Abstract
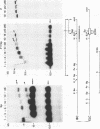
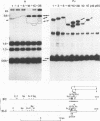
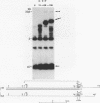
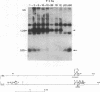
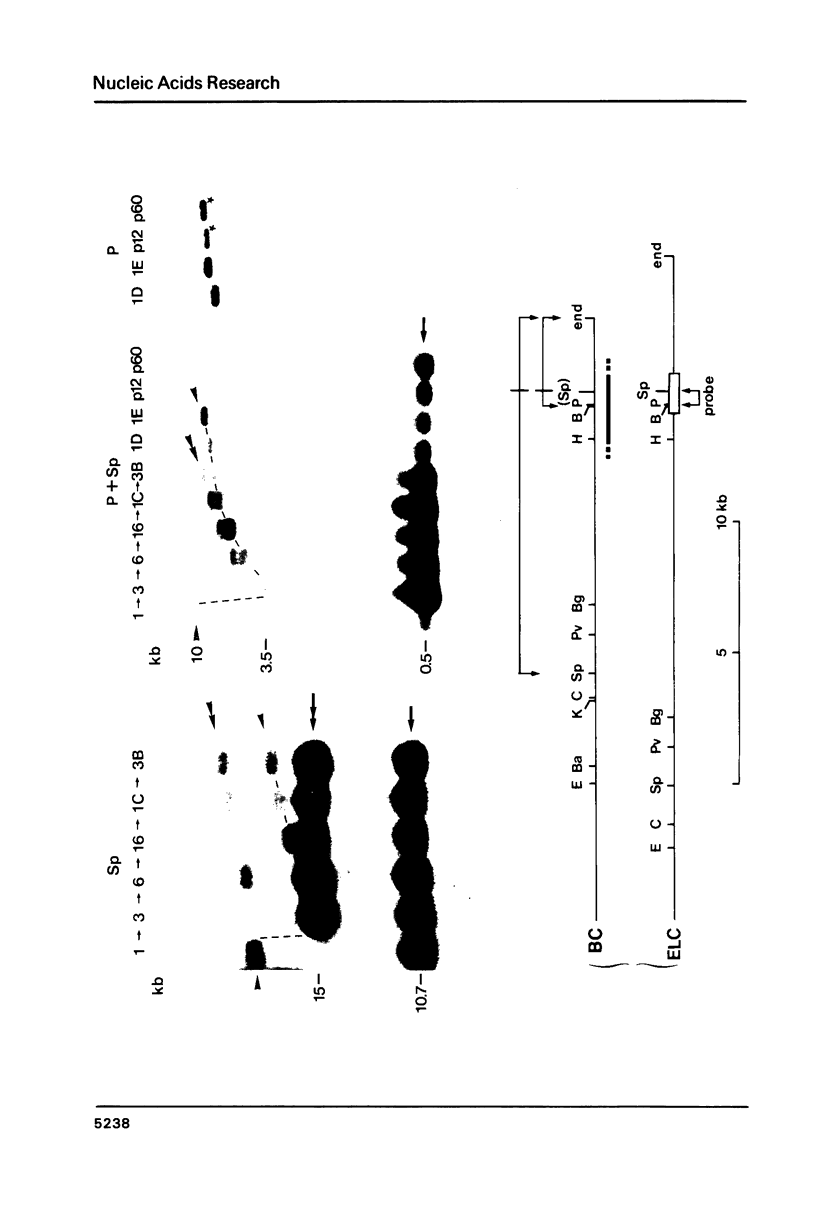
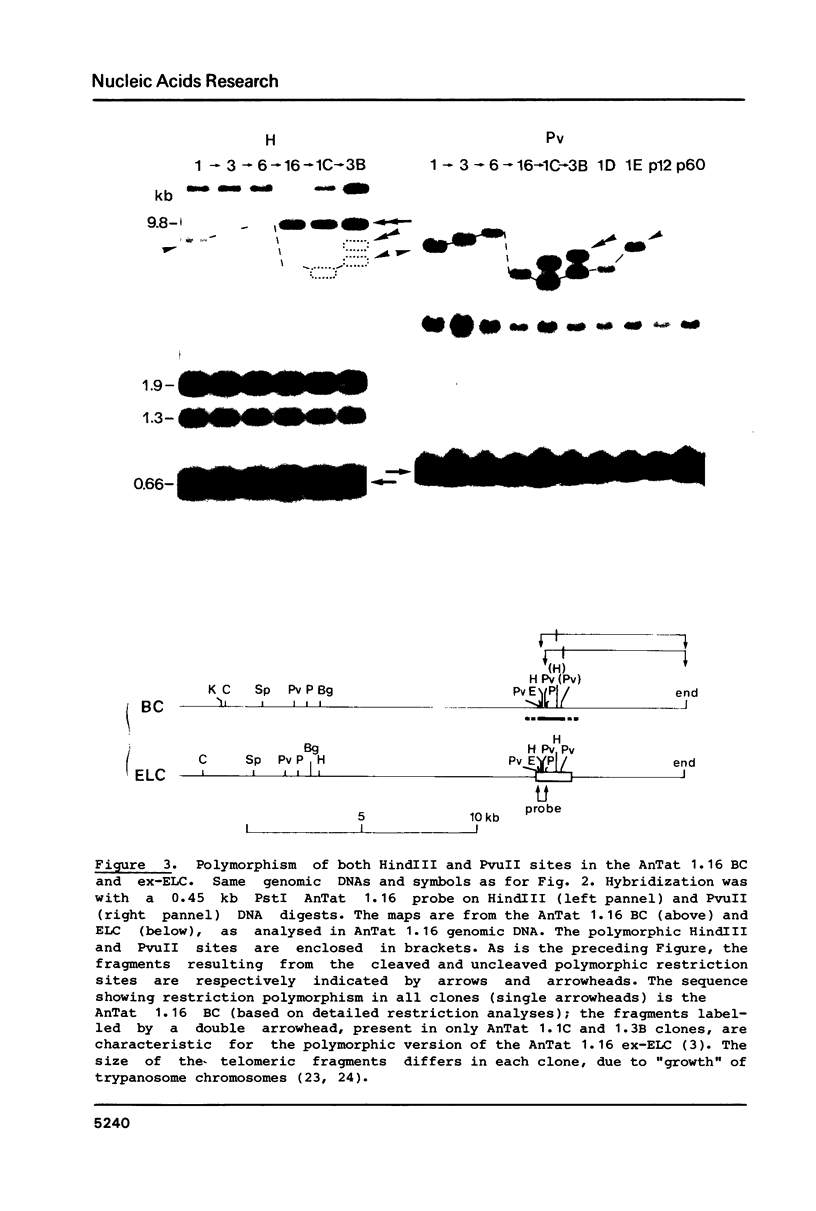
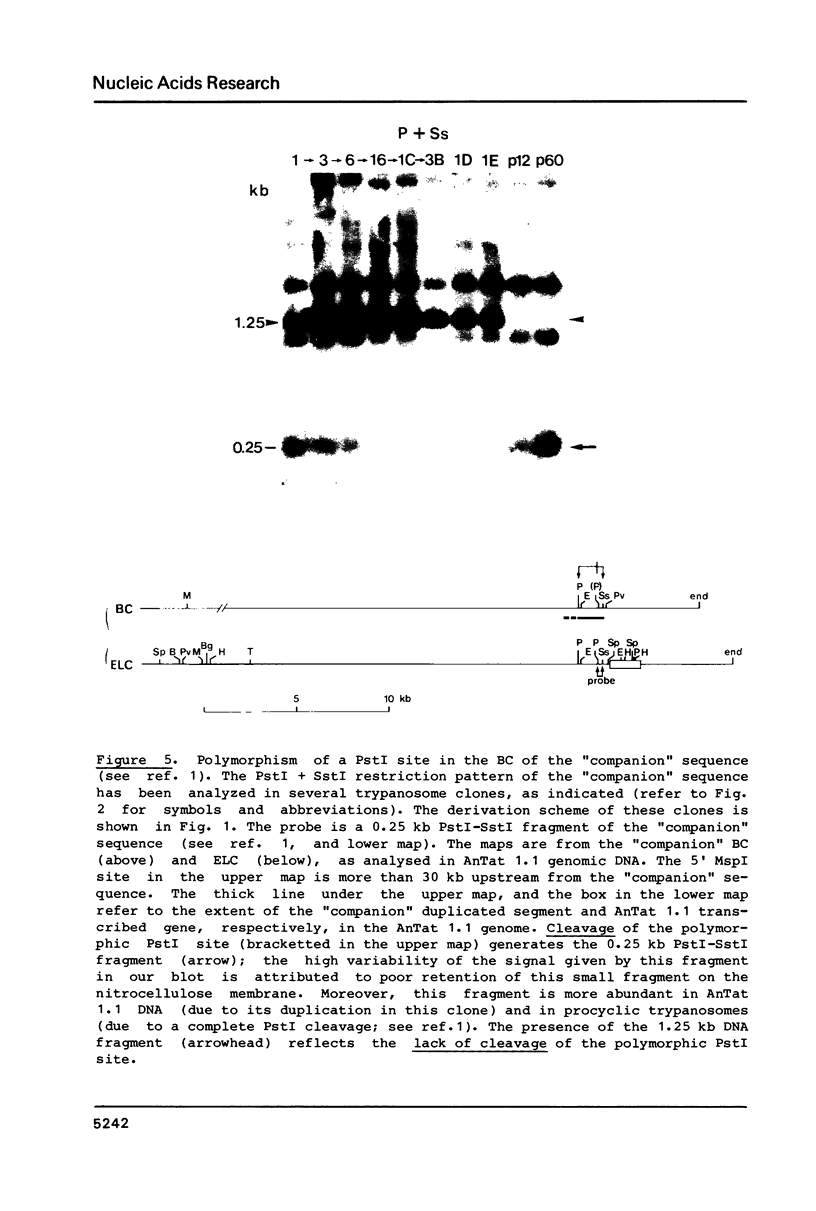
Polymorphism in restriction site cleavage (PstI, SphI, PvuII, HindIII) has been noticed in several occasions in the telomeric sequences harbouring trypanosome variant-specific antigen genes (1, 2, 3). This polymorphism has been further investigated and seems best interpreted as due to partial DNA modification in GC dinucleotides. The actively transcribed telomeric genes do not exhibit such a polymorphism; furthermore, in at least three independent cases, gene inactivation is linked to the appearance of polymorphism. It could thus be hypothesized that DNA modification prevents antigen gene transcription, or vice-versa. We report however that at least some telomeric antigen-specific sequences of the procyclic trypanosomes (in vitro culture form) are not polymorphic, although they do not synthesize any variant-specific antigen mRNA. There is thus no absolute relationship between the absence of polymorphism and antigen gene transcription.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernards A., De Lange T., Michels P. A., Liu A. Y., Huisman M. J., Borst P. Two modes of activation of a single surface antigen gene of Trypanosoma brucei. Cell. 1984 Jan;36(1):163–170. doi: 10.1016/0092-8674(84)90085-0. [DOI] [PubMed] [Google Scholar]
- Bernards A., Michels P. A., Lincke C. R., Borst P. Growth of chromosome ends in multiplying trypanosomes. Nature. 1983 Jun 16;303(5918):592–597. doi: 10.1038/303592a0. [DOI] [PubMed] [Google Scholar]
- Borst P., Cross G. A. Molecular basis for trypanosome antigenic variation. Cell. 1982 Jun;29(2):291–303. doi: 10.1016/0092-8674(82)90146-5. [DOI] [PubMed] [Google Scholar]
