Following a randomized controlled trial of methadone clinic-based directly observed therapy (DOT) versus treatment as usual, adherence and viral load improved in the DOT group. After DOT ended, differences in adherence and viral load between groups diminished by 1 month, and extinguished by 3 months.
Abstract
Background. Methadone clinic–based directly observed antiretroviral therapy (DOT) has been shown to be more efficacious for improving adherence and suppressing human immunodeficiency virus (HIV) load than antiretroviral self-administration. We sought to determine whether the beneficial effects of DOT remain after DOT is discontinued.
Methods. We conducted a post-trial cohort study of 65 HIV-infected opioid-dependent adults who had completed a 24-week randomized controlled trial of methadone clinic–based DOT versus treatment as usual (TAU). For 12 months after DOT discontinuation, we assessed antiretroviral adherence using monthly pill counts and electronic monitors. We also assessed viral load at 3, 6, and 12 months after DOT ended. We examined differences between DOT and TAU in (1) adherence, (2) viral load, and (3) proportion of participants with viral load of <75 copies/mL.
Results. At trial end, adherence was higher among DOT participants than among TAU participants (86% and 54%, respectively; P < .001), and more DOT participants than TAU participants had viral loads of <75 copies/mL (71% and 44%, respectively; P = .03). However, after DOT ended, differences in adherence diminished by 1 month (55% for DOT vs 48% for TAU; P = .33) and extinguished completely by 3 months (49% for DOT vs 50% for TAU; P = .94). Differences in viral load between DOT and TAU disappeared by 3 months after the intervention, and the proportion of DOT participants with undetectable viral load decreased steadily after DOT was stopped until there was no difference (36% for DOT and 34% for TAU; P = .92).
Conclusions. Because the benefits of DOT for adherence and viral load among HIV-infected methadone patients cease after DOT is stopped, methadone-based DOT should be considered a long-term intervention.
The feasibility and acceptability of directly observed therapy (DOT) programs have been demonstrated in numerous settings [1–11], and such programs are efficacious for improving adherence and viral suppression [12–15]. Benefits of DOT may be especially pronounced in methadone clinic settings [16]. However, few studies examining antiretroviral DOT have included a postintervention period to evaluate the persistence of the benefits of DOT, and none have evaluated the durability of the impact of directly observed antiretrovirals delivered in methadone clinics.
Methadone maintenance clinics provide a promising infrastructure for DOT because federal regulations mandate that patients receive their daily methadone dose at one clinic, and the majority of doses are directly observed by nurses. In addition, compared with community DOT programs, many methadone clinics have the potential to provide primary medical, human immunodeficiency virus (HIV) infection, and mental healthcare, as well as substance abuse treatment and other supportive services, including case management and vocational services. In such settings, it is unknown whether finite periods of DOT lead to durable improvements in adherence and HIV load or whether DOT interventions should be continued long term to maintain optimal HIV infection outcomes.
The Support for Treatment Adherence Research through Directly Observed Therapy (STAR*DOT) study was a randomized controlled trial that demonstrated that 24 weeks of antiretroviral DOT provided on-site in methadone clinics is more efficacious than self-administered antiretroviral therapy (treatment as usual [TAU]) for improving adherence and reducing viral load [17]. At the end of the 24-week intervention period, we continued to monitor participants in both the DOT and TAU treatment arms to determine whether the favorable effects of DOT were sustained during a 12-month postintervention follow-up period. We hypothesized that, among methadone-maintained current and former drug users, short-term DOT would result in long-term improvements in adherence and viral load.
METHODS
Setting, Design, and Participants
A detailed description of the STAR*DOT trial has been published elsewhere [18]. In brief, our intervention and all research visits were conducted on-site in a network of 12 methadone clinics administered by the Division of Substance Abuse (DoSA) at the Albert Einstein College of Medicine and Montefiore Medical Center in the Bronx, New York. The STAR*DOT trial was designed to include monthly research visits for an additional 12-month postintervention follow-up period, after participants had completed the 24-week trial. Frequency and length of research visits during the 12-month postintervention period were the same for participants in both the DOT and TAU treatment arms.
