Abstract
Objective
Identifying discriminatory human salivary RNA biomarkers reflective of disease in a low-cost non-invasive screening assay is crucial to salivary diagnostics. Recent studies have reported both mRNA and microRNA (miRNA) in saliva, but little information has been documented on the quality and yield of RNA collected. Therefore, the aim of the present study was to develop an improved RNA isolation method from saliva and to identify major miRNA species in human whole saliva.
Design
RNA samples were isolated from normal human saliva using a combined protocol based on the Oragene®•RNA collection kit and the mirVana™ miRNA isolation kit in tandem. RNA samples were analyzed for quality and subjected to miRNA array analysis.
Results
RNA samples isolated from twenty healthy donors ranged from 2.59–29.4 μg/ml saliva and with 1.92–2.16 OD260/280nm ratios. RNA yield and concentration of saliva samples were observed to be stable over 48 hours at room temperature. Analysis of total salivary RNA isolated from these twenty donors showed no statistical significance between sexes; however, the presence of high-, medium-, and low-yield salivary RNA producers were detected. MiRNA array analysis of salivary RNA detected five abundantly expressed miRNAs, miR-223, miR-191, miR-16, miR-203, and miR-24, that were similarly described in other published reports. Additionally, many previously undetected miRNAs were also identified.
Conclusion
High quality miRNAs can be isolated from saliva using available commercial kits, and in future studies, the availability of this isolation protocol may allow specific changes in their levels to be measured accurately in various relevant diseases.
Keywords: biomarkers, gene expression, salivary RNA
1. Introduction
The identification of biological markers of disease is a major impetus in current research. Ideal biomarkers have the capacity to identify a disease, with a strong degree of accuracy, before it can be diagnosed clinically. Thus, the search for a minimally invasive, easily accessible body medium such as saliva,1 housing biological information reflective of disease status is clinically very relevant. Recent literature has demonstrated that a class of small non-coding RNAs, known as microRNAs (miRNAs), which play crucial roles across many biological processes, exhibit differences in expression profiles between normal and diseased tissues, illustrating their usefulness for diagnostics.2 While several researchers report the use of isolated RNAs from saliva as diagnostic biomarkers, there is little information on RNA quality and yield; hence emphasizing the ambiguity of the published findings. Therefore, there is a need to improve upon the isolation of high quality RNA from saliva. This in turn will help identify discriminatory RNA biomarkers such as miRNAs with more abundance in low-cost non-invasive screening assays.
2. Materials and Methods
2.1. Saliva collection
Saliva samples were collected from 20 healthy volunteers (9 males and 11 females) with a protocol approved by the UF Institutional Review Board. The mean age of the donors was 30 years (range 20–49). Donors were negative for a history of HIV, autoimmune disorder, hepatitis, and malignancy. Saliva was obtained as previously described 3 with several modifications. First, the stringency of the collection protocol was increased. Specifically, the donors were not allowed to eat two hours prior to collection, after having undergone normal oral hygiene. Second, donors were asked to cease drinking water at least 1 hour prior to collection. Whole saliva was collected from 9–10 am each day and was preserved with the Oragene®•RNA Self-Collection Kit, according to the manufacturer’s instructions (DNA Genotek Inc., Kanata, Ontario, Canada). To save on the costs of the reagents, the Oragene®•RNA Self-Collection Kit protocol was slightly modified such that the donors were asked to spit into 50 ml conical vials instead of Oragene® containers after which 300 μl of saliva from each donor were aliquoted and then mixed with equal volumes of Oragene®•RNA solution. Samples were immediately placed on ice for subsequent RNA isolation or in some experiments left at room temperature for up to 48 hrs.
