Abstract
The lack of experimental characterization of the structures and ligand-binding motifs of therapeutic G-protein coupled receptors (GPCRs) hampers rational drug discovery. The human cannabinoid receptor 2 (hCB2R) is a class-A GPCR and promising therapeutic target for small-molecule cannabinergic agonists as medicines. Prior mutational and modeling data constitute provisional evidence that AM-841, a high-affinity classical cannabinoid, interacts with cysteine C6.47(257) in hCB2R transmembrane helix 6 (TMH6) to afford improved hCB2R selectivity and unprecedented agonist potency. We now apply bottom-up mass spectrometry (MS)-based proteomics to define directly the hCB2R-AM-841 interaction at the amino-acid level. Recombinant hCB2R, overexpressed as an N-terminal FLAG-tagged/C-terminal 6His-tagged protein (FLAG-hCB2R-6His) with a baculovirus system, was solubilized and purified by immunochromatography as functional receptor. A multiplex multiple reaction monitoring (MRM)-MS method was developed that allowed us to observe unambiguously all seven discrete TMH peptides in the tryptic digest of purified FLAG-hCB2R-6His and demonstrate that AM-841 modifies hCB2R TMH6 exclusively. High-resolution mass spectra of the TMH6 tryptic peptide obtained by Q-TOF MS/MS analysis demonstrated that AM-841 covalently and selectively modifies hCB2R at TMH6 cysteine C6.47(257). These data demonstrate how integration of MS-based proteomics into a ligand-assisted protein structure (LAPS) experimental paradigm can offer guidance to structure-enabled GPCR agonist design.
Keywords: agonist, covalent probe, drug discovery, electrospray ionization, GPCR, ligand binding domain, multiple reaction monitoring, protein structural biology
INTRODUCTION
The endocannabinoid system is a ubiquitous biosignaling network present in mammalian brain and peripheral tissues that modulates a spectrum of physiological processes including energy balance, emotional reactivity, and immune activation.1 The system’s molecular platform encompasses two principal G-protein coupled receptors (GPCRs), designated cannabinoid receptor-1 (CB1R) and cannabinoid receptor-2 (CB2R); endogenous lipid signaling molecules (endocannabinoids) that engage and activate these GPCRs; endocannabinoid biosynthesizing and inactivating enzymes that regulate cannabinergic signaling capacity; and presumptive endocannabinoid transporters.2 CB1R and CB2R are integral-membrane proteins that share with other members of the class-A (rhodopsin-like) GPCR superfamily the canonical structure of seven transmembrane α-helices (TMHs) conjoined by alternating intra- and extracellular loops.3 One of the most prevalent brain GPCRs, CB1R is also expressed at physiologically significant levels outside the central nervous system (CNS), e.g., in tissues involved with energy metabolism (liver, adipose, stomach, intestine, and endocrine pancreas) and reproduction. Detectable at only low levels in the healthy CNS, CB2R is predominantly peripheral, expressed mainly in immune, hematopoietic, and inflammatory cells. CB1R and CB2R share limited amino-acid identity (for the human receptors, 44% overall and 68% in the transmembrane helical domains) and exhibit divergences in their downstream effector pathways.3,4
Both CB1R and CB2R are well-recognized as “druggable” GPCR targets whose pharmacotherapeutic modulation holds promise for addressing important medical needs associated with substance abuse, obesity and its cardiometabolic complications, cancer, and other commonly encountered diseases.5,6 In particular, results from preclinical animal models indicate that high-affinity small-molecule ligands acting as potent agonists at human CB2R (hCB2R) could be useful hematopoietic agents and anti-inflammatory, neuroprotective, immunomodulatory, and analgesic medicines.7–11 A requirement for the success of this direct-agonist therapeutic approach, whereby engagement and activation of CB2R by a small-molecule ligand elicits a therapeutic effect with a clinically acceptable risk:benefit ratio, is selectivity of hCB2R targeting so as to circumvent the abuse liability and adverse motor, psychobehavioral, and metabolic responses associated with (central) CB1R activation.8,12,13 Protein structure-based compound design has proven to be a powerful approach for lead discovery and optimization14,15 and would greatly facilitate the acquisition of hCB2R agonists with good efficacy and minimal side-effect risk.3,16 The integral-membrane nature and dynamic flexibility of class-A GPCRs such as CB2R constitute a formidable barrier to purifying native GPCRs in sufficient quantities for direct experimental structural analysis either by nuclear magnetic resonance spectroscopy (NMR) or-- in the rare instances where the purified GPCR can be crystallized-- by X-ray crystallography.17,18 Furthermore, as integral membrane proteins, class-A GPCRs such as CB2R are highly hydrophobic overall, since they consist mainly of a transmembrane heptahelical domain. Although certain CB2R regions are indeed more polar (notably the N-terminal extracellular loop and the C-terminal cytoplasmic juxtamembrane portion), our previous proteomic characterization of hCB2R directly demonstrates the predominance of hydrophobic amino acids in the holoreceptor, which evidences particularly long stretches of hydrophobic amino acids within its TMHs.19 These factors have conspired to limit the number of GPCRs whose crystal structures have yet been obtained.18,20
The atomic structure of CB2R from any species remains elusive, and the rapid evolution of CB2R can result in marked interspecies differences with respect to ligand-binding affinity and efficacy,21,22 making it highly desirable to obtain direct structural information on functional hCB2R as guidance for drug discovery. Homology modeling and mutational studies of the holoreceptor and segmental analysis of synthetic peptides representing specific hCB2R regions have been used to interrogate hCB2R-ligand interaction domains.16,21,23,24 Although these techniques have offered some insight into the hCB2R ligand-binding pocket and the structural features of hCB2R activation, they cannot demonstrate directly the functional interactions of a ligand with hCB2R and the intermolecular basis of these interactions. To address this shortcoming, we have developed and pioneered an experimental approach termed “ligand-assisted protein structure” (LAPS) that allows direct identification of key functional residues involved in enzyme catalysis and GPCR ligand binding/signal transmission.23,25–27 The overall LAPS approach integrates four main elements: (a) pharmacologically active, high-affinity chemical probes functionalized to react covalently with specific amino acids at (or near) an enzyme active site or receptor ligand-binding domain; (b) introduction of point mutations in the target protein to determine their effect on probe binding and pharmacological activity; (c) computer modeling of the probe-protein interaction; and (d) direct identification of the site(s) of covalent ligand attachment by mass spectrometry (MS)-based proteomics. Key advantages of LAPS are the use of a functional protein target as study-object and the ability to characterize the protein’s interaction with chemically diverse ligands at the amino-acid level.
Using LAPS, this laboratory has detailed the catalytic mechanism and inhibitor profile of human monoacylglycerol lipase, a key enzymatic modulator of cannabinergic signaling.26 We have also begun to apply the LAPS approach to define hCB2R ligand-binding motifs and correlate them to particular chemical and pharmacological classes of cannabinergic ligands.23,27 In the course of that work, we generated indirect mutational and modeling data suggesting that (−)-7'-isothiocyanato-11-hydroxy-1',1'-dimethylheptylhexahydrocannabinol (AM-841), a high-affinity (apparent Ki = 1.5 nM) classical cannabinoid with a 7'-isothiocyanante (NCS) moiety at the terminus of its C-3 alkyl side chain (Figure 1a), interacts with a transmembrane helix 6 (TMH6) cysteine residue, C6.47(257)†.23,28 The interaction appears to make AM-841 a “megagonist,” i.e., an agonist that effects an unprecedented level of sustained hCB2R activation, having 40-fold greater potency than even the direct AM-841 analog without the NCS moiety.23 Furthermore, the agonist efficacy of AM-841 is some 12-fold greater at hCB2R than at human CB1 (hCB1R).23 Isothiocyanates can covalently modify proteins at the nucleophilic amino acid residues cysteine and lysine, but because under physiological conditions (pH ~ 7.4) a thiol moiety is more nucleophilic than a primary amine and the latter is protonated, selective cysteine modification is favored.29 These data suggest that a covalent interaction of AM-841 with C6.47(257) in hCB2R TMH6 may be critical to this cannabinergic ligand’s selectivity and exceptional agonist potency. Because of the implications these findings carry for the design and targeting of hCB2R agonists as potential drugs, we undertook the present work aimed at providing direct evidence that AM-841 binds covalently to hCB2R at C6.47(257). For this purpose, we elaborated upon our previously-developed strategy for hCB2R overexpression in Spodoptera frugiperda (Sf-21) cells as N-terminal FLAG-tagged/C-terminal 6His-tagged hCB2R (FLAG-hCB2R-6His), using a baculovirus expression system to generate the recombinant receptor in sufficient quantities for chromatographic purification and AM-841 binding studies on the isolated, functional receptor.19,30 We then developed a multiple reaction monitoring (MRM)-MS method31 capable of detecting all seven TMH peptides in hCB2R tryptic digests and utilized this multiplex MS mode along with high-resolution quadruple time-of-flight (Q-TOF) MS/MS for a bottom-up, MS-based proteomic analysis of the interaction between hCB2R and AM-841. The results demonstrate that AM-841 reacts covalently and selectively with hCB2R C6.47(257) and identify this TMH6 cysteine residue as a critical determinant of both the megagonist pharmacological activity of AM-841 and the ligand-binding domain for hCB2R activation. These results, along with the observation that the direct AM-841 analog unable to interact with C6.47(257) (i.e., lacking the reactive NCS functionality) is 40-fold less potent an agonist than AM-841,23 lend importance to this TMH6 cysteine residue in terms of both hCB2R-ligand interaction and agonist function, if not receptor function itself. This study also exemplifies how LAPS can be applied to advance the challenging field of MS-based plasma-membrane proteomics.
