Abstract
Treatment options for schizophrenia that address all symptom categories (positive, negative, and cognitive) are lacking in current therapies for this disorder. Compounds targeting the metabotropic glutamate (mGlu) receptors hold promise as a more comprehensive therapeutic alternative to typical and atypical antipsychotics and may avoid the occurrence of extrapyramidal side effects that accompany these treatments. Activation of the group II mGlu receptors (mGlu2 and mGlu3) and the group I mGlu5 are hypothesized to normalize the disruption of thalamocortical glutamatergic circuitry that results in abnormal glutamaterigic signaling in the prefrontal cortex (PFC). Agonists of mGlu2 and mGlu3 have demonstrated efficacy for the positive symptom group in both animal models and clinical trials with mGlu2 being the subtype most likely responsible for the therapeutic effect. Limitations in the chemical space tolerated by the orthosteric site of the mGlu receptors has led to the pursuit of compounds that potentiate the receptor’s response to glutamate by acting at less highly conserved allosteric sites. Several series of selective positive allosteric modulators (PAMs) for mGlu2 and mGlu5 have demonstrated efficacy in animal models used for the evaluation of antipsychotic agents. In addition, evidence from animal studies indicates that mGlu5 PAMs hold promise for the treatment of cognitive deficits that occur in schizophrenia. Hopefully, further optimization of allosteric modulators of mGlu receptors will yield clinical candidates that will allow full evaluation of the potential efficacy of these compounds in the treatment of multiple symptom domains in schizophrenia patients in the near future.
Keywords: metabotropic, glutamate, schizophrenia, NMDA, allosteric
1. Introduction
Schizophrenia is a complex central nervous system (CNS) disorder that affects approximately 1% of the world’s population and presents itself as a set of symptoms grouped into three categories: positive, negative, and cognitive (Lewis and Lieberman 2000). The positive symptoms include the perception of nonexistent stimuli such as visual and auditory hallucinations and feelings of paranoia. A reduced or lack of normal affect is characteristic of the negative symptoms and includes depression and social withdrawal. The cognitive deficits observed in schizophrenia include disorganized thoughts and a decreased ability to process information. Current therapies, which include typical (e.g. haloperidol and chlorpromazine) and atypical (e.g. olanzapine and clozapine) antipsychotics, have been successful at treating the positive symptoms; however, the negative and cognitive symptoms have been less responsive to these classes of drugs (Buchanan et al. 2005; Kirkpatrick et al. 2006).
An understanding of schizophrenia at both the molecular and circuit levels is necessary to validate new targets and devise new strategies for treating this disorder in a maximally effective manner. Longstanding evidence has led to the hypothesis that alterations in dopaminergic systems, specifically through D2 dopamine (DA) receptors, is a key element in schizophrenia. Supporting this hypothesis are observations that all currently available medications for schizophrenia act in some way to antagonize D2 receptors and that post-mortem analysis of brain tissue from patients with schizophrenia reveal elevated D2 receptor density (Seeman 1987; Seeman 2006). In addition, imaging studies have revealed a greater increase in DA levels upon amphetamine challenge in patients with schizophrenia compared to controls as measured by a decrease in D2/D3 receptor binding by [11C]raclopride (Breier et al. 1997) and [123I]iodobenzamide (Laruelle and Abi-Dargham 1999) with the latter study showing this difference specifically in patients with active symptoms of the disease. Although both typical and atypical antipsychotics exhibit antagonism at D2 receptors, these two classes differentiate themselves in their degree of specificity for D2 over other neurochemical targets, occupancy time at D2, and their resulting side effect profile (Seeman 2002). Although each drug offers its own distinct pharmacological profile, in general, the atypical group drugs are less selective for, bind with lower affinity to, and have a faster off rate from the D2 receptor compared to the typical antipsychotics. Related to their selectivity, atypical antipsychotics have been demonstrated to act in varying degrees at serotonergic, histaminergic, adrenergic, and muscarinic receptors (Bymaster et al. 1996; Roth et al. 1994). This diversity of profiles across both classes of antipsychotics necessitates an individualized approach in the clinic to optimize effectiveness and compliance as well as reduce risk associated with undesirable side effects (Seeman 2002).
As a more thorough understanding of this disorder is developing, additions and modifications to the dopaminergic hypothesis have been put forth. It is also becoming more evident that schizophrenia is more than the result of a change in magnitude of neurotransmitter signaling, but it is also a change in the underlying brain circuitry (Lisman et al. 2008; Marek et al. 2010). Much of this evidence points to disruptions in glutamatergic signaling via the ionotropic N-methyl-D-aspartate receptor (NMDAR) in cortical and midbrain circuits and has led investigators to pursue this avenue for therapeutic development. As will be discussed, there is evidence that these targets have potential to address the unmet needs in the treatment of schizophrenia, specifically in the cognitive deficit symptom cluster.
2. Glutamatergic circuitry in schizophrenia
A delineation of the brain regions and circuitry involved in schizophrenia has been achieved (at least partially) through association of the disrupted behaviors to the pathways known to regulate these behaviors as well as observations of 1) current therapies’ mechanisms of action, 2) identification of drugs that mimic the symptoms of schizophrenia, 3) patient brain pathophysiology, and 4) genetic models of schizophrenia. The observation that both typical and atypical antipsychotics act to antagonize D2 receptors initially implicated DA hyperfunction in the subcortical regions of the striatum and nucleus accumbens (the primary locations for D2 receptors on DA terminals) for the positive symptom group that responds to this type of therapy. This theory was later revised to include a deficit of dopamine transmission in the prefrontal cortex (PFC) at D1 receptors; a revision that resulted in a better correlation with the presence of negative and cognitive symptoms (Goldman-Rakic et al. 2000). However, additional research and observations have revealed features of schizophrenia that point to the involvement of glutamatergic pathways and signaling in ways that are not mutually exclusive to the hypothesized involvement of the dopaminergic system.
It has been known for decades that phencyclidine (PCP), as well as other NMDAR antagonists such as ketamine and MK-801, are psychotomimetic agents in healthy individuals and exacerbate all three symptom clusters in individuals with schizophrenia (Ghoneim et al. 1985; Krystal et al. 1994). These observations suggest a possible role for decreased NMDAR signaling as an explanation for the diverse symptoms observed in this disorder. NMDARs are located postsynaptically at excitatory synapses and are widely distributed in the brain with notable density in cortical and subcortical regions (Monaghan and Cotman 1985; Monaghan et al. 1988; Ozawa et al. 1998). Activation of NMDARs requires the agonists glutamate and glycine in addition to membrane depolarization, resulting in calcium influx through the receptor channel. This process is critically involved in synaptic plasticity, a mechanism required for learning and memory formation (Sweatt 2008) and is relevant to the positive symptoms and cognitive deficits observed in schizophrenia. Within regions associated with the symptoms of schizophrenia, NMDARs are located on GABAergic neurons in subcortical regions such as the nucleus accumbens and on glutamatergic neurons projecting from the mediodorsal thalamus to pyramidal neurons in the PFC. NMDARs on the GABAergic neurons receive excitatory input from glutamatergic afferents and their activation results in an inhibitory regulation of the thalamocortical pathway. A reduction in NMDAR function on these GABAergic neurons results in disinhibition of thalamocortical glutamatergic signaling to the PFC and therefore an increase in excitatory glutamatergic input to pyramidal neurons in the PFC. In support of this model, the psychotomimetic NMDAR antagonists PCP and ketamine have been shown to cause an increase in detected extracellular glutamate levels in the PFC which has been hypothesized to be linked to the effects of these agents on certain aspects of cognitive function and locomotor activity (Moghaddam et al. 1997; Moghaddam and Adams 1998). This signaling pathway as well as pertinent players within it is illustrated in Fig 1.
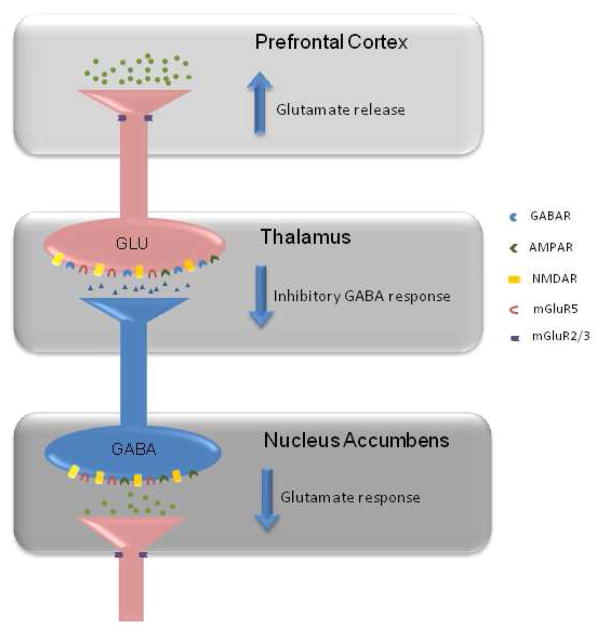
Figure 1.
A simplified schematic illustration of glutamatergic-GABAergic microcircuitry between subcortical and cortical regions. A disruption of this circuit is thought to contribute to the pathophysiology of schizophrenia. For example, a decreased glutamatergic response of inhibitory GABAergic neurons in the nucleus accumbens will result in a decreased inhibition of glutamatergic signaling to the PFC. This decreased inhibition subsequently results in hyper-glutamatergic signaling in this terminal region. The metabotropic glutamate receptors, mGlu2, mGlu3, and mGlu5, are localized in regions where their modulation of glutamatergic signaling may serve to normalize such disruptions.
The role of impaired glutamatergic circuitry and its implication for treatment options has gained momentum in the past several years (Conn et al. 2009b; Marek et al. 2010). While this does not necessarily imply a primary deficit in NMDAR function in the etiology of schizophrenia, there are several possible mechanisms by which NMDAR function could be down regulated in a manner that could contribute to schizophrenia pathology. For example, it is possible that the expression or functioning of the receptor itself may be compromised. NMDAR signal transduction could also be affected by changes in the level or activity of any of a number of proteins as well as any factor influencing glutamate availability at the postsynaptic site or the occurrence of coincident membrane depolarization. Imaging studies directed at glutamatergic targets have found reduced NMDAR binding in medication-free patients with schizophrenia (Pilowsky et al. 2005) as well as evidence of interplay between glutamatergic and dopaminergic neurotransmission (Urban and Abi-Dargham 2010). Evidence of genetic links to NMDAR functioning in schizophrenia has been found and summarized in previous reviews (Harrison and Weinberger 2004) and has served as a basis for the development of several genetic animal models that may be relevant to schizophrenia including subunit NR1 knockdown mice (Mohn et al. 1999; Ramsey 2009)and mice carrying relevant mutations within the NMDAR (Li et al. 2009).
If a change in brain circuitry is induced in a disease state such as schizophrenia, then a means for compensating for the dysfunctional signaling would be a rational strategy for treatment. Targeting the NMDAR or other ionotropic glutamate receptors directly is not considered to be a viable option due to their widespread role in fast synaptic transmission throughout the CNS and the potential toxicity of overactivation of NMDA receptors (Hirose and Chan 1993). Another approach to regulating transmission through these circuits is to target G protein-coupled metabotropic glutamate receptors which function to modulate synaptic transmission and neuronal excitability. In particular, recent attention has been focused on targeting mGlu receptor subtypes 2, 3, and 5 as novel treatment strategies for treatment of schizophrenia.
3. Metabotropic glutamate receptors
Metabotropic glutamate (mGlu) receptors are family C G-protein coupled receptors that are grouped into three classes based on sequence homology, effector coupling, and pharmacology (Niswender and Conn 2010). Group I mGluRs include mGlu1 and mGlu5 and are located primarily postsynaptically where they couple to Gq/G11. As such, activation of Group I results in phospholipase C (PLC) stimulation, an increase in phosphotinositides (PI) hydrolysis, and increases in intracellular Ca+2. Group II mGlu receptors include mGlu2 and mGlu3 which are localized both pre and postsynaptically and are primarily distributed in forebrain regions (Gu et al. 2008; Petralia et al. 1996). Group II mGlu receptors couple to Gi/o and activation of these receptors results in inhibition of adenylyl cyclase and modulation of voltage-dependent ion channels. Group III mGlu receptors include mGlu4, mGlu6, mGlu7, and mGlu8. This third group is localized predominantly presynaptically and is also coupled to Gi/o and associated signaling pathways.
