Abstract
Purpose
Cirrhosis and hepatocellular carcinoma (HCC) together form a two-disease state that affects survival of patients with HCC and dictates treatment decisions and prognostic stratification of patients in clinical trials. The study objective was to improve prognostic stratification of patients with HCC.
Patients and Methods
We prospectively collected plasma samples and baseline clinicopathologic features from 288 new patients with HCC, and plasma insulin-like growth factor-1 (IGF-1) and vascular endothelial growth factor (VEGF) levels were tested. We applied Cox regression and log-rank tests to assess association of IGF-1 and VEGF with overall survival (OS), Kaplan-Meier curves to estimate OS, and recursive partitioning to determine optimal cutoff points for IGF-1 and VEGF. Prognostic ability of conventional and molecular Barcelona Clinic Liver Cancer classifications was compared using the c-index.
Results
Lower plasma IGF-1 and higher plasma VEGF levels significantly correlated with advanced clinicopathologic parameters and poor OS, with optimal cut points of 26 ng/mL and 450 pg/mL, respectively. The combination of low IGF-1 and high VEGF predicted median OS of 2.7 months compared with 19 months for patients with high IGF-1 and low VEGF (P < .001), further refining the prognostic ability of conventional HCC staging (P < .001).
Conclusion
Baseline levels of plasma IGF-1 and VEGF correlated significantly with survival in patients with HCC. Integrating IGF-1 and VEGF into HCC staging significantly enhanced prognostic stratification of patients. If validated, these results may prove to be useful in designing strategies to personalize management approaches among these patients.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the sixth most common malignancy in the world and third most common cause of cancer mortality.1 In the United States, incidence of HCC has approximately doubled in the past three decades.2,3 HCC prognosis has remained poor, mainly because of: one, advanced tumor stage, accompanied by chronic liver disease (CLD) at diagnosis, which precludes curative treatment options, and two, lack of a universal HCC prognostic staging system. The key roles of prognostic HCC staging are to accurately predict patient survival, guide therapy decisions, and stratify patients in clinical trials. Therefore, development of better HCC prognostic stratification systems governing therapy decisions is critically needed to improve outcome in patients with HCC. Several classification systems for HCC have been developed based on multiple prognostic factors related to tumor stage and CLD status parameters.4–9 However, there is a noted heterogeneity among patients within the same HCC stage in all HCC staging systems, especially nonsurgical patients who are the focus of the clinical trials. Therefore, molecular approaches to stratifying patients with HCC, through integration of biomarkers into staging systems parameters, are expected to better predict patient survival and refine their prognostic stratification.10
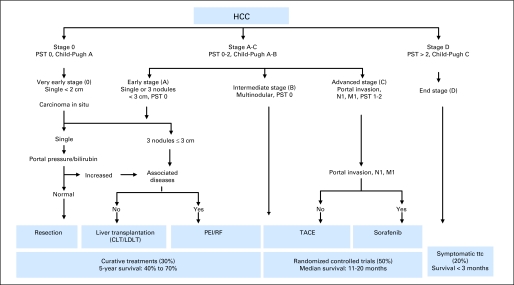
The Barcelona Clinic Liver Cancer (BCLC) staging system7and Cancer of the Liver Italian Program (CLIP) score6 are among the most commonly used HCC prognostic systems to guide therapy decisions and stratify patients in HCC clinical trials. Recent reports have indicated better prognostic ability of CLIP score compared with BCLC staging.11,12 However, the systems are conceptually different. Furthermore, BCLC staging is endorsed by the American Association for the Study of Liver Diseases and European Association for the Study of Liver Diseases clinical practice guidelines13–15 and is commonly used to guide therapy decisions in clinical practice (Fig 1). Thus, patients with unresectable HCC classified under BCLC stage C have emerged as the standard patient population to be included in HCC systemic therapy trials.15 However, there is a significant degree of heterogeneity within this group. Moreover, the Child-Pugh system—the only tool for assessing underlying liver condition under BCLC—is itself relatively quantitative and uses five empirically selected variables, including hepatic encephalopathy and ascites, which are clinically difficult to grade and may vary in severity according to medical management of patients.16
Fig 1.
Barcelona Clinic Liver Cancer staging. CLT, cadaveric liver transplantation; HCC, hepatocellular carcinoma; LDLT, living-donor liver transplantation; PEI, percutaneous ethanol injection; PST, performance status; RF, radio-frequency ablation; TACE, trans-arterial chemoembolization; ttc, treatment.
Circulating levels of insulin-like growth factor-1 (IGF-1) decrease sharply in patients with CLDs such as steatosis, chronic hepatitis C, cirrhosis, nonalcoholic steatohepatitis, and HCC,17–24 because the liver is responsible for synthesis of most of the circulating levels of IGF-1.25,26 Furthermore, HCC is a highly vascular tumor, and angiogenesis, mediated through VEGF, is thought to play a major role in development, progression, and prognosis of this cancer.27–30 Our most recent studies introduced the V-CLIP and I-CLIP scores,31,32 an integration of plasma VEGF and IGF-1 into CLIP score parameters, and showed significant improvement in prediction of survival and patient stratification.
Collectively, these data suggest that circulating levels of IGF-1 and VEGF may reflect the synthetic function of the liver and the aggressiveness of HCC tumors, respectively, and hence correlate with survival of patients with HCC and improve their prognostic stratification. This could potentially lead to more accurate classification under the BCLC system and may ultimately change treatment decisions. Therefore, our central hypothesis was that the combination of baseline plasma levels of IGF-1 and VEGF would correlate with clinicopathologic features and survival of patients with HCC and hence refine prognostic stratification of patients when added to BCLC staging parameters.
PATIENTS AND METHODS
Patients
We prospectively enrolled patients, collected their blood samples and clinical data, and retrospectively analyzed samples for plasma biomarkers. The current study is part of an ongoing HCC case-control study at The University of Texas MD Anderson Cancer Center, under an independent specific aim to study biomarkers correlating with survival and their role in refining HCC prognostic stratification. We obtained approval of the institutional review board of MDACC for this study and informed consent of patients. The study inclusion criteria were pathologically confirmed HCC and US residency. The exclusion criterion was concurrent presence of another primary liver cancer (such as fibrolamellar HCC or cholangiocarcinoma) or other types of cancers.
Baseline Plasma IGF-1 and VEGF Assay
Peripheral venous blood samples (3 to 5 mL of whole blood) were collected, anticoagulated by ethylenediaminetetraacetic acid and centrifuged at 4°C for 15 minutes (3,000 rpm). Plasma samples were removed, aliquoted, and snap frozen at −20°C until used. IGF-1 and VEGF levels were tested by enzyme-linked immunosorbent assay (ELISA) (Quantikine Human IGF-1 and VEGF ELISA Kits; R&D Systems, Minneapolis, MN). IGF-1 and VEGF were determined from a standard curve generated for each set of samples assayed, after duplicate measurements were made.
