Abstract
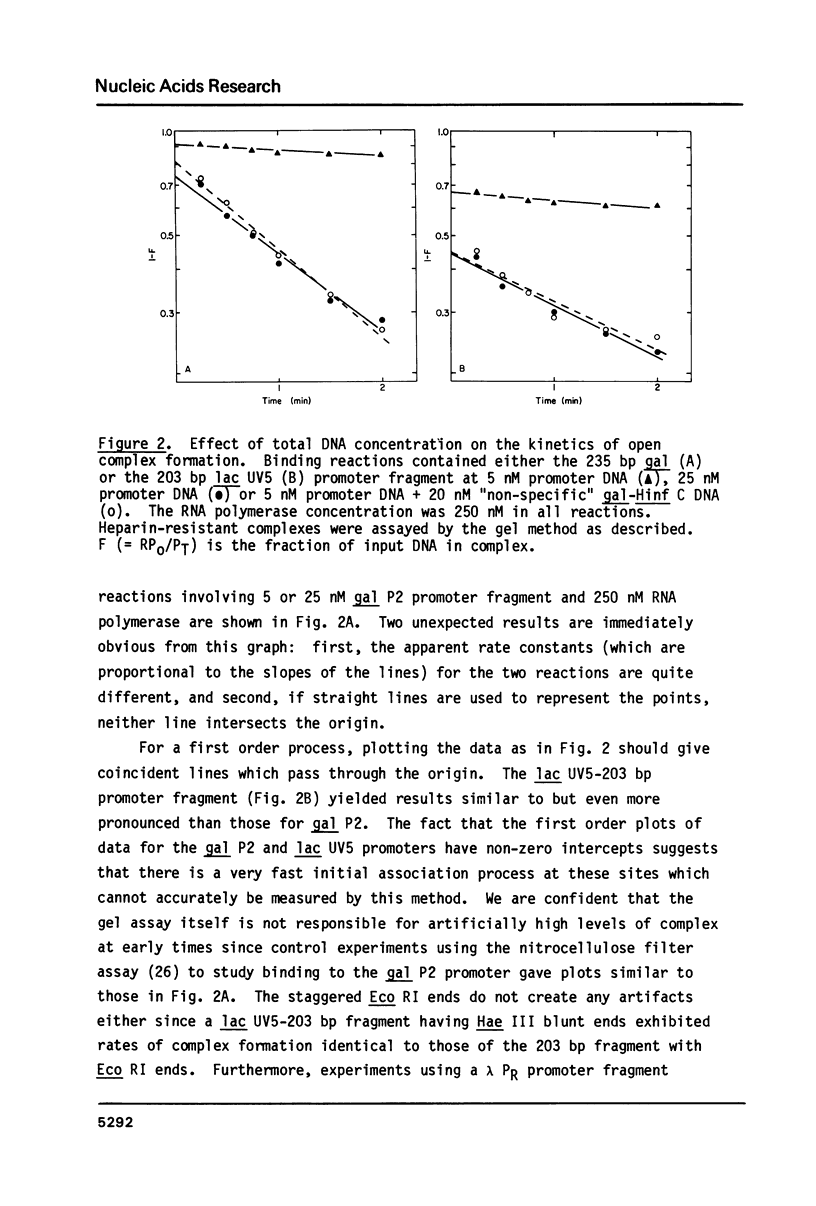
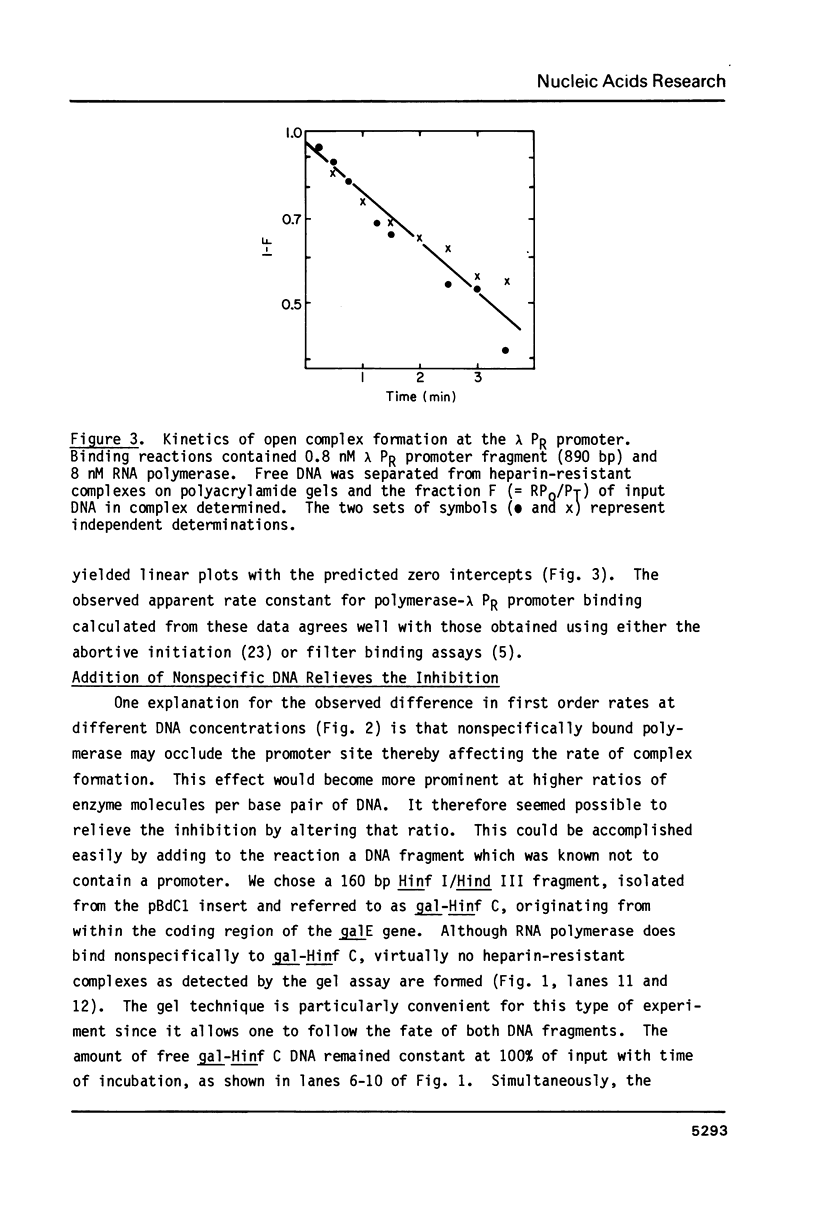
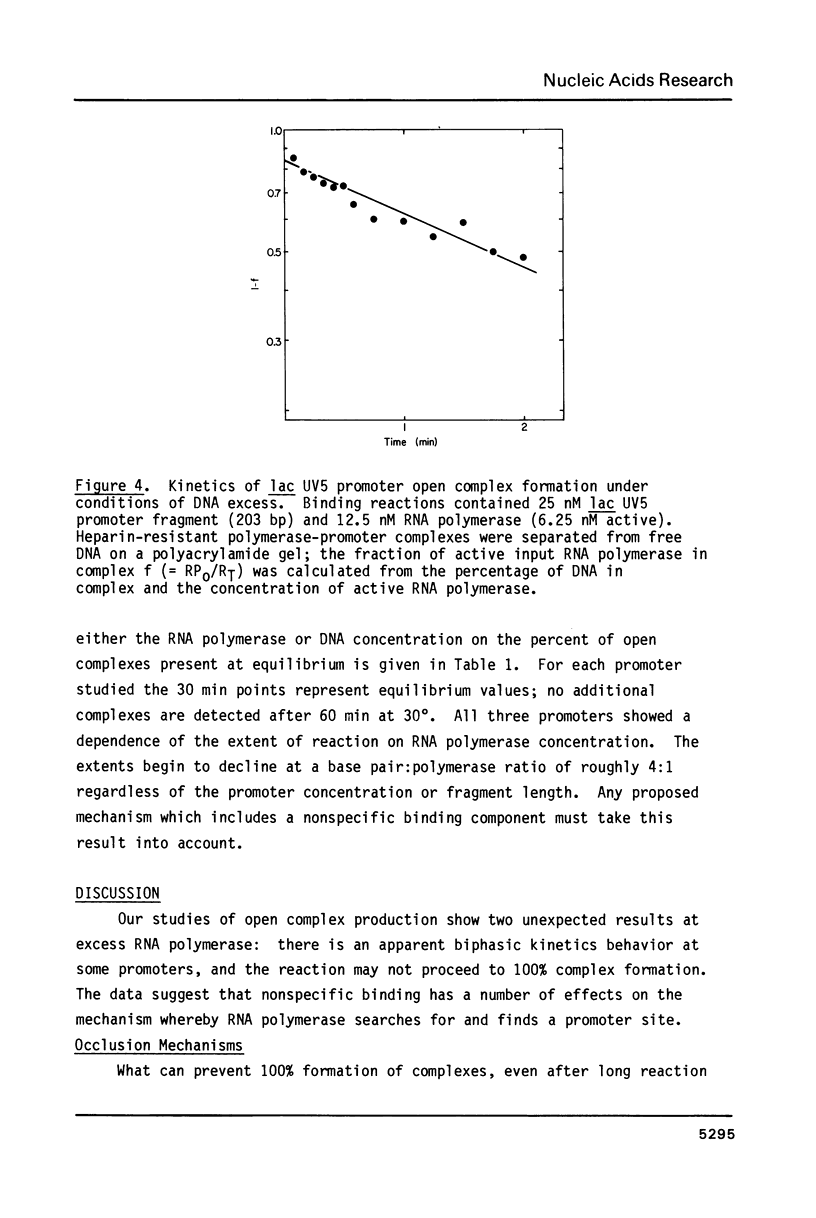
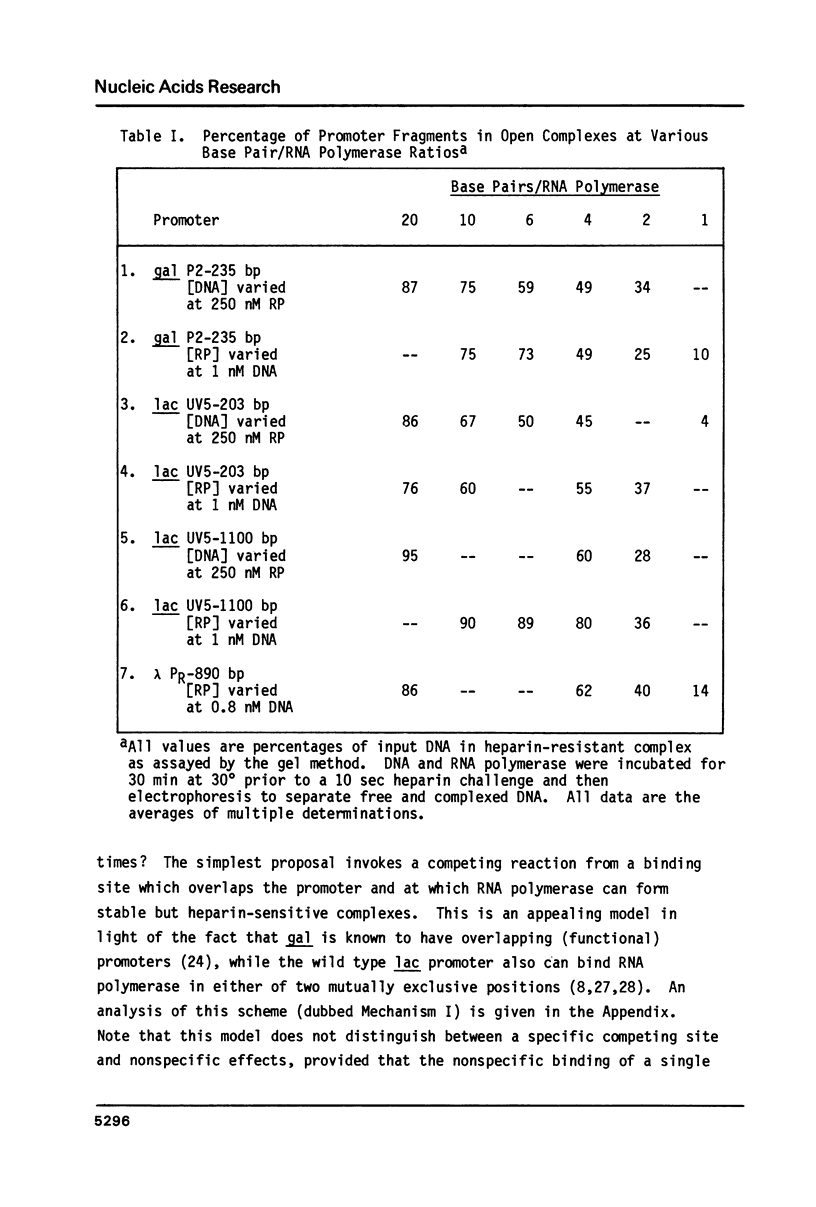
The rates of formation of RNA polymerase-promoter open complexes at the galactose P2 and lactose UV5 promoters of E. coli were studied using polyacrylamide gels to separate the heparin-resistant complexes from unbound DNA. Both the apparent rate and extent of reaction at these promoters are inhibited at excess RNA polymerase. This inhibition, which can be relieved by the addition of non-promoter DNA, is interpreted to be the result of occlusion of the promoter site by nonspecifically bound polymerase. Additionally, biphasic kinetics are observed at both gal P2 and lac UV5, but not at the PR promoter of phage lambda. This behavior disappears when the concentration of RNA polymerase in the binding reaction is less than that of the promoter fragment. It is proposed that at excess enzyme nonspecifically bound polymerase molecules sliding along the DNA may "bump" closed complexes from the promoter site thereby reducing the rate of open complex formation. Kinetics mechanisms quantifying both the occlusion and bumping phenomena are presented.