Abstract
Most recreational users of 3, 4-methylenedioxymethamphetamine (MDMA or “ecstasy”) also take cannabis, in part because cannabis can reduce the dysphoric symptoms of the ecstasy come-down such as agitation and insomnia. Although previous animal studies have examined the acute effects of co-administering MDMA and Δ9-tetrahydrocannabinol (THC), which is the major psychoactive ingredient in cannabis, research on chronic exposure to this drug combination is lacking. Therefore, the present study was conducted to investigate the effects of chronic adolescent administration of both THC and MDMA on behavior and on regional serotonin transporter (SERT) binding and serotonin (5-HT) concentrations as indices of serotonergic system integrity. Male Sprague-Dawley rats were divided into four drug administration groups: (1) MDMA alone, (2) THC alone, (3) MDMA plus THC, and (4) vehicle controls. MDMA (2 × 10 mg/kg × 4 h) was administered every fifth day from postnatal day (PD) 35 to 60 to simulate intermittent recreational ecstasy use, whereas THC (5 mg/kg) was given once daily over the same time period to simulate heavy cannabis use. THC unexpectedly produced a modest hyperthermic effect when administered alone, but in animals co-treated with both THC and MDMA, there was an attenuation of MDMA-induced hyperthermia on dosing days. Subsequent testing conducted after a drug washout period revealed that THC reduced MDMA-related behavioral changes in the emergence and social interaction tests of anxiety-like behavior and also blunted the MDMA-induced decrease in exploratory behavior in the hole-board test. THC additionally attenuated MDMA -induced decreases in 5-HT levels and in SERT binding in the frontal cortex, parietal cortex, and striatum, but not in the hippocampus. These results suggest that chronic co-administration of THC during adolescence can provide some protection against various adverse physiological, behavioral, and neurochemical effects produced by MDMA.
Keywords: MDMA, THC, behavior, neurotoxicity
1. Introduction
3,4-Methylenedioxymethamphetamine (MDMA or “ecstasy”) is used by many teenagers, especially at “rave” parties (Lyles and Cadet, 2003). Despite MDMA’s popularity, there is ample evidence that high doses of this compound produce long-lasting serotonergic deficits in rats and monkeys (Green et al., 2003), and neuroimaging studies suggest that serotonergic toxicity may also occur in heavy ecstasy users (Cowan, 2007). Moreover, a variety of neuropsychological and behavioral abnormalities have been associated with heavy ecstasy use, including cognitive impairments, heightened anxiety, and increased impulsivity (Morgan, 2000; Morgan et al., 2006; Quednow et al., 2007; Parrott, 2001). It is possible, though not yet proven, that these abnormalities stem from MDMA-induced dysfunction in serotonergic pathways in the forebrain.
Most ecstasy users also consume other drugs, with cannabis being the most popular followed by alcohol and stimulants (Lenton et al., 1997; Boys et al., 1997). Wish et al. (2006) surveyed a sample of East Coast college students and found that 98% of the ecstasy users also had taken cannabis. Moreover, toxicologic screening of urine samples collected from 198 ecstasy users between 2005 and 2007 found the Δ9-tetrahydrocannabinol (THC) metabolite 11-nor-9-carboxy-THC in approximately 62% of the samples (Black et al., 2009). The percentage of concomitant ecstasy and cannabis use is also high in other countries (Sala and Braida, 2005). Drug users report that cannabis helps reduce the dysphoric symptoms of the ecstasy come-down, such as agitation and insomnia (Strote et al., 2002; Winstock et al., 2001).
The prevalence of cannabis consumption among ecstasy users is an important potential confound in interpreting reported ecstasy-associated cognitive and neuropsychiatric abnormalities, making the cause of such abnormalities difficult to determine (Croft et al., 2001; Daumann et al., 2004; Parrott et al., 2007). Cannabis could affect MDMA action in at least three ways. First, THC may interact directly with serotonergic neurons, thereby altering their sensitivity to MDMA. This possibility is supported by the demonstration of CB1 receptor expression in at least some serotonergic neurons (Häring et al., 2007; Lau and Schloss, 2008), along with evidence that presynaptic CB1 receptor activation inhibits serotonin (5-HT) synthesis and release and reduces 5-HT turnover (Balázsa et al., 2008; Moranta et al., 2004; Nakazi et al., 2000). Second, cannabinoids also inhibit the release of other neurotransmitters, including dopamine (DA) (Nava et al., 2000; Schlicker and Kathmann, 2001). As DA is hypothesized to play a role in the serotonergic neurotoxic effects of MDMA (Breier et al., 2006; Sprague et al., 1998), THC-induced reductions in DA release might ameliorate such effects. Finally, MDMA neurotoxicity is due, in part, to drug-induced oxidative stress (Quinton and Yamamoto, 2006), whereas THC has been shown to possess antioxidant activity (Chaperon and Thiébot, 1999; van der Stelt and Di Marzo, 2005). This is a third mechanism, therefore, by which THC could alter the effects of MDMA exposure.
Despite the above mentioned evidence for possibilities of ecstasy and cannabis co-use and interaction, little is known about the modulation of MDMA effects by cannabinoids. Two previous studies reported on the effects of acute co-administration of THC and MDMA in adult rats (Morley et al., 2004; Young et al., 2005), and more recently Robledo and coworkers (2007) examined both the acute neurochemical effects of THC and MDMA co-administration and the combined effects of these compounds in a conditioned place paradigm in mice. However, to our knowledge there is no published information regarding the consequences of recurrent adolescent co-administration of these compounds in a rodent model. The present study begins to address this gap in the literature by examining the physiological, behavioral, and neurochemical effects of repeated co-administration of MDMA and THC in adolescent rats. We used an intermittent MDMA dosing regimen previously developed and characterized by our laboratory (Meyer et al., 2008; Piper and Meyer, 2004) along with daily THC dosing to simulate heavy cannabis use (Block and Ghoneim, 1993). Outcome measures included drug-induced changes in body weight, core body temperature, tests of anxiety-like and exploratory behaviors, and neurochemical measures of 5-HT neurotoxicity. All of these measures have previously been used to investigate the effects of acute and/or chronic MDMA administration in rats.
2. Materials and Methods
2.1 Animals
Male Sprague-Dawley rats were obtained from the Charles River Laboratory (Wilmington, MA) at postnatal day (PD) 25–28. Animals were pair-housed in 44.5 × 23.5 × 20.0 cm plastic tubs and habituated to the animal colony room for at least 1 week prior to the beginning of each experiment. Animals were maintained under a reversed 12-h light/dark cycle (lights off at 0800 h), with drug administration and behavioral testing performed during the dark phase of the cycle. They were provided with water and food ad libitum and maintained at a temperature of 26 ± 1°C in Experiment I and 23 ± 1°C in Experiment II. The protocol for these experiments was approved by the University of Massachusetts-Amherst Institutional Animal Care and Use Committee. Animal care conformed to the standards set forth in the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, 1996).
2.2 Drug treatments
(±) MDMA-HCL and THC (20 mg/ml in ethanol) were provided by the National Institute on Drug Abuse (NIDA). MDMA was dissolved in sterile physiological saline for administration to the animals. THC was prepared according to the procedure of Singh et al. (2005) described as follows. Briefly, a small amount of Tween-80 was added to the ethanolic THC solution, after which the mixture was placed under nitrogen gas and continuously stirred until the ethanol evaporated. The resulting residue was mixed thoroughly with saline, resulting in a fine suspension of THC in a vehicle of 0.75% Tween-80 in saline. Both MDMA and THC were freshly prepared on the day of dosing and administered at a volume of 1 ml/kg.
Animals received MDMA, THC, or their respective vehicles from PD 35 to 60, thus forming four treatment groups: (1) MDMA alone, (2) THC alone, (3) MDMA plus THC, and (4) vehicle controls. The MDMA alone group received two subcutaneous (s.c.) injections of 10 mg/kg (based on the weight of the salt) of MDMA every fifth day along with THC vehicle every day. On MDMA treatment days, the first MDMA dose was given in the morning, and the second MDMA dose was given 4 h later as previously described by Piper and Meyer (2004). The THC alone group received a single daily intraperitoneal (i.p.) injection of 5 mg/kg of THC as well as a s.c. saline injection every fifth day. The THC dose was chosen based on previous reports of cognitive impairment in rats given the same daily dose during adolescence (Cha et al., 2006; Nakamura et al., 1991). The MDMA plus THC group received THC daily and MDMA every fifth day. On MDMA/THC co-administration days, the THC injection was administered 2 h after the second MDMA dose. This procedure was adopted to simulate the use of cannabis to ameliorate the dysphoric symptoms of the ecstasy “come-down” by drug users (Strote et al., 2002; Topp et al., 1999; Winstock et al., 2001). The control group received a dosing pattern similar to that of the MDMA plus THC group but with vehicle only (i.e., daily afternoon injections of the Tween-80/saline vehicle along with two saline injections every fifth day).