Metabotropic glutamate receptors modulate synaptic neurotransmission at least in part through the binding of glutamate at its large extracellular “Venus flytrap” domain which is highly conserved across subtypes and groups. However, its signaling response to glutamate may be modulated (increased or decreased) by compounds that bind to allosteric sites located in the less highly conserved transmembrane regions. This is of major interest in drug design because the allosteric modulator approach may be more amenable to the development of compounds that are selective for a specific target (Conn et al. 2009a). Receptor subtypes from Group I (mGlu5) and Group II (mGlu2 and mGlu3) are involved in the circuitry disruptions implicated in schizophrenia and are associated with NMDARs from a co-localization and functional standpoint. These observations, as well as preclinical evidence supporting these targets as having potential therapeutic benefits in schizophrenia, have led researchers to actively pursue the development of compounds that activate and/or modulate mGlu2/3 and mGlu5 for the treatment of this disorder.
4. Group II mGlu receptors as therapeutic targets for schizophrenia
4.1 Group II agonists
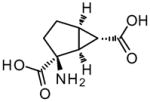
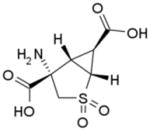
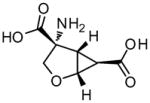
Activation of group II mGlu receptors has been shown to decrease the evoked release of glutamate and reduce excitatory synaptic transmission in multiple brain regions, including striatum, PFC, and the hippocampus (Battaglia et al. 1997; East et al. 1995; Lovinger and McCool 1995; Yoshino et al. 1996). Psychotomimetic agents have been shown to increase activity of glutamatergic synapses in the PFC (Adams and Moghaddam 1998; Lorrain et al. 2003) and several studies have confirmed that group II agonists reverse this effect (Lorrain et al. 2003; Marek et al. 2000; Moghaddam and Adams 1998). A class of group II agonists (that are essentially constrained amino acid analogues yet retain specificity at these receptors), exemplified by (1S,2S,5R,6S)-2-Aminobicyclo[3.1.0]hexane-2,6-dicarboxylic acid (LY354740) (Monn et al. 1997; Schoepp et al. 1997), has provided excellent tools for understanding the roles of group II mGlu receptor subtypes (Table 1). The neurochemical evidence for the ability of group II activation to reverse the effects of psychotomimetic agents has been supplemented by their ability to reverse the behavioral effects in several animal models that are used to predict efficacy of potential antipsychotic agents (Conn et al. 2008; Schoepp and Marek 2002) although there are exceptions to this effect depending on which compound is being studied, which behavioral paradigm is being tested, and what strain of animal is being used (Cartmell et al. 1999; Imre et al. 2006a; Imre et al. 2006b; Profaci et al. 2011; Takamori et al. 2003). For determining cognitive-enhancing properties, the effects of these agents depend on the type of learning that is being studied as well as whether or not the comparison is to a basal or disrupted level (Amitai and Markou 2010; Aultman and Moghaddam 2001; Greco et al. 2005; Higgins et al. 2004; Krystal et al. 2005; Schlumberger et al. 2009b; Spinelli et al. 2005). These compounds have also been tested in a preclinical model of schizophrenia induced by social isolation rearing, a non-pharmacological neurodevelopmental approach that results in a phenotype demonstrating hyperlocomotion and deficits in several cognitive models. Specifically, LY404039 was shown to reverse the hyperactivity induced by social isolation (Fabricius et al. 2010) and, in a separate study, LY379268 reversed the hyperactivity, deficit in novel object recognition, and disrupted PPI observed in these animals (Jones et al. 2011). However, in the latter study, LY379268 has no effect on conditional emotional response impairments. The reader is directed to Table 3 for a summary of the effects of Group II agonists in common preclinical models used for the assessment of antipsychotic activity and cognitive-enhancing properties.
Table 1.
Structures of Group II agonists and PAMs discussed. Note the narrow chemical space exhibited by the agonist compounds
Table 3.
Effects of Group II agonists, mGlu2 PAMs, and mGlu5 PAMs in common preclinical behavioral models relevant to symptom clusters in schizophrenia
| Preclinical Behavioral Model Response (rodent unless noted) | Group II Agonists | mGlu2 PAMs | mGlu5 PAMs | NOTES |
|---|---|---|---|---|
|
Antipsychotic Models
| ||||
| Attenuation of hyperlocomotion/stereotypies induced by NMDAR antagonists (PCP, Ketamine, or MK-801) | +/−[5, 7, 8, 11, 13, 20–22, 28, 29, 36, 38, 45, 48, 52] | +[13–15, 17, 33] | +/−[6, 12, 27, 39, 42] | 21 did not observe effect with LY354740 but 22 did observe effect with LY379268 27 observed effect with ADX47273, 39 did not |
| Attenuation of hyperlocomotion induced by amphetamine, methamphetamine, or apomorphine | +/−[5, 10, 11, 13, 32, 36, 37, 48, 52] | +/−[10, 13, 14] | +[24, 27, 31, 35, 37, 41, 51, 53] | 5 observed effects only at higher doses in the amphetamine model compared to the PCP model and efficacy was conditional on type of activity measured 37 did not observe effect with LY354740 48 observed effect with LY379268 at a dose that also decreased spontaneous locomotor activity 14 did not observed effect with BINA |
| Reversal of disruption of PPI induced by NMDAR antagonists (PCP, Ketamine, or MK-801) | −/+[21, 22, 34, 38, 40, 47] | +[14] | −[6] | 34 was the only study showing effect (LY354740) and was dependent on strain of mouse used 6 performed with icv administration of DFB |
| Reversal of disruption of PPI induced by amphetamine or apomorphine | −[13, 37] | +[13] | +[24, 26, 37, 39] | |
| Reduction of conditioned avoidance response | +/−[36, 46] | ND | +[27, 39, 42] | 46 observed effect with MGS compounds and LY418426 but not LY354740 |
|
Cognitive Models
| ||||
| Reduction of MK-801 impairment in active allothetic place avoidance | ND | −[50] | +[50] | |
| Increase in novel object recognition or social novelty discrimination | +[18, 23] | +[18] | +[6, 27, 49] | 18 investigated social novelty discrimination induced by neonatal PCP 23 investigated disruption by isolation rearing 49 investigated unimpaired and disruption of NOR by MK-801 and observed U-shaped dose effect |
| Improvement in five-choice serial reaction time test | −/+[1, 16, 43] | ND | +[27] | 1 and 16 looked at PCP-induced disruptions; 1 observed mixed effects; 16 observed reduction in anticipatory and perseverative responding but no improvement in accuracy in DBA/2N mice 43 performed in non-human primates and observed a decrease in sustained attention by LY354740 27 saw improvement in impulsivity |
| Improvement in set-shifting task | ND | +[30] | +[9, 44] | 30 also reported an improvement in DRL72 schedule of food reinforcement for LY487379 |
| Improvement in spatial learning and memory | −[19] | ND | +[3, 4] | |
| Improvement in working memory | −/+[2, 19, 25, 28, 38, 43] | ND | ND | 25 was performed in human subjects and 43 was performed in non-human primates All studies showed either no improvement or an impairment except for 25 and 28 |
+: compounds tested in the model and gave the indicated response
−: compounds tested in the model and did not give the indicated response
ND: compound not tested for given model
Notes column describes differences observed in each pharmacological group for the same model
Table References:
The selective group II mGlu receptor agonists in this class have been well-characterized and optimized and members of this class have entered into clinical trials for treatment of schizophrenia. The oral prodrug of (-)-(1R,4S,5S,6S)-4-amino-2-sulfonylbicyclo[3.1.0]hexane-4,6-dicarboxylic acid (LY404039), (1R,4S,5S,6S)-2-thiabicyclo[3.1.0]-hexane-4,6-dicarboxylic acid,4-[(2S)-2-amino-4-(methylthio)-1- oxobutyl]amino-, 2,2-dioxide monohydrate (LY2140023), showed significant antipsychotic efficacy for both positive and negative symptoms with no major side effects in a trial involving patients suffering from schizophrenia and also demonstrated a better metabolic profile than the comparator olanzapine (Patil et al. 2007). Unfortunately, in the follow-up study, neither LY2140023 nor the comparator olanzapine separated from the placebo group (due to higher-than expected placebo response) resulting in an inconclusive result for the second clinical study (Kinon et al. 2011). In addition, 4 of the study participants taking the test drug developed convulsions. Follow-up clinical trials with LY2140023 are ongoing (clinicaltrials.gov) and will hopefully result in a more clear resolution regarding the effectiveness of group II agonists for schizophrenia treatment. Additional studies will need to be performed with this or other group II agonist compounds to more fully understand their efficacy and the side-effect profile for this treatment option.
4.2 Group II interactions with other receptor types
Interestingly, the group II mGlu receptor agonists have also been shown to reverse stereotypies induced by PCP and head shakes induced by serotonin 2A (5-HT2A) receptor agonists (Gewirtz and Marek 2000). In addition, there is evidence from a molecular standpoint that the two receptor types are colocalized in the cortex and are jointly implicated in the neuropathology of schizophrenia (Gonzalez-Maeso et al. 2008). Activation of 5-HT2A receptors results in an increase in glutamate release in the PFC, reminiscent of the increase in excitatory drive to the PFC that is seen as a result of disinhibition of thalamocortical neurons induced by NMDAR antagonists. These effects produced by hallucinogenic 5-HT2A agonists can be blocked by 5-HT2A receptor antagonists and also by group II mGluR agonists (Marek et al. 2000) and positive allosteric modulators of mGlu2 (see section 4.4)(Benneyworth et al. 2007). Recently, it was shown that combined, sub-effective doses of the mGlu2/3 agonist (1S,2R,5R,6R)-2-amino-4- oxabicyclo[3.1.0]hexane-2,6-dicarboxylic acid (LY379268) and the 5HT2A receptor antagonist, (R)-(+)-α-(2,3-Dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl]-4-piperidinemethanol (M100907), are efficacious in an animal model predictive of antipsychotic activity (Uslaner et al. 2009a). Other intriguing associations with group II mGluRs have been discovered that are relevant to current models of schizophrenia including the upregulation of high-affinity D2 dopamine receptors in the striatum of mGlu2 and mGlu3 knockout mice (Seeman et al. 2009). Observations such as these warrant further investigation and should be kept in mind when considering a possible multi-target approach for treatment of psychosis (Wong et al. 2010).
4.3 mGlu2 is the major contributor of group II agonist effects
As mentioned above, mGlu2 and mGlu3 knockout mice have been developed and studied to better understand the receptor subtype most important for the observed antipsychotic-like effects of the group II mGlu agonists. The antipsychotic actions of group II agonists LY404039 and LY314582 were absent in mGlu2 knockout mice but present in mGlu3 knockout mice, strongly implicating mGlu2 as the predominant player in this effect of the group II agonists (Fell et al. 2008; Spooren et al. 2000). Similar results were observed for the group II agonist LY379268 in that it reversed PCP- and amphetamineevoked hyperactivity in wild type and mGlu3 but not mGlu2 knockout mice (Woolley et al. 2008). These findings highlighted mGlu2 as the target for the development of more specific compounds within this receptor group. However, targeting the orthosteric site for specificity is difficult due to the high degree of conservation across the mGlu receptor subtypes as well as the constraints that this site imposes upon the chemical space tolerated for engagement. In addition, these compounds may be limited in other ways as indicated by tolerance observed for the reversal of PCP- and amphetamine-induced hyperactivity in mice (Galici et al. 2005). As an alternative approach, advances in the development of positive modulators of mGlu2 that exert their actions through interaction with allosteric site(s) within the transmembrane region of the receptor hold promise of overcoming these limitations (Conn et al. 2009a).