Statistical Analysis
To study the correlation between baseline plasma IGF-1 and VEGF levels and various clinical characteristics and staging systems, we used Wilcoxon rank sum tests. We used a univariate Cox regression model to assess factors associated with overall survival (OS). To identify optimal IGF-1 and VEGF cutoff points, we split the data randomly into two sets: a training set (containing two thirds of data) and validation (test) set (one third of data). We applied recursive partitioning to the training set to find the optimal cutoff point maximizing the difference in OS between the groups with low and high levels. We then validated that cutoff point by fitting a Cox regression model to the test data with IGF-1 and VEGF dichotomized at optimal cutoff points. We repeated this methodology using different random training/validation splits.
We next applied the log-rank test and Kaplan-Meier analyses to multivariate Cox regression models including IGF-1 and VEGF, dichotomized at the optimal cutoff point for each, as well as the variables in the BCLC system, to evaluate whether IGF-1 and VEGF were independent prognostic factors after adjusting for other factors. Finally, we computed median OS for patients in each BCLC, I-BCLC, V-BCLC, and IV-BCLC group and compared the groups using log-rank tests to assess relative performance of the four systems. The sign test was used to assess whether groups with low IGF-1 tended to have shorter median OS than those with high IGF-1 and whether groups with high VEGF had shorter median OS than those with low VEGF within the BCLC, I-BCLC, V-BCLC, and IV-BCLC systems. We used a c-index test to compare prognostic ability of the four systems, reflecting stratification capability of each system.
RESULTS
Patient Characteristics
From January 2000 until March 2008, we enrolled 394 eligible patients; baseline plasma samples were available for 288 (73%); remaining patients did not return for blood draw. Patient characteristics are listed in Table 1. Notably, no significant differences were found between enrolled patients with and without plasma samples in demographics or clinical features. However, patients without plasma samples had a tendency to have smaller tumors (involving < 50% of liver), multinodular tumors, higher baseline α-fetoprotein (AFP) levels, and portal vein thrombosis (data not shown). For all patients, estimated median OS was 13.6 months (95% CI, 11.7 to 17.7; Kaplan-Meier curve shown in Figure 2A). Approximately two thirds of the patients (189; 66%) were classified under BCLC stage C (advanced). Using univariate Cox regression models, we found that the following were all significant predictors of poor survival in HCC: poor tumor differentiation, multinodularity, vascular invasion, distant metastasis, high serum AFP level, ALT, AST, bilirubin, and cirrhosis (Appendix Table A1, online only).
Table 1.
Patient Demographics and Clinical Characteristics
| Characteristic | Patients(n = 288) |
95% CI | |
|---|---|---|---|
| No. | % | ||
| Age, years | |||
| < 60 | 111 | 38.5 | 32.9 to 44.4 |
| ≥ 60 | 177 | 61.5 | 55.6 to 67.1 |
| Sex | |||
| Female | 89 | 30.9 | 25.6 to 36.5 |
| Male | 199 | 69.1 | 63.4 to 74.4 |
| Race | |||
| White | 199 | 69.1 | 63.4 to 74.4 |
| African American | 29 | 10.1 | 6.8 to 14.1 |
| Hispanic | 37 | 12.8 | 9.2 to 17.3 |
| Asian | 23 | 8 | 5.1 to 11.7 |
| Educational level | |||
| ≤ High school | 127 | 44.1 | 38.3 to 50 |
| Some college | 66 | 22.9 | 18.2 to 28.2 |
| College degree | 95 | 33 | 27.6 to 38.7 |
| Hepatitis infection status | |||
| HBV | 38 | 13.2 | 9.5 to 17.7 |
| HCV | 60 | 20.8 | 16.3 to 25.9 |
| HBV and HCV | 27 | 9.4 | 6.3 to 13.3 |
| None | 163 | 56.6 | 50.7 to 62.4 |
| Alcohol consumption, mL ethanol/d | |||
| None | 93 | 32.3 | 26.9 to 38.0 |
| ≤ 60 | 131 | 45.5 | 39.6 to 51.4 |
| > 60 | 64 | 22.2 | 17.6 to 27.5 |
| Cigarette smoking, packs per year | |||
| None | 91 | 31.6 | 26.2 to 37.3 |
| ≤ 20 | 76 | 26.4 | 21.4 to 31.9 |
| > 20 | 121 | 42 | 36.2 to 47.9 |
| First-degree history of liver cancer | |||
| No | 274 | 95.1 | 91.9 to 97.3 |
| Yes | 14 | 4.9 | 2.7 to 8 |
| History of diabetes, years before HCC diagnosis | |||
| None | 191 | 66.3 | 60.5 to 71.8 |
| ≤ 1 | 13 | 4.5 | 2.4 to 7.6 |
| > 1 | 84 | 29.2 | 23.9 to 34.8 |
| Serum AFP level, ng/mL | |||
| < 400 | 199 | 69.1 | 63.4 to 74.4 |
| ≥ 400 | 86 | 29.9 | 24.6 to 35.5 |
| Missing | 3 | 1 | 0.2 to 3.3 |
| Tumor differentiation | |||
| Well | 112 | 38.9 | 33.2 to 44.8 |
| Moderate | 95 | 33 | 27.6 to 38.7 |
| Poor | 50 | 17.4 | 13.2 to 22.2 |
| Unknown | 31 | 10.8 | 7.4 to 14.9 |
| Tumor size, % of liver | |||
| ≤ 50 | 191 | 66.3 | 60.5 to 71.8 |
| > 50 | 97 | 33.7 | 28.2 to 39.5 |
| Vascular invasion | |||
| Yes | 53 | 18.4 | 14.1 to 23.4 |
| No | 235 | 81.6 | 76.6 to 85.9 |
| Distant metastasis | |||
| Yes | 60 | 20.8 | 16.3 to 25.9 |
| No | 228 | 79.2 | 74 to 83.7 |
| Tumor nodularity | |||
| Uninodular | 105 | 36.5 | 30.9 to 42.3 |
| Multinodular | 183 | 63.5 | 57.7 to 69.1 |
| Lymph node involvement | |||
| Yes | 122 | 42.4 | 36.6 to 48.3 |
| No | 166 | 57.6 | 51.7 to 63.4 |
| Cirrhosis | |||
| Yes | 173 | 60.1 | 54.2 to 65.8 |
| No | 115 | 39.9 | 34.2 to 45.8 |
| Child-Pugh class | |||
| A | 206 | 71.5 | 65.9 to 76.7 |
| B | 76 | 26.4 | 21.4 to 31.9 |
| C | 6 | 2.1 | 0.7 to 4.4 |
| TNM stage | |||
| I | 45 | 15.6 | 11.6 to 20.3 |
| II | 32 | 11.1 | 7.7 to 15.3 |
| III | 157 | 54.5 | 48.6 to 60.4 |
| IV | 54 | 18.8 | 14.4 to 23.7 |
| Treatment exposure | |||
| None | 39 | 13.5 | 9.8 to 18 |
| Chemotherapy alone | 97 | 33.7 | 28.2 to 39.5 |
| Chemotherapy and others | 34 | 11.8 | 8.3 to 16.1 |
| Surgery alone | 35 | 12.2 | 8.6 to 16.5 |
| Surgery and others | 54 | 18.7 | 14.4 to 23.7 |
| Local therapies | 29 | 10.1 | 6.8 to 14.1 |
Abbreviations: AFP, α-fetoprotein; HBV, hepatitis B virus; HCV, hepatitis C virus.
