In a cohort of human immunodeficiency virus–infected adults beginning antiretroviral therapy, CD4+ lymphocyte recovery at 12 months was highest among overweight patients (body mass index, 25–30 kg/m2). Similar results were observed in the subgroups with virologic suppression and minimal weight change.
Abstract
Background. Higher body mass index (BMI) was associated with slower human immunodeficiency virus (HIV) disease progression before the availability of effective antiretroviral therapy (ART), but the relationship between pretreatment BMI and CD4+ lymphocyte recovery on ART is not well described.
Methods. We conducted an observational cohort study of HIV-infected, ART-naive adults starting treatment at a clinic affiliated with Vanderbilt University in Nashville, Tennessee. We assessed the relationship between pretreatment BMI and CD4+ lymphocyte count change from baseline to 12 months in all subjects, among those with plasma HIV-1 RNA levels <400 copies/mL for ≥6 months and those with <10% change in weight during follow-up. Linear regression models were adjusted for age, sex, race, protease inhibitor usage, year of ART initiation, and baseline CD4+ lymphocyte count and HIV-1 RNA level.
Results. A total of 915 patients met inclusion criteria; 78% were male, and their median age, BMI, and CD4+ lymphocyte count were 39 years, 24 kg/m2, and 171 cells/μL, respectively. The CD4+ lymphocyte increase at 12 months was greatest among patients with a pretreatment BMI of ∼25–30 kg/m2 and diminished above and below this range (P = .03). Similar patterns were observed in the subgroup analyses. Among patients with a pretreatment CD4+ lymphocyte count <200 cells/μL, a BMI of ∼25 kg/m2 was associated with the highest odds of reaching a CD4+ lymphocyte count >350 cells/μL at 12 months (P = .05).
Conclusions. 12-month immune reconstitution on ART was highest among patients commonly classified as overweight, suggesting there may be an optimal BMI range for immune recovery on ART.
Being overweight (ie, body mass index [BMI] 25.0–29.9 kg/m2) or obese (BMI ≥30 kg/m2) is a risk factor for several metabolic, cardiovascular, and other diseases in adults regardless of human immunodeficiency virus (HIV) infection status; but evidence from the pre–antiretroviral therapy (ART) era suggested an association between excess adiposity and reduced progression to AIDS and HIV-related mortality [1–6]. Prior analyses have examined the impact of malnutrition (BMI <18.5 kg/m2) on the response to ART in resource limited settings [7–10], but there are few studies on immune reconstitution among normal and high BMI adults in developed countries [11, 12]. In particular, the relationship between BMI and CD4+ lymphocyte recovery as continuous variables has not been described elsewhere, nor has there been an assessment for possible sex differences.
The prevalence of HIV-associated wasting in the United States declined significantly with the availability of combination ART, and the proportion of obese HIV-1 infected adults, estimated to be 20%–30%, now rivals that in the general population [13–16]. Both undernutrition and excess adiposity are associated with altered innate and adaptive immune responses, and we hypothesized that robust immune reconstitution on ART may be associated with an optimal BMI range [17–20]. In this analysis, we assess the effect of BMI at treatment initiation on 12-month CD4+ lymphocyte recovery in a cohort of HIV-infected men and women starting ART.
METHODS
We conducted a retrospective observational cohort study of adults (age ≥18 years) enrolled in care at the Comprehensive Care Center, an outpatient clinic affiliated with Vanderbilt University in Nashville, Tennessee. The study cohort included all ART-naive (defined as no prior record of any antiretroviral agent exposure) patients who started treatment (defined as a combined regimen of ≥3 antiretroviral agents) between 1 January 1998 and 31 December 2008. Women who were pregnant at ART initiation or became pregnant during the 12-month follow-up period were excluded. Data were collected from the center’s electronic medical record, which records clinical information from providers at the time of the patient encounter and automatically uploads laboratory results. Patient weight was determined at each visit using a single measurement on the same mechanical clinic scale. Laboratory data, patient deaths, and all ART use were validated by systematic chart review.
Data collected included BMI, age at ART initiation, sex, race (white or nonwhite), duration from diagnosis of HIV infection to treatment, year of ART initiation, treatment regimen, history of injection drug use, coinfection with hepatitis C virus, history of an AIDS-defining event, and pre- and post-ART initiation CD4+ lymphocyte count and plasma HIV-1 RNA measurements. Body weight nearest to ART initiation within the period from 180 days before to 30 days after treatment start was used to calculate baseline BMI (preference was given to pretreatment values). Since all patients were ≥18 years old, height at any time after enrollment in care was considered valid for calculating BMI. AIDS-defining events were identified on the basis of 1993 Centers for Disease Control and Prevention classification criteria [21], excluding diagnoses based solely on CD4+lymphocyte counts <200 cells/μL. The absolute CD4+ lymphocyte change during follow-up was calculated as the difference between the CD4+ lymphocyte count closest to ART initiation and the count at 12 months after ART (within 6 months before or after this date). A secondary analysis used multiply imputed BMI data for subjects with a known weight but missing height; height was imputed using age, sex, and baseline and 12-month CD4+ lymphocyte counts [22]. Patients who died or were unavailable for follow-up before 12 months were excluded.