Patients were eligible for inclusion in STAR*DOT if they (1) were HIV-infected, (2) were prescribed antiretroviral therapy, (3) received HIV infection medical care at their DoSA methadone clinic or at a closely affiliated site, (4) attended their methadone clinic 5 or 6 days per week to receive methadone (hereafter, 5- or 6-day pick-up schedule), (5) received a stable dose of methadone for 2 weeks prior to the baseline study visit, and (6) were genotypically sensitive to their prescribed antiretroviral regimen. Study eligibility was not based on antiretroviral treatment experience, current drug use, or adherence. Participants were excluded if they were unable or unwilling to provide informed consent, if they were monolingual Spanish speakers, if they were already receiving antiretroviral DOT, or if their primary HIV infection care provider did not agree to their participation in the study. Changes in antiretroviral regimens were allowed during both the 24-week trial and the 12-month postintervention periods. Antiretroviral therapy was available through public insurance programs for all participants, ensuring stable access throughout the 18-month study.
Intervention and Control Conditions
Participants randomized to the DOT intervention arm received 1 dose of their antiretroviral medications at the same time that they received their daily methadone dose. Methadone clinic nurses dispensed and observed ingestion of both methadone and antiretroviral medications at the usual methadone dispensing locations. Nonobserved antiretroviral doses included evening doses for participants on twice-daily regimens, Saturday doses for participants on 5-day pick-up schedules, and Sunday doses for all participants. In these instances, participants were given enough single-dose antiretroviral pillboxes, or “take home doses,” to last until their next methadone clinic visit. Participants were asked to return these take-home pillboxes to the nurses at their next clinic visit, whether or not they had taken the pills.
DOT participants were informed that the study’s DOT intervention was ending 1 month prior to its actual discontinuation, and were offered the option to discuss long-term DOT with their HIV infection care provider. At the time of DOT discontinuation, we gave DOT participants a 30-day supply of their antiretroviral medications. During the postintervention period, participants who had been in the DOT arm self-administered their antiretroviral medications. Participants in the TAU control group received antiretroviral prescriptions from their regular HIV infection care provider, and self-administered their antiretroviral medication both during and after the 24-week intervention.
Adherence counseling was available to participants in both treatment arms (DOT and TAU) during both the 24-week trial and the postintervention periods. The structure and content of our adherence counseling program have been described elsewhere [19]. All study participants were also able to receive adherence counseling at the conclusion of the 18-month study and continued to receive on-site integrated comprehensive HIV infection medical care and substance abuse treatment throughout and after the study.
Main Outcome Measures
Adherence
During the 24-week intervention period, we conducted pill counts to assess adherence in both the DOT and TAU arms. However, during the postintervention period, because all participants self-administered medications, we were also able to use Medication Event Monitoring Systems (MEMS) to monitor the backbone of the antiretroviral regimen (usually a protease inhibitor or nonnucleoside reverse transcriptase inhibitor). This allowed us to create a composite adherence measure for each participant, by computing a weighted mean average of pill count and MEMS adherence rates at each assessment point. We weighted the calculated adherence rate to account for the number of antiretroviral medications assessed with pill counts.
Our initial postintervention pill count took place at the end of the 24-week visit. At each of the subsequent 12 monthly research visits, we downloaded MEMS data and counted pills. To increase the accuracy of pill counts, interviewers documented activities that might falsely increase or decrease the pill count adherence rate, including receipt of a prescription refill, loss of any pills, or ingestion of pills from another pill source. Final pill count adherence rates for each of 5 assessment periods were derived by first computing the mean pill count adherence rate for all antiretrovirals and then computing the mean pill count adherence rate for all time points in the assessment period (eg, the month 6 pill count adherence rate represents the mean of pill count adherence rates at months 4, 5, and 6). Missing pill counts were not included in analyses. Interviewers did not perform pill counts on the medication that was being electronically monitored.