2.2. Salivary RNA extraction
Total RNA was extracted using a protocol combining the Oragene®•RNA Self-Collection Kit (DNA Genotek) and the mirVana™ miRNA isolation kit (Ambion, Austin, TX). In brief, 300 μl of whole saliva mixed with 300 μl of Oragene®•RNA solution was incubated for 1 hour at 50°C, then heated at 90°C for 15 minutes and allowed to cool to room temperature. Afterwards, 48 μl of the Oragene®•RNA Neutralizer solution was added. Samples were mixed, incubated on ice for 10 minutes, and then centrifuged at 10 000 × g for 3 minutes at room temperature. The supernatant was collected and the isolation of total RNA was completed using the mirVana™ miRNA isolation kit with modifications to the manufacturer’s protocol. First, for the lysis step, the addition of the Lysis/Binding buffer and the miRNA Homogenate Additive were bypassed and instead an equal volume of acid-phenol:chloroform was added directly to the collected supernatant. Second, only 50 μl of 95°C elution solution was used to elute RNA. RNA was quantified using a NanoDrop® ND-1000 spectrophotometer (Thermo Scientific, Wilmington, DE). An Agilent 2100 Bioanalyzer (Santa Clara, CA) was used to detect the size distribution of total RNA, as well as determine the quality of the RNA.
2.3. Real-time PCR
For 18S rRNA and GAPDH mRNA quantitation, total RNA was reverse-transcribed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). For snU6 RNA and let-7b miRNA quantification, total RNA was reverse-transcribed using TaqMan® specific RT primers and the TaqMan® microRNA Reverse Transcription Kit (Applied Biosystems). Next, quantitative real-time PCR was performed in an Applied Biosystems StepOne Real-Time PCR machine using predesigned TaqMan® gene/miRNA specific assays for 18S rRNA (catalog #4319413E), GAPDH (catalog #432317E), snU6 (catalog #1007635F), and let-7b (catalog #4427975) combined with TaqMan® Fast Universal PCR Master Mix according to the manufacturer’s instructions.
For 16S rRNA quantitation, RNA was diluted down to 30 ng/μl and reverse transcribed by the iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA) with the specific antisense 16S RT2 primer 5′-ACCCAACATCTCACGACACGAG-3′, according to the manufacturer’s protocol. Real-time PCR was carried out using the SYBR Green method4 with the use of the following primers: 16S RT1 primer 5′-CTTACCAGGTCTTGACATCCCG-3′ and the RT2 primer, which generated ~100 bp PCR product. The expression of 16S RNA was determined using an established standard curve.
2.4. miRNA array analyses
From the twenty human salivary RNA samples isolated above, twelve representing 6 males and 6 females, including high and low RNA yield producers, were selected for miRNA profiling using the TaqMan® Low Density Array Card (TLDA) Human miRNA Panel v2.0 (Applied Biosystems). The analysis of expression of the >700 miRNAs was performed by the DNA Core at the Interdisciplinary Center for Biotechnology Research Center, according to the manufacturer’s protocol except for the pre-amplification step which was omitted. The NormFinder algorithm was used to identify the optimal normalization of miRNA among the 25 most abundantly expressed miRNAs detected. The miRNA with the lowest stability value of 0.113 was miR-27a. The microarray data were submitted to the GEO archive (accession #GSE28659).
2.5. Statistical Analysis
Comparisons between inter-operator variability were performed using linear regression analysis. A value of p <0.05 was considered statistically significant.
3. Results
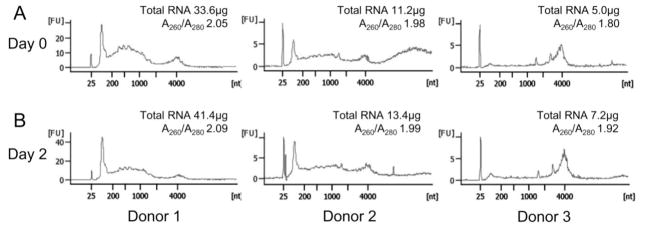
For salivary RNA to be successfully utilized as a reliable biomarker, a protocol must be established that can produce high yields of good quality RNA for subsequent downstream applications and/or analyses. Our developed protocol essentially consisted of combining and modifying the use of two kits, as described in Materials and Methods. The ability of the Oragene®•RNA solution in preserving and stabilizing RNA collected from saliva was examined from three different donors over a 48-hour period. As demonstrated in Figure 1, the RNA yield remained fairly constant between matched samples from each donor when stored for 48 hours at room temperature.