4.4 Development of mGlu2 positive allosteric modulators
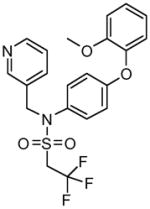
Multiple positive allosteric modulators (PAMs) that are highly selective for mGlu2 have been developed in recent years. Two of the most well studied chemical classes of mGlu2 PAMs are exemplified by N-(4-(2-methoxyphenoxy)phenyl)-N-(2,2,2-trifluoroethylsulfonyl)pyrid-3-ylmethylamine (LY487379) (Galici et al. 2005; Johnson et al. 2003; Schaffhauser et al. 2003) and 3′-(((2-cyclopentyl-6,7-dimethyl-1-oxo-2,3- dihydro- 1H-inden-5-yl)oxy)methyl)biphenyl-4-carboxylic acid (BINA) (Bonnefous et al. 2005; Galici et al. 2006; Pinkerton et al. 2005). Both are selective for mGlu2 and their activity is detected as an enhancement in the response induced by an orthosteric agonist or by a shift to the left (increased potency) of a concentration response curve of the orthosteric agonist. These PAMs engage with the receptor within the 7th transmembrane region with 3 amino acids being identified as required for their activity (Rowe et al. 2008; Schaffhauser et al. 2003). The mGlu2 PAMs potentiate the glutamatergic response to group II mGlu agonists (Benneyworth et al. 2007; Galici et al. 2006; Johnson et al. 2003; Poisik et al. 2005; Schaffhauser et al. 2003) including excitatory synaptic responses in the PFC (Benneyworth et al. 2007). Several mGlu2 PAMs from distinct chemical scaffolds have shown efficacy in animal models used for the evaluation of potential antipsychotic agents (Benneyworth et al. 2007; Duplantier et al. 2009; Galici et al. 2005; Galici et al. 2006; Govek et al. 2005; Johnson et al. 2003; Pinkerton et al. 2005) as well as in models demonstrating anxiolytic effects (Johnson et al. 2003) and enhanced cognitive flexibility (Nikiforuk et al. 2010) (see Table 3 for summary). Fell et al. recently reported a novel mGlu2 PAM, N-(4-((2-(trifluoromethyl)-3-hydroxy-4-(isobutyryl)phenoxy)methyl)benzyl)-1-methyl-1H-imidazole-4-carboxamide (THIIC), that produces anxiolytic and antidepressant effects in rodent models of stress and depression, respectively, and found evidence of a low potential for tolerance by this mechanism (Fell et al. 2011). Imaging studies have revealed that the mGlu2 PAM BINA, at doses that reverse PCP-induced hyperlocomotion, suppresses the imaging response observed in the PFC, caudate-putamen, nucleus accumbens, and mediodorsal thalamus indicating that these are likely key regions involved in BINA’s pharmacological effect (Hackler et al. 2010). Because of allosteric modulators’ dependence on the presence of a threshold level of agonist to exert their action, it has been postulated that PAMs may provide a safer, better tolerated, therapeutic profile compared to compounds that act to directly stimulate the receptor resulting in a less regulated action and greater potential receptor desensitization (Johnson et al. 2005; Urwyler 2011). While this potential advantage has not been fully established experimentally, these results are promising and support the further optimization of mGlu2 PAMs as a potential therapeutic approach for schizophrenia and possibly other psychiatric disorders (Conn et al. 2009b; Conn et al. 2008; Fraley 2009).
5. mGlu5 as a therapeutic target for schizophrenia
The group I mGlu receptor, mGlu5, has also emerged as a potential target for treatment of schizophrenia. Promising evidence suggests that selective activators of mGlu5 have potential for efficacy in treatment of positive and negative symptoms of schizophrenia and may also have cognitive-enhancing effects (Conn et al. 2009b). In contrast to mGlu2, the preclinical success for mGlu5 has been primarily in the area of allosteric modulators of this receptor as opposed to direct agonists.
5.1 mGlu5 is a close signaling partner with NMDA receptors
As discussed above, the activation of mGlu2 reduces glutamatergic excitatory synaptic transmission in the PFC which may be abnormally increased in disease states such as schizophrenia due to reduced tone at GABAergic projection neurons that help to regulate this signaling pathway. This is likely achieved through a presynaptic mechanism. Theoretically, a similar reduction of excessive activation of projections to the PFC could be achieved further upstream. For instance, potentiation of NMDAR function or other manipulations that increase synaptic excitation of midbrain GABAergic projection neurons could enhance the inhibitory regulation of thalamocortical projections. As stated earlier, inhibition of synaptic excitation of midbrain GABAergic neurons has been postulated to contribute to the psychotomimetic effects of NMDAR antagonists and enhancing transmission at these synapses could provide a beneficial effect. The mGlu5 subtype is a close signaling partner with NMDARs and it has been postulated that activation of this receptor could enhance NMDAR function without inducing the adverse effects that occur with direct activation of NMDARs. mGlu5 interacts physically with NMDARs through binding and scaffolding proteins suggesting that these receptors may be jointly involved in post-synaptic organization and signaling (Ehlers 1999). The activation of group I mGlu receptors through exogenous application of agonist (such as DHPG) potentiates NMDAR responses in forebrain regions. This potentiation has been attributed predominantly to mGlu5-, rather than mGlu1-mediated effects through the use of pharmacological approaches (Attucci et al. 2001; Awad et al. 2000; Doherty et al. 2000; Mannaioni et al. 2001; Pisani et al. 2001; Ugolini et al. 1999) and knockout mice (Pisani et al. 2001). In addition, selective PAMs of mGlu5 enhance the mGlu5-mediated potentiation of NMDAR currents in hippocampal pyramidal cells (O’Brien et al. 2004) and DHPG-induced depolarization of STN neurons (Chen et al. 2007).
5.2 mGlu5 is involved in mechanisms of learning and memory
Long-term potentiation (LTP) and long-term depression (LTD) are two forms of synaptic plasticity that are thought to play important roles in multiple forms of cognitive function. There is evidence that a balance exists between LTP and LTD and that a disruption of this balance may be characteristic of cognition-impairing pathologies such as observed in models of Fragile X (Bear et al. 2004; Godfraind et al. 1996; Huber et al. 2002; Lauterborn et al. 2007; Li et al. 2002; Nosyreva and Huber 2006; Paradee et al. 1999). Therefore, the differential effects on LTP and LTD resulting from the up- or down-regulation (either through a change in expression level, functional activity, or pharmacological manipulation) should be considered when characterizing a potential target for CNS disorders.
Genetic or pharmacological disruption of signaling through mGlu5 can lead to impaired LTP in hippocampus and other brain regions. For instance, mGlu5 knockout mice exhibit reduced NMDARmediated LTP in the hippocampus that can be recovered by stimulating PKC (Jia et al. 1998). These mice also show a reduction in the ability to perform NMDAR-dependent memory tasks (Lu et al. 1997). Thetaburst stimulation-induced LTP in hippocampal slices is blocked by the mGlu5 noncompetitive antagonist 2-Methyl-6-(phenylethynyl)pyridine (MPEP), a compound that interacts with an allosteric site on the receptor (Francesconi et al. 2004; Shalin et al. 2006). This effect is also produced in vivo where a correlation was drawn between spatial learning and both synaptic plasticity and mGlu5 expression levels in two strains of rat (Manahan-Vaughan and Braunewell 2005). In addition to the impairment of LTP by disruption of mGlu5 signaling, the stimulation of mGlu5 either through agonist application or synaptic glutamate has been observed to facilitate, or “prime”, induced LTP in area CA1 of hippocampus (Cohen et al. 1998; Raymond et al. 2000). Interestingly, DHPG, a group I agonist, induces an NMDA-independent LTD on its own which is blocked by mGlu5 antagonists (Faas et al. 2002; Gasparini et al. 1999; Huang and Hsu 2006; Huang et al. 2004; Huber et al. 2001) and is absent in mGlu5 knockout mice (Huber et al. 2001). These observations provide evidence that suggests that mGlu5 plays a role in learning and memory and may therefore be a target for the development of compounds to treat cognitive disturbances that occur in disorders such as schizophrenia. However, the pursuit of traditional agonists for this purpose has not been encouraged due to their tendency to induce LTD as well as seizure activity in hippocampal slices and in animals (Wong et al. 2005). Thus, an approach that serves to modulate the response to glutamate at mGlu5, rather than evoke it, may be more effective for achieving restoration of cognitive deficits without inducing seizure activity.
5.3 Development of mGlu5 PAMs for use in animal models of schizophrenia
Efforts to discover and optimize highly selective mGlu5 PAMs have progressed over recent years to a point where there now exists a rich set of tools with which to study and validate this mechanism. The first reported mGlu5 PAM was [(3-Fluorophenyl)methylene]hydrazone-3-fluorobenzaldehyde (DFB) (O’Brien et al. 2003) which was successful in demonstrating the feasibility of producing a compound with specificity for mGlu5 that increased the receptor’s response to orthosteric agonists without causing a response on its own. This compound was shown to interact with the MPEP site on mGlu5. However, its utility was limited to in vitro proof-of-concept due to its relatively low potency (2- 5 μM) in in vitro assays and its limited solubility. A point to note is that, within the family of compounds exemplified by DFB, there exist compounds with not only PAM activity but also compounds that act as negative allosteric modulators (NAMs) as well as neutral, or “silent” allosteric modulators. The latter class of compounds has no effect on their own but blocks the ability of PAMs and NAMs to exert their effects. This range of effects of allosteric modulators of mGlu5 within closely related compounds is observed in other series of allosteric modulators and may be exploited to more fully understand the structure-function relationship of the effect of these modulators at the allosteric site.
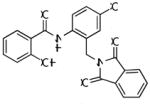
An improvement in potency compared to DFB was achieved with the discovery of N-{4-chloro-2-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)methyl]phenyl}-2-hydroxybenzamide (CPPHA), a compound which enabled further studies including the demonstration of an enhancement of NMDAR currents in hippocampal slices (O’Brien et al. 2004), a critical finding with respect to the desired effect of the compounds in relation to the hypothesized disease model. Interestingly, this compound was found to act at a site distinct from the MPEP site but within the same transmembrane domain (Chen et al. 2008). Unfortunately, with the exception of a couple of studies using icv introduction of compound (Balschun et al. 2006; Chan et al. 2008), in vivo studies could not be performed with these early compounds due to limited solubility, pharmacokinetic properties, and limited central penetration.
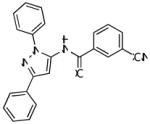
The ability to test the in vivo efficacy of mGlu5 PAMs came with the development of 3-Cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide (CDPPB) which not only provided better potency than the previous two series, but, was more soluble and centrally penetrant (Chen et al. 2007; Kinney et al. 2005; Lindsley et al. 2004). CDPPB was found to be selective for mGlu5 across mGluR subtypes as well as in a panel of 175 proteins that commonly interact with drugs. An analog of CDPPB, 4-Nitro-N-(1,3-diphenyl-1Hpyrazol- 5-yl)benzamide (VU29), was shown to selectively potentiate mGlu5-mediated responses in midbrain neurons (Chen et al. 2007) and in hippocampus (Ayala et al. 2009). This series of compounds was found to interact with the MPEP site on mGlu5 (Chen et al. 2007) and, interestingly, also demonstrate agonist-like activity at higher concentrations (Kinney et al. 2005). An exciting finding was that CDPPB demonstrated efficacy in reducing both amphetamine-induced hyperlocomotion and amphetamine-induced disruption of pre-pulse inhibition (PPI) in rats, two different animal models predictive of antipsychotic efficacy (Kinney et al. 2005). In addition, studies suggest that mGlu5 may have effects in animal models that are relevant to the negative symptoms in schizophrenia. Specifically, CDPPB attenuated the MK-801-induced deficit in sucrose preference in rats (Vardigan et al. 2010). However, although CDPPB can be used to demonstrate in vivo efficacy, it was still not optimal for formulations that are typically used for animal dosing.
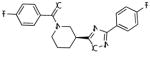
Several improved mGlu5 PAMs have since been developed that have enabled the use of these compounds in animal models for the study of their effectiveness. The first of such compounds, S-(4-fluoro-phenyl)-{3-[3-(4-fluoro-phenyl)-[1,2,4]oxadiazol-5-yl]-piperidin-1-yl}-methanone (ADX47273), is active in several different models predictive of antipsychotic efficacy as well as in cognition enhancement (Epping-Jordan et al. 2005; Liu et al. 2008) and lacks the sedative effect sometimes seen with traditional antipyschotics (Schlumberger et al. 2009a). ADX47273 has also been compared vis-à-vis the group II agonist LY354740 and the former, but not the latter, shows efficacy in reducing deficits in PPI (Schlumberger et al. 2009a).