Figure 1.
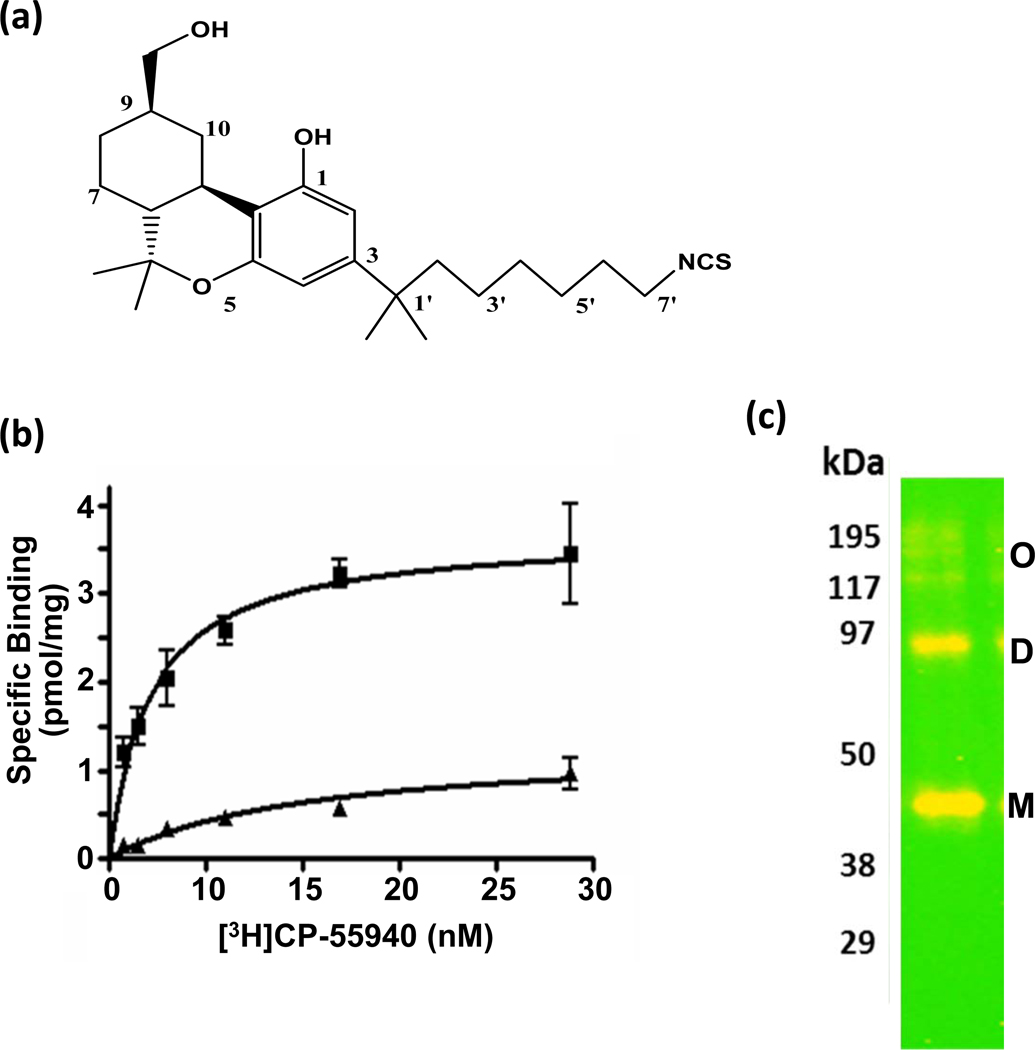
(a) Chemical structure of AM-841. (b) Saturation-binding assay of [3H]CP-55940 radioligand to FLAG-hCB2R-6His in membranes from Sf-21 cells overexpressing this receptor. Membrane incubation with AM-841 prior to [3H]CP-55940 binding (▲) reduced the receptor Bmax by ~ 75% relative to the Bmax in membranes not exposed to AM-841 (■). Data are means ± SEM of at least two independent experiments performed in duplicate. (c) Western blot analysis of the of the purified FLAG-hCB2R-6His preparation with anti-FLAG antibody detection. M, monomer; D, dimer; O, oligomers.
MATERIALS AND METHODS
Reagents and Materials
General laboratory chemicals were purchased form Sigma Chemical Co. (St. Louis, MO) and Fischer Scientific (Pittsburg, PA), unless otherwise noted. Reagents and gels for SDS-PAGE were from Bio-Rad Laboratories (Hercules, CA). Trypsin Gold, MS grade, was purchased from Promega (Madison, WI). [3H]CP-55940 was kindly supplied by the National Institute on Drug Abuse, National Institutes of Health (Bethesda, MD). AM-841 was synthesized at the Center for Drug Discovery, Northeastern University (Boston, MA). A 29-mer peptide corresponding to hCB2R TMH6 (DVRLAKTLGLVLAVLLICWFPVLALMAHS) with 26 amino acids in common with the TMH6 tryptic peptide (1 missed cleavage) was synthesized by standard methods (GenScript; Piscataway, NJ) to >95% purity according to liquid-chromatography (LC) and MS analyses.
hCB2R Expression
Sf-21 cells derived from pupal ovarian tissue of the fall armyworm Spodoptera frugiperda (Clontech, Mountainview, CA) were maintained in suspension culture at a density of 1–3 × 106 cells/mL in a shaking incubator at 27 °C in Sf900 II medium supplemented with 2% FBS (Invitrogen, Carlsbad, CA). The recombinant monoclonal baculovirus stock encoding FLAG-hCB2R-6His was generated essentially as detailed,30 except that the Bac-to-Bac® expression system (Invitrogen) was used. Cells were transfected by adding 3 mL of baculovirus stock to 100 mL of Sf-21 cell suspension (density ~ 1.5–2.0 × 106 cells/mL), the multiplicity of infection estimated at 5–10. In preliminary experiments, we established that 2 days post-infection resulted in optimal expression of functional, membrane-associated hCB2R (data not shown). The cells were then harvested by centrifugation at 500g for 5 min at 4 °C and washed twice with Dulbecco’s modified phosphate-buffered saline (MP Biomedicals, LLC; Irvine, CA) containing 1mM ethylenediaminetetraacetic acid. Cell pellets were either processed immediately or stored at −80 °C.
Membrane Isolation and Receptor-binding Assays
Following Sf-21 disruption by cavitation in a pressure cell, cellular membranes were sedimented by ultracentrifugation, as described.19,30 Membrane protein was quantified with a Bradford dye-binding method (Bio-Rad Laboratories). A standard saturation-binding assay with these membranes and [3H]CP-55940 radioligand was performed in triplicate to quantify FLAG-hCB2R-6His expression level, indexed as Bmax (Prism software; GraphPad, San Diego, CA).19 Competition-binding assays were performed essentially as detailed.23 Briefly, membranes (5.0 mL of an 0.8 mg protein/mL suspension) were preincubated with 10.0 nM AM-841, a concentration of AM-841 that is ~ 6-fold its apparent Ki of 1.5 nM and inhibits [3H]CP-55940 binding to hCB2R by ≥ 80% across a range of [3H]CP-55940 concentrations.23 Membranes were allowed to equilibrate with the AM-841 for 1 h at 30 °C with agitation and were then sedimented at 27,000g, 30 °C, and extensively buffer-washed. Saturation-binding assays were then performed with the washed membranes and [3H]CP-55940 radioligand. The resultant data were analyzed to determine the Bmax for [3H]CP-55940 as described above (Prism software) for at least two independent experiments performed in duplicate.