We compared patient demographics and clinical characteristics across BMI strata and between sexes, using Pearson χ2, Wilcoxon rank sum, or Kruskal–Wallis tests, as appropriate. The association between BMI and change in CD4+ lymphocyte count was analyzed using multiple linear regression adjusted for age, race, protease inhibitor (PI) usage, year of ART initiation, and baseline CD4+ lymphocyte count and plasma HIV-1 RNA level (log10 transformed). Sex was hypothesized to be a potential effect modifier of the relationship between BMI and CD4+ lymphocyte count change, so an interaction term between sex and BMI was included in the model. The relationship between BMI and CD4+ lymphocyte count change was fit using restricted cubic splines with 3 knots to avoid assuming a linear relationship (results were verified using 4 knots). P values were computed using likelihood ratio tests.
The primary analysis was repeated in 2 subgroups. We first limited the cohort to patients with ≥6 months of post-ART virologic suppression, determined by the sum total of days after each suppressed HIV-1 RNA measurement (<400 copies/mL) between treatment initiation and 12 months (any days after a nonsuppressed measurement were not counted). Second, we limited the cohort to patients with <10% variation in weight from treatment initiation to 12 months.
To assess the effect of BMI on reaching the clinically relevant CD4+ lymphocyte thresholds of 200 and 350 cells/μL, we used a logistic regression model and again fit BMI with restricted cubic splines. The cohort for this analysis was limited to patients with a pretreatment CD4+ lymphocyte count <200 cells/μL, and the model was adjusted for the same variables as the primary analysis. Analyses were performed using SPSS and R (version 2.12.1; www.r-project.org) software. Analysis scripts are posted at biostat.mc.vanderbilt.edu/ArchivedAnalyses. The study protocol was approved by the institutional review board of Vanderbilt University Medical Center.
RESULTS
Of 1189 ART-naive patients who started treatment between 1 January 1998 and 31 December 2008, 120 were ineligible for this study: 19 had a plasma HIV-1 RNA level <400 copies/mL at initiation of ART, 6 had a recorded therapy initiation date after the last recorded clinic visit, 58 were pregnant during the baseline window period or became pregnant during the follow-up period, 20 were missing a baseline CD4+ lymphocyte count, 7 were missing a baseline weight measurement, and 10 were missing both. Of the remaining 1069 patients, 915 had a baseline BMI measurement during the window period. Patients missing a height measurement, and thus a baseline BMI measurement, were more likely to be male (P < .01) but did not significantly differ by age, race, PI inclusion in first ART regimen, year of ART initiation, or baseline CD4+ lymphocyte count or HIV-1 RNA level (data not shown).
Demographic and clinical characteristics are shown in Table 1. Sixteen percent of patients had BMIs <20 kg/m2 at baseline and 15% had BMIs ≥30 kg/m2. There was no significant difference in age, race, or year of ART initiation between BMI categories, but patients in the lower BMI strata tended to be male, had lower baseline CD4+ lymphocyte counts and higher HIV-1 RNA measurements, and were more likely to have had an AIDS-defining event before starting treatment (P < .01 for all comparisons). When stratified by sex, women had a higher median BMI than men (25.8 vs 23.4 kg/m2) and lower HIV-1 RNA level (P < .01 for both). There were no significant sex or BMI differences in the percentage with a reported history of injection drug use (10% overall) or hepatitis C virus coinfection (13% overall; data not shown).
Table 1.
Cohort Demographic and Clinical Characteristics at Antiretroviral Therapy Initiation, Stratified by Body Mass Index and Sex
| Characteristic | All Patients (n = 915) | BMI Strata, kg/m2 |
Sex |
|||||||
| <20.0 (n = 146) | 20.0–24.9, (n = 389) | 25.0–29.9, (n = 239) | ≥30.0–39.9. (n = 126) | ≥40.0, (n = 15) | Pa | Female (n = 203) | Male (n = 712) | Pa | ||
| Female, no. (%) | 203 (22) | 25 (17) | 68 (18) | 53 (22) | 47 (37) | 10 (66) | <.01 | … | … | … |
| Age, median (IQR), years | 39 (32–45) | 38 (30–43) | 38 (32–45) | 39 (32–45) | 40 (33–46) | 43 (33–48) | .23 | 40 (31–46) | 39 (32–44) | .46 |
| Nonwhite race, no. (%) | 458 (50) | 70 (48) | 196 (50) | 112 (47) | 71 (56) | 9 (60) | .49 | 133 (66) | 325 (46) | <.01 |
