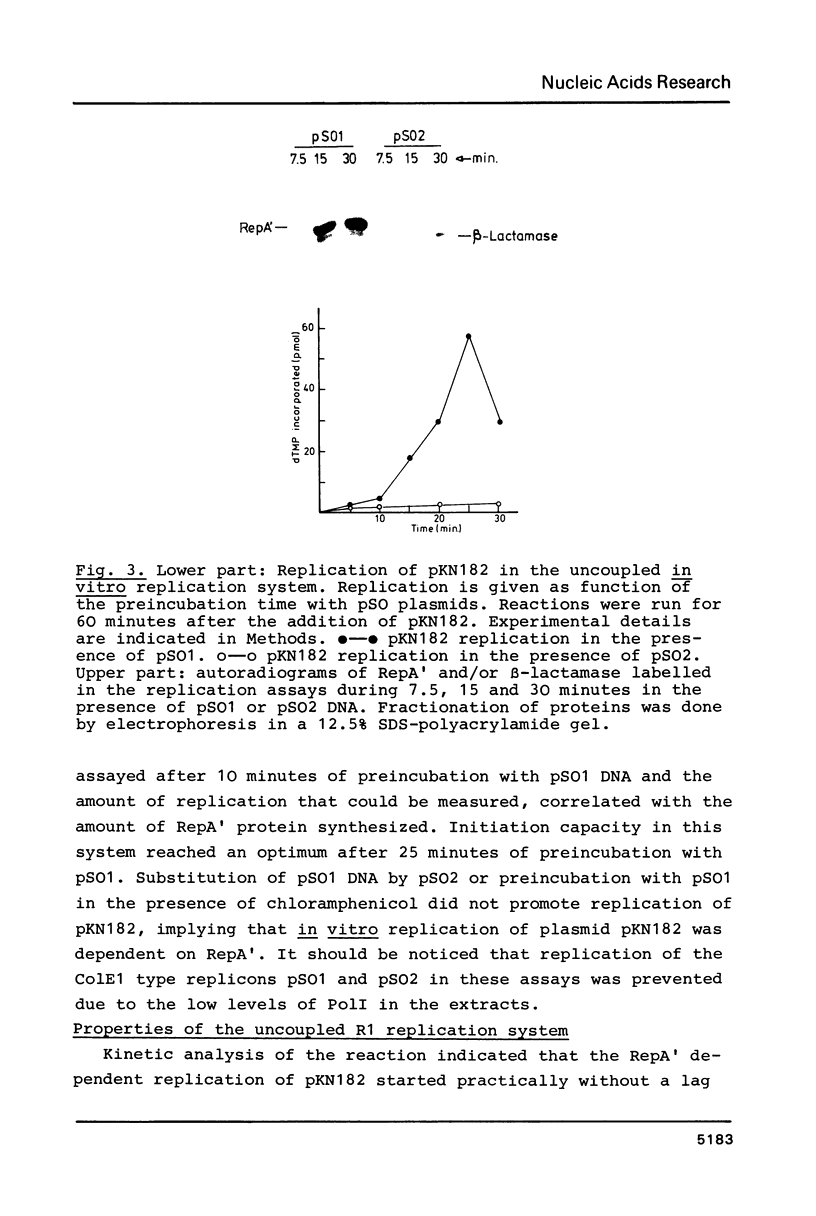
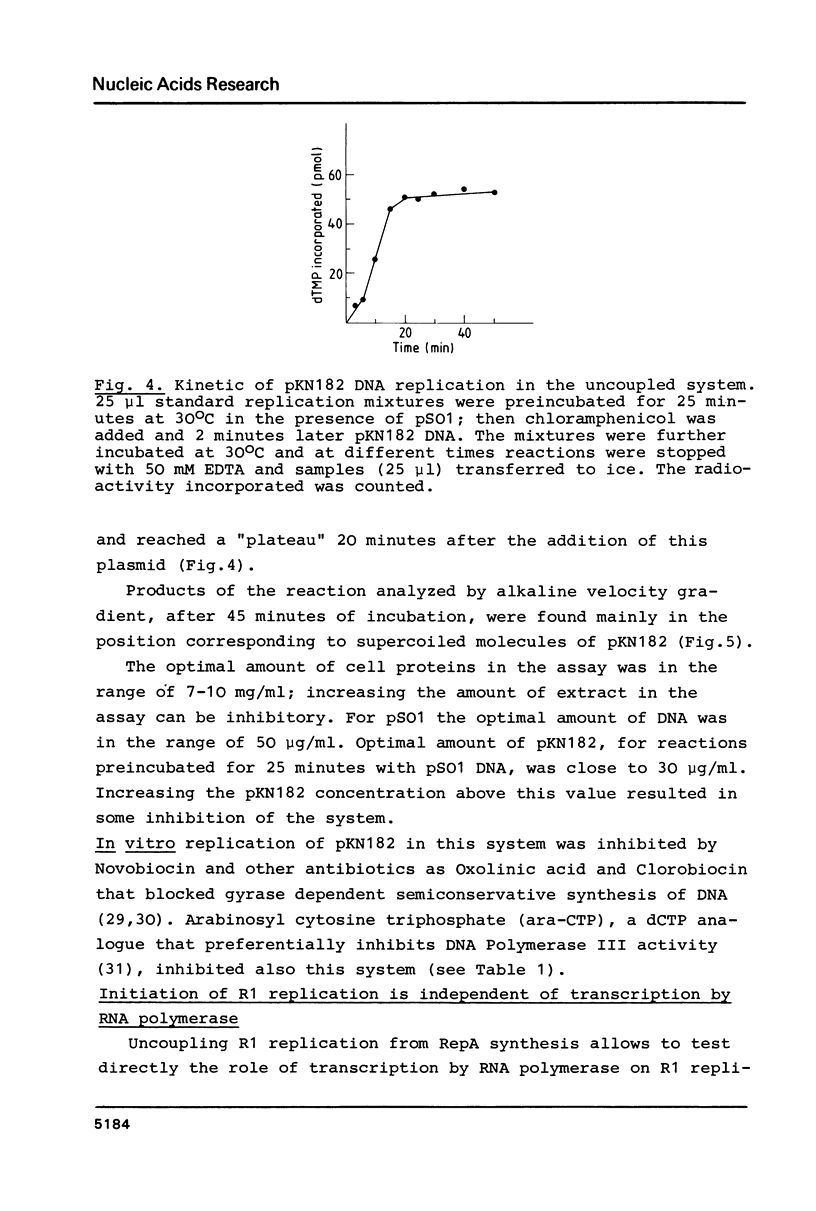
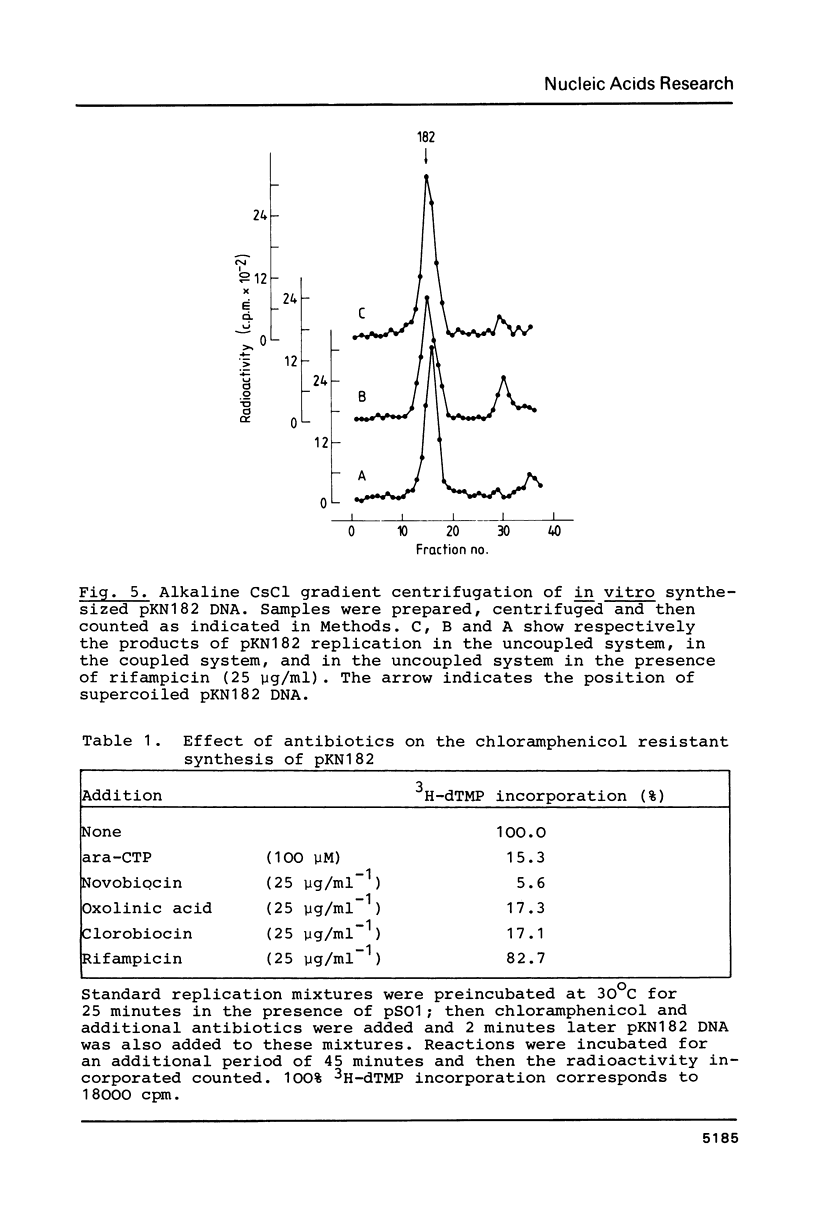
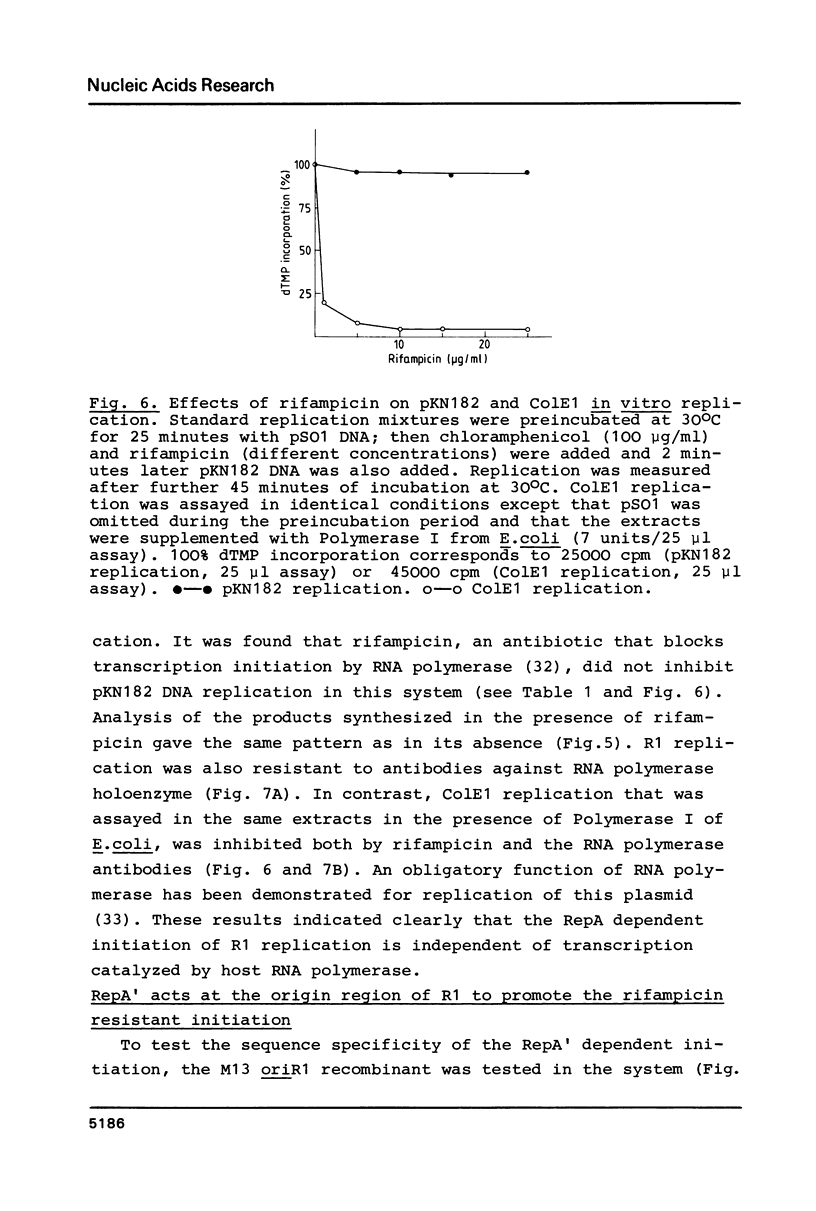
Abstract
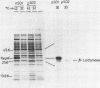
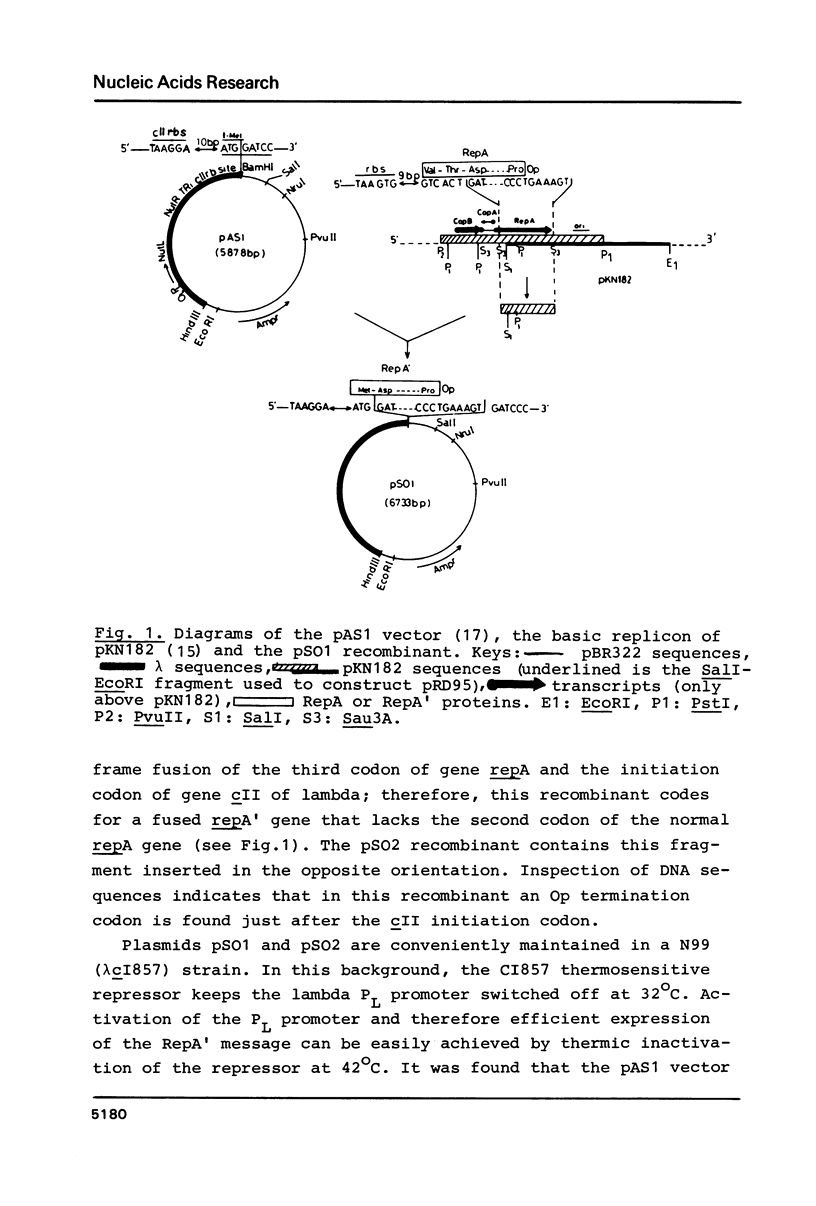
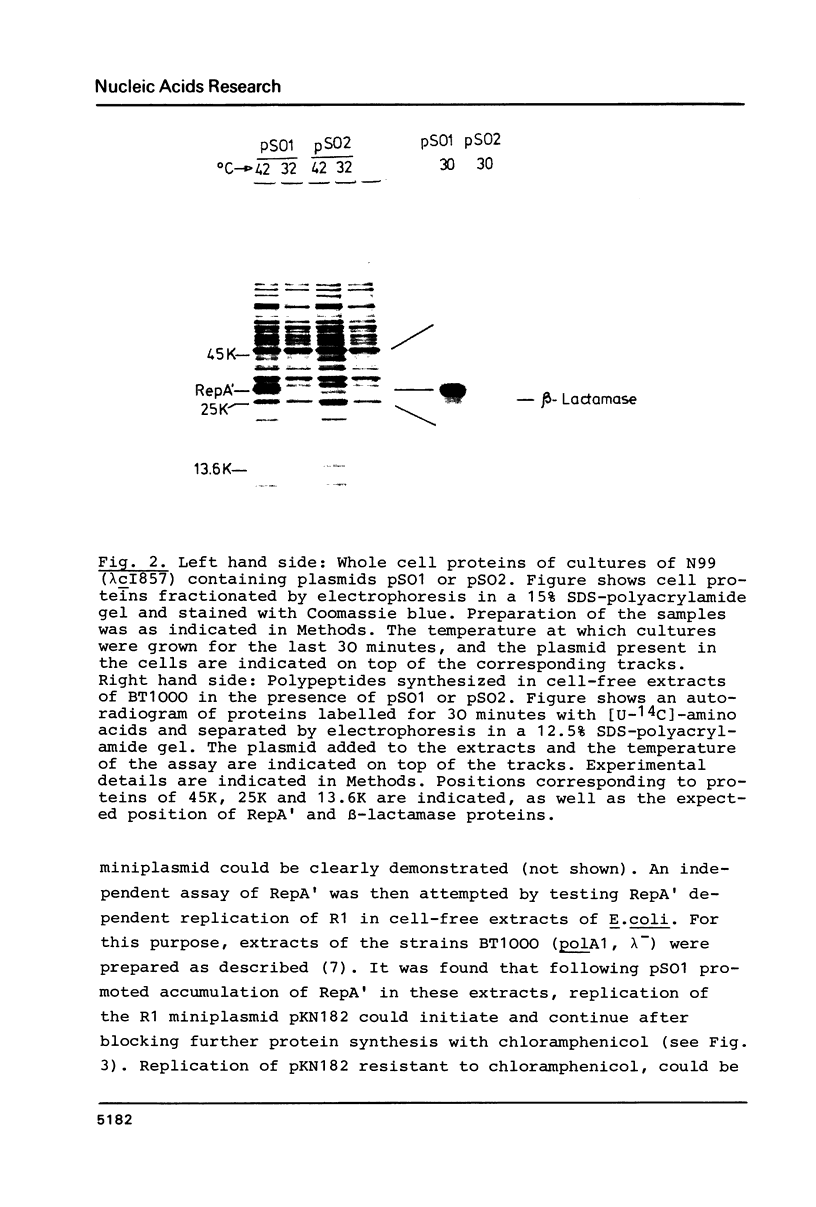
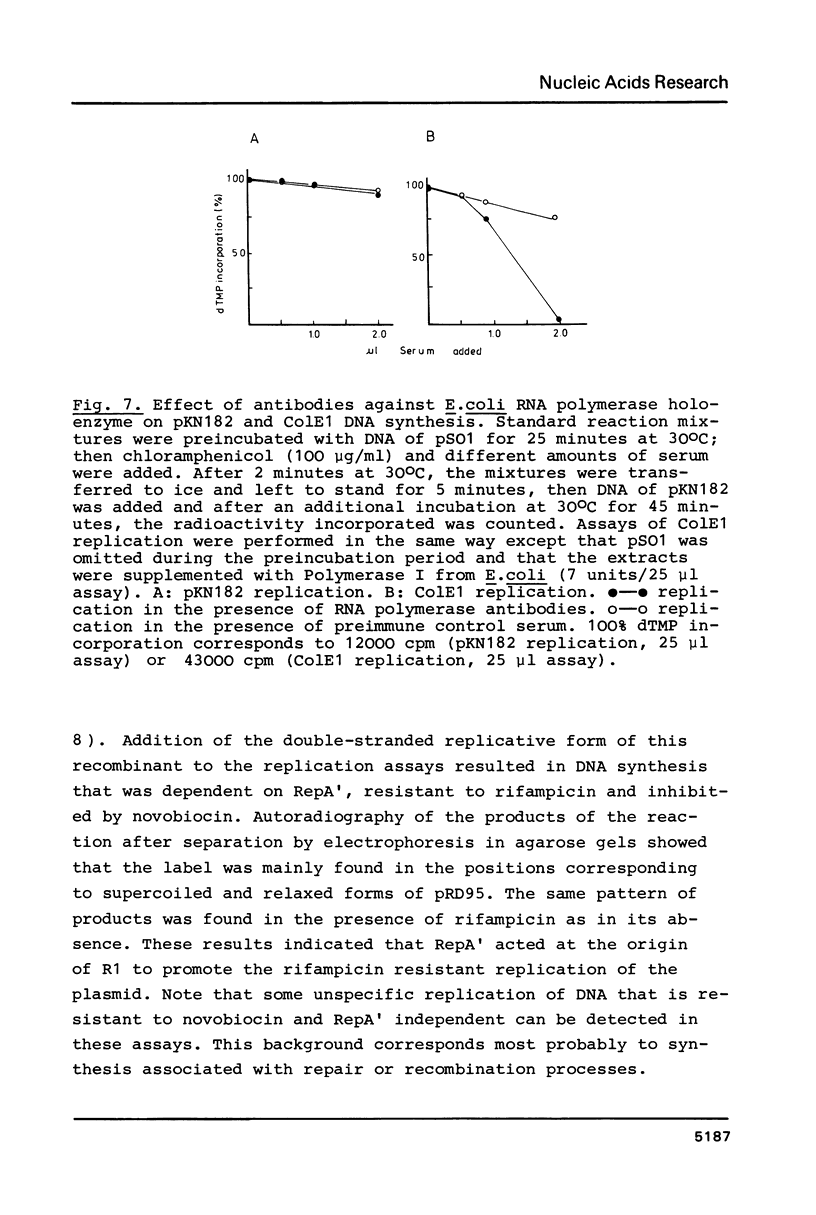
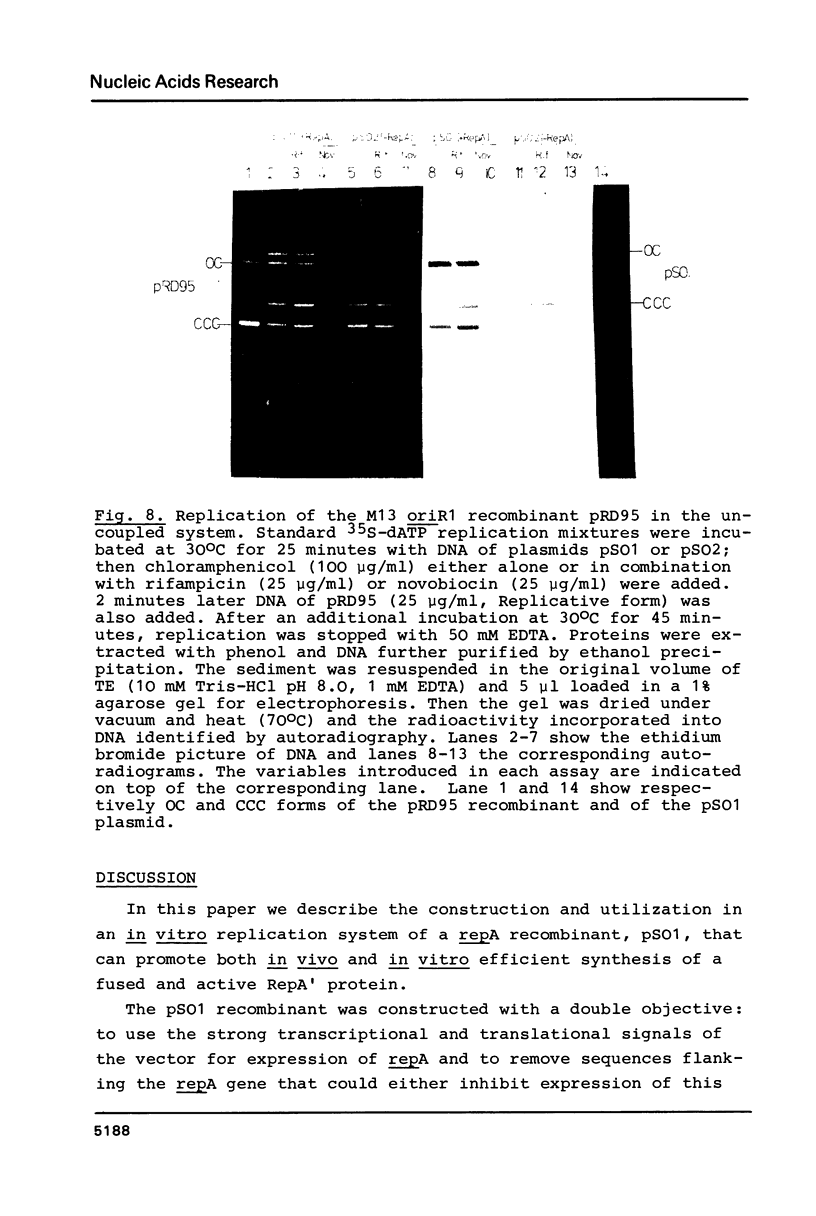
A Sau3A fragment containing most of the repA gene of plasmid R1 has been cloned in the BamH1 site of the expression vector pAS1. One of the recombinants, pSO1, codes for a fused RepA' protein which is efficiently synthesized both in vivo and in vitro from transcriptional and translational signals of the vector. It is shown that following pSO1 promoted accumulation of RepA' in cell-free extracts of E. coli, in vitro replication of the R1 miniplasmid pKN182 can initiate and proceed uncoupled from further protein synthesis. Using this uncoupled system and also a M13mp9 based ori-R1 recombinant, pRD95, it is also shown that RepA' acts at the origin region of R1 to promote initiation