Figure 1.
Stabilization of saliva for RNA isolation. Incubation of whole saliva with Oragene®•RNA solution stabilized total RNA for two days at room temperature. (A) Bioanalyzer data demonstrating the total RNA extracted from whole saliva at Day 0 from three donors with total RNA yield per ml of saliva and OD260/280 ratios indicated. (B) Bioanalyzer data of total RNA extracted from the same stabilized whole saliva samples two days later.
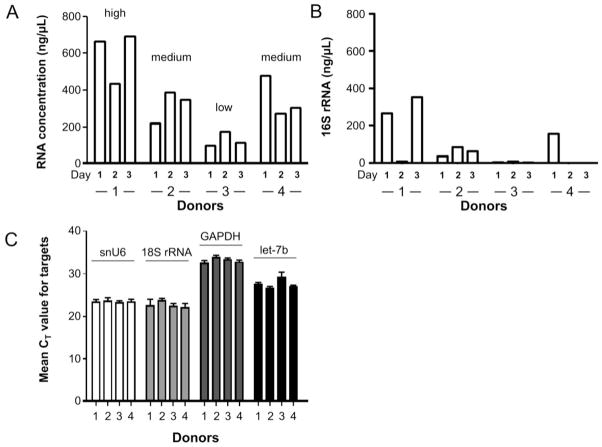
RNA concentration of the 50 μl purified samples from 1 ml whole saliva was calculated and compared for samples from four donors collected each day over a three day period. Figure 2A showed a trend that donors produced either high (donor 1), medium (donors 2 and 4), or low (donor 3) yields of total RNA over the course of three days. Next, Figure 2B demonstrated that the level of bacterial 16S rRNA in whole saliva from individual donors varied in range from 2 to 40% of total RNA. These results thus suggested that the variation in total RNA yield shown in Figure 2A might be secondary to the differences in the levels of oral bacteria across individual donors. To substantiate this, the potential variations in commonly used endogenous mRNA and miRNA normalizing controls were measured. Real-time PCR analysis of three commonly used RNA controls, snU6 small RNA, 18S rRNA, and GAPDH mRNA in the four donors over the course of three days demonstrated that the relative levels of expression for each respective target were fairly stable as observed by the small error bars which incorporated all data points across three days (Fig. 2C). Furthermore, analysis of miRNA let-7b levels also demonstrated that the relative levels of expression of this particular miRNA target were fairly stable (Fig. 2C). Whole saliva and saliva supernatants were also compared showing the total RNA content in supernatants represented ~10% of RNA from whole saliva. This is consistent with the observation described by the Park et al. study 6 which concluded that the level of RNA in saliva supernatants was very low compared to whole saliva. Indeed RNA from whole saliva of high-, medium-, and low-producers were shown to reside in pellets rather than supernatants, when whole saliva were centrifuged at 2,500 × g for 15 min. Thus, the RNA contribution distinguishing high-, medium-, and or low-producers were not from saliva supernatants, but rather from the insoluble fractions which most likely consisted of cells.
Figure 2.
The variable RNA concentration in the saliva from donors is a result of the bacterial contribution and not fluctuations in mammalian RNA. (A) Graphic representation of the RNA concentrations (in 50 μl eluted volume) normalized to 1 ml of whole saliva obtained from four donors over the course of three days. (B) The total bacterial 16S rRNA contribution across the salivary samples in the four donors over three days. (C) The mean CT values ± standard error of snU6, 18S, GAPDH, and miRNA let-7b plotted for each of the four donors across three days. Data shown were obtained from three replicates.
3.1. High RNA yield in saliva of healthy donors
Parallel comparison of the present protocol with that from an earlier report on salivary RNA purification 6 demonstrated that the present protocol generated substantially higher yields of better quality RNA. More specifically, in whole saliva obtained from four different donors, our protocol yielded a range of 0.84–25.1 μg versus 0.1–8.9 μg of total RNA per ml saliva using the published protocol. Moreover, the OD260/280 ratios ranged from 1.91–2.13 using our protocol versus 1.25–2.00 for the other protocol. 6 Having demonstrated that our protocol consistently produced higher yields of better quality RNA (as defined by the OD260/280 ratios being closer to 2.0), it became the primary method for isolating RNA from saliva for subsequent experiments.