| CD4+ lymphocyte count, median (IQR), cells/μL | 171 (50–280) | 75 (20–216) | 162 (42–280) | 210 (82–308) | 224 (90–289) | 292 (195–499) | <.01 | 189 (55–288) | 168 (50–279) | .49 |
| Log10 HIV-1 RNA, median (IQR) | 4.92 (4.52–5.48) | 5.15 (4.74–5.66) | 5.00 (4.63–5.55) | 4.83 (4.35–5.25) | 4.67 (4.27–5.06) | 4.59 (4.00–4.83) | <.01 | 4.75 (4.32–5.33) | 4.96 (4.58–5.51) | <.01 |
| Time from diagnosis of HIV infection to ART, median (IQR), years | 0.58 (0.23–2.72) | 0.46 (0.18–3.04) | 0.51 (0.23–2.50) | 0.84 (0.24–3.13) | 0.81 (0.27–2.52) | 0.40 (0.25–3.78) | .28 | 0.70 (0.26–2.47) | 0.54 (0.22–2.93) | .53 |
| AIDS-defining event before ART initiation, no. (%) | 96 (11) | 30 (21) | 53 (14) | 8 (3) | 4 (3) | 1 (7) | <.01 | 19 (9) | 77 (11) | .55 |
| Year of ART initiation, median | 2003 | 2003 | 2003 | 2004 | 2004 | 2004 | .15 | 2004 | 2003 | .22 |
| Protease inhibitor in first ART regimen, no. (%) | 407 (45) | 75 (51) | 166 (43) | 96 (40) | 63 (50) | 7 (47) | .15 | 103 (51) | 304 (43) | .04 |
| Patients achieving HIV-1 RNA levels <400 copies/mL, no. (%) | 798 (87) | 122 (84) | 336 (86) | 210 (88) | 116 (92) | 14 (93) | .26 | 173 (85) | 625 (88) | 0.34 |
| Time to HIV-1 RNA levels <400 copies/mL, median (IQR), days | 70 (36–129) | 89 (48–185) | 71 (38–129) | 65 (35–129) | 63 (33–113) | 61 (42–224) | .01 | 60 (35–117) | 73 (37–133) | .11 |
| Patients with 12-month CD4+ lymphocyte count available, no. (%) (n = 753b) | 705 (94) | 109 (92) | 294 (93) | 188 (95) | 107 (85) | 12 (80) | .92 | 152 (93) | 553 (94) | .58 |
| Time to 12-month CD4+ lymphocyte count, median (IQR), years | 0.97 (0.90–1.06) | 0.97 (0.90–1.05) | 0.98 (0.92–1.07) | 0.96 (0.88–1.06) | 0.95 (0.90–1.02) | 1.02 (0.96–1.12) | .24 | 0.96 (0.89–1.05) | 0.97 (0.91–1.06) | .55 |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; HIV, human immunodeficiency virus; IQR, interquartile range.
Comparison between baseline BMI strata or between sexes.
One patient was missing a baseline HIV-1 RNA measurement and was not included in the adjusted models.
CD4+ lymphocyte count measurements at 12 months were available for 753 patients; those without a 12-month CD4+ lymphocyte measurement were more likely to be nonwhite (P < .01; data not shown). There were no differences in the duration from baseline to the 12-month CD4+ lymphocyte measurement across BMI strata or between sexes.
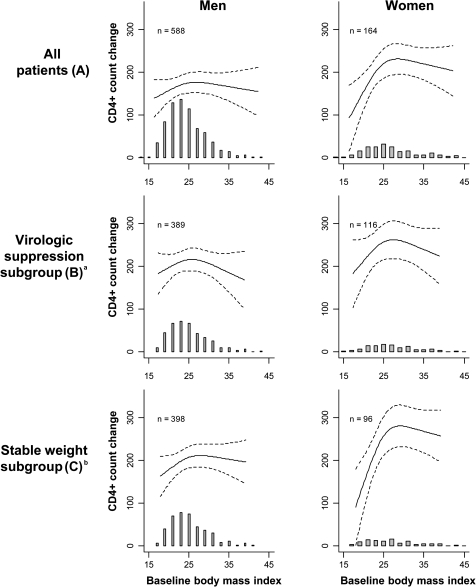
Baseline BMI was associated with 12-month CD4+ lymphocyte change after adjustment for age, race, PI usage, year of ART initiation, and baseline CD4+ lymphocyte count and HIV-1 RNA level (P = .03) (Figure 1). The relationship was nonlinear (P = .01), and diminished 12-month CD4+ lymphocyte recovery was observed at the extremes of BMI for both sexes. To facilitate comparisons between hypothetical patients, values for the change in CD4+ lymphocyte counts at the arbitrary BMI levels of 20, 25, 30, and 40 kg/m2 were extracted from Figure 1 and are shown in Table 2; however, the measure of statistical association accompanying these data is based not on any specific BMI comparison but rather on the entire relationship across all BMI levels. The reference for all comparisons in Table 2 and Table 3 is a BMI of 25 kg/m2. For example, a BMI of 20 kg/m2, compared with the reference, was associated with a reduced 12-month CD4+ lymphocyte gain among both women (−65 cells/μL) and men (−18 cells/μL). Similarly, obese women and men with a BMI of 40 kg/m2 had lower 12-month CD4+ lymphocyte gains (−12 and −17 cells/μL, respectively) compared with the reference. The interaction of sex and BMI did not appear to be an important determinant of CD4+ lymphocyte change (P = .16 for the interaction term), but male sex was associated with lower CD4+ recovery overall (P < .01).
Figure 1.
Baseline body mass index (BMI) and change in CD4+ lymphocyte count at 12 months, stratified by sex, for all patients (A), the virologic suppression subgroup (B), and the stable weight subgroup (C). Regression lines are adjusted for age, race, protease inhibitor usage, year of antiretroviral therapy (ART) initiation, and baseline CD4+ lymphocyte count and plasma HIV-1 RNA level (log10 transformed); dashed lines represent 95% confidence intervals. Shaded histogram shows the number of patients contributing data (same scale as CD4+ lymphocyte count change; each bar represents a 2 kg/m2 BMI interval). aIncludes patients with ≥6 months cumulative virologic suppression between ART initiation to 12 months. bIncludes patients with <10% change in weight between ART initiation and 12 months.