Purification of FLAG-hCB2R-6His and Receptor Incubation with AM-841
FLAG-hCB2R-6His was purified under nondenaturing conditions from Sf-21 membranes by immunoaffinity chromatography according to the following procedure modified from Zvonok et al.19 Membrane pellets were resuspended in solubilization buffer [50 mM sodium phosphate, 300 mM NaCl, 1.0% n-dodecyl-β-D-maltoside (DDM), 10% glycerol, and 0.5% protease inhibitor cocktail (P8849; Sigma), pH 7.4] and manually homogenized. Membrane solubilization was effected with gentle mixing in a rotator at 4 °C for 2 h. The solubilized membrane preparation was centrifuged at 14 000g and 4 °C for 20 min, and the supernatant was collected, diluted with an equivalent volume of solubilization buffer lacking DDM, and mixed with pre-equilibrated anti-FLAG M2 affinity resin (Sigma). After overnight incubation at 4 °C, the mixture was centrifuged at 4 °C and 700g for 4 min, and the supernatant was recovered. The mixture was packed into a gravity-flow column, and the resin was first washed three times with 5 vol of wash buffer (50 mM sodium phosphate, 300 mM NaCl, 0.1% DDM, and 10% glycerol, pH 7.4). Bound FLAG-hCB2R-6His was eluted from the column with elution buffer (50 mM sodium phosphate, 300 mM NaCl, 100 µg/mL FLAG peptide, 0.1% DDM, and 10% glycerol, pH 7.4). The eluted receptor was incubated with 30 nM AM-841 (i.e., a concentration ~ 20-fold its apparent Ki for hCB2R) for 1.5 h at 30 °C in a reciprocal shaking bath, after which time the incubation was quenched by freezing at −80 °C or immediate desalting and trypsinization (below).
SDS-PAGE and Western Blot Analysis
Protein samples were processed in Laemmli sample buffer containing 5% β-mercaptoethanol for SDS-PAGE on 10% Tris-HCl gels. For Western blotting, proteins were transferred from the SDS-PAGE gels to PVDF membranes (Bio-Rad, Trans-Blot® SD). The proteins were probed by immunodetection following procedures in the QIAexpress Detection and Assay Handbook (QIAGEN, Valencia, CA). The PVDF membranes were incubated with a 1:1000 dilution of rabbit anti-FLAG antibody (Sigma) followed by incubation with a 1:30 000 dilution of rabbit anti-mouse IgG-peroxidase conjugate (Sigma). Proteins were visualized using the ECL Western Blotting Analysis System (GE Healthcare, Piscataway, NJ).
Reduction, Cysteine Carbamidomethylation, and In-solution Digestion
To eliminate disulfide linkages that might complicate proteolytic digestion and subsequent MS analysis, FLAG-hCB2R-6His (either as the naïve receptor or after receptor incubation with AM-841) was reduced with dithiothreitol (DTT) and then carbamidomethylated with iodoacetamide (IAM) using standard procedures.19 Samples were desalted with BioSpin Columns (Bio-Rad) and subjected to overnight digestion with MS-grade trypsin at 37 °C. The digests were either analyzed immediately or stored at −80 °C until further processing.
Incubation of TMH6 Model Peptide with AM-841 and Peptide MS Analysis
The 29-mer corresponding to hCB2R TMH6 [1.5 µL of a 100-µM solution in 70% aqueous methanol] was mixed with 3 µL AM-841 (from a 100-µM solution in methanol), 1.2 µL dimethyl sulfoxide (DMSO), 1 µL ammonium bicarbonate (from a 5-mM aqueous solution), and 3 µL water. The mixture was incubated at 37 °C for 3–4 h. Both the naïve and AM-841-exposed peptide were subjected to sample preparation as above (i.e., reduction, cysteine carbamidomethylation, and in-solution trypsin digestion) prior to LC-MS/MS analysis, as described below for FLAG-hCB2R-6His tryptic peptides.
MRM-MS Analysis of FLAG-hCB2R-6His Tryptic Peptides
A MRM-MS method was developed and utilized for analyzing naïve and AM-841-modified hCB2R tryptic peptides. A 4000 QTrap hybrid mass spectrometer equipped with a nano-ESI ion source in conjunction with a Tempo Multi-Dimensional nanoLC system (Eksigent Technologies, Dublin, CA) was employed. Prior to analysis, 4 µL FLAG-hCB2R-6His tryptic digest was loaded over 3 min onto a C4 trap (5 µm, 300 Å) (Michrom Bioresources, Auburn, CA) with a flow rate of 10 µL/min using the loading solvent 0.1% aqueous formic acid-5% acetonitrile. Mobile phase A consisted of 0.1% aqueous formic acid-5% acetonitrile. Mobile phase B consisted of 0.1% formic acid in acetonitrile-5% water. Peptide separation was performed on a C4 column (150 × 0.1 mm, 3.5 µm, 120 Å) (Michrom Bioresources, Auburn, CA) with a linear gradient from 95% A:5% B to 5% A:95% B over 60 min at a flow rate of 700 nL/min. Spectra were acquired in positive-ion mode with Analyst software, version 1.4.1 (MDS Sciex/Applied Biosystems). A stainless-steel nanobore emitter tip (30 µm inner diameter) was used for nebulizing the sample (Proxeon Biosystems, San Mateo, CA). The ion source parameters were as follows: declustering potential, 70 V; entrance potential, 10 V; source temperature, 180 °C; source voltage, 2500 V; curtain gas, 20 L/min; and nebulizer gas, 10 L/min. A bioinformatics algorithm (http://www.bioinformatics.org/sms2/protein_gravy.html) was used to calculate the grand average hydropathy (GRAVY) score as an index of the hydrophilicity of each hCB2R TMH peptide.32
Q-TOF MS/MS Analysis of FLAG-hCB2R-6His Tryptic Peptides
LC-MS/MS analysis of FLAG-hCB2R-6His tryptic peptides was performed with an Agilent 6520 quadruple Q-TOF mass spectrometer (Agilent Technologies, Waldbronn, Germany) coupled to an Agilent 1200 LC chip cube interface. The LC chip consisted of an enrichment column (40 nL vol, packed with 5 µm i.d. Zorbax 300SB-C18 particles) and a separation column (75 µm i.d. × 43 mm, packed with 5-µm i.d. Zorbax 300SB-C18 particles). Sample was loaded onto the enrichment column and desalted with 2% acetonitrile-0.1% aqueous formic acid at a flow rate of 4 µL/min. Mobile phases A and B were 0.1% formic acid in water and 0.1% formic acid in acetonitrile, respectively, and a two-step gradient (2% B to 20% B over 2 min, then 20% B to 90% B over 60 min) at a flow rate of 600 nL/min was applied for peptide elution. MS data were collected in the data-dependent mode; MS scans were acquired from m/z 250 to 2000 at 5 Hz, and MS/MS scans via collision-induced dissociation (CID) were collected at 2 Hz for the three most abundant ions. For precursor selection, a dynamic exclusion window of 0.5 min was applied. Peptides were identified in the acquired spectra by using Agilent Spectrum Mill software (RevA.03.03.084) allowing for carbamidomethylated or AM-841-labeled cysteine.
Peptide Assignment
As previously detailed,19 acquired spectra were searched by Protein Pilot software (Applied Biosystems) using a single-entry database of hCB2R (accession no. P34972) to identify and sequence CB2 peptides. The Paragon search algorithm used by Protein Pilot software was set to perform a thorough identification, including over 90 biological modifications at a detected protein threshold of 1.3 (95%) for all samples.
RESULTS AND DISCUSSION
FLAG-hCB2R-6His Expression, Purification, and Functional Competency
We chose to utilize in the present work a double-tagged FLAG-hCB2R-6His construct, for we have shown that it can be robustly overexpressed in an Sf-21-baculovirus system,19 a result consistent with the known ability of this expression system to produce large amounts of recombinant protein.33,34 As quantified with a standard saturation-binding assay employing membranes from Sf-21 cells transfected with baculovirus encoding FLAG-hCB2R-6His and the synthetic cannabinergic radioligand [3H]CP-55940, the amount of FLAG-hCB2R-6His expressed for the studies to be described was calculated (as a Bmax) to be 3.2 pmol/mg membrane protein (Figure 1b), a level of hCB2R in Sf-21 membranes commensurate with previous results for this expression strategy.19
Prior empirical screening of extraction protocols19 demonstrated that FLAG-hCB2R-6His is efficiently solubilized in buffer containing the mild, non-ionic, MS-compatible surfactant DDM, a detergent with proven utility in the extraction of functional intergral membrane proteins, including GPCRs.33 Although the 6His epitope tag at the C-terminus of FLAG-hCB2R-6His would allow for immobilized-metal affinity chromatography (IMAC) as a first enrichment step prior to application of an antibody-affinity procedure,35 IMAC induced extensive and insurmountable hCB2R aggregation/precipitation under nondenaturing conditions (data not shown). However, the solubilized FLAG-hCB2R-6His was amenable to isolation under nondenaturing conditions by anti-FLAG M2 immunoaffinity chromatography, allowing complete LC-MS/MS coverage of its peptide map to be obtained.19 As a result, we elected in the current work to utilize FLAG-hCB2R-6His as isolated by immunoaffinity column chromatography. In accord with prior results,19 the preparation used herein was > 99% pure as judged from Coomassie-stained gels (data not shown), and quantitative Western-blot analysis demonstrated that this FLAG-hCB2R-6His preparation contained significant amounts of monomeric receptor. The minimal amount of immunoreactive receptor at ~ 90 kDa may reflect a naturally-occurring subpopulation of active FLAG-hCB2R-6His dimer (Figure 1c), as has been demonstrated for some other class-A GPCRs, including hCB1R.36,37 Other candidate influences on GPCR oligomeric order, such as solubilization conditions and overexpression level of the interacting protein partners, may have also played a role.38 As calculated from the input of membrane protein into the purification procedure (~ 200 mg), the saturation-binding Bmax (3.2 pmol/mg) (Figure 1b), and the FLAG-hCB2R-6His molecular mass (44 kDa), the membrane preparation contained ~ 29 µg binding-competent receptor.