of replication that is independent on transcription by host RNA polymerase. This is indicated by the insensitivity of pRD95 and pKN182 replication to rifampicin as well as to RNA polymerase antibodies. The properties of the uncoupled in vitro replication system are further described.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderl A., Klein A. Replication of lambda dv DNA in vitro. Nucleic Acids Res. 1982 Mar 11;10(5):1733–1740. doi: 10.1093/nar/10.5.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassani G., Burgess R. R., Goodman H. M., Gold L. Inhibition of RNA polymerase by streptolydigin. Nat New Biol. 1971 Apr 14;230(15):197–200. doi: 10.1038/newbio230197a0. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz R., Nordström K., Staudenbauer W. L. Plasmid R1 DNA replication dependent on protein synthesis in cell-free extracts of E. coli. Nature. 1981 Jan 22;289(5795):326–328. doi: 10.1038/289326a0. [DOI] [PubMed] [Google Scholar]
- Diaz R., Staudenbauer W. L. Origin and direction of mini-R1 plasmid DNA replication in cell extracts of Escherichia coli. J Bacteriol. 1982 Jun;150(3):1077–1084. doi: 10.1128/jb.150.3.1077-1084.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz R., Staudenbauer W. L. Replication of the broad host range plasmid RSF1010 in cell-free extracts of Escherichia coli and Pseudomonas aeruginosa. Nucleic Acids Res. 1982 Aug 11;10(15):4687–4702. doi: 10.1093/nar/10.15.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R. S., Kaguni J. M., Kornberg A. Enzymatic replication of the origin of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7370–7374. doi: 10.1073/pnas.78.12.7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel W. Studies on the initiation of plasmid DNA replication. Eur J Biochem. 1974 Jan 3;41(1):51–62. doi: 10.1111/j.1432-1033.1974.tb03243.x. [DOI] [PubMed] [Google Scholar]
- Inuzuka M., Helinski D. R. Replication of antibiotic resistance plasmid R6K DNA in vitro. Biochemistry. 1978 Jun 27;17(13):2567–2573. doi: 10.1021/bi00606a017. [DOI] [PubMed] [Google Scholar]