2.3 Experiment I
Sixty Sprague-Dawley rats were included in Experiment I. Behavioral testing began 10 days following the end of drug treatment in order to allow ample time for THC washout and for any potential acute withdrawal symptoms to subside. In this experiment, the tests consisted of an open-field test (locomotor activity), emergence and social interaction tests (anxiety-like behaviors), and the hole-board test (exploratory behavior). Testing was conducted according to the following schedule: the open-field activity and emergence tests were conducted on PD 70, the social interaction test on PD 72, and the hole-board test on PD 74. On PD 75, rats were euthanized by means of rapid decapitation under light CO2 anesthesia. Brains were removed, dissected into frontal cortex, parietal cortex, hippocampus, and striatum, and the tissues were then frozen over dry ice and stored at −70°C for subsequent analysis. Each brain region was collected from both sides of the brain, and in the case of the frontal and parietal cortices and the hippocampus, samples from the two sides were stored and analyzed separately either for SERT binding or for concentrations of 5-HT and its metabolite 5-hydroxyindoleacetic acid (5-HIAA) as indices of MDMA-induced neurotoxicity. As striatal samples were too small to permit both analyses, striatal tissues were used only for SERT binding.
2.3.1 Body weight
Body weights were recorded approximately 1 h prior to drug administration on a daily basis from PD 35 to 60, after which weights continued to be obtained at 5-day intervals for the remainder of the experiment. For simplicity, only the weight data from PD 35, 40, 45, 50, 55, 60, 65, 70, and 75 were used for presentation and statistical analysis.
2.3.2 Core body temperature
Core body temperature responses to drug administration were measured in all animals on PD 35, 45, and 60 using a rectal probe (RET-2; Physiotemp Instruments, Clifton, NJ) and a digital thermometer (TH-5; Physiotemp) according to the procedures of Piper et al. (2005). Temperature measurements were obtained at 30-min intervals beginning 30 min prior to the first MDMA or vehicle injection and ending 2 h after the THC or vehicle injection.
2.3.3 Social interaction test
Rats were placed in individual housing on PD 65, 7 days prior to the social interaction test. Testing was conducted under 40W red light in a wooden black arena measuring 60 × 60 × 30 cm. Animals were tested in pairs in the arena for 10 min. The pairs were of similar weight and were selected from the same treatment groups, but from different home cages, so that the animals were unfamiliar with each other. Each session was videotaped for later coding of the following social interactions: sniffing, adjacent lying, following, crawling, mutual grooming, and aggression. Kicking, aggressive grooming, biting, boxing, and jumping on the testing partner were combined into a single category of aggressive behavior.
2.3.4 Open-field
On PD 70, open-field testing was conducted in a black wooden apparatus measuring 60 × 60 × 30 cm according to Piper and Meyer (2004). The floor of the apparatus was marked with masking tape and divided into nine equally-sized squares (eight peripheral and one central, each measuring 20 × 20 cm). Testing was conducted for 10 min under 40W red light during which time behavior was videotaped using an overhead Sony low-light camcorder. Tapes were later scored for total grid crossings, entries into the central square, and number of rears using the freeware program JWatcher (http://www.jwatcher.ucla.edu/). In this and all subsequent behavioral tests, the apparatus was cleaned with 10% ethanol between animals. In addition, videotaped behaviors throughout the study were scored by experimenters who were blind to the animal’s treatment status.
2.3.5 Emergence test
In accordance with previous studies (Gurtman et al., 2002; Morley et al., 2001), the emergence apparatus consisted of a white painted wooden arena measuring 96 × 100 × 40 cm and a black painted hide-box measuring 24 × 40 × 15 cm with an opening on one end. The testing room was illuminated with fluorescent lights, and white noise was played in the background to mask ambient sounds. Each 5-min trial began by placing the rat in the hide-box with its nose pointed towards the end opposite to the opening. Behavior was videotaped and the following measures were later scored: numbers of emergences, latency until first emergence, duration in open-field, and duration of risk assessment behaviors. An emergence was scored when all four paws and at least part of the tail exited the hide-box. Risk assessment behavior was coded when a rat only partially exited the hide-box or when at least the tip of the nose was visible. This test was conducted on PD 72.
2.3.6 Hole-board test
On PD 74, the hole-board test of exploratory behavior was conducted using two ENV-510 activity chambers (Med Associates Inc., St. Albans, VT). Each chamber measured 27.5 × 27.5 × 20.5 cm internally and contained a steel floor insert with 16 holes, each with a diameter of 2.3 cm, arranged in a 4 × 4 pattern. Geometric images were placed on the outside of the Plexiglas walls of each chamber to enrich the hole-board spatial environment. Med Associates software automatically determined total hole entries, novel hole entries, and re-entries during the 10-min testing period.
2.3.7 Serotonin transporter binding
Striatal and one hemisphere of hippocampal and cortical tissues were placed in 40 vols of ice-cold assay buffer (10 mM sodium phosphate, 5 mM potassium chloride, and 120 mM sodium chloride, pH 7.4) and then homogenized using a Polytron. Homogenates were centrifuged at 20,000 × g for 20 min at 4°C, the supernatant was discarded, and the pellet was rehomogenized in the same buffer. This procedure was repeated twice more, with an added 20-min incubation at 30°C between the second and third centrifugations to ensure dissociation of endogenous 5-HT from SERT. The resulting washed membrane fractions were assayed in triplicate using a 1.0 nM concentration of [3H]-citalopram (79.0 Ci/mmol, New England Nuclear, Boston, MA) with or without 10 μM unlabeled fluoxetine to define total binding or nonspecific binding respectively. Following incubation for 1 h at room temperature, the reaction was terminated by rapid filtration through Whatman GF/B filters presoaked with 0.05% polyethyleneimine. Filters were washed twice with 5 ml of ice-cold buffer, placed in 4.3 ml of Scintisafe™ (Fisher Scientific, Houston, TX) and then counted in a Packard 1900CA liquid scintillation analyzer 24 h later. The BioRad DC Protein Assay (BioRad, Hercules, CA) was used to measure protein concentration, with bovine gamma globulin as the standard.
2.3.8. 5-HT and 5-HIAA concentrations
In accordance with the methods of Ali et al. (1994), high performance liquid chromatography with electrochemical detection was utilized to measure 5-HT and 5- HIAA levels in the remaining hemisphere of the cortical and hippocampal samples.
2.4 Experiment II
Fifty rats were included in Experiment II. The temperature of the colony room was maintained at 23 ± 1°C, which was several degrees lower than in Experiment I but still sufficient to ensure a hyperthermic response to MDMA. Animals were administered MDMA and THC from PD 35-60 according to the same dosing regimen as described in Experiment I. However, Experiment II used a different series of post-dosing tests than Experiment I, namely measurement of plasma corticosterone (CORT) under baseline conditions and following open-field exposure (hypothalamic-pituitary-adrenocortical stress response), and the emergence test conducted under low red light instead of bright fluorescent light. As in Experiment I, the rats were placed in individual housing following the end of drug treatment. Tests were conducted according to the following schedule: baseline plasma samples for the corticosterone assay were collected on PD 66, open-field and corticosterone stress response testing was conducted on PD 68, and the emergence test was conducted on PD 70. On PD 77, rats were euthanized by means of rapid decapitation under light CO2 anesthesia.
2.4.1 Body weight and temperature
The same procedures were used to measure body weight and core body temperature as in Experiment I.
2.4.2 Baseline plasma sampling
On PD 66, plasma was collected for baseline CORT determinations. Each animal was taken from its home cage, lightly restrained while the tip of the tail was removed with a sterile blade. Within 2 min of removal of the animal from the colony room, approximately 150 μl of blood was collected with heparinized capillary tubes. Tubes were then centrifuged and plasma was stored at −70°C for later analysis.
2.4.3 Open-field and corticosterone response
On PD 68, animals were tested for 5 min in a 60 × 60 cm open-field box with grid lines on the floor as described in Experiment I. However, in this case the test was conducted under bright fluorescent lighting instead of 40W red light. Animals were videotaped for later scoring of total ambulation, entries into the center square, and rearing. Immediately following the test session, a second blood sample was collected from the tail and processed and stored for subsequent analysis to determine the CORT response to the open field challenge.
2.4.4 Emergence test
The emergence test was conducted on PD 72 using the same apparatus and procedures described in Experiment I, except that the testing room was illuminated with 40W red lights instead of white fluorescent lights.
2.4.5. Statistical analysis
Statistical analyses were conducted using SPSS 16.0 software (SPSS Inc., Chicago, IL). Although a factorial design was used in this study, we analyzed treatment effects by means of a one-way rather than a two-way analysis of variance (ANOVA). This approach was chosen to maximize our ability to test for key group differences, since our previous experience indicates that interaction terms using the two-way ANOVA in this type of study do not always attain statistical significance despite clear differences between groups of interest. A mixed-design ANOVA (treatment x age) was used to analyze the body weight data. These data were further analyzed using Bonferroni-corrected one-way ANOVAs to determine treatment effects on each weight measurement day. Tukey post-hoc tests were used to follow up significant F values found in the primary analysis. The thermal response to drug treatment was quantified by determining the area under the temperature time curve (AUC) using Prism 4 Software (GraphPad Inc., La Jolla, CA).
3. Results
3.1 Experiment I
3.1.1 Body weight
Figure 1 shows the mean body weights of rats from each treatment group measured every fifth day from PD 35 to PD 70. A mixed-design ANOVA revealed significant main effects of treatment (F (3, 56) = 3.90, P < 0.02) and age (F (8, 448) = 5728.10, P < 0.001), and a significant treatment x age interaction (F (24, 448) = 11.56, P < 0.001). The treatment x age interaction was further analyzed using Bonferroni-corrected one-way ANOVAs on each reported weight measurement day. These analyses revealed significant group differences beginning on PD 50 (F (3, 56) = 3.53, P < 0.02) and on every measurement day thereafter. Tukey post-hoc tests revealed that the MDMA alone, THC alone, and MDMA plus THC groups all experienced significant reductions in weight compared to the vehicle control group, but none of the drug groups differed significantly from each other (Fig. 1).