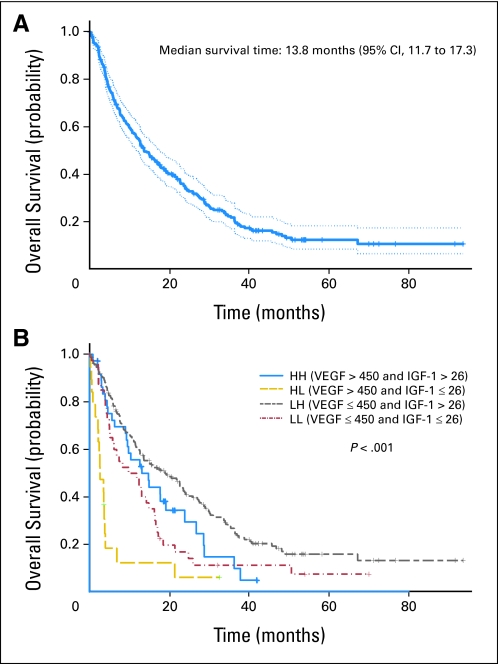
Fig 2.
Kaplan-Meier estimates of overall survival in (A) all patients (n = 288; reprinted with permission31) and (B) patients split by combination of insulin-like growth factor-1 (IGF-1) and vascular endothelial growth factor (VEGF) levels. HH, high VEGF, high IGF-1; HL, high VEGF, low IGF-1; LH, low VEGF, high IGF-1; LL, low VEGF, low IGF-1.
Baseline Plasma VEGF and IGF-1 Levels As Independent Prognostic Factors
As shown in Appendix Table A1 (online only), hazard ratios (HRs) and 95% CIs estimated from Cox regression models indicated that plasma IGF-1 (HR, 2.06; 95% CI, 1.5 to 2.81) and VEGF (HR, 1.74; 95% CI, 1.26 to 2.43) were strongly associated with OS (P < .001 for both). Table 2 describes the correlations between plasma IGF-1 and VEGF levels and patient characteristics by the Wilcoxon rank sum test. IGF-1 level was most significantly associated with Child-Pugh score, bilirubin, AST levels, tumor size and nodularity, and vascular invasion; however, the strongest association was with AST level (P < .001). VEGF level was significantly associated with tumor size, lymph node involvement, and distant metastasis; however, the strongest association was with tumor size (P < .001).
Table 2.
Correlations Between Plasma VEGF and IGF-1 Levels and Patient Characteristics by Wilcoxon Rank-Sum Test
| Characteristic | Patients (n = 288) |
Plasma VEGF Level(pg/mL) |
P | Plasma IGF-1 Level (ng/mL) |
P | |||
|---|---|---|---|---|---|---|---|---|
| No. | % | Mean | SE | Mean | SE | |||
| Age, years | .69 | .07 | ||||||
| < 60 | 111 | 38.5 | 284.77 | 390.29 | 59.37 | 45.35 | ||
| ≥ 60 | 177 | 61.5 | 290.44 | 399.55 | 50.95 | 35.57 | ||
| Race | .82 | .84 | ||||||
| Nonwhite | 89 | 30.9 | 294.34 | 355.23 | 53.51 | 42.82 | ||
| White | 199 | 69.1 | 285.53 | 412.83 | 49.55 | 35.42 | ||
| Sex | .31 | .14 | ||||||
| Female | 89 | 30.9 | 303.54 | 426.43 | 59.16 | 42.87 | ||
| Male | 199 | 69.1 | 281.42 | 381.53 | 51.97 | 35.17 | ||
| Hepatitis infection status | .27 | .047 | ||||||
| HBV | 38 | 13.2 | 375.54 | 478.99 | 56.07 | 49.16 | ||
| HCV | 60 | 20.8 | 284.21 | 341.33 | 52.33 | 32.27 | ||
| HBV + HCV | 27 | 9.4 | 220.94 | 318.27 | 35.77 | 22.24 | ||
| None | 163 | 56.6 | 280.54 | 403.96 | 57.50 | 38.07 | ||
| Serum AFP level, ng/mL | .15 | .08 | ||||||
| < 400 | 199 | 69.1 | 270.31 | 411.94 | 56.46 | 36.03 | ||
| ≥ 400 | 86 | 29.9 | 333.39 | 358.85 | 49.62 | 41.84 | ||
| Unknown | 3 | 1.0 | 184.19 | 138.13 | ||||
| Tumor differentiation | .19 | .63 | ||||||
| Well | 112 | 38.9 | 290.83 | 477.27 | 55.23 | 36.47 | ||
| Moderate | 95 | 33.0 | 280.92 | 336.95 | 56.63 | 38.19 | ||
| Poor | 50 | 17.4 | 268.85 | 355.72 | 48.29 | 38.48 | ||
| Unknown | 31 | 10.8 | 332.67 | 295.05 | 52.51 | 40.83 | ||
| Tumor nodularity | .25 | .002 | ||||||
| Uninodular | 105 | 36.5 | 261.32 | 433.68 | 63.25 | 42.01 | ||
| Multinodular | 183 | 63.5 | 303.71 | 371.91 | 49.00 | 34.19 | ||
| Tumor size, % of liver | < .001 | < .001 | ||||||
| ≤ 50 | 191 | 66.3 | 218.60 | 288.27 | 60.11 | 41.33 | ||
| > 50 | 97 | 33.7 | 425.41 | 523.55 | 42.54 | 26.10 | ||
| Vascular invasion | .94 | .016 | ||||||
| No | 235 | 81.6 | 287.43 | 397.77 | 56.73 | 39.40 | ||
| Yes | 53 | 18.4 | 291.88 | 388.02 | 42.94 | 27.19 | ||
| Lymph node involvement | .04 | .83 | ||||||
| No | 166 | 57.6 | 277.76 | 422.76 | 53.79 | 33.58 | ||
| Yes | 122 | 42.4 | 302.53 | 355.84 | 54.75 | 49.19 | ||
| Distant metastasis | .01 | .85 | ||||||
| No | 228 | 79.2 | 273.06 | 399.39 | 54.40 | 38.09 | ||
| Yes | 60 | 20.8 | 345.96 | 377.16 | 53.39 | 36.93 | ||
| Bilirubin level, mg/dL | .54 | < .001 | ||||||
| ≤ 1.6 | 260 | 90.3 | 290.02 | 408.00 | 56.84 | 38.03 | ||
| > 1.6 | 28 | 9.7 | 271.87 | 253.22 | 29.63 | 24.73 | ||
| Child-Pugh class | .05 | .0021 | ||||||
| A | 206 | 71.5 | 288.35 | 399.99 | 59.05 | 38.62 | ||
| B | 76 | 26.4 | 269.45 | 388.11 | 42.49 | 32.60 | ||
| C | 6 | 2.1 | 523.16 | 282.89 | 35.77 | 37.38 | ||
| ALT level, U/L | .27 | .02 | ||||||
| ≤ 40 | 134 | 46.5 | 284.62 | 359.07 | 59.71 | 43.40 | ||
| > 40 | 153 | 53.1 | 286.13 | 421.82 | 49.39 | 31.59 | ||
| Unknown | 1 | 0.4 | 1,099.61 | NA | ||||
| AST level, U/L | .16 | < .001 | ||||||
| ≤ 45 | 88 | 30.6 | 288.88 | 479.80 | 68.54 | 42.18 | ||
| > 45 | 179 | 62.2 | 276.28 | 346.12 | 47.26 | 33.68 | ||
| Unknown | 21 | 7.3 | 387.63 | 404.26 | 53.20 | 36.31 | ||
Identifying Optimal Cut Points of Plasma IGF-1 and VEGF