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burgess R. R., Jendrisak J. J. A procedure for the rapid, large-scall purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975 Oct 21;14(21):4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- DiLauro R., Taniguchi T., Musso R., de Crombrugghe B. Unusual location and function of the operator in the Escherichia coli galactose operon. Nature. 1979 Jun 7;279(5713):494–500. doi: 10.1038/279494a0. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G., Hirschman S. Z. A neighbor-interaction analysis of the hypochromism and spectra of DNA. J Mol Biol. 1965 Sep;13(2):407–427. doi: 10.1016/s0022-2836(65)80106-1. [DOI] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardies S. C., Patient R. K., Klein R. D., Ho F., Reznikoff W. S., Wells R. D. Construction and mapping of recombinant plasmids used for the preparation of DNA fragments containing the Escherichia coli lactose operator and promoter. J Biol Chem. 1979 Jun 25;254(12):5527–5534. [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. In vitro comparison of initiation properties of bacteriophage lambda wild-type PR and x3 mutant promoters. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6381–6385. doi: 10.1073/pnas.77.11.6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkle D. C., Chamberlin M. J. Studies of the binding of Escherichia coli RNA polymerase to DNA. I. The role of sigma subunit in site selection. J Mol Biol. 1972 Sep 28;70(2):157–185. doi: 10.1016/0022-2836(72)90531-1. [DOI] [PubMed] [Google Scholar]