Table 2.
Multiple Regression Model of CD4+ Lymphocyte Count Change at 12 Months After Antiretroviral Therapy Initiation
| All patients (n = 752) |
Virologic suppression subgroupa (n = 505) |
Stable weight subgroupb (n = 494) |
||||
| Covariate | Change in CD4+ Lymphocyte Count (95% CI), Cells/μL | P | Change in CD4+ Lymphocyte Count (95% CI), Cells/μL | P | Change in CD4+ Lymphocyte Count (95% CI), Cells/μL | P |
| Baseline BMI comparison, kg/m2c | .03d | .26d | <.01d | |||
| Female patients | ||||||
| 20 vs 25 | −65 (−109 to −21) | −44 (−95 to 6) | −111 (−174 to −48) | |||
| 30 vs 25 | 9 (−7 to 25) | 1 (−20 to 22) | 22 (2–42) | |||
| 40 vs 25 | −12 (−69 to 45) | −37 (−116 to 41) | −3 (−68 to 62) | |||
| Male patients | ||||||
| 20 vs 25 | −18 (−42 to 6) | −18 (−50 to 14) | −29 (−61 to 3) | |||
| 30 vs 25 | −1 (−15 to 13) | −9 (−28 to 10) | 3 (−12 to 17) | |||
| 40 vs 25 | −17 (−67 to 34) | −53 (−133 to 28) | −12 (−67 to 43) | |||
| Baseline CD4+ lymphocyte count, per 100 cells/μL increase | −7 (−14 to 1) | .08 | 0 (−8 to 8) | .99 | −4 (−13 to 5) | .40 |
| Baseline age, per 1-year increase | −1 (−2 to 1) | .18 | −2 (−3 to 0) | .01 | −2 (−3 to 0) | .04 |
| Baseline log10 HIV-1 RNA, per 1-unit increase | 22 (3–41) | .03 | 39 (17–61) | <.01 | 25 (1–48) | .04 |
| Nonwhite race | −38 (−62 to −15) | <.01 | −26 (−53 to 1) | .06 | −36 (−66 to −7) | .01 |
| Protease inhibitor usage | −19 (−43 to 4) | .11 | 14 (−14 to 42) | .34 | −22 (−50 to 7) | .14 |
| Year of ART initiation, per year | 5 (1–10) | <.01 | 3 (−1 to 8) | .16 | 4 (−1 to 9) | .11 |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; CI, confidence interval; HIV, human immunodeficiency virus.
The viral suppression subgroup includes patients with ≥6 months of cumulative viral suppression between ART initiation and 12-month follow-up.
The stable weight subgroup includes patients with <10% change in weight between ART initiation and 12-month follow-up.
P values for sex and BMI interaction term: all patients, P = .16; virologic suppression subgroup, P = .61; stable weight subgroup, P = .06. When the interaction term was removed, male sex was associated with a reduced CD4+ lymphocyte change compared with female sex (−38 cells/μL [95% CI, −62 to −15]; P < .01) in the full cohort.
The P values, obtained with a likelihood ratio test (5 degrees of freedom), are based on the null hypothesis that BMI and sex had no impact on CD4+ lymphocyte change (ie, the null hypothesis that the relationship between BMI and change in CD4+ lymphocyte count shown in each panel of Figure 1 is a flat line at the same level of CD4+ lymphocyte count change for male and female patients). Comparisons of CD4+ lymphocyte count changes at the arbitrary BMI levels of 20, 25, 30, and 40 kg/m2 are extracted from Figure 1 and shown in this table to help with interpretation (reference for all comparisons is a BMI of 25 kg/m2). The P values, however, are based not on any specific BMI comparison but rather on the entire relationship across all BMI levels.
Table 3.
Adjusted Odds of Attaining a 12-Month CD4+ Lymphocyte Count of >200 or >350 cells/μL Among Patients With a Baseline Count of <200 Cells/μL
| 12-month CD4+ lymphocyte count >200 cells/μL (n = 415) |
12-month CD4+ lymphocyte count >350 cells/μL (n = 415) |
|||
| Covariate | Adjusted OR (95% CI) | P | Adjusted OR (95% CI) | P |
| Baseline BMI, kg/m2 | .17a | 05a | ||
| 20 vs 25 | 0.72 (.51–1.03) | 0.58 (.36–.93) | ||
| 30 vs 25 | 0.95 (.77–1.18) | 0.80 (.55–1.16) | ||
| 40 vs 25 | 0.72 (.33–1.55) | 0.35 (.09–1.35) | ||
| Baseline CD4+ lymphocyte count, per 100 cells/μL increase | 4.87 (3.13–7.57) | <.01 | 3.78 (2.38–6.00) | <.01 |
| Baseline age, per 1-year increase | 1.00 (.97–1.03) | .99 | 0.99 (.97–1.02) | .63 |
| Baseline log10 HIV-1 RNA, per 1-unit increase | 1.33 (.91–1.96) | .14 | 1.89 (1.18–3.01) | <.01 |
| Male sexb | 0.83 (.47–1.45) | .51 | 0.28 (.16–0.51) | <.01 |
| Nonwhite race | 1.12 (.71–1.76) | .62 | 0.83 (.50–1.40) | .49 |
| Protease inhibitor usage | 1.03 (.64–1.65) | .90 | 1.12 (.66–1.89) | .68 |
| Year of ART initiation, per year | 1.07 (.99–1.17) | .09 | 1.10 (1.00–1.22) | .04 |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; CI, confidence interval; HIV, human immunodeficiency virus; OR, odds ratio.
