Abstract
Purpose
Prior results of breast-conserving therapy (BCT) have shown substantial rates of local recurrence (LR) in young patients with breast cancer (BC).
Patients and Methods
We studied 1,434 consecutive patients with invasive BC who received BCT from December 1997 to July 2006. Ninety-one percent received adjuvant systemic therapy; no patients received trastuzumab. Five BC subtypes were approximated: estrogen receptor (ER) or progesterone receptor (PR) positive, HER2 negative, and grades 1 to 2 (ie, luminal A); ER positive or PR positive, HER2 negative, and grade 3 (ie, luminal B); ER or PR positive, and HER2 positive (ie, luminal HER2); ER negative, PR negative, and HER2 positive (ie, HER2); and ER negative, PR negative, and HER2 negative (ie, triple negative). Actuarial rates of LR were calculated by using the Kaplan-Meier method.
Results
Median follow-up was 85 months. Overall 5-year cumulative incidence of LR was 2.1% (95% CI, 1.4% to 3.0%). The 5-year cumulative incidence of LR was 5.0% (95% CI, 3.0% to 8.3%) for age quartile 23 to 46 years; 2.2% (95% CI, 1.0% to 4.6%) for ages 47 to 54 years; 0.9% (95% CI, 0.3% to 2.6%) for ages 55 to 63 years; and 0.6% (95% CI, 0.1% to 2.2%) for ages 64 to 88 years. The 5-year cumulative incidence of LR was 0.8% (95% CI, 0.4% to 1.8%) for luminal A; 2.3% (95% CI, 0.8% to 5.9%) for luminal B; 1.1% (95% CI, 0.2% 7.4%) for luminal HER2; 10.8% (95% CI, 4.6% to 24.4%) for HER2; and 6.7% (95% CI, 3.6% to 12.2%) for triple negative. On multivariable analysis, increasing age was associated with decreased risk of LR (adjusted hazard ratio, 0.97; 95% CI, 0.94 to 0.99; P = .009).
Conclusion
In the era of systemic therapy and BC subtyping, age remains an independent prognostic factor after BCT. However, the risk of LR for young women appears acceptably low.
INTRODUCTION
Young patient age has been reported to be a poor prognostic factor among women with breast cancer (BC).1–4 Studies have variously defined young age as age at diagnosis younger than 35, 40, 45, or even 50 years, with reports demonstrating higher rates of local recurrence (LR) and lower survival when young women are compared with older women.5–14 Moreover, despite advances in BC survival since the 1970s among women generally, survival rates among young women continue to fall behind those of older women across all stages of BC.15,16
Although local therapy options do not generally differ for women with BC on the basis of age, breast-conserving therapy (BCT) is usually desirable among young women to preserve quality of life. Randomized trials comparing BCT to mastectomy consistently demonstrate a small but measurable increased risk of LR after BCT compared with mastectomy, estimated at 1% per year,6,17–20 but without a corresponding decrease in disease-free or overall survivals. However, the Early Breast Cancer Trialists' Collaborative Group 2005 overview analysis of randomized trials showed that LR impacts survival and suggests that, for every four LRs prevented at year 5, one fewer BC death will occur at year 15.21
Given that BC mortality appears to be influenced by LR and that higher rates of LR have been reported among young women, it is critical to accurately characterize the risk of LR among young women receiving BCT in the contemporary era. Prior results of BCT have shown substantial rates of local or locoregional recurrence among young women of at least 10% to 20% at 5 years and as high as 50% at 10 years after BCT, depending on the definition of young age, LR, and length of follow-up.7–10,22–24 A 1994 study from the Joint Center for Radiation Therapy, for example, reported rates of LR of 36% at 5 years and 51% at 10 years after BCT among women younger than 35 years, which was significantly higher than for older women.8 In the setting of observed high LR rates, some have questioned whether BCT among young women represents optimal therapy25 or have recommended treatment intensification among these women.26,27
Most of the literature examining LR rates after BCT, however, report data from randomized trials or institutional experiences with patients treated in the 1970s, 1980s, and early 1990s. This represents an era before the widespread use of adjuvant systemic therapy that has been observed to decrease the risk of LR12 and preceded improvements in BC imaging and rigorous attention to margin status that may be particularly important among young women.28 Given these advances, it is not clear that previously reported rates of LR reflect those seen in current practice. In addition, there is growing recognition that BC is a heterogeneous disease, with molecularly distinct BC subtypes identified through gene expression profiling that yield additional prognostic information.29–32 These molecular subtypes can be approximated by immunohistochemical (IHC) staining patterns for estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2),33 providing clinically useful differentiation of outcomes.34 Moreover, young women with BC appear to have tumors enriched for specific gene sets conferring a more aggressive biology compared with those of older women.35 Whether patient age remains prognostic in the setting of molecular subtype information has not been fully characterized with regard to risk of LR. In this study, we aimed to characterize the risk of LR after BCT in the current era according to both age and BC subtype.
PATIENTS AND METHODS
Patient Selection
The study cohort consisted of 1,434 consecutive women with clinical stage I or II invasive BC who received BCT between December 1997 and July 2006 at Dana-Farber Cancer Institute/Brigham and Women's Hospital (Boston, MA; n = 918) or Massachusetts General Hospital (Boston, MA; n = 516), and had information available on ER, PR, and HER2/neu status and histologic grade of their primary tumor (Table 1). Patients with prior malignancy (except nonmelanoma skin cancers), synchronous bilateral breast cancer, or treatment with preoperative systemic therapy were excluded. This investigation was approved by the Dana-Farber/Harvard Cancer Center institutional review board.
Table 1.