MS Analysis of hCB2R TMH6 Model Peptide Covalently Modified by AM-841
Although LC-MS is recognized as an established strategy for analyzing posttranslational protein modifications (either naturally occurring or induced by a site-directed chemical probe),39 the modification must withstand sample work-up and the MS procedure itself so as to generate a specific and unambiguously detectable product ion following fragmentation. Our prior mutational data suggested that, at physiological pH, the NCS moiety of AM-841 as a reactive electrophile might interact with the thiol group of hCB2R TMH6 cysteine, C6.47(257).23 Since this interaction appeared to be at the basis of AM-841’s exceptional megagonist activity,23 we sought in the present work to demonstrate this ligand-receptor interaction directly at the amino-acid level with MRM-MS- and Q-TOF MS/MS-based proteomic approaches on tryptic fragments derived from FLAG-hCB2R-6His after incubation of functional receptor with AM-841. In order to validate this MS-based approach, we first profiled a synthetic model peptide representing hCB2R TMH6 prior to and after its incubation with AM-841 so as to establish that AM-841-modified hCB2R TMH6 would be amenable to MS analysis. Specific concerns included: (a) any influence of sample preparation (i.e., reduction and carbamidomethylation) on the labeled peptide; (b) any effect of covalent protein modification by AM-841 on trypsin digestion (e.g., missed/unexpected cleavages); (c) potential loss of analyte through adherence to sample pathway components in the LC-MS instrument; and (d) the ability of a covalent bond between AM-841 and a cysteine residue, as well as AM-841 itself, to withstand MS ionization and exposure to fragmentation energy.
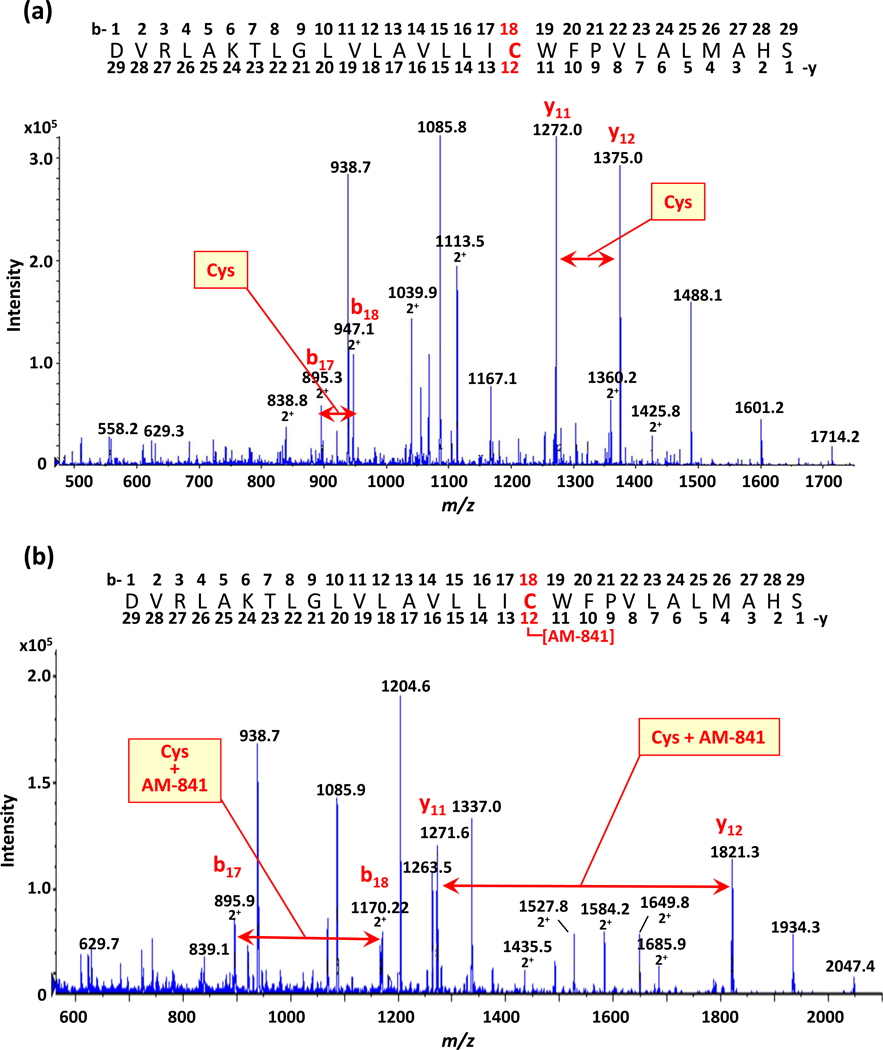
The monoisotopic mass for the unmodified synthetic hCB2R TMH6 peptide is 3161.8220 Da. We used the 3+-charged ion for fragmentation of the naïve peptide. For CID in the quadrupole, the 4+ charge state must be used for the AM-841-modified peptide because the covalent label would act as a basic residue to sequester protons and inhibit fragmentation (e.g., m/z 1204.036). In the b- and y-fragment ions for the naïve model TMH6 peptide (Figure 2a) and the peptide after incubation with AM-841 (Figure 2b), doubly-charged fragment ions are observed in the b-ion series, whereas the y-ions are singly-charged. This reflects the presence of two basic amino acids in the b-position (Arg-3 and Lys-6) which confir additional charge to the peptide, as compared to the single basic amino acid (His-28) in the C-terminal region. The mass shift (445.27 Da) in the y-12 fragment ion after incubation of the hCB2R TMH6 model peptide with AM-841 vs. the naïve peptide corresponds to that which would be expected from the covalent modification of the b-18 cysteine by AM-841. The mass shift between the b-18 labeled and unlabeled fragment ion is 223.7 Da. Since the b-ions are doubly charged, this 223.7 Da mass increase would likewise reflect covalent peptide modification by AM-841. These data establish and validate the suitability of MS-related sample workup and analytical procedures for MS-based proteomic investigation of hCB2R-AM-841 interaction.
Figure 2.
LC-MS/MS analysis of hCB2R TMH6 model peptide, either not exposed to AM-841 (panel a) or incubated with AM-841 prior to analysis (panel b). Mass shifts demonstrating covalent labeling of the peptide’s cysteine (Cys) residue by AM-841 are indicated.
MRM-MS-based Proteomic Analysis of FLAG-hCB2R-6His Modification by AM-841
Membrane preparations from HEK293or Sf-21 cells overexpressing recombinant FLAG-hCB2R-6His show specific binding of [3H]CP-55940 and other small-molecule orthosteric cannabinergic agents.19,23,40 The hCB2R-HEK293 membrane preparation avidly and irreversibly bound AM-841 under physiological conditions in a manner competitive with [3H]CP-55940 such that membrane incubation with 10 nM AM-841 (i.e., ~ 6-fold the receptor’s apparent AM-841 Ki) reduced subsequent [3H]CP-55940 binding by ~ 80%.23 Point mutation of TMH6 C6.47(257) to either alanine or serine abrogated AM-841 covalent binding to hCB2R, supporting the hypothesis that this cysteine residue is a critical constituent of the receptor’s ligand-binding domain, with which AM-841’s electrophilic NCS moiety has the potential to react covalently. When this binding assay was applied to hCB2R-Sf-21 membranes, prior membrane exposure to AM-841 likewise reduced [3H]CP-55940 Bmax by ~ 75% (Figure 1b), demonstrating an equivalent extent of AM-841 binding to recombinant FLAG-hCB2R-6His from both the HEK293 and Sf-21 expression systems. The attractive AM-841 binding profile of FLAG-hCB2R-6His in Sf-21 membrane preparations constituted a sound basis for our decision to study the interaction of AM-841 with purified FLAG-hCB2R-6His so as to enhance the signal-to-noise ratio and robustness of the subsequent MS-based proteomic analyses.
