Abstract
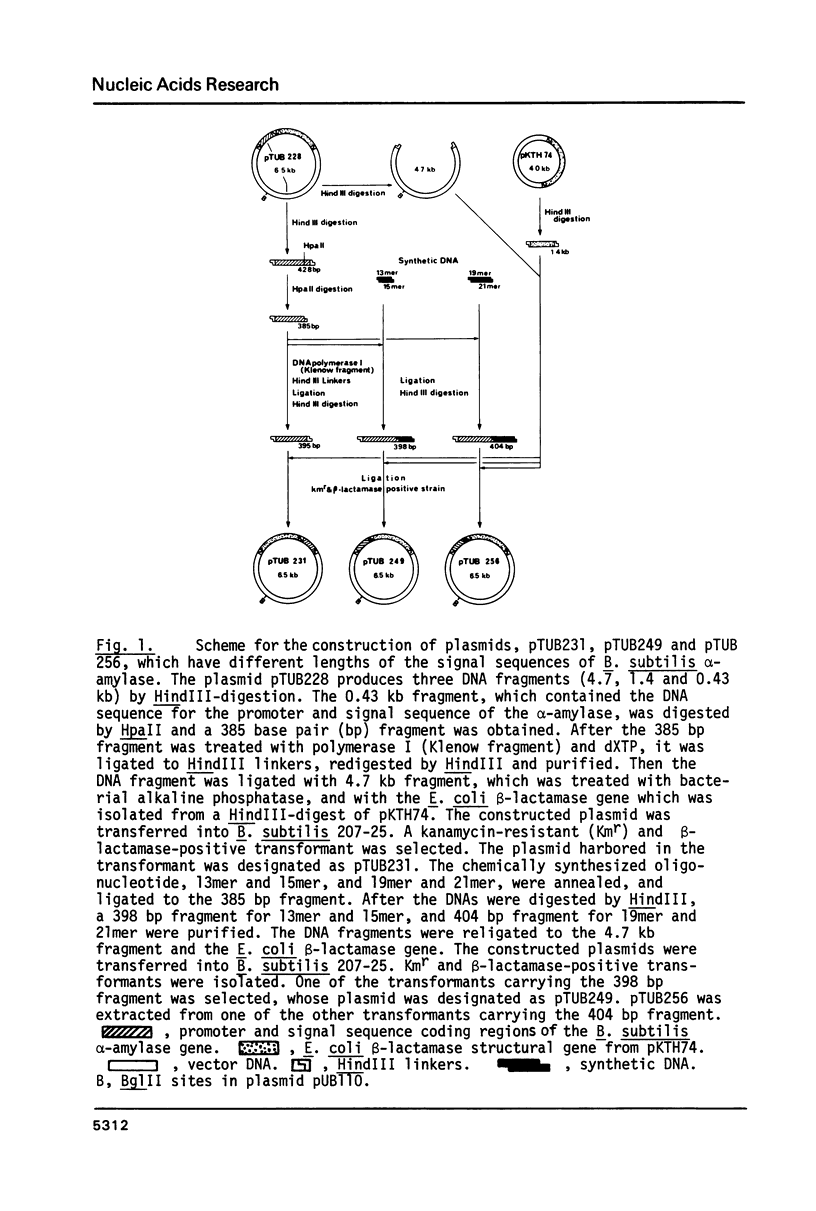
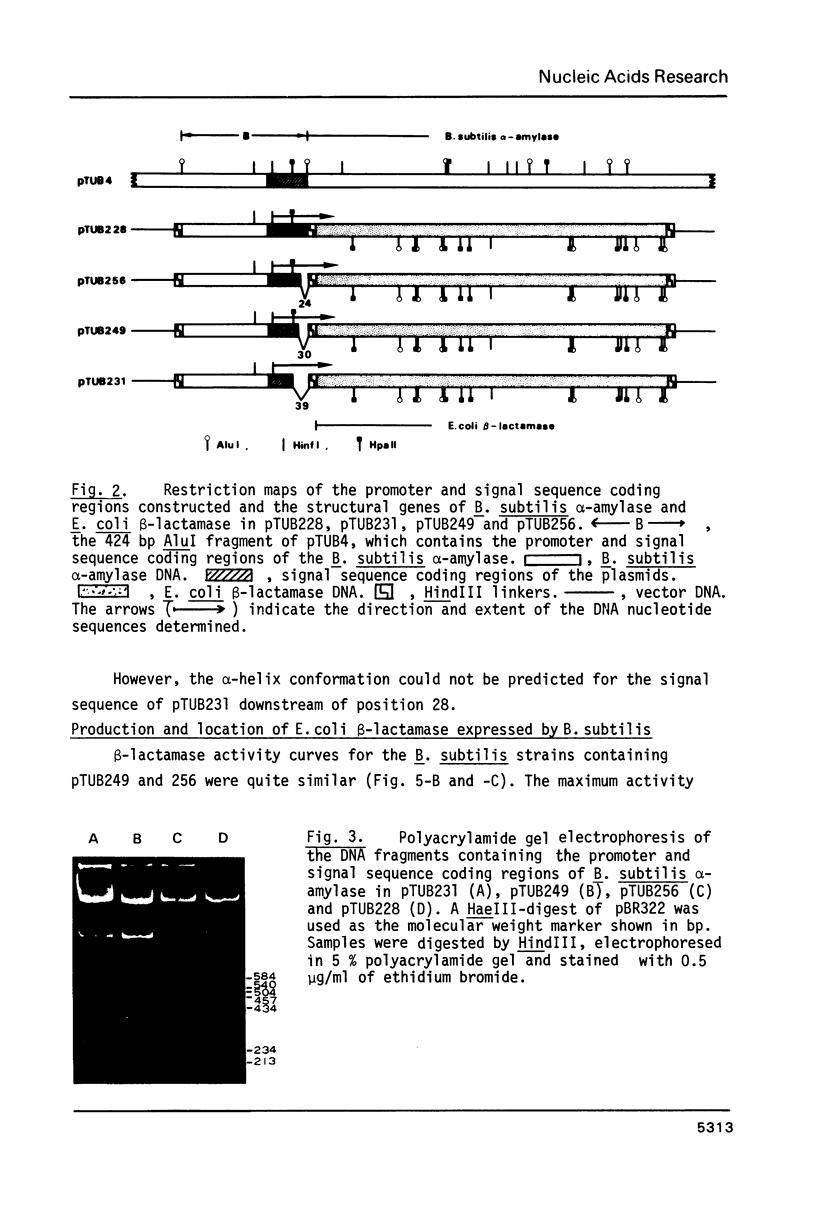
The precursor of Bacillus subtilis alpha-amylase contains an NH2-terminal extension of 41 amino acid residues as the signal sequence. The E. coli beta-lactamase structural gene was fused with the DNA for the promoter and signal sequence regions. Activity of beta-lactamase was expressed and more than 95% of the activity was secreted into the culture medium. DNA fragments coding for short signal sequences 28, 31, and 33 amino acids from the initiator Met were prepared and fused with the beta-lactamase structural gene. The sequences of 31 and 33 amino acid residues with Ala COOH-terminal amino acid were able to secrete active beta-lactamase from B. subtilis cells. However beta-lactamase was not secreted into the culture medium by the shorter signal sequence of 28 amino acid residues, which was not cleaved. Molecular weight analysis of the extracellular and cell-bound beta-lactamase suggested that the signal peptide of B. subtilis alpha-amylase was the first 31 amino acids from the initiator Met. The significance of these results was discussed in relation to the predicted secondary structure of the signal sequences.
Full text
PDF









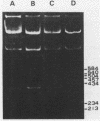
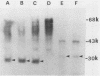
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austen B. M. Predicted secondary structures of amino-terminal extension sequences of secreted proteins. FEBS Lett. 1979 Jul 15;103(2):308–313. doi: 10.1016/0014-5793(79)81351-4. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S., Cohen S. N. High frequency transformation of Bacillus subtilis protoplasts by plasmid DNA. Mol Gen Genet. 1979 Jan 5;168(1):111–115. doi: 10.1007/BF00267940. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Cohn D. V., Smardo F. L., Jr, Morrissey J. J. Evidence for internal homology in bovine preproparathyroid hormone. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1469–1471. doi: 10.1073/pnas.76.3.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B. D., Tai P. C. The mechanism of protein secretion across membranes. Nature. 1980 Jan 31;283(5746):433–438. doi: 10.1038/283433a0. [DOI] [PubMed] [Google Scholar]