Comparisons of CD4+ lymphocyte count change at the arbitrary BMI levels of 20, 25, 30, and 40 kg/m2 are extracted from Figure 2 (reference for all comparisons is a BMI of 25 kg/m2). The P values are based not on any specific BMI comparison but rather on the entire relationship across all BMI levels.
There was not a significant interaction between BMI and sex in the models for CD4+ lymphocyte counts >200 model (0.12) or >350 (0.72) cells/μL, so the model was refit, without treating sex as a potential effect modifier.
Baseline plasma HIV-1 RNA level, nonwhite race, and year of ART initiation were also significantly associated with 12-month CD4+ lymphocyte change (P < .05). When the model was further adjusted for other potential confounders in a sensitivity analysis, a longer duration from diagnosis of HIV infection to ART initiation was associated with a lower 12-month CD4+ lymphocyte gain (P = .03), but a history of an AIDS-defining event, injection drug use, or hepatitis C coinfection was not associated with immune recovery (P = .23, 0.73, and 0.21, respectively; data not shown). The relationship between BMI and CD4+ lymphocyte change remained similar when these variables were included in the model.
In a secondary analysis, we used multiply imputed height values for subjects with a missing height but a recorded weight during the window period. An additional 154 subjects were included, which increased the cohort size to 1069. The effect of BMI on 12-month CD4+ lymphocyte count remained significant and continued to demonstrate reduced immune recovery at the extremes of BMI (P = .03), but again the interaction of sex and BMI did not seem to be an important determinant of CD4+ lymphocyte change (P = .14 for the interaction term; data not shown).
To assess the potential effects of persistent viral replication on immune reconstitution, due to either ART nonadherence or drug resistance, we performed a subgroup analysis of the 505 patients with complete data and ≥6 months of virologic suppression after ART initiation. A similar pattern of reduced CD4+ lymphocyte gains at the extremes of BMI was observed, but the statistical relationship was nonsignificant (P = .26) (Figure 1 and Table 2). To limit the potential effect of BMI changes during follow-up, we also restricted the cohort to the 494 patients with complete data and <10% change in weight from ART initiation to 12 months, and we observed a marked reduction in immune recovery among the patients with low BMI (particularly women). The overall relationship of BMI and CD4+ lymphocyte change remained statistically significant (P < .01) (Figure 1 and Table 2).
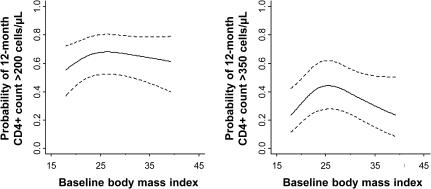
Similar trends were seen in the relationship between BMI and the probability of reaching the clinically relevant CD4+ lymphocyte thresholds of 200 and 350 cells/μL at 12 months among the patients whose pretreatment CD4+ lymphocyte count was <200 cells/μL (Figure 2 and Table 3). The interaction of sex with BMI was not significant in either model (P = .12 and P = .72 for >200 and >350 cells/μL, respectively), so the models were refit without the interaction term. Compared with patients with a BMI of 25 kg/m2, the odds of having a CD4+ lymphocyte count of >200 cells/μL at 12 months decreased for those with a BMI of 20 kg/m2 (adjusted odds ratio, 0.72) or 40 kg/m2 (adjusted odds ratio, 0.72), but the association was not significant (P = .17). However, the association between BMI and CD4+ lymphocyte counts >350 cells/μL at 12 months was significant (P = .05), with adjusted odds ratios of 0.58 or 0.35 for comparisons between patients with a BMI of 25 kg/m2 and those with a BMI of 20 or 40 kg/m2, respectively.
Figure 2.
Baseline body mass index (BMI) and the probability of attaining a 12-month CD4+ lymphocyte count >200 or >350 cells/μL. Regression lines are adjusted for age, race, protease inhibitor usage, year of antiretroviral therapy initiation, and baseline CD4+ lymphocyte count and plasma HIV-1 RNA level (log10 transformed); dashed lines represent 95% confidence intervals.
DISCUSSION
In a cohort of adults enrolled in a large HIV treatment clinic, the magnitude of immune reconstitution 12 months after ART initiation increased with rising BMI and seemed to reach a plateau in the range of BMI 25 to 30 kg/m2 but declined slightly at higher BMI among both men and women. The diminution of CD4+ lymphocyte gains in patients with higher BMIs did not seem related to prolonged viral replication, because heavier patients achieved virologic suppression more rapidly. Patterns in CD4+ lymphocyte count changes observed among those with ≥6 months virologic suppression on ART were similar, though not statistically significant. The relationships between BMI and CD4+ lymphocyte count changes persisted and the strength of association increased when the cohort was limited to those with <10% weight change, though the difference was primarily observed in the patients with low BMIs. Although further exploration of the underlying physiologic processes are needed, these epidemiologic findings suggest that a BMI in the range of 25–30 kg/m2 may be associated with optimal immune reconstitution in the first year of ART.