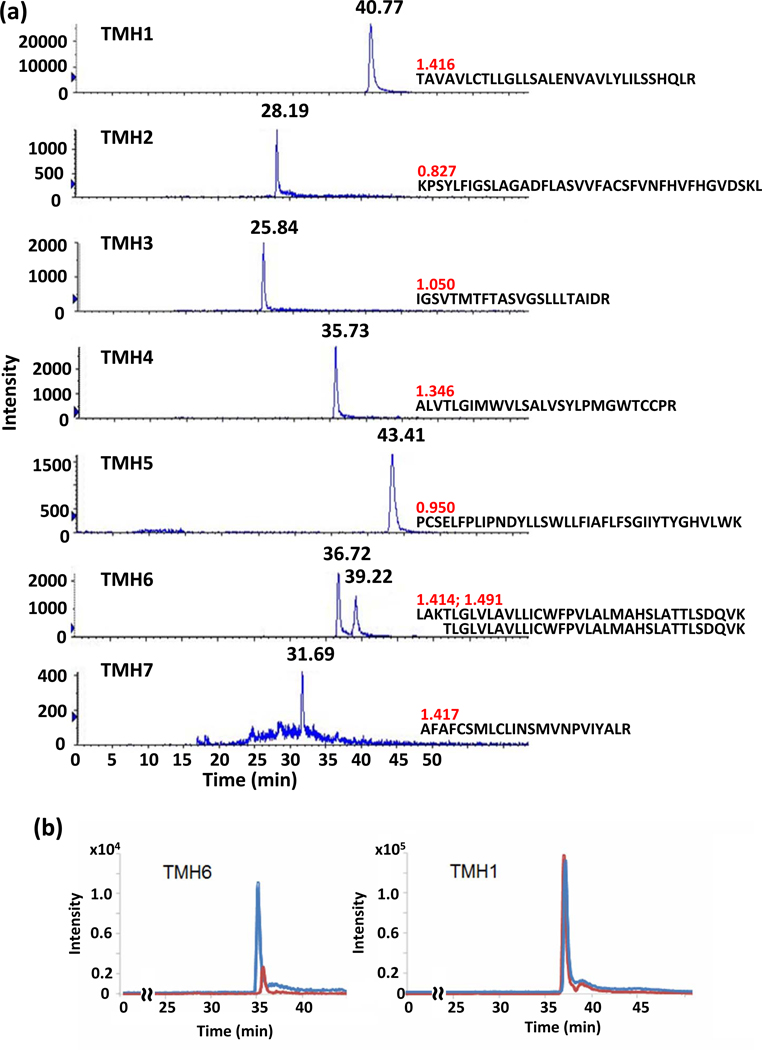
To pursue this experimental approach, we first established the functional competency of purified FLAG-hCB2R-6His, which may be defined operationally for GPCRs as the ability of detergent-solubilized receptor to recognize and bind ligand.33,34,41 However, application of a standard radiometric saturation-binding assay to purified FLAG-hCB2R-6His invites unacceptably high nonspecific background signals, a common problem likely exacerbated by incorporation of the inherently lipophilic cannabinergic radioligands into empty detergent micelles.41 As an alternative, we formulated a multiplex MRM-MS method able to detect simultaneously the seven TMH peptides in tryptic digests of isolated FLAG-hCB2R-6His. Although initially applied to small molecules, MRM-MS approaches are gaining broader acceptance in targeted, hypothesis-driven proteomics for profiling peptide/protein modifications in complex samples.31,42,43 Figure 3a depicts the MRM-MS spectrum of each of the seven hCB2R TMH peptides along with their respective intensities and elution times. As indicated by the positive GRAVY scores,32 the TMH peptides are quite hydrophobic, as might be expected given their intra-membrane localization in situ.3 The longer and/or most hydrophobic TMH peptides tended to exhibit the longest elution times. TMH1 showed appreciably greater intensity than the other TMH peptides, likely due to its superior ionization efficiency and not a relatively greater aqueous solubility, since the TMH1 peptide is very hydrophobic. Two discrete TMH6 peptides were observed, one with (retention time, 36.72 min) and one without (retention time, 39.22 min) a missed tryptic cleavage at residue-3 lysine (K245). We chose to focus on the TMH6 peptide with a missed cleavage because of its (marginally) greater intensity.
Figure 3.
Multiplex MRM-MS analysis of FLAG-hCB2R-6His tryptic digests. (a) Spectra for each of the seven hCB2R TMHs are shown, with the respective GRAVY hydrophobicity score indicated. A propensity for one missed cleavage in TMH6 is demonstrated. (b) Incubation of purified FLAG-hCB2R-6His with AM-841 reduces the TMH6-associated signal intensity by ~ 75% (red spectrum) with respect to the signal intensity of TMH6 (blue spectrum) from naïve receptor not exposed to AM-841, whereas the spectral intensities of the other TMHs remained unchanged after holoreceptor incubation with AM-841 (as exemplified by TMH1).
Since the multiplex MRM-MS method enabled us to observe unambiguously all seven discrete TMH peptides in the tryptic digest of purified FLAG-hCB2R-6His, we next applied this method to analyze purified receptor that had been incubated with AM-841. We reasoned that, with proper m/z gating, any putative covalent modification of TMH6 by AM-841 would shift the mass of the TMH6 peptide so as to prohibit its passing through Q1 and being detected, thus allowing the MRM-MS analysis to serve as an assay for FLAG-hCB2R-6His modification by AM-841. This analysis showed that incubation of purified FLAG-hCB2R-6His with AM-841 altered only peak area of the TMH6 peptide (Figure 3b), which was reduced by ~ 75% with respect to the peak area of TMH6 from the naïve FLAG-hCB2R-6His sample. The diminution in TMH6-associated signal due to AM-841 exposure suggests that ~75% of hCB2R TMH6 became covalently modified by AM-841 in a selective fashion with respect to the other hCB2 TMHs. The equivalent degree of AM-841 binding by hCB2R as isolated and in the membrane-based competition-binding assay (cf. Figure 1b) strongly suggests that receptor functionality was unimpaired by the purification protocol. The functional competency of purified FLAG-hCB2R-6His is consistent with demonstrations that Sf-21 cells constitute an environment supporting correct protein folding, targeting, and processing33,44 and that DDM-based solubilization media favor retention of activity by recombinant membrane proteins, including another class-A GPCR, the μ-opioid receptor.33,45
High-resolution Q-TOF MS/MS Analysis of AM-841 Binding to FLAG-hCB2R-6His TMH6 Cysteine
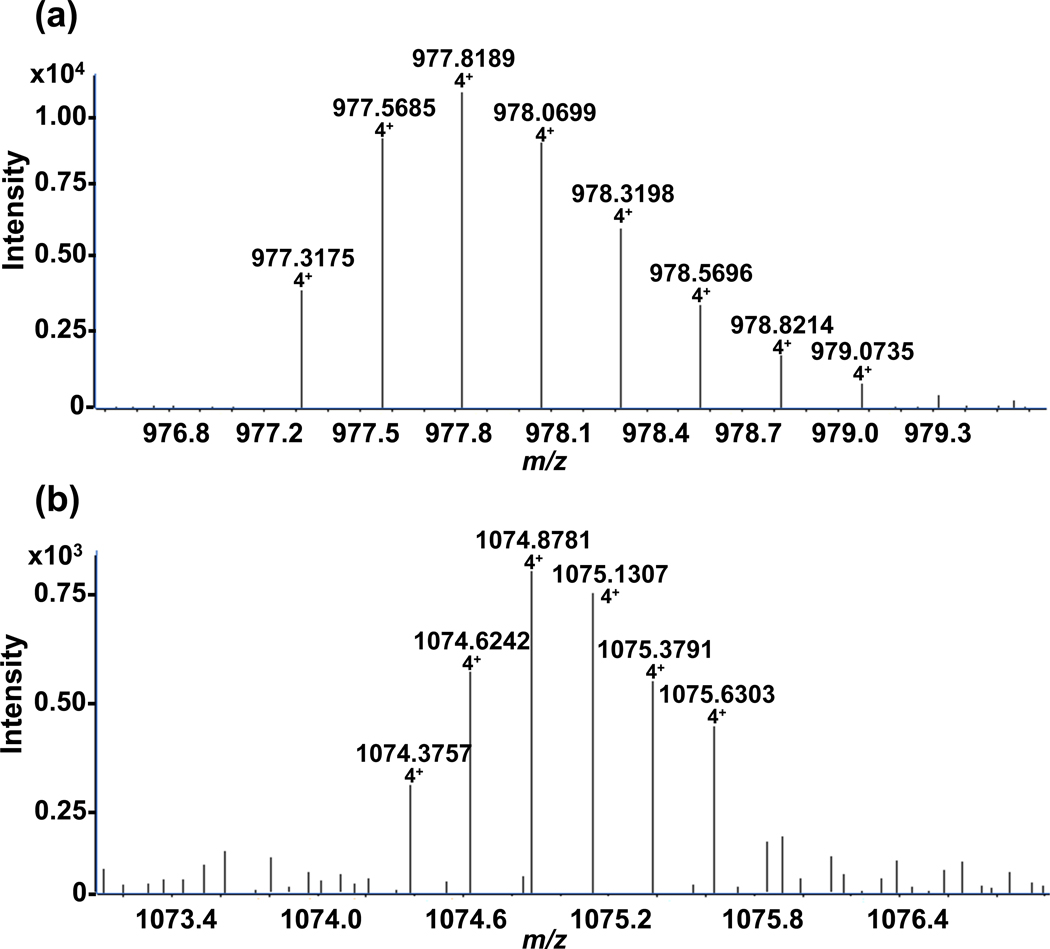
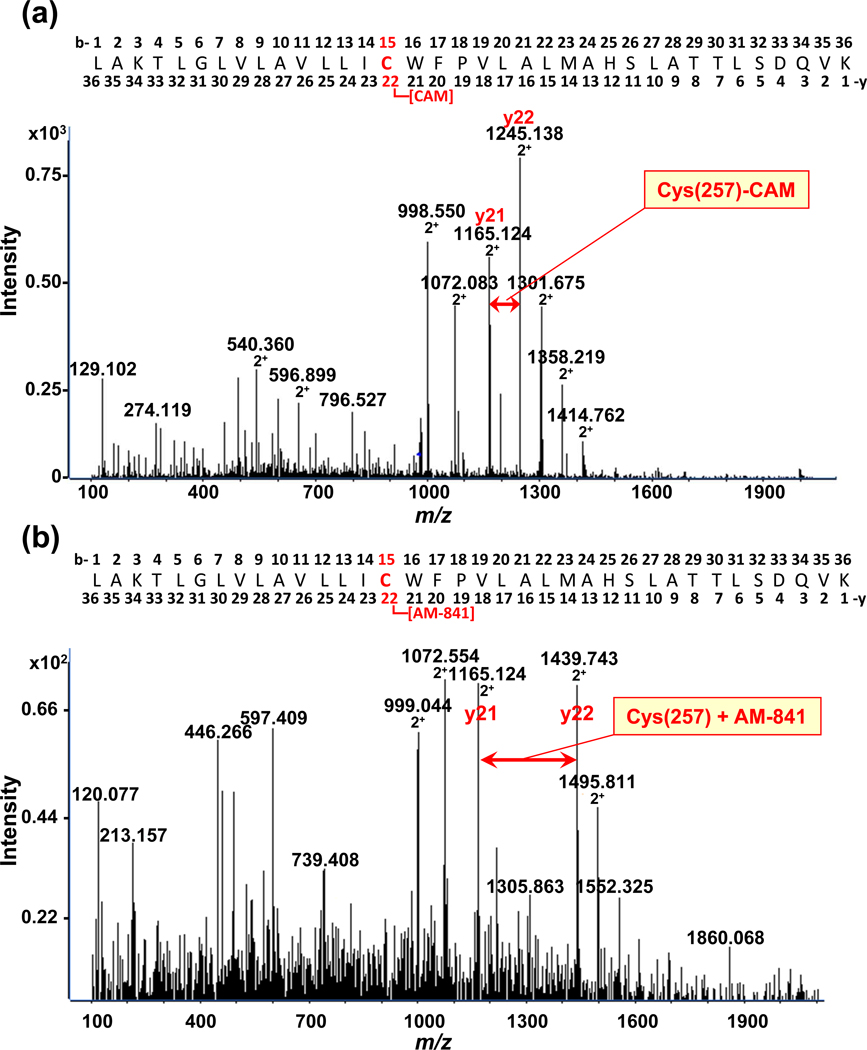