The recursive partitioning test identified optimal cut points for IGF-1 and VEGF as 26 ng/mL and 450 pg/mL, respectively. The combination of low IGF-1 and high VEGF predicted median OS of 2.7 months compared with 19 months for patients with high IGF-1 and low VEGF (P < .001; Kaplan-Meier estimates shown in Fig 2B; log-rank test on OS for combination of VEGF and IGF-1 levels listed in Table 3). When high VEGF and low IGF-1 were considered in a univariate Cox regression model fit to the entire data set, this effect was highly significant for both VEGF (P < .001; HR, 1.89; 95% CI, 1.36 to 2.65) and IGF-1 (P < .001; HR, 2.06; 95% CI, 1.50 to 2.81).
Table 3.
Log-Rank Test on OS for Combination of VEGF and IGF-1 Levels
| Combination | Patients (No.) | Deaths (No.) | Median OS (months) | 95% CI | P |
|---|---|---|---|---|---|
| Low/high (VEGF ≤ 450 pg/mL; IGF-1 > 26 ng/mL) | 191 | 135 | 19.00 | 13.64 to 23.90 | < .001 |
| High/high (VEGF > 450 pg/mL; IGF-1 > 26 ng/mL) | 37 | 29 | 13.22 | 9.60 to 26.79 | < .001 |
| Low/low (VEGF ≤ 450 pg/mL; IGF-1 ≤ 26 ng/mL) | 41 | 36 | 9.83 | 5.85 to 16.47 | < .001 |
| High/low (VEGF > 450 pg/mL; IGF-1 ≤ 26 ng/mL) | 19 | 17 | 2.70 | 2.27 to 4.04 | < .001 |
Abbreviations: IGF-1, insulin-like growth factor-1; OS, overall survival; VEGF, vascular endothelial growth factor.
Validation of BCLC Staging System and Construction of New Molecular Staging Systems by Integrating Plasma IGF-1 and VEGF Levels
We applied the BCLC system to our patient population, computed median OS duration for patients in each group, and compared groups using a log-rank test (Appendix Table A2, online only). Given the correlation that we found of low IGF-1 and high VEGF with shorter OS and with worse liver and tumor parameters, we predicted that integrating these plasma biomarkers into parameters of the BCLC system would improve patient stratification and enhance its prognostic ability. To test this, we divided patients within each BCLC stage according to whether they had low or high IGF-1 (Appendix Table A3, online only), low or high VEGF (Appendix Table A4, online only), or the combination of different IGF-1 and VEGF levels (Table 4). At each of the five BCLC stages, estimated median OS was shorter for patients with low IGF-1 and/or high VEGF than for those with high IGF-1 and/or low VEGF. Additionally, when we applied multivariate Cox regression tests of BCLC parameters after integrating VEGF, IGF-1, and both (Appendix Tables A5 to A7, online only), we found that IGF-1 was an independent strong predictor of survival using these models (P < .001; HR, 2.19; 95% CI, 1.6 to 3.0), whereas VEGF had a trend only (P = .16; HR, 1.28; 95% CI, 0.91 to 1.82).
Table 4.
Log-Rank Test on OS for BCLC Stages Split by VEGF and IGF-1 Combination
| BCLC Stage | Patients (No.) | Events (No.) | Median OS (months) | 95% CI | P |
|---|---|---|---|---|---|
| 0 | .8015 | ||||
| All patients | 21 | 7 | 49.08 | 33.99 to NA | |
| VEGF-IGF-1 | |||||
| LH | 17 | 6 | 49.08 | 33.99 to NA | |
| LL | 4 | 1 | NA | 6.84 to NA | |
| A | .8592 | ||||
| All patients | 28 | 12 | 40.67 | 27.09 to NA | |
| VEGF-IGF-1 | |||||
| HH | 3 | 1 | NA | 9.60 to NA | |
| HL | 1 | 0 | NA | NA to NA | |
| LH | 21 | 9 | 48.2 | 33.70 to NA | |
| LL | 3 | 2 | 18.51 | 12.95 to NA | |
| B | .1586 | ||||
| All patients | 29 | 23 | 22.68 | 16.14 to 28.64 | |
| VEGF-IGF-1 | |||||
| HH | 5 | 5 | 13.22 | 12.69 to NA | |
| LH | 18 | 12 | 27.39 | 19.00 to NA | |
| LL | 6 | 6 | 16.88 | 14.89 to NA | |
| C | < .001 | ||||
| All patients | 189 | 156 | 11.15 | 8.58 to 13.61 | |
| VEGF-IGF-1 | |||||
| HH | 25 | 20 | 14.83 | 9.11 to 36.3 | |
| HL | 12 | 11 | 3.55 | 2.50 to NA | |
| LH | 127 | 101 | 12.43 | 10.13 to 18.97 | |
| LL | 25 | 24 | 6.18 | 4.47 to 14.27 | |
| D | .0495 | ||||
| All patients | 21 | 19 | 2.93 | 2.14 to 9.14 | |
| VEGF-IGF-1 | |||||
| HH | 4 | 3 | 13.68 | 2.14 to NA | |
| HL | 6 | 6 | 1.86 | 0.62 to NA | |
| LH | 8 | 7 | 6.44 | 1.25 to NA | |
| LL | 3 | 3 | 2.93 | 0.53 to NA |
Abbreviations: BCLC, Barcelona Clinic Liver Cancer; HH, high/high (VEGF > 450 pg/mL; IGF-1 > 26 ng/mL); HL, high/low (VEGF > 450 pg/mL; IGF-1 ≤ 26 ng/mL); IGF-1, insulin-like growth factor-1; LH, low/high (VEGF ≤ 450 pg/mL; IGF-1 > 26 ng/mL); LL, low/low (VEGF ≤ 450 pg/mL; IGF-1 ≤ 26 ng/mL); NA, not applicable; OS, overall survival; VEGF, vascular endothelial growth factor.