Patient Baseline Characteristics
| Characteristic | Patients (N = 1,434) |
|
|---|---|---|
| No. | % | |
| T stage | ||
| T1a | 151 | 10.5 |
| T1b | 353 | 24.6 |
| T1c | 646 | 45.0 |
| T2 | 272 | 19.0 |
| T3 | 12 | 0.8 |
| No. of positive nodes | ||
| cN0 (no nodes sampled) | 99 | 6.9 |
| 0 | 964 | 67.2 |
| 1 to 3 | 303 | 21.1 |
| 4 to 9 | 54 | 3.8 |
| > 9 | 14 | 1.0 |
| Grade | ||
| 1 | 380 | 26.5 |
| 2 | 617 | 43.0 |
| 3 | 437 | 30.5 |
| ER and/or PR positive | 1,208 | 84.2 |
| HER2 positive | 160 | 11.2 |
| LVI present | 335 | 23.4 |
| EIC present | 168 | 11.7 |
| ECE present | 116 | 8.1 |
| Menopausal status | ||
| Premenopausal | 430 | 30.0 |
| Perimenopausal | 100 | 7.0 |
| Postmenopausal | 882 | 61.5 |
| Unknown | 22 | 1.5 |
| Systemic therapy | ||
| Yes | 1,302 | 90.8 |
| Node positive | 363 | 97.8 |
| Node negative | 939 | 88.3 |
| No | 132 | 9.2 |
| Node positive | 8 | 2.2 |
| Node negative | 124 | 11.7 |
| Margins | ||
| Negative | 1,282 | 89.4 |
| Close | 113 | 7.9 |
| Positive | 33 | 2.3 |
| Unknown | 6 | 0.4 |
| Age at diagnosis, years | ||
| ≤ 35 | 52 | 3.6 |
| 36-45 | 238 | 16.6 |
| 46-55 | 470 | 32.8 |
| 56-65 | 376 | 26.2 |
| 66-75 | 211 | 14.7 |
| > 75 | 87 | 6.1 |
Abbreviations: ECE, extracapsular extension; EIC, extensive intraductal component; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; LVI, lymphovascular invasion; PR, progesterone receptor.
Treatment Characteristics
All patients underwent lumpectomy, and 1,335 patients (93%) underwent surgical lymph node evaluation. All women received external-beam radiation therapy (RT) to the whole breast. Whole-breast RT was generally prescribed to 45 to 50 Gy in 1.8- to 2.0-Gy daily fractions, with a tumor-bed boost to a total dose of 60 to 61 Gy. Additional supraclavicular or axillary RT fields were not typically treated except among women with four or more positive lymph nodes. Adjuvant chemotherapy was delivered to 87% of node-positive patients (ie, 321 of 371 patients) and to 32% of node-negative patients (ie, 338 of 1,063 patients). Among ER-positive or PR-positive patients, 90% (ie, 1,089 of 1,208) received hormonal therapy. No patient received adjuvant trastuzumab.
Follow-Up and End Points
Patients were generally observed in follow-up 4 to 6 weeks after RT completion and every 6 months thereafter with annual breast imaging. Follow-up time was counted from the date of diagnosis to the date of the first event (defined herein) or last confirmed date of disease-free status. The median follow-up time was 85 months (range, 1.5 to 153 months).
The primary end point was time to LR as a first event. This end point included any ipsilateral in-breast recurrence (invasive or noninvasive) without evidence of distant metastasis. Patients diagnosed with distant metastasis within 4 months of a LR event (n = 4) were considered to have had simultaneous local and distant recurrence and, therefore, were not considered to have the primary end point.
Classification of Groups
On the basis of recent data suggesting that the tumor proliferation marker Ki-67 can additionally discriminate luminal BC subtypes,36 a tight correlation between Ki-67 and histologic grade in breast cancers,37,38 and recent consensus conference conclusions that grade is an acceptable surrogate for Ki-67 in the distinction of luminal subtypes,39 receptor status and histologic grade were used to approximate five BC subtypes: ER positive or PR positive, HER2 negative, and grade 1 or 2 (ie, subtype luminal A); ER positive or PR positive, HER2 negative, and grade 3 (ie, luminal B); ER positive or PR positive and HER2 positive (ie, luminal HER2); ER negative, PR negative, and HER2 positive (ie, HER2); and ER negative, PR negative, and HER2 negative (ie, triple negative). ER and PR status were determined by immunohistochemical (IHC) staining. Tumors were considered HER2 positive if they were scored 3+ by IHC or if they were 2+ by IHC and also HER2 amplified (ratio > 2.0) on the basis of fluorescence in situ hybridization. In the absence of positive fluorescence in situ hybridization data, tumors scored 2+ by IHC were considered negative for HER2.40,41
Statistical Methods
The χ2 test was used to compare baseline characteristics among age quartiles and BC subtypes for categoric variables, whereas the Kruskal-Wallis test was used for continuous variables. Kaplan-Meier actuarial cumulative rates of LR were calculated, and Gray's competing risks multivariable analysis42 was used to estimate associations with time to LR. Competing events were isolated regional nodal recurrence, contralateral BC, second malignancy, distant metastasis, and death without recurrence. Covariates were BC subtype with luminal A as baseline, age (continuous variable), tumor size in centimeters (continuous), number of positive lymph nodes (continuous), and whole-breast RT dose in Gy (continuous). All analyses were performed in Stata 11.1 (StataCorp, College Station, TX). All P values were two sided.
RESULTS
Baseline Distribution of Prognostic Factors According to Age Quartile and Subtype
Among the four age quartiles, there were significant differences in the distribution of histologic grade (P < .001), lymphovascular invasion (LVI; P < .001), node positivity (P < .001), pathologic T stage (P < .001), margin status (P = .04), receipt of systemic therapy (P < .001), and total RT dose (P = .02; Table 2). Compared with older patients, younger women more frequently had BC exhibiting high grade, larger size, LVI, and node positivity, and were more likely to receive adjuvant chemotherapy.
Table 2.