Our findings are consistent with a recent analysis from the US Military HIV Natural History Study (n = 607), which found that obesity (BMI, ≥30 kg/m2) was associated with a significantly lower adjusted gain in CD4+ lymphocytes after ART (−34 cells/μL; P = .01) compared with findings in normal-weight patients (BMI, 20–24.9 kg/m2) [12]. However, the cohort was composed of military recruits (mean age, 29 years; 96% male; 8% obese), and the results may not be representative of women and older, less fit populations. In contrast to an analysis of the HIV Outpatient Study cohort (n = 711), which found that being overweight or obese (BMI, ≥25.0 kg/m2) was not associated with a higher likelihood of exceeding a 100 cells/μL threshold CD4+ lymphocyte increase at 3–9 months after ART initiation, compared with findings in normal-weight controls (BMI, 18.5–24.9 kg/m2), we observed a higher likelihood of reaching a CD4+ lymphocyte threshold of 350 cells/μL among patients with a BMI of ∼25 kg/m2 and a pretreatment CD4+ lymphocyte count <200 cells/μL [11]. These discrepancies may be due to different analysis methods; prior studies treated CD4+ lymphocyte count as a stratified variable, and we treated it as a continuous variable.
It is unclear whether the varying 12-month CD4+ lymphocyte recovery observed in our cohort reflects an immunomodulatory effect of specific tissue stores (eg, adipose tissue) or whether individuals with high or low BMIs are more likely to have other health conditions or physiologic derangements that impair peripheral CD4+ lymphocyte repopulation. Undernutrition is associated with suppression of the antigen-specific arms of the immune system, decreased T-lymphocyte proliferation, and atrophy of the lymph tissues, but many of these data are from children and the relevance to immune recovery on ART is unclear [23–26]. A higher plasma HIV-1 RNA level in the lower BMI strata of our cohort probably contributed to the longer interval before virologic suppression (median, 89 days for BMIs <20 kg/m2 vs 71 days for 20.0–24.9 kg/m2), and some evidence suggests that higher HIV-1 RNA levels may promote increased cellular immune activation and altered CD4+ lymphocyte recovery kinetics, although findings of other studies differ on this association [27–29].
Patients with lower BMIs with a weight change of <10% during follow-up had markedly reduced CD4+ lymphocyte recovery, suggesting that a failure to gain needed weight may be a marker of incomplete virologic suppression, an intercurrent illness or may preclude an optimal response to ART. Indeed, 39% (22/56) of patients in the group with BMIs <20 kg/m2 did not maintain virologic suppression for ≥6 months, compared with 34% (70/205) with BMIs of 20.0–24.9 kg/m2. Additionally, 11% of the first group had an AIDS-defining event recorded in the first 12 months of ART, compared with 8% of the second group.
Although the association between BMI and CD4+ lymphocyte change did not differ significantly between men and women, the difference in CD4+ recovery between those with BMIs of 20 versus 25 kg/m2 was larger in women (−65 cells/μL) than in men (−18 cells/μL). However, the proportions of women and men achieving ≥6 months of virologic suppression did not differ significantly within the <20 kg/m2 category (55% for both men and women) or the 20.0–24.9 kg/m2 category (71% for women versus 63% for men; P = .22). Other studies have demonstrated alterations in plasma HIV-1 RNA levels and circulating CD4+ lymphocyte counts related to cyclic variations in estrogen concentration, ovulation, and pregnancy, which may be reflected in our results, but additional studies of the combined effects of sex- and body composition-related hormones on lymphocyte function are needed [30–33].
The absence of a persistently positive correlation between BMI and CD4+ lymphocyte recovery among the obese patients could represent a natural maximum plateau of CD4+ lymphocyte recovery in the first 12 months of ART, a direct effect of adipose tissue on lymphocyte proliferation and turnover or a more global physiologic impairment related to overnutrition. Although the connection to reduced immune reconstitution on ART is speculative, abdominal obesity is associated with increased cellular immune activation in HIV-uninfected individuals, and physiologic concentrations of leptin (a circulating protein produced in proportion to adipose tissue mass) promote in vitro lymphocyte activation [19, 34, 35]. On the other hand, obese individuals are at higher risk of morbidity and mortality after several bacterial and viral infections, and reduced CD4+ lymphocyte recovery may simply reflect a more global immune impairment [36–39].
Our analysis was limited by a cohort size that may have been too small to allow detection of statistical associations when limited to subgroups. Bioelectrical impedance or dual-energy X-ray absorptiometry body fat measurements would have provided more accurate estimates of body composition, but these data were not available. Both obesity and undernutrition are associated with lower socioeconomic status, which may have had an independent effect on treatment outcomes, but we did not have data on income, education, or social position (insurance type was not thought to be a reliable marker). Only 2% of our cohort was morbidly obese (≥40 kg/m2) and only 7% was malnourished (<18.5 kg/m2), which may have limited our ability to detect differences at the extremes of body composition. Finally, the median CD4+ lymphocyte count at ART initiation in our cohort was 171 cells/μL, and our results may not be generalizable to patients beginning ART at higher cell counts, according to current treatment guidelines [40].