IV-BCLC Seems to Provide More Accurate Prediction of OS and Better Stratification Than BCLC, I-BCLC, or V-BCLC Alone
The molecular systems were constructed as follows: IGF-1 score (0 if IGF-1 > 26 ng/mL; 1 if IGF-1 ≤ 26 ng/mL) and VEGF score (0 if VEGF ≤ 450 pg/mL; 1 if VEGF > 450 pg/mL). From a c-index analysis,32 we found that the IV-BCLC system was more accurate at predicting OS and provided better stratification than BCLC (P < .001), I-BCLC (P = .002), or V-BCLC (P < .001) alone. C-index for each staging system was as follows: BCLC, 0.65; V-BCLC, 0.66; I-BCLC, 0.68; and IV-BCLC, 0.68.
Notably, our analyses indicated that a majority of our patients with HCC were categorized under BCLC stage C (n = 189 [66% of all patients] median OS, 11.1 months; P < .001; Appendix Table A2, online only). We found significant differences in median OS by the log-rank test between BCLC stage C patients who were further stratified into four prognostic groups based on a combination of VEGF and IGF-1 levels, with estimated OS ranging between 14.8 and 3.5 months (P < .001; Table 4).
DISCUSSION
Notably, previous studies of plasma IGF-1 levels in patients with different types of cancers33–36 have suggested that high IGF-1 levels are associated with increased risk of cancer, secondary to activation of the downstream cascade of the IGF axis. However, the focus of our current study was to assess level of IGF-1 as an indicator of synthetic function of the liver, which reflects CLD status. Therefore, we did not assess other circulating factors related to the IGF axis, such as IGF-2 and the main IGF-1 binding protein plasma carrier, IGFBP-3. However, similar to IGF-1 data, IGFBP-3 level is also expected to be low in patients with CLD and HCC, because it is also predominantly produced by the liver. Furthermore, our results were consistent with prior reports of significantly decreased expression of IGF-1 in patients with CLD and HCC.17–24 The occurrence of trends that were not statistically significant in certain BCLC groups was not surprising, given the low power for detecting such differences because of the small number of patients with low IGF-1 and high VEGF within these BCLC stages. Interestingly, IGF-1 was found to be an independent predictor of survival in univariate and multivariate models (Appendix Tables A1, A5, and A7, online only), whereas VEGF was only significant in univariate models and showed only a trend in multivariate models (Appendix Tables A1, A6, and A7, online only), even in the largest BCLC group C (Appendix Table A4, online only). This may be the result of the presence of well-represented tumor parameters, involving number and size, within BCLC staging, although it lacks strong tools for assessment of CLD status. However, the combination of IGF-1 and VEGF further refined patient stratification, as shown in Table 4. Using a panel of noninvasive plasma biomarkers from the same sample would decrease the morbidity and mortality associated with liver sampling in the setting of cirrhosis and coagulopathy and reduce potential sampling errors and variability in assessing the degree of liver cirrhosis. Notably, although median OS of BCLC stage C patients was 11.1 months (P < .001; Appendix Table A2, online only), the integration of plasma IGF-1 and VEGF led to a significant improvement in prognostic stratification of BCLC stage C patients and separated them into four prognostic groups (Table 4). Patients with low VEGF and high IGF-1 had median OS of 12.4 months (95% CI, 10.1 to 18.9 months), and those with high VEGF and low IGF-1 had median OS of 3.5 months (P < .001; Table 4). After independent validation, this approach would lead to better prognostic stratification and more accurate interpretation of HCC systemic therapy clinical trials, because BCLC stage C patients are the standard population of these studies. Therefore, our results represent a promising step toward development of a personalized and simple prognostic stratification system for HCC.
Our study had some limitations. First, this was a single-institution study and therefore will need to be externally validated. Second, although 394 patients with HCC signed the consent form to participate in the study, baseline plasma samples were available for 288 patients only. The reason for the missed samples was mainly related to insufficient time to obtain blood samples during initial assessment in clinic. However, our analysis indicated that there were no significant differences between these two groups in terms of their epidemiologic data, and patients without blood samples tended to have more advanced tumor parameters and higher AFP levels. Therefore, even though our study population may have had better OS than patients without blood samples, our panel of biomarkers still showed statistically significant correlation with OS of our patients with HCC. Third, the study lacked a representative sampling of all BCLC stages, because a majority of our patients were categorized under BCLC stage C. However, improving prognostic stratification of patients with advanced HCC (BCLC stage C) is clinically relevant, because this patient population is the focus of HCC systemic therapy trials. Additionally, clearly a change in apparent BCLC stage from A or B to C, or from C to D, would have a substantial effect on disease management, probably leading to the selection of totally distinct treatment modalities or even symptom management (in case of stage D), whereas a change from stage 0 to stage A would have little effect, because curative treatments would be the likely option in either case. Thus, our observations could have a substantial effect on management of patients with HCC if validated. Therefore, our approach would benefit from independent validation in a more diverse patient population with HCC, not only to confirm the results but also to assess their utility in early BCLC stages. Finally, our biomarker cutoff points were based on the recursive partitioning test, which identified the best cutoff point that correlated with study patient population survival. Thus, IGF-1 and VEGF cutoff points may differ in other patient populations. However, in general, selecting circulating biomarker optimal cutoff points remains challenging because of potential daily variations, in addition to possible variations in patient genetics, sex, age, and other demographics. However, our results indicated independent prognostic information obtained from IGF-1 and VEGF cutoff points, which were consistent with previous studies and complementary to other clinically relevant prognostic indicators in our patients, including CLD status, tumor parameters, and BCLC variables. Furthermore, our results clearly showed no significant differences in mean values between patients based on age, sex, or ethnicity (Table 2). Additionally, although the commercial ELISA kits we used to measure biomarkers levels are standardized and reproducible, future independent biomarker studies in Clinical Laboratory Improvement Amendments–certified laboratories are needed before recommending their clinical use to guide management decisions.