Figure 1.
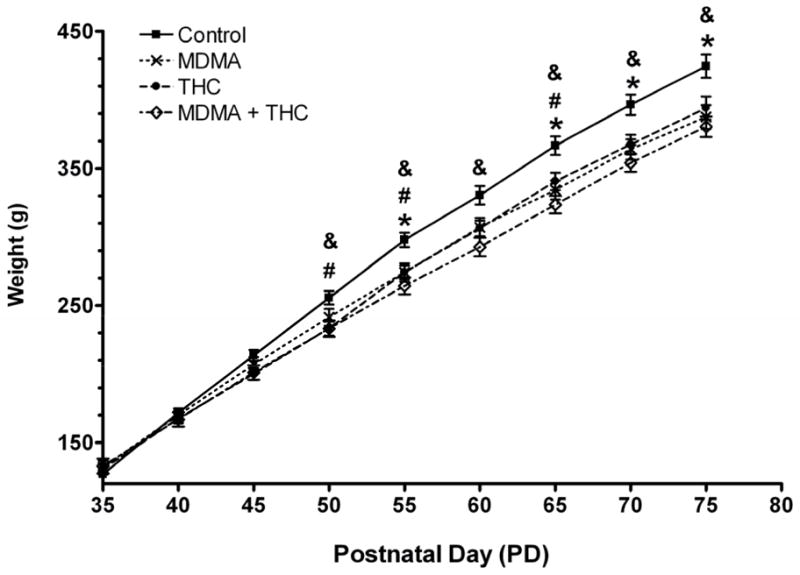
Body weight (mean ± S.E.M.) as a function of age and drug treatment (MDMA alone: 10 mg/kg × 2, s.c., 4-h interdose interval, every 5th day from PD 35 to PD 60 + THC vehicle daily; THC treatment: 5 mg/kg, i.p. daily plus saline × 2 every 5th day; MDMA + THC: combined drug treatments as described; vehicle controls: saline plus THC vehicle only; N=14–16 per group). Bonferroni-corrected one-way ANOVAs were conducted at each time point. *P < 0.05 for MDMA vs. control, #P < 0.05 for THC vs. control, and &P < 0.05 for MDMA + THC vs. control (Tukey post-hoc test).
3.1.2 Core body temperature
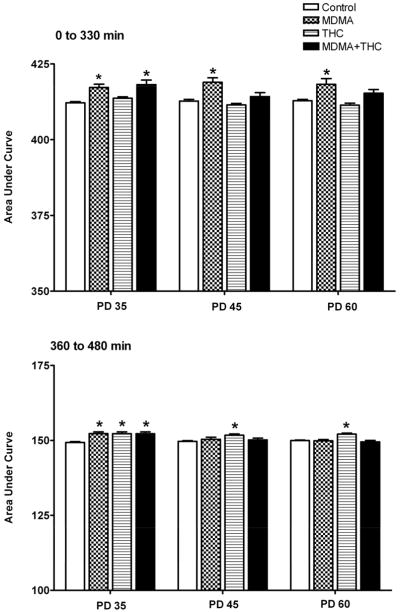
Dysregulation of core body temperature in response to drug treatment was documented on PD 35, 45, and 60, and was analyzed by means of AUC of the temperature-time curves. To determine the temperature response to the two MDMA or saline injections on those three treatment days, we calculated the AUC between 0 min (the time of first injection) and 330 min (1.5 hr after the second injection). Changes in temperature in response to the subsequent THC or vehicle injection were calculated as the AUC between 360 min (the time of the THC injection) and 480 min (2 hr after the injection). One-way ANOVAs calculated on each treatment day using all four treatment groups revealed significant overall treatment main effects for the 0–330 min AUC values on all three days tested [PD 35 (F (3,31) = 8.45, P < 0.001); PD 45 (F (3,55) = 9.95, P < 0.001); PD 60 (F (3,55) = 6.02, P < 0.001). Similar treatment main effects were obtained for the 360–480 min AUC values on each day [PD 35 (F (3,31) = 7.68, P < 0.001); PD 45 (F (3,55) = 2.92, P < 0.05); PD 60 (F (3,55) = 9.00, P < 0.001)]. Tukey post-hoc tests revealed that MDMA produced the expected hyperthermic effect compared to saline between 0 and 330 min. Chronic THC administration prevented MDMA-induced hyperthermia between 0 and 330 min on PD 45 and PD 60 to the extent that AUC values from the MDMA plus THC treatment groups were no longer significantly different from the vehicle controls. Rats given co-administration of MDMA and THC showed a hyperthermic response on PD 35 between 0 and 330 min because the temperature measurements were taken prior to the first THC administration. Tukey post hoc tests further revealed that THC produced a small but significant hyperthermic effect between 360 min and 480 min on all three treatment days. Additional Tukey post-hoc tests revealed that intermittent MDMA administration prevented THC-induced hyperthermia between 360 and 480 min on PD 45 and PD 60, but not on PD 35 (Fig. 2). This was evidenced by the fact that AUC values between 360 and 480 min in the MDMA plus THC co-administration group were no longer significantly different from the vehicle controls. AUC values from the co-administration group were not significantly different from the THC alone group.
Figure 2.
Temperature responses to drug treatments (described in Figure 1) on PD 35, PD 45 and PD 60. Data are presented as mean ± S.E.M. of the AUC over the indicated time period, *P < 0.05 vs. control (Tukey post-hoc test).
3.1.3 Social interaction test
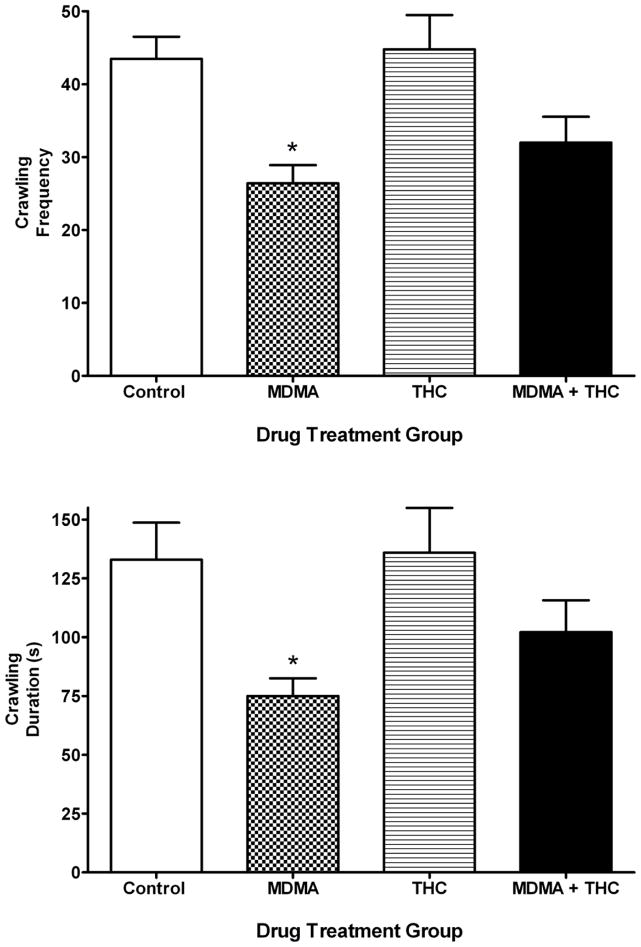
One-way ANOVA revealed an overall a significant treatment effect on the frequency (F (3,21) = 4.29, P < 0.05) and duration (F (3,21) = 6.84, P < 0.02) of crawling behavior in the social interaction test performed on PD 72. The Tukey post-hoc test revealed that the MDMA alone group showed significantly less frequency and duration of crawling behavior than the vehicle controls (Fig. 3). THC exposure by itself did not cause any changes in crawling behavior. Though frequency and duration of crawling behavior in the MDMA plus THC group were not sigificantly different from those in the MDMA alone group, THC did blunt the effect of MDMA seen in the MDMA plus THC co-administration group to the extent that crawling frequency and duration from the MDMA plus THC co-administration group were no longer statistically different from those observed in vehicle controls. Drug treatments did not alter any other behaviors in the social interaction test.
Figure 3.
Crawling frequency and duration (mean ± S.E.M.) in the social interaction test conducted on PD 72 as a function of drug treatment (described in Figure 1). *P < 0.05 vs. control (Tukey post-hoc test).
3.1.4 Open-field
There were no group differences in locomotor activity (total grid crossings) (F (3,55) = 1.69, P > 0.10), center square entries (F (3,55) = 1.06, P > 0.10), or rearing behavior (F (3,55) = 0.10, P > 0.10) in the open-field test conducted on PD 70 (data not shown).
3.1.5 Emergence test
Data from the emergence test conducted on PD 72 are summarized in Table 1. One-way ANOVA revealed an overall significant treatment effect on latency to first emergence (F (3,55) = 3.61, P < 0.02), and a nonsignificant trend in total open-field duration (F (3,55) = 2.46, P = 0.072). The Tukey post-hoc test revealed that the MDMA alone group spent significantly less time in the hide-box before first full emergence compared to the control group. THC alone had no influence on this or any measure in the emergence test. THC co-administration prevented the effect of MDMA on latency to first emergence to the extent that the significant difference between the MDMA plus THC group and the vehicle controls was no longer present. Latency to first emergence in the MDMA plus THC group was not significantly different from the MDMA alone group.
Table 1.