Patient Baseline Characteristics Stratified by Age Quartile
| Characteristic | All Patients(N = 1,434) | Patients by Age Quartile (years) |
P | |||
|---|---|---|---|---|---|---|
| < 47 (n = 341) | 47-54 (n = 360) | 55-63 (n = 370) | > 63 (n = 363) | |||
| T1, % | 80 | 72 | 81 | 85 | 83 | < .001 |
| Grade 3, % | 30 | 42 | 35 | 26 | 20 | < .001 |
| LVI, % | 23 | 34 | 23 | 22 | 15 | < .001 |
| Margins positive, % | 2.3 | 2.4 | 1.4 | 1.9 | 3.6 | .04 |
| Node positive, % | 26 | 36 | 34 | 19 | 15 | < .001 |
| ≥ 4 positive nodes, % | 5 | 5.9 | 5.6 | 5.1 | 2.5 | < .001 |
| WB dose, Gy | .2 | |||||
| Median | 45 | 45 | 45 | 45 | 45 | |
| Mean | 46.4 | 46.5 | 46.3 | 46.4 | 46.5 | |
| Range | 39.6-60.0 | 39.6-50.4 | 39.6-50.4 | 39.6-60.0 | 39.6-55.0 | |
| Total dose, Gy | .02 | |||||
| Median | 60 | 60.4 | 60 | 60 | 60 | |
| Mean | 59.1 | 59.4 | 58.2 | 59.4 | 59.7 | |
| Range | 50.0-72.0 | 50.0-68.0 | 52.0-68.0 | 51.6-70.0 | 50.0-72.0 | |
| Systemic treatment, % | 91 | 96 | 93 | 94 | 81 | < .001 |
| Hormonal treatment, % | 77 | 73 | 79 | 81 | 75 | .06 |
| Chemotherapy treatment, % | 46 | 74 | 60 | 39 | 12 | < .001 |
NOTE. All comparisons were by χ2 test except age and dose (Kruskal-Wallis test).
Abbreviations: LVI, lymphovascular invasion; WB, whole breast.
Among the five BC subtypes, there were significant differences in the distribution of patient age (P < .001), histologic grade (P < .001), LVI (P < .001), node positivity (P < .001), pathologic T stage (P < .001), receipt of systemic therapy (P < .001), whole breast RT dose (P = .002), and total RT dose (P = .004; Table 3). Compared with the other subtypes, HER2 and triple-negative subtypes more frequently demonstrated high grade and larger size, and luminal B and HER2 subtypes more frequently exhibited LVI. The triple negative subtype was most commonly observed among younger patients.
Table 3.
Patient Baseline Characteristics Stratified by Subtype
| Characteristic | All Patients(N = 1,434) | Patients by Subtype |
P | ||||
|---|---|---|---|---|---|---|---|
| Luminal A (n = 905) | Luminal B (n = 198) | Luminal-HER2 (n = 105) | HER2 (n = 55) | Triple Negative (n = 171) | |||
| Median age, years | 55 | 56 | 52 | 52 | 52 | 53 | < .001 |
| Age quartile in years, % | < .001 | ||||||
| < 47 | 24 | 19 | 31 | 31 | 27 | 34 | |
| 47-54 | 25 | 24 | 28 | 29 | 29 | 26 | |
| 55-63 | 26 | 28 | 21 | 26 | 27 | 21 | |
| > 63 | 25 | 30 | 20 | 14 | 16 | 18 | |
| T1, % | 80 | 87 | 71 | 74 | 64 | 65 | < .001 |
| Grade 3, % | 30 | 0 | 100 | 48 | 75 | 87 | < .001 |
| LVI, % | 23 | 18 | 41 | 30 | 40 | 25 | < .001 |
| Margins positive, % | 2.3 | 2.2 | 3.0 | 4.8 | 1.8 | 0.6 | .2 |
| Node positive, % | 26 | 20 | 35 | 37 | 46 | 31 | < .001 |
| ≥ 4 positive nodes, % | 5 | 3 | 7 | 8 | 11 | 8 | < .001 |
| WB dose, Gy | .002 | ||||||
| Median | 45 | 45 | 45 | 45 | 45 | 45 | |
| Mean | 46.4 | 46.4 | 46.9 | 47.2 | 45.9 | 45.8 | |
| Range | 39.6-60.0 | 39.6-60.0 | 39.6-55.0 | 39.6-50.4 | 39.6-50.0 | 39.6-50.4 | |
| Total dose, Gy | .004 | ||||||
| Median | 60 | 60 | 61 | 60 | 61 | 61 | |
| Mean | 59.1 | 59.5 | 58.6 | 59.5 | 55.7 | 58.9 | |
| Range | 50.0-72.0 | 50.0-72.0 | 51.0-68.0 | 54.2-68.0 | 53.6-61.0 | 53.0-70.0 | |
| Systemic treatment, % | 91 | 92 | 95 | 92 | 78 | 81 | < .001 |
| Hormonal treatment, % | 77 | 90 | 88 | 91 | 7 | 9 | < .001 |
| Chemotherapy treatment, % | 46 | 31 | 70 | 68 | 75 | 77 | < .001 |
NOTE. All comparisons by χ2 test except age and dose (Kruskal-Wallis test).
Abbreviations: HER2, human epidermal growth factor receptor 2; LVI, lymphovascular invasion; WB, whole breast.