In conclusion, our results suggest that plasma IGF-1 may serve as a new tool for assessing liver reserve in patients with HCC and that baseline plasma assessment of both IGF-1 and VEGF significantly improves prediction of OS and prognostic stratification of patients with HCC according to BCLC staging.
If the results of forthcoming large collaborative studies confirm our results, this simple noninvasive approach may prove beneficial in prognostic stratification of patients with HCC in clinical trials, guiding therapy decisions and ultimately improving HCC outcome. However, randomized biomarker trials are needed to determine whether this molecular staging strategy can improve HCC management compared with the conventional staging approach before wide acceptance by the scientific community.
Acknowledgment
We thank Karen Muller, PhD, for editing the manuscript and John Heymach, MD, and Lee Ellis, MD, for helpful comments on the study.
Appendix
Table A1.
Univariate Cox Proportional Hazards Regression Analysis
| Predictor | HR | 95% CI | P |
|---|---|---|---|
| Age (> 60 v ≤ 60 years) | 0.88 | 0.66 to 1.16 | .351 |
| Sex (male v female) | 1.44 | 1.06 to 1.96 | .018 |
| Race (white v nonwhite) | 0.75 | 0.56 to 1.00 | .051 |
| Hepatitis virus infection status | |||
| No infection v HBV + HCV | 0.51 | 0.32 to 0.80 | .004 |
| HBV alone v HBV + HCV | 0.76 | 0.44 to 1.32 | .334 |
| HCV alone v HBV + HCV | 0.72 | 0.43 to 1.18 | .192 |
| Serum AFP level (≥ 400 v < 400 ng/mL) | 2.26 | 1.69 to 3.02 | < .001 |
| Tumor differentiation (poor v other) | 1.63 | 1.15 to 2.31 | .006 |
| Tumor nodularity (multinodular v uninodular) | 2.28 | 1.68 to 3.11 | < .001 |
| Tumor size (> 50% v ≤ 50% of liver) | 2.92 | 2.19 to 3.90 | < .001 |
| Vascular invasion (yes v no) | 2.65 | 1.90 to 3.70 | < .001 |
| Lymph node involvement (yes v no) | 1.82 | 1.38 to 2.40 | < .001 |
| Metastasis (yes v no) | 1.76 | 1.27 to 2.45 | .001 |
| Bilirubin level (> 1.6 v ≤ 1.6 mg/dL) | 2.74 | 1.78 to 4.22 | < .001 |
| Serum ALT level (> 40 v ≤ 40 U/L) | 1.77 | 1.34 to 2.34 | < .001 |
| Serum AST level (> 45 v ≤ 45 U/L) | 2.17 | 1.57 to 3.00 | < .001 |
| Cirrhosis (yes v no) | 1.35 | 1.02 to 1.79 | .036 |
| Treatment | |||
| Chemotherapy v none | 0.56 | 0.38 to 0.84 | .0047 |
| Surgery v none | 0.19 | 0.12 to 0.31 | < .001 |
| Chemoembolization v none | 0.38 | 0.22 to 0.67 | < .001 |
| IGF-1 (≤ 26 v > 26 ng/mL) | 2.06 | 1.5 to 2.81 | < .001 |
| VEGF (> 450 v ≤ 450 pg/mL) | 1.74 | 1.26 to 2.43 | < .001 |
Table A2.
Log-Rank Test on OS for Each BCLC Stage
| BCLC Stage | Patients (No.) | Deaths (No.) | Median OS (months) | 95% CI | P |
|---|---|---|---|---|---|
| 0 | 21 | 7 | 49.08 | 33.99 to NA | < .001 |
| A | 28 | 12 | 40.67 | 27.09 to NA | < .001 |
| B | 29 | 23 | 22.68 | 16.14 to 28.64 | < .001 |
| C | 189 | 156 | 11.15 | 8.58 to 13.61 | < .001 |
| D | 21 | 19 | 2.93 | 2.14 to 9.14 | < .001 |
Abbreviations: BCLC, Barcelona Clinic Liver Cancer; NA, not applicable; OS, overall survival.
Table A3.
Log-Rank Test on OS for BCLC Stages Split by IGF-1 Level*
| BCLC Stage | Patients (No.) | Events (No.) | Median OS (months) | 95% CI | P |
|---|---|---|---|---|---|
| 0 | .8015 | ||||
| All patients | 21 | 7 | 49.08 | 33.99 to NA | |
| IGF-1 level | |||||
| 0 | 17 | 6 | 49.08 | 33.99 to NA | |
| 1 | 4 | 1 | NA | 6.84 to NA | |
| A | .52 | ||||
| All patients | 28 | 12 | 40.67 | 27.09 to NA | |
| IGF-1 level | |||||
| 0 | 24 | 10 | 40.67 | 33.7 to NA | |
| 1 | 4 | 2 | 18.51 | 12.95 to NA | |
| B | .125 | ||||
| All patients | 29 | 23 | 22.68 | 16.14 to 28.64 | |
| IGF-1 level | |||||
| 0 | 23 | 17 | 23.57 | 16.14 to 29.88 | |
| 1 | 6 | 6 | 16.88 | 14.89 to NA | |
| C | < .001 | ||||
| All patients | 189 | 156 | 11.15 | 8.58 to 13.61 | |
| IGF-1 level | |||||
| 0 | 152 | 121 | 12.43 | 10.13 to 17.75 | |
| 1 | 37 | 35 | 5.06 | 4.01 to 11.9 | |
| D | .0136 | ||||
| All patients | 21 | 19 | 2.93 | 2.14 to 9.14 | |
| IGF-1 level | |||||
| 0 | 12 | 10 | 6.44 | 2.40 to NA | |
| 1 | 9 | 9 | 2.2 | 0.62 to NA |
Abbreviations: BCLC, Barcelona Clinic Liver Cancer; IGF-1, insulin-like growth factor-1; NA, not applicable; OS, overall survival.
IGF-1 level: 0 if IGF-1 > 26 ng/mL; 1 if IGF-1 ≤ 26 ng/mL.
Table A4.