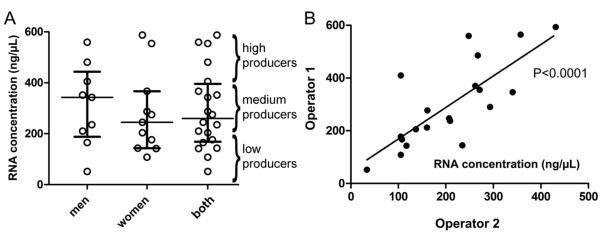
To further establish the usefulness of the present protocol, twenty randomly selected healthy donors were recruited and their salivary RNA samples were isolated and analyzed. Table 1 shows relatively high concentration yields (2.59–29.4 μg/ml saliva) of high quality RNA (OD260/280nm ratios, 1.92–2.16) were isolated. No statistical difference was observed between males and females using an unpaired t-test (Fig. 3A). There were four donors who had >400 ng/μl in the upper quartile of all donors and were considered the high RNA yield producers. Similarly, there were four donors that ranged in the bottom quartile of all donors and were considered the low RNA yield producers (<200 ng/μl). In addition, it was our experience that many donors had relatively consistent levels of total salivary RNA yields when their samples were collected multiple times over days (Figure 2A), weeks, or months (data not shown). Finally, the protocol was tested highly reproducible between two different operators as demonstrated by linear regression analysis (Fig. 3B, p <0.0001).
Table 1.
Inter-operator variability in the collection of total RNA from the saliva of 20 healthy donors.
| Donor | Operator 1 | Operator 2 | ||||
|---|---|---|---|---|---|---|
| Concentration (ng/μl) a | RNA Yield (μg) | A260/A280 ratio | Concentration (ng/μl) | RNA Yield (μg) | A260/A280 ratio | |
| 1 | 210.14 | 10.51 | 2.10 | 158.57 | 7.93 | 2.08 |
| 2 | 287.43 | 14.37 | 2.14 | 289.87 | 14.49 | 2.14 |
| 3 | 366.76 | 18.34 | 2.12 | 259.15 | 12.96 | 2.11 |
| 4 | 143.32 | 7.17 | 2.02 | 232.32 | 11.62 | 2.10 |
| 5 | 165.40 | 8.27 | 2.03 | 106.62 | 5.33 | 1.98 |
| 6 | 559.25 | 27.96 | 2.16 | 353.46 | 17.67 | 2.15 |
| 7 | 51.84 | 2.59 | 1.92 | 33.40 | 1.67 | 1.97 |
| 8 | 342.94 | 17.15 | 2.05 | 337.03 | 16.85 | 2.07 |
| 9 | 244.83 | 12.24 | 2.13 | 204.83 | 10.24 | 2.19 |
| 10 | 107.84 | 5.39 | 2.03 | 104.08 | 5.20 | 2.00 |
| 11 | 203.71 | 10.19 | 2.09 | 135.56 | 6.78 | 2.09 |
| 12 | 176.19 | 8.81 | 2.07 | 104.15 | 5.21 | 2.12 |
| 13 | 351.52 | 17.58 | 2.10 | 268.36 | 13.42 | 2.11 |
| 14 | 141.67 | 7.08 | 2.06 | 115.93 | 5.80 | 2.01 |
| 15 | 554.10 | 27.71 | 2.08 | 245.42 | 12.27 | 2.09 |
| 16 | 235.19 | 11.76 | 2.06 | 206.71 | 10.34 | 2.02 |
| 17 | 274.53 | 13.73 | 2.09 | 159.49 | 7.97 | 2.10 |
| 18 | 587.14 | 29.36 | 2.14 | 426.56 | 21.33 | 2.11 |
| 19 | 480.84 | 24.04 | 2.13 | 264.33 | 13.22 | 2.16 |
| 20 | 405.41 | 20.27 | 2.13 | 104.08 | 5.20 | 2.00 |
Concentration for 50 μl RNA samples purified from 1 ml of whole saliva.