HIV Load
We collected blood samples to measure HIV load at the 3-, 6-, and 12-month postintervention assessment points. Viral load was quantified using the Versant HIV-1 bDNA 3.0 assay (Bayer, Tarrytown, NY). Virologic outcomes during the postintervention period were the same as those used during the intervention: (1) log10 viral load and (2) proportion of participants with undetectable viral load (<75 copies/mL). We also measured CD4+ T lymphocyte count, using the BD FACSCount system (BD Biosciences, San Jose, CA).
Statistical Analyses
We compared adherence rates between participants in the DOT and TAU arms at 4 assessment points during the 24-week intervention (weeks 4, 12, 16, and 24) and at 5 assessment points during the 12-month postintervention period (months 1, 2, 3, 6, and 12 after trial end). We also compared viral load between the DOT and TAU arms during the 24-week intervention (at baseline and at weeks 8, 16, and 24) and during the 12-month postintervention period (months 3, 6, and 12 after trial end).
After the 24-week DOT intervention period, 6 participants continued receiving antiretroviral DOT at the methadone window. Of these, 4 had been randomized to DOT and 2 to TAU. As per our study design, all analyses were performed using an intent-to-treat approach, which included those participants who continued DOT after the intervention.
We fitted the repeatedly measured outcomes of composite adherence and viral load using a mixed-effects linear model. This model included an intervention effect (DOT or TAU), a time effect, and an intervention by time effect, all of which were considered fixed. Time was entered as a discreet variable, and the covariance matrix of the error term was assumed to be unstructured, to account for within-subject correlations. Subsequently, on the basis of the mixed-effects model, contrasts representing the intervention effect across the time points were constructed and tested at a 2-tailed significance level of .05. In addition, we applied χ2 tests across the time points to determine the significance of the odds of achieving viral suppression for DOT participants compared with TAU participants. Analyses were conducted using SAS software (version 9.1; SAS Institute, Cary, NC).
RESULTS
Sociodemographic and Clinical Characteristics
Trial participants were 52% male, 46% Hispanic, and 41% African American, with a mean age of 47 years. All were antiretroviral experienced, the majority were receiving protease inhibitor–based regimens, and 45% had undetectable viral load at baseline (Table 1). Their median duration of methadone treatment was 10 years (interquartile range [IQR], 5–16 years), with a median methadone dose of 130 mg (IQR, 90–180 mg). At trial enrollment, DOT and TAU participants were matched on all sociodemographic and clinical characteristics, including viral load and self-reported adherence. Sixty-five participants completed the 24-week trial, and 57 participants completed the 12-month postintervention period. Our overall retention rate was 88%. During the postintervention period, 21 participants underwent changes in antiretroviral regimens.
Table 1.
Baseline Characteristics of Participants Who Completed the 24-Week Trial
| Sociodemographic, ART regimen, HIV clinical, and substance use characteristics | No. (%) of participants (n = 65) |
| Mean age, years (SD) | 47 (7) |
| Male sex | 34 (52) |
| Race | |
| White | 7 (11) |
| African American | 27 (41) |
| Other | 31 (48) |
| Ethnicity | |
| Hispanic | 30 (46) |
| Non-Hispanic | 35 (54) |
| Education | |
| Less than high school | 32 (49) |
| High school (partial or completed) | 16 (25) |
| College (partial or completed) | 17 (26) |
| Marital status | |