- Kadesch T. R., Rosenberg S., Chamberlin M. J. Binding of Escherichia coli RNA polymerase holoenzyme to bacteriophage T7 DNA. Measurements of binding at bacteriophage T7 promoter A1 using a template competition assay. J Mol Biol. 1982 Feb 15;155(1):1–29. doi: 10.1016/0022-2836(82)90489-2. [DOI] [PubMed] [Google Scholar]
- Le Grice S. F., Matzura H. Binding of RNA polymerase and the catabolite gene activator protein within the cat promoter in Escherichia coli. J Mol Biol. 1981 Aug 5;150(2):185–196. doi: 10.1016/0022-2836(81)90448-4. [DOI] [PubMed] [Google Scholar]
- Lowe P. A., Hager D. A., Burgess R. R. Purification and properties of the sigma subunit of Escherichia coli DNA-dependent RNA polymerase. Biochemistry. 1979 Apr 3;18(7):1344–1352. doi: 10.1021/bi00574a034. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McClure W. R. Rate-limiting steps in RNA chain initiation. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5634–5638. doi: 10.1073/pnas.77.10.5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso R. E., Di Lauro R., Adhya S., de Crombrugghe B. Dual control for transcription of the galactose operon by cyclic AMP and its receptor protein at two interspersed promoters. Cell. 1977 Nov;12(3):847–854. doi: 10.1016/0092-8674(77)90283-5. [DOI] [PubMed] [Google Scholar]