LR
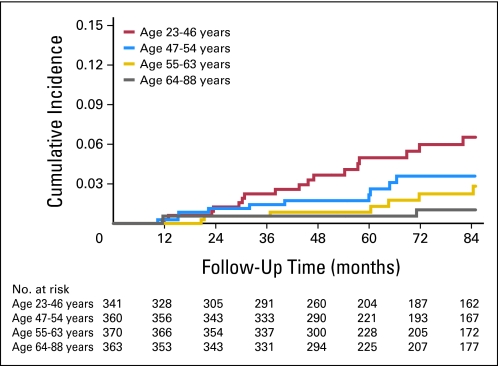
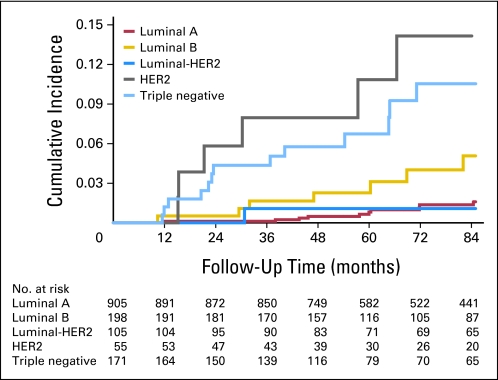
After a median follow-up of 85 months, there were 44 (isolated) LRs. The 5-year cumulative incidence of LR for all patients was 2.1% (95% CI, 1.4% to 3.0%). For patients in age quartile 23 to 46 years, the 5-year cumulative incidence of LR was 5.0% (95% CI, 3.0% to 8.3%) compared with 2.2% (95% CI, 1.0% to 4.6%) for age quartile 47 to 54 years , 0.9% (95% CI, 0.3% to 2.6%) for age quartile 55 to 63 years, and 0.6% (95% CI, 0.1% to 2.2%) for age quartile 64 to 88 years (Fig 1). For patients in the luminal A subgroup, the 5-year cumulative incidence of LR was 0.8% (95% CI, 0.4% to 1.8%) compared with 2.3% (95% CI, 0.8% to 5.9%) for luminal B, 1.1% (95% CI, 0.2% to 7.4%) for luminal HER2, 10.8% (95% CI, 4.6% to 24.4%) for HER2, and 6.7% (95% CI, 3.6% to 12.2%) for triple negative (Fig 2).
Fig 1.
Unadjusted cumulative incidence of local recurrence by age quartile on the basis of competing risks analysis.
Fig 2.
Unadjusted cumulative incidence of local recurrence by breast cancer subtype on the basis of competing risks analysis. HER2, human epidermal growth factor receptor 2.
Table 4 provides an exploratory analysis of crude rates of LR according to age quartile and BC subtype. Among the youngest age quartile, the highest rates of LR were among luminal B (8.1%), HER2 (13.3%), and triple-negative (10.2%) subtypes. In contrast, the two oldest age quartiles of ages 55 to 63 years and 64 to 88 years demonstrated LR rates of 0% and 0%, respectively, among luminal B subtypes, and 6.7% and 0%, respectively, among HER2 subtypes. Of 101 women younger than age 40 years, four (4.0%) experienced LR, of which three had luminal B subtype and one had luminal A subtype; there was one LR (2.6%) among the 39 women younger than age 35 years.
Table 4.
Crude Risk of Local Recurrence by Age Quartile and Subtype
| Age Quartile (years) | Breast Cancer Subtype |
|||||
|---|---|---|---|---|---|---|
| Luminal A | Luminal B | Luminal-HER2 | HER2 | Triple Negative | All Subtypes | |
| 23-46 | ||||||
| % | 4.7 | 8.1 | 3.0 | 13.3 | 10.2 | 6.5 |
| No. local recurrences | 8 | 5 | 1 | 2 | 6 | 22 |
| No. at risk | 172 | 62 | 33 | 15 | 59 | 341 |
| Median follow-up, months | 87.5 | 86.4 | 90.8 | 101.8 | 63.8 | 86.8 |
| 47-54 | ||||||
| % | 0.5 | 5.5 | 0 | 18.8 | 8.9 | 2.6 |
| No. local recurrences | 1 | 3 | 0 | 3 | 4 | 11 |
| No. at risk | 214 | 55 | 30 | 16 | 45 | 419 |
| Median follow-up, months | 82.2 | 84.0 | 104.0 | 69.9 | 61.2 | 84.5 |
| 55-63 | ||||||
| % | 1.6 | 0 | 0 | 6.7 | 8.3 | 2.3 |
| No. local recurrences | 4 | 0 | 0 | 1 | 3 | 8 |
| No. at risk | 250 | 42 | 27 | 15 | 36 | 343 |
| Median follow-up, months | 83.6 | 90.3 | 88.0 | 64.8 | 77.3 | 83.2 |
| 64-88 | ||||||
| % | 0.4 | 0 | 0 | 0 | 6.5 | 0.9 |
| No. local recurrences | 1 | 0 | 0 | 0 | 2 | 3 |
| No. at risk | 269 | 39 | 15 | 9 | 31 | 331 |
| Median follow-up, months | 86.4 | 56.8 | 110.3 | 83.4 | 53.7 | 84.5 |
| All ages | ||||||
| % | 1.5 | 4.0 | 1.0 | 10.9 | 8.8 | 3.1 |
| No. local recurrences | 14 | 8 | 1 | 6 | 15 | 44 |
| No. at risk | 905 | 198 | 105 | 55 | 171 | 1,434 |
| Median follow-up, months | 85.2 | 82.0 | 96.3 | 83.8 | 61.2 | 85.0 |
NOTE. Crude risk is the actual number of local recurrences per number at risk. Median follow-up in months is reported for each age quartile and breast cancer subtype combination.
Abbreviation: HER2, human epidermal growth factor receptor 2.
On multivariable analysis, increasing age at diagnosis was independently associated with decreased risk of LR (adjusted hazard ratio [AHR], 0.97 per year; 95% CI, 0.94 to 0.99; P = .009; Table A1, online only). With luminal A subtype as baseline, both HER2 (AHR, 5.2; 95% CI, 1.8 to 15; P = .003) and triple-negative (AHR, 3.9; 95% CI, 1.7 to 9; P = .001) subtypes were associated with increased risk of LR, whereas luminal B subtype (AHR, 2.1; 95% CI, 0.95 to 4.8; P = .067) showed a nonsignificant trend toward increased risk of LR, and luminal HER2 subtype (AHR, 0.48; 95% CI, 0.06 to 3.7; P = .49) was not associated with risk of LR. Increasing number of positive lymph nodes (AHR, 1.07; 95% CI, 1.00 to 1.16; P = .059) and tumor size in centimeters (AHR, 1.32; 95% CI, 0.96 to 1.80; P = .08) were not significantly associated with increased risk of LR, whereas whole-breast RT dose (AHR, 0.91; 95% CI, 0.86 to 0.98; P = .007) was associated with decreased risk of LR.