Other structurally diverse mGlu5 PAMs have become available in recent months indicating the wide chemical space tolerated within the available allosteric site(s) within mGlu5. Optimization of an HTS hit on mGlu5 resulted in N-cyclobutyl-6-((3-fluorophenyl)ethynyl)nicotinamide (VU0360172), an orally active mGlu5 PAM, and several other biphenyl acetylenes that produce a dose-dependent effect in reducing amphetamine-induced hyperlocomotion in rats (Rodriguez et al. 2010; Williams et al. 2011). It should be noted that a NAM from the same series showed efficacy in an anxiolytic rodent model. The same HTS campaign revealed a NAM that is structurally distinct from the MPEP scaffold and subsequent attempts at optimization of this N-Aryl Piperazine resulted in the identification of a PAM, 2-{4-[2-(benzyloxy)acetyl]piperazin-1-yl}benzonitrile (VU0364289), that also exhibits antipsychotic activity in preclinical models (Zhou et al. 2010). A series of 4-aryl piperazine and piperidine amides analogs has also been disclosed as mGlu5 PAM compounds (Xiong et al. 2010) with 1-(4-(2-chloro-4-fluorophenyl) 29 piperazin-1-yl)-2-(pyridin-4-ylmethoxy)ethanone (CPPZ) shown to have activity in two models predictive of antipsychotic activity (Spear et al. 2011). Interestingly, the newly disclosed mGlu5 PAM 4-butoxy-N-(2,4-difluorophenyl)benzamide (VU0357121) has been shown to interact at a structurally distinct site from MPEP and CPPHA but still functionally interacts with the MPEP site(Hammond et al. 2010), further illustrating the complexity of interactions that occur within the allosteric binding domain(s).
5.4 Effectiveness of mGlu5 PAMs for cognition enhancement
As discussed earlier, there is evidence that mGlu5 is involved in mechanisms of learning and memory. It is therefore important to establish what effect is exerted by mGlu5 PAMs at the circuit level and confirm that they do not disproportionately affect LTP or LTD, which would have a potential to impair rather than improve cognitive function. To directly address the effect of mGlu5 activation on the balance between LTD and LTP, a series of studies was performed to determine the effects of the CDPPD analog, VU29, on afferent stimulation-induced (as opposed to exogenously applied agonists) hippocampal LTP and LTD at the Schaffer collateral (SC)-CA1 synapse (Ayala et al. 2009). These studies found that the tested PAMs of mGlu5 enhanced both LTP and LTD but did not alter the normal presynaptic activity required to induce each of these forms of synaptic plasticity. The same study also revealed enhanced performance in the Morris water maze task by mice treated with the two structurally distinct mGlu5 PAMs, CDPPB and ADX47273. This study reinforced other findings of the enhancement of cognitive function in animal models by mGlu5 PAMs (Chan et al. 2008; Darrah et al. 2008; Liu et al. 2008; Uslaner et al. 2009b; Vales et al. 2010) (see Table 3 for summary).
The preclinical evidence observed with mGlu5 PAMs thus far presents exciting potential for these compounds to be developed as an alternative treatment for disorders such as schizophrenia where there is currently an unmet need in the cognitive dysfunction symptom cluster. Additionally, these compounds may improve the extrapyramidal side effect profile observed with current therapies. A recent study showing possible desensitization to repeated administration of CDPPB warrants more investigation to determine whether this mechanism will provide a robust profile for the reversal of the different symptom clusters in schizophrenia (Parmentier-Batteur et al. 2010). The next few years will be key in determining if mGlu5 PAMs will move forward for clinical evaluation.
6. Newly available tools and future directions
The possibility of addressing unmet needs in the treatment of schizophrenia and other psychiatric disorders is exciting and provides a clear motive for furthering our understanding of the metabotropic glutamate receptors in this context. Several genetic models have recently been developed that may serve as informative tools in the study of targeting mGluRs (van den Buuse 2010). The neuron-specific protein Norbin (also known as Neurochondrin) interacts with mGlu5 and serves to increase the cell surface localization of the receptor and positively regulate mGlu5 signaling (Wang et al. 2009). Norbin knockout mice show deficits in sensori-motor gating (as measured with PPI) as well as increased locomotor activity similar to those of mGlu5 knockout mice and psychotomimetic-induced models of psychosis (Wang et al. 2009). In addition, genetic models directly targeting the NMDA receptor have resulted in mice showing similar phenotypes. Mice in which the NR1 subunit of the NMDA receptor has been reduced to 5% of wild type levels show both increased motor activity and social deficits and respond to treatment with haloperidol and clozapine (Mohn et al. 1999; Ramsey 2009). Furthermore, knock-in mice bearing NMDARs in which a specific phosphorylation site known to be reduced in patients with schizophrenia (serine 897) (Li et al. 2009) is substituted with an alanine (thereby preventing phosphorylation) exhibit deficits in social interaction and sensorimotor gating. Interestingly, the group I agonist, DHPG, has been shown to induce phosphorylation at both S896 and S897 in vivo. In future studies, it will be important to determine the effects of mGlu5 PAMs in these genetic models that selectively alter specific aspects of glutamate signaling.
In conclusion, the development of novel treatments for schizophrenia based on normalizing dysfunctional glutamatergic circuitry through activation of mGlu2 and mGlu5 has shown promise both in preclinical models and initial clinical trials. Approaching these targets using allosteric modulators, as opposed to orthosteric agonists, opens up the chemical space for active compounds and may result in an improved therapeutic profile compared to direct agonism of these receptors. Further refinement and investigation will be required to determine the full potential of these agents especially in the realm of the unmet need of treating the cognitive deficits and negative symptoms that occur in schizophrenia. Specifically, results from follow-up trials of mGlu2 agonist LY2140023 that will more definitively reveal its efficacy are highly anticipated and the progression of mGlu2 and mGlu5 PAMs into clinical safety and efficacy evaluations will provide a guide for the next steps for these classes of compounds.
Table 2.
mGlu5 PAMs discussed in text
| NAME | STRUCTURE |
|---|---|
| DFB |

|
| CPPHA |
|
| CDPPB |
|
| ADX47273 |
|
| VU0360172 |
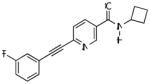
|
| CPPZ |
|
| VU0364289 |

|
Acknowledgments
The authors would like to acknowledge and thank Drs Colleen Niswender and Douglas Sheffler for their helpful comments on the manuscript and Dr Jerri M. Rook for her guidance with Table 3.
Abbreviations
- CNS
central nervous system
- DA
dopamine
- mGlu
metabotropic glutamate receptor
- PAM
positive allosteric modulator
- NAM
negative allosteric modulator
- MPEP
2-Methyl-6-(phenylethynyl)pyridine
- NMDA
N-methyl-D-aspartate
- PCP
phencyclidine
- MK-801
(5S,10R)-(+)-5-Methyl-10,11-dihydro-5H-dibenzo[a, d]cyclohepten-5,10-imine maleate
- PFC
prefrontal cortex
- DHPG
dihydroxyphenylglycine
- CDPPB
3-cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl) benzamide
- CPPHA
N-{4-chloro-2-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)methyl]phenyl}-2-hydroxybenzamide
- GPCR
Gprotein- coupled receptor
- VU0360172
N-cyclobutyl-6-((3-fluorophenyl)ethynyl)nicotinamide
- LY354740
(1S,2S,5R,6S)-2-Aminobicyclo[3.1.0]hexane-2,6-dicarboxylic acid
- LY404039
(-)-(1R,4S,5S,6S)-4-amino-2- sulfonylbicyclo[3.1.0]hexane-4,6-dicarboxylic acid
- LY2140023
(1R,4S,5S,6S)-2-thiabicyclo[3.1.0]-hexane-4,6-dicarboxylic acid,4-[(2S)-2-amino-4-(methylthio)-1-oxobutyl]amino-, 2,2-dioxide monohydrate
- LY379268
(1S,2R,5R,6R)-2-amino-4-oxabicyclo[3.1.0]hexane-2,6-dicarboxylic acid
- LY487379
N-(4-(2-methoxyphenoxy)phenyl)-N-(2,2,2-trifluoroethylsulfonyl)pyrid-3-ylmethylamine
- BINA
3′-(((2-cyclopentyl-6,7-dimethyl-1-oxo-2,3-dihydro-1H-inden-5-yl)oxy)methyl)biphenyl-4- carboxylic acid
- THIIC
N-(4-((2-(trifluoromethyl)-3-hydroxy-4-(isobutyryl)phenoxy)methyl)benzyl)-1- methyl-1H-imidazole-4-carboxamide
- DFB
[(3-Fluorophenyl)methylene]hydrazone-3- fluorobenzaldehyde
- CDPPB
3-Cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide
- VU29
4-Nitro-N-(1,3- diphenyl-1H-pyrazol-5-yl)benzamide
- ADX47273
S-(4-fluoro-phenyl)-{3-[3-(4-fluoro-phenyl)-[1,2,4]oxadiazol-5-yl]-piperidin-1-yl}-methanone
- VU0357121
4-butoxy-N-(2,4- difluorophenyl)benzamide
- LTP
Long-term potentiation
- LTD
long-term depression
- M100907
(R)-(+)-α-(2,3-Dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl]-4-piperidinemethanol
- icv
intracerebroventricular
- VU0364289
2-{4-[2-(benzyloxy)acetyl]piperazin-1-yl}benzonitrile
- NOR
novel object recognition
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams B, Moghaddam B. Corticolimbic Dopamine Neurotransmission Is Temporally Dissociated from the Cognitive and Locomotor Effects of Phencyclidine. J Neurosci. 1998;18:5545–5554. doi: 10.1523/JNEUROSCI.18-14-05545.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitai N, Markou A. Effects of metabotropic glutamate receptor 2/3 agonism and antagonism on schizophrenia-like cognitive deficits induced by phencyclidine in rats. European Journal of Pharmacology. 2010;639:67–80. doi: 10.1016/j.ejphar.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attucci S, Carlà V, Mannaioni G, Moroni F. Activation of type 5 metabotropic glutamate receptors enhances NMDA responses in mice cortical wedges. British Journal of Pharmacology. 2001;132:799–806. doi: 10.1038/sj.bjp.0703904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aultman JM, Moghaddam B. Distinct contributions of glutamate and dopamine receptors to temporal aspects of rodent working memory using a clinically relevant task. Psychopharmacology. 2001;153:353–364. doi: 10.1007/s002130000590. [DOI] [PubMed] [Google Scholar]
- Awad H, Hubert GW, Smith Y, Levey AI, Conn PJ. Activation of Metabotropic Glutamate Receptor 5 Has Direct Excitatory Effects and Potentiates NMDA Receptor Currents in Neurons of the Subthalamic Nucleus. J Neurosci. 2000;20:7871–7879. doi: 10.1523/JNEUROSCI.20-21-07871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala JE, Chen Y, Banko JL, Sheffler DJ, Williams R, Telk AN, Watson NL, Xiang Z, Zhang Y, Jones PJ, Lindsley CW, Olive MF, Conn PJ. mGluR5 Positive Allosteric Modulators Facilitate both Hippocampal LTP and LTD and Enhance Spatial Learning. Neuropsychopharmacology. 2009;34:2057–2071. doi: 10.1038/npp.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balschun D, Zuschratter W, Wetzel W. Allosteric enhancement of metabotropic glutamate receptor 5 function promotes spatial memory. Neuroscience. 2006;142:691–702. doi: 10.1016/j.neuroscience.2006.06.043. [DOI] [PubMed] [Google Scholar]