Figure 3.
Relatively high RNA yields were collected from the saliva of 20 healthy donors. (A) Scatter plot analysis of RNA concentration in samples purified from 1 ml of whole saliva from males compared to females. High, medium, and low yield producers are approximately defined as >400, 200–400, and <200 ng/μl, respectively. (B) Linear regression analysis of RNA collected from donors using two different operators. Data shown were obtained from the same starting biological samples.
3.2. Salivary miRNA array
To obtain the baseline range of salivary miRNA in healthy subjects, a miRNA array experiment was performed. Our analysis demonstrated that RNU48 and snU6 were among the best miRNA normalizers for salivary RNA because both showed the least variance in expression across the 12 samples analyzed. There was no statistical difference observed in the expression of miRNAs between males and females, similar to the findings of RNA concentrations observed between both groups. Raw data from the miRNA array analyses were normalized by subtracting marker CT values from the mean CT values of snU6 or RNU48. Interestingly, there was a ~3 cycle difference in CT values between the high and low RNA yield producers. This is important to note because if this is not corrected, the statistical differences in miRNA expression between the two groups can be skewed and register false positive. Therefore, the concept of establishing a baseline for high and low RNA yield producers between future comparisons of multiple groups becomes very important. The CT means of the 25 most abundantly expressed salivary miRNAs from the miRNA array analyses across 12 donors compared to three other published salivary miRNA reports are shown in Table 2.
Table 2.
The expression of previously undetected microRNAs and validation of previously identified miRNAs in human whole saliva.a, b
| miRNA | Current report Whole saliva CT Mean | Park et al.6 | Hanson et al.8 Dried and buccal saliva CT Mean | Michael et al.9 Parotid exosomes CT Mean | |
|---|---|---|---|---|---|
| Whole saliva CT Mean | Supernatant saliva CT Mean | ||||
| hsa-miR-223 | 19.91 | + 20.39 | + 18.23 | + ~26–28 | − |
| hsa-miR-191 | 25.00 | + 23.79 | + 21.82 | + ~35–37 | − |
| hsa-miR-16 | 25.35 | + 27.23 | + 25.53 | + ~27 | − |
| hsa-miR-203 | 25.35 | + 26.45 | + 26.34 | + ~38–40 | − |
| hsa-miR-24 | 25.54 | + 27.12 | + 25.48 | − | − |
| hsa-miR-135a* | 26.14 | − | − | − | − |
| hsa-miR-222 | 26.76 | + 30.22 | + 27.20 | − | − |
| hsa-miR-200c | 27.46 | + 26.45 | + 24.45 | + ~35–37 | − |
| hsa-miR-484 | 27.78 | − | − | + ~30 | − |
| hsa-miR-320 | 27.78 | + 31.52 | + 30.07 | − | − |
| hsa-miR-106a | 28.17 | − | − | + ~35–37 | − |
| hsa-miR-17 | 28.21 | − | − | + ~32.5 | + |
| hsa-miR-29a | 28.26 | − | − | − | − |
| hsa-miR-26a | 28.47 | + 29.48 | + 28.36 | − | − |
| hsa-miR-19b | 28.81 | + 30.25 | + 28.96 | + ~33.5 | − |
| hsa-miR-30c | 28.82 | + 32.58 | + 30.21 | − | − |
| hsa-miR-27a | 29.00 | + 34.51 | + 33.50 | − | − |
| hsa-miR-375 | 29.17 | + 32.45 | + 30.11 | − | − |
| hsa-miR-26b | 29.20 | + 30.58 | + 29.69 | + ~29–31 | − |
| hsa-miR-768-3p | 29.24 | − | − | − | − |
| hsa-miR-574-3p | 29.32 | − | − | − | − |
| hsa-miR-193b | 29.35 | − | − | − | − |
| hsa-miR-186 | 29.48 | − | − | − | − |
| hsa-miR-923 | 29.50 | − | − | − | − |
| hsa-miR-760 | 29.89 | − | − | − | + |
The 25 most abundantly expressed miRNAs in whole saliva based on the miRNA array analyses across 12 donors are presented. The data shown here compares the presence (+) or absence (−) of the particular miRNAs between other published reports, with the mean CT values listed.