- Borst P., van der Ploeg M., van Hoek J. F., Tas J., James J. On the DNA content and ploidy of trypanosomes. Mol Biochem Parasitol. 1982 Jul;6(1):13–23. doi: 10.1016/0166-6851(82)90049-4. [DOI] [PubMed] [Google Scholar]
- De Lange T., Borst P. Genomic environment of the expression-linked extra copies of genes for surface antigens of Trypanosoma brucei resembles the end of a chromosome. Nature. 1982 Sep 30;299(5882):451–453. doi: 10.1038/299451a0. [DOI] [PubMed] [Google Scholar]
- Doerfler W. DNA methylation and gene activity. Annu Rev Biochem. 1983;52:93–124. doi: 10.1146/annurev.bi.52.070183.000521. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Frasch A. C., Bernards A., Borst P., Cross G. A. Novel expression-linked copies of the genes for variant surface antigens in trypanosomes. Nature. 1980 Mar 6;284(5751):78–80. doi: 10.1038/284078a0. [DOI] [PubMed] [Google Scholar]
- Laurent M., Pays E., Delinte K., Magnus E., Van Meirvenne N., Steinert M. Evolution of a trypanosome surface antigen gene repertoire linked to non-duplicative gene activation. Nature. 1984 Mar 22;308(5957):370–373. doi: 10.1038/308370a0. [DOI] [PubMed] [Google Scholar]
- Laurent M., Pays E., Magnus E., Van Meirvenne N., Matthyssens G., Williams R. O., Steinert M. DNA rearrangements linked to expression of a predominant surface antigen gene of trypanosomes. Nature. 1983 Mar 17;302(5905):263–266. doi: 10.1038/302263a0. [DOI] [PubMed] [Google Scholar]
- Majiwa P. A., Young J. R., Englund P. T., Shapiro S. Z., Williams R. O. Two distinct forms of surface antigen gene rearrangement in Trypanosoma brucei. Nature. 1982 Jun 10;297(5866):514–516. doi: 10.1038/297514a0. [DOI] [PubMed] [Google Scholar]
- Overath P., Czichos J., Stock U., Nonnengaesser C. Repression of glycoprotein synthesis and release of surface coat during transformation of Trypanosoma brucei. EMBO J. 1983;2(10):1721–1728. doi: 10.1002/j.1460-2075.1983.tb01648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons M., Nelson R. G., Stuart K., Agabian N. Variant antigen genes of Trypanosoma brucei: genomic alteration of a spliced leader orphon and retention of expression-linked copies during differentiation. Proc Natl Acad Sci U S A. 1984 Feb;81(3):684–688. doi: 10.1073/pnas.81.3.