- Battaglia G, Monn JA, Schoepp DD. In vivo inhibition of veratridine-evoked release of striatal excitatory amino acids by the group II metabotropic glutamate receptor agonist LY354740 in rats. Neuroscience Letters. 1997;229:161–164. doi: 10.1016/s0304-3940(97)00442-4. [DOI] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends in Neurosciences. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Benneyworth MA, Xiang Z, Smith RL, Garcia EE, Conn PJ, Sanders-Bush E. A Selective Positive Allosteric Modulator of Metabotropic Glutamate Receptor Subtype 2 Blocks a Hallucinogenic Drug Model of Psychosis. Molecular Pharmacology. 2007;72:477–484. doi: 10.1124/mol.107.035170. [DOI] [PubMed] [Google Scholar]
- Bonnefous C, Vernier JM, Hutchinson JH, Gardner MF, Cramer M, James JK, Rowe BA, Daggett LP, Schaffhauser H, Kamenecka TM. Biphenyl-indanones: Allosteric potentiators of the metabotropic glutamate subtype 2 receptor. Bioorganic & Medicinal Chemistry Letters. 2005;15:4354–4358. doi: 10.1016/j.bmcl.2005.06.062. [DOI] [PubMed] [Google Scholar]
- Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, Weinberger DR, Weisenfeld N, Malhotra AK, Eckelman WC, Pickar D. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: Evidence from a novel positron emission tomography method. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:2569–2574. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan RW, Davis M, Goff D, Green MF, Keefe RSE, Leon AC, Nuechterlein KH, Laughren T, Levin R, Stover E, Fenton W, Marder SR. A Summary of the FDA-NIMH-MATRICS Workshop on Clinical Trial Design for Neurocognitive Drugs for Schizophrenia. Schizophrenia Bulletin. 2005;31:5–19. doi: 10.1093/schbul/sbi020. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Calligaro DO, Falcone JF, Marsh RD, Moore NA, Tye NC, Seeman P, Wong DT. Radioreceptor Binding Profile of the Atypical Antipsychotic Olanzapine. Neuropsychopharmacology. 1996;14:87–96. doi: 10.1016/0893-133X(94)00129-N. [DOI] [PubMed] [Google Scholar]
- Cartmell J, Monn JA, Schoepp DD. The Metabotropic Glutamate 2/3 Receptor Agonists LY354740 and LY379268 Selectively Attenuate Phencyclidine versus d-Amphetamine Motor Behaviors in Rats. Journal of Pharmacology and Experimental Therapeutics. 1999;291:161–170. [PubMed] [Google Scholar]
- Chan MH, Chiu PH, Sou JH, Chen HH. Attenuation of ketamine-evoked behavioral responses by mGluR5 positive modulators in mice. Psychopharmacology. 2008;198:141–148. doi: 10.1007/s00213-008-1103-1. [DOI] [PubMed] [Google Scholar]
- Chartoff EH, Heusner CL, Palmiter RD. Dopamine is not Required for the Hyperlocomotor Response to NMDA Receptor Antagonists. Neuropsychopharmacology. 2005;30:1324–1333. doi: 10.1038/sj.npp.1300678. [DOI] [PubMed] [Google Scholar]
- Chen Y, Goudet C, Pin JP, Conn PJ. N-{4-Chloro-2-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)methyl]phenyl}-2-hydroxybenzamide (CPPHA) Acts through a Novel Site as a Positive Allosteric Modulator of Group 1 Metabotropic Glutamate Receptors. Molecular Pharmacology. 2008;73:909–918. doi: 10.1124/mol.107.040097. [DOI] [PubMed] [Google Scholar]
- Chen Y, Nong Y, Goudet C, Hemstapat K, de Paulis T, Pin JP, Conn PJ. Interaction of Novel Positive Allosteric Modulators of Metabotropic Glutamate Receptor 5 with the Negative Allosteric Antagonist Site Is Required for Potentiation of Receptor Responses. Molecular Pharmacology. 2007;71:1389–1398. doi: 10.1124/mol.106.032425. [DOI] [PubMed] [Google Scholar]
- Clark M, Johnson BG, Wright RA, Monn JA, Schoepp DD. Effects of the mGlu2/3 receptor agonist LY379268 on motor activity in phencyclidine-sensitized rats. Pharmacology Biochemistry and Behavior. 2002;73:339–346. doi: 10.1016/s0091-3057(02)00848-1. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Raymond CR, Abraham WC. Priming of long-term potentiation induced by activation of metabotropic glutamate receptors coupled to phospholipase C. Hippocampus. 1998;8:160–170. doi: 10.1002/(SICI)1098-1063(1998)8:2<160::AID-HIPO8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Christopoulos A, Lindsley CW. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat Rev Drug Discov. 2009a;8:41–54. doi: 10.1038/nrd2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Lindsley CW, Jones CK. Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends in Pharmacological Sciences. 2009b;30:25–31. doi: 10.1016/j.tips.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Tamminga C, Schoepp DD, Lindsley C. Schizophrenia: Moving Beyond Monoamine Antagonists. Molecular Interventions. 2008;8:99–107. doi: 10.1124/mi.8.2.7. [DOI] [PubMed] [Google Scholar]
- Darrah JM, Stefani MR, Moghaddam B. Interaction of N-methyl-D-aspartate and group 5 metabotropic glutamate receptors on behavioral flexibility using a novel operant set-shift paradigm. Behavioural Pharmacology. 2008;19:225–234. doi: 10.1097/FBP.0b013e3282feb0ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty AJ, Palmer MJ, Bortolotto ZA, Hargreaves A, Kingston AE, Ornstein PL, Schoepp DD, Lodge D, Collingridge GL. A novel, competitive mGlu5 receptor antagonist (LY344545) blocks DHPG-induced potentiation of NMDA responses but not the induction of LTP in rat hippocampal slices. British Journal of Pharmacology. 2000;131:239–244. doi: 10.1038/sj.bjp.0703574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duplantier AJ, Efremov I, Candler J, Doran AC, Ganong AH, Haas JA, Hanks AN, Kraus KG, Lazzaro JT, Jr, Lu J, Maklad N, McCarthy SA, O’Sullivan TJ, Rogers BN, Siuciak JA, Spracklin DK, Zhang L. 3-Benzyl-1,3-oxazolidin-2-ones as mGluR2 positive allosteric modulators: Hit-to lead and lead optimization. Bioorganic & Medicinal Chemistry Letters. 2009;19:2524–2529. doi: 10.1016/j.bmcl.2009.03.032. [DOI] [PubMed] [Google Scholar]
- East SJ, Hill MP, Brotchie JM. Metabotropic glutamate receptor agonists inhibit endogenous glutamate release from rat striatal synaptosomes. European Journal of Pharmacology. 1995;277:117–121. doi: 10.1016/0014-2999(95)00119-6. [DOI] [PubMed] [Google Scholar]
- Ehlers MD. Synapse structure: Glutamate receptors connected by the shanks. Current Biology. 1999;9:R848–R850. doi: 10.1016/s0960-9822(00)80043-3. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, Nayak S, Derouet F, Dominguez H, Bessis AS, Le Poul E, Ludwig B, Mutel V, Poli SM, Rocher JP. In vivo characterization of mGluR5 positive allosteric modulators as novel treatments for schizophrenia and cognitive dysfunction. Neuropharmacology. 2005;49:243. [Google Scholar]
- Faas GC, Adwanikar H, Gereau RW, IV, Saggau P. Modulation of Presynaptic Calcium Transients by Metabotropic Glutamate Receptor Activation: A Differential Role in Acute Depression of Synaptic Transmission and Long-Term Depression. J Neurosci. 2002;22:6885–6890. doi: 10.1523/JNEUROSCI.22-16-06885.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricius K, Helboe L, Fink-Jensen A, Wortwein G, Steiniger-Brach B. Pharmacological characterization of social isolation-induced hyperactivity. Psychopharmacology. 2010:1–10. doi: 10.1007/s00213-010-2128-9. [DOI] [PubMed] [Google Scholar]
- Fell MJ, Svensson KA, Johnson BG, Schoepp DD. Evidence for the Role of Metabotropic Glutamate (mGlu)2 Not mGlu3 Receptors in the Preclinical Antipsychotic Pharmacology of the mGlu2/3 Receptor Agonist (−)-(1R,4S,5S,6S)-4-Amino-2-sulfonylbicyclo[3.1.0]hexane-4,6-dicarboxylic Acid (LY404039) Journal of Pharmacology and Experimental Therapeutics. 2008;326:209–217. doi: 10.1124/jpet.108.136861. [DOI] [PubMed] [Google Scholar]
- Fell MJ, Witkin JM, Falcone JF, Katner JS, Perry KW, Hart J, Rorick-Kehn L, Overshiner CD, Rasmussen K, Chaney SF, Benvenga MJ, Li X, Marlow DL, Thompson LK, Luecke SK, Wafford KA, Seidel WF, Edgar DM, Quets AT, Felder CC, Wang X, Heinz BA, Nikolayev A, Kuo MS, Mayhugh D, Khilevich A, Zhang D, Ebert PJ, Eckstein JA, Ackermann BL, Swanson SP, Catlow JT, Dean RA, Jackson K, Tauscher-Wisniewski S, Marek GJ, Schkeryantz JM, Svensson KA. N-(4-((2-(trifluoromethyl)-3-hydroxy-4-(isobutyryl)phenoxy)methyl)benzyl)-1-methyl-1H-imidazole-4-carboxamide (THIIC), a Novel Metabotropic Glutamate 2 Potentiator with Potential Anxiolytic/Antidepressant Properties: In Vivo Profiling Suggests a Link between Behavioral and Central Nervous System Neurochemical Changes. Journal of Pharmacology and Experimental Therapeutics. 2011;336:165–177. doi: 10.1124/jpet.110.172957. [DOI] [PubMed] [Google Scholar]
- Fowler SW, Ramsey AK, Walker JM, Serfozo P, Olive MF, Schachtman TR, Simonyi A. Functional interaction of mGlu5 and NMDA receptors in aversive learning in rats. Neurobiology of Learning and Memory. 2011;95:73–79. doi: 10.1016/j.nlm.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraley ME. Positive allosteric modulators of the metabotropic glutamate receptor 2 for the treatment of schizophrenia. Expert Opinion on Therapeutic Patents. 2009;19:1259–1275. doi: 10.1517/13543770903045009. [DOI] [PubMed] [Google Scholar]
- Francesconi W, Cammalleri M, Sanna PP. The metabotropic glutamate receptor 5 is necessary for late-phase long-term potentiation in the hippocampal CA1 region. Brain Research. 2004;1022:12–18. doi: 10.1016/j.brainres.2004.06.060. [DOI] [PubMed] [Google Scholar]
- Galici R, Echemendia NG, Rodriguez AL, Conn PJ. A Selective Allosteric Potentiator of Metabotropic Glutamate (mGlu) 2 Receptors Has Effects Similar to an Orthosteric mGlu2/3 Receptor Agonist in Mouse Models Predictive of Antipsychotic Activity. Journal of Pharmacology and Experimental Therapeutics. 2005;315:1181–1187. doi: 10.1124/jpet.105.091074. [DOI] [PubMed] [Google Scholar]
- Galici R, Jones CK, Hemstapat K, Nong Y, Echemendia NG, Williams LC, de Paulis T, Conn PJ. Biphenyl-indanone A, a Positive Allosteric Modulator of the Metabotropic Glutamate Receptor Subtype 2, Has Antipsychotic- and Anxiolytic-Like Effects in Mice. Journal of Pharmacology and Experimental Therapeutics. 2006;318:173–185. doi: 10.1124/jpet.106.102046. [DOI] [PubMed] [Google Scholar]
- Gasparini F, Lingenhohl K, Stoehr N, Flor PJ, Heinrich M, Vranesic I, Biollaz M, Allgeier H, Heckendorn R, Urwyler S, Varney MA, Johnson EC, Hess SD, Rao SP, Sacaan AI, Santori EM, Velicelebi G, Kuhn R. 2-Methyl-6-(phenylethynyl)-pyridine (MPEP), a potent, selective and systemically active mGlu5 receptor antagonist. Neuropharmacology. 1999;38:1493–1503. doi: 10.1016/s0028-3908(99)00082-9. [DOI] [PubMed] [Google Scholar]
- Gewirtz JC, Marek GJ. Behavioral Evidence for Interactions between a Hallucinogenic Drug and Group II Metabotropic Glutamate Receptors. Neuropsychopharmacology. 2000;23:569–576. doi: 10.1016/S0893-133X(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Ghoneim MM, Hinrichs JV, Mewaldt SP, Petersen RC. Ketamine: Behavioral Effects of Subanesthetic Doses. Journal of Clinical Psychopharmacology. 1985;5:70–77. [PubMed] [Google Scholar]