| Married or living with partner | 29 (45) |
| Widowed, separated, or divorced | 19 (29) |
| Single | 17 (26) |
| Employment status | |
| Employed | 2 (3) |
| Unemployed or unable to work | 63 (97) |
| Insurance | |
| Medicaid | 58 (89) |
| Medicare | 13 (20) |
| Private insurance | 5 (8) |
| Self-reported 7-day antiretroviral adherence rate, % | |
| 100 | 49 (75) |
| <100 | 15 (23) |
| Median duration of HIV infection, years (IQR) | 13 (9–16) |
| Duration of antiretroviral therapy, years | |
| <1 | 14 (22) |
| 1–5 | 28 (43) |
| >5 | 19 (29) |
| Antiretroviral regimen | |
| PI-containing | 61 (94) |
| NNRTI-containing | 15 (23) |
| No. of pills in antiretroviral regimena | |
| 1 | 6 (9) |
| 2 | 32 (49) |
| 3 | 22 (34) |
| 4 | 4 (6) |
| 5 | 1 (2) |
| Antiretroviral dosing regimen | |
| Once per day | 17 (26) |
| 2 or more times per day | 48 (74) |
| Viral load, copies/mL | |
| <75 | 30 (46) |
| 75–400 | 5 (8) |
| 401–10 000 | 20 (31) |
| 10 001–100 000 | 9 (14) |
| >100 000 | 1 (2) |
| Undetectable viral load of <75 copies/mL | 30 (46) |
| Mean viral load, log10 copies/mL | 2.74 |
| Median CD4+ T-cell count, cells/μL (IQR) (n = 63) | 339 (151–494) |
| Median duration of methadone maintenance, years (IQR) (n = 45) | 10 (5–16) |
| Median methadone dose, mg (IQR) (n = 63) | 130 (90–180) |
| Drug used according to end-of-intervention urine toxicology test (n = 57) | |
| Opioidb | 16 (25) |
| Cocainec | 32 (49) |
| Benzodiazepines | 4 (6) |
| Amphetamines | 1 (2) |
Data are no. (%) of participants, unless otherwise indicated.Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; IQR, interquartile range; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; SD, standard deviation.
Participants reporting <3 pills were prescribed combination pills containing multiple antiretroviral medications coformulated in 1 pill.
Other than methadone.
Including crack cocaine.
Differences in Adherence Between Groups
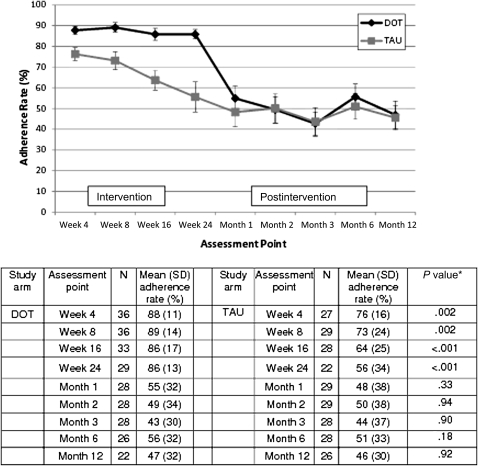
At trial end, the mean adherence rate among DOT participants was 30 percentage points higher than among TAU participants (86% vs 56%, respectively; P <.001) (Figure 1). One month after DOT discontinuation, the difference in adherence rate decreased to 8 percentage points (55% for DOT vs 48% for TAU). Three months after DOT discontinuation, adherence rates were almost identical (49% for DOT vs 50% for TAU). Differences between adherence rates were not significant during any of the 5 postintervention assessment periods (months 1, 2, 3, 6, or 12 after trial end). This finding was supported by our mixed-effects model, which demonstrated that, during the postintervention period, the group effect (DOT vs TAU) was not significant (P = .70), even though adherence rates varied over time (P = .03). Furthermore, the group effect was nonsignificant at any assessment point throughout the 12-month postintervention period (P = .36 for group-by-time effect). When we repeated these analyses after stratifying by regimen type (once daily or twice daily), results were similar.
Figure 1.
Differences in mean adherence rates between human immunodeficiency virus–infected participants in the directly observed therapy (DOT) group and those in the treatment as usual (TAU) group at each assessment point during both the 24-week intervention and the 12-month postintervention follow-up period. Fewer than 65 observations may be reported due to missing data. *P values for weeks 4, 8, 16, and 24 are based on mixed-effects models that include pill count adherence data from the 24-week intervention period; P values for months 1, 2, 3, 6, and 12 are based on mixed-effects models that include composite adherence data from the 12-month postintervention period.