Behavioral measurements (mean ± S.E.M.) in the emergence test in Experiment I.
| N | Total Number of Emergences | Latency to First Emergence (s) | Open-field Duration (s) | Risk Assessment Duration (s) | |
|---|---|---|---|---|---|
| Control | 15 | 1.5 ± 0.6 | 240.6 ± 18.0 | 31.4 ± 12.2 | 74.9 ± 6.3 |
| MDMA | 16 | 2.8 ± 0.6 | 152.1 ± 27.5* | 78.2 ± 18.5 | 60.7 ± 6.6 |
| THC | 14 | 1.7 ± 0.6 | 234.8 ± 27.0 | 42.2 ± 17.7 | 62.7 ± 7.0 |
| MDMA + THC | 14 | 1.8 ± 0.6 | 236.1 ± 20.4 | 27.1 ± 9.4 | 65.0 ± 5.7 |
3.1.6 Hole-board test
Frequencies of novel, repeated, and total hole entries during the 10-min hole-board test performed on PD 74 are shown in Table 2 (note that the N’s per group are lower in this study due to data loss from a technical difficulty). One-way ANOVA revealed significant treatment effects for both novel (F (3,31) = 3.18, P < 0.05) and total (F (3,31) = 3.16, P <0.05) hole entries, as well as a strong trend for repeat hole entries (F (3,31) = 2.87, P = 0.052). The Tukey post-hoc test revealed that prior MDMA exposure resulted in a significant decrease in novel hole entries compared to the control group. Prior THC exposure alone did not affect novel hole entries, but it did prevent the MDMA-induced decrease in this measure observed in the MDMA plus THC co-administration group (Table 2).
Table 2.
Novel, repeat, and total hole entries (mean ± S.E.M.) in the hole-board test.
| N | Novel entries | Repeat entries | Total entries | |
|---|---|---|---|---|
| Control | 9 | 13.8 ± 0.5 | 25.1 ± 3.4 | 38.9 ± 3.0 |
| MDMA | 10 | 10.8 ± 0.9* | 14.7 ± 2.8 | 25.5 ± 6.6 |
| THC | 8 | 12.6 ± 1.2 | 21.6\± 5.6 | 34.3 ± 5.1 |
| MDMA + THC | 8 | 13.6 ± 0.5 | 30.9 ± 4.4# | 44.5 ± 3.8# |
3.1.7 Serotonin transporter binding
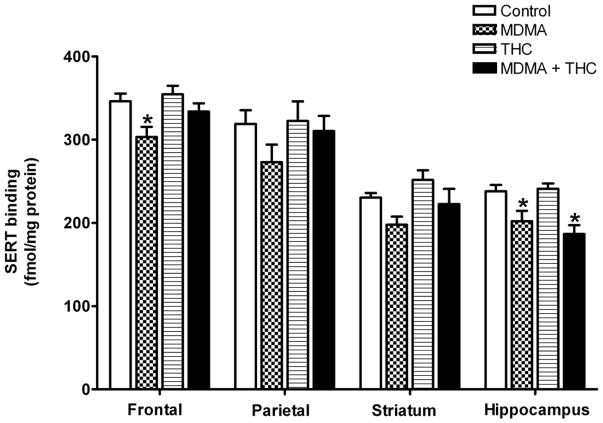
Effects of drug treatments on regional SERT binding are illustrated in Figure 4. In all brain areas examined, MDMA administration alone resulted in decreases in SERT binding ranging from approximately 12 to 15% compared to the vehicle controls. One-way ANOVA revealed an overall significant treatment effect for SERT binding in the frontal cortex (F (3,54) = 4.66, P < 0.01), parietal cortex (F (3,55) = 3.59, P < 0.02), and hippocampus (F (3,55) = 7.43, P < 0.001). Tukey post-hoc tests revealed that the MDMA-related decrease in SERT binding reached statistical significance in the frontal cortex and hippocampus, but not in the parietal cortex. THC alone did not influence SERT binding; however, co-administration of THC with MDMA prevented the significant MDMA-induced reduction in SERT binding in the frontal cortex and similarly blunted the nonsignificant effect of MDMA in the parietal cortex, but did not prevent the decrease in SERT binding in the hippocampus resulting from MDMA exposure. Reductions in SERT binding in the MDMA plus THC group were not significantly different from the MDMA alone group in any of the brain regions.
Figure 4.
Effects of drug treatments (described in Figure 1) on SERT binding (fmol/mg protein; mean ± S.E.M.) in the frontal cortex, parietal cortex, striatum, and hippocampus. *P < 0.05 vs. control (Tukey post-hoc test).
3.1.8 5-HT and 5-HIAA concentrations
Effects of drug treatments on cortical and hippocampal 5-HT and 5-HIAA concentrations are shown in Table 3. One-way ANOVA revealed an overall significant treatment effect for 5-HT levels in the frontal cortex (F (3,53) = 4.56, P < 0.01) and parietal cortex (F (3,55) = 3.18, P < 0.05), but not in the hippocampus (F (3,55) = 0.872, P > 0.10). In both cortical areas, MDMA administration alone resulted in statistically significant (Tukey tests) 20–25% reductions in 5-HT levels compared to vehicle controls. Chronic administration of THC alone did not result in any significant reduction in 5-HT levels in any of the brain region, but it blunted the MDMA-induced reductions in the cerebral cortex to the extent that the MDMA plus THC group did not differ significantly from the controls. It should be noted that 5-HT levels in the MDMA plus THC co-treatment group were not significantly different from the MDMA alone group. There were no group differences in the serotonin metabolite 5-HIAA in any of the brain regions examined (P > 0.10).
Table 3.
5-HT and 5-HIAA concentration (ng/100mg; mean ± S.E.M.).
| Frontal Cortex 5-HT | Frontal Cortex 5-HIAA | Parietal Cortex 5-HT | Parietal Cortex 5-HIAA | Hippocampal 5-HT | Hippocampal 5-HIAA | |
|---|---|---|---|---|---|---|
| Control | 39.1 ± 2.7 | 16.7 ± 1.0 | 15.2 ± 0.9 | 30.3 ± 3.4 | 23.7 ± 1.2 | 14.6 ± 1.1 |
| MDMA | 28.4 ± 2.0* | 14.6 ± 0.9 | 12.0 ± 0.7* | 30.4 ± 1.1 | 20.9 ± 1.2 | 14.7 ± 1.4 |
| THC | 35.9 ± 2.1 | 14.8 ± 1.1 | 12.7 ± 0.7 | 33.5 ± 1.8 | 21.8 ± 1.3 | 17.5 ± 2.1 |
| MDMA + THC | 30.2 ± 2.5 | 14.5 ± 0.9 | 13.5 ± 0.7 | 30.2 ± 2.2 | 21.7 ± 1.3 | 14.6 ± 1.1 |
3.2 Experiment II
3.2.1 Body weight and temperature
Drug treatments in this experiment produced similar reductions in body weight gain as were seen in Experiment 1. A mixed-design ANOVA revealed significant main effects of treatment (F (3,46) = 5.10, P < 0.01), a significant age effect (F (7,32) = 2746.53, P < 0.001), and a significant treatment x age interaction (F (7,21) = 2.08, P < 0.001). Group differences in body weight were further analyzed using one-way ANOVAs at each time point. These analyses revealed significant treatment effects beginning on PD 40 (F (3,46) = 7.63, P < 0.001) and on every measurement day thereafter except for PD 71 (data not shown).
As in Experiment 1, core temperature dysregulation in response to drug treatment was documented on PD 35, 45, and 60, and the resulting data were analyzed by means of AUC values between 0 and 330 min and again between 360 and 480 min. One-way ANOVA revealed significant overall treatment effects between 0 min and 330 min on PD 45 (F (3,46) = 8.95, P < 0.001) and PD 60 (F (3,46) = 25.78, P < 0.001), but not on PD 35 (F (3,46) = 0.16, P > 0.10). Significant treatment effects were also observed between 360 min and 480 min on PD 35 (F (3,46) = 4.18, P < 0.02), PD 45 (F (3,46) = 13.33, P < 0.001), and PD 60 (F (3,46) = 5.64, P < 0.002). Tukey post-hoc tests revealed that MDMA produced a significant hyperthermic effect between 0 min and 330 min and THC produced a significant hyperthermic effect between 360 min and 480 min on the specified treatment days. Post-hoc testing further revealed that chronic THC administration prevented MDMA-induced hyperthermia between 0 and 330 min on PD 45 and PD 60, and intermittent MDMA administration prevented THC-induced hyperthermia between 360 and 480 min on those same days (data not shown).
3.2.2 Open-field
There was no difference between the four treatment groups either in total grid crossings [mean ± SEM: Controls = 58.1 ± 3.5; MDMA alone = 55.9 ± 4.8; THC alone = 65.8 ± 2.5; MDMA + THC = 56.9 ± 4.0; (F (3,46) = 1.57, P > 0.10)] or in the number of center entries [Controls = 3.5 ± 0.7; MDMA alone = 4.2 ± 0.7; THC alone = 3.5 ± 0.8; MDMA + THC = 5.4 ± 0.8; (F (3,46) = 1.41, P > 0.10)] in the open-field test conducted on PD 70. However, there was a significant difference in rearing behavior [Controls = 18.4 ± 1.3; MDMA alone = 19.4 ± 1.1; THC alone = 23.3; MDMA + THC = 27.3; (F (3,46) = 7.21, P < 0.001)] among the treatment groups. The Tukey post-hoc test revealed that the MDMA plus THC combination treatment resulted in a significant increase in rearing behaviors compared to the controls (P < 0.001). This effect was not observed in any other treatment groups (P > 0.10).