- Devillers-Thiery A., Kindt T., Scheele G., Blobel G. Homology in amino-terminal sequence of precursors to pancreatic secretory proteins. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5016–5020. doi: 10.1073/pnas.72.12.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emr S. D., Silhavy T. J. Importance of secondary structure in the signal sequence for protein secretion. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4599–4603. doi: 10.1073/pnas.80.15.4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreil G. Transfer of proteins across membranes. Annu Rev Biochem. 1981;50:317–348. doi: 10.1146/annurev.bi.50.070181.001533. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mäntsälä P., Zalkin H. Membrane-bound and soluble extracellular alpha-amylase from Bacillus subtilis. J Biol Chem. 1979 Sep 10;254(17):8540–8547. [PubMed] [Google Scholar]
- Neugebauer K., Sprengel R., Schaller H. Penicillinase from Bacillus licheniformis: nucleotide sequence of the gene and implications for the biosynthesis of a secretory protein in a Gram-positive bacterium. Nucleic Acids Res. 1981 Jun 11;9(11):2577–2588. doi: 10.1093/nar/9.11.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J. B., Lampen J. O. Membrane-bound penicillinases in Gram-positive bacteria. J Biol Chem. 1982 Apr 25;257(8):4490–4495. [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmura K., Shiroza T., Nakamura K., Nakayama A., Yamane K., Yoda K., Yamasaki M., Tamura G. A Bacillus subtilis secretion vector system derived from the B. subtilis alpha-amylase promoter and signal sequence region, and secretion of Escherichia coli beta-lactamase by the vector system. J Biochem. 1984 Jan;95(1):87–93. doi: 10.1093/oxfordjournals.jbchem.a134607. [DOI] [PubMed] [Google Scholar]
- Ohmura K., Yamazaki H., Takeichi Y., Nakayama A., Otozai K., Yamane K., Yamasaki M., Tamura G. Nucleotide sequence of the promoter and NH2-terminal signal peptide region of Bacillus subtilis alpha-amylase gene cloned in pUB110. Biochem Biophys Res Commun. 1983 Apr 29;112(2):678–683. doi: 10.1016/0006-291x(83)91516-4. [DOI] [PubMed] [Google Scholar]
- Ohtsuka E., Ikehara M., Söll D. Recent developments in the chemical synthesis of polynucleotides. Nucleic Acids Res. 1982 Nov 11;10(21):6553–6570. doi: 10.1093/nar/10.21.6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva I., Lehtovaara P., Käriäinen L., Sibakov M., Cantell K., Schein C. H., Kashiwagi K., Weissmann C. Secretion of interferon by Bacillus subtilis. Gene. 1983 May-Jun;22(2-3):229–235. doi: 10.1016/0378-1119(83)90107-5. [DOI] [PubMed] [Google Scholar]