DISCUSSION
In this study, we found that, among 1,434 consecutive women with early-stage invasive BC who received BCT, increasing age was associated with decreased risk of LR independent of BC subtype approximation or other prognostic factors. Yet, although younger women demonstrated the highest rate of LR, the 5.0% risk of LR at 5 years we observed among the youngest age quartile was considerably lower than the 10% to 36% risk of LR at similar follow-up reported in prior studies8–10,22–24,43 from earlier treatment periods that focused on recurrence risk among young women.
The low rate of LR after BCT among young women in our series compared with earlier reports may reflect differences in treatment era. Similar to other centers,43,44 we have observed a progressive decline in LR over time. Our series included women treated with BCT from 1997 to 2006; most prior series report outcomes on women treated before 2000. Modern advances include better preoperative breast imaging and postoperative delineation of the lumpectomy cavity for radiation planning, greater attention to obtaining negative surgical margins, incorporation of a radiation boost, and—perhaps most importantly—the prevalent use of adjuvant systemic therapy. Systemic therapy substantially decreases rates of LR after BCT12,45–49; in this study, 91% of women received hormonal therapy, chemotherapy, or both. This is in contrast to rates of systemic therapy use of 20% to 35% reported for patients treated in the 1970s, 1980s, and 1990s,8,23,24 and to some contemporary series that still report systemic therapy use among less than 60% of patients,50 which may account for some of the differences observed in rates of LR.
In addition to age, we analyzed LR according to BC subtype, and we observed higher rates of LR among HER2 and triple-negative subtypes, with a trend toward higher LR among patients with luminal B subtype. We defined luminal subtypes as luminal A, luminal B, and luminal HER2 by using histologic grade in addition to hormone receptor status on the basis of data that demonstrated distinct outcomes among three luminal subtypes by incorporating tumor proliferation markers (Ki-67)36 that are now being utilized by some groups.50,51 Our results suggest that, among young women, luminal B and HER2 subtypes are associated with higher rates of LR after BCT compared with older women. Similarly, investigators in Milan reported elevated rates of locoregional recurrence for the luminal B subtype among young women after mastectomy or BCT.50 Other than the luminal B subtype, the luminal subtypes were associated with relatively low rates of LR among the youngest patients, demonstrating that many young women with hormone-positive disease have quite favorable outcomes after BCT.
The prognostic importance of age on risk of LR after BCT remains controversial. Young age has been reported as a risk factor for LR after BCT in most investigations8–12,52,53 but not in all.54–56 Younger women are more likely to present with larger, higher-grade, ER negative, LVI positive, lymph node–positive tumors.35,57,58 Thus, it is challenging to separate these clinicopathologic factors that occur more frequently among young women and are themselves prognostic from the impact of age on outcome. The multivariable analysis in this study suggests that, even in the era of BC subtype approximation, increasing age remains independently prognostic for lower risk of LR, consistent with most prior reports. However, the magnitude of increased LR risk in absolute terms among young women appears modest, and the rates of LR after BCT among the youngest age quartile, or made on the basis of age cutoffs of 35 or 40 years, appear reasonably low.
Additional study is required to understand the mechanisms underlying the prognostic value of age in BC. Anders et al35 has shown that, in addition to unfavorable clinicopathologic characteristics, BC in women 45 years of age or younger exhibited significantly lower ERα mRNA, ERβ mRNA, and PR expression but higher HER2 and epidermal growth factor receptor genomic expression, with over 350 relevant gene sets related to multiple oncogenic signaling pathways that distinguished BC in young women. Recent work suggests that, even within subtypes, there is striking heterogeneity among tumors, with transcriptome analyses identifying six subgroups within the triple-negative subtype that have divergent sensitivities to different chemotherapies or targeted inhibitors.59 Given this apparent diversity of subgroups within BC subtypes and different genomic features among young patients, ongoing research is necessary to additionally characterize what appears to be a distinct biologic expression of BC among young patients that might explain the prognostic significance of age. Indeed, another recent study by Anders et al60 evaluated the distribution of molecular BC subtypes by age to assess for potential confounding effects on the distribution of purported age-associated genes. Their work demonstrated that genes associated with intrinsic subtype and grade appeared to strongly influence the biologic differences observed among tumors in young versus older women. This suggests that, as BC continues to be better characterized at the genomic level and as therapies are selected to target molecular subtypes for individual patients, the importance of age on prognosis may eventually disappear.
There are several potential limitations to this study. Classification according to ER, PR, and HER2 status and grade are only approximations of genotype-based molecular BC subtypes, and our conclusions do not necessarily apply to genotype-based subtypes. Although our redefined BC subtypes incorporating histologic grade in addition to hormone receptor status are based on the heterogeneity of classically defined luminal B tumors61 and prognostic information gained by adding tumor proliferation marker data to classic subtypes,36 our findings that are based on these new definitions must be confirmed by other studies. Other possible limitations are the relatively small patient numbers in certain subgroups, such as for the HER2 subtype that contained only 55 patients. Additionally, no patients received trastuzumab in this cohort, but it is now the standard of care for patients with HER2-positive BC40,41; thus, the LR risk seen in the HER2 subgroup may now be lower than what we observed among women treated until 2006. In the two largest randomized trials, adjuvant trastuzumab decreased the risk of LR among HER2-positive patients by almost 50%, although LR was not a specific end point. Moreover, with a median follow-up of 7.1 years, we have reported cumulative incidence of LR at 5 years, and longer follow-up may be required to determine accurate long-term rates of LR. Finally, dosimetric analyses of whole-breast RT parameters is warranted, given that we found that increasing whole-breast RT dose was associated with reduced LR rates. Because there exists an important interplay between total RT dose, dose per fraction, and dose homogeneity within the breast,62,63 additional investigation of the impact of dosimetry on our results is necessary and has been undertaken.