3.2.3 Plasma corticosterone
One-way ANOVA found no significant effects among the four treatment groups in any of the dependent measures: basal corticosterone levels [mean ± SEM: Controls = 113.4 ± 17.4 ng/ml; MDMA alone = 126.1 ± 24.7; THC alone = 96.5 ± 11.9; MDMA + THC = 129.3 ± 29.9; (F (3,45) = 0.54, P > 0.10)], corticosterone levels after open-field exposure [Controls = 229.3 ± 22.9 ng/ml; MDMA alone = 238.7 ± 14.3; THC alone = 220.2 ± 20.2; MDMA + THC = 200.8 ± 17.2; (F (3,45) = 0.45, P > 0.10)], and corticosterone response determined by the difference between the post-exposure and basal levels [Controls = 115.9 ± 24.0 ng/ml; MDMA alone = 112.5 ± 31.2; THC alone = 123.7 ± 23.6; MDMA + THC = 71.4 ± 25.6; (F (3,40) = 0.070, P > 0.10)].
3.2.4 Emergence test
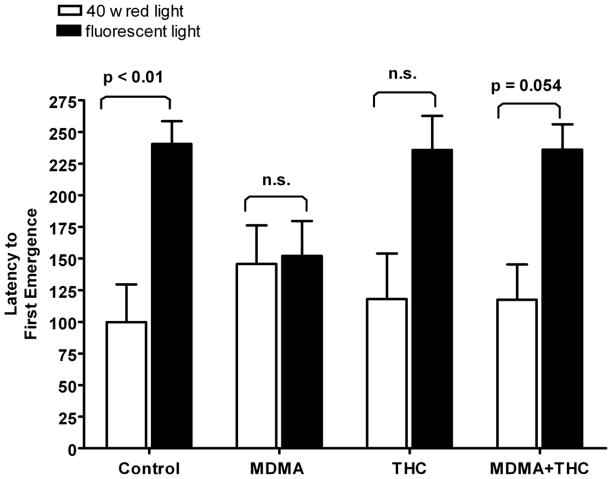
Table 4 presents the results of the emergence test conducted on PD 72. One-way ANOVA revealed no significant treatment effect on any of the dependent measures in this test: total number of emergences (F (3,40) = 0.58, P > 0.10), latency to first emergence (F (3,40) = 0.41, P > 0.10), time spent in open-field (F (3,40) = 0.051, P > 0.10), and duration of risk assessment behaviors (F (3,40) = 1.31, P > 0.10). It is important to recall that in this experiment, the emergence test was carried out under 40W red light illumination in contrast to the bright fluorescent lighting used in Experiment I. When latency to first emergence is compared across the two experiments, it can be seen that all treatment groups except for the MDMA alone group showed the expected decrease in latency under the presumed low-anxiety conditions present in Experiment II (Fig. 5). As discussed later, this finding is of importance in interpreting the significant MDMA effect on emergence latency in Experiment I.
Table 4.
Behavioral measurements in the emergence test (mean ± S.E.M.) in Experiment II.
| N | Total number of emergences | Latency to first emergence (s) | Open-field duration (s) | Risk assessment duration (s) | |
|---|---|---|---|---|---|
| Control | 12 | 3.7 ± 0.6 | 99.8 ± 29.7 | 112.7 ± 24.0 | 69.0 ± 10.2 |
| MDMA | 9 | 3.0 ± 0.6 | 145.7 ± 30.4 | 111.9 ± 25.0 | 71.7 ± 11.5 |
| THC | 11 | 4.0 ± 0.7 | 118.1 ± 35.9 | 107.2 ± 23.5 | 70.2 ± 13.0 |
| MDMA + THC | 12 | 3.2 ± 0.5 | 117.6 ± 27.9 | 101.1 ± 23.3 | 70.5 ± 13.0 |
Figure 5.
Effects of drug treatments (described in Figure 1) on latency to first emergence compared across Experiments I (bright fluorescent light) and II (40 W red light), both conducted on PD 72. The data are expressed as mean ± S.E.M.. *P < 0.05 vs. control (Tukey post-hoc test).
4. Discussion
The primary objective of the current study was to investigate the physiological, behavioral, and neurochemical effects of chronic co-administration of THC and MDMA during adolescence in rats. Co-administration of THC attenuated MDMA-induced acute hyperthermia, blunted various behavioral effects resulting from intermittent MDMA administration, and reduced the serotonergic neurotoxic effects of MDMA in the frontal and parietal cortex. On the other hand, THC provided no protection against MDMA-induced attenuation of the rate of weight gain, nor did it provide a protection against MDMA-induced neurotoxicity in the hippocampus. We will first discuss the physiological findings, and then the behavioral and neurochemical results.
4.1. Physiological effects
MDMA administration resulted in an acute hyperthermic response, which has been reported previously in both adult rats (Gurman et al., 2002; Malberg and Seiden, 1998; Morley et al., 2001) and adolescents (Piper and Meyer, 2006). However, while the majority of previous studies found that THC administration induces hypothermia (e.g., Arnold et al., 2001; Pryor et al., 1978), administration of 5 mg/kg of THC alone in the present study produced acute hyperthermia.
THC-induced hyperthermia has been previously reported in two circumstances. First, previous studies have shown that THC-induced temperature alteration is biphasic, with lower doses (0.05–0.1 mg/kg) leading to hyperthermia but higher doses (0.1–5 mg/kg) leading to a hypothermia (Taylor and Fennessy, 1977). Second, whereas initial administration of 2.5 mg/kg or 5 mg/kg of THC was found to cause hypothermia, chronic treatment with the same doses has been found to subsequently produce hyperthermia (Johansson et al., 1975). Another likely contributing factor to the present results is the higher ambient temperatures used in our study to ensure MDMA-induced hyperthermia. Most previous THC studies used ambient temperatures of 20–22°C in contrast to the present ambient temperatures that averaging 26°C and 23°C, respectively, in Experiments I and II. The influence of ambient temperature on THC-induced thermal responses has been shown in earlier studies that noted the occurrence of a hypothermic response when the experiment was conducted at 10°C or 20°C but not at 31°C (Bloom and Kiernan, 1980; Fennessy and Taylor, 1977). Morley and coworkers (2004) previously reported that concurrent administration of either THC or the synthetic cannabinoid receptor agonist CP 55,940 prevented MDMA-induced hyperthermia. More recently, a controlled study of experienced ecstasy and cannabis co-users found that THC given immediately after a single dose of MDMA and then twice more at 90-min intervals prolonged the MDMA-induced hyperthermic response (Dumont et al., 2009). Using yet a different paradigm involving daily THC treatment given after MDMA on co-administration days, the present study is the first to demonstrate a reduction of MDMA’s hyperthermic effect as a result of prior THC exposure (compare the 0–330 min AUC data on PD 45 and [in Experiment I] on PD 60 in the combination treatment group to the data from the MDMA alone group). Both the direct hypothermic effect of cannabinoid agonists found in many studies and their ability to prevent MDMA-induced hyperthermia appear to be CB1 receptor-dependent, as both effects can be reversed by administration of the CB1 receptor antagonist SR 141716 (Morley et al., 2004; Wiley et al., 2007). In contrast, the mechanisms underlying the hyperthermic effect of THC found in the present study and in the two other studies cited above remain to be elucidated.
Consistent with previous studies, repeated administration of either MDMA alone or THC alone resulted in a significant decrease in the rate of weight gain compared to control animals (Johansson et al., 1975; Piper and Meyer, 2004; Piper et al., 2005). Similar results were observed in the MDMA plus THC group, indicating a lack of either an additive or interactive effect of the two drugs on this outcome measure.
4.2 Behavioral effects
Both experiments found no significant treatment effects either in locomotor activity (i.e., grid crossings) or in anxiety-related behavior (i.e., center square entries) in the open-field test conducted on PD 70. The lack of effect on locomotor activity is particularly important for excluding changes in this parameter when interpreting drug-related effects in other behavioral tests. Although there was a statistically significant increase in open-field rearing behavior in the MDMA plus THC group in Experiment II, it may have been a false positive since the same result was not obtained in the first experiment.
Due to unexpected technical difficulties, the two experiments were carried out at slightly different ambient room temperatures (26°C in Experiment I and 23°C in Experiment II). However, we do not believe that our behavioral results were affected by the change in the ambient temperature because core body temperatures measured at PD 35, 45, and 60 were similar across the two experiments.
In Experiment I, the emergence test was conducted under bright fluorescent lighting that presumably maximized the anxiety-like state associated with leaving the hide-box. Under these conditions, treatment with MDMA alone caused a significant reduction in latency to first emergence that was reversed by THC co-administration. Previous studies using different MDMA treatment paradigms and different strains of rats have generally reported a significant increase in latency to first emergence following MDMA exposure, which has been interpreted to mean a drug-induced increase in anxiety (Gurtman et al., 2002; Morley et al., 2001; 2004). Before offering the opposite interpretation for the present results, however, it is important to note that no effect of MDMA was observed under low anxiety test conditions (i.e., red light illumination) in Experiment II. Indeed, as shown in Fig. 5, all treatment groups except for the MDMA alone group showed the expected decrease in emergence latency in Experiment II compared to Experiment I. Thus, either MDMA exposure using the present dosing paradigm causes the animals to become insensitive to anxiety-related parameters of the testing environment, or the treatment effect in Experiment I reflects a drug-induced change in a behavioral construct other than anxiety. Elsewhere we have proposed that our intermittent adolescent MDMA treatment regimen may lead to increased impulsivity (Meyer et al., 2008), which could have led to early emergence from the hide-box in Experiment I and would also be consistent with the results of several studies of ecstasy users (Karlsen et al., 2007; Morgan et al., 2000; Montoya et al., 2002). Nevertheless, this hypothesis remains speculative until MDMA-exposed animals have been studied using more traditional tests of impulsive behavior.