Differences in HIV Load Between Groups
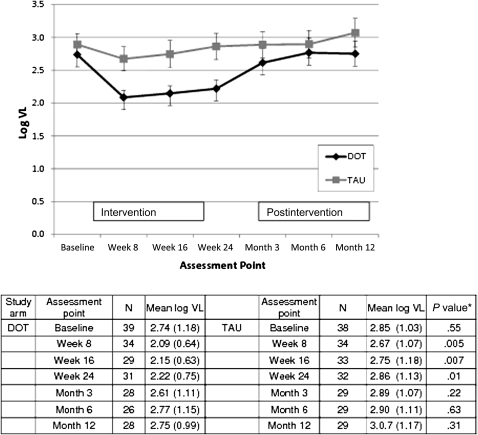
At trial end, viral load was lower among DOT participants than among TAU participants (2.22 vs 2.85 log10 copies/mL, respectively; P < .01) (Figure 2). The difference in viral load between the 2 treatment arms was nonsignificant 3 months after DOT ended (2.6 log10 copies/mL for DOT vs 2.9 log10 copies/mL for TAU) and was not significant at any postintervention assessment point. In our mixed-effects model, the group effect (DOT vs TAU) was not significant (P = .28) and remained nonsignificant at all assessment points throughout the postintervention period (P = .70 for group-by-time effect).
Figure 2.
Differences in log10 viral load (VL) between human immunodeficiency virus–infected participants in the directly observed therapy (DOT) group and those in the treatment as usual (TAU) group at each assessment point during both the 24-week intervention and the 12-month postintervention follow-up period. Fewer than 65 observations may be reported due to missing data. *P values for weeks 4, 8, 16, and 24 are based on mixed-effects models that include data from the 24-week intervention period; P values for months 3, 6, and 12 are based on mixed-effects models that include data from the 12-month postintervention period.
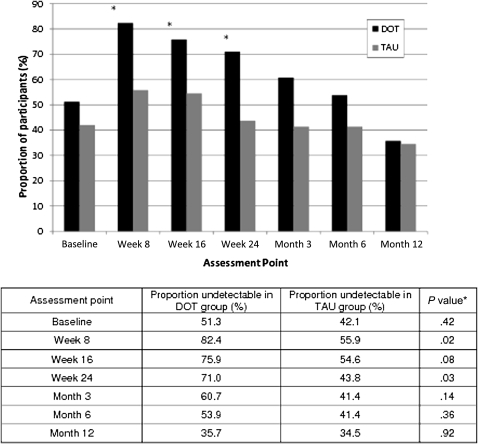
At the end of the 24-week DOT intervention, 71% of DOT participants had undetectable viral load compared with 44% of TAU participants (P = .03), and the odds of viral suppression were 3-fold greater for DOT participants than for TAU participants (unadjusted odds ratio [ORunadj], 3.14 [95% confidence interval {CI}, 1.11–5.35]) (Figure 3). However, after DOT ended, the proportion of DOT participants with undetectable viral load decreased steadily for 12 months, until there was no difference between the DOT and TAU arms (36% for DOT and 34% for TAU; P = .92), and DOT participants had the same odds as TAU participants of achieving viral suppression (ORunadj, 1.06 [95% CI, 0.36–1.84]). Similar results were observed after stratifying by regimen type (once daily or twice daily).
Figure 3.
Proportion of participants with undetectable viral load (<75 copies/mL) by study arm at each assessment point during both the 24-week intervention and the12-month postintervention follow-up period (DOT, directly observed therapy; TAU, treatment as usual). Fewer than 65 observations may be reported due to missing data. *P values for baseline and weeks 8, 16, and 24 are based on χ2 tests evaluating data from the 24-week intervention period; P values for months 3, 6, and 12 are based on χ2 tests evaluating data from the 12-month postintervention period.
DISCUSSION
In this sample of HIV-infected current and former drug users receiving methadone for opioid dependence, the beneficial effects of 24 weeks of DOT on antiretroviral adherence and HIV load were not sustained during a 12-month postintervention follow-up period. After DOT was discontinued, differences in adherence and viral load between those who had been randomized to participate in the DOT intervention and those who had been randomized to self-administer antiretrovirals decreased rapidly. Within a few months, differences between these 2 groups were indistinguishable. These results suggest that long-term DOT should be more widely available in methadone clinics.