- Park C. S., Wu F. Y., Wu C. W. Molecular mechanism of promoter selection in gene transcription. II. Kinetic evidence for promoter search by a one-dimensional diffusion of RNA polymerase molecule along the DNA template. J Biol Chem. 1982 Jun 25;257(12):6950–6956. [PubMed] [Google Scholar]
- Revzin A., Woychik R. P. Quantitation of the interaction of EScherichia coli RNA polymerase holoenzyme with double-helical DNA using a thermodynamically rigorous centrifugation method. Biochemistry. 1981 Jan 20;20(2):250–256. doi: 10.1021/bi00505a004. [DOI] [PubMed] [Google Scholar]
- Siebenlist U., Simpson R. B., Gilbert W. E. coli RNA polymerase interacts homologously with two different promoters. Cell. 1980 Jun;20(2):269–281. doi: 10.1016/0092-8674(80)90613-3. [DOI] [PubMed] [Google Scholar]
- Spassky A., Busby S., Buc H. On the action of the cyclic AMP-cyclic AMP receptor protein complex at the Escherichia coli lactose and galactose promoter regions. EMBO J. 1984 Jan;3(1):43–50. doi: 10.1002/j.1460-2075.1984.tb01759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter R. B., Berg O. G., von Hippel P. H. Diffusion-driven mechanisms of protein translocation on nucleic acids. 3. The Escherichia coli lac repressor--operator interaction: kinetic measurements and conclusions. Biochemistry. 1981 Nov 24;20(24):6961–6977. doi: 10.1021/bi00527a030. [DOI] [PubMed] [Google Scholar]