The social interaction test was also conducted in Experiment I. While no significant group differences were found in the overall duration and frequency of social interactions, rats showed significantly less frequency and duration of crawling behavior following MDMA only administration. Decreased social interaction as a result of prior MDMA exposure has been documented by previous studies (Gurtman et al., 2002; Morley et al., 2001). In the current study, decreased crawling behavior in the social interaction test is suggestive of a modest MDMA-induced increase in anxiety, thus providing further evidence that the MDMA-induced decrease in latency in the emergence test may represent enhanced impulsivity rather than decreased anxiety. THC alone did not cause significant differences in either crawling frequency or duration as compared to the control group. However, the results obtained from the MDMA plus THC co-administration group showed that THC reduced the MDMA-induced behavioral changes in the social interaction test.
On the hole-board test, an index of exploratory behavior (Makanjuola et al., 1977) that was performed in Experiment I, the MDMA alone group displayed significantly fewer novel hole entries compared to the controls. Compounds that affect the noradrenergic, dopaminergic, and serotonergic systems have all been reported to influence the outcome of the hole-board test (García-Marquez et al., 1987; Makanjuola et al., 1977; Sara et al., 1995). For example, rats treated chronically with the 5-HT reuptake inhibitor clomipramine showed reduced head dipping in this task (García-Marquez et al., 1987), and a more recent study from our laboratory found that an MDMA binge led to a subsequent reduction in novel hole entries in adult rats (Piper et al., 2008). The latter effect was blocked by citalopram pretreatment, suggesting that the MDMA-induced decrease in the animals’ exploratory behavior was related to MDMA’s effects on the serotonergic system. In the current study, the number of novel hole entries in the combined MDMA plus THC group was no longer significantly different from the controls, suggesting that chronic treatment with THC provided some protection against the MDMA-induced decrease in exploratory behavior. On the other hand, THC adminstration by itself had no effect, which contrasts with the results of Hernández-Tristán et al. (2000) showing reduced exploration in the hole-board test in rats treated acutely with THC. These discrepant findings are likely due to procedural differences in dosing (single vs. chronic administration) and the fact that the Hernández-Tristán et al. study was conducted with the drug “on board” the animals.
Experiment II additionally assessed baseline corticosterone levels and corticosterone responses to the stress of open-field exposure. No treatment effects were found on either measure, which is consistent with the previously reported lack of effect of a high-dose MDMA treatment regimen on either baseline corticosterone or the response to restraint stress (Matuszewich et al., 2002). In contrast, Gerra and coworkers (2003) reported elevated baseline cortisol and adrenocorticotropic hormone (ACTH) levels but blunted responses to a psychosocial stressor in experienced ecstasy users compared to nonusing controls. This discrepancy could have resulted from species differences in neuroendocrine reactivity to MDMA or from differences in the amount and/or pattern of MDMA exposure in human ecstasy users compared to the present study or the previous work of Matuszewich et al.
4.3 Neurochemical effects
Rats were killed 15 days after the last drug administration for assessment of treatment-related serotonergic neurotoxicity using two different markers of serotonergic fibers: radioligand binding to the plasma membrane SERT, and concentrations of 5-HT and 5-HIAA. Intermittent administration of MDMA alone caused an approximately 12–15% reduction in SERT binding in the frontal cortex, the parietal cortex, the striatum, and the hippocampus as compared to the control group. Similar reductions in SERT binding in the parietal cortex and hippocampus were reported in a previous study from our laboratory using the same MDMA dosing regimen (Piper and Meyer, 2004). Although administration of THC alone did not result in any change in SERT binding, results from the combined MDMA plus THC group indicated that THC blunted the neurotoxic effect of MDMA in the cortical areas and the striatum, but not in the hippocampus.
Group differences in cortical and hippocampal 5-HT concentrations were similar but not identical to the differences observed in SERT binding. First, the 5-HT depletion in the frontal and parietal cortices of the MDMA alone group (ranging from approximately 20 to 25%) was greater than the decrease in SERT binding in these areas. Second, the hippocampus showed only a small 5-HT depletion that failed to reach statistical significance compared to the controls. Third, although THC co-administration did produce some blunting of the MDMA-induced 5-HT depletion in the cerebral cortex, this attenuation was not as great as seen in the SERT binding data. Thus, although previous findings from many laboratories have shown large and reasonably parallel reductions in 5-HT levels and SERT binding following high-dose MDMA treatment regimens (Green et al., 2003; Piper et al., 2008), the present findings suggest that these two measures may not respond as closely to more moderate MDMA exposure and to the interaction between intermittent MDMA and chronic THC administration. The finding of MDMA and THC’s effects on serotonin and metabolite has significant clinical relevance, as human ecstasy studies often use the moderate reduction in the 5-HT and 5-HIAA levels in cerebrospinal fluid as a marker for serotonergic neurotoxicity (Green et al., 2003).
The mechanisms underlying the ability of THC and other cannabinoid agonists to at least partially ameliorate MDMA serotonergic neurotoxicity are still under investigation. In the previous rat study by Morley and coworkers (2004), THC or a high dose of the synthetic cannabinoid agonist CP 55,940 administered concurrently with a neurotoxic dosing regimen of MDMA completely blocked MDMA-induced hyperthermia and partially reduced MDMA’s 5-HT depleting effects. Co-administration of the CB1 antagonist SR 141716 with CP 55,940 and MDMA prevented the hypthermia-blocking effect of the cannabinoid agonist but had little effect on regional 5-HT levels compared to MDMA plus CP 55,940 without the antagonist. These results suggest that the thermic interactions between cannabinoids and MDMA are CB1 receptor dependent, but the protective effects of cannabinoids against MDMA neurotoxicity are not. In contrast, Touriño et al. (2010) recently found strong evidence for a critical role of CB1 receptors in the amelioration of MDMA-induced dopamine neurotoxicity by THC in mice maintained at an elevated temperature. These discrepant results raise the possibility that different mechanisms mediate the neuroprotective effects of cannabinoids on the serotonergic system of MDMA-treated rats compared to the dopaminergic system of MDMA-treated mice. Finally, it is not yet known why THC had no effect on the MDMA-induced SERT reduction in the hippocampus in the present study. We note, however, that one important difference between the hippocampus and the neocortex where THC did exert a neuroprotective action is the high density of CB1 receptors in the hippocampus (Herkenham et al., 1990). As mentioned earlier, activation of CB1 receptors inhibits 5-HT synthesis, release, and turnover in various brain areas, including the hippocampus (Balázsa et al., 2008; Moranta et al., 2004; Nakazi et al., 2000). If this CB1 receptor-mediated inhibition of serotonergic activity somehow promotes MDMA neurotoxicity, then such enhancement may have nullified the presumably CB1-independent protective effect observed in areas like the frontal and parietal cortices with lower receptor expression than the hippocampus. This hypothesis requires further investigation.
In conclusion, the current study assessed the physiological, behavioral, and neurochemical effects of intermittent MDMA administration combined with daily THC exposure during the periadolescent period in male rats. The rate of weight gain was significantly reduced by treatment with either MDMA alone, THC alone, or the two drugs combined, with no differences among treatment conditions. Administration of THC attenuated MDMA-induced acute hyperthermia in the MDMA plus THC group, though it also unexpectedly had a mild hyperthermic effect when administered on its own. While chronic THC administration alone did not cause any behavioral alterations as compared to controls, it provided some protection against the increased impulsivity, increased anxiety, and deficient exploratory behavior that resulted from intermittent MDMA administration. Protection against MDMA-induced serotonergic neurotoxicity may be responsible for at least some of THC’s ability to block various MDMA-induced behavioral deficits, as serotonergic function has been closely associated with anxiety and impulsivity (Brown et al., 1982; Owens et al., 1994; Spoont, 1992). However, this hypothesis requires further investigation. Furthermore, the protective effect of THC against MDMA-induced neurotoxicity was region specific, with the clearest protection in the cortical regions but none in the hippocampus. The failure of THC to prevent reduced SERT binding in the hippocampus leads to the prediction that THC would not blunt the adverse effects of MDMA on hippocampal-dependent cognitive tasks. This prediction is currently being tested in our laboratory.
Chronic administration of THC with MDMA prevented MDMA-induced hyperthermia.
THC protected against MDMA-induced changes in anxiety-like and exploratory behavior.
THC attenuated MDMA-induced decreases in SERT levels and 5-HT concentrations.