The few DOT trials that report adherence and viral load outcomes after DOT has ended have shown that improvements in adherence and viral load achieved during DOT wane over time [14, 20, 21]. Although these DOT programs provided additional support during the DOT intervention, this support ended when DOT ended. Unlike this model, our trial was conducted in the setting of on-site integrated comprehensive HIV infection and other medical care and substance abuse treatment. Therefore, after STAR*DOT trial participation, patients were able to receive ongoing support from healthcare providers, adherence counselors, and substance abuse and other counselors. Despite this supportive environment, we observed a rapid and marked decrease in the beneficial effects of DOT shortly after DOT discontinuation.
Although short-term DOT programs for drug users have been successfully implemented in multiple settings [9–13, 17], the feasibility of long-term DOT has not been evaluated. Several issues are likely to impact the sustainability of DOT. In particular, many programs require dedicated staff (eg, outreach workers) or space (eg, a community van), which can increase program complexity and cost [1–6]. For these reasons, feasibility and sustainability of DOT are likely to be maximized in settings with infrastructures that leverage existing staff and space and allow frequent contact, such as prisons [7], housing facilities [8], or methadone clinics [9–11, 17]. Due to the transiency of the populations served, methadone clinics may be the most promising setting to implement long-term antiretroviral DOT. In addition, because the minimum DOT dose necessary to achieve benefit has not been established, and because prepared antiretroviral pill boxes also improve adherence [22], long-term, flexible DOT delivered in methadone clinics may allow opioid-dependent HIV-infected persons the best chance of achieving optimal HIV infection outcomes. Because methadone patients who progress in substance abuse treatment come to clinics for observed methadone doses less frequently, antiretroviral DOT for highly stable patients could resemble methadone dosing (eg, 1 observed dose and 6 prepackaged take-home doses). Future research should evaluate DOT strategies with less frequent observation, to determine whether they remain effective as the frequency of observed doses decreases.
In settings where long-term, flexible DOT is not feasible, the ability to maintain adherence may be influenced by how patients transition from DOT to self-administration. Several models of DOT discontinuation have been proposed but, to our knowledge, none has been rigorously tested. The transition off DOT to self-administration could involve a supervised period during which the frequency of DOT is tapered and the patient’s ability to self-administer medications is monitored. This period of supervision would allow patients and providers to identify and address challenges to self-administration. Adherence support during the transition might include individually tailored adherence counseling, follow-up phone calls, or assistance with organizing pills. In addition, DOT programs should be flexible enough to accommodate patients who may need or want to transition back to DOT. The option of transitioning in or out of DOT may be particularly important for methadone patients, who may progress in their substance abuse treatment and acquire new self-management skills, but then may have periods of relapse to active drug use and relative social instability, during which DOT might prevent viral rebound.
Our study has several important strengths. To our knowledge, this is the first study to examine durability of effects of antiretroviral DOT in methadone clinics. Second, our DOT protocol has the potential to be sustainable: existing HIV infection care providers made all clinical decisions, we did not restrict any antiretroviral dosing regimens, and we utilized existing methadone clinic space and staff. Nonetheless, limitations should be noted. We only included patients who attended a methadone clinic 5 or 6 times per week, which resulted in a population of relatively unstable current and former drug users. This may partially explain the significant prevalence of antiretroviral regimen switching and the profound waning of benefits that we observed, and may also limit generalizability to other populations. We believe that our high-need population might be ideally suited for long-term DOT. Answering the remaining questions requires follow-up of larger populations over time and inclusion of participants at varying stages of substance abuse treatment.
In conclusion, this study adds to the literature on antiretroviral DOT programs by demonstrating a lack of sustainability of the beneficial effect of DOT among methadone-maintained current and former drug users. Future DOT trials should determine the optimal dose and duration of antiretroviral DOT and evaluate strategies for both flexible DOT programs and transition to self-administration. In addition, to inform allocation of resources, research efforts should focus on identifying the most appropriate target population for long-term DOT.