In summary, 12-month CD4+ lymphocyte recovery was greatest among patients commonly classified as overweight, suggesting an approximate pretreatment BMI range of 25–30 kg/m2 may promote optimal immune reconstitution on ART. Given the increasing epidemic of obesity worldwide, further studies of immune function in individuals with high BMIs has relevance to the treatment of both HIV infection and other infectious diseases.
Notes
Acknowledgments.
The authors acknowledge the patients and providers at the Vanderbilt Comprehensive Care Clinic, whose participation and support made this work possible. All individuals who contributed significantly to this work were included as authors.
Financial support.
This work was supported by the Vanderbilt Physician Scientist Development Program, National Institute of Allergy and Infectious Diseases (grant K24 AI065298), the Vanderbilt Meharry Center for AIDS Research (grant P30 AI054999), and the Vanderbilt Clinical and Translational Science award from the National Center for Research Resources, National Institutes of Health (grant UL1 RR024975). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028–37. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 3.Wilson PW, D'Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162:1867–72. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 4.Shor-Posner G, Campa A, Zhang G, et al. When obesity is desirable: a longitudinal study of the Miami HIV-1-infected drug abusers (MIDAS) cohort. J Acquir Immune Defic Syndr. 2000;23:81–8. doi: 10.1097/00126334-200001010-00011. [DOI] [PubMed] [Google Scholar]
- 5.Shuter J, Chang CJ, Klein RS. Prevalence and predictive value of overweight in an urban HIV care clinic. J Acquir Immune Defic Syndr. 2001;26:291–7. doi: 10.1097/00042560-200103010-00013. [DOI] [PubMed] [Google Scholar]
- 6.Jones CY, Hogan JW, Snyder B, et al. Overweight and human immunodeficiency virus (HIV) progression in women: associations HIV disease progression and changes in body mass index in women in the HIV epidemiology research study cohort. Clin Infect Dis. 2003;37(Suppl 2):S69–80. doi: 10.1086/375889. [DOI] [PubMed] [Google Scholar]
- 7.Paton NI, Sangeetha S, Earnest A, Bellamy R. The impact of malnutrition on survival and the CD4 count response in HIV-infected patients starting antiretroviral therapy. HIV Med. 2006;7:323–30. doi: 10.1111/j.1468-1293.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- 8.Koethe JR, Limbada MI, Giganti MJ, et al. Early immunologic response and subsequent survival among malnourished adults receiving antiretroviral therapy in Urban Zambia. AIDS. 2010;24:2117–21. doi: 10.1097/QAD.0b013e32833b784a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toure S, Kouadio B, Seyler C, et al. Rapid scaling-up of antiretroviral therapy in 10,000 adults in Cote d'Ivoire: 2-year outcomes and determinants. AIDS. 2008;22:873–82. doi: 10.1097/QAD.0b013e3282f768f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barth RE, van der Meer JT, Hoepelman AI, et al. Effectiveness of highly active antiretroviral therapy administered by general practitioners in rural South Africa. Eur J Clin Microbiol Infect Dis. 2008;27:977–84. doi: 10.1007/s10096-008-0534-2. [DOI] [PubMed] [Google Scholar]
- 11.Tedaldi EM, Brooks JT, Weidle PJ, et al. Increased body mass index does not alter response to initial highly active antiretroviral therapy in HIV-1-infected patients. J Acquir Immune Defic Syndr. 2006;43:35–41. doi: 10.1097/01.qai.0000234084.11291.d4. [DOI] [PubMed] [Google Scholar]
- 12.Crum-Cianflone NF, Roediger M, Eberly LE, et al. Obesity among HIV-infected persons: impact of weight on CD4 cell count. AIDS. 2010;24:1069–72. doi: 10.1097/QAD.0b013e328337fe01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Heart, Lung, and Blood Institute (NHLBI) The evidence report: clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. National Institutes of Health (NIH) publication 98-4083Bethesda, MD: NHLBI, 1998. [Google Scholar]
- 14.Wang Y, Beydoun MA. The obesity epidemic in the United States: gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- 15.Amorosa V, Synnestvedt M, Gross R, et al. A tale of 2 epidemics: the intersection between obesity and HIV infection in Philadelphia. J Acquir Immune Defic Syndr. 2005;39:557–61. [PubMed] [Google Scholar]
- 16.Crum-Cianflone N, Tejidor R, Medina S, Barahona I, Ganesan A. Obesity among patients with HIV: the latest epidemic. AIDS Patient Care STDS. 2008;22:925–30. doi: 10.1089/apc.2008.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenfield JR, Samaras K, Jenkins AB, et al. Obesity is an important determinant of baseline serum C-reactive protein concentration in monozygotic twins, independent of genetic influences. Circulation. 2004;109:3022–8. doi: 10.1161/01.CIR.0000130640.77501.79. [DOI] [PubMed] [Google Scholar]