Log-Rank Test on OS for BCLC Stages Split by VEGF Level*
| BCLC Stage | Patients (No.) | Events (No.) | Median OS (months) | 95% CI | P |
|---|---|---|---|---|---|
| 0 | NA | ||||
| All patients | 21 | 7 | 49.08 | 33.99 to NA | |
| VEGF level | |||||
| 0 | 21 | 7 | 49.08 | 33.99 to NA | |
| 1 | 0 | 0 | NA | ||
| A | .5974 | ||||
| All patients | 28 | 12 | 40.67 | 27.09 to NA | |
| VEGF level | |||||
| 0 | 24 | 11 | 40.67 | 27.09 to NA | |
| 1 | 4 | 1 | NA | 9.60 to NA | |
| B | .4007 | ||||
| All patients | 29 | 23 | 22.68 | 16.14 to 28.64 | |
| VEGF level | |||||
| 0 | 24 | 18 | 23.57 | 16.67 to 29.26 | |
| 1 | 5 | 5 | 13.22 | 12.69 to NA | |
| C | .105 | ||||
| All patients | 189 | 156 | 11.15 | 8.58 to 13.61 | |
| VEGF level | |||||
| 0 | 152 | 125 | 11.7 | 8.98 to 15.06 | |
| 1 | 37 | 31 | 9.11 | 4.04 to 17.75 | |
| D | .9357 | ||||
| All patients | 21 | 19 | 2.93 | 2.14 to 9.14 | |
| VEGF level | |||||
| 0 | 11 | 10 | 5.03 | 1.25 to NA | |
| 1 | 10 | 9 | 2.43 | 1.51 to NA |
Abbreviations: BCLC, Barcelona Clinic Liver Cancer; NA, not applicable; OS, overall survival; VEGF, vascular endothelial growth factor.
VEGF level: 0 if VEGF ≤ 450 pg/mL; 1 if VEGF > 450 pg/mL.
Table A5.
Multivariate Cox Model for BCLC Stage and IGF-1
| Variable | HR | 95% CI | P |
|---|---|---|---|
| BCLC stage | |||
| 0 | |||
| A | 1.38 | 0.54 to 3.52 | .5 |
| B | 3.36 | 1.43 to 7.90 | .006 |
| C | 5.24 | 2.43 to 11.27 | < .001 |
| D | 12.83 | 5.32 to 30.97 | < .001 |
| IGF-1 level (> 26 v ≤ 26 ng/mL) | 2.19 | 1.60 to 3.0 | < .001 |
Abbreviations: BCLC, Barcelona Clinic Liver Cancer; HR, hazard ratio; IGF-1, insulin-like growth factor-1.
Table A6.
Multivariate Cox Model for BCLC Stage and VEGF
| Variable | HR | 95% CI | P |
|---|---|---|---|
| BCLC stage | |||
| 0 | |||
| A | 1.28 | 0.50 to 3.26 | .6 |
| B | 3.26 | 1.39 to 7.66 | .007 |
| C | 4.75 | 2.21 to 10.23 | < .001 |
| D | 10.24 | 4.16 to 25.22 | < .001 |
| VEGF level (> 450 v ≤ 450 pg/mL) | 1.28 | 0.91 to 1.82 | .16 |
Abbreviations: BCLC, Barcelona Clinic Liver Cancer; HR, hazard ratio; VEGF, vascular endothelial growth factor.
Table A7.
Multivariate Cox Model for BCLC Stage, IGF-1, and VEGF
| Variable | HR | 95% CI | P |
|---|---|---|---|
| BCLC stage | |||
| 0 | |||
| A | 1.36 | 0.53 to 3.47 | .52 |
| B | 3.27 | 1.39 to 7.71 | .007 |
| C | 5.05 | 2.34 to 10.90 | < .001 |
| D | 11.28 | 4.56 to 27.89 | < .001 |
| IGF-1 level (> 26 v ≤ 26 ng/mL) | 2.17 | 1.58 to 2.98 | < .001 |
| VEGF level (> 450 v ≤ 450 pg/mL) | 1.25 | 0.88 to 1.77 | .21 |
Abbreviations: BCLC, Barcelona Clinic Liver Cancer; HR, hazard ratio; IGF-1, insulin-like growth factor-1, VEGF, vascular endothelial growth factor.
Footnotes
Supported by National Institutes of Health (NIH) Grants No. RO3 ES11481 and CA106458-01 (M.M.H.); by philanthropic funds to the Department of Gastrointestinal Medical Oncology, MD Anderson Cancer Center; and in part by NIH Support Grant No. CA016672 to MD Anderson Cancer Center.
Presented at the 8th Annual American Society of Clinical Oncology Gastrointestinal Cancers Symposium, January 20-22, 2011, San Francisco, CA.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Ahmed O. Kaseb, Sunil Krishnan
Financial support: Ahmed O. Kaseb, Jeffrey S. Morris,James L. Abbruzzese
Administrative support: Ahmed O. Kaseb, Jeffrey S. Morris, Sunil Krishnan, James L. Abbruzzese
Provision of study materials or patients: Ahmed O. Kaseb, Jeffrey S. Morris, Lianchun Xiao, Eddie K. Abdalla, Jean-Nicolas Vauthey
Collection and assembly of data: Ahmed O. Kaseb, Jeffrey S. Morris, Manal M. Hassan, Sunil Krishnan
Data analysis and interpretation: Ahmed O. Kaseb, Jeffrey S. Morris, Adnan M. Siddiqui, E Lin, Lianchun Xiao, Eddie K. Abdalla, Jean-Nicolas Vauthey, Thomas A. Aloia, Sunil Krishnan,James L. Abbruzzese
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Mortality database: WHO Statistical Information System. http://www.who.int/whosis.