- Itoh T., Tomizawa J. Formation of an RNA primer for initiation of replication of ColE1 DNA by ribonuclease H. Proc Natl Acad Sci U S A. 1980 May;77(5):2450–2454. doi: 10.1073/pnas.77.5.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S. A., Adler G. K., Novick R. P. Functional origin of replication of pT181 plasmid DNA is contained within a 168-base-pair segment. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4580–4584. doi: 10.1073/pnas.79.15.4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollek R., Oertel W., Goebel W. Site-specific deletion at the replication origin of the antibiotic resistance factor R1. Mol Gen Genet. 1980 Feb;177(3):413–419. doi: 10.1007/BF00271479. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Light J., Molin S. Post-transcriptional control of expression of the repA gene of plasmid R1 mediated by a small RNA molecule. EMBO J. 1983;2(1):93–98. doi: 10.1002/j.1460-2075.1983.tb01387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light J., Molin S. Replication control functions of plasmid R1 act as inhibitors of expression of a gene required for replication. Mol Gen Genet. 1981;184(1):56–61. doi: 10.1007/BF00271195. [DOI] [PubMed] [Google Scholar]
- Light J., Molin S. The sites of action of the two copy number control functions of plasmid R1. Mol Gen Genet. 1982;187(3):486–493. doi: 10.1007/BF00332633. [DOI] [PubMed] [Google Scholar]
- Masai H., Kaziro Y., Arai K. Definition of oriR, the minimum DNA segment essential for initiation of R1 plasmid replication in vitro. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6814–6818. doi: 10.1073/pnas.80.22.6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Molin S., Nordström K. Control of plasmid R1 replication: functions involved in replication, copy number control, incompatibility, and switch-off of replication. J Bacteriol. 1980 Jan;141(1):111–120. doi: 10.1128/jb.141.1.111-120.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molin S., Stougaard P., Light J., Nordström M., Nordström K. Isolation and characterization of new copy mutants of plasmid R1, and identification of a polypeptide involved in copy number control. Mol Gen Genet. 1981;181(1):123–130. doi: 10.1007/BF00339015. [DOI] [PubMed] [Google Scholar]