- Godfraind JM, Reyniers E, De Boulle K, D’Hooge R, De Deyn PP, Bakker CE, Oostra BA, Kooy RF, Willems PJ. Long-term potentiation in the hippocampus of fragile X knockout mice. American Journal of Medical Genetics. 1996;64:246–251. doi: 10.1002/(SICI)1096-8628(19960809)64:2<246::AID-AJMG2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Muly IEC, Williams GV. D1 receptors in prefrontal cells and circuits. Brain Research Reviews. 2000;31:295–301. doi: 10.1016/s0165-0173(99)00045-4. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, Lopez-Gimenez JF, Zhou M, Okawa Y, Callado LF, Milligan G, Gingrich JA, Filizola M, Meana JJ, Sealfon SC. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govek SP, Bonnefous C, Hutchinson JH, Kamenecka T, McQuiston J, Pracitto R, Zhao LX, Gardner MF, James JK, Daggett LP, Rowe BA, Schaffhauser H, Bristow LJ, Campbell UC, Rodriguez DE, Vernier JM. Benzazoles as allosteric potentiators of metabotropic glutamate receptor 2 (mGluR2): Efficacy in an animal model for schizophrenia. Bioorganic & Medicinal Chemistry Letters. 2005;15:4068–4072. doi: 10.1016/j.bmcl.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Greco B, Invernizzi RW, Carli M. Phencyclidine-induced impairment in attention and response control depends on the background genotype of mice: reversal by the mGLU2/3 receptor agonist LY379268. Psychopharmacology. 2005;179:68–76. doi: 10.1007/s00213-004-2127-9. [DOI] [PubMed] [Google Scholar]
- Gu G, Lorrain DS, Wei H, Cole RL, Zhang X, Daggett LP, Schaffhauser HJ, Bristow LJ, Lechner SM. Distribution of metabotropic glutamate 2 and 3 receptors in the rat forebrain: Implication in emotional responses and central disinhibition. Brain Research. 2008;1197:47–62. doi: 10.1016/j.brainres.2007.12.057. [DOI] [PubMed] [Google Scholar]
- Hackler EA, Byun NE, Jones CK, Williams JM, Baheza R, Sengupta S, Grier MD, Avison M, Conn PJ, Gore JC. Selective potentiation of the metabotropic glutamate receptor subtype 2 blocks phencyclidine-induced hyperlocomotion and brain activation. Neuroscience. 2010;168:209–218. doi: 10.1016/j.neuroscience.2010.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond AS, Rodriguez AL, Townsend SD, Niswender CM, Gregory KJ, Lindsley CW, Conn PJ. Discovery of a Novel Chemical Class of mGlu5 Allosteric Ligands with Distinct Modes of Pharmacology. ACS Chemical Neuroscience. 2010;1:702–716. doi: 10.1021/cn100051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harich S, Gross G, Bespalov A. Stimulation of the metabotropic glutamate 2/3 receptor attenuates social novelty discrimination deficits induced by neonatal phencyclidine treatment. Psychopharmacology. 2007;192:511–519. doi: 10.1007/s00213-007-0742-y. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2004;10:40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Ballard TM, Kew JNC, Grayson Richards J, Kemp JA, Adam G, Woltering T, Nakanishi S, Mutel V. Pharmacological manipulation of mGlu2 receptors influences cognitive performance in the rodent. Neuropharmacology. 2004;46:907–917. doi: 10.1016/j.neuropharm.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Hirose K, Chan PH. Blockade of glutamate excitotoxicity and its clinical applications. Neurochemical Research. 1993;18:479–483. doi: 10.1007/BF00967252. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Jackson ME, Moghaddam B. Activation of Metabotropic Glutamate 2/3 Receptors Reverses the Effects of NMDA Receptor Hypofunction on Prefrontal Cortex Unit Activity in Awake Rats. J Neurophysiol. 2005;93:1989–2001. doi: 10.1152/jn.00875.2004. [DOI] [PubMed] [Google Scholar]
- Huang CC, Hsu KS. Sustained activation of metabotropic glutamate receptor 5 and protein tyrosine phosphatases mediate the expression of (S)-3,5-dihydroxyphenylglycineinduced long-term depression in the hippocampal CA1 region. Journal of Neurochemistry. 2006;96:179–194. doi: 10.1111/j.1471-4159.2005.03527.x. [DOI] [PubMed] [Google Scholar]
- Huang C-C, You J-L, Wu M-Y, Hsu K-S. Rap1-induced p38 Mitogen-activated Protein Kinase Activation Facilitates AMPA Receptor Trafficking via the GDI·Rab5 Complex. 2004 doi: 10.1074/jbc.M312868200. [DOI] [PubMed]
- Potential Role in (S)-3,5-Dihydroxyphenylglycine-induced long term depression. The Journal of Biological Chemistry. 279:12286–12292. doi: 10.1074/jbc.M312868200. [DOI] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Roder JC, Bear MF. Chemical Induction of mGluR5- and Protein Synthesis-Dependent Long-Term Depression in Hippocampal Area CA1. J Neurophysiol. 2001;86:321–325. doi: 10.1152/jn.2001.86.1.321. [DOI] [PubMed] [Google Scholar]
- Imre G, Fokkema DS, Ter Horst GJ. Subchronic administration of LY354740 does not modify ketamine-evoked behavior and neuronal activity in rats. European Journal of Pharmacology. 2006a;544:77–81. doi: 10.1016/j.ejphar.2006.06.037. [DOI] [PubMed] [Google Scholar]
- Imre G, Salomons A, Jongsma M, Fokkema DS, Den Boer JA, Ter Horst GJ. Effects of the mGluR2/3 agonist LY379268 on ketamine-evoked behaviours and neurochemical changes in the dentate gyrus of the rat. Pharmacology Biochemistry and Behavior. 2006b;84:392–399. doi: 10.1016/j.pbb.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Jia Z, Lu Y, Henderson J, Taverna F, Romano C, Abramow-Newerly W, Wojtowicz JM, Roder J. Selective Abolition of the NMDA Component of Long-Term Potentiation in Mice Lacking mGluR5. Learning & Memory. 1998;5:331–343. [PMC free article] [PubMed] [Google Scholar]
- Johnson MP, Baez M, Jagdmann GE, Britton TC, Large TH, Callagaro DO, Tizzano JP, Monn JA, Schoepp DD. Discovery of Allosteric Potentiators for the Metabotropic Glutamate 2 Receptor Synthesis and Subtype Selectivity of N-(4-(2-Methoxyphenoxy)phenyl)-N-(2,2,2-trifluoroethylsulfonyl)pyrid-3-ylmethylamine. Journal of Medicinal Chemistry. 2003;46:3189–3192. doi: 10.1021/jm034015u. [DOI] [PubMed] [Google Scholar]
- Johnson MP, Barda D, Britton TC, Emkey R, Hornback WJ, Jagdmann GE, McKinzie DL, Nisenbaum ES, Tizzano JP, Schoepp DD. Metabotropic glutamate 2 receptor potentiators: receptor modulation, frequency-dependent synaptic activity, and efficacy in preclinical anxiety and psychosis model(s) Psychopharmacology. 2005;179:271–283. doi: 10.1007/s00213-004-2099-9. [DOI] [PubMed] [Google Scholar]
- Jones C, Brown A, Auer D, Fone K. The mGluR2/3 agonist LY379268 reverses post-weaning social isolation-induced recognition memory deficits in the rat. Psychopharmacology. 2011;214:269–283. doi: 10.1007/s00213-010-1931-7. [DOI] [PubMed] [Google Scholar]
- Kinney GG, O’Brien JA, Lemaire W, Burno M, Bickel DJ, Clements MK, Chen TB, Wisnoski DD, Lindsley CW, Tiller PR, Smith S, Jacobson MA, Sur C, Duggan ME, Pettibone DJ, Conn PJ, Williams DL. A Novel Selective Positive Allosteric Modulator of Metabotropic Glutamate Receptor Subtype 5 Has in Vivo Activity and Antipsychotic-Like Effects in Rat Behavioral Models. Journal of Pharmacology and Experimental Therapeutics. 2005;313:199–206. doi: 10.1124/jpet.104.079244. [DOI] [PubMed] [Google Scholar]
- Kinon BJ, Zhang L, Millen BA, Osuntokun OO, Williams JE, Kollack-Walker S, Jackson K, Kryzhanovskaya L, Jarkova N Group atHS. A Multicenter, Inpatient, Phase 2, Double-Blind, Placebo-Controlled Dose-Ranging Study of LY2140023 Monohydrate in Patients With DSM-IV Schizophrenia. Journal of Clinical Psychopharmacology. 2011 doi: 10.1097/JCP.1090b1013e318218dcd318215. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Fenton WS, Carpenter WT, Marder SR. The NIMH-MATRICS Consensus Statement on Negative Symptoms. Schizophrenia Bulletin. 2006;32:214–219. doi: 10.1093/schbul/sbj053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Abi-Saab W, Perry E, D’Souza DC, Liu N, Gueorguieva R, McDougall L, Hunsberger T, Belger A, Levine L, Breier A. Preliminary evidence of attenuation of the disruptive effects of the NMDA glutamate receptor antagonist, ketamine, on working memory by pretreatment with the group II metabotropic glutamate receptor agonist, LY354740, in healthy human subjects. Psychopharmacology. 2005;179:303–309. doi: 10.1007/s00213-004-1982-8. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic Effects of the Noncompetitive NMDA Antagonist, Ketamine, in Humans: Psychotomimetic, Perceptual, Cognitive, and Neuroendocrine Responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A. Dopamine as the wind of the psychotic fire: new evidence from brain imaging studies. Journal of Psychopharmacology. 1999;13:358–371. doi: 10.1177/026988119901300405. [DOI] [PubMed] [Google Scholar]
- Lauterborn JC, Rex CS, Kramar E, Chen LY, Pandyarajan V, Lynch G, Gall CM. Brain-Derived Neurotrophic Factor Rescues Synaptic Plasticity in a Mouse Model of Fragile X Syndrome. J Neurosci. 2007;27:10685–10694. doi: 10.1523/JNEUROSCI.2624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Lieberman JA. Catching Up on Schizophrenia: Natural History and Neurobiology. Neuron. 2000;28:325–334. doi: 10.1016/s0896-6273(00)00111-2. [DOI] [PubMed] [Google Scholar]
- Li B, Devidze N, Barengolts D, Prostak N, Sphicas E, Apicella AJ, Malinow R, Emamian ES. NMDA receptor phosphorylation at a site affected in schizophrenia controls synaptic and behavioral plasticity. J Neurosci. 2009;29:11965–11972. doi: 10.1523/JNEUROSCI.2109-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Pelletier MR, Perez Velazquez JL, Carlen PL. Reduced Cortical Synaptic Plasticity and GluR1 Expression Associated with Fragile X Mental Retardation Protein Deficiency. Molecular and Cellular Neuroscience. 2002;19:138–151. doi: 10.1006/mcne.2001.1085. [DOI] [PubMed] [Google Scholar]
- Lindsley CW, Wisnoski DD, Leister WH, O’Brien JA, Lemaire W, Williams DL, Burno M, Sur C, Kinney GG, Pettibone DJ, Tiller PR, Smith S, Duggan ME, Hartman GD, Conn PJ, Huff JR. Discovery of Positive Allosteric Modulators for the Metabotropic Glutamate Receptor Subtype 5 from a Series of N-(1,3-Diphenyl-1H-pyrazol-5-yl)benzamides That Potentiate Receptor Function in Vivo. Journal of Medicinal Chemistry. 2004;47:5825–5828. doi: 10.1021/jm049400d. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends in Neurosciences. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Grauer S, Kelley C, Navarra R, Graf R, Zhang G, Atkinson PJ, Popiolek M, Wantuch C, Khawaja X, Smith D, Olsen M, Kouranova E, Lai M, Pruthi F, Pulicicchio C, Day M, Gilbert A, Pausch MH, Brandon NJ, Beyer CE, Comery TA, Logue S, Rosenzweig-Lipson S, Marquis KL. ADX47273 [S-(4-Fluoro-phenyl)-{3-[3-(4-fluoro-phenyl)-[1,2,4]-oxadiazol-5-yl]-piperidin-1-yl}-methanone]: A Novel Metabotropic Glutamate Receptor 5-Selective Positive Allosteric Modulator with Preclinical Antipsychotic-Like and Procognitive Activities. Journal of Pharmacology and Experimental Therapeutics. 2008;327:827–839. doi: 10.1124/jpet.108.136580. [DOI] [PubMed] [Google Scholar]
- Lorrain DS, Baccei CS, Bristow LJ, Anderson JJ, Varney MA. Effects of ketamine and n-methyl-d-aspartate on glutamate and dopamine release in the rat prefrontal cortex: modulation by a group II selective metabotropic glutamate receptor agonist LY379268. Neuroscience. 2003;117:697–706. doi: 10.1016/s0306-4522(02)00652-8. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, McCool BA. Metabotropic glutamate receptor-mediated presynaptic depression at corticostriatal synapses involves mGLuR2 or 3. J Neurophysiol. 1995;73:1076–1083. doi: 10.1152/jn.1995.73.3.1076. [DOI] [PubMed] [Google Scholar]