Our MRM-MS data indicate that cannabinergic megagonist AM-841 exclusively modifies hCB2R TMH6. In order to define precisely this ligand-GPCR interaction at the amino-acid level, we used Q-TOF MS/MS to analyze the tryptic digests of FLAG-hCB2R-6His that had either been incubated with AM-841 or not. The high-resolution mass spectra of the TMH6 tryptic peptides from naïve and AM-841-exposed FLAG-hCB2R-6His with the sequence LAKTLGLVLAVLLIC[CAM or AM-841]WFPVLALMAHSLATTLSDQVK are given in Figure 4. The monoisotopic signal for the 4+-charged, naïve TMH6 was observed at m/z 977.3175 and corresponds to the carbamidomethylated peptide with one missed cleavage (observed and actual monoisotopic masses, 3905.27 and 3905.2285 Da, respectively; <10 ppm accuracy) (Figure 4a). The TMH6 peptide with four charges and one missed cleavage originating from FLAG-hCB2R-6His that had been exposed to AM-814 was detected as m/z 1074.3757 (Figure 4b) (observed and actual monoisotopic masses, 4293.5028 and 4293.5016 Da, respectively; <10 ppm accuracy). The observed mass shift between TMH6 from naïve and AM-841-exposed hCB2R is an increase of 388.2316 Da. When the IAM-induced carbamidomethylation at the cysteine of the naive TMH6 is taken into account, the mass shift increases to 445.2531 Da, reflecting the molecular mass of AM-841 itself. Although some 75% of hCB2R bound AM-841 with high affinity,23 our recovery of AM-841-modified TMH6 was ≈ 10%, as indicated by the relative signal intensity (vertical axes) between TMH6 and TMH6-AM-841 in Figure 4a vs. 4b, respectively, a result likely reflective of the extremely hydrophobic character of the TMH6-AM-841 complex, which invites loss due to aggregation/precipitation.
Figure 4.
High-resolution Q-TOF MS/MS spectra of the hCB2R TMH6 peptide with one missed cleavage (LAKTLGLVLAVLLICWFPVLALMAHSLATTLSDQVK) obtained from analysis of tryptic digests of (a) purified FLAG-hCB2R-6His itself (monoisotopic m/z = 977.31754+) or (b) purified FLAG-hCB2R-6His that had been incubated with AM-841 (monoisotopic m/z = 1074.37574+). The mass difference is equivalent to the mass of AM-841.
We next utilized MS/MS fragmentation to identify experimentally the specific amino-acid residue of hCB2R TMH6 modified by AM-841. The fragmentation spectra for TMH6 from either naïve hCB2R or receptor that had been incubated with AM-841 are shown in Figure 5. The observed difference for the doubly-charged y-21 to y-22 ion is m/z 80.0140 (i.e., observed actual mass 160.0280 Da) corresponding to the doubly-charged, carbamidomethylated cysteine residue [Cys(257)-CAM] (Figure 5a). For hCB2R that had been exposed to AM-841, this observed mass difference is 274.1014 (i.e., calculated actual mass, 548.2028 Da), corresponding to the mass of the hCB2R TMH6 cysteine residue and AM-841 (Figure 5b). These data directly demonstrate that AM-841 covalently modifies hCB2R by participating in a nucleophilic addition reaction with C6.47(257) of TMH6 (Figure 6), as reflected at the y22 ion. We detected some AM-841 binding to hCB2R cysteine residues in TMHs other than TMH6 to an extent that was negligible as compared to the level of TMH6 labeling (data not shown). The minor AM-841 reactivity outside of TMH6 may reflect improperly folded receptor. Although the Sf-21-baculovirus expression system produces considerable amounts of recombinant protein in a cellular environment that supports correct functional folding, the large amounts of protein generated may tax cellular quality control, commonly resulting in some conformational heterogeneity in cellular protein output.33,44 Another possibility is that AM-841 labeling of cysteine residues other than C6.47(257) might reflect minor, biologically-relevant receptor subspecies representing distinct functional forms (e.g., momo- or oligomeric states).38,39 Consequently, we presume that the docking interaction between hCB2R as isolated under nondenaturing conditions and AM-841 is representative of that in situ. This view is well supported by the concordance between our prior data in whole cells indicating that high-affinity hCB2R binding of AM-841 is abrogated upon mutation of Cys 6.47(257)23, the present, direct demonstration of this interaction and its selectivity for this cysteine residue in the isolated holoreceptor, and the comparable degree (~75%) of covalent labeling of membrane-associated (Figure 1b)23 and isolated (Figure 3b) hCB2R.
Figure 5.
High-resolution MS/MS fragmentation spectra of the hCB2R TMH6 peptide with one missed cleavage (LAKTLGLVLAVLLICWFPVLALMAHSLATTLSDQVK) obtained from analysis of tryptic digests of (a) purified FLAG-hCB2R-6His itself in which C6.47(257) was carbamidomethylated [Cys(257)-CAM] or (b) purified FLAG-hCB2R-6His that had been incubated with AM-841. The mass shift at the y-22 ion demonstrates covalent labeling of the hCB2R y-22 cysteine residue, C6.47(257), by AM-841.