- Oertel W., Kollek R., Beck E., Goebel W. The nucleotide sequence of a DNA fragment from the replication origin of the antibiotic resistance factor R1drd19. Mol Gen Genet. 1979 Mar 27;171(3):277–285. doi: 10.1007/BF00267582. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Ho Y. S., Shatzman A. The use of pKc30 and its derivatives for controlled expression of genes. Methods Enzymol. 1983;101:123–138. doi: 10.1016/0076-6879(83)01009-5. [DOI] [PubMed] [Google Scholar]
- Ryder T. B., Davidson D. B., Rosen J. I., Ohtsubo E., Ohtsubo H. Analysis of plasmid genome evolution based on nucleotide-sequence comparison of two related plasmids of Escherichia coli. Gene. 1982 Mar;17(3):299–310. doi: 10.1016/0378-1119(82)90146-9. [DOI] [PubMed] [Google Scholar]
- SUSSMAN R., JACOB F. [On a thermosensitive repression system in the Escherichia coli lambda bacteriophage]. C R Hebd Seances Acad Sci. 1962 Feb 19;254:1517–1519. [PubMed] [Google Scholar]
- Staudenbauer W. L. Letters to the editor: Novobiocin-a specific inhibitor of semiconservative DNA replication in permeabilized Escherichia coli cells. J Mol Biol. 1975 Jul 25;96(1):201–205. doi: 10.1016/0022-2836(75)90191-6. [DOI] [PubMed] [Google Scholar]
- Staudenbauer W. L. REPLICAtion of small plasmids in extracts of Escherichia coli: requirement for both DNA polymerases I and II. Mol Gen Genet. 1976 Dec 8;149(2):151–158. doi: 10.1007/BF00332883. [DOI] [PubMed] [Google Scholar]
- Staudenbauer W. L. Replication of Escherichia coli DNA in vitro: inhibition by oxolinic acid. Eur J Biochem. 1976 Mar 1;62(3):491–497. doi: 10.1111/j.1432-1033.1976.tb10183.x. [DOI] [PubMed] [Google Scholar]
- Staudenbauer W. L. Replication of small plasmids in extracts of Escherichia coli. Mol Gen Genet. 1976 Jun 15;145(3):273–280. doi: 10.1007/BF00325823. [DOI] [PubMed] [Google Scholar]
- Stougaard P., Molin S., Nordström K., Hansen F. G. The nucleotide sequence of the replication control region of the resistance plasmid R1drd-19. Mol Gen Genet. 1981;181(1):116–122. doi: 10.1007/BF00339014. [DOI] [PubMed] [Google Scholar]
- Uhlin B. E., Nordström K. A runaway-replication mutant of plasmid R1drd-19: temperature-dependent loss of copy number control. Mol Gen Genet. 1978 Oct 4;165(2):167–179. doi: 10.1007/BF00269904. [DOI] [PubMed] [Google Scholar]
- Uhlin B. E., Nordström K. A runaway-replication mutant of plasmid R1drd-19: temperature-dependent loss of copy number control. Mol Gen Genet. 1978 Oct 4;165(2):167–179. doi: 10.1007/BF00269904. [DOI] [PubMed] [Google Scholar]
- Wechsler J. A., Nüsslein V., Otto B., Klein A., Bonhoeffer F., Herrmann R., Gloger L., Schaller H. Isolation and characterization of thermosensitive Escherichia coli mutants defective in deoxyribonucleic acid replication. J Bacteriol. 1973 Mar;113(3):1381–1388. doi: 10.1128/jb.113.3.1381-1388.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa M. Identification and mapping of the replication genes of an R factor, R100-1, integrated into the chromosome of Escherichia coli K-12. J Bacteriol. 1974 Jun;118(3):1123–1131. doi: 10.1128/jb.118.3.1123-1131.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]