Average CT mean of snU6 = 23.13; Average CT mean of RNU48 = 27.08
4. Discussion
4.1. Isolation of high quality and high yield RNA from saliva
Although the detection of salivary mRNA and miRNA expression has been reported, to date, little to no information on the quality and yield of RNA collected has been presented.6–8 For example, the total RNA yield from whole saliva was reported in only a single study 9 (20.9–27.4 ng/100 μl saliva; at least a 10-fold lower yield compared to results from this report). More important than yield alone is the fact that there was no mention of the quality of RNA that was analyzed 9 whereas the current reported protocol consistently yielded OD260/280 ratios close to 2.0, which is the generally accepted standard for very high RNA purity. Other groups have reported failure in isolating good quality RNA from saliva;7,10 perhaps this is because of the experimental methodologies they used. Therefore, this study explored a protocol to improve upon the collection of RNA from human saliva for subsequent miRNA analyses.
To the best of our knowledge, this is the first report detailing the stabilization of RNA collected from saliva prior to the purification step. Testing how long RNA is stable using our protocol is important as there is often a practical waiting time needed when employed for diagnostics in basic and translational studies. RNA yield and concentration remained stable at room temperature in each of the three donors over the course of at least two days.
Alterations of miRNA expression between the saliva from healthy controls and oral squamous cell carcinoma patients has been reported 6 and salivary-specific miRNA signatures have been examined from forensically relevant biological fluids.8 However, until now, nothing has been reported as to the innate total RNA and miRNA variations in the same individual over several days. In order for miRNAs to be considered diagnostic markers in saliva, specific miRNA species should have minimal differences in expression in any one individual over multiple days with the assumption that there is no change in health status of the individual during this period. Therefore, this study not only explored the differences in the total RNA yield from donors over a three-day period, but also sought to examine the potential differences in expression between commonly used mRNA and miRNA endogenous controls. Although the total RNA from each donor varied between days, perhaps as a result of bacterial RNA contribution, the abundance of the three mammalian RNA normalizers (snU6 small RNA, 18S rRNA, GAPDH mRNA) remained relatively stable. This was also true for the let-7b miRNA. Together, the above findings demonstrated that, based on the donors analyzed, the bacterial composition in saliva can vary in one donor across several days and in comparison with other donors, as expected, but the mammalian contribution of salivary RNA remains relatively unchanged. These findings suggest that mammalian RNA harvested from saliva can therefore be used for future studies.
It was reported recently that miRNAs isolated from saliva have the clinical utility for oral cancer detection 6. However, this was achieved only after preamplification of the miRNA species. Our analysis using the current protocol across twenty healthy donors revealed similar or more abundant levels of respective miRNAs, as well as newly detected miRNAs without the need for preamplification. Non-preamplified cDNA at or above a CT value of 32 contains approximately 10–20 copies of the target of interest. Therefore, preamplification of samples containing extremely low copy numbers of target cDNAs, will increase copy number, but can pose problems such as the Poisson law of small numbers when aliquoting the cDNA into the preamplification reaction.11 For example, if there was one copy of a particular cDNA per sample, and then the sample was aliquoted into three wells, only one of the three wells would get that copy. Thus, this effect increases the variations across the samples, resulting in large unacceptable errors. For this reason, preamplification does not help if the CT values of the non-amplified cDNAs were at or above 32, which could have been the case in one study.6 Additionally, in this study, small differences of one cycle or less in the median CT values of certain miRNAs were observed between oral squamous cell carcinoma patients and healthy controls. Based on our findings, data between groups can be difficult to interpret since large variations in miRNA levels exist between high and low RNA yield producers which if not normalized properly could result in false positives.