- Palva I. Molecular cloning of alpha-amylase gene from Bacillus amyloliquefaciens and its expression in B. subtilis. Gene. 1982 Jul-Aug;19(1):81–87. doi: 10.1016/0378-1119(82)90191-3. [DOI] [PubMed] [Google Scholar]
- Palva I., Sarvas M., Lehtovaara P., Sibakov M., Käriäinen L. Secretion of Escherichia coli beta-lactamase from Bacillus subtilis by the aid of alpha-amylase signal sequence. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5582–5586. doi: 10.1073/pnas.79.18.5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman D., Halvorson H. O. A putative signal peptidase recognition site and sequence in eukaryotic and prokaryotic signal peptides. J Mol Biol. 1983 Jun 25;167(2):391–409. doi: 10.1016/s0022-2836(83)80341-6. [DOI] [PubMed] [Google Scholar]
- Rosenblatt M., Beaudette N. V., Fasman G. D. Conformational studies of the synthetic precursor-specific region of preproparathyroid hormone. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3983–3987. doi: 10.1073/pnas.77.7.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak H. F., Flavell R. A. A method for the recovery of DNA from agarose gels. Nucleic Acids Res. 1978 Jul;5(7):2321–2332. doi: 10.1093/nar/5.7.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr G. E., Black S. D., Fujita V. S., Coon M. J. Complete amino acid sequence and predicted membrane topology of phenobarbital-induced cytochrome P-450 (isozyme 2) from rabbit liver microsomes. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6552–6556. doi: 10.1073/pnas.80.21.6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki H., Ohmura K., Nakayama A., Takeichi Y., Otozai K., Yamasaki M., Tamura G., Yamane K. Alpha-amylase genes (amyR2 and amyE+) from an alpha-amylase-hyperproducing Bacillus subtilis strain: molecular cloning and nucleotide sequences. J Bacteriol. 1983 Oct;156(1):327–337. doi: 10.1128/jb.156.1.327-337.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Galizzi A., Henner D. Nucleotide sequence of the amylase gene from Bacillus subtilis. Nucleic Acids Res. 1983 Jan 25;11(2):237–249. doi: 10.1093/nar/11.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda Y., Yamane K., Maruo B. Membrane mutation related to the production of extracellular -amylase and protease in bacillus subtilis. Biochem Biophys Res Commun. 1973 Feb 5;50(3):765–770. doi: 10.1016/0006-291x(73)91310-7. [DOI] [PubMed] [Google Scholar]