Acknowledgments
Animal care was provided by Pinnie Sears. Animal behaviors were coded by Thomas R. Barnes, China N. Byrns, Erin S. Calipari, Frederico S. Fernandes, Jamie S. Richmond, Christopher D. Sacco and Alex J. Weiner. MDMA and THC were provided by the NIDA Drug Supply Program. This research was supported by NIDA grant R03 DA025839 to Dr. Jerrold S. Meyer. Erica Y. Shen was supported by NIMH training grant 5T32 MH020051.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ali SF, Newport GD, Holson RR, Slikker W, Bowyer JF. Low environmental temperature or pharmacologic agents that produce hypothermia decrease methamphetamine neurotoxicity in mice. Brain Res. 1994;658:33–38. doi: 10.1016/s0006-8993(09)90007-5. [DOI] [PubMed] [Google Scholar]
- Arnold JC, Topple AN, Mallet PE, Hunt GE, McGregor IS. The distribution of cannabinoid-induced Fos expression in rat brain: differences between the Lewis and Wistar strain. Brain Res. 2001;921:240–255. doi: 10.1016/s0006-8993(01)03127-4. [DOI] [PubMed] [Google Scholar]
- Balázsa T, Bíró J, Gullai N, Ledent C, Sperlágh B. CB1-cannabinoid receptors are involved in the modulation of non-synaptic [3H] serotonin release from the rat hippocampus. Neurochem Int. 2008;52:95–102. doi: 10.1016/j.neuint.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Black DL, Cawthon B, Robert T, Moser F, Caplan YH, Cone EJ. Multiple drug ingestion by ecstasy abusers in the United States. J Anal Toxicol. 2009;33:143–147. doi: 10.1093/jat/33.3.143. [DOI] [PubMed] [Google Scholar]
- Block RI, Ghoneim MM. Effects of chronic cannabis use on human cognition. Psychopharmacology (Berl) 1993;110:219–228. doi: 10.1007/BF02246977. [DOI] [PubMed] [Google Scholar]
- Bloom AS, Kiernan CJ. Interaction of ambient temperature with the effects of delta (9)-tetrahydrocannabinol on brain catecholamine synthesis and plasma corticosterone levels. Psychopharmacology (Berl) 1980;67:215–219. doi: 10.1007/BF00431259. [DOI] [PubMed] [Google Scholar]
- Boys A, Lenton S, Norcross K. Polydrug use at raves by a Western Australian sample. Drug Alcohol Rev. 1997;16:227–234. doi: 10.1080/09595239800187411. [DOI] [PubMed] [Google Scholar]
- Breier JM, Bankson MG, Yamamoto BK. L-Tyrosine contributes to (+) -3, 4- Methylenedioxymethamphetamine - induced serotonin depletions. J Neurosci. 2006;26:290–299. doi: 10.1523/JNEUROSCI.3353-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GL, Ebert MH, Goyer PF, Jimerson DC, Klein WJ, Bunney WE, Goodwin FK. Agression, suicide and serotonin: relationships to CSF amine metabolites. Am J Psychiatry. 1982;139:741–746. doi: 10.1176/ajp.139.6.741. [DOI] [PubMed] [Google Scholar]
- Cha YM, White AM, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of delta9-THC on learning in adolescent and adult rats. Pharmacol Biochem Behav. 2006;83:448–55. doi: 10.1016/j.pbb.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Chaperon F, Thiébot MH. Behavioral effects of cannabinoid agents in animals. Crit Rev Neurobiol. 1999;13:243–281. doi: 10.1615/critrevneurobiol.v13.i3.20. [DOI] [PubMed] [Google Scholar]
- Cowan RL. Neuroimaging research in human MDMA users: a review. Psychopharmacology (Berl) 2007;189:539–556. doi: 10.1007/s00213-006-0467-3. [DOI] [PubMed] [Google Scholar]
- Croft RJ, Mackay AJ, Mills AT, Gruzelier JG. The relative contributions of ecstasy and cannabis to cognitive impairment. Psychopharmacology (Berl) 2001;153:373–379. doi: 10.1007/s002130000591. [DOI] [PubMed] [Google Scholar]
- Daumann J, Jr, Fischermann T, Heekeren K, Thron A, Gouzoulis-Mayfrank E. Neural mechanisms of working memory in ecstasy (MDMA) users who continue or discontinue ecstasy and amphetamine use: evidence from an 18-month longitudinal functional magnetic resonance imaging study. Biol Psychiatry. 2004;56:349–355. doi: 10.1016/j.biopsych.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Dumont GJ, Kramers C, Sweep FC, Touw DJ, van Hasselt JG, de Kam M, van Gerven JM, Builtelaar JK, Verkes RJ. Cannabis coadministration potentiates the effects of “ecstasy” on heart rate and temperature in humans. Clin Pharmacol Ther. 2009;86:160–166. doi: 10.1038/clpt.2009.62. [DOI] [PubMed] [Google Scholar]
- Fennessy MR, Taylor DA. The effect of delta (9)-tetrahydrocannabinol on body temperature and brain amine concentrations in the rat at different ambient temperatures. Br J Pharmacol. 1977;60:65–71. doi: 10.1111/j.1476-5381.1977.tb16748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Marquez C, Giralt M, Armario A. Long-lasting effects of chronic chlorimipramine treatment of rats on exploratory activity on a hole-board, and on immobility in the forced swimming test. Eur J Pharmacol. 1987;142:385–389. doi: 10.1016/0014-2999(87)90077-x. [DOI] [PubMed] [Google Scholar]
- Gerra G, Bassignana S, Zaimovic A, Moi G, Bussandri M, Caccavari R, Brambilla F, Molina E. Hypothalamic-pituitary-adrenal axis responses to stress in subjects with 3,4-methylenedioxy-methamphetamine (‘ecstasy’) use history: correlation with dopamine receptor sensitivity. Psychiatry Res. 2003;120:115–124. doi: 10.1016/s0165-1781(03)00175-6. [DOI] [PubMed] [Google Scholar]
- Green AR, Mechan AO, Elliott JM, O’Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) Pharmacol Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- Gurtman CG, Morley KC, Li KM, Hunt GE, McGregor IS. Increased anxiety in rats after 3,4-methylenedioxymethamphetamine: association with serotonin depletion. Eur J Pharmacol. 2002;446:89–96. doi: 10.1016/s0014-2999(02)01820-4. [DOI] [PubMed] [Google Scholar]
- Häring M, Marsicano G, Lutz B, Monory K. Identification of the cannabinoid receptor type 1 in serotonergic cells of raphe nuclei in mice. Neuroscience. 2007;146:1212–1219. doi: 10.1016/j.neuroscience.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Tristán R, Arévalo C, Canals S, Leret ML. The effects of acute treatment with Δ9-THC on exploratory behaviour and memory in the rat. Physiol Behav. 2000;56:17–24. doi: 10.1007/BF03179772. [DOI] [PubMed] [Google Scholar]
- Johansson JO, Jarbe TU, Henriksson BG. Acute and subchronic influences of tetrahydrocannabinols on water and food intake, body weight, and temperature in rats. TIT J Life Sci. 1975;5:17–27. [PubMed] [Google Scholar]
- Karlsen SN, Spigset O, Slørdal L. The dark side of ecstasy: neuropsychiatric symptoms after exposure to 3,4-methylenedioxymethamphetamine. Basic Clin Pharmacol Toxicol. 2007;102:15–24. doi: 10.1111/j.1742-7843.2007.00159.x. [DOI] [PubMed] [Google Scholar]
- Lau T, Schloss P. The cannabinoid CB1 receptor is expressed on serotonergic and dopaminergic neurons. Eur J Pharmacol. 2008;578:137–141. doi: 10.1016/j.ejphar.2007.09.022. [DOI] [PubMed] [Google Scholar]
- Lenton S, Boys A, Norcross K. Raves, drugs and experience: drug use by a sample of people who attend raves in Western Australia. Addiction. 1997;92:1327–37. [PubMed] [Google Scholar]
- Lyles J, Cadet JL. Methylenedioxymethamphetamine (MDMA, Ecstasy) neurotoxicity: cellular and molecular mechanisms. Brain Res Reviews. 2003;42:155–168. doi: 10.1016/s0165-0173(03)00173-5. [DOI] [PubMed] [Google Scholar]
- Makanjuola RO, Hill G, Dow RC, Campbell G, Ashcroft GW. The effects of psychotropic drugs on exploratory and stereotyped behavior of rats studied on a hole-board. Psychopharmacology (Berl) 1977;55:67–74. doi: 10.1007/BF00432819. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Sabol KE, Seiden LS. Co-administration of MDMA with drugs that protect against MDMA neurotoxicity produces different effects on body temperature in the rat. J Pharmacol Exp Ther. 1996;278:258–267. [PubMed] [Google Scholar]
- Martínez-Orgado J, Fernández-Frutos B, González R, Romero E, Urigüen L, Romero J, Viveros MP. Neuroprotection by the cannabinoid agonist WIN-55212 in an in vivo newborn rat model of acute severe asphyxia. Brain Res Mol Brain Res. 2003;114:132–139. doi: 10.1016/s0169-328x(03)00163-3. [DOI] [PubMed] [Google Scholar]
- Matuszewich L, Filon ME, Finn DA, Yamamoto BK. Altered forebrain neurotransmitter responses to immobilization stress following 3,4-methylenedioxymethamphetamine. Neuroscience. 2002;110:41–48. doi: 10.1016/s0306-4522(01)00539-5. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Piper BJ, Vancollie VE. Development and characterization of a novel animal model of intermittent MDMA (“ecstasy”) exposure during adolescence. Ann N Y Acad Sci. 2008;1139:151–163. doi: 10.1196/annals.1432.029. [DOI] [PubMed] [Google Scholar]