- Lu Y-M, Jia Z, Janus C, Henderson JT, Gerlai R, Wojtowicz JM, Roder JC. Mice Lacking Metabotropic Glutamate Receptor 5 Show Impaired Learning and Reduced CA1 Long-Term Potentiation (LTP) But Normal CA3 LTP. J Neurosci. 1997;17:5196–5205. doi: 10.1523/JNEUROSCI.17-13-05196.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Braunewell KH. The Metabotropic Glutamate Receptor, mGluR5, is a Key Determinant of Good and Bad Spatial Learning Performance and Hippocampal Synaptic Plasticity Cerebral Cortex. 2005;15:1703–1713. doi: 10.1093/cercor/bhi047. [DOI] [PubMed] [Google Scholar]
- Mannaioni G, Marino MJ, Valenti O, Traynelis SF, Conn PJ. Metabotropic Glutamate Receptors 1 and 5 Differentially Regulate CA1 Pyramidal Cell Function. J Neurosci. 2001;21:5925–5934. doi: 10.1523/JNEUROSCI.21-16-05925.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek GJ, Behl B, Bespalov AY, Gross G, Lee Y, Schoemaker H. Glutamatergic (N-Methyl-D-aspartate Receptor) Hypofrontality in Schizophrenia: Too Little Juice or a Miswired Brain? Molecular Pharmacology. 2010;77:317–326. doi: 10.1124/mol.109.059865. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Wright RA, Schoepp DD, Monn JA, Aghajanian GK. Physiological Antagonism between 5-Hydroxytryptamine2A and Group II Metabotropic Glutamate Receptors in Prefrontal Cortex. Journal of Pharmacology and Experimental Therapeutics. 2000;292:76–87. [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of Glutamatergic Neurotransmission by Ketamine: A Novel Step in the Pathway from NMDA Receptor Blockade to Dopaminergic and Cognitive Disruptions Associated with the Prefrontal Cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Adams BW. Reversal of Phencyclidine Effects by a Group II Metabotropic Glutamate Receptor Agonist in Rats. Science. 1998;281:1349–1352. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with Reduced NMDA Receptor Expression Display Behaviors Related to Schizophrenia. Cell. 1999;98:427–436. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- Monaghan D, Cotman C. Distribution of N-methyl-D-aspartate-sensitive L-[3H]glutamate-binding sites in rat brain. J Neurosci. 1985;5:2909–2919. doi: 10.1523/JNEUROSCI.05-11-02909.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan DT, Olverman HJ, Nguyen L, Watkins JC, Cotman CW. Two classes of N-methyl-D-aspartate recognition sites: differential distribution and differential regulation by glycine. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:9836–9840. doi: 10.1073/pnas.85.24.9836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monn JA, Valli MJ, Massey SM, Wright RA, Salhoff CR, Johnson BG, Howe T, Alt CA, Rhodes GA, Robey RL, Griffey KR, Tizzano JP, Kallman MJ, Helton DR, Schoepp DD. Design, Synthesis, and Pharmacological Characterization of (+)-2-Aminobicyclo[3.1.0]hexane-2,6-dicarboxylic Acid (LY354740): A Potent, Selective, and Orally Active Group 2 Metabotropic Glutamate Receptor Agonist Possessing Anticonvulsant and Anxiolytic Properties. Journal of Medicinal Chemistry. 1997;40:528–537. doi: 10.1021/jm9606756. [DOI] [PubMed] [Google Scholar]
- Nakazato A, Kumagai T, Sakagami K, Yoshikawa R, Suzuki Y, Chaki S, Ito H, Taguchi T, Nakanishi S, Okuyama S. Synthesis, SARs, and Pharmacological Characterization of 2-Amino-3 or 6-fluorobicyclo[3.1.0]hexane-2,6-dicarboxylic Acid Derivatives as Potent, Selective, and Orally Active Group II Metabotropic Glutamate Receptor Agonists. Journal of Medicinal Chemistry. 2000;43:4893–4909. doi: 10.1021/jm000346k. [DOI] [PubMed] [Google Scholar]
- Nikiforuk A, Popik P, Drescher KU, van Gaalen MM, Relo AL, Mezler M, Marek G, Schoemaker H, Gross G, Bespalov A. Effects of a positive allosteric modulator of mGlu2 receptors LY487379 on cognitive flexibility and impulsive-like responding in rats. Journal of Pharmacology and Experimental Therapeutics. 2010;335:665–673. doi: 10.1124/jpet.110.170506. [DOI] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. Metabotropic Glutamate Receptors: Physiology, Pharmacology, and Disease. Annual Review of Pharmacology and Toxicology. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosyreva ED, Huber KM. Metabotropic Receptor-Dependent Long-Term Depression Persists in the Absence of Protein Synthesis in the Mouse Model of Fragile X Syndrome. J Neurophysiol. 2006;95:3291–3295. doi: 10.1152/jn.01316.2005. [DOI] [PubMed] [Google Scholar]
- O’Brien JA, Lemaire W, Chen TB, Chang RSL, Jacobson MA, Ha SN, Lindsley CW, Schaffhauser HJ, Sur C, Pettibone DJ, Conn PJ, Williams DL. A Family of Highly Selective Allosteric Modulators of the Metabotropic Glutamate Receptor Subtype 5. Molecular Pharmacology. 2003;64:731–740. doi: 10.1124/mol.64.3.731. [DOI] [PubMed] [Google Scholar]
- O’Brien JA, Lemaire W, Wittmann M, Jacobson MA, Ha SN, Wisnoski DD, Lindsley CW, Schaffhauser HJ, Rowe B, Sur C, Duggan ME, Pettibone DJ, Conn PJ, Williams DL. A Novel Selective Allosteric Modulator Potentiates the Activity of Native Metabotropic Glutamate Receptor Subtype 5 in Rat Forebrain. Journal of Pharmacology and Experimental Therapeutics. 2004;309:568–577. doi: 10.1124/jpet.103.061747. [DOI] [PubMed] [Google Scholar]
- Ozawa S, Kamiya H, Tsuzuki K. Glutamate receptors in the mammalian central nervous system. Progress in Neurobiology. 1998;54:581–618. doi: 10.1016/s0301-0082(97)00085-3. [DOI] [PubMed] [Google Scholar]
- Paradee W, Melikian HE, Rasmussen DL, Kenneson A, Conn PJ, Warren ST. Fragile X mouse: strain effects of knockout phenotype and evidence suggesting deficient amygdala function. Neuroscience. 1999;94:185–192. doi: 10.1016/s0306-4522(99)00285-7. [DOI] [PubMed] [Google Scholar]
- Parmentier-Batteur S, Obrien JA, Doran S, Nguyen SJ, Flick RB, Uslaner JM, Chen H, Finger EN, Williams TM, Jacobson MA, Hutson PH. Differential effects of the mGluR5 positive allosteric modulator CDPPB in the cortex and striatum following repeated administration. Neuropharmacology. 2010 doi: 10.1016/j.neuropharm.2010.11.013. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, Andreev BV, Avedisova AS, Bardenstein LM, Gurovich IY, Morozova MA, Mosolov SN, Neznanov NG, Reznik AM, Smulevich AB, Tochilov VA, Johnson BG, Monn JA, Schoepp DD. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med. 2007;13:1102–1107. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- Pehrson A, Moghaddam B. Impact of metabotropic glutamate 2/3 receptor stimulation on activated dopamine release and locomotion. Psychopharmacology. 2010;211:443–455. doi: 10.1007/s00213-010-1914-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Niedzielski AS, Wenthold RJ. The metabotropic glutamate receptors, MGLUR2 and MGLUR3, show unique postsynaptic, presynaptic and glial localizations. Neuroscience. 1996;71:949–976. doi: 10.1016/0306-4522(95)00533-1. [DOI] [PubMed] [Google Scholar]
- Pilowsky LS, Bressan RA, Stone JM, Erlandsson K, Mulligan RS, Krystal JH, Ell PJ. First in vivo evidence of an NMDA receptor deficit in medication-free schizophrenic patients. Mol Psychiatry. 2005;11:118–119. doi: 10.1038/sj.mp.4001751. [DOI] [PubMed] [Google Scholar]
- Pinkerton AB, Cube RV, Hutchinson JH, James JK, Gardner MF, Rowe BA, Schaffhauser H, Rodriguez DE, Campbell UC, Daggett LP, Vernier JM. Allosteric potentiators of the metabotropic glutamate receptor 2 (mGlu2). Part 3: Identification and biological activity of indanone containing mGlu2 receptor potentiators. Bioorganic & Medicinal Chemistry Letters. 2005;15:1565–1571. doi: 10.1016/j.bmcl.2005.01.077. [DOI] [PubMed] [Google Scholar]
- Pisani A, Gubellini P, Bonsi P, Conquet F, Picconi B, Centonze D, Bernardi G, Calabresi P. Metabotropic glutamate receptor 5 mediates the potentiation of N-methyl--aspartate responses in medium spiny striatal neurons. Neuroscience. 2001;106:579–587. doi: 10.1016/s0306-4522(01)00297-4. [DOI] [PubMed] [Google Scholar]
- Poisik O, Raju DV, Verreault M, Rodriguez A, Abeniyi OA, Conn PJ, Smith Y. Metabotropic glutamate receptor 2 modulates excitatory synaptic transmission in the rat globus pallidus. Neuropharmacology. 2005;49:57–69. doi: 10.1016/j.neuropharm.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Profaci C, Krolikowski K, Olszewski R, Neale J. Group II mGluR agonist LY354740 and NAAG peptidase inhibitor effects on prepulse inhibition in PCP and D-amphetamine models of schizophrenia. Psychopharmacology. 2011:1–9. doi: 10.1007/s00213-011-2200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey AJ. NR1 knockdown mice as a representative model of the glutamate hypothesis of schizophrenia. In: Sawa A, editor. Progress in Brain Research. Vol. 179. Elsevier; 2009. pp. 51–58. [DOI] [PubMed] [Google Scholar]
- Raymond CR, Thompson VL, Tate WP, Abraham WC. Metabotropic Glutamate Receptors Trigger Homosynaptic Protein Synthesis to Prolong Long-Term Potentiation. J Neurosci. 2000;20:969–976. doi: 10.1523/JNEUROSCI.20-03-00969.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez AL, Grier MD, Jones CK, Herman EJ, Kane AS, Smith RL, Williams R, Zhou Y, Marlo JE, Days EL, Blatt TN, Jadhav S, Menon UN, Vinson PN, Rook JM, Stauffer SR, Niswender CM, Lindsley CW, Weaver CD, Conn PJ. Discovery of Novel Allosteric Modulators of Metabotropic Glutamate Receptor Subtype 5 Reveals Chemical and Functional Diversity and In Vivo Activity in Rat Behavioral Models of Anxiolytic and Antipsychotic Activity. Molecular Pharmacology. 2010;78:1105–1123. doi: 10.1124/mol.110.067207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorick-Kehn L, Johnson B, Knitowski K, Salhoff C, Witkin J, Perry K, Griffey K, Tizzano J, Monn J, McKinzie D, Schoepp D. In vivo pharmacological characterization of the structurally novel, potent, selective mGlu2/3 receptor agonist LY404039 in animal models of psychiatric disorders. Psychopharmacology. 2007;193:121–136. doi: 10.1007/s00213-007-0758-3. [DOI] [PubMed] [Google Scholar]
- Roth BL, Craigo SC, Choudhary MS, Uluer A, Monsma FJ, Shen Y, Meltzer HY, Sibley DR. Binding of typical and atypical antipsychotic agents to 5-hydroxytryptamine-6 and 5-hydroxytryptamine-7 receptors. Journal of Pharmacology and Experimental Therapeutics. 1994;268:1403–1410. [PubMed] [Google Scholar]
- Rowe BA, Schaffhauser H, Morales S, Lubbers LS, Bonnefous C, Kamenecka TM, McQuiston J, Daggett LP. Transposition of Three Amino Acids Transforms the Human Metabotropic Glutamate Receptor (mGluR)-3-Positive Allosteric Modulation Site to mGluR2, and Additional Characterization of the mGluR2-Positive Allosteric Modulation Site. Journal of Pharmacology and Experimental Therapeutics. 2008;326:240–251. doi: 10.1124/jpet.108.138271. [DOI] [PubMed] [Google Scholar]
- Schaffhauser H, Rowe BA, Morales S, Chavez-Noriega LE, Yin R, Jachec C, Rao SP, Bain G, Pinkerton AB, Vernier JM, Bristow LJ, Varney MA, Daggett LP. Pharmacological Characterization and Identification of Amino Acids Involved in the Positive Modulation of Metabotropic Glutamate Receptor Subtype 2. Molecular Pharmacology. 2003;64:798–810. doi: 10.1124/mol.64.4.798. [DOI] [PubMed] [Google Scholar]