Notes
Acknowledgments.
The authors thank Metta Cantlo, Amanda Carter, Hillel Cohen, Nina Cooperman, Elise Duggan, Uri Goldberg, Harris Goldstein, Joseph Hecht, Laxmi Modali, Jennifer Mouriz, Francesca Parker, Megha Ramaswamy, and Maite Villanueva for assistance with protocol development, pharmacy coordination, data collection and management, and laboratory analyses. The trial was dependent on the cooperation of the medical providers, nurses, and patients at the Albert Einstein College of Medicine and Montefiore Medical Center Division of Substance Abuse. We also thank the Center for AIDS Research of the Albert Einstein College of Medicine and Montefiore Medical Center, and staff members at Melrose Pharmacy, Bendiner & Schlesinger, and Bio-Reference Laboratories.
Financial support.
This work was supported by the National Institute of Drug Abuse at the National Institutes of Health (grants R01 DA015302 and R25 DA14551 to J. H. A., K23 DA021087 to K. M. B.); and the Center for AIDS Research (grant P30 AI051519 to the Albert Einstein College of Medicine of Yeshiva University).
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Behforouz HL, Kalmus A, Scherz CS, Kahn JS, Kadakia MB, Farmer PE. Directly observed therapy for HIV antiretroviral therapy in an urban US setting. J Acquir Immune Defic Syndr. 2004;36:642–5. doi: 10.1097/00126334-200405010-00016. [DOI] [PubMed] [Google Scholar]
- 2.Altice FL, Mezger JA, Hodges J, et al. Developing a directly administered antiretroviral therapy intervention for HIV-infected drug users: implications for program replication. Clin Infect Dis. 2004;38:S376–87. doi: 10.1086/421400. [DOI] [PubMed] [Google Scholar]
- 3.Mitty JA, Macalino GE, Bazerman LB, et al. The use of community-based modified directly observed therapy for the treatment of HIV-infected persons. J Acquir Immune Defic Syndr. 2005;39:545–50. [PubMed] [Google Scholar]
- 4.Khanlou H, Kandula VR, Yeh V, et al. Pilot study of directly observed therapy in highly nonadherent HIV-infected patients in an urban community-based institution. J Acquir Immune Defic Syndr. 2003;33:651–3. doi: 10.1097/00126334-200308150-00017. [DOI] [PubMed] [Google Scholar]
- 5.Ma M, Brown BR, Coleman M, Kibler JL, Loewenthal H, Mitty JA. The feasibility of modified directly observed therapy for HIV-seropositive African American substance users. AIDS Patient Care STDS. 2008;22:139–46. doi: 10.1089/apc.2007.0063. [DOI] [PubMed] [Google Scholar]
- 6.Wohl AR, Garland WH, Valencia R, et al. A randomized trial of directly administered antiretroviral therapy and adherence case management intervention. Clin Infect Dis. 2006;42:1619–27. doi: 10.1086/503906. [DOI] [PubMed] [Google Scholar]
- 7.Babudieri S, Aceti A, D'Offizi GP, Carbonara S, Starnini G. Directly observed therapy to treat HIV infection in prisoners. JAMA. 2000;284:179–80. doi: 10.1001/jama.284.2.179. [DOI] [PubMed] [Google Scholar]
- 8.Tinoco I, Giron-Gonzalez JA, Gonzalez-Gonzalez MT, et al. Efficacy of directly observed treatment of HIV infection: experience in AIDS welfare homes. Eur J Clin Microbiol Infect Dis. 2004;23:331–5. doi: 10.1007/s10096-003-1099-8. [DOI] [PubMed] [Google Scholar]
- 9.Clarke S, Keenan E, Ryan M, Barry M, Mulcahy F. Directly observed antiretroviral therapy for injection drug users with HIV infection. AIDS Read. 2002;12:305–6. [PubMed] [Google Scholar]
- 10.Conway B, Prasad J, Reynolds R, et al. Directly observed therapy for the management of HIV-infected patients in a methadone program. Clin Infect Dis. 2004;38:S402–8. doi: 10.1086/421404. [DOI] [PubMed] [Google Scholar]
