Abstract
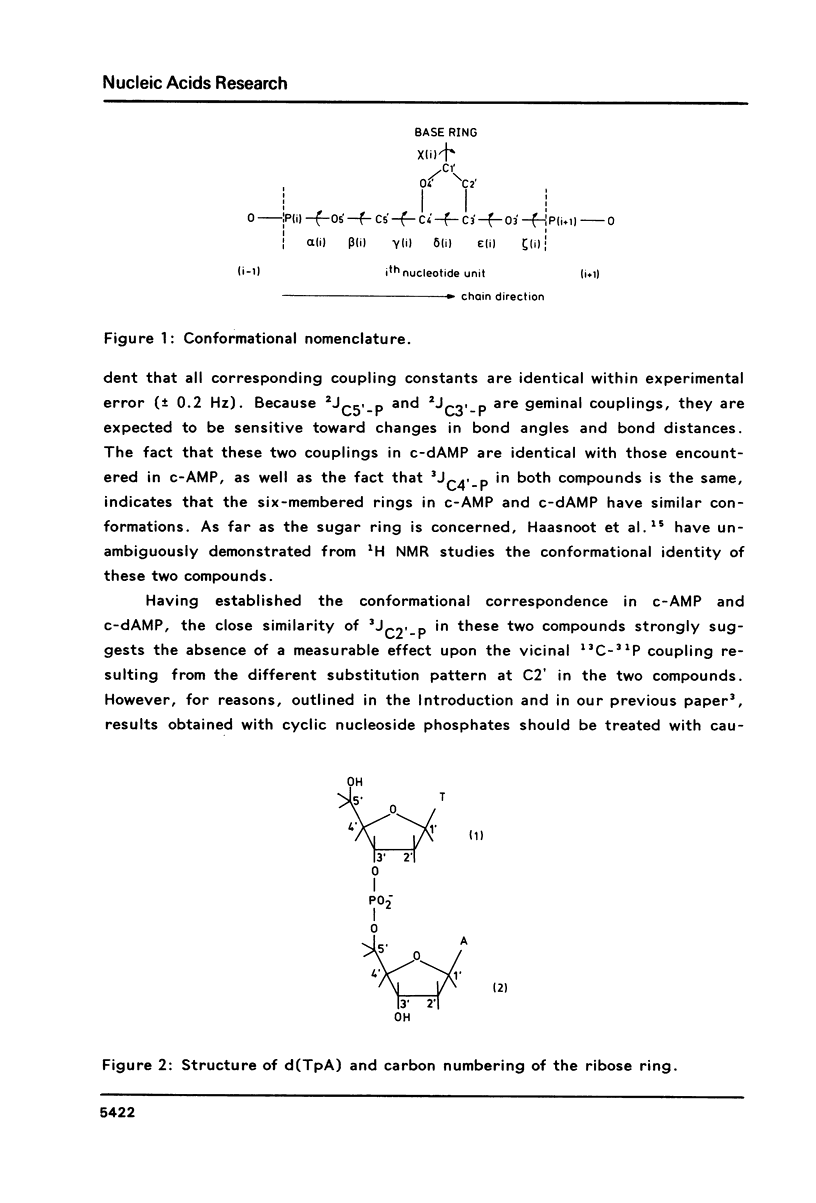
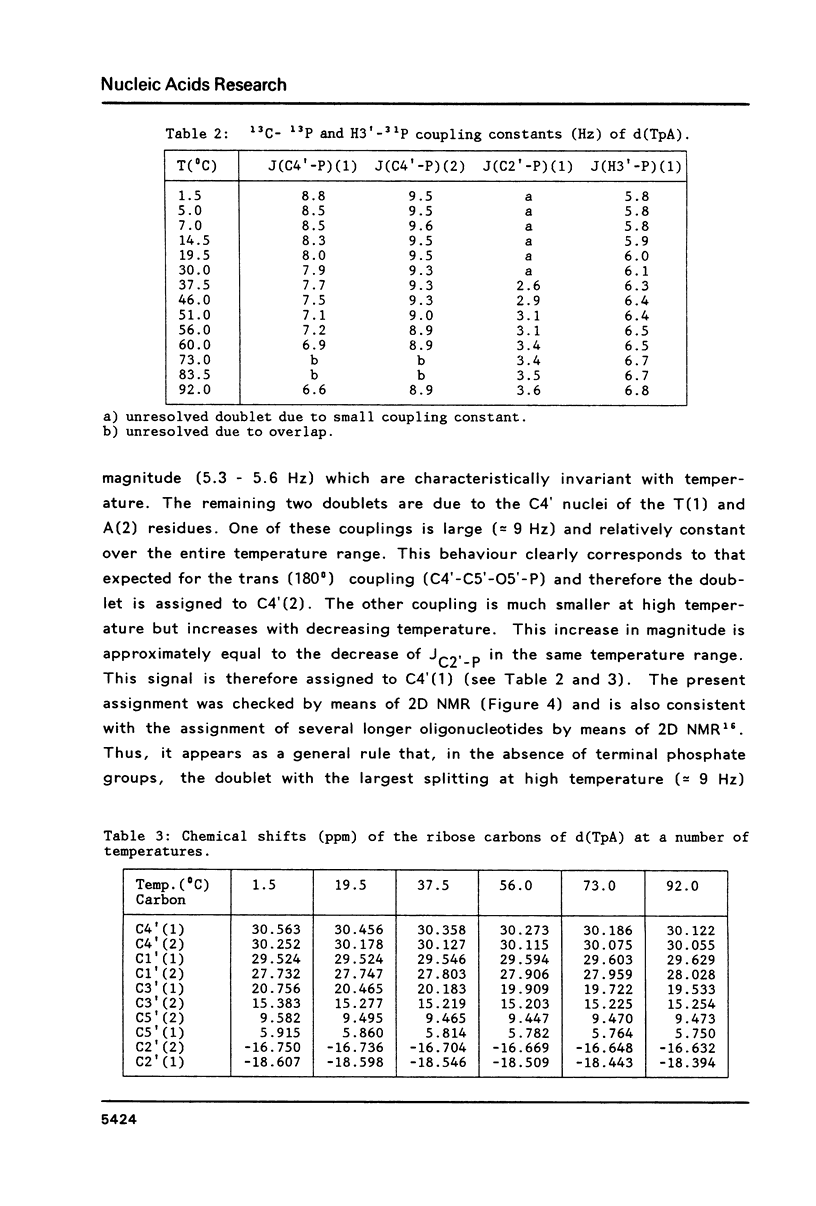
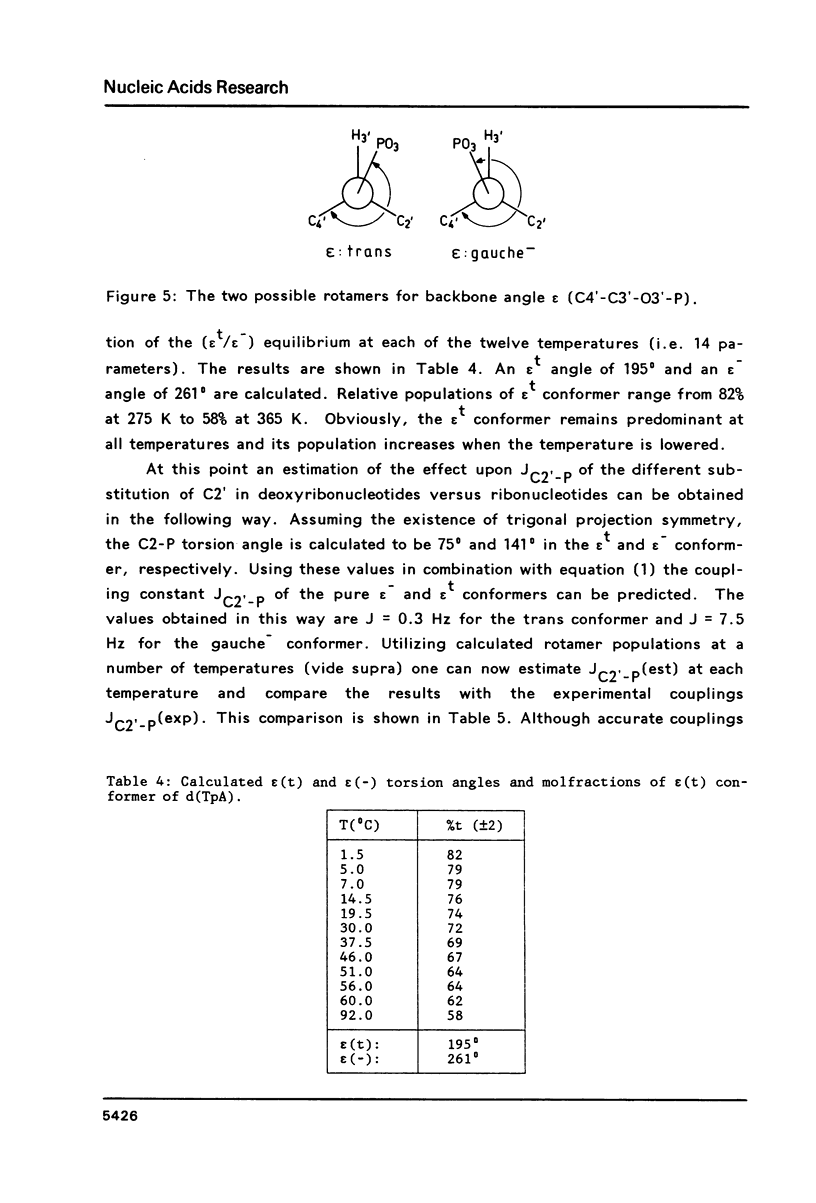
Carbon-13 NMR spectra of the deoxyribonucleotide d(TpA), 3',5'-cyclic AMP and 3',5'-cyclic dAMP were measured. It is shown that the different substitution of C2' in deoxyribonucleotides versus ribonucleotides does not affect the vicinal C2'-C3'-O3'-P coupling to a measurable extent. Therefore, the same set of Karplus parameters may be used for the C2'-C3-O3'-P couplings in ribonucleotides and in deoxyribonucleotides. Vicinal carbon-phosphorus and proton-phosphorus coupling constants are used to calculate the magnitude of the torsion angle epsilon (C4'-C3'-O3'-P), which amounts to 195(0) in the trans conformer and to 261(0) in the gauche(-) conformer.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hartel A. J., Lankhorst P. P., Altona C. Thermodynamics of stacking and of self-association of the dinucleoside monophosphate m2(6)A-U from proton NMR chemical shifts: differential concentration temperature profile method. Eur J Biochem. 1982 Dec 15;129(2):343–357. doi: 10.1111/j.1432-1033.1982.tb07057.x. [DOI] [PubMed] [Google Scholar]
- Lankhorst P. P., Erkelens C., Haasnoot C. A., Altona C. Carbon-13 NMR in conformational analysis of nucleic acid fragments. Heteronuclear chemical shift correlation spectroscopy of RNA constituents. Nucleic Acids Res. 1983 Oct 25;11(20):7215–7230. doi: 10.1093/nar/11.20.7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama S., Inagaki F., Miyazawa T. Advanced nuclear magnetic resonance lanthanide probe analyses of short-range conformational interrelations controlling ribonucleic acid structures. Biochemistry. 1981 May 12;20(10):2981–2988. doi: 10.1021/bi00513a041. [DOI] [PubMed] [Google Scholar]