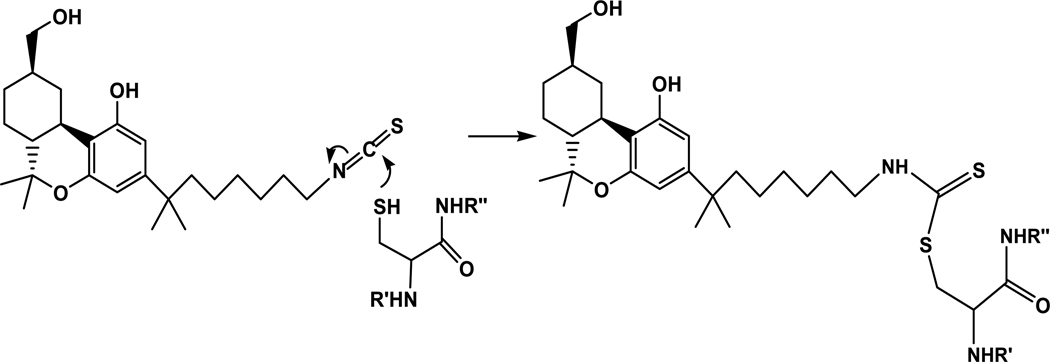
Figure 6.
Schematic illustration of the reaction between AM-841 and the -SH moiety of hCB2R C6.47(257). R′ and R″ denote the portions of the TMH6 peptide chain flanking the reactive C6.47(257) residue.
CONCLUSION
By demonstrating experimentally the covalent binding of AM-841 to hCB2R C6.47(257), the current work markedly advances prior site-directed mutagenesis and computational modeling data indirectly implicating this TMH6 cysteine in the ligand-binding motif of hCB2R. Since the direct AM-841 analog without the reactive NCS moiety is a much weaker hCB2R agonist than AM-841,23 this covalent interaction appears critical to the exceptional megagonist potency of AM-841. The present work thus identifies a small-molecule chemotype important to hCB2R activation that represents a useful guide to the rational design of and virtual screening for drug-like hCB2R agonists.
In membrane-mimetic environments, hCB2R TMH6 assumes the structure of a discontinuous α-helix interrupted by a nonhelical region containing a CWFP motif, of which C6.47(257) is a constituent residue.24 The CWxP motif is highly conserved among class-A GPCRs and has been proposed to act as a flexible hinge or microswitch that accommodates structural adjustments associated with ligand docking and receptor activation.46 These aggregate data invite speculation that covalent binding of AM-841 to hCB2R C6.47(257) may induce and/or stabilize conformational change in this receptor region to elicit structural features highly advantageous to hCB2R activation. Successful crystallization of AM-841-liganded hCB2R followed by X-ray analysis of the complex would be an interesting, if at present technologically elusive,3,47 means of characterizing what such a highly-activated hCB2R structure might represent.
Important underpinnings to this work were the use of an hCB2R TMH6 model peptide to validate the MS-related sample preparation and analytical methodologies and the development of an MRM-MS method affording the multiplex detection of all seven TMH peptides in hCB2R tryptic digests. These experiments allowed us to apply reliably bottom-up, MS-based proteomics to analyze holoreceptor trypsin digests for AM-841-induced hCB2R modification. Since hCB2R is a well-recognized drug target whose pharmacotherapeutic activation could help address such common health problems as neurodegeneration, inflammation, and pain,7–11 our data provide direct insight into functional residues for hCB2R activation and thus constitute valuable information supporting structure-enabled drug design/discovery targeted to this GPCR. The study has also introduced a MRM-MS-based proteomic method for the multiplex analysis of all seven hCB2R TMHs. More generally, we demonstrate how site-directed labeling with a designer covalent probe coupled to MS-based proteomics in the LAPS experimental paradigm can be used to identify unambiguously GPCR amino-acid signatures critical to ligand interaction and pharmacology.
ACKNOWLEDGMENT
This work has been supported by grants DA 3801 and DA 9158 from the USA National Institutes of Health, National Institute on Drug Abuse. DWS was supported in part by an Integrative Graduate Education and Research Traineeship (IGERT) from the National Science Foundation. We greatly appreciate helpful discussions with John Kirsch (Harvard University, Cambridge, Massachusetts) regarding the megagonist terminology.
Footnotes
Amino acid residues in hCB2R TMHs are formally designated using a modified Ballesteros and Weinstein numerical descriptor system.28 The most highly conserved residue in each TMH is assigned a locant of 50. This number is preceded by the TMH number and followed in parentheses by the sequence number. All other residues in any given TMH are numbered relative to this residue.
REFERENCES
- 1.Mouslech Z, Valla V. Endocannabinoid system: an overview of its potential in current medical practice. Neuro. Endocrinol. Lett. 2009;30:153–179. [PubMed] [Google Scholar]
- 2.Di Marzo V. The endocannabinoid system: its general strategy of action, tools for its pharmacological manipulation and potential therapeutic application. Pharmacol. Res. 2009;60:77–84. doi: 10.1016/j.phrs.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Reggio PH. Endocannabinoid binding to the cannabinoid receptors: what is known and what remains unknown. Curr. Med. Chem. 2010;17:1468–1486. doi: 10.2174/092986710790980005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demuth DG, Molleman A. Cannabinoid signaling. Life Sci. 2006;78:549–563. doi: 10.1016/j.lfs.2005.05.055. [DOI] [PubMed] [Google Scholar]
- 5.Janero DR, Makriyannis A. Cannabinoid receptor antagonists: pharmacological opportunities, clinical experience, and translational prognosis. Expert Opin. Emerg. Drugs. 2009;14:43–65. doi: 10.1517/14728210902736568. [DOI] [PubMed] [Google Scholar]
- 6.Janero DR, Vadivel SK, Makriyannis A. Pharmacotherapeutic modulation of the endocannabinoid signaling system in psychiatric disorders: drug-discovery strategies. Int. Rev. Psychiatry. 2009;21:122–133. doi: 10.1080/09540260902782778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim K, Moore DH, Makriyannis A, Abood ME. AM1241, a cannabinoid CB2 receptor selective compound, delays disease progression in a mouse model of amyotrophic lateral sclerosis. Eur. J. Pharmacol. 2006;542:100–105. doi: 10.1016/j.ejphar.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 8.Rahn EJ, Thakur GA, Wood JA, Zvonok AM, Makriyannis A, Hohmann AG. Pharmacological characterization of AM1710, a putative cannabinoid CB(2) agonist from the cannabilactone class: antinociception without central nervous system side-effects. Pharmacol. Biochem. Behav. 2011;98:493–502. doi: 10.1016/j.pbb.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorantla S, Makarov E, Roy D, Finke-Dwyer J, Murrin LC, Gendelman HE, Poluektova L. Immunoregulation of a CB2 receptor agonist in a murine model of neuroAIDS. J. Neuroimmune. Pharmacol. 2010;5:456–468. doi: 10.1007/s11481-010-9225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel KD, Davison JS, Pittman QJ, Sharkey KA. Cannabinoid CB(2) receptors in health and disease. Curr. Med. Chem. 2010;17:1393–1410. doi: 10.2174/092986710790980041. [DOI] [PubMed] [Google Scholar]
- 11.Patinkin D, Milman G, Breuer A, Fride E, Mechoulam R. Endocannabinoids as positive or negative factors in hematopoietic cell migration and differentiation. Eur. J. Pharmacol. 2008;595:1–6. doi: 10.1016/j.ejphar.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Pertwee RG. Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br. J. Pharmacol. 2009;156:397–411. doi: 10.1111/j.1476-5381.2008.00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashton JC, Wright JL, McPartland JM, Tyndall JD. Cannabinoid CB1 and CB2 receptor ligand specificity and the development of CB2-selective agonists. Curr. Med. Chem. 2008;15:1428–1443. doi: 10.2174/092986708784567716. [DOI] [PubMed] [Google Scholar]
- 14.Alkhalfioui F, Magnin T, Wagner R. From purified GPCRs to drug discovery: the promise of protein-based methodologies. Curr. Opin. Pharmacol. 2009;9:629–635. doi: 10.1016/j.coph.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Congreve M, Marshall F. The impact of GPCR structures on pharmacology and structure-based drug design. Br. J. Pharmacol. 2010;159:986–996. doi: 10.1111/j.1476-5381.2009.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poso A, Huffmann JW. Targeting the cannabinoid CB2 receptor: modeling and structural determinants of CB2 selective ligands. Br. J. Pharmacol. 2008;153:335–346. doi: 10.1038/sj.bjp.0707567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tikhonova IG, Costanzi S. Unraveling the structure and function of G protein-coupled receptors through NMR spectroscopy. Curr. Pharm. Des. 2009;15:4003–4016. doi: 10.2174/138161209789824803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cherezov A, Abola E, Stevens RC. Recent progress in the structure determination of GPCRs, a membrane protein family with high potential as pharmaceutical targets. Methods Mol. Biol. 2010;654:141–168. doi: 10.1007/978-1-60761-762-4_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zvonok N, Yaddanapudi S, Williams J, Dai S, Dong K, Rejtar T, Karger BL, Makriyannis A. Comprehensive proteomic mass spectrometric characterization of human cannabinoid CB2 receptor. J. Proteome. Res. 2007;6:2068–2079. doi: 10.1021/pr060671h. [DOI] [PubMed] [Google Scholar]