- 18.Schaible UE, Kaufmann SH. Malnutrition and infection: complex mechanisms and global impacts. PLoS Med. 2007;4:e115. doi: 10.1371/journal.pmed.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thewissen MM, Damoiseaux JG, Duijvestijn AM, et al. Abdominal fat mass is associated with adaptive immune activation: the CODAM study. Obesity (Silver Spring) 2011;19:1690–98. doi: 10.1038/oby.2010.337. [DOI] [PubMed] [Google Scholar]
- 20.Chandra RK. 1990 McCollum Award lecture. Nutrition and immunity: lessons from the past and new insights into the future. Am J Clin Nutr. 1991;53:1087–101. doi: 10.1093/ajcn/53.5.1087. [DOI] [PubMed] [Google Scholar]
- 21.1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 22.Little RJA, Rubin DB. Statistical analysis with missing data. Hoboken, NJ: Wiley; 1987. [Google Scholar]
- 23.Keusch G. Malnutrition and the thymus gland. In: Cunningham-Rundles S, editor. Nutrient modulation of the immune response. New York, NY: Marcel Dekker.; 1993. pp. 283–99. [Google Scholar]
- 24.Gershwin M, Beach R, Hurley L. Nutrition and immunity. New York, NY: Academic Press; 1984. [Google Scholar]
- 25.Najera O, Gonzalez C, Cortes E, Toledo G, Ortiz R. Effector T lymphocytes in well-nourished and malnourished infected children. Clin Exp Immunol. 2007;148:501–6. doi: 10.1111/j.1365-2249.2007.03369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMurray DN. Cellular immune changes in undernourished children. Prog Clin Biol Res. 1981;67:305–18. [PubMed] [Google Scholar]
- 27.Benito JM, Lopez M, Lozano S, et al. Differential upregulation of CD38 on different T-cell subsets may influence the ability to reconstitute CD4+ T cells under successful highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2005;38:373–81. doi: 10.1097/01.qai.0000153105.42455.c2. [DOI] [PubMed] [Google Scholar]
- 28.Hunt PW, Martin JN, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–43. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 29.Srinivasula S, Lempicki RA, Adelsberger JW, et al. Differential effects of HIV viral load and CD4 counts on proliferation of naive and memory CD4 and CD8 T lymphocytes. Blood. 2011;118:262–70. doi: 10.1182/blood-2011-02-335174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melekhin VV, Shepherd BE, Stinnette SE, et al. Antiretroviral therapy initiation before, during, or after pregnancy in HIV-1-infected women: maternal virologic, immunologic, and clinical response. PLoS One. 2009;4:e6961. doi: 10.1371/journal.pone.0006961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prieto GA, Rosenstein Y. Oestradiol potentiates the suppressive function of human CD4 CD25 regulatory T cells by promoting their proliferation. Immunology. 2006;118:58–65. doi: 10.1111/j.1365-2567.2006.02339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arruvito L, Sanz M, Banham AH, Fainboim L. Expansion of CD4+CD25+and FOXP3+ regulatory T cells during the follicular phase of the menstrual cycle: implications for human reproduction. J Immunol. 2007;178:2572–8. doi: 10.4049/jimmunol.178.4.2572. [DOI] [PubMed] [Google Scholar]
- 33.Greenblatt RM, Ameli N, Grant RM, Bacchetti P, Taylor RN. Impact of the ovulatory cycle on virologic and immunologic markers in HIV-infected women. J Infect Dis. 2000;181:82–90. doi: 10.1086/315207. [DOI] [PubMed] [Google Scholar]
- 34.Martin-Romero C, Santos-Alvarez J, Goberna R, Sanchez-Margalet V. Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell Immunol. 2000;199:15–24. doi: 10.1006/cimm.1999.1594. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez-Margalet V, Martin-Romero C, Santos-Alvarez J, Goberna R, Najib S, Gonzalez-Yanes C. Role of leptin as an immunomodulator of blood mononuclear cells: mechanisms of action. Clin Exp Immunol. 2003;133:11–9. doi: 10.1046/j.1365-2249.2003.02190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fezeu L, Julia C, Henegar A, et al. Obesity is associated with higher risk of intensive care unit admission and death in influenza A (H1N1) patients: a systematic review and meta-analysis. Obes Rev. 2011;12:653–59. doi: 10.1111/j.1467-789X.2011.00864.x. [DOI] [PubMed] [Google Scholar]
- 37.Louie JK, Acosta M, Samuel MC, et al. A novel risk factor for a novel virus: obesity and 2009 pandemic influenza A (H1N1) Clin Infect Dis. 2011;52:301–12. doi: 10.1093/cid/ciq152. [DOI] [PubMed] [Google Scholar]
- 38.Huttunen R, Laine J, Lumio J, Vuento R, Syrjanen J. Obesity and smoking are factors associated with poor prognosis in patients with bacteraemia. BMC Infect Dis. 2007;7:13. doi: 10.1186/1471-2334-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bochicchio GV, Joshi M, Bochicchio K, Tracy K, Scalea TM. A time-dependent analysis of intensive care unit pneumonia in trauma patients. J Trauma. 2004;56:296–301. doi: 10.1097/01.TA.0000109857.22312.DF. discussion 301–3. [DOI] [PubMed] [Google Scholar]
- 40.Department of Health and Human Services. Panel on antiretroviral guidelines for adults and adolescents: guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Available at: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed 10 January 2011. [Google Scholar]