- Montoya AG, Sorrentino R, Lukas SE, Price BH. Long-term neuropsychiatric consequences of “ectasy” (MDMA): a review. Harv Rev Psychiatry. 2002;10:212–220. [PubMed] [Google Scholar]
- Moranta D, Esteban S, Garcia-Sevilla JA. Differential effects of acute cannabinoid drug treatment, mediated by CB1 receptors, on the in vivo activity of tyrosine and tryptophan hydroxylase in the rat brain. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:516–524. doi: 10.1007/s00210-004-0921-x. [DOI] [PubMed] [Google Scholar]
- Morgan MJ. Ecstasy (MDMA): a review of its possible persistent psychological effects. Psychopharmacology (Berl) 2000;152:230–248. doi: 10.1007/s002130000545. [DOI] [PubMed] [Google Scholar]
- Morgan MJ, Impallomeni LC, Pirona A, Rogers RD. Elevated impulsivity and impaired decision-making in abstinent Ecstasy (MDMA) users compared to polydrug and drug-naïve controls. Neuropsychopharmacology. 2006;31:1562–1573. doi: 10.1038/sj.npp.1300953. [DOI] [PubMed] [Google Scholar]
- Morley KC, Gallate JE, Hunt GE, Mallet PE, McGregor IS. Increased anxiety and impaired memory in rats 3 months after administration of 3,4-methylenedioxymethamphetamine (“ecstasy”) Eur J Pharmacol. 2001;433:91–99. doi: 10.1016/s0014-2999(01)01512-6. [DOI] [PubMed] [Google Scholar]
- Morley KC, Li KM, Hunt GE, Mallet PE, McGregor IS. Cannabinoids prevent the acute hyperthermia and partially protect against the 5-HT depleting effects of MDMA (“Ecstasy”) in rats. Neuropharmacology. 2004;46:954–65. doi: 10.1016/j.neuropharm.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Nakamura EM, da Silva EA, Concilio GV, Wilkinson DA, Masur J. Reversible effects of acute and long-term administration of delta-9-tetrahydrocannabinol (THC) on memory in the rat. Drug Alcohol Depend. 1991;28:167–75. doi: 10.1016/0376-8716(91)90072-7. [DOI] [PubMed] [Google Scholar]
- Nakazi M, Bauer U, Nickel T, Kathmann M, Schlicker E. Inhibition of serotonin release in the mouse brain via presynaptic cannabinoid CB1 receptors. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:19–24. doi: 10.1007/s002109900147. [DOI] [PubMed] [Google Scholar]
- Nava F, Carta G, Battasi AM, Gessa GL. D(2) dopamine receptors enable delta(9)-tetrahydrocannabinol induced memory impairment and reduction of hippocampal extracellular acetylcholine concentration. Br J Pharmacol. 2000;130:1201–1210. doi: 10.1038/sj.bjp.0703413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens WJ, Nemeroff CB. Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter. Clin Chem. 1994;40:288–295. [PubMed] [Google Scholar]
- Parrott AC. Human psychopharmacology of Ecstasy (MDMA): a review of 15 years of empirical research. Hum Psychopharmacol. 2001;16:557–577. doi: 10.1002/hup.351. [DOI] [PubMed] [Google Scholar]
- Parrott AC, Milani RM, Gouzoulis-Mayfrank E, Daumann J. Cannabis and Ecstasy/MDMA (3,4-methylenedioxymethamphetamine): an analysis of their neuropsychobiological interactions in recreational users. J Neural Transm. 2007;114:959–968. doi: 10.1007/s00702-007-0715-7. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Piper BJ, Fraiman JB, Owens CB, Ali SF, Meyer JS. Dissociation of the neurochemical and behavioral toxicology of MDMA (‘Ecstasy’) by citalopram. Neuropsychopharmacology. 2008;33:1192–1205. doi: 10.1038/sj.npp.1301491. [DOI] [PubMed] [Google Scholar]
- Piper BJ, Fraiman JB, Meyer JS. Repeated MDMA (“Ecstasy”) exposure in adolescent male rats alters temperature regulation, spontaneous motor activity, attention, and serotonin transporter binding. Dev Psychobiol. 2005;47:145–157. doi: 10.1002/dev.20085. [DOI] [PubMed] [Google Scholar]
- Piper BJ, Meyer JS. Memory deficit and reduced anxiety in young adult rats given repeated intermittent MDMA treatment during the periadolescent period. Pharmacol Biochem Behav. 2004;79:723–731. doi: 10.1016/j.pbb.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Piper BJ, Meyer JS. Increased responsiveness to MDMA in adult rats treated neonatally with MDMA. Neurotoxicol Teratol. 2006;28:95–102. doi: 10.1016/j.ntt.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Pryor GT, Larsen FF, Husain S, Braude MC. Interactions of delta9-tetrahydrocannabinol with d-amphetamine, cocaine, and nicotine in rats. Pharmacol Biochem Behav. 1978;8:295–318. doi: 10.1016/0091-3057(78)90320-9. [DOI] [PubMed] [Google Scholar]
- Quednow BB, Kühn KU, Hoppe C, Westheide J, Maier W, Daum I, Wagner M. Elevated impulsivity and impaired decision-making cognition in heavy users of MDMA (“Ecstasy”) Psychopharmacology (Berl) 2007;189:517–530. doi: 10.1007/s00213-005-0256-4. [DOI] [PubMed] [Google Scholar]
- Quinton MS, Yamamoto BK. Causes and consequences of methamphetamine and MDMA toxicity. AAPS J. 2006;8:337–347. doi: 10.1007/BF02854904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robledo P, Trigo JM, Panayi F, de la Torre R, Maldonado R. Behavioural and neurochemical effects of combined MDMA and THC administration in mice. Psychopharmacology (Berl) 2007;195:255–264. doi: 10.1007/s00213-007-0879-8. [DOI] [PubMed] [Google Scholar]
- Sala M, Braida D. Endocannabinoids and 3,4-methylenedioxymethamphetamine (MDMA) interaction. Pharmacol Biochem Behav. 2005;81:407–16. doi: 10.1016/j.pbb.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Dyon-Laurent C, Hervé A. Novelty seeking behavior in the rat is dependent upon the integrity of the noradrenergic system. Cogn Brain Res. 1995;2:181–187. doi: 10.1016/0926-6410(95)90007-1. [DOI] [PubMed] [Google Scholar]
- Schlicker E, Kathmann M. Modulation of transmitter release via presynaptic cannabinoid receptors. Trends Pharmacol Sci. 2001;22:565–572. doi: 10.1016/s0165-6147(00)01805-8. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Lê AD. Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacology (Berl) 2006;186:201–208. doi: 10.1007/s00213-006-0373-8. [DOI] [PubMed] [Google Scholar]
- Singh ME, McGregor IS, Mallet PE. Repeated exposure to delta (9)-tetrahydrocannabinol alters heroin-induced locomotor sensitization and Fos- immunoreactivity. Neuropharmacology. 2005;49:1189–1200. doi: 10.1016/j.neuropharm.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Sprague JE, Everman SL, Nichols DE. An integrated hypothesis for the serotonergic axonal loss induced by 3,4-methylenedioxymethamphetamine. Neurotoxicology. 1998;19:427–441. [PubMed] [Google Scholar]
- Spoont MR. Modulatory role of serotonin in neural information processing: implications of human psychopathology. Psychol Bull. 1992;112:330–350. doi: 10.1037/0033-2909.112.2.330. [DOI] [PubMed] [Google Scholar]
- Strote J, Lee JE, Wechsler H. Increasing MDMA use among college students: results of a national survey. J Adolesc Health. 2002;30:64–72. doi: 10.1016/s1054-139x(01)00315-9. [DOI] [PubMed] [Google Scholar]
- Taylor DA, Fennessy MR. Biphasic nature of the effects of Δ9-tetrahydrocannabinol on body temperature and brain amines of the rat. Eur J Pharmacol. 1977;46:93–99. doi: 10.1016/0014-2999(77)90244-8. [DOI] [PubMed] [Google Scholar]
- Topp L, Hando J, Dillon P, Roche A, Solowij N. Ecstasy use in Australia: patterns of use and associated harm. Drug Alcohol Depend. 1999;55:105–115. doi: 10.1016/s0376-8716(99)00002-2. [DOI] [PubMed] [Google Scholar]
- Touriño C, Zimmer A, Valverde O. THC prevents MDMA neurotoxicity in mice. PLoS One. 2010;5:e9143. doi: 10.1371/journal.pone.0009143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Stelt M, Di Marzo V. Cannabinoid receptors and their role in neuroprotection. Neuromolecular Med. 2005;7:37–50. doi: 10.1385/NMM:7:1-2:037. [DOI] [PubMed] [Google Scholar]
- Wiley JL, O’connell MM, Tokarz ME, Wright MJ., Jr Pharmacological effects of acute and repeated administration of delta(9)-tetrahydrocannabinol in adolescent and adult rats. J Pharmacol Exp Ther. 2007;320:1097–105. doi: 10.1124/jpet.106.108126. [DOI] [PubMed] [Google Scholar]
- Winstock AR, Griffiths P, Stewart D. Drugs and the dance music scene: a survey of current drug use patterns among a sample of dance music enthusiasts in the UK. Drug Alcohol Depend. 2001;64:9–17. doi: 10.1016/s0376-8716(00)00215-5. [DOI] [PubMed] [Google Scholar]
- Wish ED, Fitzelle DB, O’Grady KE, Hsu MH, Arria AM. Evidence for significant polydrug use among ecstasy-using college students. J Am Coll Health. 2006;55:99–104. doi: 10.3200/JACH.55.2.99-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JM, McGregor IS, Mallet PE. Co-administration of THC and MDMA (‘ecstasy’) synergistically disrupts memory in rats. Neuropsychopharmacology. 2005;30:1475–1482. doi: 10.1038/sj.npp.1300692. [DOI] [PubMed] [Google Scholar]