high-density DNA microarrays. Neoplasia. 2004;6:611–622. doi: 10.1593/neo.04295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gust KM, et al. RHAMM (CD168) is overexpressed at the protein level and may constitute an immunogenic antigen in advanced prostate cancer disease. Neoplasia. 2009;11:956–963. doi: 10.1593/neo.09694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zlobec I, Baker K, Terracciano LM, Lugli A. RHAMM, p21 combined phenotype identifies microsatellite instability-high colorectal cancers with a highly adverse prognosis. Clin Cancer Res. 2008;14:3798–3806. doi: 10.1158/1078-0432.CCR-07-5103. [DOI] [PubMed] [Google Scholar]
- 8.Ota T, et al. Complete sequencing and characterization of 21,243 full-length human cDNAs. Nat Genet. 2004;36:40–45. doi: 10.1038/ng1285. [DOI] [PubMed] [Google Scholar]
- 9.Sohr S, Engeland K. RHAMM is differentially expressed in the cell cycle and downregulated by the tumor suppressor p53. Cell Cycle. 2008;7:3448–3460. doi: 10.4161/cc.7.21.7014. [DOI] [PubMed] [Google Scholar]
- 10.Tolg C, et al. Rhamm-/- fibroblasts are defective in CD44-mediated ERK1,2 motogenic signaling, leading to defective skin wound repair. J Cell Biol. 2006;175:1017–1028. doi: 10.1083/jcb.200511027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monslow J, Sato N, Mack JA, Maytin EV. Wounding-induced synthesis of hyaluronic acid in organotypic epidermal cultures requires the release of heparin-binding egf and activation of the EGFR. J Invest Dermatol. 2009;129:2046–2058. doi: 10.1038/jid.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392:190–193. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- 13.Ciardiello F, et al. Antitumor effect and potentiation of cytotoxic drugs activity in human cancer cells by ZD-1839 (Iressa), an epidermal growth factor receptor-selective tyrosine kinase inhibitor. Clin Cancer Res. 2000;6:2053–2063. [PubMed] [Google Scholar]
- 14.Chen C-H, et al. FLJ10540-elicited cell transformation is through the activation of PI3-kinase/AKT pathway. Oncogene. 2007;26:4272–4283. doi: 10.1038/sj.onc.1210207. [DOI] [PubMed] [Google Scholar]
- 15.Chen C-H, et al. Expression of FLJ10540 is correlated with aggressiveness of oral cavity squamous cell carcinoma by stimulating cell migration and invasion through increased FOXM1 and MMP-2 activity. Oncogene. 2009;28:2723–2737. doi: 10.1038/onc.2009.128. [DOI] [PubMed] [Google Scholar]
- 16.Hofmann M, et al. Problems with RHAMM: A new link between surface adhesion and oncogenesis? Cell. 1998;95:591–592. doi: 10.1016/s0092-8674(00)81628-1. author reply 592–593. [DOI] [PubMed] [Google Scholar]
- 17.Maxwell CA, McCarthy J, Turley E. Cell-surface and mitotic-spindle RHAMM: Moonlighting or dual oncogenic functions? J Cell Sci. 2008;121:925–932. doi: 10.1242/jcs.022038. [DOI] [PubMed] [Google Scholar]
- 18.Nolan-Stevaux O, et al. Differential contribution to neuroendocrine tumorigenesis of parallel Egfr signaling in cancer cells and pericytes. Genes Cancer. 2010;1:125–141. doi: 10.1177/1947601909358722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li W, Liang X, Kellendonk C, Poli V, Taub R. STAT3 contributes to the mitogenic response of hepatocytes during liver regeneration. J Biol Chem. 2002;277:28411–28417. doi: 10.1074/jbc.M202807200. [DOI] [PubMed] [Google Scholar]
- 20.Podsypanina K, et al. Seeding and propagation of untransformed mouse mammary cells in the lung. Science. 2008;321:1841–1844. doi: 10.1126/science.1161621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanahan D. Heritable formation of pancreatic beta-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature. 1985;315:115–122. doi: 10.1038/315115a0. [DOI] [PubMed] [Google Scholar]
- 22.Maxwell CA, et al. RHAMM expression and isoform balance predict aggressive disease and poor survival in multiple myeloma. Blood. 2004;104:1151–1158. doi: 10.1182/blood-2003-11-4079. [DOI] [PubMed] [Google Scholar]
- 23.Fekete DM, Cepko CL. Replication-competent retroviral vectors encoding alkaline phosphatase reveal spatial restriction of viral gene expression/transduction in the chick embryo. Mol Cell Biol. 1993;13:2604–2613. doi: 10.1128/mcb.13.4.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang Y, et al. Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc Natl Acad Sci USA. 2005;102:13909–13914. doi: 10.1073/pnas.0506517102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du YC, Klimstra DS, Varmus H. Activation of PyMT in beta cells induces irreversible hyperplasia, but oncogene-dependent acinar cell carcinomas when activated in pancreatic progenitors. PLoS ONE. 2009;4:e6932. doi: 10.1371/journal.pone.0006932. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.