4.2. miRNA signatures in human saliva
From the perspective of average CT values for the 25 most abundantly expressed miRNAs from this study in comparison to the other published reports (Table 2), the usefulness and superiority of the current protocol for the isolation of high quality RNA from whole saliva is reaffirmed. Furthermore, the data coincide with the expression of miRNAs from the other reports, validating our results, but nearly one third of the miRNAs (Table 2) are not described in other studies, suggesting an unsurpassed resolution in using the current saliva RNA isolation protocol. Of note, despite the lack of preamplification for the current study, the mean CT values for miR-223 were 19.91, whereas they were 20.39 in the Park et al. study which used preamplification.6 The ability to discern and identify miRNAs in saliva previously undetected from other reports may prove useful for understanding molecular events that may have been overlooked in the past. Eighteen of the 25 most abundantly expressed miRNAs from this report have been collectively observed from other published reports.8, 9 However, it must be noted that since the sample collection protocols were different in these reports, any direct comparison as shown in Table 2 must be made with the following considerations: the current study used whole saliva, whereas the Park et al. study used both whole and supernatant saliva;6 the Hanson et al. study used saliva obtained from the buccal mucosa;8 and finally the Michael et al. study collected saliva directly from the submandibular gland.9 Regardless, as discussed above, the main point is that the current protocol using whole saliva is superior when compared to whole saliva data obtained from the Park et al. study.6
Recently, it was reported that, in serum, miRNAs can be detected in un-degraded forms within structures termed exosomes, which are membrane-bound secretory granules containing certain proteins, mRNAs, and miRNAs.12 Salivary miRNAs and mRNAs have also been isolated from exosomes found within the saliva.9,13 Interestingly, three of the top five miRNAs, miR-223, miR-16, and miR-24 that were abundantly expressed in the healthy donors based on the miRNA array analysis have been reported to be involved in hematopoietic stem cell differentiation. Similarly, other reports have found these same miRNAs to also be copiously expressed in saliva.6, 8 In this study, the overabundance of miR-223 topped even the expression of snU6, a small nuclear RNA commonly used as a normalizer for miRNA studies (Table 2). Moreover, the crucial role of miR-223 during myelopoiesis has been well documented and it has also been reported to positively regulate granulocytic differentiation.14–16 Similarly, miR-16 and miR-24 are found to be abundantly expressed in CD34+ cells and are also implicated in controlling erythropoiesis.17, 18 Clearly, however, more work is needed to identify the cellular origin of the major miRNA species found within whole saliva.
In conclusion, based on the samples analyzed, our present study demonstrated that high quality and high yields of transcriptomic miRNA information can be isolated from saliva without the need for preamplification. Moreover, previously unidentified salivary miRNA species were discovered using the current protocol. In addition, this methodology has the capacity to isolate high quality total RNA from saliva stored at room temperature for two days after the addition of Oragene®•RNA solution. This work further implies that high and low producers of salivary total RNA must be taken into consideration prior to studying the differential expression between any healthy and diseased states. Lastly, the sheer abundance of major salivary miRNA signatures and the high resolution of miRNAs compared to previously published reports along with a very significant correlation of the methodology between users make the current protocol a useful resource for employing salivary RNA diagnostics. The results of the present study potentially could help identify miRNA signatures exclusive to disease states that not only could lead to early detection, but also facilitate disease treatments.
Acknowledgments
Funding
This work was supported by a UF/Moffitt Collaborative Initiative grant (EKLC, JQC), NIDCR grant 5K99DE018191 (AJ), T32 DE007200 (RP), and the Andrew J. Semesco Foundation, Ocala, FL.
We thank Bryan Korithoski for his help with the bacterial RNA analyses, and Sharon Norton from the Interdisciplinary Center for Biotechnology Research for technical help with the PCR microRNA array.
Abbreviations used in this paper
- CT
cycle threshold
- miRNAs
microRNAs
Footnotes
Ethical approval
Our samples were collected from healthy volunteers with a protocol approved by the UF Institutional Review Board.