In conclusion, this study demonstrates that, in an era of routine use of systemic therapy and clinical BC subtype approximation, young age remains an independent risk factor for LR, with variability in LR risk for young patients according to BC subtype. More important, however, is our observation of the low overall risk of LR among the youngest age group in our study. The 5.0% risk of LR at 5 years we found among the youngest age quartile is substantially lower than the risk of LR reported in prior series from earlier treatment periods, and it constitutes an acceptably low risk of LR after BCT among young women in the current era.
Appendix
Table A1.
Multivariable Analysis of Time to Local Recurrence
| Predictor | AHR | 95% CI | P |
|---|---|---|---|
| Age, years | 0.97 | 0.94 to 0.99 | .009 |
| BC subtype | |||
| Luminal A | 1 (reference) | — | — |
| Luminal B | 2.14 | 0.95 to 4.85 | .067 |
| Luminal HER2 | 0.48 | 0.06 to 3.73 | .49 |
| HER2 | 5.15 | 1.76 to 15.05 | .003 |
| Triple negative | 3.94 | 1.72 to 9.01 | .001 |
| No. of positive nodes | 1.07 | 1.00 to 1.16 | .059 |
| Tumor size, cm | 1.32 | 0.96 to 1.80 | .08 |
| WB dose, Gy | 0.91 | 0.86 to 0.98 | .007 |
Abbreviations: AHR, adjusted hazard ratio; BC, breast cancer; HER2, human epidermal growth factor receptor 2; WB, whole breast.
Footnotes
Supported in part by award No. R01CA139118 (A.G.T.) and award No. P50CA089393 (A.G.T.) from the National Cancer Institute; also supported in part by the Jane Mailloux Research Fund, the Blanche Montesi Fund, and the Tim Levy Fund for breast cancer research.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Nils D. Arvold, Alphonse G. Taghian, Andrzej Niemierko, Paul L. Nguyen, Jay R. Harris
Financial support: Alphonse G. Taghian, Jay R. Harris
Administrative support: Rita F. Abi Raad, Meera Sreedhara
Provision of study materials or patients: Alphonse G. Taghian, Jennifer R. Bellon, Julia S. Wong, Barbara L. Smith, Jay R. Harris
Collection and assembly of data: Nils D. Arvold, Rita F. Abi Raad, Meera Sreedhara, Paul L. Nguyen
Data analysis and interpretation: Nils D. Arvold, Alphonse G. Taghian, Andrzej Niemierko, Jennifer R. Bellon, Julia S. Wong, Barbara L. Smith, Jay R. Harris
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Kroman N, Jensen MB, Wohlfahrt J, et al. Factors influencing the effect of age on prognosis in breast cancer: Population based study. BMJ. 2000;320:474–478. doi: 10.1136/bmj.320.7233.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albain KS, Allred DC, Clark GM. Breast cancer outcome and predictors of outcome: Are there age differentials. J Natl Cancer Inst Monogr. 1994;16:35–42. [PubMed] [Google Scholar]
- 3.Swanson GM, Lin CS. Survival patterns among younger women with breast cancer: The effects of age, race, stage, and treatment. J Natl Cancer Inst Monogr. 1994;16:69–77. [PubMed] [Google Scholar]
- 4.Han W, Kim SW, Park IA, et al. Young age: An independent risk factor for disease-free survival in women with operable breast cancer. BMC Cancer. 2004;4:82. doi: 10.1186/1471-2407-4-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher ER, Anderson S, Redmond C, et al. Ipsilateral breast tumor recurrence and survival following lumpectomy and irradiation: Pathological findings from NSABP protocol B-06. Semin Surg Oncol. 1992;8:161–166. [PubMed] [Google Scholar]
- 6.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 7.Voogd AC, Nielsen M, Peterse JL, et al. Differences in risk factors for local and distant recurrence after breast-conserving therapy or mastectomy for stage I and II breast cancer: Pooled results of two large European randomized trials. J Clin Oncol. 2001;19:1688–1697. doi: 10.1200/JCO.2001.19.6.1688. [DOI] [PubMed] [Google Scholar]
- 8.Nixon AJ, Neuberg D, Hayes DF, et al. Relationship of patient age to pathologic features of the tumor and prognosis for patients with stage I or II breast cancer. J Clin Oncol. 1994;12:888–894. doi: 10.1200/JCO.1994.12.5.888. [DOI] [PubMed] [Google Scholar]
- 9.Elkhuizen PH, van de Vijver MJ, Hermans J, et al. Local recurrence after breast-conserving treatment for invasive breast cancer: High incidence in young patients and association with poor survival. Int J Radiat Oncol Biol Phys. 1998;40:859–867. doi: 10.1016/s0360-3016(97)00917-6. [DOI] [PubMed] [Google Scholar]
- 10.Kroman N, Holtveg H, Wohlfahrt J, et al. Effect of breast-conserving therapy versus radical mastectomy on prognosis for young women with breast carcinoma. Cancer. 2004;100:688–693. doi: 10.1002/cncr.20022. [DOI] [PubMed] [Google Scholar]
- 11.Kurtz JM, Jacquemier J, Amalric R, et al. Why are local recurrences after breast-conserving therapy more frequent in younger patients. J Clin Oncol. 1990;8:591–598. doi: 10.1200/JCO.1990.8.4.591. [DOI] [PubMed] [Google Scholar]
- 12.Anderson SJ, Wapnir I, Dignam JJ, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five National Surgical Adjuvant Breast and Bowel Project protocols of node-negative breast cancer. J Clin Oncol. 2009;27:2466–2473. doi: 10.1200/JCO.2008.19.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stotter AT, McNeese MD, Ames FC, et al. Predicting the rate and the extent of locoregional failure after breast conservation therapy for early breast cancer. Cancer. 1989;64:2217–2225. doi: 10.1002/1097-0142(19891201)64:11<2217::aid-cncr2820641106>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 14.Mate TP, Carter D, Fischer DB, et al. A clinical and histopathologic analysis of the results of conservation surgery and radiation therapy in stage I and II breast carcinoma. Cancer. 1986;58:1995–2002. doi: 10.1002/1097-0142(19861101)58:9<1995::aid-cncr2820580907>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 15.Ries LAG, Melbert D, Krapcho M, et al., editors. Surveillance, Epidemiology, and End Results Cancer Statistics Review, 1975-2005. Bethesda, MD: National Cancer Institute; 2008. [Google Scholar]