- 20.Cristalli G, Lambertucci C, Marucci G, Volpini R, Dal Ben D. A2A adenosine receptor and its modulators: overview on a druggable GPCR and on structure-activity relationship analysis and binding requirements of agonists and antagonists. Curr. Pharm. Des. 2008;14:1525–1552. doi: 10.2174/138161208784480081. [DOI] [PubMed] [Google Scholar]
- 21.Durdagi S, Papadopoulos MG, Zoumpoulakis PG, Kokoulista C, Mavromoustakos T. A computational study on cannabinoid receptors and potent bioactive cannabinoid ligands: homology modeling, docking, de novo drug design and molecular dynamics analysis. Mol. Divers. 2010;14:257–276. doi: 10.1007/s11030-009-9166-4. [DOI] [PubMed] [Google Scholar]
- 22.McPartland JM, Glass M, Pertwee RG. Meta-analysis of cannabinoid ligand binding affinity and receptor distribution: interspecies differences. Br. J. Pharmacol. 2007;152:589–593. doi: 10.1038/sj.bjp.0707399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pei Y, Mercier RW, Anday JK, Thakur GA, Zvonok AM, Hurst D, Reggio PH, Janero DR, Makriynannis AM. Ligand-binding architecture of human CB2 cannabinoid receptor: evidence for receptor subtype-specific binding motif and modeling GPCR activation. Chem. Biol. 2008;15:1207–1219. doi: 10.1016/j.chembiol.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tiburu EK, Tyukhtenko S, Deshmukh L, Vinogradova O, Janero DR, Makriyannis A. Structural biology of human cannabinoid receptor-2 helix 6 in membrane-mimetic environments. Biochem. Biophys. Res. Commun. 2009;384:243–248. doi: 10.1016/j.bbrc.2009.04.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howlett AC, Wilken GH, Pigg JJ, Houston DB, Lan R, Liu Q, Makriyannis A. Azido- and isothiocayano-substituted aryl pyrazoles bind covalently to the CB1 cannabinoid receptor and impair signal transduction. J. Neurochem. 2000;74:2174–2181. doi: 10.1046/j.1471-4159.2000.0742174.x. [DOI] [PubMed] [Google Scholar]
- 26.Zvonok N, Pandarinathan L, Williams J, Johnston M, Karageorgos I, Janero DR, Krishnan SC, Makriyannis A. Covalent inhibitors of human monoacylglycerol lipase: ligand-assisted characterization of the catalytic site by mass spectroscopy and mutational analysis. Chem. Biol. 2008;15:854–862. doi: 10.1016/j.chembiol.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mercier RW, Pei Y, Pandarinathan L, Janero DR, Zhang J, Makriyannis A. hCB2 ligand-interaction landscape: cysteine residues critical to biarylpyrazole antagonist binding motif and receptor modulation. Chem. Biol. 2010;17:1132–1142. doi: 10.1016/j.chembiol.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ballasteros JA, Weinstein H. Integrated methods for the construction three dimensional models and computational probing of structure function relations in G protein-coupled receptors. In: Conn PM, Sealfon SM, editors. Methods in Neuroscience. Vol. 25. San Diego, CA: Academic Press; 1995. pp. 366–428. [Google Scholar]
- 29.Baslé E, Joubert N, Pucheault M. Protein chemical modification on endogenous amino acids. Chem. Biol. 2010;17:213–227. doi: 10.1016/j.chembiol.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Filppula S, Yaddanapudi S, Mercier R, Xu W, Pavlopoulos S, Cai J, Pierce WM, Markiyannis A. Purification and mass spectroscopic analysis of human CB2 cannabinoid receptor expressed in baculovirus system. J. Peptide Res. 2004;64:225–236. doi: 10.1111/j.1399-3011.2004.00188.x. [DOI] [PubMed] [Google Scholar]
- 31.Yocum AK, Chinnaiyan AM. Current affairs in quantitative targeted proteomics: multiple reaction monitoring-mass spectrometry. Brief. Funct. Genomic. Proteomic. 2008;8:145–157. doi: 10.1093/bfgp/eln056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kyle J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 33.Chiu M, Tsang C, Grihalde N, MacWIlliams MP. Over-expression, solubilization, and purification of G protein-coupled receptors for structural biology. Comb. Chem. High Throughput Screen. 2008;11:439–462. doi: 10.2174/138620708784911456. [DOI] [PubMed] [Google Scholar]
- 34.Sarramenga V, Muller I, Milon A, Talmont F. Recombinant G protein-coupled receptors from expression to renaturation: a challenge toward structure. Cell. Mol. Life Sci. 2006;63:1149–1164. doi: 10.1007/s00018-005-5557-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Block H, Maertens B, Spriestersbach A, Brinker N, Kubicek J, Fabis R, Labahn J, Schäfer F. Immobilized-metal affinity chromatography (IMAC): a review. Methods Enzymol. 2009;463:439–473. doi: 10.1016/S0076-6879(09)63027-5. [DOI] [PubMed] [Google Scholar]
- 36.Wager-Miller J, Westenbroek R, Mackie K. Dimerization of G protein-coupled receptors: CB1 cannabinoid receptors as an example. Chem. Phys. Lipids. 2002;121:83–89. doi: 10.1016/s0009-3084(02)00151-2. [DOI] [PubMed] [Google Scholar]
- 37.Fanelli F, Felline A. Dimerization and ligand binding affect the structure network of A2A adenosine receptor. Biochim. Biophys. Acta. 2011;1808:1256–1266. doi: 10.1016/j.bbamem.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 38.Kristiansen K. Molecular mechanisms of ligand binding, signaling, and regulation within the superfamily of G-protein-coupled receptors: molecular modeling and mutagenesis approaches to receptor structure and function. Pharmacol. Ther. 2004;103:21–80. doi: 10.1016/j.pharmthera.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Cutillas PR, Timms JF. Approaches and applications of quantitative LC-MS for proteomics and activitomics. Methods Mol. Biol. 2010;658:3–17. doi: 10.1007/978-1-60761-780-8_1. [DOI] [PubMed] [Google Scholar]
- 40.Picone RP, Khanolkar AD, Wei X, Ayotte LA, Thakur GA, Hurst DP, Abood ME, Reggio PH, Fournier DJ, Makriyannis A. (−)-7'-Isothiocyanato-11-hydroxy-1',1'-dimethylheptylhexahydrocannabinol (AM-841), a high-affinity electrophilic ligand, interacts covalently with a cysteine in helix six and activates the CB1 cannabinoid receptor. Mol. Pharmacol. 2005;68:1623–1635. doi: 10.1124/mol.105.014407. [DOI] [PubMed] [Google Scholar]
- 41.Grisshammer R. Purification of recombinant G-protein-coupled receptors. Methods Enzymol. 2009;463:631–645. doi: 10.1016/S0076-6879(09)63036-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuzyk MA, Smith D, Yang J, Cross TJ, Jackson AM, Hardie DB, Anderson NL, Borchers CH. Multiple reaction monitoring-based, multiplexed, absolute quantitation of 45 proteins in human plasma. Mol. Cell. Proteomics. 2009;8:1860–1877. doi: 10.1074/mcp.M800540-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kitteringham NR, Jenkins RE, Lane CS, Elliot VL, Park BK. Multiple reaction monitoring for quantitative biomarker analysis in proteomics and metabolomics. J. Chromatogr. B. 2009;877:1229–1239. doi: 10.1016/j.jchromb.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 44.Nettleship JE, Assenberg R, Diprose JM, Rahman-Huq N, Owens RJ. Recent advances in the production of proteins in insect and mammalian cells for structural biology. J. Struct. Biol. 2010;172:55–65. doi: 10.1016/j.jsb.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Christoffers KH, Li H, Keenan SM, Howells RD. Purification and mass spectrometric analysis of the mu opioid receptor. Brain Res. Mol. Brain Res. 2003;118:119–131. doi: 10.1016/j.molbrainres.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 46.Holst B, Nygaard R, Valentin-Hansen L, Bach A, Engelstoft MS, Petersen PS, Frimurer TM, Schwartz TW. A conserved aromatic lock for the tryptophan rotameric switch in TM-VI of seven-transmembrane receptors. J. Biol. Chem. 2010;285:3973–3985. doi: 10.1074/jbc.M109.064725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Langelaan DN, Ngweniform P, Rainey JK. Biophysical characterization of G-protein coupled receptor-peptide ligand binding. Biochem. Cell Biol. 2011;89:98–105. doi: 10.1139/o10-142. [DOI] [PMC free article] [PubMed] [Google Scholar]