Conflict of interest statement
None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schipper RG, Silletti E, Vingerhoeds MH. Saliva as research material: biochemical, physicochemical and practical aspects. Arch Oral Biol. 2007;52:1114–35. doi: 10.1016/j.archoralbio.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Dalmay T. MicroRNAs and cancer. J Intern Med. 2008;263:366–75. doi: 10.1111/j.1365-2796.2008.01926.x. [DOI] [PubMed] [Google Scholar]
- 3.Navazesh M. Methods for collecting saliva. Ann N Y Acad Sci. 1993;694:72–7. doi: 10.1111/j.1749-6632.1993.tb18343.x. [DOI] [PubMed] [Google Scholar]
- 4.Ahn SJ, Lemos JA, Burne RA. Role of HtrA in growth and competence of Streptococcus mutans UA159. J Bacteriol. 2005;187:3028–38. doi: 10.1128/JB.187.9.3028-3038.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersen CL, Jensen JL, Orntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64:5245–50. doi: 10.1158/0008-5472.CAN-04-0496. [DOI] [PubMed] [Google Scholar]
- 6.Park NJ, Zhou H, Elashoff D, Henson BS, Kastratovic DA, Abemayor E, et al. Salivary microRNA: discovery, characterization, and clinical utility for oral cancer detection. Clin Cancer Res. 2009;15:5473–7. doi: 10.1158/1078-0432.CCR-09-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zubakov D, Hanekamp E, Kokshoorn M, van Ijcken W, Kayser M. Stable RNA markers for identification of blood and saliva stains revealed from whole genome expression analysis of time-wise degraded samples. Int J Legal Med. 2008;122:135–42. doi: 10.1007/s00414-007-0182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanson EK, Lubenow H, Ballantyne J. Identification of forensically relevant body fluids using a panel of differentially expressed microRNAs. Anal Biochem. 2009;387:303–14. doi: 10.1016/j.ab.2009.01.037. [DOI] [PubMed] [Google Scholar]
- 9.Michael A, Bajracharya SD, Yuen PS, Zhou H, Star RA, Illei GG, et al. Exosomes from human saliva as a source of microRNA biomarkers. Oral Dis. 2010;16:34–8. doi: 10.1111/j.1601-0825.2009.01604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar SV, Hurteau GJ, Spivack SD. Validity of messenger RNA expression analyses of human saliva. Clin Cancer Res. 2006;12:5033–9. doi: 10.1158/1078-0432.CCR-06-0501. [DOI] [PubMed] [Google Scholar]
- 11.Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169–93. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- 12.Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–6. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palanisamy V, Sharma S, Deshpande A, Zhou H, Gimzewski J, Wong DT. Nanostructural and transcriptomic analyses of human saliva derived exosomes. PLoS One. 2010;5:e8577. doi: 10.1371/journal.pone.0008577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fazi F, Rosa A, Fatica A, Gelmetti V, De Marchis ML, Nervi C, et al. A minicircuitry comprised of microRNA-223 and transcription factors NFI-A and C/EBPalpha regulates human granulopoiesis. Cell. 2005;123:819–31. doi: 10.1016/j.cell.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 15.Nervi C, Fazi F, Rosa A, Fatica A, Bozzoni I. Emerging role for microRNAs in acute promyelocytic leukemia. Curr Top Microbiol Immunol. 2007;313:73–84. doi: 10.1007/978-3-540-34594-7_5. [DOI] [PubMed] [Google Scholar]
- 16.Garzon R, Pichiorri F, Palumbo T, Visentini M, Aqeilan R, Cimmino A, et al. MicroRNA gene expression during retinoic acid-induced differentiation of human acute promyelocytic leukemia. Oncogene. 2007;26:4148–57. doi: 10.1038/sj.onc.1210186. [DOI] [PubMed] [Google Scholar]
- 17.Bruchova H, Yoon D, Agarwal AM, Mendell J, Prchal JT. Regulated expression of microRNAs in normal and polycythemia vera erythropoiesis. Exp Hematol. 2007;35:1657–67. doi: 10.1016/j.exphem.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Q, Huang Z, Xue H, Jin C, Ju XL, Han JD, et al. MicroRNA miR-24 inhibits erythropoiesis by targeting activin type I receptor ALK4. Blood. 2008;111:588–95. doi: 10.1182/blood-2007-05-092718. [DOI] [PubMed] [Google Scholar]