- 16.American Cancer Society. Cancer Facts and Figures 2010. Atlanta, GA: American Cancer Society; 2010. [Google Scholar]
- 17.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 18.Jacobson JA, Danforth DN, Cowan KH, et al. Ten-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancer. N Engl J Med. 1995;332:907–911. doi: 10.1056/NEJM199504063321402. [DOI] [PubMed] [Google Scholar]
- 19.Blichert-Toft M, Rose C, Andersen JA, et al. Danish randomized trial comparing breast conservation therapy with mastectomy: Six years of life-table analysis—Danish Breast Cancer Cooperative Group. J Natl Cancer Inst Monogr. 1992;11:19–25. [PubMed] [Google Scholar]
- 20.van Dongen JA, Voogd AC, Fentiman IS, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000;92:1143–1150. doi: 10.1093/jnci/92.14.1143. [DOI] [PubMed] [Google Scholar]
- 21.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomized trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 22.Boyages J, Recht A, Connolly JL, et al. Early breast cancer: Predictors of breast recurrence for patients treated with conservative surgery and radiation therapy. Radiother Oncol. 1990;19:29–41. doi: 10.1016/0167-8140(90)90163-q. [DOI] [PubMed] [Google Scholar]
- 23.Bollet MA, Sigal-Zafrani B, Mazeau V, et al. Age remains the first prognostic factor for loco-regional breast cancer recurrence in young (< 40 years) women treated with breast conserving surgery first. Radiother Oncol. 2007;82:272–280. doi: 10.1016/j.radonc.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Vrieling C, Collette L, Fourquet A, et al. Can patient-, treatment- and pathology-related characteristics explain the high local recurrence rate following breast-conserving therapy in young patients. Eur J Cancer. 2003;39:932–944. doi: 10.1016/s0959-8049(03)00123-0. [DOI] [PubMed] [Google Scholar]
- 25.Coulombe G, Tyldesley S, Speers C, et al. Is mastectomy superior to breast-conserving treatment for young women. Int J Radiat Oncol Biol Phys. 2007;67:1282–1290. doi: 10.1016/j.ijrobp.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 26.McCormick B. Selection criteria for breast conservation: The impact of young and old age and collagen vascular disease. Cancer. 1994;74:430–435. doi: 10.1002/cncr.2820741331. [DOI] [PubMed] [Google Scholar]
- 27.White JR, Halberg FE, Rabinovitch R, et al. American College of Radiology appropriateness criteria on conservative surgery and radiation: Stages I and II breast carcinoma. J Am Coll Radiol. 2008;5:701–713. doi: 10.1016/j.jacr.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 28.Jobsen JJ, van der Palen J, Ong F, et al. The value of a positive margin for invasive carcinoma in breast-conservative treatment in relation to local recurrence is limited to young women only. Int J Radiat Oncol Biol Phys. 2003;57:724–731. doi: 10.1016/s0360-3016(03)00644-8. [DOI] [PubMed] [Google Scholar]
- 29.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 30.Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oh DS, Troester MA, Usary J, et al. Estrogen-regulated genes predict survival in hormone receptor-positive breast cancers. J Clin Oncol. 2006;24:1656–1664. doi: 10.1200/JCO.2005.03.2755. [DOI] [PubMed] [Google Scholar]
- 32.Rouzier R, Perou CM, Symmans WF, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005;11:5678–5685. doi: 10.1158/1078-0432.CCR-04-2421. [DOI] [PubMed] [Google Scholar]
- 33.Tang P, Skinner KA, Hicks DG. Molecular classification of breast carcinomas by immunohistochemical analysis: Are we ready. Diagn Mol Pathol. 2009;18:125–132. doi: 10.1097/PDM.0b013e31818d107b. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen PL, Taghian AG, Katz MS, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast conserving therapy. J Clin Oncol. 2008;26:2373–2378. doi: 10.1200/JCO.2007.14.4287. [DOI] [PubMed] [Google Scholar]
- 35.Anders CK, Hsu DS, Broadwater G, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26:3324–3330. doi: 10.1200/JCO.2007.14.2471. [DOI] [PubMed] [Google Scholar]
- 36.Cheang MC, Chia SK, Voduc D, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736–750. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trihia H, Murray S, Price K, et al. Ki-67 expression in breast carcinoma: Its association with grading systems, clinical parameters, and other prognostic factors—A surrogate marker. Cancer. 2003;97:1321–1331. doi: 10.1002/cncr.11188. [DOI] [PubMed] [Google Scholar]
- 38.Spyratos F, Ferrero-Poüs M, Trassard M, et al. Correlation between MIB-1 and other proliferation markers: Clinical implications of the MIB-1 cutoff value. Cancer. 2002;94:2151–2159. doi: 10.1002/cncr.10458. [DOI] [PubMed] [Google Scholar]