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pays E., Delauw M. F., Van Assel S., Laurent M., Vervoort T., Van Meirvenne N., Steinert M. Modifications of a Trypanosoma b. brucei antigen gene repertoire by different DNA recombinational mechanisms. Cell. 1983 Dec;35(3 Pt 2):721–731. doi: 10.1016/0092-8674(83)90105-8. [DOI] [PubMed] [Google Scholar]
- Pays E., Delronche M., Lheureux M., Vervoort T., Bloch J., Gannon F., Steinert M. Cloning and characterization of DNA sequences complementary to messenger ribonucleic acids coding for the synthesis of two surface antigens of Trypanosoma brucei. Nucleic Acids Res. 1980 Dec 20;8(24):5965–5981. doi: 10.1093/nar/8.24.5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pays E., Laurent M., Delinte K., Van Meirvenne N., Steinert M. Differential size variations between transcriptionally active and inactive telomeres of Trypanosoma brucei. Nucleic Acids Res. 1983 Dec 10;11(23):8137–8147. doi: 10.1093/nar/11.23.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pays E., Van Assel S., Laurent M., Darville M., Vervoort T., Van Meirvenne N., Steinert M. Gene conversion as a mechanism for antigenic variation in trypanosomes. Cell. 1983 Sep;34(2):371–381. doi: 10.1016/0092-8674(83)90371-9. [DOI] [PubMed] [Google Scholar]
- Pays E., Van Assel S., Laurent M., Dero B., Michiels F., Kronenberger P., Matthyssens G., Van Meirvenne N., Le Ray D., Steinert M. At least two transposed sequences are associated in the expression site of a surface antigen gene in different trypanosome clones. Cell. 1983 Sep;34(2):359–369. doi: 10.1016/0092-8674(83)90370-7. [DOI] [PubMed] [Google Scholar]