- 11.Lucas GM, Weidle PJ, Hader S, Moore RD. Directly administered antiretroviral therapy in an urban methadone maintenance clinic: a nonrandomized comparative study. Clin Infect Dis. 2004;38:S409–13. doi: 10.1086/421405. [DOI] [PubMed] [Google Scholar]
- 12.Macalino GE, Hogan JW, Mitty JA, et al. A randomized clinical trial of community-based directly observed therapy as an adherence intervention for HAART among substance users. AIDS. 2007;21:1473–7. doi: 10.1097/QAD.0b013e32811ebf68. [DOI] [PubMed] [Google Scholar]
- 13.Altice FL, Maru DS, Bruce RD, Springer SA, Friedland GH. Superiority of directly administered antiretroviral therapy over self-administered therapy among HIV-infected drug users: a prospective, randomized, controlled trial. Clin Infect Dis. 2007;45:770–8. doi: 10.1086/521166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarna A, Luchters S, Geibel S, et al. Short- and long-term efficacy of modified directly observed antiretroviral treatment in Mombasa, Kenya: a randomized trial. J Acquir Immune Defic Syndr. 2008;48:611–9. doi: 10.1097/QAI.0b013e3181806bf1. [DOI] [PubMed] [Google Scholar]
- 15.Pearson CR, Micek MA, Simoni JM, et al. Randomized control trial of peer-delivered, modified directly observed therapy for HAART in Mozambique. J Acquir Immune Defic Syndr. 2007;46:238–44. doi: 10.1097/QAI.0b013e318153f7ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hart JE, Jeon CY, Ivers LC, et al. Effect of directly observed therapy for highly active antiretroviral therapy on virologic, immunologic, and adherence outcomes: a meta-analysis and systematic review. J Acquir Immune Defic Syndr. 2010;54:167–79. doi: 10.1097/QAI.0b013e3181d9a330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berg KM, Litwin AH, Li X, Heo M, Arnsten JH. Directly observed antiretroviral therapy improves adherence and viral load in drug users attending methadone maintenance clinics: a randomized controlled trial. Drug Alcohol Depend. 2011;113:192–9. doi: 10.1016/j.drugalcdep.2010.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berg KM, Mouriz J, Li X, Duggan E, Goldberg U, Arnsten JH. Rationale, design, and sample characteristics of a randomized controlled trial of directly observed antiretroviral therapy delivered in methadone clinics. Contemp Clin Trials. 2009;30:481–9. doi: 10.1016/j.cct.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooperman NA, Parsons JT, Chabon B, Berg KM, Arnsten JH. The development and feasibility of an intervention to improve HAART adherence among HIV-positive patients receiving primary care in methadone clinics. J HIV AIDS Soc Serv. 2007;6:101–20. [Google Scholar]
- 20.Gross RF, Tierney CF, Andrade AF, et al. Modified directly observed antiretroviral therapy compared with self-administered therapy in treatment-naive HIV-1-infected patients: a randomized trial. Arch Intern Med. 2009;169:1224–32. doi: 10.1001/archinternmed.2009.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maru DS, Bruce RD, Walton M, Springer SA, Altice FL. Persistence of virological benefits following directly administered antiretroviral therapy among drug users: results from a randomized controlled trial. J Acquir Immune Defic Syndr. 2009;50:176–81. doi: 10.1097/QAI.0b013e3181938e7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalichman SC, Cain D, Cherry C, Kalichman M, Pope H. Pillboxes and antiretroviral adherence: prevalence of use, perceived benefits, and implications for electronic medication monitoring devices. AIDS Patient Care STDS. 2005;19:833–9. doi: 10.1089/apc.2005.19.833. [DOI] [PubMed] [Google Scholar]