- 39.Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes: Dealing with the diversity of breast cancer—Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER-2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 41.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER-2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 42.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 43.Cabioglu N, Hunt KK, Buchholz TA, et al. Improving local control with breast-conserving therapy: A 27-year single-institution experience. Cancer. 2005;104:20–29. doi: 10.1002/cncr.21121. [DOI] [PubMed] [Google Scholar]
- 44.Pass H, Vicini FA, Kestin LL, et al. Changes in management techniques and patterns of disease recurrence over time in patients with breast carcinoma treated with breast-conserving therapy at a single institution. Cancer. 2004;101:713–720. doi: 10.1002/cncr.20410. [DOI] [PubMed] [Google Scholar]
- 45.Fisher B, Redmond C, Poisson R, et al. Eight-year results of a randomized clinical trial comparing total mastectomy and lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med. 1989;320:822–828. doi: 10.1056/NEJM198903303201302. [DOI] [PubMed] [Google Scholar]
- 46.Fisher B, Redmond C, Dimitrov NV, et al. A randomized clinical trial evaluating sequential methotrexate and fluorouracil in the treatment of patients with node-negative breast cancer who have estrogen-receptor-negative tumors. N Engl J Med. 1989;320:473–478. doi: 10.1056/NEJM198902233200801. [DOI] [PubMed] [Google Scholar]
- 47.Buchholz TA, Tucker SL, Erwin J, et al. Impact of systemic treatment on local control for patients with lymph node-negative breast cancer treated with breast-conservation therapy. J Clin Oncol. 2001;19:2240–2246. doi: 10.1200/JCO.2001.19.8.2240. [DOI] [PubMed] [Google Scholar]
- 48.Fisher B, Dignam J, Bryant J, et al. Five versus more than five years of tamoxifen for lymph node-negative breast cancer: Updated findings from the National Surgical Adjuvant Breast and Bowel Project B-14 randomized trial. J Natl Cancer Inst. 2001;93:684–690. doi: 10.1093/jnci/93.9.684. [DOI] [PubMed] [Google Scholar]
- 49.Fisher B, Bryant J, Dignam JJ, et al. Tamoxifen, radiation therapy, or both for prevention of ipsilateral breast tumor recurrence after lumpectomy in women with invasive breast cancers of one centimeter or less. J Clin Oncol. 2002;20:4141–4149. doi: 10.1200/JCO.2002.11.101. [DOI] [PubMed] [Google Scholar]
- 50.Cancello G, Maisonneuve P, Rotmensz N, et al. Prognosis and adjuvant treatment effects in selected breast cancer subtypes of very young women (< 35 years) with operable breast cancer. Ann Oncol. 2010;21:1974–1981. doi: 10.1093/annonc/mdq072. [DOI] [PubMed] [Google Scholar]
- 51.Voduc KD, Cheang MC, Tyldesley S, et al. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28:1684–1691. doi: 10.1200/JCO.2009.24.9284. [DOI] [PubMed] [Google Scholar]
- 52.Leong C, Boyages J, Jayasinghe UW, et al. Effect of margins on ipsilateral breast tumor recurrence after breast conservation therapy for lymph node-negative breast carcinoma. Cancer. 2004;100:1823–1832. doi: 10.1002/cncr.20153. [DOI] [PubMed] [Google Scholar]
- 53.Recht A, Connolly JL, Schnitt SJ, et al. The effect of young age on tumor recurrence in the treated breast after conservative surgery and radiotherapy. Int J Radiat Oncol Biol Phys. 1988;14:3–10. doi: 10.1016/0360-3016(88)90043-0. [DOI] [PubMed] [Google Scholar]
- 54.Solin LJ, Fowble B, Schultz DJ, et al. Age as a prognostic factor for patients treated with definitive irradiation for early stage breast cancer. Int J Radiat Oncol Biol Phys. 1989;16:373–381. doi: 10.1016/0360-3016(89)90333-7. [DOI] [PubMed] [Google Scholar]
- 55.van Dongen JA, Bartelink H, Fentiman IS, et al. Factors influencing local relapse and survival and results of salvage treatment after breast-conserving therapy in operable breast cancer: EORTC trial 10801, breast conversation compared with mastectomy in TNM stage I and II breast cancer. Eur J Cancer. 1992;28A:801–805. doi: 10.1016/0959-8049(92)90118-l. [DOI] [PubMed] [Google Scholar]
- 56.Clarke DH, Lê MG, Sarrazin D, et al. Analysis of local-regional relapses in patients with early breast cancers treated by excision and radiotherapy: Experience of the Institut Gustave-Roussy. Int J Radiat Oncol Biol Phys. 1985;11:137–145. doi: 10.1016/0360-3016(85)90372-4. [DOI] [PubMed] [Google Scholar]
- 57.Fowble BL, Schultz DJ, Overmoyer B, et al. The influence of young age on outcome in early stage breast cancer. Int J Radiat Oncol Biol Phys. 1994;30:23–33. doi: 10.1016/0360-3016(94)90515-0. [DOI] [PubMed] [Google Scholar]
- 58.Klauber-DeMore N. Tumor biology of breast cancer in young women. Breast Dis. 2005-2006;23:9–15. doi: 10.3233/bd-2006-23103. [DOI] [PubMed] [Google Scholar]
- 59.Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anders CK, Fan C, Parker JS, et al. Breast carcinomas arising at a young age: Unique biology or a surrogate for aggressive intrinsic subtypes. J Clin Oncol. 2011;29:e18–e20. doi: 10.1200/JCO.2010.28.9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 62.Cheng CW, Das IJ, Tang W, et al. Dosimetric comparison of treatment planning systems in irradiation of breast with tangential fields. Int J Radiat Oncol Biol Phys. 1997;38:835–842. doi: 10.1016/s0360-3016(97)00078-3. [DOI] [PubMed] [Google Scholar]
- 63.Delaney G, Beckham W, Veness M. Three-dimensional dose distribution of tangential breast irradiation: Results of a multicentre phantom dosimetry study. Radiother Oncol. 2000;57:61–68. doi: 10.1016/s0167-8140(00)00262-0. [DOI] [PubMed] [Google Scholar]