- Schlumberger C, Pietraszek M, Gravius A, Danysz W. Effects of a positive allosteric modulator of mGluR5 ADX47273 on conditioned avoidance response and PCP-induced hyperlocomotion in the rat as models for schizophrenia. Pharmacology Biochemistry and Behavior. 2010;95:23–30. doi: 10.1016/j.pbb.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Schlumberger C, Pietraszek M, Gravius A, Klein KU, Greco S, More L, Danysz W. Comparison of the mGlu5 receptor positive allosteric modulator ADX47273 and the mGlu2/3 receptor agonist LY354740 in tests for antipsychotic-like activity. European Journal of Pharmacology. 2009a;623:73–83. doi: 10.1016/j.ejphar.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Schlumberger C, Schafer D, Barberi C, More L, Nagel J, Pietraszek M, Schmidt WJ, Danysz W. Effects of a metabotropic glutamate receptor group II agonist LY354740 in animal models of positive schizophrenia symptoms and cognition. Behavioural Pharmacology. 2009b;20:56–66. doi: 10.1097/FBP.0b013e3283242f57. [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Johnson BG, Wright RA, Salhoff CR, Mayne NG, Wu S, Cockerham SL, Paul Burnett J, Belegaje R, Bleakman D, Monn JA. LY354740 is a Potent and Highly Selective Group II Metabotropic Glutamate Receptor Agonist in Cells Expressing Human Glutamate Receptors. Neuropharmacology. 1997;36:1–11. doi: 10.1016/s0028-3908(96)00160-8. [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Marek GJ. Preclinical Pharmacology of mGlu2 / 3 Receptor Agonists: Novel Agents for Schizophrenia? Current Drug Targets-CNS & Neurological Disorders. 2002;1:215–225. doi: 10.2174/1568007024606177. [DOI] [PubMed] [Google Scholar]
- Schreiber R, Lowe D, Voerste A, De Vry J. LY354740 affects startle responding but not sensorimotor gating or discriminative effects of phencyclidine. European Journal of Pharmacology. 2000;388:R3–R4. doi: 10.1016/s0014-2999(99)00844-4. [DOI] [PubMed] [Google Scholar]
- Seeman P. Dopamine receptors and the dopamine hypothesis of schizophrenia. Synapse. 1987;1:133–152. doi: 10.1002/syn.890010203. [DOI] [PubMed] [Google Scholar]
- Seeman P. Atypical Antipsychotics: Mechanism of Action. Canadian Journal of Psychiatry. 2002;47:27–38. [PubMed] [Google Scholar]
- Seeman P. Targeting the dopamine D2 receptor in schizophrenia. Expert Opinion on Therapeutic Targets. 2006;10:515–531. doi: 10.1517/14728222.10.4.515. [DOI] [PubMed] [Google Scholar]
- Seeman P, Battaglia G, Corti C, Corsi M, Bruno V. Glutamate receptor mGlu2 and mGlu3 knockout striata are dopamine supersensitive, with elevated D2High receptors and marked supersensitivity to the dopamine agonist (+)PHNO. Synapse. 2009;63:247–251. doi: 10.1002/syn.20607. [DOI] [PubMed] [Google Scholar]
- Shalin SC, Hernandez CM, Dougherty MK, Morrison DK, Sweatt JD. Kinase Suppressor of Ras1 Compartmentalizes Hippocampal Signal Transduction and Subserves Synaptic Plasticity and Memory Formation. Neuron. 2006;50:765–779. doi: 10.1016/j.neuron.2006.04.029. [DOI] [PubMed] [Google Scholar]
- Sharma S, Kedrowski J, Rook JM, Smith RL, Jones CK, Rodriguez AL, Conn PJ, Lindsley CW. Discovery of Molecular Switches That Modulate Modes of Metabotropic Glutamate Receptor Subtype 5 (mGlu5) Pharmacology in Vitro and in Vivo within a Series of Functionalized, Regioisomeric 2- and 5-(Phenylethynyl)pyrimidines. Journal of Medicinal Chemistry. 2009;52:4103–4106. doi: 10.1021/jm900654c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear N, Gadient RA, Wilkins DE, Do M, Smith JS, Zeller KL, Schroeder P, Zhang M, Arora J, Chhajlani V. Preclinical profile of a novel metabotropic glutamate receptor 5 positive allosteric modulator. European Journal of Pharmacology. 2011 doi: 10.1016/j.ejphar.2011.02.003. In Press, Uncorrected Proof. [DOI] [PubMed] [Google Scholar]
- Spinelli S, Ballard T, Gatti-McArthur S, Richards GJ, Kapps M, Woltering T, Wichmann J, Stadler H, Feldon J, Pryce CR. Effects of the mGluR2/3 agonist LY354740 on computerized tasks of attention and working memory in marmoset monkeys. Psychopharmacology. 2005;179:292–302. doi: 10.1007/s00213-004-2126-x. [DOI] [PubMed] [Google Scholar]
- Spooren WPJM, Gasparini F, van der Putten H, Koller M, Nakanishi S, Kuhn R. Lack of effect of LY314582 (a group 2 metabotropic glutamate receptor agonist) on phencyclidine-induced locomotor activity in metabotropic glutamate receptor 2 knockout mice. European Journal of Pharmacology. 2000;397:R1–R2. doi: 10.1016/s0014-2999(00)00269-7. [DOI] [PubMed] [Google Scholar]
- Stefani MR, Moghaddam B. Activation of type 5 metabotropic glutamate receptors attenuates deficits in cognitive flexibility induced by NMDA receptor blockade. European Journal of Pharmacology. 2010;639:26–32. doi: 10.1016/j.ejphar.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson CJ, Schoepp DD. The Group II Metabotropic Glutamate Receptor Agonist (−)-2-Oxa-4-aminobicyclo[3.1.0.]hexane-4,6-dicarboxylate (LY379268) and Clozapine Reverse Phencyclidine-Induced Behaviors in Monoamine-Depleted Rats. Journal of Pharmacology and Experimental Therapeutics. 2002;303:919–927. doi: 10.1124/jpet.102.038422. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. In: The NMDA Receptor. Learning and Memory: A Comprehensive Reference. John HB, editor. Oxford: Academic Press; 2008. pp. 409–426. [Google Scholar]
- Takamori K, Hirota S, Chaki S, Tanaka M. Antipsychotic action of selective group II metabotropic glutamate receptor agonist MGS0008 and MGS0028 on conditioned avoidance responses in the rat. Life Sciences. 2003;73:1721–1728. doi: 10.1016/s0024-3205(03)00509-5. [DOI] [PubMed] [Google Scholar]
- Takatsu Y, Fujita Y, Tsukamoto T, Slusher BS, Hashimoto K. Orally active glutamate carboxypeptidase II inhibitor 2-MPPA attenuates dizocilpine-induced prepulse inhibition deficits in mice. Brain Research. 2011;1371:82–86. doi: 10.1016/j.brainres.2010.11.048. [DOI] [PubMed] [Google Scholar]
- Ugolini A, Corsi M, Bordi F. Potentiation of NMDA and AMPA responses by the specific mGluR5 agonist CHPG in spinal cord motoneurons. Neuropharmacology. 1999;38:1569–1576. doi: 10.1016/s0028-3908(99)00095-7. [DOI] [PubMed] [Google Scholar]
- Urban N, Abi-Dargham A. Neurochemical Imaging in Schizophrenia. In: Swerdlow NR, editor. Behavioral Neurobiology of Schizophrenia and Its Treatment. Vol. 4. Springer: Berlin Heidelberg; 2010. pp. 215–242. [DOI] [PubMed] [Google Scholar]
- Urwyler S. Allosteric Modulation of Family C G-Protein-Coupled Receptors: from Molecular Insights to Therapeutic Perspectives. Pharmacological Reviews. 2011;63:59–126. doi: 10.1124/pr.109.002501. [DOI] [PubMed] [Google Scholar]
- Uslaner J, Smith S, Huszar S, Pachmerhiwala R, Hinchliffe R, Vardigan J, Hutson P. Combined administration of an mGlu2/3 receptor agonist and a 5-HT 2A receptor antagonist markedly attenuate the psychomotor-activating and neurochemical effects of psychostimulants. Psychopharmacology. 2009a;206:641–651. doi: 10.1007/s00213-009-1644-y. [DOI] [PubMed] [Google Scholar]
- Uslaner JM, Parmentier-Batteur S, Flick RB, Surles NO, Lam JSH, McNaughton CH, Jacobson MA, Hutson PH. Dose-dependent effect of CDPPB, the mGluR5 positive allosteric modulator, on recognition memory is associated with GluR1 and CREB phosphorylation in the prefrontal cortex and hippocampus. Neuropharmacology. 2009b;57:531–538. doi: 10.1016/j.neuropharm.2009.07.022. [DOI] [PubMed] [Google Scholar]
- Vales K, Svoboda J, Benkovicova K, Bubenikova-Valesova V, Stuchlik A. The difference in effect of mGlu2/3 and mGlu5 receptor agonists on cognitive impairment induced by MK-801. European Journal of Pharmacology. 2010;639:91–98. doi: 10.1016/j.ejphar.2009.11.067. [DOI] [PubMed] [Google Scholar]
- van den Buuse M. Modeling the Positive Symptoms of Schizophrenia in Genetically Modified Mice: Pharmacology and Methodology Aspects. Schizophrenia Bulletin. 2010;36:246–270. doi: 10.1093/schbul/sbp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardigan JD, Huszar SL, McNaughton CH, Hutson PH, Uslaner JM. MK-801 produces a deficit in sucrose preference that is reversed by clozapine, d-serine, and the metabotropic glutamate 5 receptor positive allosteric modulator CDPPB: Relevance to negative symptoms associated with schizophrenia? Pharmacology Biochemistry and Behavior. 2010;95:223–229. doi: 10.1016/j.pbb.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Wang H, Westin L, Nong Y, Birnbaum S, Bendor J, Brismar H, Nestler E, Aperia A, Flajolet M, Greengard P. Norbin Is an Endogenous Regulator of Metabotropic Glutamate Receptor 5 Signaling. Science. 2009;326:1554–1557. doi: 10.1126/science.1178496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R, Manka JT, Rodriguez AL, Vinson PN, Niswender CM, Weaver CD, Jones CK, Conn PJ, Lindsley CW, Stauffer SR. Synthesis and SAR of centrally active mGlu5 positive allosteric modulators based on an aryl acetylenic bicyclic lactam scaffold. Bioorganic & Medicinal Chemistry Letters. 2011;21:1350–1353. doi: 10.1016/j.bmcl.2011.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EHF, Tarazi FI, Shahid M. The effectiveness of multi-target agents in schizophrenia and mood disorders: Relevance of receptor signature to clinical action. Pharmacology & Therapeutics. 2010;126:173–185. doi: 10.1016/j.pharmthera.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Wong RKS, Bianchi R, Chuang SC, Merlin LR. Group I mGluR-induced Epileptogenesis: Distinct and Overlapping Roles of mGluR1 and mGluR5 and Implications for Antiepileptic Drug Design. Epilepsy Currents. 2005;5:63–68. doi: 10.1111/j.1535-7597.2005.05207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley M, Pemberton D, Bate S, Corti C, Jones D. The mGlu2 but not the mGlu3 receptor mediates the actions of the mGluR2/3 agonist, LY379268, in mouse models predictive of antipsychotic activity. Psychopharmacology. 2008;196:431–440. doi: 10.1007/s00213-007-0974-x. [DOI] [PubMed] [Google Scholar]
- Xiong H, Brugel TA, Balestra M, Brown DG, Brush KA, Hightower C, Hinkley L, Hoesch V, Kang J, Koether GM, McCauley JP, Jr, McLaren FM, Panko LM, Simpson TR, Smith RW, Woods JM, Brockel B, Chhajlani V, Gadient RA, Spear N, Sygowski LA, Zhang M, Arora J, Breysse N, Wilson JM, Isaac M, Slassi A, King MM. 4-Aryl piperazine and piperidine amides as novel mGluR5 positive allosteric modulators. Bioorganic & Medicinal Chemistry Letters. 2010;20:7381–7384. doi: 10.1016/j.bmcl.2010.10.036. [DOI] [PubMed] [Google Scholar]
- Yoshino M, Sawada S, Yamamoto C, Kamiya H. A metabotropic glutamate receptor agonist DCG-IV suppresses synaptic transmission at mossy fiber pathway of the guinea pig hippocampus. Neuroscience Letters. 1996;207:70–72. doi: 10.1016/0304-3940(96)12486-1. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Manka JT, Rodriguez AL, Weaver CD, Days EL, Vinson PN, Jadhav S, Hermann EJ, Jones CK, Conn PJ, Lindsley CW, Stauffer SR. Discovery of N-Aryl Piperazines as Selective mGluR5 Potentiators with Improved In Vivo Utility. ACS Medicinal Chemistry Letters. 2010;1:433–438. doi: 10.1021/ml100181a. [DOI] [PMC free article] [PubMed] [Google Scholar]