- 2.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Lau M, Eschbach K, et al. Epidemiology of hepatocellular carcinoma in Hispanics in the United States. Arch Intern Med. 2007;167:1983–1989. doi: 10.1001/archinte.167.18.1983. [DOI] [PubMed] [Google Scholar]
- 4.Pons F, Varela M, Llovet JM. Staging systems in hepatocellular carcinoma. HPB (Oxford) 2005;7:35–41. doi: 10.1080/13651820410024058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score) J Gastroenterol. 2003;38:207–215. doi: 10.1007/s005350300038. [DOI] [PubMed] [Google Scholar]
- 6.A new prognostic system for hepatocellular carcinoma: A retrospective study of 435 patients. Hepatology. 1998;28:751–755. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- 7.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: The BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 8.Tateishi R, Yoshida H, Shiina S, et al. Proposal of a new prognostic model for hepatocellular carcinoma: An analysis of 403 patients. Gut. 2005;54:419–425. doi: 10.1136/gut.2003.035055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okuda K, Ohtsuki T, Obata H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment: Study of 850 patients. Cancer. 1985;56:918–928. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 10.Wilson SR, Greig P, Kaseb AO. Pretreatment assessment of hepatocellular cancer: Expert consensus conference. HPB (Oxford) 2010;12:300–301. doi: 10.1111/j.1477-2574.2010.00187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collette S, Bonnetain F, Paoletti X, et al. Prognosis of advanced hepatocellular carcinoma: Comparison of three staging systems in two French clinical trials. Ann Oncol. 2008;19:1117–1126. doi: 10.1093/annonc/mdn030. [DOI] [PubMed] [Google Scholar]
- 12.Huitzil-Melendez FD, Capanu M, O'Reilly EM, et al. Advanced hepatocellular carcinoma: Which staging systems best predict prognosis? J Clin Oncol. 2010;28:2889–2895. doi: 10.1200/JCO.2009.25.9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 14.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma: Conclusions of the Barcelona-2000 EASL conference—European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 15.Llovet JM, Di Bisceglie AM, Bruix J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 16.Durand F, Valla D. Assessment of the prognosis of cirrhosis: Child-Pugh versus MELD. J Hepatol. 2005;42(suppl):S100–S107. doi: 10.1016/j.jhep.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 17.Buzzelli G, Dattolo P, Pinzani M, et al. Circulating growth hormone and insulin-like growth factor-I in nonalcoholic liver cirrhosis with or without superimposed hepatocarcinoma: Evidence of an altered circadian rhythm. Am J Gastroenterol. 1993;88:1744–1748. [PubMed] [Google Scholar]
- 18.Luo SM, Tan WM, Deng WX, et al. Expression of albumin, IGF-1, IGFBP-3 in tumor tissues and adjacent non-tumor tissues of hepatocellular carcinoma patients with cirrhosis. World J Gastroenterol. 2005;11:4272–4276. doi: 10.3748/wjg.v11.i27.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Su TS, Liu WY, Han SH, et al. Transcripts of the insulin-like growth factors I and II in human hepatoma. Cancer Res. 1989;49:1773–1777. [PubMed] [Google Scholar]
- 20.Stuver SO, Kuper H, Tzonou A, et al. Insulin-like growth factor 1 in hepatocellular carcinoma and metastatic liver cancer in men. Int J Cancer. 2000;87:118–121. doi: 10.1002/1097-0215(20000701)87:1<118::aid-ijc17>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 21.Su WW, Lee KT, Yeh YT, et al. Association of circulating insulin-like growth factor 1 with hepatocellular carcinoma: One cross-sectional correlation study. J Clin Lab Anal. 2010;24:195–200. doi: 10.1002/jcla.20320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elsammak MY, Amin GM, Khalil GM, et al. Possible contribution of serum activin A and IGF-1 in the development of hepatocellular carcinoma in Egyptian patients suffering from combined hepatitis C virus infection and hepatic schistosomiasis. Clin Biochem. 2006;39:623–629. doi: 10.1016/j.clinbiochem.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 23.García-Galiano D, Sánchez-Garrido MA, Espejo I, et al. IL-6 and IGF-1 are independent prognostic factors of liver steatosis and non-alcoholic steatohepatitis in morbidly obese patients. Obes Surg. 2007;17:493–503. doi: 10.1007/s11695-007-9087-1. [DOI] [PubMed] [Google Scholar]
- 24.Mazziotti G, Sorvillo F, Morisco F, et al. Serum insulin-like growth factor I evaluation as a useful tool for predicting the risk of developing hepatocellular carcinoma in patients with hepatitis C virus-related cirrhosis: A prospective study. Cancer. 2002;95:2539–2545. doi: 10.1002/cncr.11002. [DOI] [PubMed] [Google Scholar]
- 25.Yakar S, Liu JL, Stannard B, et al. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci U S A. 1999;96:7324–7329. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daughaday WH. The possible autocrine/paracrine and endocrine roles of insulin-like growth factors of human tumors. Endocrinology. 1990;127:1–4. doi: 10.1210/endo-127-1-1. [DOI] [PubMed] [Google Scholar]
- 27.Yao DF, Wu XH, Zhu Y, et al. Quantitative analysis of vascular endothelial growth factor, microvascular density and their clinicopathologic features in human hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2005;4:220–226. [PubMed] [Google Scholar]
- 28.Tseng CS, Lo HW, Chen PH, et al. Clinical significance of plasma D-dimer levels and serum VEGF levels in patients with hepatocellular carcinoma. Hepatogastroenterology. 2004;51:1454–1458. [PubMed] [Google Scholar]
- 29.Poon RT, Ho JW, Tong CS, et al. Prognostic significance of serum vascular endothelial growth factor and endostatin in patients with hepatocellular carcinoma. Br J Surg. 2004;91:1354–1360. doi: 10.1002/bjs.4594. [DOI] [PubMed] [Google Scholar]
- 30.Kamel L, Nessim I, Abd-el-Hady A, et al. Assessment of the clinical significance of serum vascular endothelial growth factor and matrix metalloproteinase-9 in patients with hepatocellular carcinoma. J Egypt Soc Parasitol. 2005;35:875–890. [PubMed] [Google Scholar]
- 31.Kaseb AO, Hassan MM, Lin E, et al. V-CLIP: Integrating plasma vascular endothelial growth factor into a new scoring system to stratify patients with advanced hepatocellular carcinoma for clinical trials. Cancer. 2010;117:2478–2488. doi: 10.1002/cncr.25791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaseb AO, Abbruzzese JL, Vauthey JN, et al. I-CLIP: Improved stratification of advanced hepatocellular carcinoma patients by integrating plasma IGF-1 into CLIP score. Oncology. 2011;80:373–381. doi: 10.1159/000329040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan JM, Stampfer MJ, Giovannucci E, et al. Plasma insulin-like growth factor-I and prostate cancer risk: A prospective study. Science. 1998;279:563–566. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- 34.Hankinson SE, Willett WC, Colditz GA, et al. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet. 1998;351:1393–1396. doi: 10.1016/S0140-6736(97)10384-1. [DOI] [PubMed] [Google Scholar]
- 35.Ma J, Pollak MN, Giovannucci E, et al. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer Inst. 1999;91:620–625. doi: 10.1093/jnci/91.7.620. [DOI] [PubMed] [Google Scholar]
- 36.Yu H, Spitz MR, Mistry J, et al. Plasma levels of insulin-like growth factor-I and lung cancer risk: A case-control analysis. J Natl Cancer Inst. 1999;91:151–156. doi: 10.1093/jnci/91.2.151. [DOI] [PubMed] [Google Scholar]