- Pays E., Van Meirvenne N., Le Ray D., Steinert M. Gene duplication and transposition linked to antigenic variation in Trypanosoma brucei. Proc Natl Acad Sci U S A. 1981 May;78(5):2673–2677. doi: 10.1073/pnas.78.5.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raibaud A., Gaillard C., Longacre S., Hibner U., Buck G., Bernardi G., Eisen H. Genomic environment of variant surface antigen genes of Trypanosoma equiperdum. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4306–4310. doi: 10.1073/pnas.80.14.4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait A. Evidence for diploidy and mating in trypanosomes. Nature. 1980 Oct 9;287(5782):536–538. doi: 10.1038/287536a0. [DOI] [PubMed] [Google Scholar]
- Van Meirvenne N., Janssens P. G., Magnus E. Antigenic variation in syringe passaged populations of Trypanosoma (Trypanozoon) brucei. 1. Rationalization of the experimental approach. Ann Soc Belg Med Trop. 1975;55(1):1–23. [PubMed] [Google Scholar]
- Van der Ploeg L. H., Liu A. Y., Borst P. Structure of the growing telomeres of Trypanosomes. Cell. 1984 Feb;36(2):459–468. doi: 10.1016/0092-8674(84)90239-3. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Valerio D., De Lange T., Bernards A., Borst P., Grosveld F. G. An analysis of cosmid clones of nuclear DNA from Trypanosoma brucei shows that the genes for variant surface glycoproteins are clustered in the genome. Nucleic Acids Res. 1982 Oct 11;10(19):5905–5923. doi: 10.1093/nar/10.19.5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. O., Young J. R., Majiwa P. A. Genomic environment of T. brucei VSG genes: presence of a minichromosome. Nature. 1982 Sep 30;299(5882):417–421. doi: 10.1038/299417a0. [DOI] [PubMed] [Google Scholar]
- Young J. R., Donelson J. E., Majiwa P. A., Shapiro S. Z., Williams R. O. analysis of genomic rearrangements associated with two variable antigen genes of Trypanosoma brucei. Nucleic Acids Res. 1982 Feb 11;10(3):803–819. doi: 10.1093/nar/10.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]